Abstract
The olfactory system of salmonids is sensitive to the adverse effects of metals such as copper and cadmium. In the current study, we analyzed olfactory-mediated alarm responses, epithelial injury and recovery, and a suite of olfactory molecular biomarkers encoding genes critical in maintaining olfactory function in juvenile coho salmon receiving acute exposures to cadmium (Cd). The molecular biomarkers analyzed included four G-protein coupled receptors (GPCRs) representing the two major classes of odorant receptors (salmon olfactory receptor sorb and vomeronasal receptors svra, svrb, and gpr27), as well as markers of neurite outgrowth (nrn1) and antioxidant responses to metals, including heme oxygenase 1 (hmox1), and peroxiredoxin 1 (prdx1). Coho received acute (8–168 hr) exposures to 3.7 ppb and 347 ppb Cd, and a subset of fish was analyzed following a 16-day depuration. Coho exposed to 347 ppb Cd over 48 hrs exhibited a reduction in freeze responses, and an extensive loss of olfaction accompanied by histological injury to the olfactory epithelium. The olfactory injury in coho exposed to 347 ppb Cd was accompanied at the gene level by significant decreases in expression of the olfactory GPCRs and increased expression of hmox1. Persistent behavioral deficits, histological injury and altered expression of a subset of olfactory biomarkers were still evident in Cd-exposed coho following a 16-day depuration in clean water. Exposure to 3.7 ppb Cd also resulted in reduced freeze responses and histological changes to the olfactory epithelium within 48 hrs of Cd exposure, although the extent of olfactory injury was less severe than observed for fish in the high dose Cd group. Furthermore adverse behavioral effects were present in some coho receiving the low dose of Cd following a 16-day depuration. In summary, acute exposures to environmental levels of Cd can cause olfactory injury in coho salmon that may persist following depuration. Mechanism-based biomarkers of oxidative stress and olfactory structures can augment the evaluation of olfactory injury manifested at the physiological level.
Keywords: biomarkers, Coho salmon, olfaction, cadmium, G-protein coupled receptors, oxidative stress, alarm response
1.0 Introduction
Salmon populations have declined in the western United States, with several species being listed as extinct or endangered (Williams et al., 1991; Brown et al., 1994; NOAA, 2011). One of the factors implicated in these population declines is inhibition of olfactory processes associated with exposures to waterborne pollutants, including metals (Brown et al., 1994; Domagalski, 1996; Sandahl et al., 2007; Baldwin et al., 2009; Feist et al., 2011; Scholz et al., 2011). The olfactory sensory epithelium is in direct contact with the water column, making it highly sensitive to metal uptake and metal-induced olfactory injury (Julliard et al., 1996; Beyers et al., 2001; Moore et al., 2001; Baldwin et al., 2003; Scott et al., 2003; Carreau et al., 2005; Kalmakov et al., 2009). Exposures to environmentally relevant concentrations of Cd, in particular, have been shown to alter important behavioral and physiological functions associated with the detection of olfactory odorants (Scott et al., 2003; Sloman et al., 2003; Matz et al., 2007; Kusch et al., 2008). Even a transient impairment of olfaction can lead to increased mortality through a loss of competence and increased susceptibility to predation (Beyers et al., 2001; Hamdani et al., 2007; Sandahl et al., 2007). Collectively, these observations indicate that fish olfactory injury can serve as a relevant adverse outcome pathway (Carvan et al., 2008; Ankley et al., 2010) in assessing the effects of aquatic pollution.
At the cellular level, the salmon olfactory epithelium contains specialized olfactory receptor neurons (ORNs) of two sub-systems. ORNs in the olfactory system are responsive to amino acids, prostaglandins, steroids and bile acid odorants, whereas ORNs in the vomeronasal system are responsive to amino acids and nucleotide odorants (Sato and Suzuki, 2001). These odorants bind to G-protein coupled receptors (GPCRs), located on the apical cilia and microvilli of the olfactory and vomeronasal ORNs (Hamdani et al., 2007). GPCRs play a central role in olfactory signal transduction and thus have the potential to serve as sensitive biomarkers of metal induced olfactory injury. However, olfactory GPCRs remain significantly under studied in the context of biomarker development and application.
The goals of the present study were to characterize the effects of acute Cd exposures on olfactory-mediated behaviors, histological injury, and molecular biomarkers in coho salmon, we employed a mechanism-based biomarker approach using gene markers associated with olfactory injury and regeneration to link histological and behavioral impacts from exposures. Our biomarker genes included those encoding two major classes of odorant receptors (Dukes et al., 2004; Dukes et al., 2006), and biomarkers of antioxidant responses. These aforementioned pathways have been quantitatively altered in the olfactory tissues of fish following metal exposures (Tilton et al., 2008; Berg et al., 2010; Espinoza et al., 2012; Wang and Gallagher, 2013). As regeneration of lost ORNs is critical for olfactory function, we also utilized a biomarker of ORN neurite regeneration (Naeve et al., 1997; Marron et al., 2005). Our laboratory exposure paradigm modeled the scenario in which juvenile salmon out-migrating through contaminated waterways could be transiently exposed to metals followed by transition to unpolluted water (Ruggerone and Volk, 2003).
2.0 Materials and Methods
2.1. Chemicals
MS-222 (Tricaine methanesulfonate) was purchased from Argent Chemical Laboratories (Redmond, WA). Analytical grade cadmium chloride was purchased from Mallinckrodt Baker (Phillipsburg, NJ). RNeasy® mini kit was obtained from Qiagen (Valencia, CA). TRIzol® reagent and SuperScript® First-Strand Synthesis System were purchased from Invitrogen (Carlsbad, CA). Finnzymes® DyNAmo® SYBR Green 2-Step qPCR Kit was purchased from New England Biolabs, Inc. (Ipswich, MA). Quantitative real time PCR (qPCR) primers were obtained from Eurofins MWG Operon (Huntsville, AL).
