Abstract
Background
Serologic response to influenza vaccination declines with age. Few other host factors are known to be associated with serologic response. Our objective was to determine whether obesity and vulnerability independently predicted serologic response to influenza vaccination.
Methods
Adults ≥ 50 yrs were recruited during the 2008-09 influenza season. Subjects provided pre- and post-vaccination sera for measuring antibody titers to 2008-09 vaccine components. Body mass index (BMI) was calculated as weight (kg) ÷ height (m)2. Data were collected on vulnerability using the Vulnerable Elders Survey (VES13). Logistic regression evaluated the associations between obesity and vulnerability and the serologic response to vaccination (both seroprotection andseroconversion), adjusting for gender, age, comorbidities, pre-vaccination titer, and site.
Results
Mean (± standard deviation) age of 415 study subjects was 65 ± 10 yrs; 40% were obese. Mean BMI was 29 ± 5.6 kg/m2; mean VES13 was 1.6 ± 1.8. The proportions of subjects who seroconverted and had seroprotective titers were 40% and 49%, respectively, for A/Brisbane/59 (H1N1); 73% and 80% for A/Brisbane/10 (H3N2); and 34% and 94% for B/Florida. Modified VES-13 (score 0 to 10, with 10 being most vulnerable) was not associated with seroprotection against H1N1 or H3N2, and VES-13 was directly associated with seroconversion to H1N1 but not H3N2 or B. Obesity (BMI ≥ 30 kg/m2 vs. BMI 18.5 – 30) was not associated with seroprotection for H1N1 or H3N2; obesity was directly associated with seroconversion to H3N2 but not H1N1 or B. Age was inversely associated with seroprotection and seroconversion against H1N1 and with seroconversion to influenza B.
Conclusion
Based on this sample of older healthy subjects, there were no consistent relationships between VES 13 or obesity and either seroprotection or seroconversion to three influenza vaccine antigens.
Introduction
The current standard for evaluating immunogenicity of influenza vaccination is the measurement of hemagglutination inhibition (HAI). In young healthy adults an HAI titer of 1:40 is the level of antibody that protects 50% of the population against influenza infection. [1] Among adults ≥50 years, HAI titers tend to be lower than those of young adults and the level of protection provided by an HAI titer of 1:40 or greater is unknown. A recent meta-analysis found that rates of seroprotection declined with advancing age, while previous vaccination, high pre-vaccination titers and institutional residence were all associated with higher rates of seroprotection. [2] The age-related decrease in antibody responses to vaccination is likely due to immunosenescence, but comprehensive studies of the immune response to influenza vaccine in older adults are complicated by simultaneous changes in different arms of the immune system.[3] It is crucial to understand the immune responses in older adults since the same immune senescence that causes poor vaccine response also likely increases the risk of serious complications from influenza, including hospitalizations, placement in nursing homes, and death. Influenza-associated morbidity begins to increase around age of 50.[4]
Obesity and risk of health deterioration (vulnerability) may also influence immune response to influenza vaccination. The role of an elevated body mass index (BMI) in the immune response to infectious pathogens and vaccines has received recent attention with studies showing an increase in serious disease and complications due to 2009 pandemic H1N1 influenza A in obese patients. [5, 6] Obesity produces a chronic inflammatory state associated with dysregulated cytokine production, reduced natural killer cell activity, altered CD4:CD8 T cell balance, and decreased response to antigen stimulation [7], which could affect response to vaccination. Obesity may also be a complicating factor for delivery of vaccine due to inadequate needle length that prevents deposition of the vaccine intramuscularly, thus limiting antigen exposure to the immune system. [8] Antibody responses to hepatitis B and tetanus vaccination are reduced in obese subjects. [7] One recent study found that while serologic response to influenza vaccination may not initially be impaired in obese versus non-obese subjects, there is a greater decline in influenza antibodies after 12 months.[9]
Recent work has shown that frailty may be a better predictor of immune response in older adults than chronologic age. Frailty is the conceptualization of a phenoptye of poor physiologic reserve and poor resistance to stressors and hence is associated with a high risk of morbidity and death from diseases [10] and can be measured by two different mechanisms: either by measuring grip strength and walking speed and querying about weight loss, exhaustion, and physical activity [11] or by a questionnaire used to identify medical disorders and difficulties with activities of daily living.[12] Frailty has been shown to predict vaccine response to the polysaccharide pneumococcal vaccine better than age [13], and frailty has been identified as a confounder in some influenza vaccine efficacy studies, being associated with both likelihood of vaccination and likelihood of hospitalization and/or death.[14, 15] Vulnerability is a concept, similar to frailty, and is measured by a self-rated scale which assesses physical function and health in order to identify older adults at risk for health deterioration. Vulnerability, as measured by the Vulnerable Elders Survey (VES13),[16] has been shown to predict mortality one year post-hospital discharge [17], 5 year functional decline and mortality in ambulatory settings [18], and utilization of medical care and receipt of influenza vaccine.[19]
The objective of the present study was to determine whether obesity and vulnerability are associated with serologic response to influenza vaccination in adults ≥ age 50, after adjusting for age, comorbidities, and pre-vaccination antibody titer.
