Abstract
Age related macular degeneration (AMD) is the leading cause of vision loss of those over the age of 65 in the industrialized world. The prevalence and need to develop effective treatments for AMD has lead to the development of multiple animal models. AMD is a complex and heterogeneous disease that involves the interaction of both genetic and environmental factors with the unique anatomy of the human macula. Models in mice, rats, rabbits, pigs and non-human primates have recreated many of the histological features of AMD and provided much insight into the underlying pathological mechanisms of this disease. In spite of the large number of models developed, no one model yet recapitulates all of the features of human AMD. However, these models have helped reveal the roles of chronic oxidative damage, inflammation and immune dysregulation, and lipid metabolism in the development of AMD. Models for induced choroidal neovascularization have served as the backbone for testing new therapies. This article will review the diversity of animal models that exist for AMD as well as their strengths and limitations.
1. Introduction
Age related macular degeneration (AMD) is the leading cause of blindness in the industrialized world of adults older than 65 years (Klein et al., 2002). AMD is a heterogeneous disease, which first manifests in the macula with the appearance of pigmentary changes and subretinal deposits called drusen. AMD can progress to a “dry”, non-neovascular form leading to geographic atrophy of the retinal pigment epithelium (RPE), choriocapillaris, and photoreceptors, or to a more rapid “wet”, neovascular form, which occurs when new blood vessels invade from the choroid and penetrate Bruch's membrane resulting in vascular leakage, hemorrhage, and scarring. Dry AMD is much more common than wet, but choroidal neovascularization (CNV) in wet AMD accounts for the majority of the vision loss (Bressler et al., 1988).
Accurate animal models of a disease can assist greatly in the development of new therapies. The ideal model of AMD would be inexpensive, recapitulate the histological and functional changes, but evolve in a rapid time course to allow more efficient studies. As shown in Table 1, numerous models mimic several of the important pathological features seen in AMD, but none recreated all of its characteristics. Developing a model that mimics both the early and late features of AMD has been challenging due to several obstacles. First, AMD is a complex process involving both genetic and environmental factors. Rather than being caused by a single genetic defect, numerous genetic polymorphisms have been implicated in contributing increased risk for AMD. Oxidative stress, inflammation, and lipid and carbohydrate metabolism have all been implicated in the pathology of AMD. Second, anatomical differences between the species used for AMD models and the human retina have lent further complexity to the task.
Table 1.

|
Models of AMD have been created in mice, rats, rabbits, pigs, and non-human primates. Rodent models offer the advantages of low cost, disease progression on a relatively quick time scale, and the ability to perform genetic manipulation. However, one distinct disadvantage of mice and rats is the lack of an anatomical macula. On the other end of the spectrum, non-human primates offer the closest anatomy to humans, but are quite difficult to manipulate genetically, costly to maintain, and have a slow time course of disease progression. In spite of these limitations, numerous animal models for AMD have been created and have revealed many important aspects about the underlying pathology of the disease. This review attempts to provide a comprehensive and updated report on animal models of AMD by building on several other reviews previously published on the subject (Edwards and Malek, 2007; Grossniklaus et al., 2010; Marmorstein and Marmorstein, 2007; Rakoczy et al., 2006; Ramkumar et al., 2010; Zeiss, 2010).
2. Rodent models of dry macular degeneration
To evaluate a given animal model, it is important to determine which features need to be present to consider it a good model. Some of the histological features that have been reported from the eyes of patients with AMD include thickening of Bruch's membrane (BM), sub-RPE basal laminar deposits and basal linear deposits (i.e. drusen), changes in the RPE including loss of the basal infoldings, atrophy, and hyperplasia, accumulation of immune cells such as macrophages or microglia, deposition of activated complement proteins, photoreceptor atrophy, retinal or choroidal neovascularization and fibrosis (Green, 1999; Green and Enger, 1993; Sarks, 1976). Additionally, accumulation of lipofuscin in RPE cells with increased levels of A2E and corresponding increases in autofluorescence have been demonstrated in AMD patients (Sparrow et al., 2003). Functional changes include decreased signals on electroretinograms (ERGs) reflecting photoreceptor atrophy (Gerth, 2009). This paper will review many animal models with the features of human AMD, but it is important to realize that most of these are laboratory creations and may not be an etiological representation of human disease.
