Abstract
Background
Immunoglobulin A (IgA) autoantibodies to tissue transglutaminase (tTG) are commonly used for screening and diagnosing of celiac disease (CD). Seroreactivity for anti-Saccharomyces cerevisiae antibody (ASCA) and bacterial antigens have also been detected in CD patients. The aim of this study was to examine prospectively serologic responses to microbial targets in adult CD patients at the time of diagnosis and during a gluten-free diet (GFD). Further, we wanted to evaluate whether these serologic specificities could provide new tools for the follow-up of CD patients.
Methods
Data on 55 adult biopsy-proven CD patients were available for follow-up study. Upper gastrointestinal endoscopy was performed on all patients. Sera from patients were tested for antibodies to tTG and ASCA and additionally analyzed with IgA enzyme-linked immunosorbent assays to Pseudomonas fluorescens-associated sequence, I2, and to a Bacteroides caccae TonB-linked outer membrane protein, OmpW.
Results
At the time of diagnosis, 91% of CD cases were positive for tTG and 49% for ASCA; positive seroreactivity to I2 was found in 86% and to OmpW in 60% of CD patients at the time of diagnosis. The frequency of seropositivity and serum levels of these antibodies decreased during GFD. Moreover, we found that the decline in the serum levels was significant in all of these markers (p<0.005). Interestingly, we also found that serum levels of ASCA correlated with the grade of mucosal morphology (p=0.021), as the ASCA serum levels declined in accordance with mucosal healing.
Conclusions
Commensal enteric bacteria seem to play a role in the small intestinal mucosal damage in CD. This was proven by the serological responses to different microbial antigens shown in this study. Serum levels of ASCA, anti-I2, and anti-OmpW antibodies decreased significantly during GFD, indicating that these serologic markers are gluten dependent in CD patients. These specificities could provide new tools in the follow-up of CD patients.
Keywords: Celiac disease, gluten-free diet, ASCA, I2, OmpW
Introduction
Celiac disease (CD) is characterized as a gluten-induced disease with manifest small bowel mucosal damage with villous shortening, crypt hyperplasia, and inflammation recovering on a gluten-free diet (GFD) [1–3]. The presence of distinct autoantibodies is typical for the condition. Immunoglobulin A (IgA) autoantibodies to tissue transglutaminase (tTG) are commonly used for the screening and diagnosing of CD [4–7]. Recently, the Saccharomyces cerevisiae antibody (ASCA) positivity was also observed in CD patients [8–9]. Commensal luminal bacteria are involved in activating dysregulated mucosal immune responses [10]. We have recently pointed out that the majority of CD patients are seropositive for Pseudomonas fluorescens-associated sequence I2 and to a Bacteroides caccae TonB-linked outer membrane protein, OmpW [11]. These findings reveal that CD patients do indeed express distinct mosaics of anti-microbial serology, which supports an unexpected contribution of anti-microbial host responses in CD pathogenesis.
tTG antibodies are valuable in the follow-up of CD as antibody levels decline with GFD [12–14]. Previously, it has been shown that with GFD, ASCA positivity disappeared in most of the CD cases, and the phenomenon was more pronounced in children [15]. By contrast, Toumi et al. did not find a statistically significant difference in ASCA frequency between untreated and treated CD patients [16].
The aim of this study was to assess ASCA and host serologic responses to additional bacterial targets (the P. fluorescens-associated sequence I2 and to a B. caccae TonB-linked outer membrane protein, OmpW) in adult CD patients at the time of diagnosis and during GFD. In addition, we wanted to evaluate whether these serologic specificities could provide new tools in the follow-up of CD patients and whether they correlated with the mucosal morphology.
Materials and Methods
Patients
Fifty-five adult CD patients (43 women, 12 men, median age; 44.0 years, range 21.0–68.0 years) referred to the Department of Gastroenterology and Alimentary Tract Surgery, Tampere University Hospital during the period 1998–1999 were available for the follow-up study. Upper gastrointestinal endoscopies with multiple biopsies from the duodenum at the time of diagnosis and during GFD were performed to all. At the same time, sera were collected for antibody testing at the time of primary diagnosis and at follow-up. Mean duration of the follow-up period was 349 days (SD 41 days). The CD diagnosis was based on small bowel mucosal severe partial or subtotal villous atrophy with crypt hyperplasia [1].
