Abstract
Flagellin is a highly effective adjuvant, but the cellular mechanism underlying this activity remains uncertain. More specifically, no consensus exists as to whether flagellin activates dendritic cells (DC) directly or indirectly. Intramuscular immunization with flagellin-OVA fusion protein resulted in enhanced in vivo T cell clustering in draining lymph nodes and IL-2 production by OVA-specific CD4+ T cells. Immunization with flagellin-OVA also triggered greater levels of Ag-specific CD4+ T cell proliferation than immunization with flagellin and OVA as separate proteins. To determine whether flagellin, in the context of a fusion protein with OVA, was acting directly on DC, we used a combination of CD4+ T cell adoptive transfers and bone marrow chimera mice in which the presence or absence of potential tlr5+/+ CD11c+ cells was controlled by injection of diphtheria toxin. The Ag-specific CD4+ T cell response in mice with CD11c+ cells from a tlr5−/− background and mixed populations of all other hematopoietic cells was dramatically reduced in comparison to mice that had DC from tlr5−/− and wild-type backgrounds. Immunization of MyD88−/−tlr5+/+ mice revealed that the enhanced response following immunization with flagellin-OVA is dependent on signaling via the TLR5-MyD88 pathway as well as enhanced Ag uptake and processing resulting from Ag targeting via TLR5. In summary, our data are consistent with the conclusion that direct stimulation of tlr5+/+ CD11c+ cells is necessary for the adjuvant activity of a flagellin fusion protein and that this adjuvant effect requires signaling through TLR5.
Flagellin, the major structural protein of bacterial flagella (1) and the ligand for TLR5 (2–4), is a potent systemic and mucosal adjuvant (5–13). Although the adjuvant activity of flagellin is widely accepted to stem from its ability to induce dendritic cell (DC)3 maturation (10, 14–16), the mechanism, either direct or indirect, has not been demonstrated. Indeed, some researchers have concluded that flagellin does not exert a direct effect on DC (15, 17).
Numerous studies have examined the effect of treatment with flagellin on DC. Didierlaurent et al. (14) demonstrated that incubation of splenic and bone marrow-derived murine DC (BMDC) with flagellin results in modest up-regulation of MHC class II, CD86, CD80, and CD40 and that immunization of mice adoptively transferred with OVA-specific CD4+ T cells with flagellin and OVA promoted proliferation of OVA-specific cells. Ablation of CD80 or CD86 expression reduced the ability of murine DC to promote Ag-specific CD4+ T cell proliferation and Ab production following immunization with flagellin (10). These findings support the hypothesis that flagellin activates DC and that DC are a crucial cell type in the cellular mechanism of flagellin adjuvant activity, but these findings do not directly address the route of activation.
Several groups have reported that flagellin can promote the activation of myeloid-derived human DC in vitro (15, 18–20). Although Means et al. (15) found that flagellin could promote the in vitro activation of human DC, they also reported that incubation of murine BMDC with flagellin did not trigger up-regulation of CD80, CD86, or CCR7 and concluded that flagellin does not mature murine DC. In vivo experiments examining the effect of i.v. injection of flagellin revealed slight up-regulation of CD80 and CD40 expression by CD11b+ and CD8α+ splenic DC as well as substantial up-regulation of CD86 expression by CD8α+ DC (17). On the basis of this finding and the observation that splenic DC from mice that were irradiated and reconstituted with MyD88−/−bone marrow showed reduced modulation of CD80, CD86, and CD40 expression following i.v. injection of flagellin, Salazar-Gonzalez et al. (17) concluded that the in vivo effect of flagellin on DC occurs through an indirect mechanism.
To date, no published study has demonstrated an ability of flagellin to directly stimulate DC in vivo. Experiments on in vitro-generated, BMDC are potentially a direct test, but the weak response generated in these cells raises the possibility that BMDC are not sufficiently differentiated to have acquired flagellin responsiveness. Additionally, some studies have used concentrations of flagellin that are far in excess of what other have found to be required for a maximal response (10−10 M). Consequently, the responses in these studies may result from stimulation with contaminating endotoxins or nucleic acids. In view of these shortcomings, we developed an experimental model to determine the requirement for direct stimulation of tlr5+/+ DC in the in vivo immune response.
Materials and Methods
Mice
C57BL/6, CD11c-diptheria toxin receptor (DTR)/GFP (21), and B6.PL-Thyla (CD90.1+) mice were obtained from The Jackson Laboratory. TCR transgenic OT-II mice (22), which recognize residues 323–339 of chicken OVA in the context of I-Ab, were provided by Dr. E. Hiltbold (Wake Forest University School of Medicine). MyD88−/− (23) and tlr5−/− (24) mice have been described previously. All mice were housed in the Wake Forest University School of Medicine animal facility in accordance with institutional and U.S. Department of Agriculture guidelines. All mouse experiments were approved by the Institutional Animal Care and Use Committee.
Immunogens
Recombinant his-tagged Salmonella FliC (flagellin) and the nonsignaling flagellin truncation 229 were produced as described previously (9, 25). Recombinant his-tagged flagellin-OVA fusion protein was produced by replacing the hypervariable region of flagellin with OVA and expressing the fusion protein in E. coli using a pET29a expression vector. All recombinant proteins were purified using a metal-affinity resin and Acrodisc Mustang Q and E membranes (Pall), which remove nucleic acids and endotoxins. Contaminating endotoxin levels were verified to be <30 pg LPS/µg protein by Limulus amebocyte lysate assay (Associates of Cape Cod). Activity of flagellin and flagellin-OVA were verified by measuring TNF-α production in cultures of RAW424 cells stably transfected with mouse TLR5 (26). OVA was purchased from Sigma-Aldrich. Contaminating nucleic acids and endotoxins were removed from the OVA by treatment with Acrodisc Mustang Q and E membranes.
BMDC cultures
Bone marrow was harvested from the femurs and tibias of C57BL/6 mice. RBC were lysed with ammonium chloride lysing buffer (Bio-Whittaker), and the bone marrow cells were plated in a 24-well plates at a density of 5 × 105 cells/well. Cells were cultured in recombinant mouse GM-CSF for 6 days with media changes on days 2, 4, and 5. On day 6, cells were harvested, counted, and replated in a 48-well plate at 5 × 105 cells/well. Cells were stimulated with flagellin, flagellin 229, flagellin-OVA, E. coli LPS (Sigma-Aldrich), or poly(I:C) (InvivoGen). Twenty-four hours later, supernatant was harvested for analysis by ELISA using the OptEIA kit IL-6 (BD Biosciences). Cells were harvested for flow cytometric analysis of CD80 (16-10A1) and CD86 (GL1) expression using Abs from BD Biosciences.
Generation of bone marrow chimeras and OT-II adoptive transfers
Six- to 8-wk-old, wild-type, female C57BL/6 mice received whole-body irradiation of 900 rad from a 127Cs irradiator and then were injected with 1 × 106 bone marrow cells from CD11c-DTR/GFP mice (21) and 1 × 106 bone marrow cells from wild-type or tlr5−/− mice. All donor and recipient mice were sex-matched. Bone marrow chimera mice were maintained on acid water (pH 2.7) for 4 wk following irradiation. Twelve weeks following bone marrow cell transfer, chimera mice were injected via the tail vein with 3 × 106 CFSE-labeled OT-II cells. Twenty-four hours following OT-II cell transfer, chimera mice were injected with 10 ng of diphtheria toxin (DT) per gram of body weight. This dose of toxin resulted in elimination of 87% of splenic CD11c+ cells in CD11c-DTR/GFP mice (data not shown). Eighteen hours after injection of DT, adoptive transfer recipient mice were immunized by i.m. route with 1 × 10−11 mol (0.8 µg) of flagellin-OVA. CD4+ T cells were enriched by negative selection before adoptive transfer in all experiments using bone marrow chimera mice, MyD88−/−, and tlr5−/− mice. Immunizations were performed 1 day following cell transfer for all experiments except ones in which mice were treated with DT.
Immunofluoresence
Tissue samples were prepared as described previously (11). OT-II cells were identified on the basis of CD90.1 expression using the anti-CD90.1FITC (OX-7 from BD Biosciences) and rabbit anti-FITCAF594 (In-vitrogen) Abs. DC were revealed by staining with the CD11c-specific Ab N418 directly conjugated to AF647 (Biolegend), and CD4+ cells were identified by staining with RMN-4 directly conjugated to AF488 (BD Biosciences). CD19+ cells were identified with the mAb 1D3, and CD3+ cells were identified with 145-2C11 (from BD Biosciences). Slides were imaged using a Nikon Eclipse TE300 microscope and a Retiga EX camera. Overlays were composed using Adobe PhotoShop 7.0, and cell counts were performed using ImageJ.
