Abstract
Vitamin A has diverse biological functions and is essential for human survival. STRA6 is the high-affinity membrane receptor for plasma retinol binding protein (RBP), the principle and specific carrier of vitamin A (retinol) in the blood. It was previously shown that STRA6 couples to lecithin retinol acyltransferase (LRAT) and cellular retinol binding protein I (CRBP-I), but poorly to CRBP-II, for retinol uptake from holo-RBP. STRA6 catalyzes both retinol release from holo-RBP, which is responsible for its retinol uptake activity, and the loading of free retinol into apo-RBP, which can cause retinol efflux. Although STRA6-catalyzed retinol efflux into apo-RBP can theoretically deplete cells of retinoid, it is unclear to what extent this efflux happens and in what context. We show here that STRA6 can couple strongly to both CRBP-I and CRBP-II for retinol efflux to apo-RBP. Strikingly, pure apo-RBP can cause almost complete depletion of retinol taken up by CRBP-I in a STRA6-dependent manner. However, if STRA6 encounters both holo-RBP and apo-RBP (as in blood), holo-RBP blocks STRA6-mediated retinol efflux by competing with apo-RBP's binding to STRA6 and by counteracting retinol efflux with influx. We also found that STRA6 catalyzes efficient retinol exchange between intracellular CRBP-I and extracellular RBP, even in the presence of holo-RBP. STRA6's retinol exchange activity may serve to refresh the intracellular retinoid pool. This exchange is also a previously unknown function of CRBP-I and distinguishes CRBP-I from LRAT.
Keywords: Keywords Membrane receptor, Membrane transport, Retinoid, Vitamin A transport
Vitamin A performs diverse physiological functions including its essential roles in vision (Crouch et al. 1996; Dowling 1966; Travis et al. 2007; Wald 1968), embryonic development (Niederreither and Dolle 2008), immune cell maturation (Stephensen 2001), and the adult nervous system (Drager 2006; Maden 2007). Vitamin A mediates these physiological functions through its derivatives called retinoids (Evans 1994; Mark et al. 2006; Napoli 1999). Despite the ability of free vitamin A and most of its derivatives to diffuse systemically, virtually all vitamin A in the blood under physiological conditions is bound to plasma retinol binding protein (RBP), the principle and specific high-affinity carrier of vitamin A (Blomhoff et al. 1990; Goodman 1984; Newcomer and Ong 2000; Quadro et al. 2003, 2005; Zanotti and Berni 2004). RBP mediates vitamin A transport from sites of storage (especially the liver) to target organs. Unlike other retinoids in the blood, which fluctuate depending on dietary intake, vitamin A–bound RBP (holo-RBP) is maintained stably at micromolar concentrations and serves as a buffer for fluctuations in dietary intake of retinoids to maintain a stable supply for tissues that depend on vitamin A (Blomhoff et al. 1990).
Although free vitamin A can diffuse through membranes as a result of its hydrophobic nature, vitamin A bound to RBP becomes membrane impermeable. It was first proposed in the 1970s that there exists an RBP receptor that mediates cellular vitamin A uptake (Heller 1975; Heller and Bok 1976; Rask and Peterson 1976). Despite strong evidence accumulated over three decades for the existence of this receptor, this hypothesis was heatedly debated as a result of a belief that RBP can freely release retinol, which can pass through membrane without a receptor (Noy 2000). This belief that supports the nonexistence of the RBP receptor suggests that retinol acutely mixed with RBP is identical to holo-RBP. Because free retinol can diffuse through membranes, there is indeed no need for the RBP receptor to exist if this belief is true.
The RBP receptor was recently identified as a multitransmembrane domain protein called STRA6 through unbiased biochemical purification and mass spectrometry (Kawaguchi et al. 2007). The fact that STRA6 promotes cellular vitamin A uptake from holo-RBP (Golczak et al. 2008; Isken et al. 2008; Kawaguchi et al. 2007) strongly argues against the belief that the RBP receptor does not exist and that retinol can freely be released by RBP. STRA6 was originally known as a retinoic acid-stimulated cell-surface protein in cancer cells (Bouillet et al. 1997; Szeto et al. 2001). STRA6 has nine transmembrane domains (Kawaguchi et al. 2008b) and an essential RBP binding domain on an extracellular loop (Kawaguchi et al. 2008a). In addition to RBP binding, STRA6 catalyzes both retinol release from holo-RBP, which is responsible for its retinol uptake activity, and the highly efficient loading of free retinol into apo-RBP in the absence of LRAT or CRBP-I (Kawaguchi et al. 2011). STRA6-mediated retinol uptake is perhaps most similar to Scavenger Receptor Class B Type I (SR-BI)-mediated cholesterol uptake from high-density lipoprotein (HDL) (Acton et al. 1996) because both membrane proteins take up molecules bound to extracellular carrier proteins while the carrier proteins remain outside of the cell after uptake. STRA6 and SR-BI have no similarity in sequence, and STRA6 has nine transmembrane domains (Kawaguchi et al. 2008b), while SR-BI has two (Babitt et al. 1997; Connelly et al. 2001). Although a few possible mechanisms have been proposed for SR-BI (Connelly 2009; Connelly and Williams 2004), STRA6's vitamin A uptake mechanism is different from these mechanisms.
LRAT is known to enhance STRA6's vitamin A uptake activity (Golczak et al. 2008; Isken et al. 2008; Kawaguchi et al. 2007). However, it has been shown that the reverse reaction of vitamin A loading into apo-RBP still happens in cells that express both STRA6 and LRAT to lead to cellular retinol loss (Isken et al. 2008). This suggests the possibility that STRA6 can cause retinol efflux (Isken et al. 2008). It was also found that apo-RBP efficiently takes up retinol in a STRA6-dependent manner in the absence of LRAT (Kawaguchi et al. 2011). This activity is consistent with the hypothesis that STRA6 might mediate cellular retinol efflux by loading retinol into extracellular apo-RBP. SR-BI, the HDL receptor, is known to mediate cellular cholesterol loss (Connelly and Williams 2004). Can the RBP receptor STRA6 mediate cellular retinol loss, in analogy to HDL receptor-mediated cholesterol loss?
Although apo-RBP in the blood is removed by kidney filtration, blood still contains a small fraction of apo-RBP in addition to holo-RBP (Mills et al. 2008). If STRA6 encounters both holo-RBP and apo-RBP, does it mediate cellular vitamin A uptake or loss? Does retinol efflux mediated by apo-RBP happen in human blood? If STRA6 mediates cellular vitamin A loss when it is exposed to the blood, how does it function as the RBP receptor to mediate cellular vitamin A uptake?
Excessive RBP in the blood has also been found to be a signal in insulin resistance (Yang et al. 2005). The average RBP level in healthy people is 2.67 μM (Mills et al. 2008), and this level does not cause insulin resistance. What is the signaling receptor that senses RBP levels in excess of 2.67 μM to cause insulin resistance? It was hypothesized that STRA6, the nanomolar affinity RBP receptor that mediates vitamin A uptake (Kawaguchi et al. 2007), is also the signaling receptor that mediates insulin resistance, and that holo-RBP, but not apo-RBP, binds to STRA6 to initiate the signaling (Berry et al. 2011). This study was based on a commercial antibody that does not correctly recognize STRA6 (Supplementary Fig. 1) and the belief that retinol acutely mixed with RBP is identical to holo-RBP. A subsequent report reached the opposite conclusion that apo-RBP, not holo-RBP, binds to STRA6 to initiate signal transduction (Chen et al. 2012). Does STRA6 bind only holo-RBP or only apo-RBP or both? If it binds both, can its interaction with holo-RBP influence its interaction with apo-RBP?
We show here that both holo-RBP and apo-RBP bind to STRA6. Holo-RBP inhibits STRA6-catalyzed retinol loading into apo-RBP. Although STRA6 couples to CRBP-I and LRAT (but poorly to CRBP-II) in retinol uptake from holo-RBP, STRA6 couples to both CRBP-I and CRBP-II (but poorly to LRAT) in retinol efflux to pure apo-RBP. Despite the ability of pure apo-RBP to cause retinol efflux, STRA6 mediates retinol uptake, not retinol loss, when exposed to the blood, which contains both holo-RBP and apo-RBP. We also found that STRA6 catalyzes retinol exchange between intracellular holo-CRBP-I and extracellular holo-RBP and between RBP molecules. These findings reveal new biochemical activities of STRA6, in addition to its role as the RBP receptor to mediate retinol uptake.
Materials and Methods
Production of Holo-RBP, Apo-RBP, and 3H-retinol/RBP
Holo-RBP was produced, refolded, and purified as described (Kawaguchi et al. 2007). It is necessary to use high-performance liquid chromatography (HPLC) in order to remove imperfectly folded RBP, and to purify holo-RBP 100 % loaded with retinol. High quality holo-RBP is essential because poorly folded holo-RBP has low affinity for retinol and can release retinol easily. Apo-RBP was prepared from holo-RBP by extracting retinol as described (Heller and Horwitz 1973). For production of 3H-retinol/RBP, 3H-retinol (PerkinElmer) was incubated with apo-RBP (with 6XHis tag at the N-terminus) overnight at 4 °C. 3H-retinol/RBP was incubated with Ni-NTA resin (Qiagen) for 3 h. After three washes with phosphate-buffered saline (PBS), the complex was eluted by 100 mM imidazole in PBS. The eluted 3H-retinol/RBP was used immediately for cellular uptake assays.