Materials and Methods
Subjects
As part of a larger study designed to investigate immune response to influenza vaccination, subjects were enrolled at two sites, Vanderbilt University Medical Center (Nashville, TN) and Marshfield Clinic Research Foundation (Marshfield, WI), during the months of September through October 2008. Subjects were eligible for recruitment if they were ≥50 years of age at Vanderbilt and ≥65 years of age at Marshfield. At Vanderbilt, advertisements through email and flyers were given to all employees. At Marshfield letters were sent to older adults who had received an influenza vaccine in the year prior. Due to the enrollment strategy, this was a cohort with a high prevalence of influenza vaccination in prior years. For the current study, all subjects were vaccinated either by their usual caregiver, special influenza vaccine clinics, or by study staff. Vaccine components for the 2008-09 season included A/Brisbane/59/2007 (H1N1), A/Brisbane/10/2007 (H3N2), and B/Florida/4/2006. All vaccine administered to participants at Marshfield Clinic was Fluzone. At Vanderbilt, 22% of participants received Fluviron and 76% received Fluzone. All subjects donated serum pre- and 21 - 28 days post-vaccination. Due to interest in vulnerability and obesity, all subjects were invited to return for a third visit in April-May 2009 where additional patient information was collected. Study procedures, informed consent documents and data collection forms were reviewed and approved by Institutional Review Boards at each of the study sites.
Data Collection
Age, co-morbid conditions, sex, and race were ascertained from participant interview. Recent chemotherapy, radiation therapy, or use of immunomodulating medications were ascertained by self-report or chart review. During the third visit, height and weight were measured by research study staff and BMI was calculated as weight (kg) ÷ height (m)2. The calculated BMI value was categorized as obese (BMI ≥ 30.0 kg/m2) or not obese (BMI <30 kg/m2) according to CDC guidelines [20]. Also during the third visit, vulnerability was measured using the VES13 questionnaire which is a screening tool to assess physical function and self-rated health for the purpose of identifying older adults at risk for health deterioration. [16] Questions focus on the participant’s perception of his/her own health, the ability to stoop or crouch, to lift or carry heavy objects, the ability to walk a quarter of a mile, perform heavy housework, pay bills, walk across the room, manage money, and to self-bathe. The Scale ranges from 0 to 10 with 10 being the most vulnerable and 0 being the least vulnerable. A score of ≥3 has 4.2 times the risk of death or function decline over a 2-year period compared to those with scores <3.[16] For the present analysis, a modified vulnerability score was used which excluded age (considered separately) from the questionnaire, resulting in a 0 to 7 scale.