Flow cytometric analysis
CFSE labeling was performed by incubating 2.5 × 106 cells/ml serum-free PBS containing 2 µM CFSE (Invitrogen) for 10 min at room temperature. Adoptively transferred OT-II cells were discriminated on the basis of CD90.1 (OX-7) and CD4 (RM4-5) expression. For restimulation experiments, lymph node cell suspensions generated from the draining lymph nodes were restimulated in vitro with 30 µg/ml OVA323–339 in RPMI 1640 with 10% FBS for 5 h. Brefeldin A (BD Biosciences) was added for the last 2.5 h of culture. Staining for IL-2 was performed using cytofix/cytoperm solution (BD Biosciences) following by staining with mouse IL-2-specific Ab (JES6-5H4). Data were analyzed using FloJo 7.2.5 (Tree Star). Absolute cell numbers were determined by flow cytometric counting (27).
Statistics
Statistical analysis of data was performed with SigmaStat 3.10 (Systat Software) or GraphPad Prism 5 for Windows (GraphPad Software). For normally distributed data sets, significance was determined using the Student’s t test. The significance of data sets, which were not normally distributed or of unequal variance, was determined using the Mann-Whitney rank-sum test. Values of p < 0.05 were considered significant. Error bars represent the SEM.
Results
In vitro stimulation of BMDC
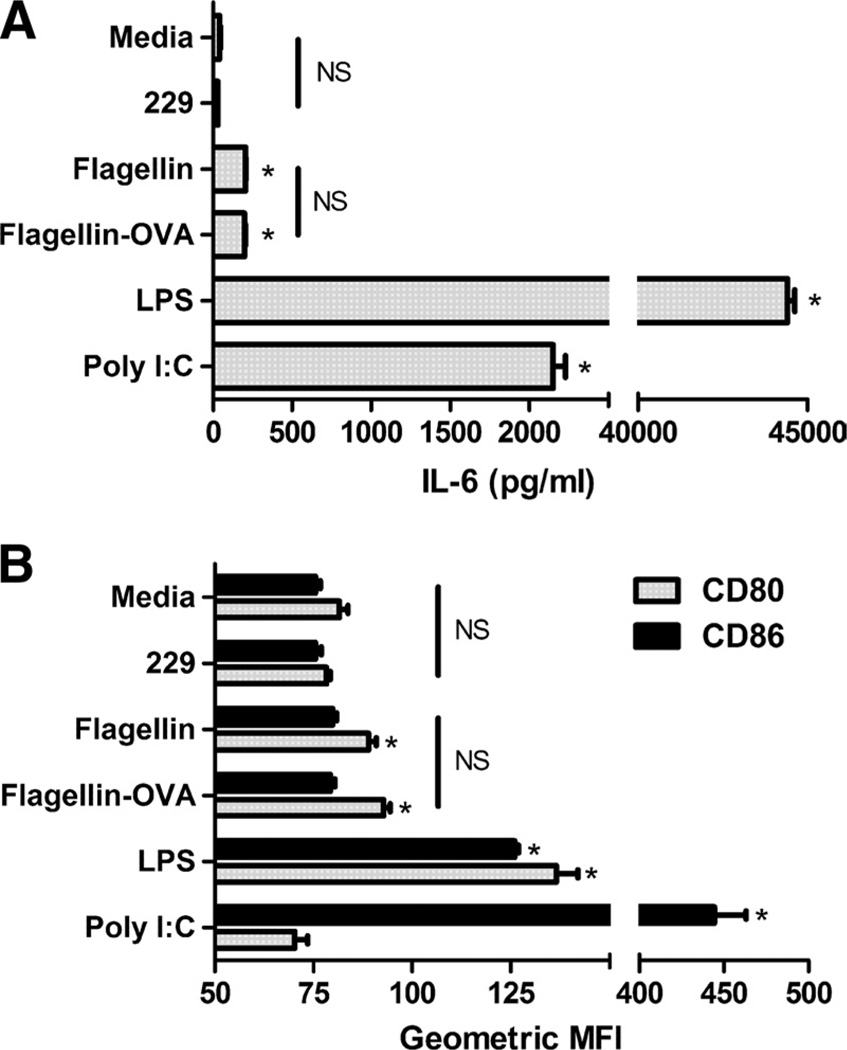
Since conflicting evidence regarding the effect of flagellin on BMDC might be due to contaminants in the flagellin preparations used in some of these studies, we first determined that our flagellin and flagellin-OVA did not signal in a TLR5-independent fashion. Incubation of TLR5-negative RAW 264.7 cells with flagellin or flagellin-OVA over a broad concentration range did not result in TNF-α production (data not shown; see also Refs. 13 and 26). Having established that our proteins did not contain stimulatory factors other than flagellin, we proceeded with experiments on the effect of flagellin on BMDC stimulated in vitro. This approach has the benefit of eliminating the effect of non-DC cell types on DC activation. Bone marrow cells were cultured for 6 days with murine GM-CSF before stimulation with flagellin (10−9 M), flagellin-OVA (10−9 M), inactive flagellin 229 (10−9 M), LPS (250 ng/ml), or poly(I:C) (25 µg/ml). BMDC stimulated with flagellin or flagellin-OVA produced equivalent and extremely low levels of IL-6 compared with BMDC stimulated with the other TLR agonists but slightly higher than with media or flagellin 229 (p < 0.001) (Fig. 1A). Stimulation with flagellin or flagellin-OVA resulted in slight up-regulation of CD80 but not CD86. As with cytokine production, the effect on expression of CD80 and CD86 was less than that seen following stimulation with other TLR agonists, although stimulation with poly(I: C) did not effect expression of CD80 (Fig. 1 B). These results are consistent with either of two hypotheses. Flagellin activates DC in vivo by an indirect mechanism, or alternatively, flagellin can directly activate DC in vivo but that in vitro-generated DC have not reached the point at which they have acquired flagellin responsiveness—either for lack of TLR5 expression or required intracellular signaling components. Since low levels of IL-6 production and slight up-regulation of CD80 and CD86 were observed (Fig. 1), it is likely that the cultures do indeed contain flagellin-responsive cells, but they represent only a small fraction of the total cell population. In either case, BMDC are clearly a poor model for studying the effect of flagellin on DC.
FIGURE 1.
Flagellin triggers low-level IL-6 production and slight up-regulation of CD80 by BMDC. Bone marrow cells were cultured for 6 days in GM-CSF, before replating and stimulation with flagellin 229 (10−9 M), flagellin (10−9 M), flagellin-OVA (10−9 M), LPS (250 ng/ml), or poly(I:C) (25 µg/ml). A, IL-6 production following in vitro stimulation of BMDC. B, Modulation of CD80 and CD86 following in vitro stimulation of BMDC. *, p < 0.05 compared with flagellin 229 control. NS indicates no statistically significant difference between conditions.
Immunization with flagellin-OVA promotes clustering and IL-2 production by Ag-specific CD4+ T cells
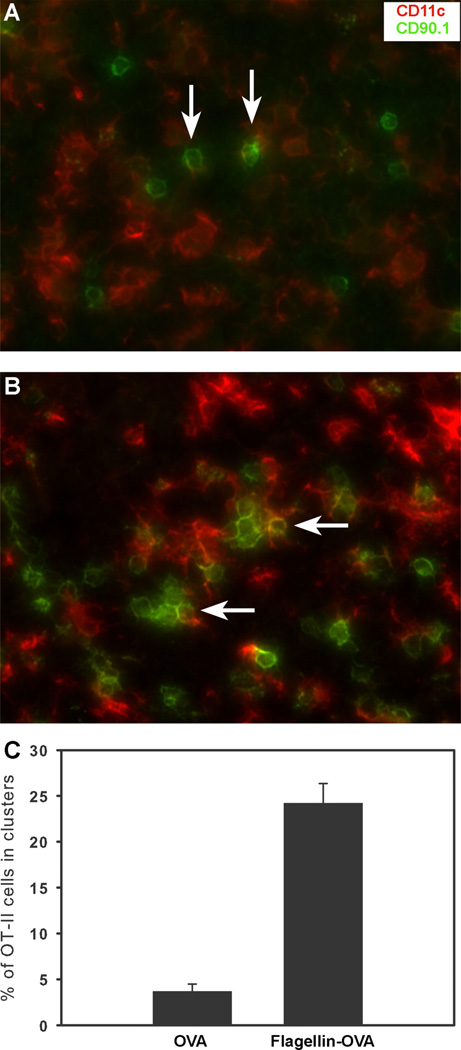
Ingulli et al. (28) showed that 24 h following immunization, Ag-specific CD4+ T cells cluster around Ag-loaded DC. Subsequent intravital microscopy studies have revealed that these clusters represent the second of three distinct phases of DC-mediated, CD4+ T cell priming during which phase-stable T cell-APC synapses form (29, 30). We hypothesized that immunization with flagellin-OVA would result in maturation of Ag-loaded DC and that these mature DC would be more effective at promoting stable T cell-DC interactions than DC in mice, which were immunized with an equal dose of OVA. C57BL/6 mice received 3 × 106 OT-II CD4+ T cells and 1 day later were immunized with 1 × 10−11 mol of flagellin-OVA or OVA only. Twenty-four hours after immunization, the draining popliteal lymph nodes were harvested and frozen in OCT. Frozen tissue sections were cut and stained for CD4, CD90.1, and CD11c. The minimum requirement for a cluster was three contiguous CD90.1+ cells in the CD4+ region of the lymph node cortex. Representative images for OVA and flagellin-OVA immunized mice are shown in Fig. 2, A and B. At least 200 CD90.1+ cells were counted from each lymph node. In mice that were immunized with flagellin-OVA, 24% of the CD90.1+ cells were in clusters. By contrast only, 4% of the OVA-specific T cells in the draining lymph nodes of mice immunized with OVA alone were in clusters (Fig. 2C).