Assays for 3H-retinol Uptake or Efflux Using 3H-retinol/RBP
The COS-1 cell is chosen for this assay because it adheres to the cell culture dish strongly and does not detach during repeated washes and media changes in this live cell assay. The other commonly used cell type HEK293 cell is not useful for this assay because of its weak adherence that can cause complete cell loss after a few washes. Transfected COS-1 cells or untransfected control cells were washed once with Hanks balanced salt solution (HBSS) and incubated in serumfree medium (SFM) 24 h after transfection. Cellular 3H-retinol uptake assay from 3H-retinol/RBP was performed 48 h after transfection. Cells were incubated with 3H-retinol/RBP diluted in SFM for 1 h at 37 °C. To assay for retinol efflux into apo-RBP or normal human serum (NHS) (Innovative Research), the cells were then washed with HBSS and incubated with SFM for 2 h to dissociate cell-surface-bound RBP. Apo-RBP or NHS diluted in SFM was then added to the cells for an additional 2 h of incubation. Bovine serum albumin (BSA), a nonspecific retinol binding protein, was used as a control. After a further HBSS wash, the cells were solubilized in 1 % Triton X-100 in PBS. 3H-retinol remaining in the cells was measured with a scintillation counter. All experiments were done in triplicate. Conditions specific to each experiment are described in the figure captions.
Assays for 3H-retinol Efflux into Apo-RBP or Human Serum Using Free 3H-retinol
For experiments involving cells that cannot effectively take up retinol from holo-RBP due to the absence of STRA6 (e.g., CRBP-I-only cells or CRBP-II-only cells), free 3H-retinol was loaded to the cells instead of 3H-retinol/RBP. Loading cells with free 3H-retinol makes it possible to demonstrate STRA6-independent 3H-retinol efflux. After cells were loaded with free 3H-retinol for 2 h, the cells were washed with HBSS before apo-RBP or NHS was added to cause 3H-retinol efflux. BSA was used as a negative control. After a further HBSS wash, the cells were solubilized in 1 % Triton X-100 in PBS. 3H-retinol remaining in cells was measured with a scintillation counter. All experiments were done in triplicate. Conditions specific to each experiment are described in the figure captions.
HPLC-based Assay for Retinol Uptake from Human Serum and Efflux into Extracellular Apo-RBP
For retinol uptake assay using human serum as the source of holo-RBP, we first incubated COS-1 cells transfected with STRA6/CRBP-I or STRA6/LRAT with 25 % NHS diluted in SFM for 4 h. To assess the ability of apo-RBP to extract retinol taken up by cells, 1 μM of apo-RBP in SFM, 25 % NHS in SFM or SFM alone was added to HBSS-washed cells and incubated for another 4 h. At the end of each experiment, cells were washed once with HBSS and collected for HPLC analysis, as described below.
Production and Purification of Enhanced Green Fluorescent Protein-RBP Fusion Protein (EGFP-RBP)
It was known previously that N-terminal tagging of RBP does not interfere with its binding to its receptor (Kawaguchi et al. 2007). To create EGFP-tagged RBP, EGFP was tagged to the N-terminus of RBP right after the secretion signal (EGFP is connected to RBP through a 3-glycine linker). A 6XHis tag was also added to the protein to allow nickel affinity purification. Six hours after transfection with the expression plasmid, COS-1 cells were washed with HBSS, and the culture medium was changed to SFM. The medium containing secreted EGFP-RBP was collected 24 h later. After concentrating the medium, EGFP-RBP was purified using Ni-NTA resin (Qiagen).
HPLC Analysis of Vitamin A Uptake from Human Serum
Retinol from cells was extracted using hexane containing 1 mM 2,6-Di-tert-butyl-4-methylphenol (BHT). Retinol was dried under nitrogen and resolubilized in 100 % methanol. Before HPLC analysis, 10 % water was added to the solution. Samples were analyzed on the Agilent 1100 series liquid chromatography system with a photo-diode array detector. Retinol was separated using a Zorbax Rx-SIL column (Agilent) and using 90 % methanol as the mobile phase. Retinyl ester was extracted by adding methanol containing 1 mM BHT. Retinyl ester was separated using a Zorbax Eclipse EDB-C18 column (Agilent). The isopropanol concentration was increased from 0 to 100 % against methanol during the 10 min run, followed by a 5 min wash with 100 % isopropanol. Both retinol and retinyl esters were detected by absorbance at 325 nm.
Fluorescence Assay for STRA6-Catalyzed Retinol Loading into Apo-RBP and Retinol Release from Holo-RBP
Real-time monitoring of retinol fluorescence was measured using POLARstar Omega (BMG Labtech) with the excitation filter 320ex and the emission filter 460–10. The black Microfluor-2 plate (Thermo) was coated with Blocker Casein (Pierce) overnight at 4 °C and was washed once with PBS before addition of membrane suspension to prevent nonspecific sticking of holo-RBP to the plastic wall. Membranes resuspended in PBS and mixed with various proteins were transferred to the plate for fluorescent measurement. The signal from each time points is the average of 10 measurements. Samples were shaken for 10 s at 500 rpm using double-orbital shaking before each measurement. The fluorescent signals before the addition of retinol or holo-RBP at 0 min are considered background signals and were subtracted from the final fluorescence signals at each time point.
Fluorescence Resonance Transfer Assay for STRA6-Catalyzed Retinol Transfer between RBP Molecules
To study retinol transfer between RBP molecules, we tagged EGFP to the N-terminus of RBP right after its natural secretion signal to create N-EGFP-RBP. The fluorescence resonance transfer (FRET) between retinol and EGFP has been demonstrated previously (Kawaguchi et al. 2011). The transfer reaction was initiated by adding holo-RBP to N-EGFP-RBP premixed with membrane purified from STRA6 expressing COS-1 cells. Membranes from COS-1 cells expressing both STRA6 and LRAT and cells that express neither STRA6 nor LRAT were used as controls. Real-time retinol-EGFP FRET was measured with the excitation filter 320ex and the emission filters 460–10 and 510–10 using simultaneous duel emission optics in POLARstar Omega. The signal from each time point of each reaction was the average of 10 measurements. The fluorescence of each reaction was measured before holo-RBP was added at 0 min to initiate the reaction. The equation to calculate this ratio is [(510t – 510b)/(460t –460b)], where 510t, 510b, 460t, and 460b represent emissions at 510 nm after initiation of the reaction (t = time point), at 510 nm before holo-RBP is added (b = background), at 460 nm after initiation of the reaction (t = time point), and at 460 nm before holo-RBP is added (b = background), respectively.
Statistical Analysis of Experimental Results
All statistical analysis was performed by Minitab 15 software. Student's t test was used for a single set of comparison. The data sets containing multiple data points were analyzed by one-way ANOVA followed by Tukey's multiple comparison (1 and 5 % error rate were used).
Results
Both Holo-RBP and Apo-RBP Bind STRA6
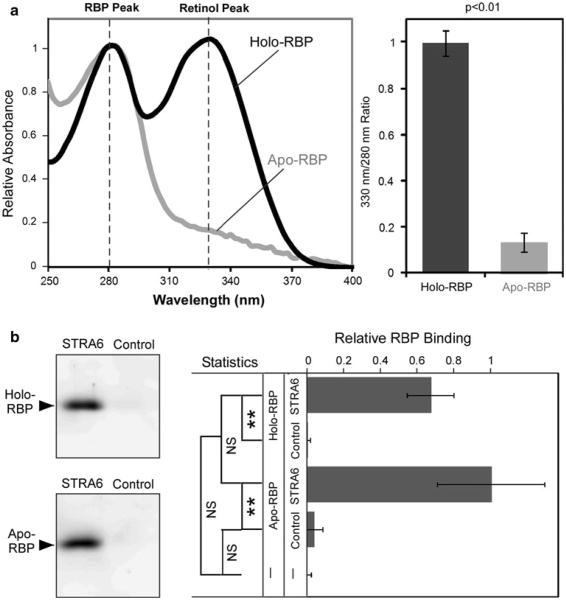
Because both retinol influx and retinol efflux mediated by STRA6 theoretically depend on STRA6 interacting with holo-RBP and apo-RBP, it is imperative to confirm these interactions. Two previous reports reached opposite conclusions on the interactions of holo-RBP and apo-RBP with STRA6 (Berry et al. 2011; Chen et al. 2012). To resolve the issue of STRA6's interaction with RBP, we used HPLC to purify correctly folded and fully loaded holo-RBP (as demonstrated by the retinol peak similar in height as the protein peak in the absorption spectrum), and produced apo-RBP from this correctly folded RBP (Fig. 1a). These reagents are different from the previous reports, which used either free retinol mixed with apo-RBP in solution (not holo-RBP) (Berry et al. 2011) or urine RBP combined with retinoic acid as holo-RBP (Chen et al. 2012). Using these reagents, we compared the binding of holo-RBP and apo-RBP to STRA6 and found that they both bind to STRA6 (Fig. 1b).
Fig. 1.