2.1. Complement factor pathway
The discovery of components of the complement cascade in drusen from the eyes of patients with AMD suggested an important role for inflammation in this disease (Johnson et al., 2000; Mullins et al., 2000). Further work demonstrated that polymorphisms in the gene for complement factor H (CFH) are associated with an increased risk of AMD (Edwards et al., 2005; Hageman et al., 2005; Haines et al., 2005; Klein et al., 2005; Thakkinstian et al., 2006; Zareparsi et al., 2005). The Y402H polymorphism in CFH increases the risk of AMD five to sevenfold, and at least 50% of AMD cases are associated with this polymorphism, making it the single largest genetic risk factor (ibid). Polymorphisms in other complement factors (such as Factor B, C2, C3) have also been associated with conferring protection or susceptibility to the development and progression of AMD (Gold et al., 2006; Maller et al., 2007; Montes et al., 2009; Reynolds et al., 2009; Yates et al., 2007). Recent work has begun to link together the models of oxidative damage to those with defects in the innate immune system. For example, the Y402H polymorphism in complement factor H results in a reduced ability of this protein to bind to malondialdehyde, a product of lipid peroxidation which is present in AMD (Weismann et al., 2011). Such loss of binding would impair the ability of CFH to down regulate complement activation in surfaces coated by proteins adducted with malondialdehyde. In the last several years, a number of animal models have emerged to examine the role of complement in the development of AMD.
2.1.1. Cfh−/− mice
Complement factor H (CFH) plays an important regulatory role in the alternative pathway by preventing the binding of C3b with factor B and blocking the formation of C3 convertase (Pickering and Cook, 2008). Lack of CFH function leads to dysregulation of the alternative pathway resulting in low systemic levels of C3, deposition of C3 in glomerular basement membranes, and ultimately membranoproliferative glomerulonephritis (MPGN) Type II (Pickering and Cook, 2008). Interestingly, these patients develop macular drusen similar to those seen in AMD (Duvall-Young et al., 1989a, b). Mice genetically engineered to lack complement factor H also develop MPGN and retinal abnormalities reminiscent of AMD (Coffey et al., 2007; Pickering et al., 2002). At two years of age, these animals demonstrated decreased visual acuity as measured by water maze, reduction in rod-driven ERG a- and b-wave responses, increased subretinal autofluorescence, complement deposition in the retina, and disorganization of photoreceptor outer segments. One feature of these animals, which was not typical compared to other models of AMD, was thinning of the Bruch's membrane. The authors hypothesized that unregulated C3 activation may lead to an increase in phagocytic activity thereby resulting in decreased thickness.
2.1.2. Transgenic CFH Y402H mice
To further elucidate the mechanisms by which CFH mutations contribute to AMD, transgenic mouse lines expressing the Y402H polymorphism under control of the human ApoE promoter were constructed (Ufret-Vincenty et al., 2010). The ApoE gene codes for apolipoprotein E, which is important in forming lipoproteins for lipid transport. At one year of age, these animals demonstrated a larger number of drusen-like deposits than seen in wild-type mice or Cfh−/− mice. Immunohistochemistry revealed increased numbers of microglial and macrophages in the subretinal space and electron microscopy showed thickening of Bruch's membrane and basement membrane deposition of C3d. Compared to the Cfh−/− mice, transgenic CFH Y402H mice did not show photoreceptor atrophy. This could be due to the younger age at which these mice were studied (one year versus two years) or may be secondary to the presence of some functional activity of CFH protein that prevented a more severe dysregulation of the alternative pathway. With the recent studies by Weismann et al. (2011) demonstrating the loss of CFH Y402H to bind malondialdehyde, it will be quite intriguing to study the effects of lipid peroxidation products in CFH Y402H mice.
2.1.3. Transgenic mice overexpressing C3
Complement factor C3 plays a pivotal role in the activation of complement by integrating signals from the three distinct complement-activating pathways. Cleavage of C3 to C3a and C3b can activate further downstream components including C5a and lead to the formation of the membrane attack complex, which is composed of C5b through C9. Since C3 plays such a central role in regulating the cascade, it is reasonable to expect that overexpression of this protein might lead to accelerated activation of complement and the development of retina pathology. Mice transfected with a C3-expressing adenovirus regulated under the CMV promoter expressed C3 at higher levels than normal (Cashman et al., 2011). Near the areas of injected virus, these animals showed several features of AMD including proliferation and migration of endothelial cells within the retina, disruption of the RPE with migration of pigmented cells into the retina, complement deposition, and atrophy of the photoreceptor outer segments. In AMD, new vessels typically appear in the form of choroidal neovascularization, but some patients can develop new vessels in the retina in a process known as retinal angiomatous proliferation (RAP). The migration of endothelial cells in the retina could be analogous to the development of RAP. However, compared to animals injected with a GFP vector, the C3-overexpressing animals demonstrated increased incidence of retinal detachments, a feature not consistent with AMD. This change as well as evidence that the adenovirus itself could contribute to the pathological features might be limitations of this model.