2.2. Fish husbandry and exposures
Juvenile coho salmon (1 yr of age, 15.0 g ± 5.7 mg) were raised in large cylindrical tanks with 8–10 °C recirculating freshwater from Lake Washington, Seattle under a natural photoperiod. The fish were fed BioVita Fry Feed (Bio-Oregon) and water quality conditions were typically 80–120 mg/L total hardness as calcium carbonate, pH 7.4 ± 0.2, 3mg/L DOC, and 9.1 mg/L dissolved oxygen content. Water chemistry was similar to those of other salmon-bearing rivers and streams in the Pacific Northwest (McIntyre et al., 2008). Twenty-four hours prior to exposure the fish were transferred to 120 L glass aquaria, containing aerated lake water inside a large chilled water bath for acclimation. For the exposures, 8–12 juvenile coho per treatment were exposed for 8–168 hrs, with an equal number of control animals receiving carrier (DI water). The concentrations of waterborne Cd used were 3.7 ppb, a concentration below the 5 ppb EPA drinking water standard (EPA, water.epa.gov/drink/contaminants) and representative of urban storm water runoff concentrations (WDOE, 2008), and 347 ppb, representative of heavily polluted waterways (Srinivasa Gowd and Govil, 2008; Maceda-Veiga et al., 2011). Total waterborne Cd concentrations were analyzed prior and post exposures by ICP-MS (Espinoza et al., 2012). Background levels of total Cd in lake water were below the limit of detection (0.1 ppb). Although the nominal Cd concentrations were 3.1 and 310 ppb, the measured total Cd concentrations (3.7 and 347 ppb) are used in all tables and figures. A 90% static renewal approach was implemented with water containing Cd replaced after a 24 hr period. Exposures were staggered for each of the four experimental periods (8, 24, 48, and 168 hrs) so that equal numbers of fish were continually exposed for the treatments, and to avoid ammonia buildup. Nitrate and nitrite levels were below detection for all treatment groups, and ammonia levels were ≤ 0.25 ppm during the exposures.
For the depuration study, Cd-exposed coho (and controls) were treated as described above with the following modification. Following 48 hrs of exposure, fish underwent behavioral testing before being returned to the large cylindrical tanks and allowed to recover for 16 days in clean water. At the termination of the 16-day depuration, the coho were transferred to 120 L glass aquaria and held in lake water for 48 hrs prior to behavioral testing and tissue collection.
2.3. Neurobehavioral analysis of olfactory function
Olfactory-mediated behavioral analysis was conducted using a Y-maze with a black curtain enclosure to avoid frightening the fish during testing. The Y-maze was 100 × 40 × 25 cm and consisted of two arms, each of which measured 50 cm long and 20 cm wide. The arms terminated at a holding chamber, which was 40 × 40 cm. A transparent perforated gate separated the maze arms from the holding chamber. The maze received lake water at a constant flow rate of 8.3 L/min. A dye test was performed to confirm no mixing occurred between the arms. Coho from each Cd exposure group (n=12) were tested in groups of 4 and were acclimated for 15 minutes in the holding chamber prior to stimulus addition (skin extract or DI water). Stimuli were delivered via a peristaltic pump at 10 ml/min, and the gate was lifted 2 minutes post addition of the stimuli. Video recordings for behavioral responses were initiated 10 minutes prior to the gate lifting to establish baseline behavior, and for 5 minutes after gate opening to establish the behavioral response. The maze was flushed with clean water between trials and the arm receiving the skin extract was alternated.
For alarm testing, a coho skin extract was prepared by collecting 10 grams of skin tissue from 4 juvenile coho. The skin was rinsed in ice-cold PBS and homogenized in 40 ml distilled water on ice and filtered. The skin extract was diluted in 1 L of DI water to provide a final stock concentration of 10 mg/ml. Behavioral endpoints analyzed were associated with alarm responses and included freeze responses and odor avoidance. The freeze response is a classic stress/alarm response which is characterized by the lack of movement for >2 sec (Egan et al., 2009). Odor avoidance was characterized as time spent in the maze arm receiving DI water vs. time in the arm receiving the skin extract.
2.4. Histological analysis of olfactory injury
Coho head tissues collected for histological analyses were fixed in 4% paraformaldehyde (PFA) at 4 °C. Olfactory rosettes were excised and embedded in a 2:1 mixture of 20% sucrose to Tissue-Tek® OCT™. Tissue sections (6 μm) were mounted on VWR® glass Superfrost Plus micro-slides. Sections were stained with Mayer’s hematoxylin and eosin (H&E) and the slides were examined using a Nikon LABOPHOT-2 microscope. Images were captured using Nuance 3.0 imaging software. Histological sections were scored based on a 4-grade scale as follows; grade 0 (no visible difference relative to controls), grade 1 (clearly visible increase in the number of goblet cells), grade 2 (clearly visible increase in the number of goblet cells and decreased nuclei near the apical epithelial surface), grade 3 (clearly visible increase in the number of goblet cells, decreased nuclei near the apical epithelial surface and the presence of condensed nuclei). Three tissue sections were analyzed for each individual coho, and 3 coho were analyzed within each exposure group.
2.5. Cellular apoptosis (TUNEL assay)
Detection of apoptosis in the olfactory epithelium was performed by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) (In Situ Cell Death Detection Kit; Roche Diagnostics, Indianapolis, IN) using olfactory tissue sections. Images were collected using a Nikon LABOPHOT-2 microscope with a red filter set (Nikon G-1B, EX540/10, DM580, BA590) and captured at 610 nm with Nuance 3.0.1 software (Caliper Life Science, Hopkinton, MA). Image analysis was performed with Metamorph® software (Molecular Devices, Sunnyvale, CA). TUNEL positive nuclei detected above the threshold value (determined by negative controls) were counted and expressed as number of positive nuclei per 100 μm2 of olfactory epithelium. Three tissue sections were analyzed for each fish and 3 individual coho were analyzed in each exposure group.
2.6. Quantitative PCR analysis of olfactory genes
Eight individual coho were collected for analysis of molecular biomarkers. The fish were anesthetized with MS-222 prior to cervical dislocation, and the olfactory rosettes were quickly excised, transferred to TRIzol® reagent, frozen in liquid nitrogen and stored at −80° C. Total RNA isolation and cDNA synthesis procedures have been described previously (Espinoza et al., 2012). Candidate olfactory biomarker gene primers were generated by multiple sequence alignment of rainbow trout (Oncorhynchus mykiss), Atlantic salmon (Salmo salar), and zebrafish (Danio rerio) target sequences. Primers for hmox1, prdx1, nrn1 and gpr27 were then designed using Oligo® Software, Version 6.71 (Cascade, CO). Dukes et al., (2006) previously published the svra, svrb and sorb primer sequences used in the present study (Primer sequences are presented in table 1). All PCR products were confirmed by sequencing. In addition to the above molecular markers, fish receiving the sub-chronic 168 hr exposure to 3.7 ppb Cd, as well as depurated fish, were also analyzed for olfactory metallothionein-1a (mt1a) mRNA expression. We have previously shown mt1a to be responsive to acute Cd exposures (8–48 hr), but had not analyzed mRNA expression following longer-term low dose Cd exposures (Espinoza et al., 2012). Quantitative PCR analysis for all genes was conducted in a 96-well format with SYBR Green using the relative standard curve method (Espinoza et al., 2012). β-actin expression did not differ between treatments and was used for normalization purposes.