Serum Antibody Tests
Sera from patients were tested for antibodies to tTG (Celikey tTG IgA, Phadia, Freiburg, Germany) and/or endomysium antibodies (Ema) [17] and concentrations 1:≥5 U/ml were considered positive. An enzyme immunoassay (EIA) kit (QUANTA Lite™ ASCA, INOVA Diagnostics, San Diego, CA, USA) was used for the determination of ASCA of both IgG and IgA isotypes from the sera. The kit included positive and negative controls and was used according to the manufacturer’s instructions. Quantitative results in arbitrary EIA units were obtained from standard curves defined by the manufacturer, but the results were statistically handled as qualitative. Ambiguous and borderline results were interpreted as negative. Results exceeding 25 U for IgG or IgA ASCA were regarded as positive.
Sera for the determination of anti-I2 and anti-OmpW IgA levels were stored at −70°C until testing. In our laboratory, Escherichia coli XL-1-blue and E. coli BL-21 (Stratagene, La Jolla, CA, USA) strains were used for all cloning and recombinant expression experiments. I2-GST and OmpW were produced by using previously reported antigen purification techniques [18, 19]. Sera were analyzed with IgA enzymelinked immunosorbent assays (ELISA) to I2 and OmpW. The cut-off level for positivity in the IgA-class ELISA test was set at 0.5 (for I2-GST) and at 1.0 (for OmpW) [11, 19].
Biopsy Specimen Processing and Immunohistochemical Staining
The biopsy specimen was processed by routine histological methods, and sections of formalin-fixed and paraffin-embedded specimens were stained with hematoxylin and eosin. Small bowel mucosal morphology (ratio of villous height and crypt depth) was determined in all patients as previously described [20].
Statistical Analysis
Optical densities of antibody ELISA tests were expressed as means with 95% confidence intervals of mean. Other continuous variables were expressed as medians with range due to the skewed distribution and tested by Wilcoxonsigned ranks test. The differences between categorical variables were tested using McNemar test or Fisher’s exact test. The associations between the change in serum antibody titers and duration of GFD were tested by Spearman correlation test. Statistical calculations were carried out with SPSS for Windows (version 14.0.2; SPSS, Chicago IL, USA).
Ethical Considerations
The study protocol was approved by the Ethical Committee of Tampere University Hospital, and informed consent was obtained from the patients.
Results
Of the CD patients, 91% were positive for tTG antibodies and 49% for ASCA at the time of diagnosis. The respective percentages for seroreactivity for bacterial antigens I2 and OmpW were the majority of CD patients (86% and 60%, respectively).
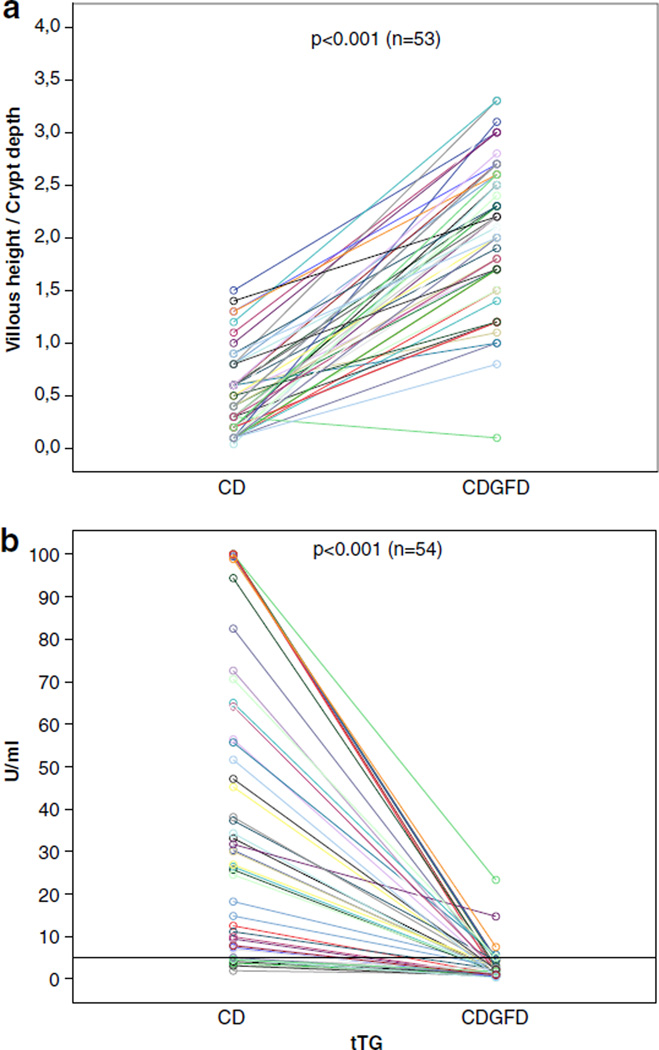
The response to gluten-free diet was evident in the majority of our patients. The small intestinal villous height and crypt depth ratio increased, and serum tTG antibody titers declined in line with mucosal recovery (Fig. 1).
