Abstract
Background : The major cause of peptic ulcer disease is helicobacter pylori (Hp), and it is also implicated in the pathogenesis of adenocarcinoma of the distal stomach and gastric lymphoma. The incidence of peptic ulcer disease, atrophic gastritis, and gastric adenocarcinoma are more common in people infected of cagA positive strains of Hp. The aim of this study was to determine the prevalence of the anti-Hp and anti-cagA antibodies among healthy persons in Golestan province-North of Iran.
Methods : The blood samples of 1028 healthy people were collected all over Golestan province by cluster sampling. A demographic questionnaire was completed and body mass index (BMI) was calculated for each case. Hp-IgG (Pishtaz teb Co. Iran) and anti- cagA (DIA.PRO Italy) titer were evaluated by Elisa method. Data were collected and analyzed.
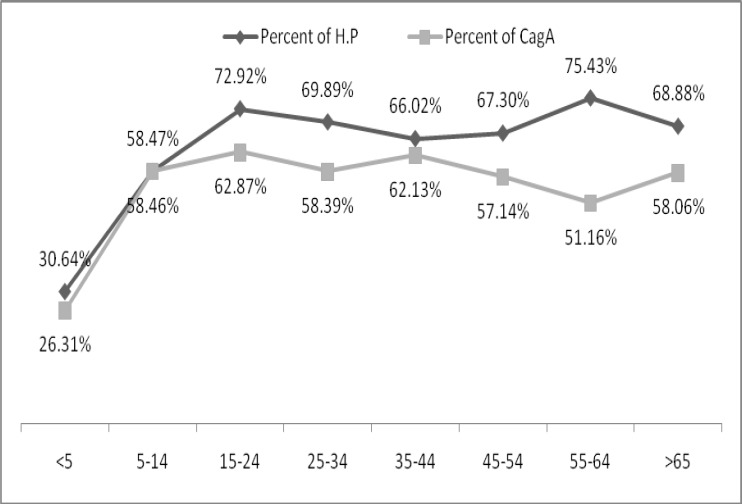
Results : Six hundred-eighty three individuals (66.4%) were positive for Hp and 395 (57.8%) of them were cagA positive. Hp positive cases were (66.3%) and (66.6%) in male and female, respectively. Prevalence of cagA was 56.3% and 58.9%, respectively. The most seropositivity of Helicobacter Pylori (75.4%) was in 55-64 years old (p<0.001). Prevalence of cagA (63.4%) was more in age between 15-24 years.
Conclusion: Prevalence of anti-Hp antibody and strains of cagA seropositive in healthy persons of this province of Iran were relatively high. Preventive protocol for reducing of the infection is recommended.
Key Words: Prevalence, Helicobacter Pylori, CagA, Antibody
Helicobacter Pylori (Hp) is one of the gram-negative bacteria that colonize the human stomach. The prevalence rates of this bacterium range from 25% in Western countries and over 90% in developing countries (1). The major cause of peptic ulcer disease is Helicobacter pylori, and it is also implicated in the pathogenesis of adenocarcinoma of the distal stomach and gastric lymphoma, especially the mucosa-associated lymphoid tissue-type lymphoma (MALT lymphoma) (3). The bacteria’s ability to cause a disease has been associated with the expression of several virulence factors, including VacA cytotoxin, BabA, the neutrophil- activating protein (HP-NAP), outer inflammatory protein A (OipA), the duodenal ulcer promoting gene (dupA), and cag pathogenicity Island (cagPAI) (4). HP clinical isolates are classified into two types according to their pathogenicity: Type I is associated with severe disease pathology expresses functional vacA (vacuolating cytotoxin A) and contains cagA (cytotoxin-associated gene), Type II lacks cagPAI, harbors a non-toxic form of vacA and is regarded as less virulent (5). There are clustes of genes (pathogenicity island) of about 35 kilo base pairs which cagA is considered as a marker for these genes present in more than 50% of the Hp strains and encodes the120–140 KDa (6).
cagA positive strains are more commonly associated with peptic ulcer disease, atrophic gastritis, and gastric adenocarcinoma than are cagA- negative strains (7). The aim of this study was to determine the prevalence of the anti-Hp antibody and anti-cagA antibody among the healthy subjects in Golestan province-northeast of Iran.