Laboratory Methods
Blood samples were processed, stored, and shipped by each institution’s local Sample Processing Core. Whole blood was drawn in a tube without anticoagulant and left at room temperature for a minimum of 30 minutes and maximum of 2 hours. The samples were then placed at 4°C and processed within 24 hours of collection. Tubes were placed in a table top centrifuge and serum was clarified by centrifugation at 3000 rpm for 10 minutes at 4°C. Serum was aliquotted into labeled cryovials and stored at −80°C in labeled fiberboard boxes until shipping or testing was performed. HAI testing was performed by Focus Diagnostics Inc. (Cypress, CA)
Statistical Analysis
Seroprotection was defined as an HAI titer of ≥1:40. Seroconversion was defined as a four-fold rise in HAI post-influenza vaccination compared to pre-vaccination. A logistic regression model evaluated the association between seroprotection and seroconversion and 1) BMI analyzed as both a continuous and categorical variable (obese, BMI ≥30 kg/m2 vs. not obese, BMI <30 kg/m2) 2) vulnerability score analyzed as a continuous variable and 3) age analyzed as a continuous variable with restricted cubic spline. Using a restricted cubic spline function is a flexible approach for modeling non-linear relationships between age and log-odds of seroprotection or seroconversion. To evaluate the magnitude of the post-vaccination HAI response, a linear regression of log-transformed post-vaccination HAI titer was performed with the same predictor variables included in the model. All models were adjusted for study site, gender, comorbidities (as a single variable: high risk conditions, yes/no), and pre-vaccination antibody titer. All calculations were done using R version 2.12.2 with Hmisc and rms packages.
Results
Descriptive Results
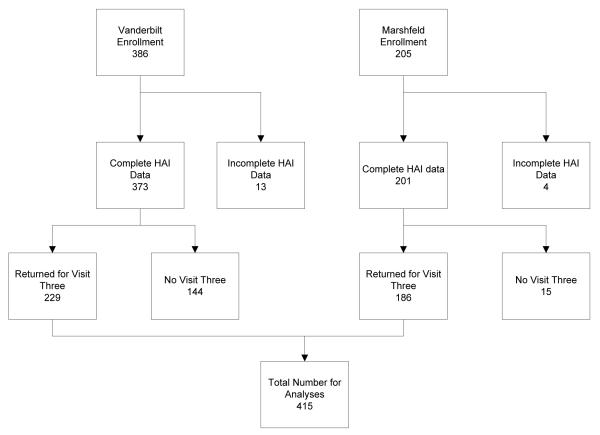
A total of 591 subjects were enrolled between the two sites, of whom 415 (70%) returned for a third, post-influenza season, visit (Figure 1). Subjects who returned for the third visit were slightly older but otherwise similar to the original enrollment group (Supplementary Table 1). Among the 415 subjects who completed a post-influenza season visit, the mean age was 65 ± 10 years; 60% were female and 40% were obese. Mean VES13 was tightly clustered at the non-vulnerable portion of the VES scale (1.6 ± 1.8) and mean BMI was 29.0± 5.6 kg/m2 (Table 1). Both the mean and median BMI in non-obese (mean: 25.5 kg/m2; median: 26.1 kg/m2) and obese (mean: 34.5 kg/m2; median: 33.4 kg/m2) subjects were significantly different (p< 0.001). Pre-vaccination geometric mean titers (GMT) were 11.0, 15.1, and 53.3 for H1N1, H3N2, and influenza B respectively (Supplementary Table 2). GMT pre-vaccination did not differ by age, gender, BMI, or VES13 score in this cohort for any of the three influenza viruses.
Figure 1.
Reasons for incomplete post-vaccination HAI data include inadequate blood sample or study withdrawal between pre-and post-vaccination visits. 7% of Marshfield participants and 39% of Vanderbilt participants did not complete 3rd study visits since this was not an original part of the study protocol and some individuals were not available.
Table 1.