Fig. 1.
Mucosal healing in 55 adult celiac disease patients during a GFD according to small bowel mucosal morphology (a) and the levels of serum tissue transglutaminase antibodies (b)
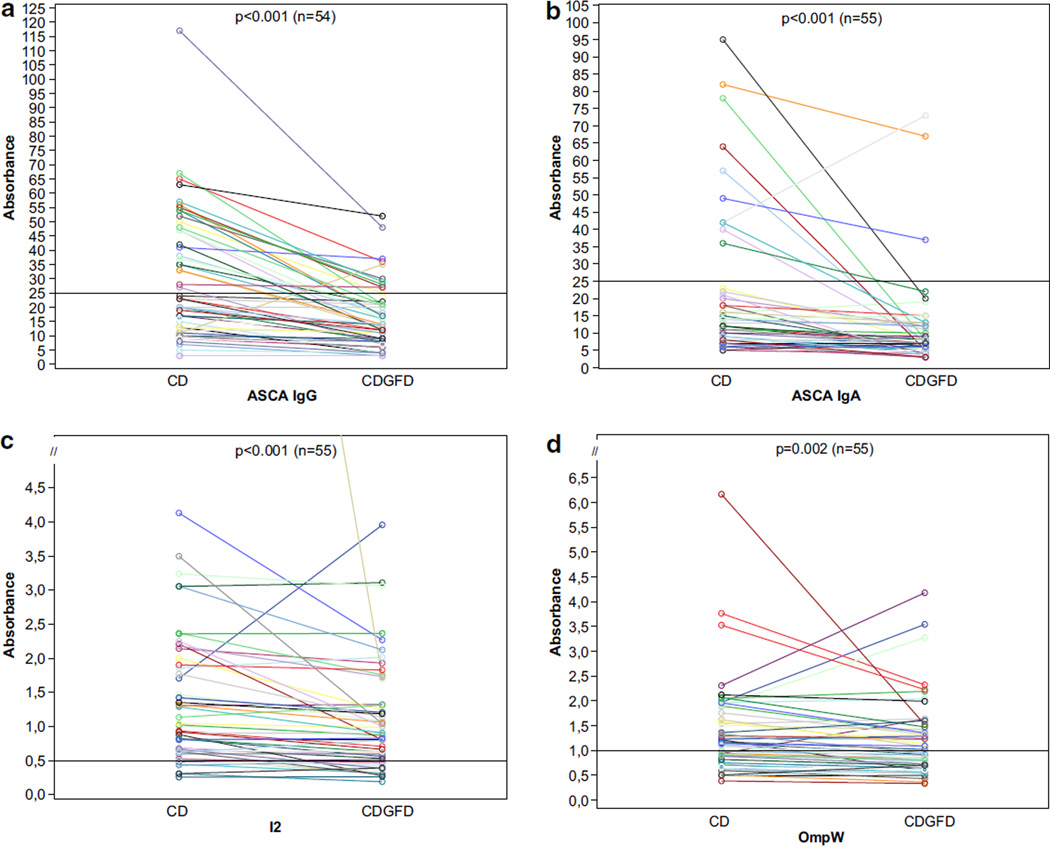
Serum IgA and IgG ASCA and serum anti-I2 and anti-OmpW levels (median absorbances) decreased significantly during GFD (follow-up period 4–14 months; Fig. 2). The frequencies of positive seroreactivity also declined during GFD (Table I). However, we found no association between the decrease in serum antibody titers and duration of GFD, and no statistical difference was found either when making the analysis according to gender. The frequency of ASCA positivity was associated with mucosal morphology (p=0.021), and the ratio of villous height and crypt depth declined in line with rising ASCA positivity. No similar association between seropositivity of bacterial antigens (I2 and OmpW) and mucosal morphology was observed (p=0.464 and p=0.334, respectively).
