Abstract
The development of therapeutic strategies that promote functional recovery is a major goal of multiple sclerosis (MS) research. Neuroscientific and methodological advances have improved our understanding of the brain’s recovery from damage, generating novel hypotheses for potential targets or modes of intervention and laying the foundation for the development of scientifically informed strategies promoting recovery in interventional studies. This Review aims to encourage the transition from characterization of recovery mechanisms to the development of strategies that promote recovery in MS. We discuss current evidence for functional reorganization that underlies recovery and its implications for development of new recovery-oriented strategies in MS. Promotion of functional recovery requires an improved understanding of recovery mechanisms modulated by interventions and the development of reliable measures of therapeutic effects. As imaging methods can be used to measure functional and structural alterations associated with recovery, this Review discusses their use as reliable markers to measure the effects of interventions.
Introduction
Inflammatory demyelination and axonal loss are considered major determinants of neurological deficits in Multiple Sclerosis (MS)1. Functional recovery in MS is achieved and sustained by repair of damage through remyelination with resolution of inflammation and functional reorganisation. Remyelination is an important mechanism of restoration of axonal function after acute inflammatory demyelination2. Functional reorganisation relies on molecular and cellular mechanisms to induce changes in systems-level functional responses, which are the proximal effectors of perception, action and cognition. This review focuses on systems-level adaptive functional reorganisation in MS as measured by functional MRI (fMRI), discussing mechanisms of functional recovery and ways to enhance them.
The overall aim of this Review is to stimulate progress from studies characterising recovery mechanisms to studies developing strategies to promote recovery in MS. In the first section, we summarize evidence from imaging studies that shows adaptation of functional systems to damage to emphasise principles of adaptive functional reorganization. In the second section, we propose ways in which this understanding can be translated into new recovery-oriented strategies for MS, supported by related findings in other neurological conditions. As our understanding of recovery mechanisms and the development of interventions are influenced by our ability to measure the desired effects, the third section discusses the opportunities and limitations of imaging methods that are used to measure neuroplasticity underlying functional recovery in order to improve their application as reliable and quantitative measures of therapeutic interventions3,4. This will extend opportunities for neurorepair to other disabling neurological conditions.
Adaptive functional reorganization in MS
Evidence for reorganisation of brain function underlying functional recovery comes from studies of focal ischaemic brain damage, where systems-level reorganisation reflects molecular, synaptic and cellular events and constitutes post-injury brain plasticity5,6. Perilesional remapping of cortical representations, functional reorganisation in intact regions of the damaged hemisphere and activation of cortical areas in the undamaged hemisphere accompany functional recovery after stroke5. Several lines of evidence show that such reorganization is behaviourally relevant for stroke recovery as (a) it is associated with preserved or completely recovered behaviour7; (b) the extent of functional changes correlates with the associated pathology8; (c) similar changes can be induced through learning or rehabilitation9; (d) potential for recovery increases with facilitated reorganization9; (e) functional impairment results from interference with such processes10 (Box 1). Evidence across brain systems supports a similar adaptive role of functional reorganization in MS despite widespread pathology by showing that functional reorganization accompanying recovery in this disease limits the impact of damage on behaviour11-17.
Box 1.
Criteria for the definition of behaviourally relevant brain functional reorganization after brain damage. Recovery studies in the fields of stroke and MS suggest that behaviourally meaningful functional reorganization - that is, adaptive functional reorganization - can be defined if:
There is a relationship between extent of changes of functional patterns and associated pathology
Altered patterns of functional activation accompanied by preserved or completely recovered behaviour
Learning or rehabilitation induce similar changes in functional activation
Facilitation of reorganization increases the rate of, or the potential for recovery
Interruption of reorganization processes and/or maladaptation results in functional impairment
Evidence across functional systems
In this section, we focus on three psychological domains of perception, action and cognition to discuss evidence for functional reorganization leading to functional recovery in MS.
Perception
Visual recovery after acute demyelinating optic neuritis typically occurs within weeks despite permanent axonal loss18,19. Plasticity in the visual system contributes to recovery, as the effects of lesions on the optic nerve spread both pathologically20-22 and functionally23-30 through the visual pathway. fMRI studies in patients following onset of optic neuritis show reduced activation in the visual cortex in response to visual stimulation of the affected eye23,24,26-31. Consistent with adaptive functional reorganization that promotes clinical recovery28,32, this cortical response increases within 2–6 weeks of disease onset, but remains below that of the unaffected eye28.
Adaptive functional reorganization occurs at various levels along the visual pathways. During the early period after onset of optic neuritis, activation of the lateral geniculate nucleus (LGN) and visual cortical areas is lower in response to visual stimulation of the affected eye compared with that of the unaffected eye31. Later, during recovery, this difference progressively diminishes in both the LGN and the visual cortex31. These changes may reflect remyelination of the optic nerve that re-establishes a normal visual input or functional reorganization within LGN that compensates for an impaired optic nerve input to the primary visual cortex. Adaptive changes in early or higher visual areas can also assist in maintaining normal visual function29-31. Cortical reorganization within extrastriate visual areas occurs early after onset of optic neuritis and is associated with better visual function28 and longer-term visual outcome32. This early reorganization is associated with recovery independently of other markers of damage in anterior or posterior visual pathways32. Orbitofrontal and lateral temporal cortices can be transiently involved in recovery after optic neuritis as part of a dynamic reorganization of visual function in the occipital cortex28.
Action
Altered functional patterns of sensorimotor activation constitute a disease trait across different forms of MS33-35. The extent and type of motor reorganization varies across phases12,36,37 and stages38,39 of the disease. After a clinically isolated syndrome (CIS), patients show more widespread recruitment of sensorimotor networks than do healthy volunteers38. This functional pattern persists in patients who progress to clinically definite MS37 and characterizes the acute phases of the disease12,36. As the disease advances towards secondary progression, patterns of functional reorganization show an increasingly bilateral distribution and, even for simple motor tasks, involve higher-control sensorimotor areas that in healthy controls are recruited for novel or complex tasks39.
The magnitude and extent of functional reorganization depends on the extent and severity of lesional13,40 and extralesional11,41 brain and spinal cord42 damage. In patients with normal motor function, greater lesion volume and microstructural damage are associated with more-widespread activation of brain areas11,13,43. The increased, bilateral recruitment of sensorimotor areas may represent an adaptive mechanism that limits the functional impact of MS damage11. Alternatively, such changes may be a consequence of reduced ipsilateral deactivation with impaired interhemispheric inhibition owing to callosal damage40,44. In either case, the bilateral pattern of sensorimotor recruitment re-lateralises on the contralateral (affected) hemisphere with functional recovery after a relapse. A persistent recruitment of sensorimotor cortex on the ipsilateral (unaffected) hemisphere is associated with poor clinical recovery36. Lateralized brain activity with preservation of motor function is a consistent finding across age groups in MS33. In addition to the hemispheric re-lateralization, adaptive functional reorganization seems to follow a hierarchy within the motor system, with primary sensorimotor regions being recruited in the benign forms34 and in the initial stages39 of MS, whereas secondary motor45 and multimodal nonmotor35 areas are involved in the progressive forms of the disease. While damage prompts adaptive functional changes17,43, disability is associated with a specific altered pattern of hand movement that can reflect maladaptation17.
Cognition
Deficits in cognitive performance46-52 and their evolution53,54 correlate with MS damage. Functional studies investigating cognitive processes such as memory, efficiency of information processing, attention and executive functions55 have consistently shown that these processes are associated with the activity of wider and more bilateral networks of task-specific regions in patients with MS than in healthy individuals56-58. The extent of this recruitment increases progressively with an increased cognitive load59-61 and becomes more prominent as MS progresses59, when activity can involve regions outside the specific cognitive domain. Compared with healthy individuals, the magnitude of activation of task-specific networks in patients with MS is reported to be greater in some studies62, but lower in others63. Within cognitive networks, changes in perfusion64-66 and metabolism67, as well as in functional and structural connectivity63,68,69 correlate with cognitive performance. Stronger interhemispheric functional and structural interactions are observed in patients than in controls63,68,69. This increased strength of connectivity is associated with damage to specific, task-relevant white matter tracts69.
Factors influencing adaptive functional reorganization
In MS, individual-specific and disease-related factors influence adaptive functional reorganization and its measurements with imaging methods. Age at disease onset may influence the premorbid cognitive functional reserve61. After disease onset, a different capacity for brain plasticity70 and remyelination71 may help to explain the effect of age on cortical reorganisation and functional connectivity33,72 that underlie recovery in MS. Sex of the patient also affects damage and repair mechanisms in MS73 through the effects of sex hormones74,75 and helps to explain clinically relevant sex-specific differences in brain functional connectivity that are observed in MS76.
The type12,36,37, location13,30,42,77, extent34,39 and severity11,50,78 of MS damage influences adaptive reorganization. Acute inflammation alters functional brain responses12,28,36,38, with magnitudes that vary depending on the functional system involved12,28,36,38, as well as on the role of individual brain regions within networks40,44. These altered responses return to baseline activity with resolution of inflammation12,36,37, but a chronic inflammatory state can produce sustained reorganization of function across brain systems39 through interference with local mechanisms of brain plasticity79. Depending on its location, damage can either interfere with80 or initiate13,28,42,77,81 functional reorganization. The extent of brain damage affects substrates for functional functional reorganization39, potentially with clinically relevant consequences34,82. The extent of damage can also affect the regional and network efficiency83,84. Clinically, this may be apparent with a higher occurrence of cognitive deficits in the progressive phase of the disease55,61. Whereas factors related to brain damage initiate functional reorganization11, more extensive and irreversible tissue loss is associated with reduced capacity for functional reorganization12,78,85, which is reflected in a worse clinical outcome86,87.
Functional reorganization can be maladaptive6. Maladaptation with chronic limb disuse contributes to disability17 and may explain the functional differences that are observed among clinical stages39,88 and among forms34,80 of MS, beyond the adaptive functional reorganization. Maladaptive plasticity triggered and sustained by disuse may involve multiple functional systems and contribute to disability in multiple functional domains89. Although maladaptation may contribute to disability, establishing whether insufficient adaptive reorganization is the basis for disability, and distinguishing between insufficient and maladaptive plasticity is difficult. Future interventional studies that interfere with cortical function or studies assessing concurrent structural changes may disambiguate the relative contributions of maladaptation versus insufficient adaptive plasticity.
Promotion of functional reorganization in MS
Adaptive brain plasticity offers a flexible substrate for functional reorganization in MS through local re-mapping of cortical representation13, increased activation in relevant higher-order areas28,30,77,90 and a shift in interhemispheric lateralization towards the ipsilateral hemisphere90,91. A substantial preservation of brain structural architecture allows these mechanisms to act, although at lower efficiency83, even when MS damage or task demand increase92-94.