Enrolled participant characteristics by study site and risk factors*
| Vanderbilt (N=229) |
Marshfield (N=186) |
Combined (N=415) |
||
|---|---|---|---|---|
| Age | Median (IQR) | 57.0 (55.0, 63.0) |
73.0 (69.0,77.8) |
67.0 (57.0, 74.0) |
| Age category | 50-59 | 59% | 0% | 33% |
| 60-69 | 26% | 26% | 26% | |
| 70+ | 15% | 74% | 41% | |
| Sex | Female | 67% | 52% | 60% |
| Male | 33% | 48% | 40% | |
| Race | White | 94% | 100% | 97% |
| Black | 5% | 0% | 3% | |
| Comorbid conditions | Heart disease | 7% | 9% | 7% |
| Lung disease | 10% | 8% | 9% | |
| Immunosuppressed** | 2% | 8% | 4% | |
| VES-13*** | Mean ±SD | 1.1±1.3 | 2.2±2.1 | 1.6±1.8 |
| BMI | Mean ±SD | 28.8± 5.7 | 29.4± 5.4 | 29.0± 5.6 |
| ≥ 30 kg/m2 | 38% | 42% | 40% |
includes subjects who completed 3rd study visit, at which time data related to BMI and vulnerability were assessed
Immunosuppressed includes history of transplant, immune dysfunction (including HIV), use of systemic steroid medications, receipt of chemotherapy, or use of other immune modulating medications.
Higher VES scores is associated with higher vulnerability.
Univariate Results
Post-vaccination, seroprotection rates were highest for influenza B and lowest for H1N1, but seroconversion rates were highest for H3N2 (Supplementary Table 2). Univariate analysis showed no association between VES13 score or obesity (yes/no) and seroconversion or seroprotection.
Multivariate Results
In logistic regression models, obesity was not associated with post-vaccination seroprotection against either H1N1 or H3N2. Seroprotection against influenza B was not analyzed due to the high proportion (94%) of seroprotected subjects. Obesity was positively associated with seroconversion to H3N2 but not H1N1 or B (data not shown). VES13 (Figures 2 and 3) was not associated with seroprotection against either H1N1 or H3N2. VES13 was positively associated with seroconversion to H1N1 but not H3N2 or B. Gender did not impact seroprotection against H1N1 (0.90, 95%CI: 0.55, 1.47) or H3N2 (1.37, 95%CI: 0.78, 2.41) nor seroconversion to H1N1 (1.05, 95%CI: 0.66, 1.68), H3N2 (1.39, 95%CI: 0.90, 2.14) or B (0.70, 95%CI: 0.44, 1.10). Comorbid conditions also had no impact on seroconversion to H1N1 (1.19, 95%CI: (0.73, 1.94), H3N2 (0.90, 95%CI: 0.57, 1.42), or B (1.51, 95%CI: 0.95, 2.41).
Figure 2.
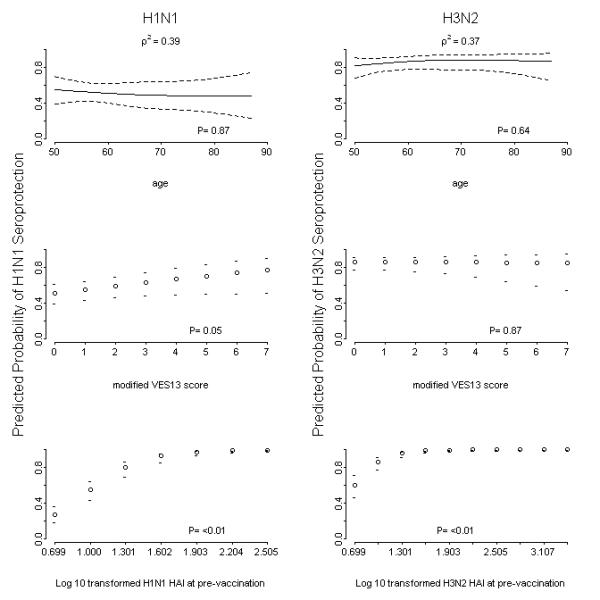
Predicted probability using logistic regression models for seroprotection (hemaglutination inhibition titer ≥40) for either age or modified Vulnerable Elders Survey (VES-13) score for the H1N1 and H3N2 vaccine strains. The model controlled for gender, age, body mass index (BMI), VES13 score, and pre-vaccination HAI titers. Seroprotection for influenza B was not analyzed due to almost complete seroconversion in this population for this vaccine strain.
Figure 3.