FIGURE 2.
Immunization with flagellin-OVA fusion protein promotes clustering of OVA-specific CD4+ T cells. Draining popliteal lymph nodes were harvested from adoptive transfer recipient mice 24 h following immunization. Adoptively transferred CD4+ OVA-specific cells were identified by staining lymph node tissue sections with CD90.1-specific Ab (green). CD11c+ cells were identified by staining with mouse CD11c-specific Ab (red). Arrows indicate examples of single CD90.1+ OVA-specific T cells from OVA-immunized mice (A) and clusters of CD90.1+ OVA-specific cells from flagellin-OVA-immunized mice (B). C, Clustering of Ag-specific cells was quantified by counting at least 200 cells from each lymph node. Immunization with flagellin-OVA promoted significantly more clustering than immunization with OVA alone (p < 0.001). The data in this experiment were obtained from three mice per group.
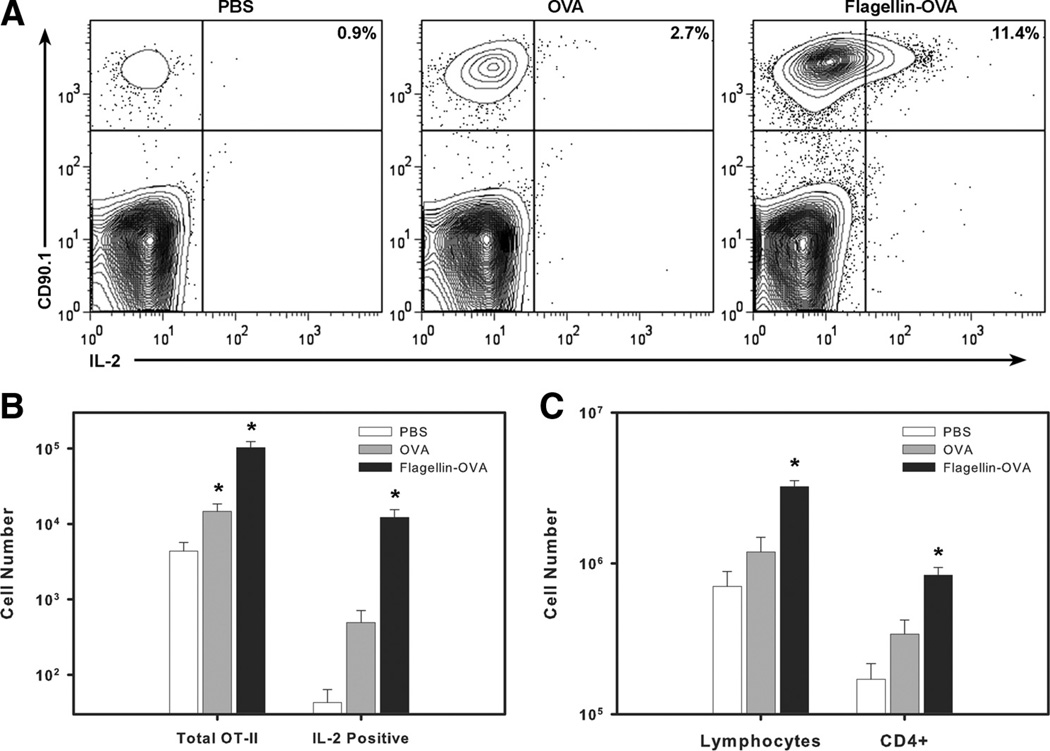
On the basis of the enhanced clustering of OVA-specific cells in mice immunized with flagellin-OVA, we hypothesized that immunization with the fusion protein would also enhance IL-2 production and, consequently, clonal expansion by the responding CD4+ T cell population. OT-II cells were transferred into C57BL/6 mice as described above and immunized with OVA, flagellin-OVA, or PBS (as a control). Mice were sacrificed 3 days after immunization, and cell suspensions generated from the draining popliteal lymph nodes were restimulated in vitro with 30 µg/ml OVA323–339 peptide for 5 h. IL-2 production was measured by intracellular cytokine staining, and the total numbers of lymphocytes, CD4+ T cells, and CD90.1+ cells were also determined using flow cytometry-based measurement in conjunction with fluorescent microspheres (27). Immunization with flagellin-OVA stimulated an increase in the total number of lymphocytes and CD4+ cells recovered from the draining node (Fig. 3C). This finding is consistent with the results of a prior study (11) in which we demonstrated that flagellin is a potent inducer of T and B lymphocyte recruitment to draining lymph nodes following immunization. Consistent with the enhanced clustering of cells in mice immunized with fusion protein, we observed a 4- to 5-fold increase in the percentage of IL-2-producing cells within the OT-II population recovered from mice immunized with flagellin-OVA compared with OVA alone (Fig. 3A). The percent increase, when compounded with the increase in the total number of OVA-specific cells, resulted in a 25-fold increase in the absolute number of IL-2+ cells recovered from mice immunized with flagellin-OVA compared with mice immunized with OVA alone (Fig. 3B). IL-2 production was restricted to cells that had divided (data not shown). The enhanced T cell clustering, IL-2 production, and proliferation seen in mice immunized with flagellin-OVA is consistent with but not formal proof of the hypothesis that flagellin-OVA activates DC by a direct mechanism that involves TLR5-dependent uptake of the fusion protein and thus increased levels of processed Ag for presentation to OVA-specific T cells.
FIGURE 3.
Immunization with flagellin-OVA fusion protein enhances IL-2 production by OVA-specific CD4+ T cells. C57BL/6 mice were i.v. injected with 3 × 106 CD4+CD90.1 + OT-II T cells. Twenty-four hours later, mice were immunized i.m. with 1 × 10−11 mol of flagellin-OVA fusion protein. Mice were sacrificed 3 days following immunization, and cells recovered from the draining popliteal lymph nodes were restimulated in vitro with 30 µg/ml OVA323–339 for 5 h. Brefel-din A was added for the final 2.5 h of culture. A, IL-2 production was determined by intracellular cytokine staining. Plots are gated on CD4+ cells. Total numbers of CD90.1+CD4+ and CD90.1+CD4+IL-2+ cells (B) and lymphocytes (C) and polyclonal CD4+ cells were determined by flow cytometric quantification. The data in this experiment were obtained from four mice per group. The experiment was repeated twice with similar results.