Neuroplasticity offers a substrate for interventions that promote functional recovery in MS, but stability of networks is also necessary for adaptive patterns to be retained70. Different functional changes are observed in the motor system in childhood-onset versus adult-onset MS, which could be explained by age-related differences in the plastic properties of the brain functional systems72. In addition, distinct neural systems can have different requirements for plasticity versus stability across the lifespan70. Functional reorganisation in the extra-striate cortex after ON provides an example of this phenomenon within the visual system30,70.
Interventions to drive adaptive functional reorganisation
Interventions to drive adaptive plasticity can promote functional restoration by inducing adaptive changes or by predisposing functional systems to plasticity (Figure 1 and Figure 2). Stroke recovery research suggests that functional recovery after brain damage is associated with normalization of patterns of functional reorganization95-97. Despite the effects of chronic inflammation on brain plasticity98, this situation holds true in MS recovery, in both the short-term14,28,36,37 and the longer-term14,34.
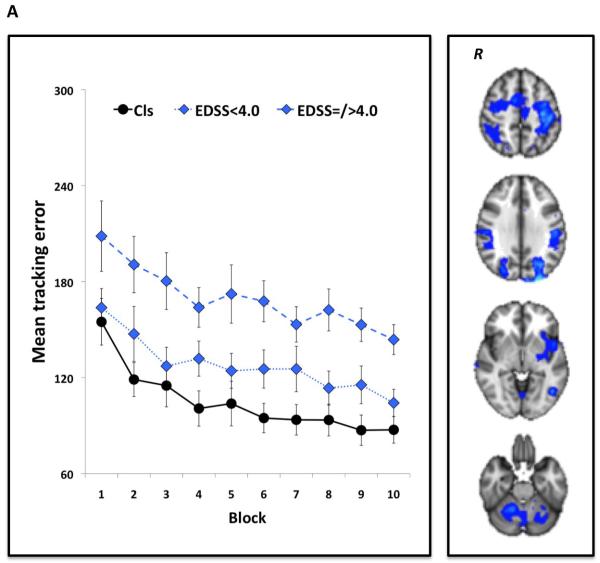
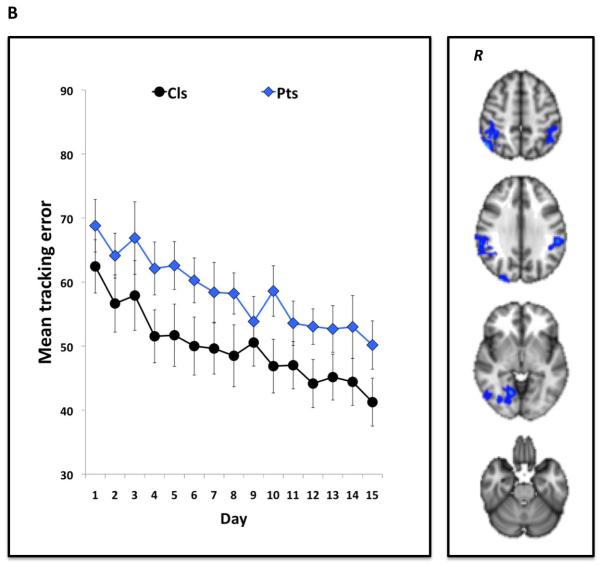
Figure 1.
Non-pharmacological modulation of brain plasticity in MS14,94. Patients with MS and healthy volunteers performed a visuomotor task in which they tracked a continuously moving bar on a computer screen by altering pressure applied to a handle held in the right hand. The task was performed in blocks of 38 sec. Participants practised the task in short-term (10 blocks for a total of ~25 min) and in the longer-term (daily for 15 days consecutively) settings. Performance was measured as the mean tracking error across each block (short-term) or day (longer-term) of practice. During the first and last session, participants underwent fMRI scanning. As depicted in the graphs, short-term (a) and longer-term (b) task practice significantly improved visuomotor performance in both healthy controls and patients with MS, across levels of disability according to EDSS scores. As shown in the fMRI scans, these performance improvements were associated with a reduction in blood oxygenation level-dependent signal in brain regions involved in visuomotor integration. Abbreviations: EDSS, Expanded Disability Status Scale; fMRI, functional MRI; MS, multiple sclerosis; R, right hemisphere. Permission obtained from SAGE Publications © Tomassini, V. et al. Mult. Scler. 17, 103–115 (2011), and Tomassini, V. et al. Neurorehabil. Neural Repair 26, 581–593 (2012).
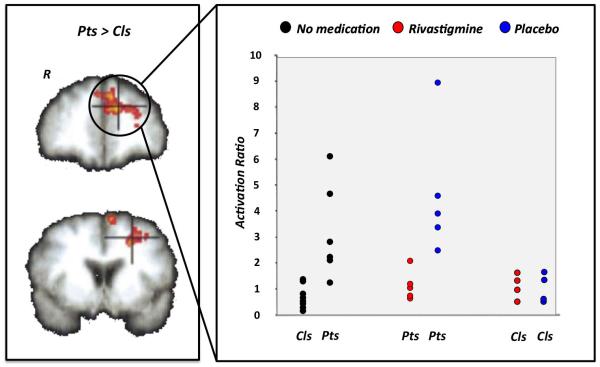
Figure 2.
Pharmacological modulation of brain plasticity in MS16. Patients with MS and healthy volunteers underwent a counting Stroop task during fMRI scanning. Patients had comparable cognitive performance to controls, but a significantly greater BOLD signal change in the left prefrontal cortex - a difference that reflects functional reorganization. BOLD signal changes in these regions correlated with cognitive performance and brain volume. A functional score, the activation ratio (AR), representing the ratio between the magnitude of prefrontal cortex activation on the left (found in MS patients) relative to right hemisphere, was calculated to test the effect of pharmacological modulation of brain adaptive plasticity with rivastigmine, a cholinesterase inhibitor. Before rivastigmine administration or following administration of placebo, mean AR in patients was greater than in controls. After rivastigmine administration, mean AR in patients was reduced to within the range of controls. Abbreviations: BOLD, blood oxygenation level-dependent; fMRI, functional MRI; MS, multiple sclerosis; R, right hemisphere. Permission obtained from Oxford University Press © Parry, A. M. et al. Brain 126,2750–2760 (2003).
Training-based interventions
Interference with maladaptation caused by learned disuse17,99 may be the mechanism through which physical therapy can limit the impact of MS disability14,100. Short-term right-hand practice (over minutes) of visuomotor tasks in patients with MS can induce performance improvements that are associated with functional reorganization of ipsilateral (right) sensorimotor regions14, whose activity is associated with clinical disability17. This finding suggests that plasticity changes spreading across functional systems in MS may reflect maladaptation that sustains disability and may be a therapeutic target for recovery-oriented interventions. Longer-term practice (over weeks) of visuomotor tasks also induces performance improvements in patients with MS14. The improvements are associated with functional reorganization in cognitive systems that are not involved in visuomotor performance improvements in healthy controls.
Constraint-induced movement therapy is based on overcoming learned disuse. This approach is under evaluation in the treatment of MS101, supported by its successful application in stroke recovery102, where it can induce behaviourally meaningful functional changes in the sensorimotor regions of the hemisphere contralateral to the hand moved9. The preserved potential for neuroplasticity14 and motor performance improvements94 even at higher levels of disease burden suggests that patients with MS patients could benefit from neurorehabilitation irrespective of the initial severity of motor dysfunction14 (Figure 1), although cognitive systems different from those acting for the same practice in healthy subjects likely contribute to this plasticity in patients14.
Studies on cognitive rehabilitation in MS that compared the effects of a specific versus a nonspecific cognitive treatment have reported conflicting results103,104. However, evidence that brain functional patterns subserving an increasing load of cognitive performance before and after cognitive training are comparable in patients with mild or severe cognitive impairment105 suggests that cognitive training can be beneficial in MS15 irrespective of the severity of cognitive dysfunction. This finding also suggests that functional plasticity can be enhanced by neuropsychological intervention. Beyond the effect on cognitive dysfunction, such interventions may have the potential to expand the brain’s functional reserve61, especially in childhood MS106.
Other forms of intervention have been tested in recovery from CNS damage107-110. Motor imagery practice (MIP) involves mental repetition of movements, with the aim of improving motor execution111. The rationale for MIP is evidence that mentally simulated and physically executed actions, both simple and complex112, share similar mechanisms of motor control111. Through this overlap of neural substrates, MIP may predispose the motor system to the effects of physical therapy113. In stroke recovery, MIP provides sufficient repetitive practice to increase use of the affected arm 110 and to change patterns of brain function114. Although factors in MS such as cognitive dysfunction and limb disuse could reduce the capacity for mentally simulated actions115,116, the ability of MIP to drive reorganization of sensorimotor function independently of movement117 may find clinical application in disabling forms of the disease, in which motor output is severely impaired.
Application of device-based therapies, such as neuroprosthesis for recovery of motor function and computer-based interfaces for cognitive rehabilitation, to rehabilitation of complex behaviours and severe forms of disability is becoming increasingly feasible15,104,118. Substantial preservation of brain plasticity in patients across levels of disease burden14,94 encourages use of these devices for rehabilitation in MS.
Pharmacological and electrical modulation
The rationale for pharmacological and electrical modulation in MS rehabilitation lies in the substrates and mechanisms of brain plasticity119. A rich network of intracortical connections can support many organizational structures, allowing for formation of new cortical representations with learning120,121 or for functional remapping with recovery13,122. Persistent changes in the efficacy of intra-cortical connections require a stable form of synaptic modification that is achieved through activity-dependent alteration of the excitatory-inhibitory synaptic balance. These changes constitute synaptic plasticity123, which permits neuronal interconnections to be continuously adjusted as a consequence of their exposure to particular activity patterns. Synaptic plasticity is the basis of network plasticity. Induction of plastic processes depends critically on changes within glutamatergic and γ-aminobutyric acid (GABA)-ergic interneurons124,125. Although neuromodulators induce little or no change in basal neuronal activity, they can potentiate or attenuate responses evoked by such neurotransmitters126.
Pharmacological interventions in recovery strategies can increase or prolong the efficacy of rehabilitation by increasing the susceptibility of relevant nodes or systems to the effects of physical or cognitive interventions107. Modulation of glutamatergic activity with potassium-channel blockers enhances the excitability of the motor cortex and conduction along corticospinal pathways in patients with MS127,128, providing a rationale for testing the effects of modulation of glutamatergic tone in motor recovery129. Cholinergic agonism modulates synaptic plasticity in the hippocampus130,131. Modulation of cholinergic tone through acetylcholinesterase inhibition enhances cognitive function in MS patients with memory deficits132. Functional changes in the prefrontal cortex, as well as changes in its functional connectivity, may underlie the efficacy of this intervention16,133 (Figure 2). Dopamine modulates cortical excitability via changes in synaptic plasticity134 that are relevant for motivational and motor aspects of learning135. As in stroke recovery136, modulation of dopaminergic frontal projections in MS might potentiate aspects of motor recovery and memory consolidation135. Serotonin also regulates synaptic plasticity and cortical excitability137-140. Use of serotonin-reuptake inhibitors in association with physical therapy has produced beneficial effects on motor outcomes in patients who are moderately impaired after stroke141. Modulation of multiple neurotransmitter systems to promote stroke recovery has been attempted with amphetamines,107 which act primarily through noradrenaline and dopamine signalling and enhance arousal and attention that is relevant for learning and recovery142. L-amphetamine sulphate has been tested for treatment of cognitive dysfunction in MS. They significantly improved performance in learning and memory tasks143,144, as well as speed of processing and working memory145.