Methods
From 2008 to 2009, this cross-sectional seroprevalence study was carried out among the healthy people in Golestan province, northeast of Iran. Totally, 1028 persons were recruited (489 males, 539 females) aged 1-83 years. Demographic questionnaires including age, sex, residency, and ethnicity, were completed. Blood samples were stored in -70oC. After measuring weight and the height, body mass index was determined and the subjects were classified into 4 groups:
less than 18.5 kg/m2 = thin
24/5-25 kg/m2 = normal
29/5-25 kg/m2 =.overweight
30-39 kg/m2 =.obese
More than 40 kg/m2 = severe obesity (8).
Other variables included consuming fish, vegetables and fruits per day, past history of diabetes, smoking and blood pressure. Golestan province is classified into 3 different climates: humid mediterranean, humid semi- mediterranean and semi - dry mediterranean. We evaluated the prevalence of cagA in 3 groups.
The presence of anti- Hp antibody was evaluated by Elisa method and Hp -IgG kit (Pishtaz teb Co. Iran), and positive samples were tested for anti- cagA antibody, IgG class by anti-cagA antibody kit (DIA.PRO Italy). The data were analyzed with SPSS version 16. The prevalence of anti-Hp and anti-cagA were determined.
Results
In this study, prevalence of Hp infection was 66.4% (683/1028), which 57.7% (395/683) of them were infected to cagA. Rate of Hp-positive cases in males was (66.3%) and in females (66.6%). Prevalence of cagA in men and women were (56.3%) and (58.9%), respectively. The difference was not statistically significant. We saw minimum rates (47.7%) of Hp- cagA positive cases in the western part of the province and maximum rates (74.4%) in the eastern part of the province.
The seroprevalence of both anti- Hp and anti- cagA antibodies increased with age, Hp infection varied from (30.6%) in children under 5 years and 71.1% above 65 years and it was most prevalent in 55-64 years (75.4%). There was significant relationship between infection of Hp and age (p <0.001).
Maximum percentage of cagA positive was reported in 15-24 years (63.4%). No significant difference was seen (figure 1).
Figure1.
Age distribution of Helicobacter pylori infection and cagA-seropositive in Hp+
There was no significant relationship between the subjects with regard to consuming fish, fruits and vegetables per day (table 1).
Table 1.
Distribution of H.p and cagA seropositive regards to different variables
| Factors | Positive cases of HP (%) | Positive cases of cagA* (%) |
|---|---|---|
| consumption of fruits and vegetables a day Less than once a day 1-2 portions a day 2-4 portions a day More than 4 portions a day |
69.70 66.50 70.30 66.70 |
55.80 62.20 57.30 40.60 |
| Diabetes mellitus Yes No |
60.50 70.00 |
47.80 57.90 |
| BMI Thin Normal Overweight Obese Morbid obesity |
56.60 70.70 69.90 61.90 68.90 |
43.90 39.30 43.10 41.10 54.80 |
| Smoking Yes No |
57.90 68.70 |
54.34 57.34 |
| fish consumption in a week No 1 time 2 times 3times |
75.50 67.40 67.10 68.30 |
60.00 51.00 53.60 59.30 |
People infected with Helicobacter Pylori, were classified into three categories regarding ethnicity: Sistani, Turkman and Fars which had 70.5%, 71.2% and 61.3% prevalence, respectively (p=0.009). Distribution of cagA positive cases were reported as 67.2%, 57. 2%, 53.6%, in the above mentioned ethnicity, respectively (p =0.075).