Fig. 2.
Serological responses to microbial antigens (IgG and IgA ASCA, I2, and OmpW) in celiac disease (CD) patients during a gluten-free diet (GFD). Horizontal lines denote the cut-off value for seropositivity (see text). p values compare median absorbances at the time of diagnosis and after follow-up period in CD patients, using the Mann–Whitney U test
Table I.
The Frequency of Positive Seroreactivity to Tissue Transglutaminase (tTG) Anti-Saccharomyces cerevisiae (ASCA), I2, and OmpW among Patients (55) with Celiac Disease at the Time of Diagnosis and After Gluten-free Diet
| CD at the time of dg | CD GFD | p value | |
|---|---|---|---|
| tTG | 50/55 (90.9%) | 7/54 (13.0%) | <0.001 |
| ASCA IgA and/or IgG | 27/55 (49.1%) | 12/55 (21.8%) | <0.001 |
| I2 | 47/55 (85.5%) | 41/55 (74.5%) | 0.070 |
| OmpW | 35/55 (60%) | 27/55 (49.1%) | 0.070 |
Discussion
Here, we report different microbial and autoantibodies in 55 adult CD patients during GFD. This is the first study assessing the serum anti-I2 and anti-OmpW levels in CD patients during GFD. Interestingly, the serum levels of I2 and OmpW (median absorbances) declined significantly during GFD. However, frequency of I2 and OmpW positivity was not associated with the grade of villous morphology. The measurement of serological responses to I2 and OmpW may provide new valuable information on the pathogenesis of CD and tools in the follow-up of small intestinal damage and CD.
Previously, in CD patients, ASCA positivity was shown to be evident in up to 40–60% of cases [8, 9, 11]. In addition, we showed previous that serological responses to bacterial antigens, the P. fluorescens-associated sequence I2 and to a B. caccae TonB-linked outer membrane protein OmpW, are significantly elevated not only in IBD patients but, interestingly, also in CD patients [11, 18]. These findings may provide new evidence for a contribution of such responses to the pathogenetic mechanisms of inflammation of CD [11]. In the present study, tTG antibodies decreased during GFD, which addresses the specificity of tTG for follow-up as previously described [12, 13]. Controversial reports concerning behavior of ASCA during GFD have been published [15, 16]. Our finding about frequency of ASCA positivity decreasing significantly is in agreement with the results of previous studies, which showed that ASCA positivity disappeared in most CD cases with GFD [15]. However, in contrast to their conclusions, we also found a correlation between serum levels of ASCA and the grade of mucosal morphology.
Seroreactivity to microbial components in Crohn’s disease has been shown to be associated with disease severity and progression [21–24]. Furthermore, IBD patients with the highest levels of serum reactivity toward an increasing number of microbiota have been reported to show the greatest frequency of complications [21]. As in IBD, our earlier findings in CD suggest that immune responses to commensal enteric bacteria may play a role in inducing mucosal damage [11]. If this is the case, microbial seroreactivity may correlate with immune reaction and inflammation of the small intestine in CD patients or patients with other intestinal symptoms [11]. Altered permeability in the small intestine could explain the frequent detection of seroreactivity for different microbial antigens in patients with CD [25–30].