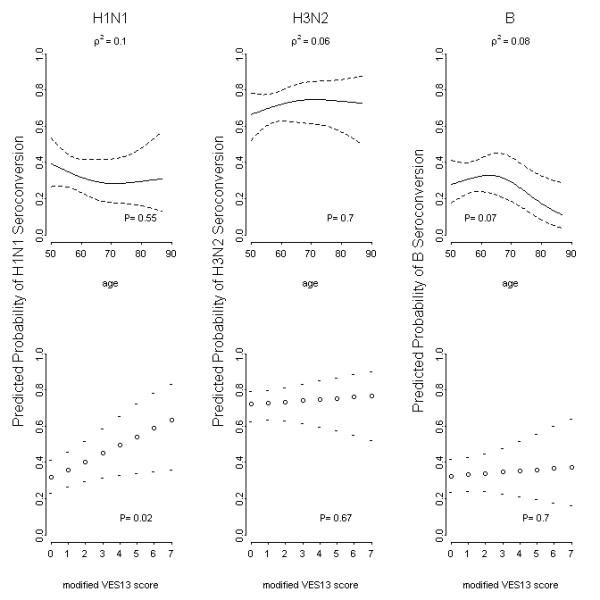
Predicted probability using logistic regression models of serconversion (four-fold rise in hemagglutination inhibition titer) for each vaccine strain. Modeling controlled for gender, age, body mass index, and modified Vulnerable Elders Survey score.
Older age was generally associated with lower rates of seroprotection and seroconversion (Supplemental Table 2, Figures 2 and 3). In fact, for both seroprotection and seroconversion, the odds ratio decreased non-linearly with increasing age. Consistent with the univariate anlyses, there were no differences in vaccine response for males vs. females, or for subjects with vs. without high risk medical conditions that confer an increased risk of influenza complications.
In linear regression models, VES13 was associated with higher post-vaccination HAI for H1N1 but not for H3N2 or B (data not shown). Age was inversely associated with the post-vaccination HAI titer. Obesity, gender, and high-risk conditions had no measurable impact. Table 2 summarizes the association of multiple risk factors and antibody response by influenza vaccine strain.
Table 2.
Summary of associations between host factors and serologic response by influenza subtype
| H1N1 | H3N2 | B | |
|---|---|---|---|
| Seroconversion | 28% | 66% | 31% |
| VES-13 | + | NS | NS |
| BMI ≥ 30 kg/m2 | NS | + | NS |
| Age | NS | NS | NS |
| Gender | NS | NS | NS |
| Comorbidities | NS | NS | NS |
| Seroprotection | 44% | 78% | 95% |
| VES-13 | NS | NS | ND |
| BMI ≥ 30 kg/m2 | NS | NS | ND |
| Age | NS | NS | ND |
| Gender | NS | NS | ND |
| Comorbidities | NS | NS | ND |
| Post-vaccination HAI titer | |||
| VES-13 | + | NS | NS |
| BMI ≥ 30 kg/m2 | NS | + | NS |
| Age | NS | NS | − |
| Gender | NS | − | NS |
| Comorbidities | NS | NS | NS |
NS not significant
ND not done due to high proportion of seroprotected subjects
+ direct association
Discussion
In this population of community-dwelling adults ≥ 50 years old, neither obesity nor vulnerability score, a measure of physical function and self-rated health, was associated with post-vaccination seroprotection (HAI titer ≥ 40) against any of the influenza vaccine components, including A/Brisbane/59/2007 (H1N1), A/Brisbane/10/2007 (H3N2), and B/Florida/4/2006. Results for seroconversion did not show a consistent picture for either obesity or vulnerability, although we did observe an increase in seroconversion against the H3N2 subtype only, among obese individuals.