Both holo-RBP and apo-RBP bind to STRA6. a Absorption spectra of HPLC-purified holo-RBP fully loaded with retinol and apo-RBP produced from fully loaded RBP. The absorption at 280 nm is defined as 1. Statistical analysis of the difference between holo-RBP and apo-RBP in 330/280 nm ratio is shown on the right. Error bars indicates standard deviation (n = 3) for the ratio of absorption at 330 and 280 nm. Statistical significance is determined by Student's t test with equal variance. b Both holo-RBP and apo-RBP bind to STRA6. Holo-RBP (0.2 μM) or apo-RBP (0.2 μM) was incubated with STRA6-expressing cells or control cells that do not express STRA6. After 1 h binding, the cells were washed twice to remove unbound proteins. RBP associated with cells was detected by Western blot analysis. Western blot was recorded using Fujifilm LAS3000 Luminescent Image Analyzer and analyzed by Multi-Gauge software. Statistical analysis (n = 3) was conducted by Tukey's multiple comparison test (**p < 0.01; NS not significant)
STRA6 Catalyzes Retinol Transport from CRBP-I to Apo-RBP
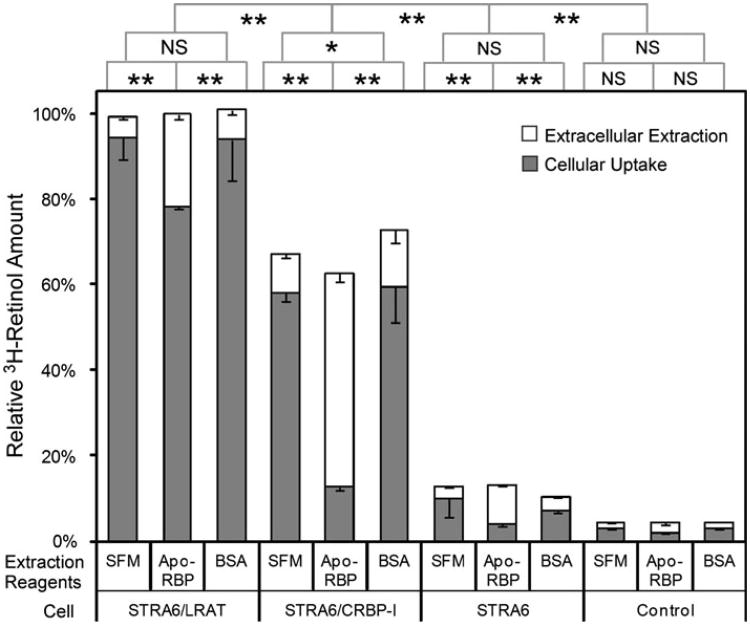
It was previously shown that STRA6 couples to CRBP-I for retinol uptake from holo-RBP. Can STRA6 also mediate retinol efflux into apo-RBP if retinol is bound to CRBP-I intracellularly? To test this possibility, we first incubated STRA6/CRBP-I cells with holo-RBP to allow retinol uptake by CRBP-I and then incubated the cells with pure apo-RBP. Interestingly, apo-RBP did cause retinol efflux and an almost complete loss of retinol taken up by CRBP-I (Fig. 2). This effect is apo-RBP dependent because BSA, a nonspecific retinol binding protein, did not have this effect. As a control, apo-RBP removed little retinol from STRA6/LRAT cells. This result revealed a difference in retinol uptake mediated by CRBP-I from retinol uptake mediated by LRAT in that retinol taken up through CRBP-I can be much more readily lost to extracellular apo-RBP.
Fig. 2.
Preferential loss of retinol taken up by STRA6/CRBP-I cells in an apo-RBP-dependent manner. After cells take up 3H-retinol from 3H-retinol/RBP for 1 h, incubation with apo-RBP for 1 h can remove more 3H-retinol from STRA6/CRBP-I than STRA6/LRAT or STRA6-only cells. BSA and SFM were used as controls for apo-RBP. 3H-retinol extracted during the 1 h incubation with apo-RBP, BSA, or SFM is shown in the white column. 3H-retinol remaining in the 0cells after extraction is shown in the gray column. The total amount of 3H-retinol taken up by cells after the first 1 h incubation is represented by combining the white column and blue column for each reaction. The highest activity by STRA6/LRAT cells is defined as 100 %. Statistical significance of the comparison of the extracted 3H-retinol is shown on the top of the graph (n = 3). Statistical significance was determined by Tukey's multiple comparison test (*p < 0.05; **p < 0.01; NS not significant)
Comparison of STRA6 Catalyzed Retinol Efflux from Retinol/CRBP-I and Retinol/CRBP-II
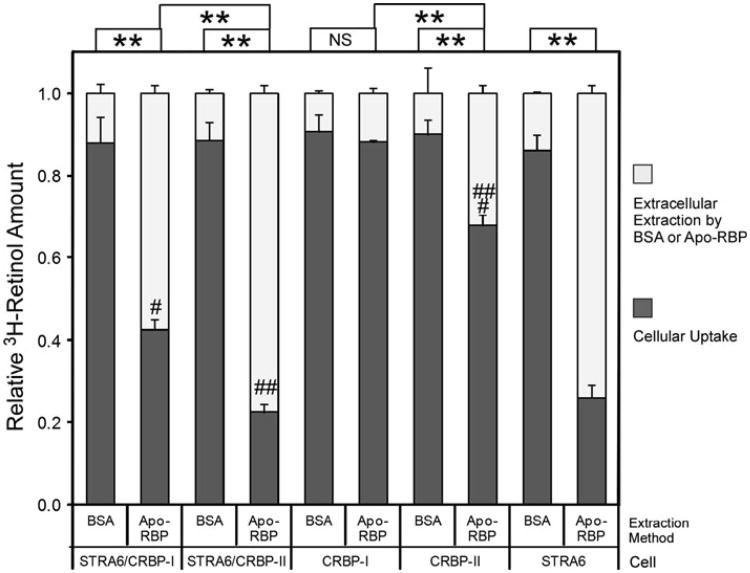
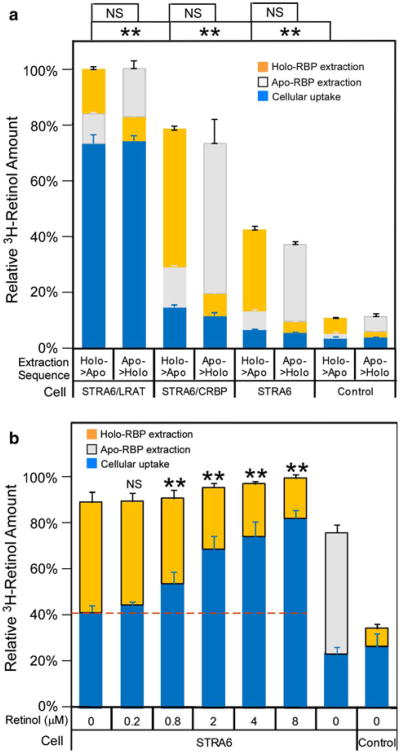
In contrast to CRBP-I, CRBP-II couples poorly to STRA6 in mediating cellular retinol uptake from holo-RBP (Kawaguchi et al. 2011). Can STRA6 mediate retinol efflux to extracellular apo-RBP from retinol taken up by CRBP-II? To compare CRBP-I and CRBP-II in retinol efflux, we first loaded cells with free 3H-retinol as has been done to study STRA6/LRAT in efflux previously (Isken et al. 2008) and then compared the ability of apo-RBP to cause retinol efflux from different cells. This method effectively loads cells with retinol in the absence of STRA6 and overcomes the difficulty of CRBP-II's ineffectiveness in coupling to STRA6 to take up retinol from 3H-retinol/RBP and CRBP-I's dependence on STRA6 to efficiently take up retinol from 3H-retinol/RBP. This method of loading retinol makes it possible to study STRA6 dependence of retinol efflux. Interestingly, STRA6 mediates retinol efflux from both STRA6/CRBP-I cells and STRA6/CRBP-II cells (Fig. 3). In addition, CRBP-II cells lose retinol to extracellular apo-RBP more readily than CRBP-I in the absence of STRA6. These experiments offer unique insight into the relationship between cellular retinol binding proteins and STRA6-mediated retinol efflux and the difference in STRA6-mediated retinol influx and efflux.
Fig. 3.