As a conclusion, on the present results, we suggest that commensal enteric bacteria play a role in the small intestinal mucosal damage of CD. This was proven by the serological responses to different microbial antigens shown in this study. Serum levels of ASCA, anti-I2, and anti-OmpW antibodies decreased significantly during GFD; ASCA positivity was also associated with grade of villous morphology. This indicates that these serologic specificities could in the future help in the non-invasive evaluation of small intestinal mucosal damage at the time of diagnosis and during GFD.
Acknowledgments
This study was supported by grants from the Paediatric Research Foundation, The Competitive Research Funding of the Pirkanmaa Hospital District, and NIH PO1-DK 46763 (J.B.). The authors would like to acknowledge Ms Leena Ripsaluoma for excellent assistance in recruitment of the patients and Ms Kaija Laurila and Ms Marja-Leena Koskinen for technical support.
Contributor Information
Sara Ashorn, Paediatric Research Centre and Medical School, University of Tampere and Tampere University Hospital, Tampere, Finland.
Tuuli Välineva, Institute of Medical Technology, University of Tampere, Tampere, Finland.
Katri Kaukinen, Paediatric Research Centre and Medical School, University of Tampere and Tampere University Hospital, Tampere, Finland; Department of Gastroenterology and Alimentary Tract Surgery, Tampere University Hospital, Tampere, Finland.
Merja Ashorn, Paediatric Research Centre and Medical School, University of Tampere and Tampere University Hospital, Tampere, Finland; Department of Paediatrics, Tampere University Hospital, Tampere, Finland.
Jonathan Braun, Department of Pathology and Laboratory Medicine, University of California, Los Angeles, CA, USA.
Hanna Raukola, Department of Microbiology, Tampere University Hospital, Tampere, Finland.
Immo Rantala, Department of Pathology, Tampere University Hospital, Tampere, Finland.
Pekka Collin, Department of Gastroenterology and Alimentary Tract Surgery, Tampere University Hospital, Tampere, Finland.
Markku Mäki, Paediatric Research Centre and Medical School, University of Tampere and Tampere University Hospital, Tampere, Finland; Department of Paediatrics, Tampere University Hospital, Tampere, Finland.
Tiina Luukkaala, Science Center, Pirkanmaa University Hospital District, Tampere, Finland; Tampere School of Public Health, University of Tampere, Tampere, Finland.
Sari Iltanen, Email: sari.iltanen@uta.fi, Paediatric Research Centre and Medical School, University of Tampere and Tampere University Hospital, Tampere, Finland.
References
- 1.Walker-Smith JA, Guandalini S, Schmitz J. Revised criteria for diagnosis of coeliac disease. Arch Dis Child. 1990;65:909–911. doi: 10.1136/adc.65.8.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mäki M, Holm K, Koskimies S, Hällström O, Visakorpi JK. Normal small bowel biopsy followed by coeliac disease. Arch Dis Child. 1990;65:1137–1141. doi: 10.1136/adc.65.10.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marsh MN. Gluten major histocompatibility complex, the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’) Gastroenterology. 1992;102:330–354. [PubMed] [Google Scholar]
- 4.Dieterich W, Ehnis T, Bauer M, Donner P, Volta U, Riecken EO, et al. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med. 1997;3:797–801. doi: 10.1038/nm0797-797. [DOI] [PubMed] [Google Scholar]
- 5.Troncone R, Maurano F, Rossi M, Micillo M, Greco L, Auricchio R, et al. IgA antibodies to tissue transglutaminase: an effective diagnostic test for celiac disease. J Pediatr. 1999;134:166–171. doi: 10.1016/s0022-3476(99)70410-5. [DOI] [PubMed] [Google Scholar]