Immunization with a single flagellin-OVA fusion protein is superior to immunization with flagellin plus OVA as separate proteins
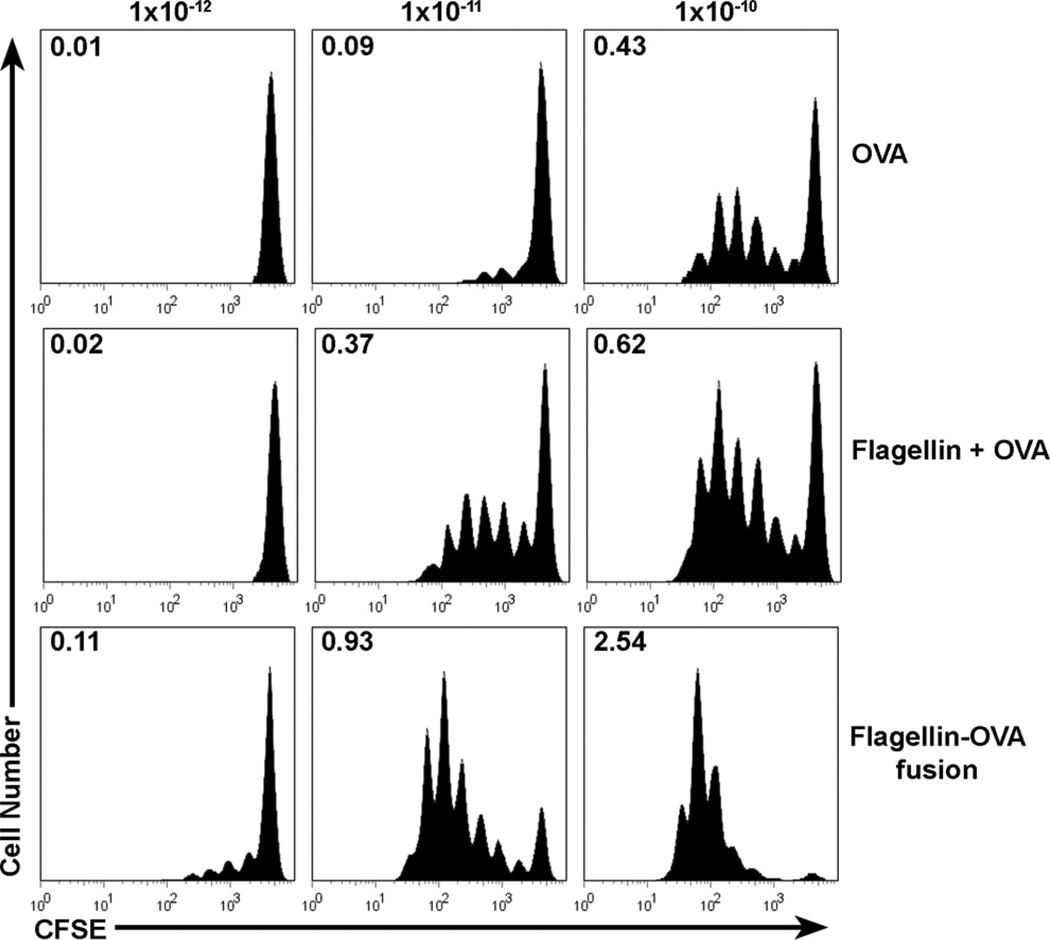
If flagellin directly stimulates DC in vivo, then flagellin-OVA should be significantly more potent than flagellin and OVA as separate proteins in the induction of OVA-specific CD4+ T cell proliferation since the DC that bind flagellin will also receive OVA. Thus, Ag uptake should be far more efficient than obtained with just OVA. However, if the mechanism of DC activation by flagellin is indirect, flagellin-OVA and flagellin plus OVA should exhibit equal potency. To test these possibilities, C57BL/6 mice were injected via the tail vein with 3 × 106 CFSE-labeled, OVA-specific cells and i.m. immunized the following day with 1 × 10−12, 1 × 10−11, or l × 10−10 mol of OVA, flagellin plus OVA, or flagellin-OVA. Three days later, the mice were sacrificed, and OVA-specific cell proliferation was measured in cells recovered from the draining popliteal lymph nodes.
Immunization with flagellin-OVA fusion protein results in enhanced Ag-specific cell division compared with immunization with flagellin plus OVA or OVA alone (Fig. 4). Immunization with 1 × 10−10 mol of OVA alone stimulated an average of 0.43 divisions/cell in the original starting population. However, a similar level of cell division, an average of 0.37 divisions/cell, was achieved following immunization with one-tenth as much (1 × 10−11 mol) flagellin plus OVA. In contrast immunization with 1 × 10−11 mol of flagellin-OVA resulted in an average of 0.93 cell divisions by the original starting population. Although the addition of flagellin as a separate protein has a clear adjuvant effect, the effect is not as great as immunization with flagellin fusion protein. Immunization with 1 × 10−12 mol of the fusion protein resulted in detectable levels of proliferation by OVA-specific cells, but at this low dose, immunization with flagellin plus OVA or OVA alone did not stimulate OVA-specific cell proliferation. Notably, after immunization with the middle and high doses, the fraction of cells in the undivided population is much smaller in mice immunized with fusion protein than in mice immunized with flagellin plus OVA or OVA alone. These results clearly demonstrate that immunization with flagellin-OVA fusion protein promotes a stronger response than immunization with equal doses of flagellin plus OVA as separate proteins. This finding is consistent with, but not conclusive evidence for, a direct effect of flagellin-OVA on DC.
FIGURE 4.
In vivo T cell proliferation in response to immunization with OVA, flagellin plus OVA, or flagellin-OVA fusion protein. C57BL/6 mice were i.v. injected with 3 × 106 CFSE-labeled, CD4+CD90.1+ OT-II T cells. Twenty-four hours later, mice were immunized i.m. with 1 × 10−12, 1 × 10−11, or 1 × 10−10 mol of OVA, flagellin and OVA, or flagellin-OVA fusion protein. Mice were sacrificed 3 days following immunization, and proliferation by the OT-II population in the draining, popliteal lymph node was compared based on CFSE dilution. Numbers indicate the division index, which is the average number of divisions that a cell present in the starting population has undergone. Histograms are gated on CD4+CD90.1+ cells. The data in this experiment were obtained from four mice per group. The experiment was repeated twice with similar results.
tlr5+/+ DC are necessary for the in vivo adjuvant effect of flagellin
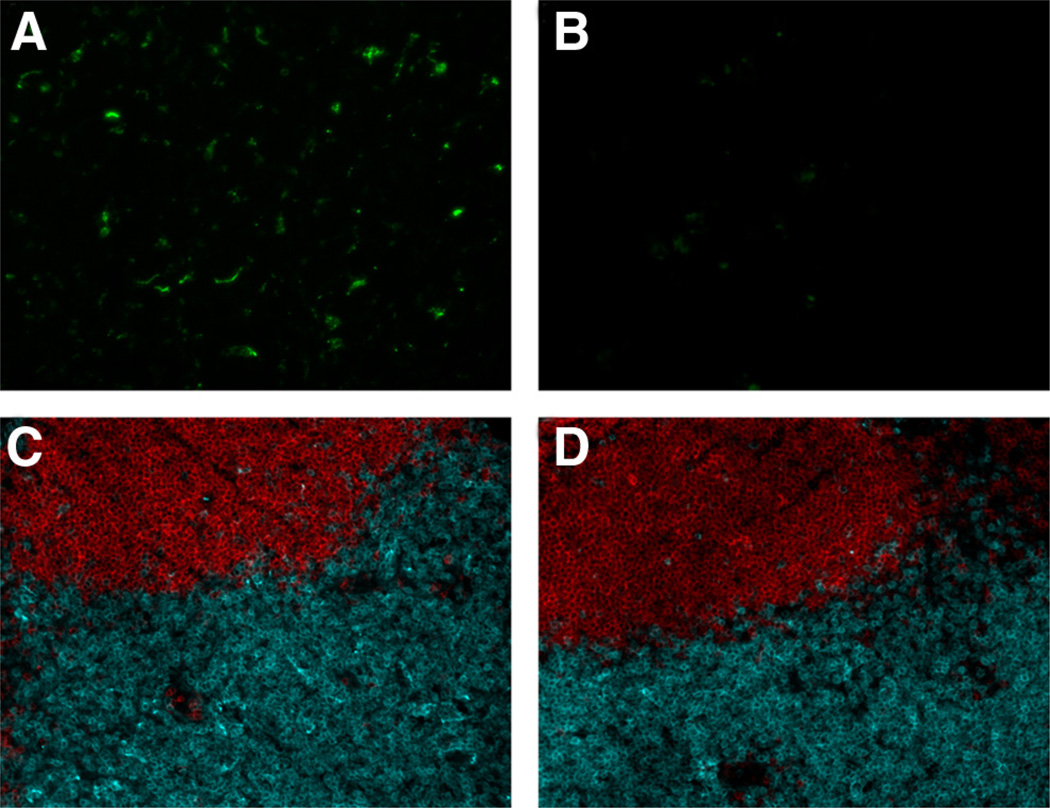
To determine whether the in vivo adjuvant effect of flagellin-OVA involves TLR5-positive DC and thus a direct effect of flagellin-OVA, we developed a bone marrow chimera system in which irradiated mice were reconstituted with a combination of CD 11c-DTR/GFP and wild-type bone marrow or CD11c-DTR/GFP and tlr5−/− bone marrow. Although wild-type murine cells are insensitive to treatment with DT (31), CD11c-DTR/GFP mice express the primate DTR under the control of the CD11c promoter (21), and thus, CD11c+ cells in these mice are fully sensitive to the toxic effect of DT. The histological effects of DT treatment of these mice has been thoroughly examined (32) and confirmed the original report that injection of these mice with DT results in the transient depletion of CD11c+ cells. In confirmation of prior published studies, we found that DT treatment resulted in 85–90% depletion of CD11c+ cells in CD11c-DTR/GFP mice as measured by flow cytometry (data not shown). Depletion of GFP+ cells was also confirmed by histological analysis (Fig. 5, A and B) as well as preservation of normal T and B lymphocyte microenvironments in lymph nodes of CD11c-DTR/GFP mice treated with PBS (Fig. 5C) or DT (Fig. 5D). Bone marrow from the CD11c-DTR/GFP mice used in combination with bone marrow from tlr5−/− mice afford the unique opportunity to create a chimera mouse in which, following treatment with DT, the vast majority of the CD11c+ cells are from a tlr5−/− background, whereas CD11c− hemopoietic cell populations in these mice should be 50% tlr5−/− and 50% tlr5+/+. For these studies, we have assumed equal engraftment of the different types of bone marrow used to reconstitute irradiated mice. Due to the very low levels of TLR5 surface expression by flagellin responsive cells (our unpublished observations), we were unable to detect TLR5 expression by cells from normal C57BL/6 or bone marrow chimera mice. However, if TLR5-expressing DC are present in draining lymph nodes, then such cells and the adjuvant effect of flagellin would be lost in mice reconstituted with CD11c-DTR/GFP plus tlr5−/− bone marrow and treated with DT. If lymph node DC do not normally express TLR5, then DT treatment should have no significant effect on the adjuvant activity of flagellin in these chimera mice.