Comparison of CRBP-I and CRBP-II in STRA6-mediated retinol efflux. After cells were loaded with free 3H-retinol for 2 h, 1 μM of apo-RBP was added to cause 3H-retinol depletion for 2 h. BSA (1 μM) of was used as a control. 3H-retinol extracted during the 2-h incubation with apo-RBP or BSA is shown in the white column. 3H-retinol remaining in the cells after extraction is shown in the dark gray column. The total amount of 3H-retinol taken up by cells before the extraction is represented by combining the white column and dark gray column and is defined as 100 % for each reaction. Apo-RBP caused strong 3H-retinol depletion of both STRA6/CRBP-I cells (#) and STRA6/CRBP-II cells (##). In contrast to CRBP-I, CRBP-II loses retinol more readily to extracellular apo-RBP independently of STRA6 (###). Statistical significance of the comparison of the extracted 3H-retinol is shown on the top of the graph (n = 3). Statistical significance was determined by Tukey's multiple comparison test (**p < 0.01; NS not significant)
Apo-RBP to Holo-RBP Ratio Affects STRA6-mediated Retinol Efflux from Retinol/CRBP-I
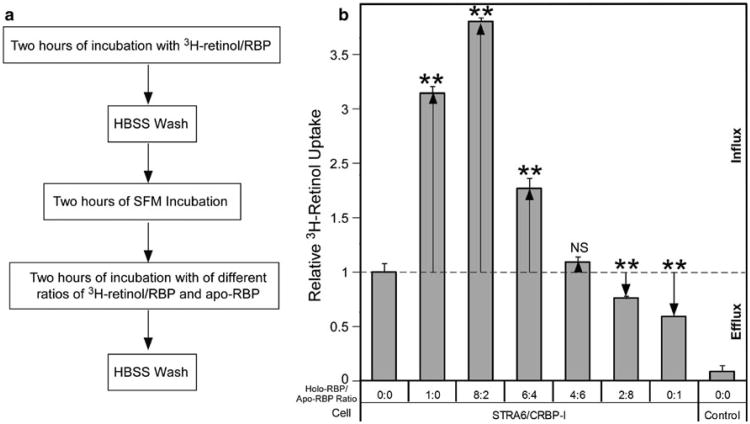
It was also shown previously that pure apo-RBP can cause a fraction of retinol to be lost from STRA6/LRAT cells (Isken et al. 2008). The above experiments showed that STRA6 can cause strong retinol efflux from retinol/CRBP-I to pure apo-RBP and an almost complete loss of retinol taken up by CRBP-I. However, the blood is known to contain only a small fraction of apo-RBP compared to holo-RBP. Whether apo-RBP causes retinol efflux in the presence of holo-RBP has not been studied. When we added holo-RBP in apo-RBP-dependent retinol efflux reactions, net flux depended on the ratio of holo-RBP to apo-RBP (Fig. 4). Retinol efflux only happened when there was an overwhelming amount of apo-RBP relative to holo-RBP. When the holo-RPB concentration was similar to or higher than the apo-RBP concentration, retinol influx instead of efflux happened. The effect of holo-RBP on STRA6-catalyzed retinol efflux is likely the combined result of holo-RBP's competition with apo-RBP in binding to STRA6 and the counteracting effect of STRA6-mediated retinol influx.
Fig. 4.
The ratio of holo-RBP and apo-RBP determines the ability of apo-RBP to cause net retinol efflux from STRA6/CRBP-I cells. a Diagram of experimental design. After taking up 3H-retinol from 3H-retinol/RBP for 2 h, the cells were washed with HBSS and incubated with SFM for 2 h to dissociate cell-surface bound RBP. Apo-RBP with different amounts of 3H-retinol/RBP was incubated with the cells for 2 h. After a further HBSS wash, the 3H-retinol remaining in cells was counted. b Effect of different ratios of holo-RBP to apo-RBP on the ability of apo-RBP to cause retinol efflux. Holo-RBP to apo-RBP ratio is shown on the X-axis. For all reactions in which apo-RBP was added (“8:2,” “6:4,” “4:6,” “2:8,” “0:1” reactions), the same amount of apo-RBP (0.12 μM) was used. Reaction “0:0” means neither holo-RBP nor apo-RBP was added, while “1:0” means only holo-RBP was added and “0:1” means only apo-RBP was added. The dashed line indicates basal level of 3H-retinol uptake without efflux or influx after the 2-h uptake (no additional apo-RBP or holo-RBP added). This basal level of 3H-retinol uptake is defined as 1. The downward arrow indicates net retinol efflux. The upward arrow indicates net retinol influx. Statistical analysis (n = 3) was determined by Tukey's multiple comparison test against the control reaction without holo-RBP/apo-RBP added in the last 2 h as indicated by “0:0” (**p < 0.01; NS not significant)
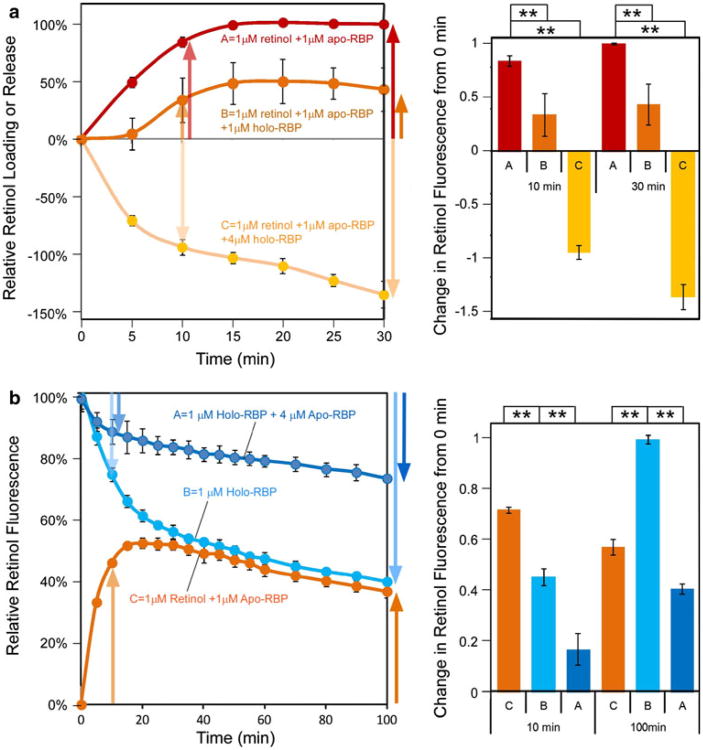
STRA6-Catalyzed Retinol Loading Is Inhibited by Holo-RBP and STRA6-Catalyzed Retinol Release Is Inhibited by Apo-RBP
It was previously known that STRA6 catalyzes efficient loading of retinol into apo-RBP in the absence of LRAT or CRBP-I. How is this loading reaction affected by holo-RBP? Using a retinol fluorescence assay established previously (Kawaguchi et al. 2011), we found that holo-RBP inhibits STRA6-catalyzed retinol loading into apo-RBP, although STRA6 catalyzes retinol loading into pure apo-RBP (Fig. 5a). This is consistent with results above using radioactive assays. When STRA6 is exposed to a mixture of RBP with a holo-RBP to apo-RBP ratio similar to that of the blood, STRA6 catalyzes net retinol release not loading. Conversely, apo-RBP inhibits STRA6-catalyzed retinol release from holo-RBP (Fig. 5b). In this experiment, we also examined a long time course of STRA6-catalyzed retinol loading and STRA6-catalyzed retinol release involving the same concentrations of retinol and RBP. STRA6-catalyzed retinol loading is not simply a mirror image of STRA6-catalyzed retinol release. These fundamental properties of STRA6's interactions with holo-RBP and apo-RBP determine STRA6-mediated retinol release and loading in the presence of a mixture of holo-RBP and apo-RBP. These experiments are consistent with the previous experiment (Fig. 1b) showing that both holo-RBP and apo-RBP bind to STRA6.
Fig. 5.
Inhibition of STRA6-catalyzed retinol loading into apo-RBP by holo-RBP and inhibition of STRA6-catalyzed retinol release from holo-RBP by apo-RBP. a Although STRA6-catalyzed retinol loading into pure apo-RBP and caused an increase in retinol fluorescence (reaction A), the presence of holo-RBP inhibited net retinol loading (reactions B and C). When holo-RBP to apo-RBP ratio approached that of blood (reaction C), STRA6 catalyzed net retinol release, not retinol loading. To visualize and compare time-dependent changes, retinol fluorescence at time 0 min is defined as 0 for all reactions, and the highest retinol fluorescence change achieved in the retinol loading reaction (red reaction) is defined as 100 %. Statistical significance of the 10 min and 30 min data points (n = 3) was determined by Tukey's multiple comparison test (**p < 0.01). b Apo-RBP inhibits STRA6-catalyzed retinol release. STRA6 catalyzed retinol release from 1 μM holo-RBP (reaction A) is strongly inhibited by the presence of 4 μM apo-RBP (reaction B). STRA6-catalyzed retinol loading (reaction C) is not a mirror image of STRA6-catalyzed retinol release (reaction B), although they contain the same concentration of retinol and RBP. For all reactions, fluorescence before the addition of holo-RBP or apo-RBP is defined as 0. Retinol fluorescence of 1 μM holo-RBP is defined as 100 %. Statistical significance of the 10 and 100 min data points (n = 3) was determined by Tukey's multiple comparison test (**p < 0.01)
STRA6 Catalyzes Retinol Exchange Between Intracellular Holo-CRBP-I and Extracellular Holo-RBP
There are two possible mechanisms to explain the lack of net retinol efflux caused by apo-RBP in the presence of holo-RBP. It is possible that retinol efflux does not happen in the presence of holo-RBP, but it is more likely that retinol influx counteracts retinol efflux to prevent net retinol loss in the presence of holo-RBP. To test the second possibility, we distinguished intracellular retinol from the extracellular retinol by preloading STRA6/CRBP-I cells with 3H-retinol from 3H-retinol/RBP and then incubating cells with cold holo-RBP or apo-RBP (Fig. 6a). Amazingly, intracellular retinol clearly still gets out of the cell in a STRA6- and RBP-dependent manner in the presence of holo-RBP. Cold holo-RBP is as effective as apo-RBP in causing retinol efflux from STRA6/CRBP-I cells (Fig. 6a). This experiment demonstrates that retinol efflux still happens in the presence of holo-RBP. Although retinol influx in the presence of holo-RBP prevents net retinol efflux, the efflux reaction can exchange retinol between intracellular CRBP-I and extracellular RBP. The existence of the retinol exchange activity suggests that cold holo-RBP causes 3H-retinol loss from STRA6 expressing cells not only by removing 3H-retinol/RBP bound to cell-surface STRA6 but also through retinol exchange between 3H-retinol and cold retinol. To further demonstrate the existence of retinol exchange, we added unlabeled retinol before addition of holo-RBP but after STRA6 cells were incubated with 3H- retinol/RBP. Indeed, unlabeled retinol blocked the ability of holo-RBP to remove 3H-retinol from STRA6 cells (Fig. 6b). This result is consistent with the occurrence of retinol exchange.