2.1.4. C3a and C5a receptor−/− mice
Both complement components C3 and C5 have been found in drusen (Johnson et al., 2000; Mullins et al., 2000). Aside from their activities in opsonization and formation the membrane attack complex, these proteins can also elicit additional biological responses through activation of their endogenous receptors. Mice lacking the receptors for C3a or C5a exhibit smaller lesions in the laser-induced CNV model (described below), decreased levels of VEGF expression, and impaired leukocyte recruitment (Nozaki et al., 2006a). This evidence suggests that activation of the C3a and C5a receptors plays a role in the development of CNV in AMD.
2.2. Chemokines
Chemotactic cytokines, or chemokines, are a diverse set of small molecular weight proteins that mediate the migration of leukocytes (Graves and Jiang, 1995). Chemokines can be pro-inflammatory or homeostatic and are divided into four families: CXC, CX3C, CC, and C depending on their conserved cysteine residues (Murphy et al., 2000; Zlotnik and Yoshie, 2000). The receptors for chemokines are G-protein coupled receptors that can mediate diverse second messenger signaling resulting in the recruitment of inflammatory cells to a target. In AMD, there is activation of macrophages and microglia resulting in the migration of both into the subretinal space, which is normally devoid of these cells (Combadiere et al., 2007; Gupta et al., 2003; van der Schaft et al., 1993; Xu et al., 2009). The CCL2/CCR2 and CX3CL1/CX3CR1 ligand/receptor pairs in particular have been implicated in the pathogenesis of AMD both through the occurrence of at-risk SNPs as well as by pathology in animal models (Raoul et al., 2010). The T280 M allele of Cx3cr1 gene has been shown to be associated with AMD in several patient populations (Chan et al., 2005; Combadiere et al., 2007; Tuo et al., 2004; Yang et al., 2010). Knockout mice for these ligand/receptor pairs have created models with many features of AMD.
2.2.1. Ccl2−/− and Ccr2−/− mice
The CCL2/CCR2 signaling axis is known to play an important role in macrophage mobilization. Ccl2, also known as monocyte chemoattractant protein 1 (MCP-1), mediates the binding of monocytes to blood vessels for subsequent tissue extravasation (Kuziel et al., 1997; Lu et al., 1998). RPE cells under oxidative stress can upregulate Ccl2 (Higgins et al., 2003). Interestingly, mice lacking either the CC-motif ligand (Ccl2−/−) or receptor (Ccr2−/−) have been found to have changes suggestive of AMD. After nine months of age, these animals demonstrate subretinal drusen-like accumulations, thickening of Bruch's membrane, an increase in autofluorescence and lipofuscin granules, photoreceptor malfunction, and the occurrence of CNV (Ambati et al., 2003). These findings led to the hypothesis that dysfunctional phagocytic clearance might play a key role in AMD. However, screening of the Ccl2 and Ccr2 loci have yet to reveal any at risk alleles associated with the development of AMD (Despriet et al., 2008). Additional studies from Ccl2−/− mice have challenged the interpretation of some of the initial retinal findings (Luhmann et al., 2009). The drusen-like subretinal deposits initially observed were demonstrated to be accumulations of swollen macrophages. Luhmann et al. found no difference between thickening of Bruch's membrane, RPE and photoreceptor atrophy, and ERG amplitudes compared to age matched wild-type animals. Additionally, they did not observe development of CNV in any Ccl2−/− mice and found these animals to be less susceptible to laser induced CNV. Thus, the exact contribution of the CCL2/CCR2 pathway to AMD pathogenesis remains to be clarified.