Table 1.
Sequences of PCR primers used in this study
| Gene | Primers (5′ to 3′) | (Source Species) accession # / reference |
|---|---|---|
| β-actin | Forward: GACCCACACAGTGCCCATCT | (O. mykiss) AF157514/ Matsuo et al. (2008) |
| Reverse: GTG CCCATCTCCTGCTCAAA | ||
| svra | Forward: ATGGCCTTCAGGGCTACGCT | (S. salar) DQ375532/ Dukes et al. (2006) |
| Reverse: AGGCAGCTTCCGAGCCAGAA | ||
| svrb | Forward: ATAGCTTTCCAGGCCACAAT | (S. salar) DQ375537.1/ Dukes et al. (2006) |
| Reverse: AGGCAGCTTCCGAGCCAGAA | ||
| sorb | Forward: TGGCCATAGTCTTAGTGGGG | (S. salar) DQ375529/ Dukes et al. (2006) |
| Reverse: GTCAAATGTGTGCTGCAGGT | ||
| gpr27 | Forward: GGGATGCATTTGTATCACCA | (D. rerio) NM_001114434 |
| Reverse: GCCTAGGCCTATGTCAATTCT | ||
| nrn1 | Forward: GCTCTCACCGCCTGTAGCAG | (S. salar) BT058878 |
| Reverse: TGCCCAGACCTCAACATCGTT | ||
| prdx1 | Forward: TTCTTCTTCTACCCGCTGGA | (S. salar) NM_001140823.2 |
| Reverse: CTGGTCCTCCTTCAGCACTC | ||
| hmox1 | Forward: GATGCTGGCCTACCAGAGAG | (S. salar) BT046987 |
| Reverse: GACTCCAGCCGTGCTAGTTC | ||
| mt1a | Forward: CAAGTGCTCCAACTGTGCAT | (S. salar) BT059876/ Espinoza et al. (2012) |
| Reverse: TACACCAGGCCTCACTGACA |
2.7. Statistical Analysis
Behavioral, histological and gene expression data were inspected for homogeneity of variances using D’Agostino and Pearson omnibus normality testing. Gene expression, histological and behavioural data data conforming to normal distributions were assessed for significance relative to control animals using a one-way ANOVA followed by a Dunnett’s test. In some cases, data conformed to a non-parametric distribution, and these datasets were assessed using Kruskal-Wallis one-way ANOVA test followed by Dunn’s non-parametric post-hoc test. All treatment-related differences relative to controls were considered statistically significant at p ≤ 0.05. All statistical analyses were conducted using GraphPad Prism Ver. 5.0 (Graph Pad Software Inc., San Diego, CA, USA).
3.0 Results
3.1. Effects of Cd on olfactory mediated alarm behaviors
As observed in Fig. 1, coho receiving 8 hr exposure to 347 ppb Cd exhibited significantly reduced freeze responses to skin extract (p<0.05). This trend continued, reaching a maximum 92% reduction in freezing behavior at 48 hrs of exposure (p<0.05, Fig. 1). Additionally, coho exposed to 347 ppb Cd for 48 hrs exhibited significantly impaired skin extract avoidance behaviors, which were not evident following a 16-day depuration (p<0.05, Fig. 1). Although coho exposed to 3.7 ppb Cd exhibited significantly reduced freeze responses after 24 hrs of exposure, there were no observable effects on avoidance behavior (p<0.05, Fig. 1). Following the 16-day depuration, coho exposed to both the high and low doses of Cd continued exhibiting significantly reduced freeze responses to skin extract relative to control animals (p<0.05, Fig. 1). Behavioral responses were not recorded for the 168 hr Cd exposures due to mortality following 96 hrs of exposure.
Fig. 1.
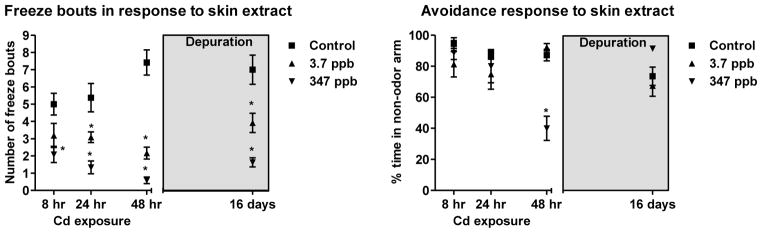
Behavioral responses to a skin extract following acute exposures to 3.7 and 347 ppb Cd and after a depuration (grey box). (A) Freeze responses. (B) Analysis of avoidance to the skin extract. The 0, 3.7 and 347 ppb datasets are represented as squares, triangles and upside-down triangles, respectively, in the figures. All data represent the mean ± SEM of n=12 individuals. * indicates statistically significant differences relative to controls (p <0.05).