FIGURE 5.
Treatment of CD11c-DTR mice with DT causes depletion of GFP+ cells in lymph nodes but does not affect normal lymph node architecture 18 h after treatment. CD11c+ cells are revealed by GFP expression in CD11-DTR mice treated with PBS (A). The level of GFP detected was greatly reduced in mice treated with DT (B). Sections stained with CD19-specific (red) and CD3-specific (blue) Abs revealed no difference in normal lymph node architecture in T and B lymphocyte zones between PBS control mice (C) and DT-treated mice (D). The data in this experiment were obtained from four mice per group.
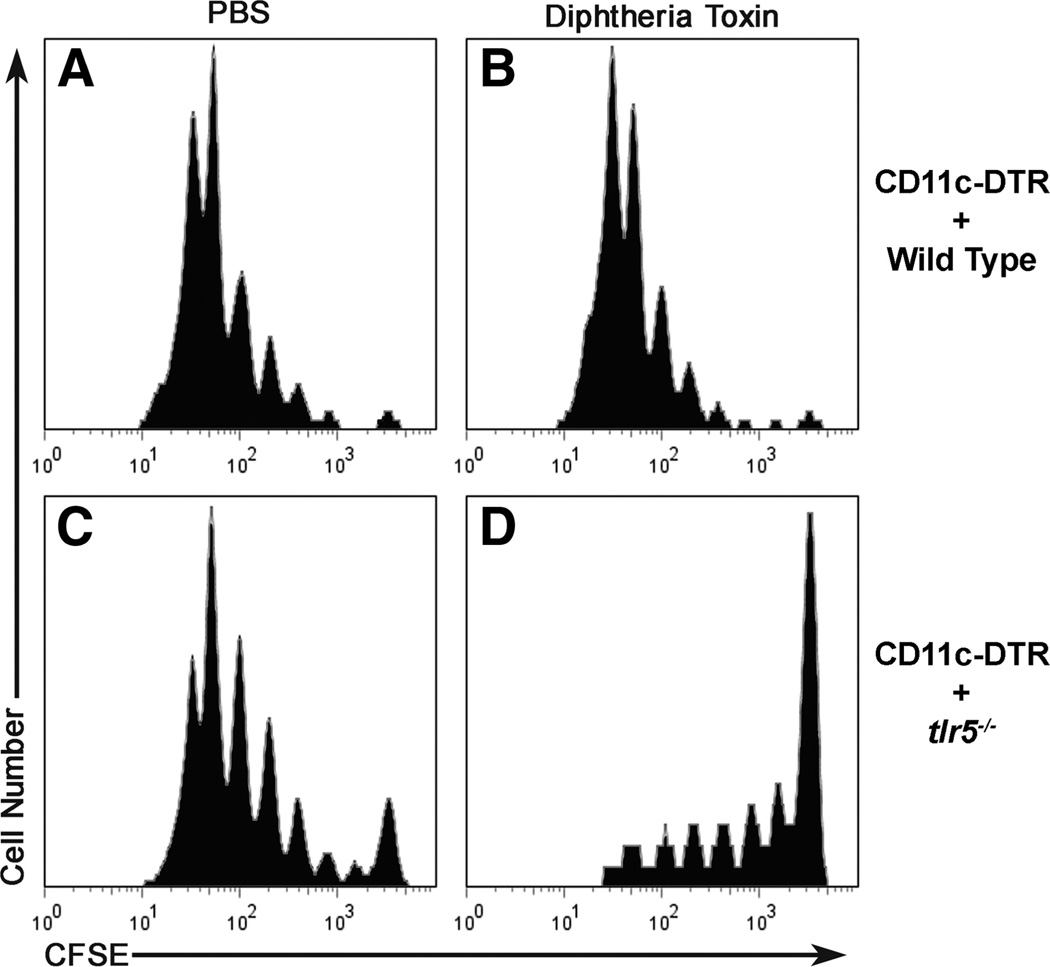
Twelve weeks following irradiation and reconstitution, chimera mice received 3 × 106 CD4+ enriched, CFSE-labeled OT-II cells and the following day were injected with DT or PBS (as a control). This combination of reconstitution and toxin treatment resulted in four groups of mice, three of which had populations of DC from tlr5+/+ mice (C57BL/6 and/or CD11c-DTR/GFP) and one of which that had DC from a tlr5−/− background. Each group contained four mice. Eighteen hours after injection with DT or PBS, mice were immunized with 1 × 10−11 mol of flagellin-OVA. Three days after immunization, CFSE dilution by OVA-specific OT-II cells recovered from the draining lymph node was measured by flow cytometry. OVA-specific cells recovered from the group of mice that were reconstituted with CD11c-DTR/GFP plus wild-type bone marrow and treated with PBS (Fig. 6A) or DT (Fig. 6B) exhibited substantial levels of OVA-specific T cell proliferation. A similar pattern of OVA-specific T cell proliferation was also observed with mice reconstituted with CD1 lc-DTR/GFP and tlr5−/−bone marrow and treated with PBS (Fig. 6C). In striking contrast, OVA-specific T cell proliferation was dramatically reduced in mice reconstituted with CD11c-DTR/GFP and tlr5−/− bone marrow and treated with DT (Fig. 6D). The small extent of proliferation is probably due to the incomplete (85–90%) deletion of the CD11c-DTR/GFP DC in the presence of DT. The dramatic difference in proliferation by OVA-specific cells in mice containing tlr5+/+ DC and mice with DC from a tlr5−/− background clearly demonstrates that the adjuvant effect of flagellin-OVA requires tlr5+/+ DC. Similar results were obtained in a separate experiment following intranasal immunization with flagellin-OVA (three mice per group).
FIGURE 6.
Deletion of tlr5+/+ CD11c+ cells severely impairs OVA-specific CD4+ T cell proliferation in mice immunized with flagellin-OVA fusion protein. Irradiated C57BL/6 mice were reconstituted with 50% CD11c-DTR bone marrow and 50% WT (A and B) or 50% tlr5−/− BM (C and D) and rested for 12 wk. Chimera mice then received 3 × 106 CD4+-enriched, CFSE-labeled CD4+CD90.1+ OT-II cells and were injected with PBS (A and C) or DT (B and D) 1 day following cell transfer. Eighteen hours after toxin treatment mice were immunized i.m. with 1 × 10−11 mol of flagellin-OVA and sacrificed 3 days following immunization. Proliferation by the OT-II population in the draining, popliteal lymph node was compared based on CFSE dilution. Plots are gated on CD4+CD90.1+ cells. The data in this experiment were obtained from four mice per group.
Enhanced efficacy of flagellin-OVA fusion protein requires expression of TLR5 and MyD88
The requirement for a TLR5-expressing DC might simply be due to enhanced uptake of flagellin-OVA by a TLR5-dependent endo-cytic mechanism and not because of any significant contribution of TLR5 signaling. Indeed, in other systems, targeting Ags to specific populations of DC has been shown to significantly enhance the in vivo CD4+ T cell response (33, 34), albeit at higher Ag doses and in combination with adjuvants. To address these possibilities, we compared the response to immunization with flagellin-OVA fusion protein in tlr5−/− and MyD88−/− mice since flagellin signaling via TLR5 is MyD88 dependent (35, 36).
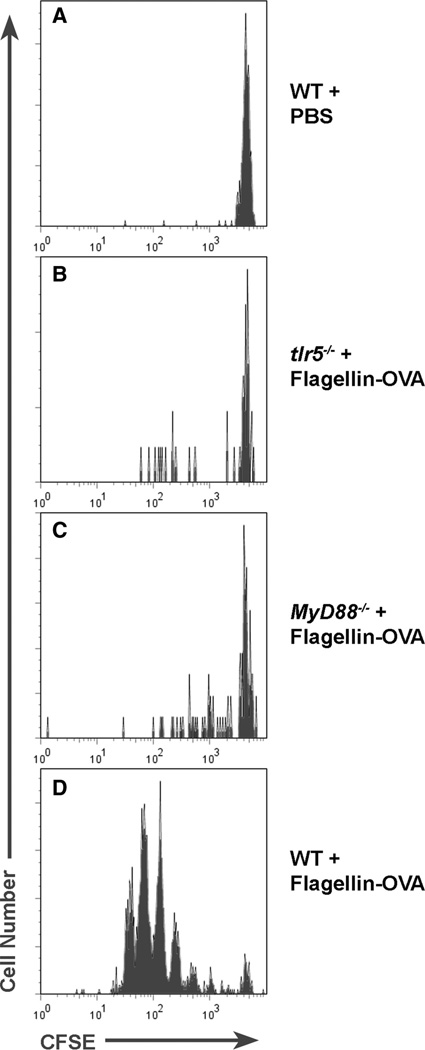
In the first set of experiments, CFSE-labeled OT-II cells were transferred into C57BL/6, tlr5−/−, or MyD88−/− mice. One day following transfer, mice were immunized i.m. with 1 × 10−11 mol of flagellin-OVA or PBS. Mice were sacrificed 3 days later, and CFSE dilution was compared among OVA-specific cells recovered from the draining lymph nodes of mice from each group. In confirmation of the results presented in Fig. 6, flagellin-OVA induced T cell proliferation was dramatically reduced in immunized tlr5−/− mice relative to wild-type mice (Fig. 7).