2.2.2. Cx3cr1−/− mice
The ligand, CX3CL1, is heavily expressed on retinal endothelial cells as well as Muller and ganglion cells, while its receptor, CX3CL1, has only been found in microglial cells in the retina (Cardona et al., 2006; Combadiere et al., 2007; Silverman et al., 2003). Studies of two independently generated Cx3cr1 deficient mice (Combadiere et al., 2003; Jung et al., 2000) have revealed changes consistent with AMD (Combadiere et al., 2007). At 12 months of age, Cx3cr1−/− mice demonstrated drusen-like deposits that were not apparent in age matched wild-type mice. Similar to the deposits in Ccl2−/− mice, these deposits were composed of accumulations of subretinal microglia, however these cells were lipid laden. At 18 months of age, Cx3cr1−/− mice showed significant retinal thinning (40%) compared to wild-types. Additionally, laser induced CNV in Cx3cr1−/− mice lead to twice the area of neovascularization as in control animals. Lack of CX3CL1 function on retinal microglia may have led to deficiencies in egress of these cells from the retina leading to the formation of subretinal deposits.
2.2.3. Ccl2−/− Cx3cr1−/− double knockout mice
Ccl2−/− and Cx3cr1−/− single knockout mice exhibit many features of AMD, but do not manifest these changes until older ages (16 and 18 months, respectively) and the phenotype is not fully penetrant. To determine if loss of both CCL2 and CX3CR1 might have a synergistic effect, Ccl2−/− and Cx3cr1−/− double knockout mice were generated (Tuo et al., 2007). Combination Ccl2−/− Cx3cr1−/− mice developed retinal changes as early as 6 weeks providing a more convenient model to study than the single knockout animals. By 9 weeks of age, all Ccl2−/− Cx3cr1−/− mice demonstrated subretinal drusen, which enlarged with age. Immunostaining revealed increased complement in Bruch's membrane, RPE, and choroidal capillaries (Ross et al., 2008). Knockout mice had focal thickening of Bruch's membrane, increased levels of A2E, infiltration of microglial, and photoreceptor atrophy. In older animals, areas of atrophy and chorioretinal scars were observed. In about 15% of eyes, CNV was observed with the earliest occurrence at 12 weeks. Double knockout mice fed a diet high in omega-3 fatty acids had a smaller number of retinal lesions than animals fed a deficient diet (Chan et al., 2008). This correlates with clinical evidence that high dietary intake of fish or omega-3 fatty acids decrease the likelihood of developing AMD (SanGiovanni and Chew, 2005; SanGiovanni et al., 2007). All of these findings seem to indicate that Ccl2−/− Cx3cr1−/− mice are a useful model for AMD. However, Raoul and coworkers have questioned the validity of the severe findings in the Ccl2−/− Cx3cr1−/− mice developed by Tuo et al., because their independently developed lines of Ccl2−/− Cx3cr1−/− double knockout mice do not exhibit severe features and are not phenotypically different from Ccl2−/− or Cx3cr1−/− single knockout mice (Raoul et al., 2010). They suggest that selective breeding of animals with the worst drusen may have inadvertently selected for AMD factors independent of CCL2 or CXCR1. Furthermore, recent studies reveal that these mouse lines are contaminated with mutations in CRB1, which confound interpretation of the phenotypes (Matapalli et al. 2012).
2.3. Oxidative damage models
There are several lines of evidence to suggest that oxidative damage might play a role in the development of AMD. The retina is particularly susceptible to oxidative damage due its high metabolic demand, high concentration of oxidizable polyunsaturated fatty acids, and the presence of photosensitive molecules such as rhodopsin or lipofuscin that can produce reactive oxygen intermediates when exposed to light (Cai et al., 2000). Epidemiological studies have suggested that oxidative damage, such as from smoking or sunlight contribute to the risk of AMD (Cruickshanks et al., 1997; Vingerling et al., 1996; Wenzel et al., 2005). Individuals with AMD have significantly higher levels of lipid peroxidation products compared to controls (Nowak et al., 2003). Finally, dietary supplements that include anti-oxidants have been shown to reduce the risk of progression from dry to wet AMD (Group, 2001). Animal models that lack intrinsic anti-oxidant mechanisms or those where additional oxidative stress is applied demonstrate many of the features of AMD.