- 6.Salmi TT, Collin P, Järvinen O, Haimila K, Partanen J, Laurila K, et al. Immunoglobulin A autoantibodies against transglutaminase 2 in the small intestinal mucosa predict forthcoming celiac disease. Aliment Pharmacol Ther. 2006;24:541–552. doi: 10.1111/j.1365-2036.2006.02997.x. [DOI] [PubMed] [Google Scholar]
- 7.Nemec G, Ventura A, Stefano M, Di Leo G, Baldas V, Tommasini A, et al. Looking for celiac disease: diagnostic accuracy of two rapid commercial assays. Am J Gastroenterol. 2006;101:1597–1600. doi: 10.1111/j.1572-0241.2006.00597.x. [DOI] [PubMed] [Google Scholar]
- 8.Damoiseaux JG, Bouten B, Linders AM, Austen J, Roozendaal C, Russel MG, et al. Diagnostic value of anti-Saccharomyces cerevisiae and antineutrophil cytoplasmic antibodies for inflammatory bowel disease: high prevalence in patients with celiac disease. J Clin Immunol. 2002;22:281–288. doi: 10.1023/a:1019926121972. [DOI] [PubMed] [Google Scholar]
- 9.Granito A, Zauli D, Muratori P, Muratori L, Grassi A, Bortolotti R, et al. Anti-saccaharomyces cerevisiae and perinuclear antineutrophil cytoplasmic antibodies in coeliac disease before and after gluten-free diet. Aliment Pharmacol Ther. 2005;21:881–887. doi: 10.1111/j.1365-2036.2005.02417.x. [DOI] [PubMed] [Google Scholar]
- 10.Sartor RB. Induction of mucosal immune responses by bacteria and bacterial components. Curr Opin Gastroenterol. 2001;17:555–561. doi: 10.1097/00001574-200111000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Ashorn S, Raukola H, Välineva T, Ashorn M, Wei B, Braun J, et al. Elevated serum anti-Saccharomyces Cerevisiae, anti-I2 and anti-OmpW antibody levels in Patients with suspicion of celiac disease. J Clin Immunol. 2008;28:486–494. doi: 10.1007/s10875-008-9200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bürgin-Wolff A, Dahlbom I, Hadziselimovic F, Petersson CJ. Antibodies against human tissue transglutaminase and endomysium in diagnosing and monitoring coeliac disease. Scand J Gastroenterol. 2002;37:685–691. doi: 10.1080/00365520212496. [DOI] [PubMed] [Google Scholar]
- 13.Di Domenico MR, Annaluisa S, Pluvio R, Iovine C, Rea F. The role of anti-endomysium and anti-transglutaminase antibodies in the diagnosis and follow-up of celiac disease. Pediatr Med Chir. 2002;24:208–212. [PubMed] [Google Scholar]
- 14.Kaukinen K, Collin P, Laurila K, Kaartinen T, Partanen J, Mäki M. Resurrection of gliadin antibodies in coeliac disease. Deamidated gliadin peptide antibody test provides additional diagnostic benefit. Scand J Gastroenterol. 2007;19:1–6. doi: 10.1080/00365520701452217. [DOI] [PubMed] [Google Scholar]
- 15.Mallant-Hent RCh, Mary B, von Blomberg E, Yüksel Z, Wahab PJ, Gundy C, et al. Disappearance of anti-Saccharomyces cerevisiae antibodies in coeliac disease during gluten-free diet. Eur J Gastroenterol Hepatol. 2006;18:75–78. doi: 10.1097/00042737-200601000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Toumi D0, Mankaï A, Belhadj R, Ghedira-Besbes L, Jeddi M, Ghedira I. Anti-Saccharomyces cerevisiae antibodies in coeliac disease. Scand J Gastroenterol. 2007;42:821–826. doi: 10.1080/00365520601154996. [DOI] [PubMed] [Google Scholar]
- 17.Collin P, Kaukinen K, Vogelsang H, Korponay-Szabó I, Sommer R, Schreier E, et al. Antiendomysial and antihuman recombinant tissue transglutaminase antibodies in the diagnosis of coeliac disease: a biopsy-proven European multicentre study. Eur J Gastroenterol Hepatol. 2005;17:85–91. doi: 10.1097/00042737-200501000-00017. [DOI] [PubMed] [Google Scholar]