Repetitive transcranial magnetic stimulation (rTMS) interferes with or potentiates the function of specific cortical regions146. In stroke recovery, rTMS can improve motor function by re-establishing the functional interhemispheric balance through reduction of interhemispheric inhibition or by increasing the excitability of damaged circuits147. In MS, rTMS may limit the effect of functional interhemispheric imbalance between motor regions and may induce remote effects on the excitability of spinal circuits in patients suffering from spasticity148,149. Transcranial direct current stimulation (tDCS) modulates synaptic plasticity by altering cortical excitability150. Decreased GABAergic tone, which releases latent cortico-cortical projections from tonic inhibition, is a mechanism of rapid cortical plasticity that can facilitate recovery120. tDCS can predispose brain plasticity mechanisms to learning151 and recovery152 through modulation of this GABAergic tone. The therapeutic potential of electrical stimulation is under investigation for stroke recovery, but its possible application to MS recovery remains to be explored.
The presence of cortical pathology in MS challenges attempts to develop pharmacological and electrical interventions that modulate the function of specific brain systems as pathological changes may alter cortical excitability and thus interfere with the desired effects of interventions.
Beyond pharmacological and electrical interventions, the question remains open as to what effect pharmacological modulation of inflammation with disease modifying treatment has on mechanisms of brain plasticity79.
Imaging adaptive functional reorganization in MS
Promotion of functional restoration requires optimization of methods to detect the effects of interventions and to improve the efficiency of studies. fMRI has been widely used in studies on recovery in MS153. It characterizes functional reorganization at the systems level154, as generation of an fMRI signal correlates with neural activity. However, the fMRI signal is only indirectly neural in origin, and disease-related factors and therapeutic interventions can further complicate interpretation of the signal3 (Figure 3). Therefore, the use and interpretation of fMRI as a measure of neural activity in studies on neuroplasticity and recovery requires methodological consideration3.
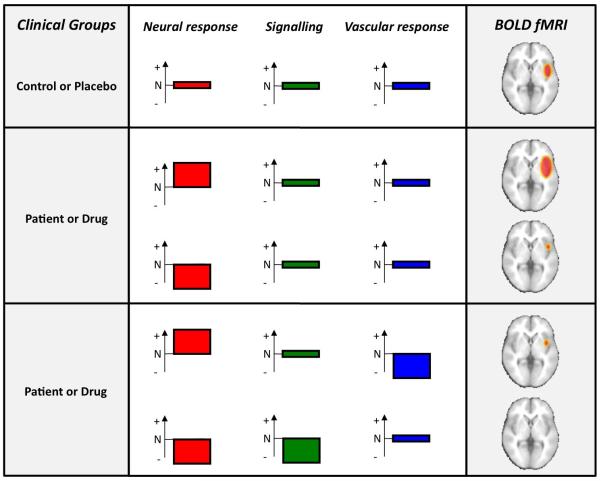
Figure 3.
Effects of disease and pharmacological interventions on generation of BOLD fMRI signal3. The graphs illustrate normal (N), elevated (+) or reduced (−) levels in processes that generate the measured BOLD signal. These processes include neural and vascular factors such as signalling to the vasculature and vascular responsiveness. (a) A schematic fMRI activation map in a control group or under placebo administration. (b) Changes in neural activity induced by disease or drugs are correctly reflected in the final statistical map when the confounding effects of signalling and vascular responses are taken into account. (c) Changes in neural activity induced by disease or drugs are incorrectly reflected in the final statistical map because of the intervening confounds of altered neurovascular signalling or vascular responsiveness. Abbreviations: BOLD, blood oxygenation level-dependent; fMRI, functional MRI. Permission obtained from Elsevier Ltd © Iannetti, G. D. & Wise, R. G. Magn. Reson. Imaging 25, 978–988 (2007).
Interpretation of fMRI signal in disease and in interventional studies
The blood oxygenation level-dependent (BOLD) signal is the image contrast most commonly used in fMRI studies (Box 2). Comparison with electrophysiological measurements suggests that the BOLD signal most closely corresponds to pre- and postsynaptic processing of incoming afferent signals and intracortical processing, such as are represented in local field potentials155-157 rather than the spiking output of a particular region. Proportional increases or decreases in local excitation and inhibition are likely to lead to increases or decreases, respectively, in the local energy demand and, therefore, in the BOLD signal155-157. Net excitation is also likely to lead to BOLD signal increases, whereas the fMRI response to a net inhibition is probably more circuit-dependent owing to lower energy demands of reduced excitation but increased energy requirement for the inhibitory processes155-157.
Box 2.
The neurophysiological basis of functional MRI. Functional MRI (fMRI) has millimetre-scale spatial resolution, providing a large-scale average of neural activity. The parameter measured by this imaging technique is the blood oxygenation level-dependent (BOLD) signal, which is principally affected by changes in the local balance between neuronal excitation and inhibition. Increased neural activity results in increased cerebral metabolic rate of oxygen (CMRO2) and local vasodilatation. As a consequence, cerebral blood flow increases. The fractional increase in blood flow is greater than the fractional increase in CMRO2. This difference reduces the quantity of (paramagnetic) deoxy-haemoglobin in the veins and is equivalent to increased oxygenation, which increases local magnetic field homogeneity around capillaries and veins and, thereby, increases net signal intensity in that area. This change in BOLD signal varies depending on the magnetic field strength, the brain region and the underlying physiology or pathology. Within a scan, periods of active stimulation (‘ON’ condition) are contrasted with rest periods (‘OFF’ condition). The choice of baseline is crucial in the interpretation of fMRI data.
The most powerful experimental designs use equal-duration alternating ON and OFF periods (block design), each lasting 10-60 s. Brief stimuli can be used in event-related designs where the functions under investigation dictate. Investigations of resting-state activity, in which the volunteer does not perform a particular task, have grown in popularity in recent years, especially in patient populations, removing the potential complication of disease-associated impaired task performance. These investigations seek to identify temporally co-varying BOLD signals, which are thought to reflect dynamically co-varying levels of neural activity, from different brain regions. Such signals are thought to represent functional connectivity between the brain regions in question. This approach can identify networks of brain regions that could underlie a specific function, such as motor output or vision192, and can be used to detect their potential modulation in disease.
The origin of the BOLD signal is vascular, so neurovascular coupling must remain intact for fMRI to provide a faithful representation of alterations in neural activity. Not only should the chemical signalling between neurons, astrocytes and cerebral arterioles be preserved, but so should the biophysical coupling between the vascular response and the BOLD signal. This coupling is embodied in the concept of vascular reactivity, defined as the capacity of the vasculature to augment blood flow and generate a BOLD response following a vascular stimulus.
Alteration of the physiological properties of the BOLD signal can occur with age, in chronic inflammatory states or with therapeutic interventions158-160 (Figure 3). Age can affect the fMRI response independently of other pathological factors. A reduction of task-induced BOLD contrast associated with reduced baseline cerebral blood flow (CBF) and baseline cerebral metabolic rate of oxygen (CMRO2) has been demonstrated in the ageing brain161. Baseline CBF has been shown to modulate task-related BOLD signal162. Vascular reactivity assessment using carbon dioxide (CO2) has suggested a reduced ability of blood vessels to respond in the ageing and diseased brain163,164. In addition to alterations in vascular behaviour, neurodegenerative diseases and even a genetic predisposition for such diseases are likely to modify CBF, cerebral blood volume (CBV) and CMRO2 and, therefore, the BOLD response165,166.
Disease or interventions can induce changes in baseline neural activity and vascular response, which are likely to modulate the BOLD signal in response to a task167, leading to either over- or underestimation of their true modulatory effects on brain activity (Figure 3). Altered fMRI responses have been demonstrated in circumstances of altered underlying cerebral physiology168. In MS, vascular and metabolic changes have been described169,170. Abnormal perfusion occurs in enhancing171 and nonenhancing172 MS lesions, as well as in normal-appearing brain tissue in patients with MS169. Both white and grey matter can be affected by perfusional changes that result from damage. This perfusional changes can differ across disease phases and forms173. Baseline CMRO2 and venous CBV can also be reduced in MS174. Furthermore, a more systemic vascular dysregulation can arise from production of inflammatory molecules and from astrocyte dysfunction169, which can alter neurovascular coupling through a vasoconstrictive effect169 or through impaired buffering of ions and neurotransmitters175. In addition to disease-associated alterations, therapeutic interventions can induce changes in fMRI responses through their effects on brain plasticity. Their effects on BOLD response may also differ from those in healthy individuals, as therapeutic interventions can interact with damage.
Given the complexity of the processes leading to generation of BOLD signal and the additional confounders generated by factors such as age, pathology or interventions, methods to improve the interpretability of the fMRI signal are needed to characterise mechanisms and aid in the development of interventions for functional recovery in MS.
Improving interpretability of fMRI signal
Controlling for factors that modulate the BOLD response to neural activity improves the interpretability of fMRI3 (Table 1). However, use of a control task to explore the functional system specificity of an intervention is useful for ruling out global modulation of signalling or vascular reactivity induced by the disease or the intervention. Also, resting fMRI provides an alternative approach to studying functional plasticity that avoids the confounding effect of task-related performance. This approach has been used to explore spontaneous and intervention-driven functional reorganization in MS15,80,81,176-178. It provides a powerful tool in recovery studies as changes in local versus distant connectivity can be characterized despite inter-individual differences in spontaneous or intervention-driven behavioural changes. Resting fMRI can also help to disentangle the contribution of insufficient adaptive versus undesirable maladaptive plasticity to clinical status and to changes in clinical status with intervention. However, given the absence of associated behavioural information in resting fMRI, use of this approach in disease and interventional studies requires similar methodological consideration to task-based fMRI.
Table 1.