Fig. 6.
STRA6-catalyzed retinol exchange. a Holo-RBP and apo-RBP are similarly effective in their abilities to remove 3H-retinol from STRA6/CRBP-I cells. Both apo-RBP and cold holo-RBP extract much more 3H-retinol from STRA6/CRBP-I cells than from STRA6/LRAT cells. After cells took up 3H-retinol from 3H-retinol/RBP for 1 h, unlabeled holo-RBP and apo-RBP were sequentially added to cells (1 h incubation each) to extract 3H-retinol. The amounts of 3H-retinol extracted by holo-RBP (yellow column), 3H-retinol extracted by apo-RBP (white column), or 3H-retinol remaining in cells (blue column) are shown for each reaction. The sequence of addition is indicated on the bottom of each reaction. The total amount of 3H-retinol taken up by cells after the first 1 h incubation is represented by combining the white, yellow, and blue columns for each reaction. The highest total activity by STRA6/LRAT cells is defined as 100 %. Statistical significance (n = 3) of the comparison between the first extraction by holo-RBP or apo-RBP was determined by Tukey's multiple comparison test (**p < 0.01; NS not significant). b Unlabeled retinol suppressed the ability of unlabeled holo-RBP to extract 3H-retinol. After cells took up 3H-retinol from 3H-retinol/RBP for 1 h, they were washed with SFM. Different amounts of cold retinol were added to cells incubated in SFM for 10 min. After removal of medium, unlabeled holo-RBP or apo-RBP was added to cells (1 h incubation) to extract 3H-retinol. The amount of 3H-retinol extracted by holo-RBP (yellow column), 3H-retinol extracted by apo-RBP (white column), or 3H-retinol remaining in cells (blue column) are shown. The total amount of 3H-retinol taken up by cells after the first 1 h incubation is represented by combining extracted amount (yellow or white column) with cellular uptake after extraction (blue column) for each reaction. The highest activity by STRA6 cells is defined as 100 %. Statistical significance of the comparison between the retinol addition reactions and the control reaction with no retinol added (n = 3) was determined by Tukey's multiple comparison test (**p < 0.01; NS not significant)
STRA6 Catalyzes Net Retinol Influx, Not Efflux in the Presence of Human Serum
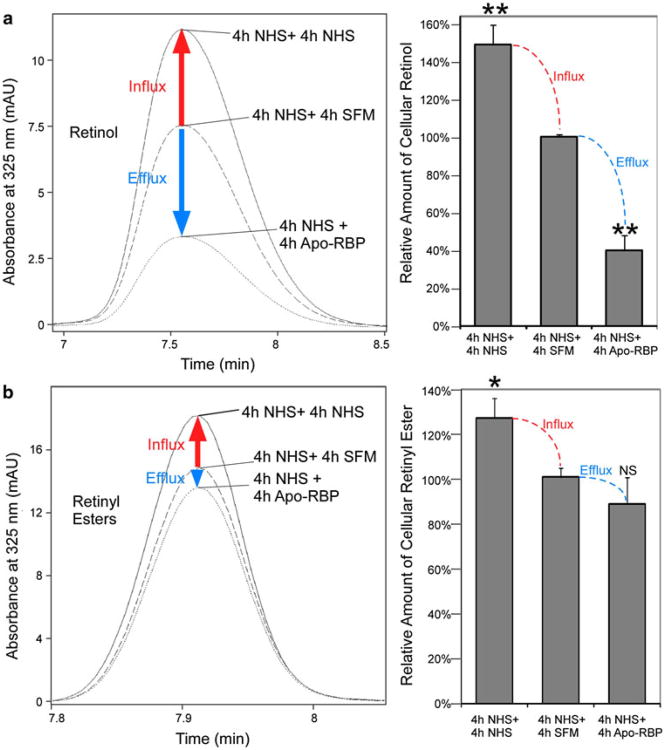
Human serum contains RBP in the form of holo-RBP/Transthyretin (TTR) complex and a fraction of apo-RBP (Mills et al. 2008; Zanotti and Berni 2004). Does the apo-RBP in human serum cause retinol efflux from STRA6/LRAT or STRA6/CRBP-I cells? Using HPLC assays, we found that strong retinol efflux to pure extracellular apo-RBP did happen for STRA6/CRBP-I cells that have taken retinol from human serum (Fig. 7a). However, human serum, which contains both holo-RBP and apo-RBP, caused net retinol influx, not retinol efflux for either STRA6/LRAT cells or STRA6/CRBP-I cells that have previously taken up retinol (Fig. 7a, b). This result is consistent with the inhibitory effect of holo-RBP on apo-RBP-mediated retinol efflux from STRA6-expressing cells.
Fig. 7.
HPLC analyses demonstrating that STRA6 mediates net vitamin A influx not efflux when exposed to serum. a HPLC assays for retinol efflux from STRA6/CRBP-I cells. After STRA6/CRBP-I cells took up retinol from NHS for 4 h, they are further incubated with SFM, NHS, or 1 μM apo-RBP for another 4 h. Although apo-RBP caused significant retinol loss compared to the SFM control, NHS caused further retinol uptake, not loss. Left representative retinol peaks from the HPLC analyses. Right average data from 3 independent experiments. Statistical significance (n = 3) of the comparison between SFM incubation with apo-RBP incubation (efflux) or with NHS incubation (influx) was determined by Tukey's multiple comparison test (**p < 0.01). The retinol level in the SFM control is defined as 100 %. b HPLC assays for retinol efflux from STRA6/LRAT cells. After STRA6/LRAT cells took up retinol from NHS for 4 h, they are further incubated with SFM, NHS, or 1 μM apo-RBP for another 4 h. Left panel shows representative retinyl ester peaks from the HPLC analyses. Right panel shows the average data from three independent experiments. The level of retinyl ester in the SFM control is defined as 100 %. Statistical significance (n = 3) of the comparison between SFM incubation with apo-RBP incubation (efflux) or with NHS incubation (influx) was determined by Tukey's multiple comparison test (*p < 0.05; NS not significant)
STRA6 Catalyzes Retinol Exchange between CRBP-I and Human Serum
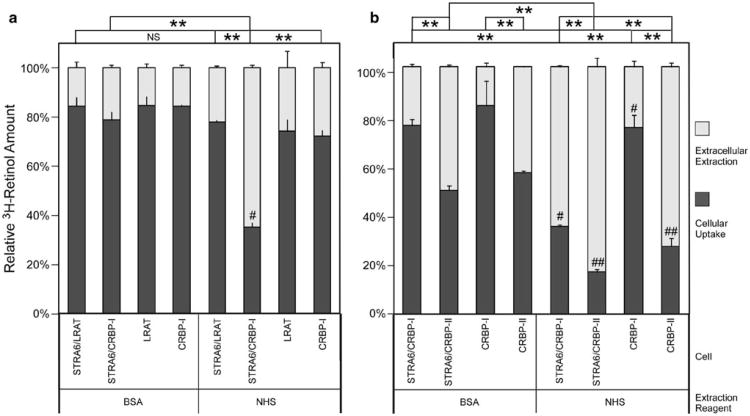
Given the retinol exchange reaction mentioned above, is it possible that intracellular retinol exchanges with retinol in human serum despite the ability of serum to cause net retinol influx in STRA6-expressing cells? To study retinol exchange, we preloaded cells with 3H-retinol and found that human serum does cause strong STRA6-dependent 3H-retinol efflux from STRA6/CRBP-I cells (Fig. 8a), consistent with STRA6-catalyzed retinol exchange between intracellular CRBP-I and extracellular RBP. This STRA6-dependent 3H-retinol efflux is much more efficient from STRA6/CRBP-I cells than STRA6/LRAT cells. This experiment demonstrates that STRA6 mediates retinol exchange between intracellular CRBP-I and the serum, although holo-RBP in the serum prevents net retinol efflux from cells. We also compared the ability of serum in causing 3H-retinol efflux from intracellular CRBP-I and CRBP-II. Although STRA6 promotes 3H-retinol efflux from both CRBP-I and CRBP-II cells, 3H-retinol efflux caused by human serum is much less STRA6-dependent for CRBP-II cells than CRBP-I cells (Fig. 8b).
Fig. 8.