- 18.Amundsen SS, Adamovic S, Hellqvist A, Nilsson S, Gudjónsdóttir AH, Ascher H, et al. A comprehensive screen for SNP associations on chromosome region 5q31-33 in Swedish/Norwegian celiac disease families. Eur J Hum Genet. 2007;15:980–987. doi: 10.1038/sj.ejhg.5201870. [DOI] [PubMed] [Google Scholar]
- 19.Landers CJ, Cohavy O, Misra R, Yang H, Lin YC, Braun J, et al. Selected loss of tolerance evidenced by Crohn’s disease-associated immune responses to auto- and microbial antigens. Gastroenterology. 2002;123:689–699. doi: 10.1053/gast.2002.35379. [DOI] [PubMed] [Google Scholar]
- 20.Holm K, Maki M, Savilahti E, Lipsanen V, Laippala P, Koskimies S. Intraepithelial gamma delta T-cell-receptor lymphocytes and genetic susceptibility to coeliac disease. Lancet. 1992;339:1500–1503. doi: 10.1016/0140-6736(92)91262-7. [DOI] [PubMed] [Google Scholar]
- 21.Papp M, Norman GL, Altorjay I, Lakatos PL. Utility of serological markers in inflammatory bowel diseases: gadget or magic? World J Gastroenterol. 2007;13:2028–2036. doi: 10.3748/wjg.v13.i14.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papp M, Altorjay I, Norman GL, Shums Z, Palatka K, Vitalis Z, et al. Seroreactivity to microbial components in Crohn’s disease is associated with ileal involvement, noninflammatory disease behavior and NOD2/CARD15 genotype, but not with risk for surgery in a Hungarian cohort of IBD patients. Inflamm Bowel Dis. 2007;13:984–992. doi: 10.1002/ibd.20146. [DOI] [PubMed] [Google Scholar]
- 23.Arnott ID, Landers CJ, Nimmo EJ, Drummond HE, Smith BK, Targan SR, et al. Sero-reactivity to microbial components in Crohn’s disease is associated with disease severity and progression, but not NOD2/CARD15 genotype. Am J Gastroenterol. 2004;99:2376–2384. doi: 10.1111/j.1572-0241.2004.40417.x. [DOI] [PubMed] [Google Scholar]
- 24.Mow WS, Vasiliauskas EA, Lin YC, Fleshner PR, Papadakis KA, Taylor KD, et al. Association of antibody responses to microbial antigens and complications of small bowel Crohn’s disease. Gastroenterology. 2004;126:414–424. doi: 10.1053/j.gastro.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 25.Peeters M, Geypens B, Claus D, Nevens H, Ghoos Y, Verbeke G, et al. Clustering of increased small intestinal permeability in families in Crohn’s disease. Gasteroenterology. 1997;113:802–807. doi: 10.1016/s0016-5085(97)70174-4. [DOI] [PubMed] [Google Scholar]
- 26.Clemente MG, De Virgiliis S, Kang JS, Macatagney R, Musu MP, Di Pierro MR, et al. Early effects of gliadin on enterocyte intracellular signalling involved in intestinal barrier function. Gut. 2003;52:218–223. doi: 10.1136/gut.52.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaila B, Orr K, Bernstein CN. The anti-Saccharomyces cerevisiae antibody assay in a province-wide practice: accurate in identifying cases of Crohn’s disease and predicting inflammatory disease. Can J Gastroenterol. 2005;19:717–721. doi: 10.1155/2005/147681. [DOI] [PubMed] [Google Scholar]
- 28.van Elburg RM, Uil JJ, Mulder CJ, Heymans HS. Intestinal permeability in patients with coeliac disease and relatives of patients with coeliac disease. Gut. 1993;34:354–357. doi: 10.1136/gut.34.3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sander GR, Cummins AG, Henshall T, Powell BC. Rapid disruption of intestinal barrier function by gliadin involves altered expression of apical junctional proteins. FEBS Lett. 2005;579:4851–4855. doi: 10.1016/j.febslet.2005.07.066. [DOI] [PubMed] [Google Scholar]
- 30.Ciccocioppo R, Finamore A, Ara C, Di Sabatino A, Mengheri E, Corazza GR. Altered expression, localization, and phosphorylation of epithelial junctional proteins in celiac disease. Am J Clin Pathol. 2006;125:502–511. doi: 10.1309/DTYR-A91G-8R0K-TM8M. [DOI] [PubMed] [Google Scholar]