Potential confounding factors affecting BOLD signal generation in MS studies and strategies to overcome them.
| Source of fMRI signal | Confounding factors | Strategy to control for confounding factors |
|---|---|---|
| Neural signalling | Disease-related or intervention-related increases or decreases in brain activity | Simultaneous or delayed electrophysiological recording |
| Neurovascular coupling | Disease-related or intervention-related effect on vascular response to changes of neural activity | Measure vascular reactivity (for example, with carbon dioxide challenge) |
| Vascular compartment | Disease-related or intervention-related differences in baseline perfusion levels | Measure baseline perfusion Measure perfusion response to a task |
Measurement of baseline perfusion and of perfusion responses to a task can help to control for differences in baseline BOLD signal161,162,165,179, which is relevant when assessing the effect of interventions in patient groups that are affected by different levels of inflammation or are undergoing different types of pharmacological interventions. Vascular reactivity, tested using a vascular stimulus such as CO2180, can be factored into a subsequent analysis of task-related BOLD signal changes181 to separate the effect of disease or intervention on the vascular versus the neuronal component of the BOLD signal159. Combination of fMRI approaches with simultaneous or delayed electrophysiological recording - that is, electroencephalography, magnetoencephalography or TMS - can further contribute to elucidation of the origin of BOLD signal changes3. This combination approach is particularly useful for clarifying the neural correlates of an increased or decreased BOLD signal128 and, thereby, the mechanisms underlying therapeutic interventions. Calibrated fMRI, in which task-related fractional changes in CMRO2 are derived from calibration of BOLD signal relative to changes in CBF182,183, has been used in pharmacological studies in the healthy brain159,184 with the expectation that CMRO2 changes reflect the underlying neural activity better than BOLD signal alone. In addition to controlling for potential confounding factors, the measurement of cerebral physiology might provide novel markers of recovery or treatment effects. Arterial-spin labelling measures of CBF, for example, are more stable markers of resting levels of brain activity over long time periods than are BOLD signal measures185, which is relevant to disease evolution and treatment158. Furthermore, regional measures of vascular reactivity163 and CBF may help to determine inflammatory status172 and thus assess the effects of anti-inflammatory treatments and their effects on brain plasticity in MS.
Efficient study designs would facilitate the development of interventions to promote recovery. Multicentre fMRI studies are feasible and reliably informative in MS91. In multicentre settings, longitudinal studies, which are required when testing interventions to promote recovery186, can provide reproducible fMRI measures187.
Several studies in motor recovery in MS have analysed functional and effective connectivity using sophisticated statistical approaches to establish the strength of activation and synchrony between specific brain areas77,178. Graph theory approaches that model effectiveness of information transfer within brain networks can enable assessment of the effect of individual factors, disease and intervention in dynamically changing brain systems76,83,188.
Functional changes in specific regions can be particularly informative in assessment of restorative therapies. ‘Recovery-weighted’ maps, in which patient-specific28,30,63 and performance-related91 functional responses are associated with a favourable clinical status63 or outcome33,34, can be useful for testing the effects of interventions9. Similarly, the development of high-resolution methods to study difficult-to-access anatomical regions relevant for recovery such as the LGN can help in understanding aspects of recovery that can be manipulated early after acute damage31.
In studies on recovery, fMRI is often combined with structural information in an attempt to capture brain plasticity. Models that combine visual responses as measured on fMRI with optic nerve structure and measures of visual function can determine the contribution of functional reorganization to clinical function after accounting for structural factors28. Combination of functional connectivity measures with measures of structural damage to specific white matter tracts is also used to investigate the relationship between structural and functional abnormalities in patients with MS90. Structural imaging can be used in combination with functional imaging in recovery studies to investigate the bases for individual variation in neuroplasticity189 and recovery190, to demonstrate structural plasticity accompanying functional plasticity and to characterize the time course of these concurrent changes191. A detailed discussion of structural imaging methods to investigate structural repair is beyond the scope of this Review. Given the close interplay between systems-level functional and structural plasticity, opportunities and limitations of structural imaging methods, which may be relevant to investigate structural repair in MS153 and may be similarly affected by disease or interventions, are briefly discussed in Supplementary Box 1 online.
Conclusions and future directions
Despite substantial progress in the field of functional recovery, MS continues to be the major cause of chronic neurological disability in young adults, and development of therapeutic strategies to promote functional recovery remains challenging. Review of the Literature highlights difficulties of confidently interpreting results from single studies or of combining results from different studies because of uncertainties about the homogeneity of patient groups, standardization of interventions, whose biological effects can be characterized and quantified using both clinical scales and objective (imaging or electrophysiological) measures, a lack of sensitive surrogate markers of recovery or an understanding of expected treatment effect sizes that could contribute to prospective powering of studies. A path forward will involve development of new kind of study designs, optimized for assessing specific mechanisms of recovery and incorporating clinically relevant outcomes, with testing of specific hypotheses related to the underlying neurobiological mechanisms with which interventions promote recovery. A combined strategy involving a strong biological rationale and monitoring of functional and structural reorganization using brain imaging methods should form the basis for scientifically informed neurorehabilitation in MS. Using this approach, effects of interventions can be quantified and compared with clinically-relevant, sensitive and reproducible measures in selected clinical cohorts (Box 3). As in stroke research, restorative strategies in MS are building on emerging understanding of neural plasticity. Their progress, therefore, is inherently cross-disciplinary and relies on more complex, multimodal approaches, beyond the purely behaviour-centred studies.
Box 3.
Considerations for future studies to promote functional recovery in MS.
Type of study
Hypothesis-driven studies are preferable when targets of intervention are known. Translational studies, from bench to bedside, should be encouraged. Exploratory methodologies can be used for identification of potential new therapies or targets.
Design
Optimized trial designs (for example, sequential, adaptive or enrichment methodologies) should be prioritized over traditional trial designs. Appropriate control groups are essential. A post-intervention study phase is desirable to confirm the effect of interventions and to test for sustained effects.
Groups
Cohorts with disabilities should be prioritized when investigating the potential benefits of new interventions. Nondisabled cohorts can be studied to define biological mechanisms of successful recovery. They may be also considered for studies of interventions that have potential to increase the capacity for recovery by delaying accrual of disability Eligibility criteria for study group should be based on “rehabilitation criteria” (performance over disease characteristics), when effects of interventions are tested, or on “standard clinical criteria” (disease characteristics over performance), when the influence of specific disease characteristics on effects of interventions is to be tested.
Sample size
Fixed sample size or adaptive sample size re-estimation can be considered, depending on the type of study design.
End point
The study end point should be clinically relevant. Both clinical and paraclinical measures such as imaging should be collected to define mechanisms of therapeutic benefit. Efforts should be accelerated to better validate imaging or other paraclinical measures of brain recovery. Patient-related outcome measures provide an important complementary perspective.
Interventions
Behavioural, pharmacological or electrophysiological interventions should all be considered. Interventions and key aspects of imaging or other paraclinical measures should be standardized as much as possible to allow comparisons between studies. To facilitate the development of such standardised methods, the scientific community should work towards sharing of methodology and data.
Analysis methods
Hypothesis testing and exploratory studies should be clearly identified as such and appropriate statistical approaches used for each. Confidence intervals should be regularly reported and consideration should be given to the potential effects of heterogeneity in patient populations. When imaging is combined with behavioural studies, multimodal approaches are desirable.
Experience gained from other neurological conditions provides a powerful framework in which models of recovery and neurorehabilitation can be constructed and tested154. Development of new strategies to promote recovery, and of imaging markers to measure effects of therapeutic intervention, however, needs to take place within the specific pathological context of MS. The chronic and diffuse nature of MS pathology poses challenges, as effects of interventions need to be sustained and to operate across multiple brain systems. In addition to adaptive plasticity, maladaptive reorganization accompanying chronic disuse can occur, presenting a further challenge to recovery. Limited, direct evidence from studies in MS encourages manipulation of adaptive plasticity with therapeutic interventions, but our knowledge of brain plasticity in MS derives mainly from observational studies without external inducement of plasticity. Further testing in controlled interventional studies is essential if we are to develop an understanding of how to effectively promote adaptive plasticity in MS and how to translate such methods into clinical practice.
Supplementary Material
Search strategy and selection criteria.
References for this Review were identified through searches of PubMed using the search terms “functional reorganization”, “brain plasticity”, “recovery”, “rehabilitation”, “pharmacological modulation” and “MRI” from January 1949 until April 2012. Articles were also identified through searches of the authors’ own files. Only papers published in English were reviewed. The final reference list was generated on the basis of originality and relevance to this Review.
Key points.
Evidence across brain systems supports a behaviourally relevant role for neuroplasticity in multiple sclerosis (MS) across ages, stages and phases of the disease, which is preserved despite widespread pathology.
Together with adaptive plasticity, maladaptive plasticity can occur owing to disuse with impairment and may contribute to disability.
Interventions that drive neuroplasticity can promote functional restoration by inducing adaptive changes or by predisposing functional systems to adaptive plasticity.
Individual and disease-related factors influence both spontaneous and intervention-driven adaptive functional reorganization, as well as its assessment using imaging.
Improving the interpretability of functional MRI measures is important for characterization and quantification of the effects of recovery interventions and, thereby for development of recovery-oriented strategies.
Acknowledgements
V. Tomassini is supported by the MS Society Italy, the MS International Federation and the Italian Ministry of Health. D. F. receives research funding from The Lundbeck Foundation Centre for Neurovascular Signalling. H. Johansen-Berg is funded by the Wellcome Trust. R. G. Wise receives research funds from the UK Medical Research Council. This review reflects the outcome of an international workshop held in 2010 in Warwick, UK, by the MAGNIMS Network (http://www.magnims.eu/). V.T. has received an International Meeting Grant from the MS International Federation (http://www.msif.org/en/) in 2010 to support the organisation of the Workshop.
Footnotes
Competing interests [printed version]
P. M. Matthews declares an association with GlaxoSmithKline. A. J. Thompson declares associations with the following companies: Biogen, BTG, Eisai, Merck-Serono, Novartis, Teva. See the article online for full details of the relationships. The other authors declare no competing interests.
Competing interests [online version]
P. M. Matthews is a part-time employee of GlaxoSmithKline Research and Development and holds stocks and options in GlaxoSmithKline. A. J. Thompson has received honoraria for consultancy and support for travel from BTG, Eisai, Teva, Biogen, Merck-Serono, and Novartis. The other authors declare no competing interests
Author contributions
V. Tomassini, D. Fuglø, J. J. Geurts, D. K. Jones, M. A. Rocca and R. G. Wise researched data for the article. All authors provided substantial contribution to discussion of the article content and writing of the article. V. Tomassini, P. M. Matthews, A. J. Thompson, J. J. Geurts, R. G. Wise and F. Barkhof contributed to review and/or editing of the manuscript before submission.