STRA6 catalyzes retinol exchange between intracellular CRBP and the serum. To study retinol exchange, intracellular retinol is distinguished from extracellular retinol using 3H-retinol. a After cells were loaded with free 3H-retinol for 2 h, NHS or BSA was added to cause 3H-retinol depletion. NHS caused strong 3H-retinol depletion of STRA6/CRBP-I cells (#), as compared to STRA6/LRAT cells. The strong 3H-retinol depletion from CRBP-I expression cells is both STRA6 dependent and NHS dependent (the control is 5 mg/ml BSA). b Comparison of CRBP-I and CRBP-II in retinol efflux. After cells were loaded with free 3H-retinol (2 h), NHS or BSA was added to cause 3H-retinol depletion (2 h). Although NHS-mediated depletion of CRBP-I cells is dependent on STRA6 (compare the 2 reactions marked with #), NHS can strongly deplete 3H-retinol from CRBP-II cells with or without STRA6 (compare the 2 reactions marked with ##). Even BSA (5 mg/ml) is sufficient to cause large 3H-retinol efflux from CRBP-II-expressing cells. In both (a) and (b), 3H-retinol extracted by NHS or BSA is shown in the white column and 3H-retinol remaining in the cells after extraction is shown in the dark gray column. The total activity of each reaction before extraction is defined as 100 %. Statistical significance (n = 3) of the extracted 3H-retinol was determined by Tukey's multiple comparison test. Comparison is shown on the top of each figure (**p < 0.01; NS not significant)
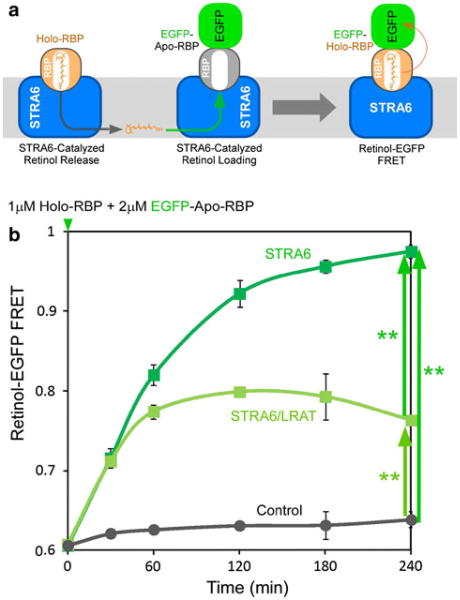
STRA6 Catalyzes Retinol Exchange between RBP Molecules
Given the mechanism of STRA6's action, its abilities to catalyze retinol release from RBP and loading into RBP should be responsible for its ability to catalyze retinol exchange between RBP and CRBP-I. If that is the case, the exchange reaction should also apply to RBP molecules. To test this possibility, we used fluorescence resonance transfer (FRET) between retinol and EGFP by tagging RBP with EGFP. The FRET pair of retinol and EGFP has been demonstrated previously (Kawaguchi et al. 2011). We tagged EGFP at the N-terminus of RBP because we found previously that N-terminus tag does not interfere with RBP's binding to STRA6 (Kawaguchi et al. 2007). By incubating untagged holo-RBP with EGFP-RBP, we found that STRA6 indeed catalyzes retinol transport between RBP molecules as shown by the time-dependent and STRA6-dependent increase in retinol-EGFP FRET signals (Fig. 9). LRAT suppresses this retinol transfer. This experiment revealed another perspective of STRA6's function in vitamin A uptake. STRA6 constantly catalyzes retinol exchange between holo-RBP and apo-RBP. During this exchange reaction, holo-RBP becomes apo-RBP and apo-RBP becomes holo-RBP. LRAT couples to STRA6 for vitamin A uptake by stopping this exchange reaction. This retinol exchange activity is a further support of the proposed mechanism of STRA6 (Kawaguchi et al. 2011) and argues against the absolute dependence of STRA6 for LRAT or CRBP-I for its activity.
Fig. 9.
Retinol-EGFP FRET demonstrating STRA6-catalyzed retinol exchange between RBP molecules. a Schematic diagram of the proposed mechanism of STRA6-catalyzed retinol exchange between RBP molecules. To demonstrate STRA6-catalyzed exchange between RBP molecules, apo-RBP is tagged with EGFP. Retinol binding to EGFP-RBP would lead to fluorescence resonance energy transfer between retinol and EGFP. b STRA6 catalyzes the transfer of retinol from untagged holo-RBP to EGFP-apo-RBP in a time-dependent manner (dark green), as shown by the increase in retinol-EGFP FRET. This transfer reaction does not happen in the absence of STRA6 (black) and is suppressed by LRAT (light green). Statistical significance (n = 3) was determined by Tukey's multiple comparison test (**p < 0.01)
Discussion
LRAT was the first protein shown to enhance STRA6's vitamin A uptake activity (Golczak et al. 2008; Isken et al. 2008; Kawaguchi et al. 2007). It was also shown that the reverse reaction of vitamin A loading into apo-RBP still happens in cells that express both STRA6 and LRAT to lead to cellular retinol loss (Isken et al. 2008). This finding suggests that STRA6's action can lead to retinol efflux (Isken et al. 2008). The activity of STRA6-catalyzed retinol loading into apo-RBP is much stronger without LRAT (Kawaguchi et al. 2011). Can STRA6 mediate both retinol uptake and retinol efflux, analogous to the role of HDL receptor SR-BI's role in cholesterol uptake and efflux (Connelly and Williams 2004)? If STRA6 can do both, what are the factors that determine these activities? If STRA6 mediates retinol efflux to cause vitamin A loss, how does it function to take up vitamin A?
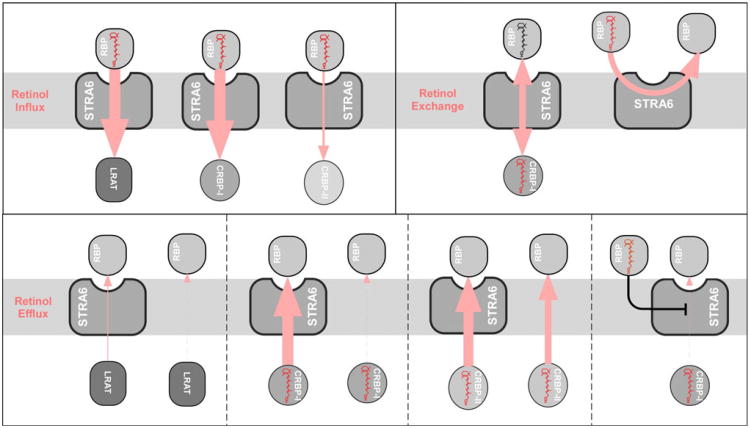
To answer these questions, we systematically examined the roles of individual components involved in these activities to dissect STRA6's mechanism (Table 1). For example, natural blood contains both holo-RBP and apo-RBP. If only natural blood is used as a source of RBP (no purified component), it is not possible to evaluate apo-RBP's binding to STRA6, to study apo-RBP's role in retinol efflux, to compare STRA6's binding to holo-RBP and apo-RBP, or to reveal their mutual competition during STRA6-mediated retinol influx and efflux. Using this strategy, we found that STRA6's ability to mediate retinol efflux is determined by both RBP species (apo-RBP to holo-RBP ratio) and intracellular retinol storage mechanisms (CRBP-I or LRAT) (Table 1; Fig. 10). Holo-RBP inhibits STRA6-mediated retinol efflux by apo-RBP. Similarly, apo-RBP inhibits STRA6-mediated retinol influx from holo-RBP. However, holo-RBP permits retinol exchange between intracellular CRBP and extracellular RBP (Table 1). In addition, experiments presented here (e.g., STRA6-catalyzed retinol exchange between RBP molecules) provide further support for the proposed mechanism of STRA6 (Kawaguchi et al. 2011) and argue against the alternative models. For example, they argue against the models that propose a strict dependence of STRA6 on CRBP-I or LRAT for its activity. Lastly, this study also helps to explain the opposite conclusions of STRA6's interaction with holo-RBP and apo-RBP from two previous reports (Berry et al. 2011; Chen et al. 2012) (Supplementary Discussion and Supplementary Fig. 1).
Table 1. Summary of key experimental conditions to dissect STRA6-dependent retinol influx, efflux, and exchange.
| Extracellular | Intracellular | STRA6-dependent retinol transport | |||
|---|---|---|---|---|---|
|
|
|
||||
| Holo-RBP | Apo-RBP | Holo-CRBP-I | Apo-CRBP-I | LRAT | |
| + | − | − | + | − | Influx |
| − | + | + | − | − | Efflux |
| + | − | + | − | − | Exchange |
| + | − | − | − | + | Influx |
| +a | + | − | + | − | Influx |
| +a | + | − | − | + | Influx |
| +a | + | + | − | − | Exchange |
| + | + | − | − | − | Exchange |
Holo-RBP to apo-RBP ratio similar to that of blood
Fig. 10.
Schematic diagrams comparing STRA6-mediated retinol influx with STRA6-mediated retinol exchange and retinol efflux. The directions of retinol transport are indicated by light red arrows. The efficiency of retinol transport is indicated by the thickness of each arrow (the thicker the arrow, the stronger the flow)
There are three major findings of this study. First, pure extracellular apo-RBP can effectively cause retinol efflux from STRA6/CRBP-I cells and can deplete almost all retinol taken up by STRA6/CRBP-I cells. A simple hypothesis to explain the previous finding that CRBP-I effectively couples to STRA6 for retinol uptake from holo-RBP is that CRBP-I's affinity for retinol is higher (or much higher) than that of RBP bound to STRA6. This hypothesis is consistent with STRA6's ability to catalyze retinol release from holo-RBP and suggests that STRA6 may simply functions like a “bottle opener” (if RBP is analogous to a “bottle” that holds vitamin A) to let retinol out. However, STRA6's ability to catalyze retinol efflux from CRBP-I to apo-RBP, as shown in the study, argues against this hypothesis and suggests that CRBP-I's affinity for retinol is similar to that of RBP bound to STRA6. The structural basis of STRA6's ability to fine-tune the transport of retinol into and out of RBP so that it catalyzes both retinol influx from RBP to CRBP-I and retinol efflux from CRBP-I to RBP is still unknown.