2.3.1. Immunization with carboxyethylpyrrole adducted proteins
The oxidation of docosahexaenoic acid (DHA), one of the most prevalent fatty acids in the retina, can lead to the formation of carboxyethylpyrrole (CEP) adducted proteins. These modified proteins are found in drusen and are more prevalent in the RPE/Bruch's membrane of patients with AMD (Crabb et al., 2002; Hollyfield et al., 2003). Additionally, compared to age-match donor eyes from patients without AMD, those with AMD demonstrate higher plasma levels of CEP-adducted proteins as well as demonstrate antibodies to these conjugated proteins (Gu et al., 2003). To test the hypothesis that oxidative damage leading to formation of CEP adducted proteins could be the initiating event in AMD, Hollyfield et al. created a mouse model whereby animals were immunized with CEP-adducted mouse serum albumin (Hollyfield et al., 2008, 2010). Animals were divided in two groups: a short-term group received a strong immunological challenge over three months, while the long-term group was inoculated with a weaker challenge over one year. Both groups developed antibodies to CEP-adducted albumin. At 3 months, the short-term group exhibited deposition of complement component C3d in Bruch's membrane, sub-RPE deposits, RPE swelling and lysis, invasion of macrophages, and pyknosis of overlying photoreceptors. Long-term animals demonstrated a 3 to 5-fold thickening of Bruch's membrane. Furthermore, the severity of pathology correlated with the levels of CEP-specific antibodies. No neovascularization was observed in either the short-term or long-term groups of mice demonstrating the usefulness of this model for dry AMD, but not wet AMD. One advantage of this model is that genetic manipulation or extreme lighting conditions were not needed to induce the phenotype. It will be interesting to see how animals that have been genetically altered to carry human polymorphisms related to AMD, such as Cfh Y402H mice, respond to immunization with CEP-adducted proteins.
2.3.2. Ceruloplasmin/hephaestin−/− mice
Iron is a potent generator of oxidative stress and its levels increase in the body, including the retina, with aging (Dunaief, 2006). Ceruloplasmin is a ferroxidase that is thought to mediate iron export from cells.
Humans with ceruloplasminemia, who lack functional ceruloplasmin, develop drusen and retinal pigmentary changes later in life (Miyajima et al., 1987; Morita et al., 1995; Yamaguchi et al., 1998). Mice lacking ceruloplasmin, demonstrate at most mild retinal changes, likely because a second ferroxidase, hephaestin, can compensate for the loss (Harris et al., 1999; Patel et al., 2002; Vulpe et al., 1999). To analyze the potential contribution of iron overload to AMD pathology, mice lacking both ceruloplasmin and hephaestin were created (Hahn et al., 2004). After 6–9 months, these animals displayed focal areas of RPE hypertrophy and hypopigmentation in the midperipheral retina, subretinal deposits, photoreceptor atrophy, and subretinal neovascularization. Studies from mice older than 12 months demonstrated infiltration of macrophages, focal areas of hyperautofluorescence, and complement deposition (Hadziahmetovic et al., 2008). One limitation of this model is that most double knockout animals die young from a movement related disorder. This limits the ability to study long terms effects in senescent mice. Recently, treatment with an iron chelator, deferiprone, has been shown to decrease iron levels in these double knockout mice and ameliorate retinal degeneration (Hadziahmetovic et al., 2011).
2.3.3. Sod1−/− mice
Super oxide dismutase (SOD) is a powerful anti-oxidant that is expressed in three forms. SOD1, which has the highest expression in retina, localizes primarily to the cytosol, while SOD2 and SOD3 localize to the mitochondria and extracellular space, respectively (Behndig et al., 1998). Prior to 7 months of age, retinas from Sod1−/− mice were indistinguishable from age matched wild-type animals (Imamura et al., 2006). After 7 months of age, knockout animals displayed yellowish drusen-like deposits between the RPE and Bruch's membrane. These deposits stained for several biomarkers of drusen including: vitronectin, carboxymethyl lysine, and tissue inhibitor metalloproteinase 3 (TIMP3) (Crabb et al., 2002; Imamura et al., 2006). There was evidence of oxidative damage to the RPE and thickening of Bruch's membrane and light exposure in these animals hastened the appearance of drusen in a dose dependent manner. About 10% of Sod1−/− mice demonstrated evidence of CNV by fundus examination or histology. Senescent Sod1−/− mice demonstrated evidence of necrotic death of cells in the INL and reduced ERGs (Hashizume et al., 2008).