FIGURE 7.
TLR5 and MyD88 are necessary for the in vivo adjuvant effect of flagellin. C57BL/6 (A and D), tlr5−/− (B), and MyD88−/− (C) mice were i.v. injected with 1.5 × 106 CD4+-enriched CFSE-labeled, CD4+CD90.1+ OT-II T cells. Twenty-four hours later, mice were immunized i.m. with 1 × 10−11 mol of flagellin-OVA fusion protein. Mice were sacrificed 3 days following immunization, and proliferation by the OT-II population in the draining, popliteal lymph node was compared based on CFSE dilution. Plots are gated on CD4+CD90.1+ cells. The data in this experiment were obtained from four mice per group.
To address the individual roles of enhanced Ag uptake via TLR5 and TLR5 signaling in the effect of flagellin-OVA, we evaluated the proliferation of OT-II cells in immunized MyD88−/− mice. Since MyD88−/− mice have normal TLR5 expression, if Ag targeting is sufficient to fully account for the enhanced potency of flagellin-OVA vs flagellin plus OVA, then OVA-specific cells recovered from MyD88−/− mice should exhibit Ag-specific proliferation that is equivalent to that observed in wild-type mice. However, if TLR5 signaling is critical, then OVA-specific cells recovered from MyD88−/− mice should exhibit lower levels of Ag-specific T cell proliferation than observed in wild-type mice. Like the situation in tlr5−/− mice, OT-II proliferation was quite low in MyD88−/− mice (Fig. 7 C). Although these results are consistent with the hypothesis that the enhanced effect of immunization with flagellin-OVA fusion protein is dependent on signaling through TLR5, it is possible that the lack of MyD88 might have a negative effect on Ag processing that is independent of TLR5 (37, 38). To address this question, we used the assay for in vivo T cell Ag-specific clustering described in Fig. 2 to assess the ability of OT-II cells to interact with DC in MyD88−/− mice. We found that 9% of adoptively transferred OVA-specific T cells were engaged in clusters in the draining lymph nodes of MyD88−/− mice 24 h after immunization (as opposed to 24% in wild-type mice; Fig. 2). By comparison, <2% OVA-specific T cells were engaged in clusters in MyD88−/− immunized with just OVA. These findings are consistent with the hypothesis that the enhanced effectiveness of flagellin-OVA as opposed to flagellin plus OVA is due to enhanced Ag uptake via TLR5 as well as signaling through MyD88. However, it is important to emphasize that the precise contributions of each of these mechanisms must await future studies in which we are able to measure the activation of the DC within the lymph node itself.
Discussion
The results presented in this study are consistent with the conclusion that the adjuvant effect of flagellin is dependent, at least in part, on a high-affinity interaction with TLR5 on CD11c+ cells that facilitate extremely efficient uptake of Ag (when the Ag is part of a flagellin fusion protein) via TLR5 (Figs. 6 and 7). On the basis of the retention of normal patterns of T and B lymphocyte staining following DT treatment (Fig. 5), we believe that the DT-sensitive CD11c+ cell that is required for the adjuvant effect of flagellin is a DC. The observation that immunization with flagellin-OVA is more effective at promoting an Ag-specific immune response than immunization with equimolar doses of flagellin and OVA given as separate proteins is clearly consistent with this conclusion and can be explained by two actions of flagellin-enhanced efficiency of Ag uptake via TLR5 and signaling via TLR5 that promotes DC activation. Our results (Figs. 5 and 6) are consistent with the hypothesis that both of these actions are important in the overall adjuvant effect of flagellin in the context of a flagellin-Ag fusion protein. On the basis of the observation that 24 h following immunization with flagellin-OVA, 9% of OT-II cells in MyD88−/− are in clusters compared with 24% in wild-type mice, we estimate that approximately one-third of the adjuvant effect of flagellin results from Ag targeting to DC and two-thirds from signaling through TLR5 and MyD88. However, as noted previously, additional studies are required to quantitate the contributions of each mechanism to the overall stimulatory effect of DC on T cell activation. MyD88 deficiency has been shown to negatively affect phagocytosis (38) and phagosome maturation (37). However, on the basis of the comparisons with our observations, processing of Ag acquired by TLR5-mediated endocytosis appears to be less dependent on MyD88 function than does processing of Ag acquired by phagocytosis.
It is important to emphasize that flagellin can also function as an adjuvant when the Ag is not part of the flagellin protein (9, 11, 39), but the response requires significantly higher doses of Ag and flagellin (∼ 10-fold; Fig. 4). Given the extremely high affinity of flagellin for TLR5 (25, 40), the uptake of flagellin-OVA via TLR5-dependent endocytosis is likely to be far more efficient than the uptake of OVA by itself. As noted earlier, others have shown that targeting Ag for uptake by DC can enhance the Ag-specific in vivo immune response (33, 34). If as expected, uptake and processing of flagellin-OVA fusion protein is much more efficient by TLR5-expressing DC than uptake of OVA alone, the enhanced response to fusion protein could simply result from concentration of Ag by TLR5 + DC and presentation of a greater number of MHC class II molecules loaded with the cognate peptide as opposed to enhanced uptake and TLR5 signaling. Immunization with high doses of OVA alone can trigger proliferation of OVA-specific CD4+ T cell. Thus, if the adjuvant effect of flagellin fused to Ag is mediated predominantly through enhanced uptake and processing following TLR5 ligation and not signaling via TLR5, tlr5+/+MyD88−/−mice should generate a significantly more robust response to flagellin-OVA than tlr5−/−MyD88+/+ mice. Our finding that MyD88−/− and tlr5−/− mice respond similarly to immunization with flagellin-OVA (Fig. 7) provides strong evidence in support of the conclusion that TLR5 and MyD88 are necessary for the adjuvant effect of flagellin and that Ag concentration by TLR5+ APC may not fully account for the adjuvant effect of flagellin. Our experimental approach has relied heavily on the OVA-specific TCR transgenic OT-II cells, a widely accepted model for studying CD4+ T cell biology. Several groups have demonstrated that the increased precursor frequency in adoptive transfer model systems impacts the dynamics of the immune response (41–43); thus, it is possible that polyclonal wild-type cells could exhibit slightly different response than seen in this model system.
In confirmation of other reports (15, 44), we have shown that flagellin does not significantly activate murine BMDC. Although others have arrived at the opposite conclusion (14), those results were generated using doses of flagellin 30–100× in excess of what we have found to be a maximally active concentration (10−10 M) and could result from contaminating nucleic acids not removed by endotoxin depletion. Since the responsiveness to flagellin of monocytes (45) and human myeloid-derived DC (C. L. Hickman, J. T. Bates, and S. B. Mizel; unpublished observations) are dependent on their differentiation state, it is quite likely that the inability of murine BMDC to respond to flagellin reflects their degree of maturation rather than a general property of mature murine DC. Ue-matsu et al. (24, 46) identified a population of TLR5+ DC in the lamina propria of the murine small intestine that is clearly responsive to flagellin. However, Salazar-Gonzalez et al. (17) concluded that flagellin does not directly activate murine splenic DC. Our results as well as those of these investigators support the idea that responsiveness to flagellin varies among DC populations and that the environment in which DC terminally differentiate can significantly modify or induce their responsiveness to flagellin.
Sanders et al. (47) recently reported that flagellin is capable of promoting Ag-specific humoral immunity by a mechanism that is independent of TLR5. The flagellin doses used in their studies are markedly higher than those used in our studies (0.8 µg in our studies vs 50 µg used by Sanders et al.). Indeed, the doses used by Sanders et al. (47) are higher than what is required to drive a maximal response in nonhuman primates (13) and likely reflects a nonspecific effect of high-dose flagellin. It should be noted, however, that Sanders et al. also found that cytokine production in the innate immune response and activation of DC was severely limited in TLR5-deficient mice.