CRBP-I's behavior is certainly not the only possible scenario of how cellular retinol binding proteins may couple to STRA6. Unlike the more widely expressed CRBP-I, CRBP-II is most highly expressed in the small intestine and functions to facilitate the uptake of free retinol by enterocytes (Harrison 2005). Because both CRBP-I and CRBP-II are cellular retinol binding proteins and CRBP-II binds retinol with lower affinity (Ong 1994), we included CRBP-II here and in a previous study (Kawaguchi et al. 2011) as a control for CRBP-I. CRBP-II couples poorly with STRA6 in taking up retinol from holo-RBP, but it can couple to STRA6 for retinol efflux into apo-RBP.
The effective coupling of LRAT and CRBP-I to STRA6 for retinol influx distinguishes them from CRBP-II, but the effective coupling of CRBP-I and CRBP-II to STRA6 for retinol efflux distinguishes them from LRAT and the degree of dependence on STRA6 distinguishes CRBP-I from CRBP-II in retinol efflux (CRBP-II depends less on STRA6 for retinol efflux). Although retinyl ester formation by LRAT prevents STRA6-catalyzed retinol efflux into apo-RBP, it is possible that there exist a still unidentified complex regulatory mechanism that promotes retinol efflux into apo-RBP by native cells that express both STRA6 and LRAT. This mechanism needs to balance retinyl ester formation, which prevents retinol efflux, and retinyl ester hydrolysis, which prevents vitamin A storage as retinyl esters.
Second, holo-RBP inhibits STRA6-mediated retinol efflux into apo-RBP. Holo-RBP promotes STRA6-mediated retinol release, which counteracts retinol loading. For healthy individuals, the blood contains on average 2.67 μM RBP, which includes about 90 % holo-RBP and 10 % apo-RBP (Mills et al. 2008). It was also known previously that the residence time for RBP uncomplexed with TTR is only 1.2–1.3 h in the blood (Vahlquist et al. 1973). We have shown that holo-RBP inhibits STRA6-catalyzed retinol loading into apo-RBP. At the ratio of holo-RBP to apo-RBP found in the blood, STRA6 mediates net retinol release from RBP, not retinol loading into RBP. In the presence of human serum, STRA6 mediates net vitamin A uptake (not loss), despite the presence of a small fraction of apo-RBP in serum.
Consistent with STRA6's function in vitamin A uptake, retinoid analyses have shown that STRA6 knockdown in zebrafish (Isken et al. 2008) or STRA6 knockout in mice (Ruiz et al. 2012) both lead to loss of tissue vitamin A uptake, not excessive vitamin A accumulation. If STRA6's primary function is retinol efflux, excessive vitamin A accumulation is expected. STRA6 knockout in mice leads to a loss of more than 90 % of retinyl ester, the major form of stored vitamin A, in the retinal pigment epithelium (RPE), the primary cell type responsible for vitamin A uptake for vision (Ruiz et al. 2012). Because RPE-specific knockout of LRAT leads to a similar decrease in retinyl ester levels in the RPE (LRAT-independent retinyl ester in the RPE is a result of cellular uptake of retinyl ester directly) (Ruiz et al. 2007), STRA6 is responsible for virtually all retinol from the blood that is accessible to LRAT in the RPE. To prevent excessive accumulation of cellular retinoid, it is possible that there exist unknown cellular proteins that might mediate retinol efflux into extracellular apo-RBP with or without STRA6's involvement.
Third, STRA6 catalyzes efficient retinol exchange between extracellular RBP molecules and between extracellular RBP and intracellular CRBP-I. Unlike apo-RBP-dependent retinol efflux, STRA6-catalyzed retinol exchange between intracellular CRBP-I and extracellular RBP applies to holo-RBP and is not inhibited by holo-RBP. We have demonstrated that the exchange happens in human serum, which consists largely of holo-RBP. It is likely that this retinol exchange between extracellular and intracellular binding proteins functions to “refresh” the intracellular stores of retinol to guard against depletion due to gradual oxidation occurring during long-term storage. The CRBP-I and STRA6-dependent retinol exchange is a newly identified role of CRBP-I in retinol homeostasis and distinguishes CRBP-I from LRAT although both proteins can couple to STRA6 for retinol uptake.
This finding may also help to explain a few interesting and puzzling earlier findings on vitamin A transport. Kinetic models of vitamin A metabolism predicted that 50–70 % of the total input of retinol into the circulation comes from extrahepatic tissues (Blomhoff et al. 1991). This result is seemingly counterintuitive given the role of the liver as the major site of holo-RBP secretion. Another interesting puzzle is that muscle-expressed human RBP can rescue RBP null mice's vision defect (Quadro et al. 2002), although muscle-expressed human RBP cannot mobilize liver stored vitamin A (Quadro et al. 2004). STRA6's potent retinol exchange activity between RBP and CRBP-I discovered in this study is a likely source of retinol input from extrahepatic tissues and may contribute to the retinol in human RBP that rescues the vision defect.
Supplementary Material
Acknowledgments
Supported by National Institutes of Health Grant 5R01EY018144.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00232-012-9463-1) contains supplementary material, which is available to authorized users.
Contributor Information
Riki Kawaguchi, Department of Physiology, Jules Stein Eye Institute and Howard Hughes Medical Institute, David Geffen School of Medicine, University of California, Los Angeles, CA, USA.
Ming Zhong, Department of Physiology, Jules Stein Eye Institute and Howard Hughes Medical Institute, David Geffen School of Medicine, University of California, Los Angeles, CA, USA.
Miki Kassai, Department of Physiology, Jules Stein Eye Institute and Howard Hughes Medical Institute, David Geffen School of Medicine, University of California, Los Angeles, CA, USA.
Mariam Ter-Stepanian, Department of Physiology, Jules Stein Eye Institute and Howard Hughes Medical Institute, David Geffen School of Medicine, University of California, Los Angeles, CA, USA.
Hui Sun, Email: hsun@mednet.ucla.edu, Department of Physiology, Jules Stein Eye Institute and Howard Hughes Medical Institute, David Geffen School of Medicine, University of California, Los Angeles, CA, USA.
References
- Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- Babitt J, Trigatti B, Rigotti A, Smart EJ, Anderson RG, Xu S, Krieger M. Murine SR-BI, a high density lipoprotein receptor that mediates selective lipid uptake, is N-glycosylated and fatty acylated and colocalizes with plasma membrane caveolae. J Biol Chem. 1997;272:13242–13249. doi: 10.1074/jbc.272.20.13242. [DOI] [PubMed] [Google Scholar]
- Berry DC, Jin H, Majumdar A, Noy N. Signaling by vitamin A and retinol-binding protein regulates gene expression to inhibit insulin responses. Proc Natl Acad Sci USA. 2011;108:4340–4345. doi: 10.1073/pnas.1011115108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomhoff R, Green MH, Berg T, Norum KR. Transport and storage of vitamin A. Science. 1990;250:399–404. doi: 10.1126/science.2218545. [DOI] [PubMed] [Google Scholar]
- Blomhoff R, Green MH, Green JB, Berg T, Norum KR. Vitamin A metabolism: new perspectives on absorption, transport, and storage. Physiol Rev. 1991;71:951–990. doi: 10.1152/physrev.1991.71.4.951. [DOI] [PubMed] [Google Scholar]
- Bouillet P, Sapin V, Chazaud C, Messaddeq N, Decimo D, Dolle P, Chambon P. Developmental expression pattern of Stra6, a retinoic acid–responsive gene encoding a new type of membrane protein. Mech Dev. 1997;63:173–186. doi: 10.1016/s0925-4773(97)00039-7. [DOI] [PubMed] [Google Scholar]
- Chen CH, Hsieh TJ, Lin KD, Lin HY, Lee MY, Hung WW, Hsiao PJ, Shin SJ. Increased unbound retinol binding protein 4 concentration induced apoptosis through its receptor-mediated signaling. J Biol Chem. 2012;287:9694–9707. doi: 10.1074/jbc.M111.301721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly MA. SR-BI-mediated HDL cholesteryl ester delivery in the adrenal gland. Mol Cell Endocrinol. 2009;300:83–88. doi: 10.1016/j.mce.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Connelly MA, Williams DL. Scavenger receptor BI: a scavenger receptor with a mission to transport high density lipoprotein lipids. Curr Opin Lipidol. 2004;15:287–295. doi: 10.1097/00041433-200406000-00008. [DOI] [PubMed] [Google Scholar]
- Connelly MA, de la Llera-Moya M, Monzo P, Yancey PG, Drazul D, Stoudt G, Fournier N, Klein SM, Rothblat GH, Williams DL. Analysis of chimeric receptors shows that multiple distinct functional activities of scavenger receptor, class B, type I (SR-BI), are localized to the extracellular receptor domain. Biochemistry. 2001;40:4259–5259. doi: 10.1021/bi002825r. [DOI] [PubMed] [Google Scholar]