3.2. Histological effects of Cd on the olfactory epithelium
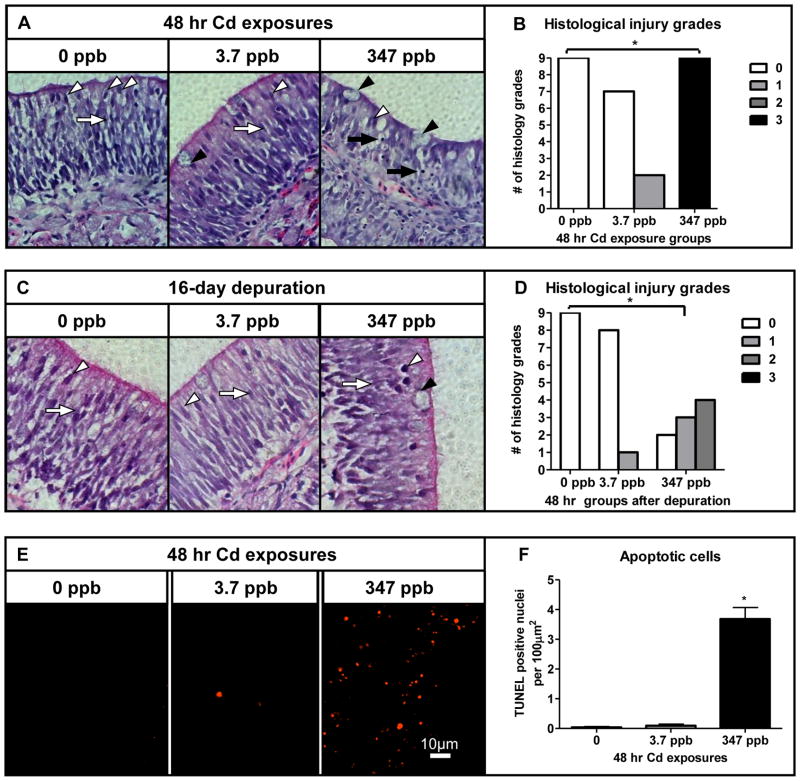
Histological examination of the olfactory epithelium revealed that exposure to 347 ppb Cd caused a decrease in ORN and sustentacular (SUS) cells. All tissue sections in the high dose group were scored grade 3, indicating a reduced olfactory epithelial cell population and an increased number of condensed nuclei compared to controls (p<0.05, Fig. 2A, B). TUNEL analysis confirmed a significant number of apoptotic cells in fish receiving exposure to 347 ppb Cd (p<0.05, Fig. 2E, F). Similar to the behavioral observations, continued histological recovery in the high dose group was still evident after the depuration, with several fish exhibiting grade 2 characteristics (Fig. 2C, D). By contrast, the olfactory epithelium of coho continually exposed to 3.7 ppb Cd for up to 168 hrs showed relatively minor histological injury as evidenced by an increase in the number of goblet cells in the olfactory epithelia (Fig. 2A, D and Fig. 3A, B).
Fig. 2.
Histological effects of acute Cd exposure on the coho olfactory epithelium. (A and C) Light microphotograph (40× magnification) of cross-sectioned olfactory epithelium of coho exposed to 0, 3.7 and 347 ppb Cd for 48 hrs and following a 16 day depuration. SUS cells (white arrow heads), ORNs (white arrows), goblet cells (black arrowheads) and condensed nuclei (black arrows). (B and D) Histology injury grades of olfactory tissue sections following acute Cd exposures as represented in A and C. (E) Apoptosis in cross-sectioned olfactory epithelium of coho exposed to 0, 3.7 and 347 ppb Cd for 48 hrs (20× magnification). (F) Quantification of TUNEL positive nuclei per 100μm2 of olfactory epithelium. All data represent the mean ± SEM of n = 3 individuals. * indicates statistically significant differences in histological injury relative to controls (p <0.05).
Fig. 3.
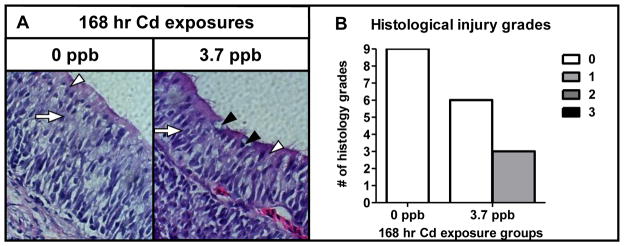
Histological effects of sub-chronic Cd exposure on the olfactory epithelium. (A) Light micrograph (40× magnification) of cross-sectioned olfactory epithelium of coho exposed to 0 and 3.7 ppb Cd for 168 hrs. SUS cells (white arrows), ORNs (white arrows), and goblet cells (black arrowheads). (B) Histology injury grades of olfactory tissue sections following sub-chronic Cd exposures represented in (A). All data represent the mean ± SEM of n = 3 individuals. * indicates statistically significant differences in histological injury relative to controls (p <0.05). (A).
3.3. Effects of Cd on the olfactory molecular biomarkers
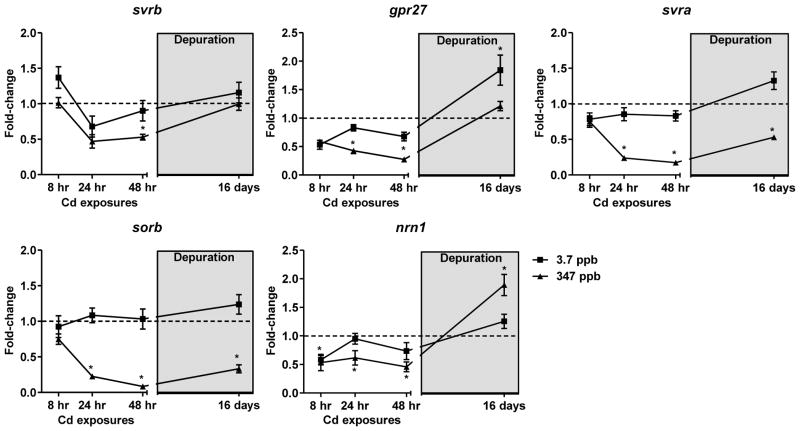
Similar to the observed behavioral deficits and histological injury, coho exposed to 347 ppb Cd exhibited a time-dependent decrease of mRNAs encoding olfactory and vomeronasal receptors (Fig. 4). Of the 4 olfactory GPCRs analyzed, sorb and svra were the most sensitive to Cd, with maximal 90% and 80% decreases in expression observed at 48 hrs of exposure (Fig. 4). Following the 16-day depuration, the expression of sorb and svra remained decreased, although some partial recovery of expression was observed (p<0.05, Fig. 4). Svrb expression was not as sensitive to Cd as the other olfactory receptor markers, although a loss of expression was observed following 48 hrs of exposure to 347 ppb Cd (p<0.05, Fig. 4). Expression of gpr27 significantly decreased maximally to 25% of control levels in coho exposed to 347 ppb Cd for 48 hrs (p<0.05, Fig. 4). The mRNA expression levels of svrb and gpr27 following the depuration were not different from controls (Fig. 4). By contrast, exposure to the low dose of Cd caused only minor changes in mRNA expression of the 4 olfactory GPCRs (Fig. 4 and Table 2).