Historically, interest in nutritional status and immune response has focused on the role of low BMI, or malnutrition, in influencing immunity. More recently, however, the effect of high BMI on vaccine response has become of greater interest with the increased attention now focused on the obesity epidemic. Improving nutritional status, broadly defined, has been studied as a means to improve immune response to influenza vaccination, particularly among older individuals who may have reduced antibody response and poor nutritional status compared to younger individuals. Most studies have involved supplementation with a combination nutritional formula, primarily antioxidant-based, but results have been mixed with some studies describing a beneficial effect on antibody response [21, 22] and others reporting no effect [23, 24]. In general, these studies were of relatively short duration, and included frail or elderly subjects residing in nursing homes or long term care facilities; these studies did not investigate the independent effect of weight status per se, making it difficult to draw comparisons with our results. One observational study found that serologic response to influenza vaccination was not associated with either BMI (analyzed as a continuous variable) or functional status in a group of elderly residents of a long-term care facility, but this study was limited by a high degree of frailty and poor nutritional status (nearly one-half of the subjects had a BMI < 25th percentile; 14% had a BMI < 5th percentile) and very low rates of seroconversion among subjects overall [25].
A limited number of studies have shown an association between higher BMI (categorized in two-unit increments) and poor antibody response to hepatitis B among health-care workers [26] or high BMI (overweight vs. normal weight) and lower anti-tetanus IgG antibodies [27] in adolescents. Only one study, to our knowledge, has explored the association between obesity and serologic response to influenza vaccination. [9] Sheridan et al recently reported an elevated antibody response to influenza vaccination among obese participants, however, in that study the increased response was found for influenza B, not influenza A H3N2 as in the current study. We cannot explain the increased response to different specific antigensin obese subjects, but it may be due to changes in vaccine components. From 2007-08 to 2008-09, the H1 and H3 vaccine components were changed, but from 2008-09 to 2009-10 the H1 and H3 vaccine components remained the same. The B strain, however, did change from 2008-09 to 2009-10, suggesting that differences in response among obese participants could be due to this vaccine change. The increased risk of serious influenza with obesity may be due to other factors including measures of immunity not reflected by HAI titers.[28] We did not measure response at 12-months so cannot compare whether or not the duration of response was decreased in our population as it was in the study by Sheridan. Sheridan et al. [9] also found that CD8 T cell responses to influenza were decreased in obese individuals, likely explaining the increased susceptibility of obese individuals during the 2009 influenza pandemic.
One potential link between obesity and immune response could occur via leptin. Leptin, an adipocyte-derived cytokine, has complex actions that increase inflammation and yet also protect against infection.[7] Our observation that obese subjects had increased seroconversion to the H3N2 sub-type could potentially be related to increased leptin levels leading to a more robust serologic response. Why this occurred for H3N2 only, however, is difficult to explain but could be related to the antigenicity of that particular vaccine component or chance.
Several factors may have limited our ability to detect consistent differences in antibody response to vaccination by nutritional factors. Study participants were all non-institutionalized, relatively healthy, adults over age 50 years old. The majority of individuals were overweight or obese, limiting comparisons by nutritional and functional status. Only one subject in our study was underweight, with a BMI < 18.5; 4.3% had morbid obesity, with a BMI ≥ 40. The subjects that participated in this study agreed to attend multiple visits on the medical center campus. Most were highly functional individuals since we recruited a convenience sample in each community. Indeed, vulnerability scores clustered at the low end of the range and only 10% had a VES13 score ≥3. The limited number of persons with high vulnerability scores limits our inferences on the effect of vulnerability on antibody responses.
This study was not designed a priori to examine obesity or vulnerability as predictors of vaccine response. Hence, data on BMI and vulnerability were collected after vaccination. In addition, each site had slightly different protocols so that the age distribution, the specific influenza vaccine administered, and prior vaccination status differed modestly by site. We dealt with these known and unknown between-site differences by adjusting for them in our model, and by including a “site” variable which was expected to encompass any differences not otherwise accounted for. Regarding the different time points at which obesity/vulnerability were identified and when vaccination was administered, we did not expect meaningful changes in either obesity status or vulnerability during that relatively short (approximately 6 month) period of time. Strong tracking of obesity has been clearly demonstrated, with only 2-6% of adults over 65 years having more than a 10% change in body weight over 3 years.[29] Despite these limitations, this post-hoc analysis helps fill the gap in knowledge about the relationship of obesity and response to influenza vaccination.