- Crouch RK, Chader GJ, Wiggert B, Pepperberg DR. Retinoids and the visual process. Photochem Photobiol. 1996;64:613–621. doi: 10.1111/j.1751-1097.1996.tb03114.x. [DOI] [PubMed] [Google Scholar]
- Dowling JE. Night blindness. Sci Am. 1966;215:78–84. doi: 10.1038/scientificamerican1066-78. [DOI] [PubMed] [Google Scholar]
- Drager UC. Retinoic acid signaling in the functioning brain. Sci STKE. 2006;2006:pe10. doi: 10.1126/stke.3242006pe10. [DOI] [PubMed] [Google Scholar]
- Evans RM. The molecular basis of signaling by vitamin A and its metabolites. Harvey Lect. 1994;90:105–117. [PubMed] [Google Scholar]
- Golczak M, Maeda A, Bereta G, Maeda T, Kiser PD, Hunzelmann S, von Lintig J, Blaner WS, Palczewski K. Metabolic basis of visual cycle inhibition by retinoid and nonretinoid compounds in the vertebrate retina. J Biol Chem. 2008;283:9543–9554. doi: 10.1074/jbc.M708982200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman DS. Plasma retinol-binding protein. In: Sporn MB, Boberts AB, Goodman DS, editors. The Retinoids. Academic Press; New York: 1984. pp. 41–88. [Google Scholar]
- Harrison EH. Mechanisms of digestion and absorption of dietary vitamin A. Annu Rev Nutr. 2005;25:87–103. doi: 10.1146/annurev.nutr.25.050304.092614. [DOI] [PubMed] [Google Scholar]
- Heller J. Interactions of plasma retinol-binding protein with its receptor. Specific binding of bovine and human retinol-binding protein to pigment epithelium cells from bovine eyes. J Biol Chem. 1975;250:3613–3619. [PubMed] [Google Scholar]
- Heller J, Bok D. Transport of retinol from the blood to the retina: involvement of high molecular weight lipoproteins as intracellular carriers. Exp Eye Res. 1976;22:403–410. doi: 10.1016/0014-4835(76)90178-0. [DOI] [PubMed] [Google Scholar]
- Heller J, Horwitz J. Conformational changes following interaction between retinol isomers and human retinol-binding protein and between the retinol-binding protein and prealbumin. J Biol Chem. 1973;248:6308–6316. [PubMed] [Google Scholar]
- Isken A, Golczak M, Oberhauser V, Hunzelmann S, Driever W, Imanishi Y, Palczewski K, von Lintig J. RBP4 disrupts vitamin A uptake homeostasis in a stra6-deficient animal model for Matthew-Wood syndrome. Cell Metab. 2008;7:258–268. doi: 10.1016/j.cmet.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wiita P, Bok D, Sun H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science. 2007;315:820–825. doi: 10.1126/science.1136244. [DOI] [PubMed] [Google Scholar]
- Kawaguchi R, Yu J, Wiita P, Honda J, Sun H. An essential ligand-binding domain in the membrane receptor for retinol-binding protein revealed by large-scale mutagenesis and a human polymorphism. J Biol Chem. 2008a;283:15160–15168. doi: 10.1074/jbc.M801060200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi R, Yu J, Wiita P, Ter-Stepanian M, Sun H. Mapping the membrane topology and extracellular ligand binding domains of the retinol binding protein receptor. Biochemistry. 2008b;47:5387–5395. doi: 10.1021/bi8002082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi R, Yu J, Ter-Stepanian M, Zhong M, Cheng G, Yuan Q, Jin M, Travis GH, Ong D, Sun H. Receptor-mediated cellular uptake mechanism that couples to intracellular storage. ACS Chem Biol. 2011;6:1041–1051. doi: 10.1021/cb200178w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maden M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat Rev Neurosci. 2007;8:755–765. doi: 10.1038/nrn2212. [DOI] [PubMed] [Google Scholar]
- Mark M, Ghyselinck NB, Chambon P. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu Rev Pharmacol Toxicol. 2006;46:451–480. doi: 10.1146/annurev.pharmtox.46.120604.141156. [DOI] [PubMed] [Google Scholar]
- Mills JP, Furr HC, Tanumihardjo SA. Retinol to retinol-binding protein (RBP) is low in obese adults due to elevated apo-RBP. Exp Biol Med (Maywood) 2008;233:1255–1261. doi: 10.3181/0803-RM-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli JL. Interactions of retinoid binding proteins and enzymes in retinoid metabolism. Biochim Biophys Acta. 1999;1440:139–162. doi: 10.1016/s1388-1981(99)00117-1. [DOI] [PubMed] [Google Scholar]
- Newcomer ME, Ong DE. Plasma retinol binding protein: structure and function of the prototypic lipocalin. Biochim Biophys Acta. 2000;1482:57–64. doi: 10.1016/s0167-4838(00)00150-3. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Dolle P. Retinoic acid in development: towards an integrated view. Nat Rev Genet. 2008;9:541–553. doi: 10.1038/nrg2340. [DOI] [PubMed] [Google Scholar]
- Noy N. Retinoid-binding proteins: mediators of retinoid action. Biochem J. 2000;348(pt 3):481–495. [PMC free article] [PubMed] [Google Scholar]
- Ong DE. Cellular transport and metabolism of vitamin A: roles of the cellular retinoid-binding proteins. Nutr Rev. 1994;52:S24–S31. doi: 10.1111/j.1753-4887.1994.tb01383.x. [DOI] [PubMed] [Google Scholar]
- Quadro L, Blaner WS, Hamberger L, Van Gelder RN, Vogel S, Piantedosi R, Gouras P, Colantuoni V, Gottesman ME. Muscle expression of human retinol-binding protein (RBP). Suppression of the visual defect of RBP knockout mice. J Biol Chem. 2002;277:30191–30197. doi: 10.1074/jbc.M205046200. [DOI] [PubMed] [Google Scholar]
- Quadro L, Hamberger L, Colantuoni V, Gottesman ME, Blaner WS. Understanding the physiological role of retinol-binding protein in vitamin A metabolism using transgenic and knockout mouse models. Mol Aspects Med. 2003;24:421–430. doi: 10.1016/s0098-2997(03)00038-4. [DOI] [PubMed] [Google Scholar]
- Quadro L, Blaner WS, Hamberger L, Novikoff PM, Vogel S, Piantedosi R, Gottesman ME, Colantuoni V. The role of extrahepatic retinol binding protein in the mobilization of retinoid stores. J Lipid Res. 2004;45:1975–1982. doi: 10.1194/jlr.M400137-JLR200. [DOI] [PubMed] [Google Scholar]
- Quadro L, Hamberger L, Gottesman ME, Wang F, Colantuoni V, Blaner WS, Mendelsohn CL. Pathways of vitamin A delivery to the embryo: insights from a new tunable model of embryonic vitamin A deficiency. Endocrinology. 2005;146:4479–4490. doi: 10.1210/en.2005-0158. [DOI] [PubMed] [Google Scholar]
- Rask L, Peterson PA. In vitro uptake of vitamin A from the retinol-binding plasma protein to mucosal epithelial cells from the monkey's small intestine. J Biol Chem. 1976;251:6360–6366. [PubMed] [Google Scholar]
- Ruiz A, Ghyselinck NB, Mata N, Nusinowitz S, Lloyd M, Dennefeld C, Chambon P, Bok D. Somatic ablation of the lrat gene in the mouse retinal pigment epithelium drastically reduces its retinoid storage. Invest Ophthalmol Vis Sci. 2007;48:5377–5387. doi: 10.1167/iovs.07-0673. [DOI] [PubMed] [Google Scholar]
- Ruiz A, Mark M, Jacobs H, Klopfenstein M, Hu J, Lloyd M, Habib S, Tosha C, Radu RA, Ghyselinck NB, Nusinowitz S, Bok D. Retinoid content, visual responses and ocular morphology are compromised in the retinas of mice lacking the retinol-binding protein receptor, STRA6. Invest Ophthalmol Vis Sci. 2012;53:3027–3039. doi: 10.1167/iovs.11-8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephensen CB. Vitamin A, infection, and immune function. Annu Rev Nutr. 2001;21:167–192. doi: 10.1146/annurev.nutr.21.1.167. [DOI] [PubMed] [Google Scholar]
- Szeto W, Jiang W, Tice DA, Rubinfeld B, Hollingshead PG, Fong SE, Dugger DL, Pham T, Yansura DG, Wong TA, Grimaldi JC, Corpuz RT, Singh JS, Frantz GD, Devaux B, Crowley CW, Schwall RH, Eberhard DA, Rastelli L, Polakis P, Pennica D. Overexpression of the retinoic acid–responsive gene Stra6 in human cancers and its synergistic induction by Wnt-1 and retinoic acid. Cancer Res. 2001;61:4197–4205. [PubMed] [Google Scholar]
- Travis GH, Golczak M, Moise AR, Palczewski K. Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Annu Rev Pharmacol Toxicol. 2007;47:469–512. doi: 10.1146/annurev.pharmtox.47.120505.105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahlquist A, Peterson PA, Wibell L. Metabolism of the viatmin A transporting protein complex. I. Turnover studies in normal persons and in patients with chronic renal failure. Eur J Clin Invest. 1973;3:352–362. doi: 10.1111/j.1365-2362.1973.tb00362.x. [DOI] [PubMed] [Google Scholar]
- Wald G. Molecular basis of visual excitation. Science. 1968;162:230–239. doi: 10.1126/science.162.3850.230. [DOI] [PubMed] [Google Scholar]
- Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- Zanotti G, Berni R. Plasma retinol-binding protein: structure and interactions with retinol, retinoids, and transthyretin. Vitam Horm. 2004;69:271–295. doi: 10.1016/S0083-6729(04)69010-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.