Fig. 4.
Fold-change in mRNA expression of the olfactory GPCRs and nrn1 following acute Cd exposures and a depuration (light grey box). The 3.7 ppb and 347 ppb datasets are represented as squares and triangles, respectively. Data represent the mean ± SEM of n=8 individuals normalized to the expression of β-actin mRNA, and expressed as fold-change to control levels. * indicates statistically significant differences in gene expression relative to controls (p <0.05).
Table 2.
Fold-change in mRNA expression of the olfactory biomarkers following 168 hr exposures to 3.7 ppb Cd
| mRNA biomarker | Fold-change ± SEM1 |
|---|---|
| svra | 1.0 ± 0.07 |
| svrb | 1.3 ± 0.16 |
| sorb | 1.1 ± 0.12 |
| gpr27 | 1.2 ± 0.15 |
| nrn1 | 1.2 ± 0.17 |
| hmox1 | 1.0 ± 0.09 |
| prdx1 | 1.0 ± 0.15 |
| mt1a | 7.1 ± 0.60* |
values indicate changes in expression relative to controls. mRNA expression data normalized to that of β-actin
indicates statistically significant differences relative to controls (p<0.05).
Expression of nrn1 decreased in a time and dose dependent manner, with a maximum 68% reduction relative to control coho following 48 hrs of exposure to 347 ppb Cd (p<0.05, Fig. 4). After depuration, nrn1 expression was still elevated by 1.9-fold over control levels (p<0.05, Fig. 4). Expression of nrn1 in coho exposed to 3.7 ppb Cd rapidly decreased by 48% at 8 hr of exposure, but was similar to that of controls at the 24, 48, 168 hr exposures, and after depuration (Fig. 4 and Table 2). hmox1 mRNA expression was induced on exposure to 347 ppb Cd at all time points analyzed, with a maximal 7-fold induction observed at 24 hrs (p<0.05, Fig. 5). By contrast, exposure to 347 ppb Cd markedly decreased mRNA expression of prdx1 to 19% of control levels after 48 hrs (p<0.05, Fig. 5). Following depuration, the expression of both hmox1 and prdx1 mRNA in the 347 ppb Cd exposure groups did not differ from controls (Fig. 5). Conversely, expression of mt1a mRNA in the depurated 347 ppb Cd group remained highly induced (21-fold over controls, Fig. 5). Similarly, the expression of mt1a was induced 7-fold and 2-fold above controls following 168 hrs of exposure to 3.7 ppb Cd and the depuration, respectively (Table 2 and Fig. 5). Although some minor modulation of hmox1 and prdx1 expression were observed in coho receiving exposure to 3.7 ppb Cd, these effects were not statistically significant (Fig. 5).
Fig. 5.
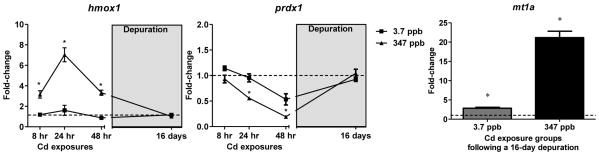
Fold-change in mRNA expression of biomarkers of oxidative stress following acute Cd exposure and depuration. The hmox1 and prdx1 data for the: 3.7 ppb and 347 ppb exposure groups are represented as squares and triangles, respectively with depuration data in the grey box. mt1a mRNA expression in fish following a 16 day depuration after to 3.7 and 347 ppb Cd, presented as grey and black bars, respectively. All data represent the mean ± SEM of n=8 individuals normalized to the expression of β-actin mRNA, and expressed as fold-change to control levels. * indicates statistically significant differences in gene expression relative to controls (p <0.05).
4.0 Discussion
To understand the cellular events underlying metal-induced olfactory injury, it is critically important to provide a substantial linkage among events at the molecular level to effects manifested at the behavioral and physiological levels. These linkages are especially important in the development of fish olfactory injury as an adverse outcome pathway for applications to the field of ecotoxicology (Ankley et al., 2010). Our approach in the current study was directed towards filling these data gaps using a representative salmonid and a model olfactory toxicant relevant to field exposures. It also included an exposure scenario that modeled the environmental scenario, which salmon may receive transient metal exposures followed by a period in which exposures do not occur (Ruggerone and Volk, 2003). Cadmium serves as a well-established olfactory toxicant and environmental pollutant in which exposures as low as 2 ppb in rainbow trout may impair behavior (Sloman et al., 2003). The fact that we observed a rapid inhibition of olfactory behavior as manifested by freezing in the presence of an alarm signal in coho represents a novel finding that supports the sensitivity of salmonids to Cd. While the ecotoxicological consequence of this behavioral impairment was not established in the present study, freezing behavior is an important anti-predator behavior that can affect predator avoidance and survival (Smith, 1992; Sandahl et al., 2007). In this regard, McIntyre et al., recently reported similar neurobehavioral effects in juvenile coho exposed to environmental levels of copper that led to an increased susceptibility to predation (McIntyre et al., 2012).
The impaired alarm responses observed in coho exposed to high, but environmental levels of Cd, was associated with histological injury to the olfactory epithelium, induction of apoptosis, and the extensive loss of GPCR gene expression. Among the biomarkers tested, the olfactory GPCR biomarkers sorb and svra proved to be the most strongly associated to neurobehavioral injury. These observations are consistent with those of our previous microarray analysis of olfactory gene expression in olfactory tissues of zebrafish (Tilton et al., 2008) that indicated GPCRs as potential targets of copper, supporting the utility of these endpoints as effects-based biomarkers. The fact that exposure to the low 3.7 ppb Cd dose did not cause extensive damage to the olfactory epithelium, but partially blocked alarm behavior suggests a relevant adverse outcome for salmon migrating through metal contaminated waterways (Mirza et al., 2009). It is possible that the effects of Cd were partially mitigated by the strong inductive response by mt1a and also by the increase in goblet cells associated with increased mucus production, which are classic olfactory responses to metals (Julliard et al., 1996; Tallkvist et al., 2002; Bettini et al., 2006; Espinoza et al., 2012). In particular, increased mucus production can slow the diffusion of odorants, decreasing olfactory sensitivity, and protects against Cd inhibition of sodium and calcium ion channels essential for olfactory signal transduction (Pärt and Lock, 1983; Elinder and Arhem, 2003; DeMaria and Ngai, 2010; Tierney et al., 2010).