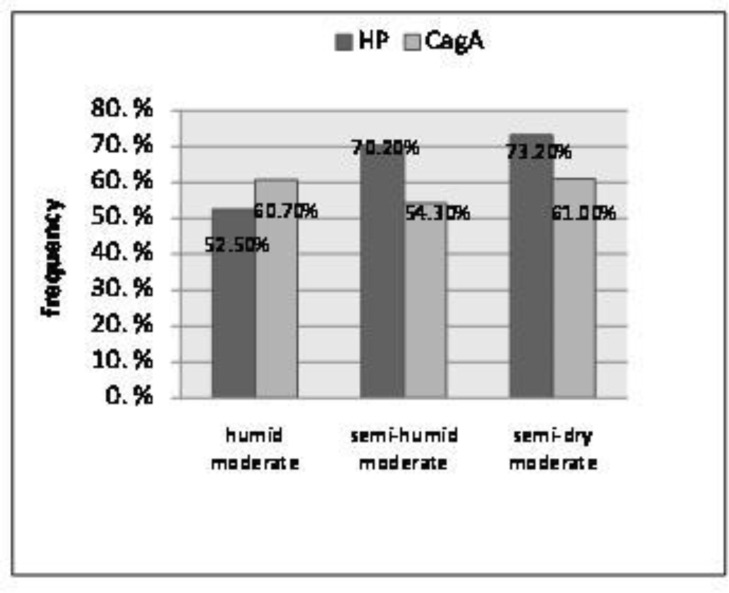
Based on the classified climate condition, it was found that the frequency of positive Hp in humid areas was less than others (52.5%), and the frequency of cagA cases in semi- dry temperate regions are more than the other areas (61%) (figure 2).
Figure 2.
Frequency distribution Hp + (healthy individuals) and cagA+ (Hp +cases) based on the Climate
Positive Hp cases were not different in rural and urban areas (p>0.05). In cagA-positive cases, rural residents showed more prevalence than urban residents (34%) but no significant differences were found (p= 0.435).
Discussion
Hp is one of the common infectious diseases in the world (9). Approximately 20% of persons infected to Hp would progress to gastro-duodenal ulcer (10). Rising the life standards, the prevalence of Hp infection has been declined. So that contamination with this infection in developed countries were less than developing countries (11). The prevalence of this bacterium was 23.1% in Canada (12) and 32.5% in the USA (12). In developing countries, it had a higher prevalence. In several developing countries such as Albania, Kazakhstan, Vietnam and Bangladesh, the prevalence of Hp were 70%, 74%, 74.6% and 92%, respectively (14-17). In Iran, studies showed an overall prevalence of infection to be 36-71%, our study denoted that 66.8% of people living in Golestan province have IgG anti-Hp antibody and the other studies in Iran on healthy individuals have been shown the same. Prevalence of Hp was estimated 64.2% in Sari and 61.6% in Kerman (18, 19). Therefore, the pattern in our country is similar to the other developing countries. In several studies, it was shown that the prevalence of infection increased with age (17, 20, 21).
Seropositivity in studied cases in Golestan province varied from 30.6% in people under 5 to 75.4% in people aged 55-64 years. The result of the present study showed that the age trend in our region was similar to the others and infection is high in lower age. In some studies, men had more risk of Hp infection. According to the findings in Frah Naja in Canada, infection in men was 29.4% and in women was14.9% (12). However, it was not in agreement to other studies, like Kazakhstan, Saudi Arabia and Kerman which showed no correlation with gender (16, 18, 22).
The role of nutrition in Hp infection in our study was not significant. That was consistent with the study of Khan et al. in 2007 (22). Frah Naja study showed that prevalence of infection in those who have high consumption of vegetables was less than the others (12).
In a study in Canada, no significant relationship between BMI and the prevalence of infection was seen (12). Our results showed that thin individuals (BMI less than 18.5) prevalence is lower than other groups (p=0.02).
In our study, no significant relationship was seen between infection and smoking. This result was consistent with Farah Naja results (12). But the study in UK by Glasyow, it showed that in smokers the prevalence of this infection was higher than non-smokers (23). The reason of high prevalence of infection in smokers is its shared use which transfers the infection in oral- oral root (24). Hp virulence factors are important (18). In some studies in the presence of cytotoxic-associated gene in Hp strains have been attributed with gastric ulcer and cancer (25). Therefore, several researches have been done on the importance of cagA, on patients with gastric ulcer or cancer.