References
- 1.Tomassini V, Palace J. Multiple sclerosis lesions: insights from imaging techniques. Expert Rev Neurother. 2009;9:1341–1359. doi: 10.1586/ern.09.83. [DOI] [PubMed] [Google Scholar]
- 2.Irvine KA, Blakemore WF. Remyelination protects axons from demyelination-associated axon degeneration. Brain. 2008;131:1464–1477. doi: 10.1093/brain/awn080. [DOI] [PubMed] [Google Scholar]
- 3.Iannetti GD, Wise RG. BOLD functional MRI in disease and pharmacological studies: room for improvement? Magn Reson Imaging. 2007;25:978–988. doi: 10.1016/j.mri.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 4.Jones DK. Challenges and limitations of quantifying brain connectivity in vivo with diffusion MRI. Imaging Med. 2010;2:341–355. [Google Scholar]
- 5.Cramer SC. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann Neurol. 2008;63:272–287. doi: 10.1002/ana.21393. [DOI] [PubMed] [Google Scholar]
- 6.Nudo RJ. Plasticity. NeuroRx. 2006;3:420–427. doi: 10.1016/j.nurx.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fridman EA, et al. Reorganization of the human ipsilesional premotor cortex after stroke. Brain. 2004;127:747–758. doi: 10.1093/brain/awh082. [DOI] [PubMed] [Google Scholar]
- 8.Bosnell RA, et al. Motor practice promotes increased activity in brain regions structurally disconnected after subcortical stroke. Neurorehabilitation and neural repair. 2011;25:607–616. doi: 10.1177/1545968311405675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johansen-Berg H, et al. Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain : a journal of neurology. 2002;125:2731–2742. doi: 10.1093/brain/awf282. [DOI] [PubMed] [Google Scholar]
- 10.Johansen-Berg H, et al. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci U S A. 2002;99:14518–14523. doi: 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reddy H, et al. Evidence for adaptive functional changes in the cerebral cortex with axonal injury from multiple sclerosis. Brain : a journal of neurology. 2000;123(Pt 11):2314–2320. doi: 10.1093/brain/123.11.2314. [DOI] [PubMed] [Google Scholar]
- 12.Reddy H, et al. Relating axonal injury to functional recovery in MS. Neurology. 2000;54:236–239. doi: 10.1212/wnl.54.1.236. [DOI] [PubMed] [Google Scholar]
- 13.Lee M, et al. The motor cortex shows adaptive functional changes to brain injury from multiple sclerosis. Ann Neurol. 2000;47:606–613. [PubMed] [Google Scholar]
- 14.Tomassini V, et al. Relating Brain Damage to Brain Plasticity in Patients With Multiple Sclerosis. Neurorehabil Neural Repair. 2012 doi: 10.1177/1545968311433208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filippi M, et al. Multiple sclerosis: effects of cognitive rehabilitation on structural and functional MR imaging measures--an explorative study. Radiology. 2012;262:932–940. doi: 10.1148/radiol.11111299. [DOI] [PubMed] [Google Scholar]
- 16.Parry AM, Scott RB, Palace J, Smith S, Matthews PM. Potentially adaptive functional changes in cognitive processing for patients with multiple sclerosis and their acute modulation by rivastigmine. Brain : a journal of neurology. 2003;126:2750–2760. doi: 10.1093/brain/awg284. [DOI] [PubMed] [Google Scholar]
- 17.Reddy H, et al. Functional brain reorganization for hand movement in patients with multiple sclerosis: defining distinct effects of injury and disability. Brain. 2002;125:2646–2657. doi: 10.1093/brain/awf283. [DOI] [PubMed] [Google Scholar]
- 18.Kupersmith MJ, Alban T, Zeiffer B, Lefton D. Contrast-enhanced MRI in acute optic neuritis: relationship to visual performance. Brain : a journal of neurology. 2002;125:812–822. doi: 10.1093/brain/awf087. [DOI] [PubMed] [Google Scholar]
- 19.Trip SA, et al. Optic nerve atrophy and retinal nerve fibre layer thinning following optic neuritis: evidence that axonal loss is a substrate of MRI-detected atrophy. Neuroimage. 2006;31:286–293. doi: 10.1016/j.neuroimage.2005.11.051. [DOI] [PubMed] [Google Scholar]
- 20.Ciccarelli O, et al. Optic radiation changes after optic neuritis detected by tractography-based group mapping. Hum Brain Mapp. 2005;25:308–316. doi: 10.1002/hbm.20101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenkins TM, et al. Early pericalcarine atrophy in acute optic neuritis is associated with conversion to multiple sclerosis. J Neurol Neurosurg Psychiatry. 2011 doi: 10.1136/jnnp.2010.239715. [DOI] [PubMed] [Google Scholar]
- 22.Pfueller CF, et al. Metabolic changes in the visual cortex are linked to retinal nerve fiber layer thinning in multiple sclerosis. PLoS One. 2011;6:e18019. doi: 10.1371/journal.pone.0018019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gareau PJ, et al. Reduced visual evoked responses in multiple sclerosis patients with optic neuritis: comparison of functional magnetic resonance imaging and visual evoked potentials. Mult Scler. 1999;5:161–164. doi: 10.1177/135245859900500304. [DOI] [PubMed] [Google Scholar]
- 24.Langkilde AR, Frederiksen JL, Rostrup E, Larsson HB. Functional MRI of the visual cortex and visual testing in patients with previous optic neuritis. Eur J Neurol. 2002;9:277–286. doi: 10.1046/j.1468-1331.2002.00399.x. [DOI] [PubMed] [Google Scholar]
- 25.Levin N, Orlov T, Dotan S, Zohary E. Normal and abnormal fMRI activation patterns in the visual cortex after recovery from optic neuritis. Neuroimage. 2006;33:1161–1168. doi: 10.1016/j.neuroimage.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 26.Rombouts SA, et al. Visual activation patterns in patients with optic neuritis: an fMRI pilot study. Neurology. 1998;50:1896–1899. doi: 10.1212/wnl.50.6.1896. [DOI] [PubMed] [Google Scholar]
- 27.Russ MO, et al. Functional magnetic resonance imaging in acute unilateral optic neuritis. J Neuroimaging. 2002;12:339–350. doi: 10.1111/j.1552-6569.2002.tb00142.x. [DOI] [PubMed] [Google Scholar]
- 28.Toosy AT, et al. Adaptive cortical plasticity in higher visual areas after acute optic neuritis. Ann Neurol. 2005;57:622–633. doi: 10.1002/ana.20448. [DOI] [PubMed] [Google Scholar]
- 29.Toosy AT, et al. Functional magnetic resonance imaging of the cortical response to photic stimulation in humans following optic neuritis recovery. Neurosci Lett. 2002;330:255–259. doi: 10.1016/s0304-3940(02)00700-0. [DOI] [PubMed] [Google Scholar]
- 30.Werring DJ, et al. Recovery from optic neuritis is associated with a change in the distribution of cerebral response to visual stimulation: a functional magnetic resonance imaging study. J Neurol Neurosurg Psychiatry. 2000;68:441–449. doi: 10.1136/jnnp.68.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korsholm K, Madsen KH, Frederiksen JL, Skimminge A, Lund TE. Recovery from optic neuritis: an ROI-based analysis of LGN and visual cortical areas. Brain : a journal of neurology. 2007;130:1244–1253. doi: 10.1093/brain/awm045. [DOI] [PubMed] [Google Scholar]
- 32.Jenkins TM, et al. Neuroplasticity predicts outcome of optic neuritis independent of tissue damage. Ann Neurol. 2010;67:99–113. doi: 10.1002/ana.21823. [DOI] [PubMed] [Google Scholar]
- 33.Rocca MA, et al. Is a preserved functional reserve a mechanism limiting clinical impairment in pediatric MS patients? Hum Brain Mapp. 2009;30:2844–2851. doi: 10.1002/hbm.20712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rocca MA, et al. Preserved brain adaptive properties in patients with benign multiple sclerosis. Neurology. 2010;74:142–149. doi: 10.1212/WNL.0b013e3181c91a00. [DOI] [PubMed] [Google Scholar]
- 35.Rocca MA, et al. Evidence for widespread movement-associated functional MRI changes in patients with PPMS. Neurology. 2002;58:866–872. doi: 10.1212/wnl.58.6.866. [DOI] [PubMed] [Google Scholar]
- 36.Mezzapesa DM, Rocca MA, Rodegher M, Comi G, Filippi M. Functional cortical changes of the sensorimotor network are associated with clinical recovery in multiple sclerosis. Hum Brain Mapp. 2008;29:562–573. doi: 10.1002/hbm.20418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pantano P, et al. A longitudinal fMRI study on motor activity in patients with multiple sclerosis. Brain : a journal of neurology. 2005;128:2146–2153. doi: 10.1093/brain/awh549. [DOI] [PubMed] [Google Scholar]
- 38.Pantano P, et al. Cortical motor reorganization after a single clinical attack of multiple sclerosis. Brain : a journal of neurology. 2002;125:1607–1615. doi: 10.1093/brain/awf164. [DOI] [PubMed] [Google Scholar]
- 39.Rocca MA, et al. Cortical adaptation in patients with MS: a cross-sectional functional MRI study of disease phenotypes. Lancet Neurol. 2005;4:618–626. doi: 10.1016/S1474-4422(05)70171-X. [DOI] [PubMed] [Google Scholar]
- 40.Lenzi D, et al. Effect of corpus callosum damage on ipsilateral motor activation in patients with multiple sclerosis: a functional and anatomical study. Hum Brain Mapp. 2007;28:636–644. doi: 10.1002/hbm.20305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Stefano N, et al. Brain damage as detected by magnetization transfer imaging is less pronounced in benign than in early relapsing multiple sclerosis. Brain : a journal of neurology. 2006;129:2008–2016. doi: 10.1093/brain/awl152. [DOI] [PubMed] [Google Scholar]
- 42.Rocca MA, et al. The level of spinal cord involvement influences the pattern of movement-associated cortical recruitment in patients with isolated myelitis. Neuroimage. 2006;30:879–884. doi: 10.1016/j.neuroimage.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 43.Rocca MA, et al. Adaptive functional changes in the cerebral cortex of patients with nondisabling multiple sclerosis correlate with the extent of brain structural damage. Ann Neurol. 2002;51:330–339. doi: 10.1002/ana.10120. [DOI] [PubMed] [Google Scholar]
- 44.Manson SC, Palace J, Frank JA, Matthews PM. Loss of interhemispheric inhibition in patients with multiple sclerosis is related to corpus callosum atrophy. Exp Brain Res. 2006;174:728–733. doi: 10.1007/s00221-006-0517-4. [DOI] [PubMed] [Google Scholar]
- 45.Rocca MA, et al. A functional magnetic resonance imaging study of patients with secondary progressive multiple sclerosis. Neuroimage. 2003;19:1770–1777. doi: 10.1016/s1053-8119(03)00242-8. [DOI] [PubMed] [Google Scholar]
- 46.Amato MP, et al. Neocortical volume decrease in relapsing-remitting MS patients with mild cognitive impairment. Neurology. 2004;63:89–93. doi: 10.1212/01.wnl.0000129544.79539.d5. [DOI] [PubMed] [Google Scholar]
- 47.Benedict RH, et al. Diffusion-weighted imaging predicts cognitive impairment in multiple sclerosis. Mult Scler. 2007;13:722–730. doi: 10.1177/1352458507075592. [DOI] [PubMed] [Google Scholar]