Although the molecular mechanisms underlying metal-induced olfactory epithelial injury have not been fully established, it is apparent that oxidative stress is a major factor in this process (Ercal et al., 2001; He et al., 2008; Cuypers et al., 2010; Espinoza et al., 2012; Wang and Gallagher, 2013). In this regard, hmox1 is a potent antioxidant enzyme that is highly inducible during oxidative stress (Ryter et al., 2002; Alam et al., 2003). The rapid increase in hmox1 expression in coho exposed to Cd in our study indicates an olfactory antioxidant response that implicates a role for oxidative stress in olfactory cell injury. Similarly, the decline of prdx1 expression may represent an overwhelming of antioxidant defenses, associated with the loss of SUS cells on Cd exposure, as these cells preferentially express prdx1 (Novoselov et al., 1999; Yu et al., 2005). Loss of prdx1 could be especially deleterious to managing the harmful effects of increased ROS production on olfactory signal transduction processes (Rhee et al., 2005; Maher, 2006).
Recovery of olfactory function and olfactory epithelial injury occurs in fish following exposure to environmental pollutants and is a mechanism to ensure survival (Beyers et al., 2001; Hamdani et al., 2007; Sandahl et al., 2007). The relatively slow, or only partial, recovery of olfactory function observed following Cd exposures has ramifications for predator avoidance in the wild. Sloman et al., reported that brief exposures to Cd as low as 2 ppb can impair social behaviors for a period of up to 5 days (Sloman et al., 2003). Others have reported olfactory epithelial recovery within two weeks of chemical exposures (Sloman et al., 2003; Bettini et al., 2006), however longer recovery periods may occur following sub-chronic exposure to metals (Saucier et al., 1991). Our findings of acute Cd exposure causing a poor recovery of the coho olfactory epithelium was somewhat surprising, albeit consistent with effects at the molecular level associated with decreased expression of the GPCRs, elevated nrn1 and mt1a mRNA in several of our exposure groups. Rapid bioaccumulation of waterborne Cd into the olfactory system has been previously reported (Scott et al., 2003) and may explain the lack of a full olfactory recovery following acute Cd exposures. Collectively, these aforementioned observations support using molecular biomarkers in conjunction with analysis of effects at the physiological level to evaluate olfactory function in wild fish exposed to metals.
5.0 Conclusion
In summary, our findings indicate that acute Cd exposures can have both rapid and persistent effects on olfactory neurobehavioral function. Similar neurobehavioral effects have been linked to impaired survival and increased susceptibility to predation in salmonids. Our approach involving molecular biomarkers, histological analysis, and behavior indicate that impairment of key olfactory sensory neurons and G-protein coupled receptors and the losses of ORN and non-neural supporting (SUS) cells may underlie impaired alarm responses. In the context of biomonitoring for olfactory injury in the field, the molecular biomarkers mt1a and hmox1 may be of particular relevance for detecting metal exposures and the ability of fish olfactory tissues to mount a protective antioxidant response within the olfactory epithelium.
Highlights.
Low Cd exposures elicited significant olfactory mediated behavioral changes independent of histological injury.
The olfactory behavioral deficits persisted following a 16-day depuration.
Olfactory molecular biomarkers expression was strongly linked to injury to the olfactory epithelium.
Cd induced a strong antioxidant response in the Coho salmon olfactory system.
Results suggest a sensitivity of salmonids to waterborne Cd.
Acknowledgments
This study was supported in part by the University of Washington NIEHS Superfund Basic Sciences Grant NIEHS P42004696, the UW NIEHS EP/T training program T32ES007032-35. The authors appreciate the technical assistance of Herbert M. Espinoza and Dr. Lu Wang, Jon Wittouck at the University of Washington hatchery, and Dexter Morin and Kristen Cosselman who provided technical comments on the manuscript. The authors also acknowledge the assistance of Dr. Brian Beckman and Abby Tillotson at NOAA fisheries, and Heather Bartlett and John Kerwin (Washington Department of Fish and Wildlife) who provided the juvenile coho salmon used in the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alam J, Cook JL. Transcriptional regulation of the heme oxygenase-1 gene via the stress response element pathway. Curr Pharm Des. 2003;9:2499–2511. doi: 10.2174/1381612033453730. [DOI] [PubMed] [Google Scholar]
- Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK, Serrrano JA, Tietge JE, Villeneuve DL. Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem. 2010;29:730–741. doi: 10.1002/etc.34. [DOI] [PubMed] [Google Scholar]
- Baldwin DH, Sandahl JF, Labenia JS, Scholz NL. Sublethal effect of copper on Coho salmon: impacts on nonoverlapping receptor pathways in the peripheral olfactory nervous system. Environ Toxicol Chem. 2003;22:2266–2274. doi: 10.1897/02-428. [DOI] [PubMed] [Google Scholar]
- Baldwin DH, Spromberg JA, Collier TK, Scholz NL. A fish of many scales: extrapolating sublethal pesticide exposures to the productivity of wild salmon populations. Ecol Appl. 2009;19:2004–2015. doi: 10.1890/08-1891.1. [DOI] [PubMed] [Google Scholar]