In this study, the prevalence of anti- cagA antibody in cases of Hp was 57.7% and it was 38.1% in the participants. High percentage of anti- cagA antibody in this region with high gastrointestinal cancers will discuss the possibility of relationship between contamination to cagA- positive Hp and cancer and also gastritis. Therefore, future researches are recommended to evaluate the relationship between gastrointestinal cancers, especially in gastric cancer cagA positive strains in this region. Age distribution of cagA antibody is similar to the distribution of Hp antibody, and was lower in the age group under 5 years-old.
One of the important findings of this study was a significant relationship between climate with existence of antibodies against cagA and Hp. Our findings showed that the probability of infection in humid climate is lower than the others. Possible causes that influence these findings are not clear to us and for confirming this authenticity and finding reasons, further studies of the climate are recommended to be able to discuss more confidently about this area.
Acknowledgments
The authors thank all their colleagues in Health Center of Golestan University of Medical Sciences for their help in data collection.
References
- 1.Baglan PH, Bozdayi G, Ozkan M, et al. Clarithromycin Resistance Prevalence and Icea Gene Status in Helicobacter Pylori Clinical Isolates in Turkish Patients with Duodenal Ulcer and Functional Dyspepsia. J Microbiol. 2006;44:409–16. [PubMed] [Google Scholar]
- 2.Aramã SS, Cochino AV, Aramã V. Helicobacter Pylori Infection in Children. INFO Medica. 2007;4:8–12. [Google Scholar]
- 3.Ferrández A, Benito R, Arenas J, et al. CagA-positive Helicobacter pylori infection is not associated with decreased risk of Barrett's esophagus in a population with high H. pylori infection rate. BMC Gastroenterol. 2006;6:7. doi: 10.1186/1471-230X-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salehi Z, Jelodar MH, Rassa M, et al. Helicobacter pylori cagA Status and Peptic Ulcer Disease in Iran. Dig Dis Sci. 2009;54:608–13. doi: 10.1007/s10620-008-0378-8. [DOI] [PubMed] [Google Scholar]
- 5.Mattar R, Marques SB, Monteiro MS, et al. Helicobacter pylori cag pathogenicity island genes: clinical relevance for peptic ulcer disease development in Brazil. J Med Microbiol. 2007;56:9–14. doi: 10.1099/jmm.0.46824-0. [DOI] [PubMed] [Google Scholar]
- 6.Safaei HG, Tavakkol H, Mojtahedi A, et al. Correlation of cagA positive Helicobacter pylori Infection with clinical outcomes in Alzahra hospital, Isfahan, Iran. J Res Med Sci. 2008;13:196–201. [Google Scholar]
- 7.Siavoshi F, Malekzadeh R, Daneshmand M, Ashktorab H. Helicobacter pylori Endemic and Gastric Dis. Dig Dis Sci. 2005;50:2075–80. doi: 10.1007/s10620-005-3010-1. [DOI] [PubMed] [Google Scholar]
- 8.WHO. Global Database on Body Mass Index. An interactive surveillance tool for monitoring nutrition. Available at: http://apps.who.int/bmi . Accessed last update December 10, 2009.
- 9.Naja F, Kreiger N, Sullivan T. Helicobacter pylori infection in Ontario: Prevalence and risk factors. Can J Gastroenterol. 2007;21:501–6. doi: 10.1155/2007/462804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Douraghi M, Mohammadi M, Shirazi MH, et al. Assessment the relationship of cagA gene with different gastroduodenal diseases in Helicobacter pylori infected patients. Iranian J Med Microbiol. 2008;1:31–6. [Google Scholar]
- 11.Windsor HM, Abioye-Kuteyi EA, Leber JM, et al. Prevalence of Helicobacter pylori in Indigenous Western Australians: comparison between urban and remote rural populations. Med J Aust. 2005;182:210–3. [PubMed] [Google Scholar]
- 12.Farah Naja. Prevalence, Risk Factors and Effect on the Bioavailability of Vitamins C and E. PhD thesis. https://tspace.libraryutoronto/bitstream/1807/16810/1/Naja_Farah_200806.