- 48.Brass SD, Benedict RH, Weinstock-Guttman B, Munschauer F, Bakshi R. Cognitive impairment is associated with subcortical magnetic resonance imaging grey matter T2 hypointensity in multiple sclerosis. Mult Scler. 2006;12:437–444. doi: 10.1191/135248506ms1301oa. [DOI] [PubMed] [Google Scholar]
- 49.Calabrese M, et al. Cortical lesions and atrophy associated with cognitive impairment in relapsing-remitting multiple sclerosis. Arch Neurol. 2009;66:1144–1150. doi: 10.1001/archneurol.2009.174. [DOI] [PubMed] [Google Scholar]
- 50.Filippi M, et al. Changes in the normal appearing brain tissue and cognitive impairment in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2000;68:157–161. doi: 10.1136/jnnp.68.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lazeron RH, et al. Neuropsychological impairment in multiple sclerosis patients: the role of (juxta)cortical lesion on FLAIR. Mult Scler. 2000;6:280–285. doi: 10.1177/135245850000600410. [DOI] [PubMed] [Google Scholar]
- 52.Roosendaal SD, et al. Accumulation of cortical lesions in MS: relation with cognitive impairment. Mult Scler. 2009;15:708–714. doi: 10.1177/1352458509102907. [DOI] [PubMed] [Google Scholar]
- 53.Benedict RH, et al. Neocortical atrophy, third ventricular width, and cognitive dysfunction in multiple sclerosis. Arch Neurol. 2006;63:1301–1306. doi: 10.1001/archneur.63.9.1301. [DOI] [PubMed] [Google Scholar]
- 54.Pelletier J, et al. A longitudinal study of callosal atrophy and interhemispheric dysfunction in relapsing-remitting multiple sclerosis. Arch Neurol. 2001;58:105–111. doi: 10.1001/archneur.58.1.105. [DOI] [PubMed] [Google Scholar]
- 55.Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008;7:1139–1151. doi: 10.1016/S1474-4422(08)70259-X. [DOI] [PubMed] [Google Scholar]
- 56.Audoin B, et al. Compensatory cortical activation observed by fMRI during a cognitive task at the earliest stage of MS. Hum Brain Mapp. 2003;20:51–58. doi: 10.1002/hbm.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chiaravalloti N, et al. Cerebral activation patterns during working memory performance in multiple sclerosis using FMRI. J Clin Exp Neuropsychol. 2005;27:33–54. doi: 10.1080/138033990513609. [DOI] [PubMed] [Google Scholar]
- 58.Mainero C, et al. fMRI evidence of brain reorganization during attention and memory tasks in multiple sclerosis. Neuroimage. 2004;21:858–867. doi: 10.1016/j.neuroimage.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 59.Loitfelder M, et al. Reorganization in cognitive networks with progression of multiple sclerosis: insights from fMRI. Neurology. 2011;76:526–533. doi: 10.1212/WNL.0b013e31820b75cf. [DOI] [PubMed] [Google Scholar]
- 60.Penner IK, Rausch M, Kappos L, Opwis K, Radu EW. Analysis of impairment related functional architecture in MS patients during performance of different attention tasks. J Neurol. 2003;250:461–472. doi: 10.1007/s00415-003-1025-0. [DOI] [PubMed] [Google Scholar]
- 61.Sumowski JF, Wylie GR, Deluca J, Chiaravalloti N. Intellectual enrichment is linked to cerebral efficiency in multiple sclerosis: functional magnetic resonance imaging evidence for cognitive reserve. Brain : a journal of neurology. 2010;133:362–374. doi: 10.1093/brain/awp307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wishart HA, et al. Brain activation patterns associated with working memory in relapsing-remitting MS. Neurology. 2004;62:234–238. doi: 10.1212/01.wnl.0000103238.91536.5f. [DOI] [PubMed] [Google Scholar]
- 63.Cader S, Cifelli A, Abu-Omar Y, Palace J, Matthews PM. Reduced brain functional reserve and altered functional connectivity in patients with multiple sclerosis. Brain : a journal of neurology. 2006;129:527–537. doi: 10.1093/brain/awh670. [DOI] [PubMed] [Google Scholar]
- 64.Inglese M, et al. Perfusion magnetic resonance imaging correlates of neuropsychological impairment in multiple sclerosis. J Cereb Blood Flow Metab. 2008;28:164–171. doi: 10.1038/sj.jcbfm.9600504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lycke J, Wikkelso C, Bergh AC, Jacobsson L, Andersen O. Regional cerebral blood flow in multiple sclerosis measured by single photon emission tomography with technetium-99m hexamethylpropyleneamine oxime. Eur Neurol. 1993;33:163–167. doi: 10.1159/000116926. [DOI] [PubMed] [Google Scholar]
- 66.Pozzilli C, et al. SPECT, MRI and cognitive functions in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1991;54:110–115. doi: 10.1136/jnnp.54.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blinkenberg M, et al. Cortical cerebral metabolism correlates with MRI lesion load and cognitive dysfunction in MS. Neurology. 2000;54:558–564. doi: 10.1212/wnl.54.3.558. [DOI] [PubMed] [Google Scholar]
- 68.Audoin B, et al. Structure of WM bundles constituting the working memory system in early multiple sclerosis: a quantitative DTI tractography study. Neuroimage. 2007;36:1324–1330. doi: 10.1016/j.neuroimage.2007.04.038. [DOI] [PubMed] [Google Scholar]
- 69.Rocca MA, et al. Structural and functional MRI correlates of Stroop control in benign MS. Hum Brain Mapp. 2009;30:276–290. doi: 10.1002/hbm.20504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wandell BA, Smirnakis SM. Plasticity and stability of visual field maps in adult primary visual cortex. Nat Rev Neurosci. 2009;10:873–884. doi: 10.1038/nrn2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Franklin RJ, Zhao C, Sim FJ. Ageing and CNS remyelination. Neuroreport. 2002;13:923–928. doi: 10.1097/00001756-200205240-00001. [DOI] [PubMed] [Google Scholar]
- 72.Rocca MA, et al. Functional and structural connectivity of the motor network in pediatric and adult-onset relapsing-remitting multiple sclerosis. Radiology. 2010;254:541–550. doi: 10.1148/radiol.09090463. [DOI] [PubMed] [Google Scholar]
- 73.Pozzilli C, et al. ‘Gender gap’ in multiple sclerosis: magnetic resonance imaging evidence. Eur J Neurol. 2003;10:95–97. doi: 10.1046/j.1468-1331.2003.00519.x. [DOI] [PubMed] [Google Scholar]
- 74.Tomassini V, et al. Sex hormones modulate brain damage in multiple sclerosis: MRI evidence. J Neurol Neurosurg Psychiatry. 2005;76:272–275. doi: 10.1136/jnnp.2003.033324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gregg C, et al. White matter plasticity and enhanced remyelination in the maternal CNS. J Neurosci. 2007;27:1812–1823. doi: 10.1523/JNEUROSCI.4441-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schoonheim MM, et al. Gender-related differences in functional connectivity in multiple sclerosis. Mult Scler. 2011 doi: 10.1177/1352458511422245. [DOI] [PubMed] [Google Scholar]
- 77.Rocca MA, et al. Abnormal connectivity of the sensorimotor network in patients with MS: a multicenter fMRI study. Hum Brain Mapp. 2009;30:2412–2425. doi: 10.1002/hbm.20679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Audoin B, et al. Magnetic resonance study of the influence of tissue damage and cortical reorganization on PASAT performance at the earliest stage of multiple sclerosis. Hum Brain Mapp. 2005;24:216–228. doi: 10.1002/hbm.20083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Min SS, et al. Chronic brain inflammation impairs two forms of long-term potentiation in the rat hippocampal CA1 area. Neurosci Lett. 2009;456:20–24. doi: 10.1016/j.neulet.2009.03.079. [DOI] [PubMed] [Google Scholar]
- 80.Rocca MA, et al. Default-mode network dysfunction and cognitive impairment in progressive MS. Neurology. 2010;74:1252–1259. doi: 10.1212/WNL.0b013e3181d9ed91. [DOI] [PubMed] [Google Scholar]
- 81.Roosendaal SD, et al. Structural and functional hippocampal changes in multiple sclerosis patients with intact memory function. Radiology. 2010;255:595–604. doi: 10.1148/radiol.10091433. [DOI] [PubMed] [Google Scholar]
- 82.De Stefano N, et al. Brain damage as detected by magnetization transfer imaging is less pronounced in benign than in early relapsing multiple sclerosis. Brain. 2006;129:2008–2016. doi: 10.1093/brain/awl152. [DOI] [PubMed] [Google Scholar]
- 83.He Y, et al. Impaired small-world efficiency in structural cortical networks in multiple sclerosis associated with white matter lesion load. Brain : a journal of neurology. 2009;132:3366–3379. doi: 10.1093/brain/awp089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dineen RA, et al. Disconnection as a mechanism for cognitive dysfunction in multiple sclerosis. Brain. 2009;132:239–249. doi: 10.1093/brain/awn275. [DOI] [PubMed] [Google Scholar]
- 85.Filippi M, et al. Correlations between structural CNS damage and functional MRI changes in primary progressive MS. Neuroimage. 2002;15:537–546. doi: 10.1006/nimg.2001.1023. [DOI] [PubMed] [Google Scholar]
- 86.Kolappan M, et al. Assessing structure and function of the afferent visual pathway in multiple sclerosis and associated optic neuritis. J Neurol. 2009;256:305–319. doi: 10.1007/s00415-009-0123-z. [DOI] [PubMed] [Google Scholar]
- 87.Leone MA, et al. Factors predicting incomplete recovery from relapses in multiple sclerosis: a prospective study. Mult Scler. 2008;14:485–493. doi: 10.1177/1352458507084650. [DOI] [PubMed] [Google Scholar]
- 88.DeLuca J, Chelune GJ, Tulsky DS, Lengenfelder J, Chiaravalloti ND. Is speed of processing or working memory the primary information processing deficit in multiple sclerosis? J Clin Exp Neuropsychol. 2004;26:550–562. doi: 10.1080/13803390490496641. [DOI] [PubMed] [Google Scholar]
- 89.Bruce JM, Bruce AS, Arnett PA. Mild visual acuity disturbances are associated with performance on tests of complex visual attention in MS. J Int Neuropsychol Soc. 2007;13:544–548. doi: 10.1017/S1355617707070658. [DOI] [PubMed] [Google Scholar]
- 90.Rocca MA, et al. Pyramidal tract lesions and movement-associated cortical recruitment in patients with MS. Neuroimage. 2004;23:141–147. doi: 10.1016/j.neuroimage.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 91.Wegner C, et al. Relating functional changes during hand movement to clinical parameters in patients with multiple sclerosis in a multi-centre fMRI study. Eur J Neurol. 2008;15:113–122. doi: 10.1111/j.1468-1331.2007.02027.x. [DOI] [PubMed] [Google Scholar]
- 92.Mancini L, et al. Short-term adaptation to a simple motor task: a physiological process preserved in multiple sclerosis. Neuroimage. 2009;45:500–511. doi: 10.1016/j.neuroimage.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 93.Morgen K, et al. Training-dependent plasticity in patients with multiple sclerosis. Brain. 2004;127:2506–2517. doi: 10.1093/brain/awh266. [DOI] [PubMed] [Google Scholar]