- Berg K, Puntervoll P, Valdersnes S, Goksøyr A. Responses in the brain proteome of Atlantic cod (Gadus morhua) exposed to methylmercury. Aquat Toxicol. 2010;100:51–65. doi: 10.1016/j.aquatox.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Bettini S, Ciani F, Franceschini V. Recovery of the olfactory receptor neurons in the African Tilapia mariae following exposure to low copper level. Aquat Toxicol. 2006;76:321–328. doi: 10.1016/j.aquatox.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Beyers DW, Farmer MS. Effects of copper on olfaction of Colorado pikeminnow. Environ Toxicol Chem. 2001;20:907–912. [PubMed] [Google Scholar]
- Brown RB, Moyle PB, Yoshiyama RM. Historical decline and current status of Coho salmon in California. N Am J Fish Manage. 1994;14:237–261. [Google Scholar]
- Carreau ND, Pyle GG. Effect of copper exposure during embryonic development on chemosensory function of juvenile fathead minnows (Pimephales promelas) Ecotoxicol Environ Safety. 2005;61:1–6. doi: 10.1016/j.ecoenv.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Carvan MJ, Incardona JP, Rise ML. Meeting the challenges of aquatic vertebrate ecotoxicology. BioScience. 2008;58:1015–1025. [Google Scholar]
- Cuypers A, Plusquin M, Remans T, Jozefczak M, Keunen E, Gielen H, Opdenakker K, Nair AR, Munters E, Artois TJ, Nawrot T, Vangronsveld J, Smeets K. Cadmium stress: an oxidative challenge. Biometals. 2010;23:927–940. doi: 10.1007/s10534-010-9329-x. [DOI] [PubMed] [Google Scholar]
- DeMaria S, Ngai J. The cell biology of smell. J Cell Biol. 2010;191:443–452. doi: 10.1083/jcb.201008163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domagalski J. Pesticides and pesticide degradation products in stormwater runoff: Sacramento River Basin, California. Water Res Bull. 1996;32:953–964. [Google Scholar]
- Dukes JP, Deaville R, Bruford MW, Youngson AF, Jordan WC. Odorant receptor gene expression changes during the parr-smolt transformation in Atlantic salmon. Mol Ecol. 2004;13:2851–2857. doi: 10.1111/j.1365-294X.2004.02252.x. [DOI] [PubMed] [Google Scholar]
- Dukes JP, Deaville R, Gottelli D, Neigel JE, Bruford MW, Jordan WC. Isolation and characterization of main olfactory and vomeronasal receptor gene families from the Atlantic salmon (Salmo salar) GENE. 2006;317:257–267. doi: 10.1016/j.gene.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Egan RJ, Bergner CL, Hart PC, Cachat JM, Canavello PR, Elegante MF, Elkhayat SI, Bartels BK, Tien AK, Tien DH, Mohnot S, Beeson E, Glasgow E, Amri H, Zukowska Z, Kalueff AV. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav Brain Res. 2009;205(14):38–44. doi: 10.1016/j.bbr.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elinder F, Arhem P. Metal ion effects on ion channel gating. Q Rev Biophys. 2003;36:373–427. doi: 10.1017/s0033583504003932. [DOI] [PubMed] [Google Scholar]
- Ercal N, Gurer-Orhan H, Aykin-Burns N. Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem. 2001;1:529–539. doi: 10.2174/1568026013394831. [DOI] [PubMed] [Google Scholar]
- Espinoza HM, Williams CR, Gallagher EP. Effect of cadmium on glutathione S-transferase and metallothionein gene expression in Coho salmon liver, gill and olfactory tissues. Aquat Toxicol. 2012;110–111:37–44. doi: 10.1016/j.aquatox.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feist BE, Buhle ER, Arnold P, Davis JW, Scholz NL. Landscape ecotoxicology of coho salmon spawner mortality in urban streams. PLoS One. 2011;6:e23424. doi: 10.1371/journal.pone.0023424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdani EH, Døving KB. The functional organization of the fish olfactory system. Prog Neurobiol. 2007;82:80–86. doi: 10.1016/j.pneurobio.2007.02.007. [DOI] [PubMed] [Google Scholar]
- He X, Chen MG, Ma Q. Activation of nrf2 in defense against cadmium-induced oxidative stress. Chem Res Toxicol. 2008;21:1375–1383. doi: 10.1021/tx800019a. [DOI] [PubMed] [Google Scholar]
- Julliard AK, Saucier D, Astic L. Time-course of apoptosis in the olfactory epithelium of rainbow trout exposed to a low copper level. Tissue Cell. 1996;28:367–377. doi: 10.1016/s0040-8166(96)80023-1. [DOI] [PubMed] [Google Scholar]
- Kusch RC, Krone PH, Chivers DP. Chronic exposure to low concentrations of waterborne cadmium during embryonic and larval development results in long-term hindrance of antipredator behavior in zebrafish. Environ Toxicol Chem. 2008;27:705–710. doi: 10.1897/07-273.1. [DOI] [PubMed] [Google Scholar]
- Maceda-Veiga A, Monroy M, de Sostoa A. Metal bioaccumulation in the Mediterranean barbel (Barbus meridionalis) in a Mediterranean River receiving effluents from urban and industrial wastewater treatment plants. Ecotoxicol Environ Saf. 2011;76:93–101. doi: 10.1016/j.ecoenv.2011.09.013. [DOI] [PubMed] [Google Scholar]
- Maher P. Redox control of neural function: background, mechanisms, and significance. Antioxid Redox Signaling. 2006;8:1941–1970. doi: 10.1089/ars.2006.8.1941. [DOI] [PubMed] [Google Scholar]
- Marron TU, Guerini V, Rusmini P, Sau D, Brevini TA, Martini L, Poletti A. Androgen-induced neurite outgrowth is mediated by nrn1 in motor neurons. J Neurochem. 2005;92:10–20. doi: 10.1111/j.1471-4159.2004.02836.x. [DOI] [PubMed] [Google Scholar]
- Matz CJ, Krone PH. Cell death, stress-responsive transgene activation, and deficits in the olfactory system of larval zebrafish following cadmium exposure. Environ Sci Technol. 2007;41:5143–5148. doi: 10.1021/es070452c. [DOI] [PubMed] [Google Scholar]
- McIntyre JK, Baldwin DH, Meador JP, Scholz NL. Chemosensory deprivation in juvenile coho salmon exposed to dissolved copper under varying water chemistry conditions. Environ Sci Technol. 2008;42 (4):1352–1358. doi: 10.1021/es071603e. [DOI] [PubMed] [Google Scholar]
- McIntyre JK, Baldwin DH, Beauchamp DA, Scholz NL. Low-level copper exposures increase visibility and vulnerability of juvenile coho salmon to cutthroat trout predators. Ecol Appl. 2012;22(5):1460–1471. doi: 10.1890/11-2001.1. [DOI] [PubMed] [Google Scholar]