In summary, neither obesity nor vulnerability was independently associated with post-influenza vaccination seroprotection against any of the 2008-09 influenza vaccine components in our sample of adults ≥ age 50. We observed an increase in seroconversion to the H3N2 subtype among obese subjects and to the H1N1 subtype among those who were more vulnerable. The significance of these findings is unknown. Future studies that include more frail elderly populations are needed in order to advance our understanding of the effects of nutritional and functional status on influenza vaccine response.
Supplementary Material
Acknowledgements
This research was supported by: K23 AI074863-01A1 (PI, Talbot HK), CDC 1 U18 IP000184-01 (PI, Griffin MR), CDC 5 U18 IP000183-02 (PI, Belongia EA), 1 UL1 RR024975 from NCRR/NIH, and the Atlantic Philanthropies (USA) Inc, the Infectious Diseases Society of America, the John A. Hartford Foundation, Inc., and the Association of Specialty Professors.
References
- [1].Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond) 1972;70(4):767–77. doi: 10.1017/s0022172400022610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24(8):1159–69. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- [3].Aspinall R, Del Giudice G, Effros RB, Grubeck-Loebenstein B, Sambhara S. Challenges for vaccination in the elderly. Immun Ageing. 2007;4:9. doi: 10.1186/1742-4933-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. Jama. 2003;289(2):179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- [5].Louie JK, Acosta M, Samuel MC, Schechter R, Vugia DJ, Harriman K, et al. A novel risk factor for a novel virus: obesity and 2009 pandemic influenza A (H1N1) Clin Infect Dis. 2011;52(3):301–12. doi: 10.1093/cid/ciq152. [DOI] [PubMed] [Google Scholar]
- [6].Yu H, Feng Z, Uyeki TM, Liao Q, Zhou L, Feng L, et al. Risk factors for severe illness with 2009 pandemic influenza A (H1N1) virus infection in China. Clin Infect Dis. 2011;52(4):457–65. doi: 10.1093/cid/ciq144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Karlsson EA, Beck MA. The burden of obesity on infectious disease. Exp Biol Med (Maywood) 2010;235(12):1412–24. doi: 10.1258/ebm.2010.010227. [DOI] [PubMed] [Google Scholar]
- [8].Coalition IA. How to Administer IM and SC Vaccine Injections to Adutls. 2010 [Google Scholar]
- [9].Sheridan PA, Paich HA, Handy J, Karlsson EA, Hudgens MG, Sammon AB, et al. Obesity is associated with impaired immune response to influenza vaccination in humans. Int J Obes (Lond) 2011 doi: 10.1038/ijo.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Barker WH, Mullooly JP. Impact of epidemic type A influenza in a defined adult population. Am J Epidemiol. 1980;112(6):798–811. doi: 10.1093/oxfordjournals.aje.a113052. [DOI] [PubMed] [Google Scholar]
- [11].Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- [12].Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. Cmaj. 2005;173(5):489–95. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Barker WH, Mullooly JP. Pneumonia and influenza deaths during epidemics: implications for prevention. Arch Intern Med. 1982;142(1):85–9. [PubMed] [Google Scholar]
- [14].Jackson LA, Jackson ML, Nelson JC, Neuzil KM, Weiss NS. Evidence of bias in estimates of influenza vaccine effectiveness in seniors. Int J Epidemiol. 2006;35(2):337–44. doi: 10.1093/ije/dyi274. [DOI] [PubMed] [Google Scholar]
- [15].Jackson LA, Nelson JC, Benson P, Neuzil KM, Reid RJ, Psaty BM, et al. Functional status is a confounder of the association of influenza vaccine and risk of all cause mortality in seniors. Int J Epidemiol. 2006;35(2):345–52. doi: 10.1093/ije/dyi275. [DOI] [PubMed] [Google Scholar]