- 94.Tomassini V, et al. Preservation of motor skill learning in patients with multiple sclerosis. Mult Scler. 2010;17:103–115. doi: 10.1177/1352458510381257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Corbetta M, Kincade MJ, Lewis C, Snyder AZ, Sapir A. Neural basis and recovery of spatial attention deficits in spatial neglect. Nat Neurosci. 2005;8:1603–1610. doi: 10.1038/nn1574. [DOI] [PubMed] [Google Scholar]
- 96.Warburton E, Price CJ, Swinburn K, Wise RJ. Mechanisms of recovery from aphasia: evidence from positron emission tomography studies. J Neurol Neurosurg Psychiatry. 1999;66:155–161. doi: 10.1136/jnnp.66.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of outcome after stroke: a cross-sectional fMRI study. Brain : a journal of neurology. 2003;126:1430–1448. doi: 10.1093/brain/awg145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 99.Nudo RJ. Adaptive plasticity in motor cortex: implications for rehabilitation after brain injury. J Rehabil Med. 2003:7–10. doi: 10.1080/16501960310010070. [DOI] [PubMed] [Google Scholar]
- 100.Thompson AJ. Neurorehabilitation in multiple sclerosis: foundations, facts and fiction. Curr Opin Neurol. 2005;18:267–271. doi: 10.1097/01.wco.0000169743.37159.a0. [DOI] [PubMed] [Google Scholar]
- 101.Mark VW, et al. Constraint-Induced Movement therapy can improve hemiparetic progressive multiple sclerosis. Preliminary findings. Mult Scler. 2008;14:992–994. doi: 10.1177/1352458508090223. [DOI] [PubMed] [Google Scholar]
- 102.Wolf SL, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. Jama. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 103.Lincoln NB, et al. Evaluation of cognitive assessment and cognitive intervention for people with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2002;72:93–98. doi: 10.1136/jnnp.72.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Solari A, et al. Computer-aided retraining of memory and attention in people with multiple sclerosis: a randomized, double-blind controlled trial. J Neurol Sci. 2004;222:99–104. doi: 10.1016/j.jns.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 105.Penner IK, Kappos L, Rausch M, Opwis K, Radu EW. Therapy-induced plasticity of cognitive functions in MS patients: insights from fMRI. J Physiol Paris. 2006;99:455–462. doi: 10.1016/j.jphysparis.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 106.Portaccio E, et al. Cognitive rehabilitation in children and adolescents with multiple sclerosis. Neurol Sci. 2010;31:S275–278. doi: 10.1007/s10072-010-0377-3. [DOI] [PubMed] [Google Scholar]
- 107.Cramer SC. Repairing the human brain after stroke. II. Restorative therapies. Ann Neurol. 2008;63:549–560. doi: 10.1002/ana.21412. [DOI] [PubMed] [Google Scholar]
- 108.Dobrossy M, et al. Neurorehabilitation with neural transplantation. Neurorehabilitation and neural repair. 2010;24:692–701. doi: 10.1177/1545968310363586. [DOI] [PubMed] [Google Scholar]
- 109.Heremans E, et al. Motor imagery ability in patients with early- and mid-stage Parkinson disease. Neurorehabilitation and neural repair. 2011;25:168–177. doi: 10.1177/1545968310370750. [DOI] [PubMed] [Google Scholar]
- 110.Page SJ, Levine P, Leonard A. Mental practice in chronic stroke: results of a randomized, placebo-controlled trial. Stroke. 2007;38:1293–1297. doi: 10.1161/01.STR.0000260205.67348.2b. [DOI] [PubMed] [Google Scholar]
- 111.Jackson PL, Lafleur MF, Malouin F, Richards C, Doyon J. Potential role of mental practice using motor imagery in neurologic rehabilitation. Archives of physical medicine and rehabilitation. 2001;82:1133–1141. doi: 10.1053/apmr.2001.24286. [DOI] [PubMed] [Google Scholar]
- 112.Szameitat AJ, Shen S, Sterr A. Motor imagery of complex everyday movements. An fMRI study. Neuroimage. 2007;34:702–713. doi: 10.1016/j.neuroimage.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 113.Lotze M, Cohen LG. Volition and imagery in neurorehabilitation. Cogn Behav Neurol. 2006;19:135–140. doi: 10.1097/01.wnn.0000209875.56060.06. [DOI] [PubMed] [Google Scholar]
- 114.Page SJ, Szaflarski JP, Eliassen JC, Pan H, Cramer SC. Cortical plasticity following motor skill learning during mental practice in stroke. Neurorehabilitation and neural repair. 2009;23:382–388. doi: 10.1177/1545968308326427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Malouin F, et al. Effects of practice, visual loss, limb amputation, and disuse on motor imagery vividness. Neurorehabilitation and neural repair. 2009;23:449–463. doi: 10.1177/1545968308328733. [DOI] [PubMed] [Google Scholar]
- 116.Rocca MA, et al. The “mirror-neuron system” in MS: A 3 tesla fMRI study. Neurology. 2008;70:255–262. doi: 10.1212/01.wnl.0000284667.29375.7e. [DOI] [PubMed] [Google Scholar]
- 117.Cramer SC, Orr EL, Cohen MJ, Lacourse MG. Effects of motor imagery training after chronic, complete spinal cord injury. Exp Brain Res. 2007;177:233–242. doi: 10.1007/s00221-006-0662-9. [DOI] [PubMed] [Google Scholar]
- 118.Nicolelis MA, Lebedev MA. Principles of neural ensemble physiology underlying the operation of brain-machine interfaces. Nat Rev Neurosci. 2009;10:530–540. doi: 10.1038/nrn2653. [DOI] [PubMed] [Google Scholar]
- 119.Sanes JN, Donoghue JP. Plasticity and primary motor cortex. Annu Rev Neurosci. 2000;23:393–415. doi: 10.1146/annurev.neuro.23.1.393. [DOI] [PubMed] [Google Scholar]
- 120.Jacobs KM, Donoghue JP. Reshaping the cortical motor map by unmasking latent intracortical connections. Science. 1991;251:944–947. doi: 10.1126/science.2000496. [DOI] [PubMed] [Google Scholar]
- 121.Rioult-Pedotti MS, Friedman D, Hess G, Donoghue JP. Strengthening of horizontal cortical connections following skill learning. Nat Neurosci. 1998;1:230–234. doi: 10.1038/678. [DOI] [PubMed] [Google Scholar]
- 122.Pineiro R, Pendlebury S, Johansen-Berg H, Matthews PM. Functional MRI detects posterior shifts in primary sensorimotor cortex activation after stroke: evidence of local adaptive reorganization? Stroke. 2001;32:1134–1139. doi: 10.1161/01.str.32.5.1134. [DOI] [PubMed] [Google Scholar]
- 123.Hebb DO. Organization of behavior. Wiley; New York: 1949. [Google Scholar]
- 124.Castro-Alamancos MA, Donoghue JP, Connors BW. Different forms of synaptic plasticity in somatosensory and motor areas of the neocortex. J Neurosci. 1995;15:5324–5333. doi: 10.1523/JNEUROSCI.15-07-05324.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Trepel C, Racine RJ. Long-term potentiation in the neocortex of the adult, freely moving rat. Cereb Cortex. 1998;8:719–729. doi: 10.1093/cercor/8.8.719. [DOI] [PubMed] [Google Scholar]
- 126.Barchas JD, Akil H, Elliott GR, Holman RB, Watson SJ. Behavioral neurochemistry: neuroregulators and behavioral states. Science. 1978;200:964–973. doi: 10.1126/science.25486. [DOI] [PubMed] [Google Scholar]
- 127.Judge SI, Bever CT., Jr. Potassium channel blockers in multiple sclerosis: neuronal Kv channels and effects of symptomatic treatment. Pharmacol Ther. 2006;111:224–259. doi: 10.1016/j.pharmthera.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 128.Mainero C, et al. Enhanced brain motor activity in patients with MS after a single dose of 3,4-diaminopyridine. Neurology. 2004;62:2044–2050. doi: 10.1212/01.wnl.0000129263.14219.a8. [DOI] [PubMed] [Google Scholar]
- 129.Bever CT, Judge SI. Sustained-release fampridine for multiple sclerosis. Expert Opin Investig Drugs. 2009;18:1013–1024. doi: 10.1517/13543780903002082. [DOI] [PubMed] [Google Scholar]
- 130.Ge S, Dani JA. Nicotinic acetylcholine receptors at glutamate synapses facilitate long-term depression or potentiation. J Neurosci. 2005;25:6084–6091. doi: 10.1523/JNEUROSCI.0542-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ji D, Lape R, Dani JA. Timing and location of nicotinic activity enhances or depresses hippocampal synaptic plasticity. Neuron. 2001;31:131–141. doi: 10.1016/s0896-6273(01)00332-4. [DOI] [PubMed] [Google Scholar]
- 132.Christodoulou C, et al. Effects of donepezil on memory and cognition in multiple sclerosis. J Neurol Sci. 2006;245:127–136. doi: 10.1016/j.jns.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 133.Cader S, Palace J, Matthews PM. Cholinergic agonism alters cognitive processing and enhances brain functional connectivity in patients with multiple sclerosis. J Psychopharmacol. 2009;23:686–696. doi: 10.1177/0269881108093271. [DOI] [PubMed] [Google Scholar]
- 134.Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 135.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 136.Geminiani G, Bottini G, Sterzi R. Dopaminergic stimulation in unilateral neglect. J Neurol Neurosurg Psychiatry. 1998;65:344–347. doi: 10.1136/jnnp.65.3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dam M, et al. Effects of fluoxetine and maprotiline on functional recovery in poststroke hemiplegic patients undergoing rehabilitation therapy. Stroke. 1996;27:1211–1214. doi: 10.1161/01.str.27.7.1211. [DOI] [PubMed] [Google Scholar]
- 138.Edagawa Y, Saito H, Abe K. Stimulation of the 5-HT1A receptor selectively suppresses NMDA receptor-mediated synaptic excitation in the rat visual cortex. Brain Res. 1999;827:225–228. doi: 10.1016/s0006-8993(99)01300-1. [DOI] [PubMed] [Google Scholar]
- 139.Kojic L, Gu Q, Douglas RM, Cynader MS. Serotonin facilitates synaptic plasticity in kitten visual cortex: an in vitro study. Brain Res Dev Brain Res. 1997;101:299–304. doi: 10.1016/s0165-3806(97)00083-7. [DOI] [PubMed] [Google Scholar]
- 140.Pariente J, et al. Fluoxetine modulates motor performance and cerebral activation of patients recovering from stroke. Ann Neurol. 2001;50:718–729. doi: 10.1002/ana.1257. [DOI] [PubMed] [Google Scholar]
- 141.Chollet F, et al. Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. Lancet Neurol. 2011;10:123–130. doi: 10.1016/S1474-4422(10)70314-8. [DOI] [PubMed] [Google Scholar]
- 142.Barbay S, Nudo RJ. The effects of amphetamine on recovery of function in animal models of cerebral injury: a critical appraisal. NeuroRehabilitation. 2009;25:5–17. doi: 10.3233/NRE-2009-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Morrow SA, et al. The effects of L-amphetamine sulfate on cognition in MS patients: results of a randomized controlled trial. J Neurol. 2009;256:1095–1102. doi: 10.1007/s00415-009-5074-x. [DOI] [PubMed] [Google Scholar]
- 144.Sumowski JF, et al. L-amphetamine improves memory in MS patients with objective memory impairment. Mult Scler. 2011 doi: 10.1177/1352458511404585. [DOI] [PubMed] [Google Scholar]