- Mirza RS, Green WW, Connor S, Weeks ACW, Wood CM, Pyle GG. Do you smell what I smell? Olfactory impairment in wild yellow perch from metal-contaminated waters. Ecotox Environ Safe. 2009;72(3):677–683. doi: 10.1016/j.ecoenv.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Moore A, Waring CP. The effects of a synthetic pyrethroid pesticide on some aspects of reproduction in Atlantic salmon (Salmo salar L.) Aquat Toxicol. 2001;52:1–12. doi: 10.1016/s0166-445x(00)00133-8. [DOI] [PubMed] [Google Scholar]
- Naeve GS, Ramakrishnan M, Kramer R, Hevroni D, Citri Y, Theill LE. Nrn1: A gene induced by neural activity and neurotrophins that promotes neuritogenesis. Proc Natl Acad Sci U S A. 1997;94:2648–2653. doi: 10.1073/pnas.94.6.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Oceanic and Atmospheric Administration. Snapshot of Salmon and Steelhead ESA Status. Seattle, WA: 2011. http://www.nwr.noaa.gov/ESA-Salmon-Listings/salmon-trends.cfm. [Google Scholar]
- Novoselov SV, Peshenko IV, Popv VI, Novoselov VI, Bystrova MF, Evdokimov VJ, Kamzalov SS, Merkulovs MI, Shuvaeva TM, Lipkin VM, Fesenko EE. Localization of 28-kDa peroxiredoxin in rat epithelial tissues and its antioxidant properties. Cell Tissue Res. 1999;298:471–480. doi: 10.1007/s004419900115. [DOI] [PubMed] [Google Scholar]
- Pärt P, Lock RAC. Diffusion of calcium, cadmium and mercury in a mucous solution from rainbow trout. Comp Biochem Physiol. 1983;76:259–263. doi: 10.1016/0742-8413(83)90075-0. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Kang SW, Jeong W, Chang TS, Yang KS, Woo H. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr Opin Cell Biol. 2005;17(2):183–189. doi: 10.1016/j.ceb.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Ruggerone GT, Volk EC. Residence time and growth of natural and hatchery chinook salmon in the Duwamish Estuary and Elliott Bay, Washington, based on otolith chemical and structural attributes. I. Natural Resources Consultants; Seattle, Washington: 2003. [Google Scholar]
- Ryter SW, Choi AMK. Heme oxygenase-1: molecular mechanisms of gene expression in oxygen-related stress. Antioxid Redox Signaling. 2002;4:625–632. doi: 10.1089/15230860260220120. [DOI] [PubMed] [Google Scholar]
- Sandahl JF, Baldwin DH, Jenkins JJ, Scholz NL. A Sensory system at the interface between urban stormwater runoff and salmon survival. Environ Sci Technol. 2007;41:2998–3004. doi: 10.1021/es062287r. [DOI] [PubMed] [Google Scholar]
- Sato K, Suzuki N. Whole-cell response characteristics of ciliated and microvillous olfactory receptor neurons to amino acids, pheromone candidates and urine in rainbow trout. Chem senses. 2001;26(9):1145–1156. doi: 10.1093/chemse/26.9.1145. [DOI] [PubMed] [Google Scholar]
- Saucier D, Astic L. Morpho-functional alterations in the olfactory system of rainbow trout (Oncorhynchus mykiss) and possible acclimation in response to long-lasting exposure to low copper levels. Comp Biochem Physiol. 1995;112:273–284. [Google Scholar]
- Scott GR, Sloman KA, Rouleau C, Wood CM. Cadmium disrupts behavioural and physiological responses to alarm substance in juvenile rainbow trout (Oncorhynchus mykiss) Journal Exp Biol. 2003;206:1779–1790. doi: 10.1242/jeb.00353. [DOI] [PubMed] [Google Scholar]
- Scholz NL, Myers MS, McCarthy SG, Labenia JS, McIntyre JK, Ylitalo GM, Rhodes LD, Laetz CA, Stehr CM, French BL, McMillan B, Wilson D, Reed L, Lynch KD, Damm S, Davis JW, Collier TK. Recurrent die-offs of adult Coho salmon returning to spawn in Puget Sound lowland urban streams. PLoS ONE. 2011;6:e28013. doi: 10.1371/journal.pone.0028013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloman KA, Scott GR, Diao Z, Rouleau C, Wood CM, McDonald DG. Cadmium affects the social behaviour of rainbow trout, Oncorhynchus mykiss. Aquat Toxicol. 2003;65:171–185. doi: 10.1016/s0166-445x(03)00122-x. [DOI] [PubMed] [Google Scholar]
- Smith RJF. Alarm signals in fish. Rev Fish Biol Fish. 1992;2(1):33–63. [Google Scholar]
- Srinivasa Gowd S, Govil PK. Distribution of heavy metals in surface water of Ranipet industrial area in Tamil Nadu, India. Environ Monit Assess. 2008;136:197–207. doi: 10.1007/s10661-007-9675-5. [DOI] [PubMed] [Google Scholar]
- Tallkvist J, Persson E, Henriksson J, Tjälve H. Cadmium-metallothionein interactions in the olfactory pathways of rats and pikes. Toxicol Sci. 2002;67:108–113. doi: 10.1093/toxsci/67.1.108. [DOI] [PubMed] [Google Scholar]
- Tierney KB, Baldwin DH, Hara TJ, Ross PS, Scholz NL, Kennedy CJ. Olfactory toxicity in fishes. Aquat Toxicol. 2010;96(1):2–26. doi: 10.1016/j.aquatox.2009.09.019. [DOI] [PubMed] [Google Scholar]
- Tilton F, Tilton SC, Bammler TK, Beyer R, Farin F, Stapleton PL, Gallagher EP. Transcriptional biomarkers and mechanisms of copper-induced olfactory injury in zebrafish. Environ Sci Technol. 2008;42:9404–9411. doi: 10.1021/es801636v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Gallagher EP. Role of Nrf2 antioxidant defense in mitigating cadmium-induced oxidative stress in the olfactory system of zebrafish. Toxicol Appl Pharmacol. 2012;266(2):177–186. doi: 10.1016/j.taap.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Washington State Department of Ecology. Control of toxic chemicals in Puget Sound - Phase 2: Improved estimates of loadings from surface runoff and roadways. 2008 Retrieved from: https://fortress.wa.gov/ecy/publications/summarypages/0810084.html.
- Williams JE, Nehlsen W, Lichatowich JA. Pacific salmon at the crossroads: stocks at risk from California, Oregon, Idaho, and Washington. Fisheries. 1991;16:4–21. [Google Scholar]
- Yu TT, McIntyre JC, Bose SC, Hardin D, Owen MC, McClintock TS. Differentially expressed transcripts from phenotypically identified olfactory sensory neurons. J Comp Neurol. 2005;483:251–262. doi: 10.1002/cne.20429. [DOI] [PMC free article] [PubMed] [Google Scholar]