- [16].Saliba D, Elliott M, Rubenstein LZ, Solomon DH, Young RT, Kamberg CJ, et al. The Vulnerable Elders Survey: a tool for identifying vulnerable older people in the community. J Am Geriatr Soc. 2001;49(12):1691–9. doi: 10.1046/j.1532-5415.2001.49281.x. [DOI] [PubMed] [Google Scholar]
- [17].Arora VM, Fish M, Basu A, Olson J, Plein C, Suresh K, et al. Relationship between quality of care of hospitalized vulnerable elders and postdischarge mortality. J Am Geriatr Soc. 2010;58(9):1642–8. doi: 10.1111/j.1532-5415.2010.03024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Min L, Yoon W, Mariano J, Wenger NS, Elliott MN, Kamberg C, et al. The vulnerable elders-13 survey predicts 5-year functional decline and mortality outcomes in older ambulatory care patients. J Am Geriatr Soc. 2009;57(11):2070–6. doi: 10.1111/j.1532-5415.2009.02497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].McGee HM, O’Hanlon A, Barker M, Hickey A, Montgomery A, Conroy R, et al. Vulnerable older people in the community: relationship between the Vulnerable Elders Survey and health service use. J Am Geriatr Soc. 2008;56(1):8–15. doi: 10.1111/j.1532-5415.2007.01540.x. [DOI] [PubMed] [Google Scholar]
- [20].Prevention CfDCa Healthy Weight - its not a diet, it’s a lifestyle. Atlanta. 2011 [Google Scholar]
- [21].Langkamp-Henken B, Wood SM, Herlinger-Garcia KA, Thomas DJ, Stechmiller JK, Bender BS, et al. Nutritional formula improved immune profiles of seniors living in nursing homes. J Am Geriatr Soc. 2006;54(12):1861–70. doi: 10.1111/j.1532-5415.2006.00982.x. [DOI] [PubMed] [Google Scholar]
- [22].Wouters-Wesseling W, Rozendaal M, Snijder M, Graus Y, Rimmelzwaan G, De Groot L, et al. Effect of a complete nutritional supplement on antibody response to influenza vaccine in elderly people. J Gerontol A Biol Sci Med Sci. 2002;57(9):M563–6. doi: 10.1093/gerona/57.9.m563. [DOI] [PubMed] [Google Scholar]
- [23].Allsup SJ, Shenkin A, Gosney MA, Taylor S, Taylor W, Hammond M, et al. Can a short period of micronutrient supplementation in older institutionalized people improve response to influenza vaccine? A randomized, controlled trial. J Am Geriatr Soc. 2004;52(1):20–4. doi: 10.1111/j.1532-5415.2004.52005.x. [DOI] [PubMed] [Google Scholar]
- [24].Provinciali M, Montenovo A, Di Stefano G, Colombo M, Daghetta L, Cairati M, et al. Effect of zinc or zinc plus arginine supplementation on antibody titre and lymphocyte subsets after influenza vaccination in elderly subjects: a randomized controlled trial. Age Ageing. 1998;27(6):715–22. doi: 10.1093/ageing/27.6.715. [DOI] [PubMed] [Google Scholar]
- [25].Potter JM, O’Donnel B, Carman WF, Roberts MA, Stott DJ. Serological response to influenza vaccination and nutritional and functional status of patients in geriatric medical long-term care. Age Ageing. 1999;28(2):141–5. doi: 10.1093/ageing/28.2.141. [DOI] [PubMed] [Google Scholar]
- [26].Weber DJ, Rutala WA, Samsa GP, Santimaw JE, Lemon SM. Obesity as a predictor of poor antibody response to hepatitis B plasma vaccine. Jama. 1985;254(22):3187–9. [PubMed] [Google Scholar]
- [27].Eliakim A, Schwindt C, Zaldivar F, Casali P, Cooper DM. Reduced tetanus antibody titers in overweight children. Autoimmunity. 2006;39(2):137–41. doi: 10.1080/08916930600597326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hassantoufighi A, Zhang H, Sandbulte M, Gao J, Manischewitz J, King L, et al. A practical influenza neutralization assay to simultaneously quantify hemagglutinin and neuraminidase-inhibiting antibody responses. Vaccine. 2010;28(3):790–7. doi: 10.1016/j.vaccine.2009.10.066. [DOI] [PubMed] [Google Scholar]
- [29].Newman AB, Yanez D, Harris T, Duxbury A, Enright PL, Fried LP. Weight change in old age and its association with mortality. J Am Geriatr Soc. 2001;49(10):1309–18. doi: 10.1046/j.1532-5415.2001.49258.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.