- 145.Benedict RH, et al. Effects of l-amphetamine sulfate on cognitive function in multiple sclerosis patients. J Neurol. 2008;255:848–852. doi: 10.1007/s00415-008-0760-7. [DOI] [PubMed] [Google Scholar]
- 146.Lefaucheur JP. Stroke recovery can be enhanced by using repetitive transcranial magnetic stimulation (rTMS) Neurophysiol Clin. 2006;36:105–115. doi: 10.1016/j.neucli.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 147.Khedr EM, Fetoh NA. Short- and long-term effect of rTMS on motor function recovery after ischemic stroke. Restor Neurol Neurosci. 2010;28:545–559. doi: 10.3233/RNN-2010-0558. [DOI] [PubMed] [Google Scholar]
- 148.Centonze D, et al. Repetitive transcranial magnetic stimulation of the motor cortex ameliorates spasticity in multiple sclerosis. Neurology. 2007;68:1045–1050. doi: 10.1212/01.wnl.0000257818.16952.62. [DOI] [PubMed] [Google Scholar]
- 149.Manson SC, et al. Impairment of movement-associated brain deactivation in multiple sclerosis: further evidence for a functional pathology of interhemispheric neuronal inhibition. Exp Brain Res. 2008;187:25–31. doi: 10.1007/s00221-008-1276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. 2011;17:37–53. doi: 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- 151.Floyer-Lea A, Wylezinska M, Kincses T, Matthews PM. Rapid modulation of GABA concentration in human sensorimotor cortex during motor learning. J Neurophysiol. 2006;95:1639–1644. doi: 10.1152/jn.00346.2005. [DOI] [PubMed] [Google Scholar]
- 152.Reis J, et al. Consensus: Can transcranial direct current stimulation and transcranial magnetic stimulation enhance motor learning and memory formation? Brain Stimul. 2008;1:363–369. doi: 10.1016/j.brs.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 153.Barkhof F, Calabresi PA, Miller DH, Reingold SC. Imaging outcomes for neuroprotection and repair in multiple sclerosis trials. Nat Rev Neurol. 2009;5:256–266. doi: 10.1038/nrneurol.2009.41. [DOI] [PubMed] [Google Scholar]
- 154.Matthews PM, Johansen-Berg H, Reddy H. Non-invasive mapping of brain functions and brain recovery: applying lessons from cognitive neuroscience to neurorehabilitation. Restor Neurol Neurosci. 2004;22:245–260. [PubMed] [Google Scholar]
- 155.Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- 156.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 157.Rauch A, Rainer G, Logothetis NK. The effect of a serotonin-induced dissociation between spiking and perisynaptic activity on BOLD functional MRI. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:6759–6764. doi: 10.1073/pnas.0800312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mozolic JL, Hayasaka S, Laurienti PJ. A cognitive training intervention increases resting cerebral blood flow in healthy older adults. Front Hum Neurosci. 2010;4:16. doi: 10.3389/neuro.09.016.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.St Lawrence KS, Ye FQ, Lewis BK, Frank JA, McLaughlin AC. Measuring the effects of indomethacin on changes in cerebral oxidative metabolism and cerebral blood flow during sensorimotor activation. Magn Reson Med. 2003;50:99–106. doi: 10.1002/mrm.10502. [DOI] [PubMed] [Google Scholar]
- 160.Lindauer U, et al. Pathophysiological interference with neurovascular coupling - when imaging based on hemoglobin might go blind. Front Neuroenergetics. 2010;2 doi: 10.3389/fnene.2010.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ances BM, et al. Effects of aging on cerebral blood flow, oxygen metabolism, and blood oxygenation level dependent responses to visual stimulation. Hum Brain Mapp. 2009;30:1120–1132. doi: 10.1002/hbm.20574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Brown GG, et al. BOLD and perfusion response to finger-thumb apposition after acetazolamide administration: differential relationship to global perfusion. J Cereb Blood Flow Metab. 2003;23:829–837. doi: 10.1097/01.WCB.0000071887.63724.B2. [DOI] [PubMed] [Google Scholar]
- 163.Cantin S, et al. Impaired cerebral vasoreactivity to CO(2) in Alzheimer’s disease using BOLD fMRI. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2011.06.070. [DOI] [PubMed] [Google Scholar]
- 164.Riecker A, et al. Relation between regional functional MRI activation and vascular reactivity to carbon dioxide during normal aging. J Cereb Blood Flow Metab. 2003;23:565–573. doi: 10.1097/01.WCB.0000056063.25434.04. [DOI] [PubMed] [Google Scholar]
- 165.Fleisher AS, et al. Cerebral perfusion and oxygenation differences in Alzheimer’s disease risk. Neurobiol Aging. 2009;30:1737–1748. doi: 10.1016/j.neurobiolaging.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Johnson NA, et al. Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology. 2005;234:851–859. doi: 10.1148/radiol.2343040197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hyder F, Rothman DL, Shulman RG. Total neuroenergetics support localized brain activity: implications for the interpretation of fMRI. Proc Natl Acad Sci U S A. 2002;99:10771–10776. doi: 10.1073/pnas.132272299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cohen ER, Ugurbil K, Kim SG. Effect of basal conditions on the magnitude and dynamics of the blood oxygenation level-dependent fMRI response. J Cereb Blood Flow Metab. 2002;22:1042–1053. doi: 10.1097/00004647-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 169.De Keyser J, Steen C, Mostert JP, Koch MW. Hypoperfusion of the cerebral white matter in multiple sclerosis: possible mechanisms and pathophysiological significance. J Cereb Blood Flow Metab. 2008;28:1645–1651. doi: 10.1038/jcbfm.2008.72. [DOI] [PubMed] [Google Scholar]
- 170.Paling D, Golay X, Wheeler-Kingshott C, Kapoor R, Miller D. Energy failure in multiple sclerosis and its investigation using MR techniques. J Neurol. 2011 doi: 10.1007/s00415-011-6117-7. [DOI] [PubMed] [Google Scholar]
- 171.Wuerfel J, et al. Changes in cerebral perfusion precede plaque formation in multiple sclerosis: a longitudinal perfusion MRI study. Brain : a journal of neurology. 2004;127:111–119. doi: 10.1093/brain/awh007. [DOI] [PubMed] [Google Scholar]
- 172.Ge Y, et al. Dynamic susceptibility contrast perfusion MR imaging of multiple sclerosis lesions: characterizing hemodynamic impairment and inflammatory activity. AJNR Am J Neuroradiol. 2005;26:1539–1547. [PMC free article] [PubMed] [Google Scholar]
- 173.Rashid W, et al. Abnormalities of cerebral perfusion in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2004;75:1288–1293. doi: 10.1136/jnnp.2003.026021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Holst B, et al. T2’ imaging indicates decreased tissue metabolism in frontal white matter of MS patients. Mult Scler. 2009;15:701–707. doi: 10.1177/1352458509103713. [DOI] [PubMed] [Google Scholar]
- 175.Schummers J, Yu H, Sur M. Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science. 2008;320:1638–1643. doi: 10.1126/science.1156120. [DOI] [PubMed] [Google Scholar]
- 176.Roosendaal SD, et al. Resting state networks change in clinically isolated syndrome. Brain. 2010;133:1612–1621. doi: 10.1093/brain/awq058. [DOI] [PubMed] [Google Scholar]
- 177.Lowe MJ, et al. Resting state sensorimotor functional connectivity in multiple sclerosis inversely correlates with transcallosal motor pathway transverse diffusivity. Hum Brain Mapp. 2008;29:818–827. doi: 10.1002/hbm.20576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.De Luca M, Smith S, De Stefano N, Federico A, Matthews PM. Blood oxygenation level dependent contrast resting state networks are relevant to functional activity in the neocortical sensorimotor system. Exp Brain Res. 2005;167:587–594. doi: 10.1007/s00221-005-0059-1. [DOI] [PubMed] [Google Scholar]
- 179.Pattinson KT, et al. Opioids depress cortical centers responsible for the volitional control of respiration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:8177–8186. doi: 10.1523/JNEUROSCI.1375-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Pattinson KT, Rogers R, Mayhew SD, Tracey I, Wise RG. Pharmacological FMRI: measuring opioid effects on the BOLD response to hypercapnia. J Cereb Blood Flow Metab. 2007;27:414–423. doi: 10.1038/sj.jcbfm.9600347. [DOI] [PubMed] [Google Scholar]
- 181.Murphy K, Harris AD, Wise RG. Robustly measuring vascular reactivity differences with breath-hold: normalising stimulus-evoked and resting state BOLD fMRI data. Neuroimage. 2011;54:369–379. doi: 10.1016/j.neuroimage.2010.07.059. [DOI] [PubMed] [Google Scholar]
- 182.Davis TL, Kwong KK, Weisskoff RM, Rosen BR. Calibrated functional MRI: mapping the dynamics of oxidative metabolism. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:1834–1839. doi: 10.1073/pnas.95.4.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hoge RD, et al. Linear coupling between cerebral blood flow and oxygen consumption in activated human cortex. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:9403–9408. doi: 10.1073/pnas.96.16.9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Griffeth VE, Perthen JE, Buxton RB. Prospects for quantitative fMRI: Investigating the effects of caffeine on baseline oxygen metabolism and the response to a visual stimulus in humans. Neuroimage. 2011;57:809–816. doi: 10.1016/j.neuroimage.2011.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wang J, et al. Arterial spin labeling perfusion fMRI with very low task frequency. Magn Reson Med. 2003;49:796–802. doi: 10.1002/mrm.10437. [DOI] [PubMed] [Google Scholar]
- 186.Levin HS, Grafman J, editors. Cerebral reorganization of function after brain damage. Oxford University Press; Oxford: 2000. [Google Scholar]
- 187.Bosnell R, et al. Reproducibility of fMRI in the clinical setting: implications for trial designs. Neuroimage. 2008;42:603–610. doi: 10.1016/j.neuroimage.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 188.Bassett DS, Bullmore ET. Human brain networks in health and disease. Curr Opin Neurol. 2009;22:340–347. doi: 10.1097/WCO.0b013e32832d93dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tomassini V, et al. Structural and functional bases for individual differences in motor learning. Hum Brain Mapp. 2011;32:494–508. doi: 10.1002/hbm.21037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Stinear CM, et al. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130:170–180. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- 191.Dijkhuizen RM, et al. Functional MRI and Diffusion Tensor Imaging of Brain Reorganization After Experimental Stroke. Transl Stroke Res. 2012;3:36–43. doi: 10.1007/s12975-011-0143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.