Our findings, in conjunction with what was previously known about flagellin, are consistent with the conclusion that the potent adjuvant effect of flagellin results from the synergy of three distinct processes: direct activation of TLR5+ DC (Figs. 6 and 7), cytokine and chemokine production by non-DC (12, 48–50), and activation of the vascular endothelium (51). Binding of flagellin by TLR5 + DC leads to activation of NF-κB-regulated genes. A number of these genes are critical to mounting an effective immune response. Several reported outcomes of NF-κB activation in DC include enhanced Ag processing and presentation (52, 53), up-regulation of comstimulatory molecules (53), and cytokine (53–55) and chemokine (56) production. Notably, NF-κB activation in human DC results in up-regulation of ICAM-1, ICAM-3, and LFA-1, which are important molecules in facilitating DC-T cell interactions (53). Collectively, activation of NF-κB can have a significant adjuvant effect on the activity of DC in vivo (57). Consequently, in our system, DC that have been activated by flagellin and simultaneously pulsed with cognate Ag are especially effective at promoting an Ag-specific immune response. However, crucial to this outcome is the activation of cytokine and chemokine production by non-DC and also of the vascular endothelium. Activation of these cell populations is likely responsible for the increased flux of lymphocytes into draining lymph nodes soon after immunization (Fig. 3 and Ref. 11). An increase in the number of lymphocytes entering the lymph node and possibly prolonged retention in the node maximize the likelihood that Ag-specific lymphocytes will encounter their cognate Ag. For T cells, presentation of that Ag by an activated DC ensures that they will receive Ag and costimulation sufficient to mount an immune response.
Acknowledgments
We thank Kristen Delaney, Aaron Graff, James Phipps, and Eric Weimer for their generous assistance.
Footnotes
This work was supported by a grant from the National Institutes of Health (P01 AI 60642; to S.B.M.).
Abbreviations used in this paper: DC, dendritic cell; BMDC, bone marrow-derived murine DC; DTR, diptheria toxin receptor.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Lowy J, Hanson J. Structure of Bacterial Flagella. Nature. 1964;202:538–540. doi: 10.1038/202538a0. [DOI] [PubMed] [Google Scholar]
- 2.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 3.Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 2001;167:1882–1885. doi: 10.4049/jimmunol.167.4.1882. [DOI] [PubMed] [Google Scholar]
- 4.Mizel SB, Snipes JA. Gram-negative flagellin-induced self-tolerance is associated with a block in interleukin-1 receptor-associated kinase release from Toll-like receptor 5. J. Biol. Chem. 2002;277:22414–22420. doi: 10.1074/jbc.M201762200. [DOI] [PubMed] [Google Scholar]
- 5.McEwen J, Levi R, Horwitz RJ, Arnon R. Synthetic recombinant vaccine expressing influenza haemagglutinin epitope in Salmonella flagellin leads to partial protection in mice. Vaccine. 1992;10:405–411. doi: 10.1016/0264-410x(92)90071-q. [DOI] [PubMed] [Google Scholar]
- 6.Levi R, Arnon R. Synthetic recombinant influenza vaccine induces efficient long-term immunity and cross-strain protection. Vaccine. 1996;14:85–92. doi: 10.1016/0264-410x(95)00088-i. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Yedidia T, Tarrab-Hazdai R, Schechtman D, Arnon R. Intranasal administration of synthetic recombinant peptide-based vaccine protects mice from infection by Schistosoma mansoni . Infect. Immun. 1999;67:4360–4366. doi: 10.1128/iai.67.9.4360-4366.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McSorley SJ, Ehst BD, Yu Y, Gewirtz AT. Bacterial flagellin is an effective adjuvant for CD4+ T cells in vivo. J. Immunol. 2002;169:3914–3919. doi: 10.4049/jimmunol.169.7.3914. [DOI] [PubMed] [Google Scholar]
- 9.Honko AN, Sriranganathan N, Lees CJ, Mizel SB. Flagellin is an effective adjuvant for immunization against lethal respiratory challenge with Yersinia pestis . Infect. Immun. 2006;74:1113–1120. doi: 10.1128/IAI.74.2.1113-1120.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pino O, Martin M, Michalek SM. Cellular mechanisms of the adjuvant activity of the flagellin component FljB of Salmonells enterica serovar typhimurium to potentiate mucosal and systemic responses. Infect. Immun. 2005;73:6763–6770. doi: 10.1128/IAI.73.10.6763-6770.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bates JT, Honko AN, Graff AH, Kock ND, Mizel SB. Mucosal adjuvant activity of flagellin in aged mice. Mech. Ageing Dev. 2008;129:271–281. doi: 10.1016/j.mad.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SE, Kim SY, Jeong BC, Kim YR, Bae SJ, Ahn OS, Lee JJ, Song HC, Kim JM, Choy HE, et al. A bacterial flagellin, Vibrio vulnificus FlaB, has a strong mucosal adjuvant activity to induce protective immunity. Infect. Immun. 2006;74:694–702. doi: 10.1128/IAI.74.1.694-702.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mizel SB, Graff AH, Sriranganathan N, Ervin S, Lees CJ, Lively MO, Hantgan RR, Thomas MJ, Wood J, Bell B. Flagellin-Fl-V fusion protein is an effective plague vaccine in mice and two species of nonhuman primates. Clin. Vaccine Immunol. 2009;16:21–28. doi: 10.1128/CVI.00333-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Didierlaurent A, Ferrero I, Otten LA, Dubois B, Reinhardt M, Carlsen H, Blomhoff R, Akira S, Kraehenbuhl JP, Sirard JC. Flagellin promotes myeloid differentiation factor 88-dependent development of Th2-type response. J. Immunol. 2004;172:6922–6930. doi: 10.4049/jimmunol.172.11.6922. [DOI] [PubMed] [Google Scholar]
- 15.Means TK, Hayashi F, Smith KD, Aderem A, Luster AD. The Toll-like receptor 5 stimulus bacterial flagellin induces maturation and chemo-kine production in human dendritic cells. J. Immunol. 2003;170:5165–5175. doi: 10.4049/jimmunol.170.10.5165. [DOI] [PubMed] [Google Scholar]
- 16.Tsujimoto H, Uchida T, Efron PA, Scumpia PO, Verma A, Matsumoto T, Tschoeke SK, Ungaro RF, Ono S, Seki S, et al. Flagellin enhances NK cell proliferation and activation directly and through dendritic cell-NK cell interactions. J. Leukocyte Biol. 2005;78:888–897. doi: 10.1189/jlb.0105051. [DOI] [PubMed] [Google Scholar]
- 17.Salazar-Gonzalez RM, Srinivasan A, Griffin A, Muralimohan G, Ertelt JM, Ravindran R, Vella AT, McSorley SJ. Salmonella flagellin induces bystander activation of splenic dendritic cells and hinders bacterial replication in vivo. J. Immunol. 2007;179:6169–6175. doi: 10.4049/jimmunol.179.9.6169. [DOI] [PubMed] [Google Scholar]
- 18.Arimilli S, Johnson JB, Clark KM, Graff AH, Alexander-Miller MA, Mizel SB, Parks GD. Engineered expression of the TLR5 ligand flagellin enhances paramyxovirus activation of human dendritic cell function. J. Virol. 2008;82:10975–10985. doi: 10.1128/JVI.01288-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merlo A, Calcaterra C, Menard S, Balsari A. Cross-talk between Toll-like receptors 5 and 9 on activation of human immune responses. J. Leukocyte Biol. 2007;82:509–518. doi: 10.1189/jlb.0207100. [DOI] [PubMed] [Google Scholar]
- 20.Agrawal S, Agrawal A, Doughty B, Gerwitz A, Blenis J, Van Dyke T, Pulendran B. Cutting edge: different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J. Immunol. 2003;171:4984–4989. doi: 10.4049/jimmunol.171.10.4984. [DOI] [PubMed] [Google Scholar]
- 21.Jung S, Unutmaz D, Wong P, Sano G, Delos Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based α- and β-chain genes under the control of heterologous regulatory elements. Immunol. Cell. Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 23.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the. MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 24.Uematsu S, Jang MH, Chevrier N, Guo Z, Kumagai Y, Yamamoto M, Kato H, Sougawa N, Matsui H, Kuwata H, et al. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat. Immunol. 2006;7:868–874. doi: 10.1038/ni1362. [DOI] [PubMed] [Google Scholar]
- 25.McDermott PF, Ciacci-Woolwine F, Snipes JA, Mizel SB. High-affinity interaction between Gram-negative flagellin and a cell surface polypeptide results in human monocyte activation. Infect. Immun. 2000;68:5525–5529. doi: 10.1128/iai.68.10.5525-5529.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.West AP, Dancho BA, Mizel SB. Gangliosides inhibit flagellin signaling in the absence of an effect on flagellin binding to Toll-like receptor 5. J. Biol. Chem. 2005;280:9482–9488. doi: 10.1074/jbc.M411875200. [DOI] [PubMed] [Google Scholar]