- 13.Everhart JE, Kruszon-Moran D, Perez-Perez GI, Tralka TS, McQuillan G. Seroprevalence and Ethnic Differences in Helicobacter pylori Infection among Adults in the United States. The J Infect Dis. 2000;181:1359–63. doi: 10.1086/315384. [DOI] [PubMed] [Google Scholar]
- 14.Hoang TT, Bengtsson C, Phung DC, Sörberg M, Granström M. Seroprevalence of Helicobacter pylori Infection in Urban and Rural. Vietnam Clin Diagn Lab Immunol. 2005;12:81–5. doi: 10.1128/CDLI.12.1.81-85.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tadege T, Mengistu Y, Desta K, Asrat D. Seroprevalence of Helicobacter pylori Infection in and its Relationship with ABO Blood Groups. Ethiop J Health Dev. 2005;19:55–9. [Google Scholar]
- 16.Nurgalieva ZZ, Malaty HM, Graham DY, et al. Helicobacter pylori infection in Kazakhstan: effect of water source and houseold hygiene. Am J Trop Med Hyg. 2002;67:201–6. doi: 10.4269/ajtmh.2002.67.201. [DOI] [PubMed] [Google Scholar]
- 17.Monno R, Volpe A, Basho M, et al. Helicobacter pylori Seroprevalence in selected groups of Albanian volunteers. Infection. 2008;36:345–50. doi: 10.1007/s15010-008-6338-6. [DOI] [PubMed] [Google Scholar]
- 18.Zahedi MJ, Darvish S, Hayatbakhsh M, Atapoor M. Relative frequency of Helicobacter Pylori infection in the city of Kerman in 2000. J Kerman Univ Med Sci. 2002;9:140–5. [Google Scholar]
- 19.Babamahmoodi F, Ajemi A, Kalhor M, Shfiei GH R, Khalilian AR. A seroepidemiological study of Helicobacter Pylori infection in Sari in 2001-02. J Mazandaran Univ Med Sci. 2004;14:39–48. [Google Scholar]
- 20.EL Sherbini EM, Zakaria S, EL Raziky MS, et al. Low Seroprevalence of Anti-CagA Antibodies Inspite of High Seroprevalence of Anti-Hpantibodies in Rural Egyptian Community. European Helicobacter study grou. XX th international workshop on helicobater and related Bacteria in chronic Digestive inflammation. Ostanbul, Turkey: 2007. pp. 20–22. [Google Scholar]
- 21.Majidi M, Rezaei S, Hassanzadeh N, et al. The correlation between helicobacter pylori infection and squamous cell carcinoma of larynx and hypopharynx. Iranian J Otorhinolaryngol . 2007;19:89–94. [In Persian] [Google Scholar]
- 22.Khan MA, Ghazi HO. Helicobacter pylori infection in asymptomatic subjects in Mekka, Saudi Arabia. J Pak Med Assoc. 2007;57:114–7. [PubMed] [Google Scholar]
- 23.Woodward M, Morrison C, McColl K. An investigation into the factors associated with Helicobacter pylori infection. J Clin Epidemiol. 2000;53:175–81. doi: 10.1016/s0895-4356(99)00171-7. [DOI] [PubMed] [Google Scholar]
- 24.Kurata JH, Nogawa AN. Meta-analysis of risk factors for peptic ulcer. Nonsteroidal anti-inflammatory drugs, Helicobacter pylori, and smoking. J Clin Gasteoenterol. 1997;24:2–17. doi: 10.1097/00004836-199701000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Zamani A, Bahremand Sh, Ojaghi Haghighi SM, Tirgari F, Ghasemi M. Endoscopic findings in children with Helicobacter pylori infection and abdominal tenderness. Tehran Univ Med J. 2008;65:60–5. [Google Scholar]