- 27.Schlenke P, Frohn C, Kluter H, Saballus M, Hammers HJ, Zajac SR, Kirchner H. Evaluation of a flow cytometric method for simultaneous leukocyte phenotyping and quantification by fluorescent microspheres. Cytometry. 1998;33:310–317. doi: 10.1002/(sici)1097-0320(19981101)33:3<310::aid-cyto4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 28.Ingulli E, Mondino A, Khoruts A, Jenkins MK. In vivo detection of dendritic cell antigen presentation to CD4+ T cells. J. Exp. Med. 1997;185:2133–2141. doi: 10.1084/jem.185.12.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mempel TR, Henrickson SE, Von Andrian UH. T cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;421:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 30.Miller MJ, Safrina O, Parker I, Cahalan MD. Imaging the single cell dynamics of CD4+ T cell activation by dendritic cells in lymph nodes. J. Exp. Med. 2004;200:847–856. doi: 10.1084/jem.20041236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pappenheimer AM, Jr., Harper AA, Moynihan M, Brockes JP. Diphtheria toxin and related proteins: effect of route of injection on toxicity and the determination of cytotoxicity for various cultured cells. J. Infect. Dis. 1982;145:94–102. doi: 10.1093/infdis/145.1.94. [DOI] [PubMed] [Google Scholar]
- 32.Probst HC, Tschannen K, Odermatt B, Schwendener R, Zinkernagel RM, Van Den Broek M. Histological analysis of CD11c-DTR/GFP mice after in vivo depletion of dendritic cells. Clin. Exp. Immunol. 2005;141:398–404. doi: 10.1111/j.1365-2249.2005.02868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii S, Soares H, Brimnes MK, Moltedo B, Moran TM, Steinman RM. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J. Exp. Med. 2004;199:815–824. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Do Y, Park CG, Kang YS, Park SH, Lynch RM, Lee H, Powell BS, Steinman RM. Broad T cell immunity to the LcrV virulence protein is induced by targeted delivery to DEC - 20 5/CD2 05-positive mouse dendritic cells. Eur. J. Immunol. 2008;38:20–29. doi: 10.1002/eji.200737799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rhee SH, Kim H, Moyer MP, Pothoulakis C. Role of MyD88 in phosphatidylinositol 3-kinase activation by flagellin/Toll-like receptor 5 engagement in colonic epithelial cells. J. Biol. Chem. 2006;281:18560–18568. doi: 10.1074/jbc.M513861200. [DOI] [PubMed] [Google Scholar]
- 36.Bagchi A, Herrup EA, Warren HS, Trigilio J, Shin HS, Valentine C, Hellman J. MyD88-dependent and MyD88-independent pathways in synergy, priming, and tolerance between TLR agonists. J. Immunol. 2007;178:1164–1171. doi: 10.4049/jimmunol.178.2.1164. [DOI] [PubMed] [Google Scholar]
- 37.Yates RM, Russell DG. Phagosome maturation proceeds independently of stimulation of Toll-like receptors 2 and 4. Immunity. 2005;23:409–417. doi: 10.1016/j.immuni.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 38.Shin OS, Isberg RR, Akira S, Uematsu S, Behera AK, Hu LT. Distinct roles for MyD88 and Toll-like receptors 2, 5, and 9 in phagocytosis of Borrelia burgdorferi and cytokine induction. Infect. Immun. 2008;16:2341–2351. doi: 10.1128/IAI.01600-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Honko AN, Mizel SB. Effects of flagellin on innate and adaptive immunity. Immunol. Res. 2005;33:83–101. doi: 10.1385/IR:33:1:083. [DOI] [PubMed] [Google Scholar]
- 40.Mizel SB, West AP, Hantgan RR. Identification of a sequence in human Toll-like receptor 5 required for the binding of Gram-negative flagellin. J. Biol. Chem. 2003;278:23624–23629. doi: 10.1074/jbc.M303481200. [DOI] [PubMed] [Google Scholar]
- 41.Bates JT, Bucy RP. Enhanced responsiveness to antigen contributes more to immunological memory in CD4 T cells than increases in the number of cells. Immunology. 2005;116:318–327. doi: 10.1111/j.1365-2567.2005.02227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith AL, Wikstrom ME, Fazekas de St Groth B. Visualizing T cell competition for peptide/MHC complexes: a specific mechanism to minimize the effect of precursor frequency. Immunity. 2000;13:783–794. doi: 10.1016/s1074-7613(00)00076-5. [DOI] [PubMed] [Google Scholar]
- 43.Foulds KE, Shen H. Clonal competition inhibits the proliferation and differentiation of adoptively transferred TCR transgenic CD4 T cells in response to infection. J. Immunol. 2006;176:3037–3043. doi: 10.4049/jimmunol.176.5.3037. [DOI] [PubMed] [Google Scholar]
- 44.Dearman RJ, Cumberbatch M, Maxwell G, Basketter DA, Kimber I. Toll-like receptor ligand activation of murine bone marrow-derived dendritic cells. Immunology. 2008;126:475–484. doi: 10.1111/j.1365-2567.2008.02922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ciacci-Woolwine F, McDermott PF, Mizel SB. Induction of cytokine synthesis by flagella from Gram-negative bacteria may be dependent on the activation or differentiation state of human monocytes. Infect. Immun. 1999;61:5176–5185. doi: 10.1128/iai.67.10.5176-5185.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uematsu S, Fujimoto K, Jang MH, Yang BG, Jung YJ, Nishiyama M, Sato S, Tsujimura T, Yamamoto M, Yokota Y, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat. Immunol. 2008;9:769–776. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- 47.Sanders CJ, Franchi L, Yarovinsky F, Uematsu S, Akira S, Nunez G, Gewirtz AT. Induction of adaptive immunity by fiagellin does not require robust activation of innate immunity. Eur. J. Immunol. 2009;39:359–371. doi: 10.1002/eji.200838804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sierro F, Dubois B, Coste A, Kaiserlian D, Kraehenbuhl JP, Sirard JC. Fiagellin stimulation of intestinal epithelial cells triggers CCL20-mediated migration of dendritic cells. Proc. Natl. Acad. Sci. USA. 2001;98:13722–13727. doi: 10.1073/pnas.241308598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Honko AN, Mizel SB. Mucosal administration of fiagellin induces innate immunity in the mouse lung. Infect. Immun. 2004;72:6676–6679. doi: 10.1128/IAI.72.11.6676-6679.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pegu A, Qin S, Fallert Junecko BA, Nisato RE, Pepper MS, Reinhart TA. Human lymphatic endothelial cells express multiple functional TLRs. J. Immunol. 2008;180:3399–3405. doi: 10.4049/jimmunol.180.5.3399. [DOI] [PubMed] [Google Scholar]
- 51.Maaser C, Heidemann J, von Eiff C, Lugering A, Spahn TW, Binion DG, Domschke W, Lugering N, Kucharzik T. Human intestinal microvascular endothelial cells express Toll-like receptor 5: a binding partner for bacterial fiagellin. J. Immunol. 2004;172:5056–5062. doi: 10.4049/jimmunol.172.8.5056. [DOI] [PubMed] [Google Scholar]
- 52.Lind EF, Ahonen CL, Wasiuk A, Kosaka Y, Becher B, Bennett KA, Noelle RJ. Dendritic cells require the NF-κB2 pathway for cross-presentation of soluble antigens. J. Immunol. 2008;181:354–363. doi: 10.4049/jimmunol.181.1.354. [DOI] [PubMed] [Google Scholar]
- 53.Yoshimura S, Bondeson J, Foxwell BM, Brennan FM, Feldmann M. Effective antigen presentation by dendritic cells is NF-κB dependent: coordinate regulation of MHC, co-stimulatory molecules and cytokines. Int. Immunol. 2001;13:675–683. doi: 10.1093/intimm/13.5.675. [DOI] [PubMed] [Google Scholar]
- 54.Moore F, Buonocore S, Aksoy E, Ouled-Haddou N, Goriely S, Lazarova E, Paulart F, Heirman C, Vaeremans E, Thielemans K, et al. An alternative pathway of NF-κB activation results in maturation and T cell priming activity of dendritic cells overexp res sing a mutated IκB α. J. Immunol. 2007;178:1301–1311. doi: 10.4049/jimmunol.178.3.1301. [DOI] [PubMed] [Google Scholar]
- 55.Baltathakis I, Alcantara O, Boldt DH. Expression of different NF-κB pathway genes in dendritic cells (DCs) or macrophages assessed by gene expression profiling. J. Cell. Biochem. 2001;83:281–290. doi: 10.1002/jcb.1231. [DOI] [PubMed] [Google Scholar]
- 56.Pietila TE, Veckman V, Lehtonen A, Lin R, Hiscott J, Julkunen I. Multiple NF-κB and IFN regulatory factor family transcription factors regulate CCL19 gene expression in human monocyte-derived dendritic cells. J Immunol. 2007;178:253–261. doi: 10.4049/jimmunol.178.1.253. [DOI] [PubMed] [Google Scholar]
- 57.Andreakos E, Williams RO, Wales J, Foxwell BM, Feldmann M. Activation of NF-κB by the intracellular expression of NF-κB-inducing kinase acts as a powerful vaccine adjuvant. Proc. Natl. Acad. Sci. USA. 2006;103:14459–14464. doi: 10.1073/pnas.0603493103. [DOI] [PMC free article] [PubMed] [Google Scholar]