2.3.4. Sod2−/− and Sod2 knockdown mice
Polymorphisms in the Sod2 gene have been associated with increased risk of the development of AMD (Kimura et al., 2000). Unfortunately, Sod2−/− mice die soon after birth from a dilated cardiomyopathy, limiting their use as a model for AMD (Li et al., 1995). Retinal histology from some of the few animals that survived to 3 weeks demonstrated thinning primarily of the inner retina and evidence of mitochondrial dysfunction (Sandbach et al., 2001). To study, the effects of Sod2 on retinal function, Justilien and coworkers developed a mouse model in which the retina and RPE were transfected by an adenovirus to expresses a ribozyme that degrades Sod2 (Justilien et al., 2007). These mice demonstrated increased markers of oxidative damage including nitrated and carboxyethylpyrrole-modified proteins. Histology showed thickening of Bruch's membrane, degeneration of the RPE, increased autofluorescence, elevation of A2E levels and atrophy of the photoreceptors. However, the development of CNV was not observed in this model.
2.3.5. Cigarette smoke/hydroquinone+/− high fat diet+/− blue light
Cigarette smoke contains over 4000 potentially harmful substances including many pro-oxidants such as nitric oxide, carbon monoxide, and hydroquinone (Smith and Hansch, 2000). Smoking is the most significant preventable risk factor for AMD (Evans, 2001; Klein et al., 2008; Seddon et al., 1996; Smith et al., 2001; Thornton et al., 2005). The role of cigarette smoke to induce or worsen AMD has been studied in several animal models as well as the additive risk of oxidation from blue light and high fat diets. Espinosa-Heidmann and colleagues studied exposing mice to combinations of high fat diets, blue light, and whole cigarette smoke versus oral hydroquinone (Cousins et al., 2002; Espinosa-Heidmann et al., 2006). They found that animals stressed with blue light and on a high fat diet developed thickening of Bruch's membrane and significantly increased basal laminar deposits. Mice exposed to cigarette smoke or hydroquinone in their diet developed similar changes to Bruch's membrane and had increased basal laminar deposits. Some animals showed invasion of the choriocapillaris into Bruch's membrane reminiscent of early AMD. Wild-type mice treated with hydroquinone in their drinking water develop decreased expression of Ccl-2 in the RPE and choroid and demonstrated altered ratios of pro-angiogenic VEGF versus anti-angiogenic PEDF (Pons and Marin-Castano, 2011). These studies suggest that hydroquinone can further the risk of CNV by altering the balance of these angiogenic factors. However, Weikel et al., 2011 found that hydroquinone exposure did not enhance age-related retinal phenotypes of mice fed high glycemic index diets. The exact relationship between hydroquinone and the development of AMD requires further study.
2.3.6. The OXYS rat
The OXY rats are an inbred strain of Wistar rats that were selected for susceptibility or resistance to the early development of cataracts when placed on a cataractogenic diet rich in galactose (Salganik et al., 1994b). The susceptible line (OXYS) demonstrates high levels of hydroxyl radical generation and lipid peroxidation resulting in mitochondrial oxidative damage and a senescence accelerated model. These animals exhibit multiple signs of premature aging including the development of cataracts, emphysema, scoliosis, tumors, and myocardiopathy (Kolosova et al., 2003; Marsili et al., 2004; Salganik et al., 1994a,b). OXYS rats first demonstrated fundoscopic changes at 1.5 months of age with the appearance of atrophic areas in the RPE and choriocapillaris (Markovets et al., 2011). Older rats developed thickening of Bruch's membrane, drusen and RPE detachments. By 12 months, some animals demonstrated photoreceptor atrophy, decreased ERGs, destruction of the choriocapillaris with fibrosis and in some cases hemorrhagic detachment of the retina due to neovascularization (Markovets et al., 2011; Neroev et al., 2008; Salganik et al., 1994a).
2.4. Lipid/glucose metabolism
Lipid metabolism and more recently glucose metabolism have long been suspected to play a role in the development of AMD. Parallels have been drawn between the deposition of cholesterol and lipids in atherosclerotic plaques and the material that accumulates in Bruch's membrane. There has been disagreement as to how strongly cardiovascular disease and AMD are related with some studies demonstrating a positive relationship (Klein et al., 2003; van Leeuwen et al., 2003) and others not (Hyman et al., 2000; Tomany et al., 2004). Deciphering the exact relationship between dietary fat and cholesterol intake and the development of AMD has been complex. Lipids and cholesterol are known to accumulate within Bruch's membrane with advancing age and are thought to interfere with the transport of metabolites between the RPE and the choriocapillaris (Curcio et al., 2001; Pauleikhoff et al., 1990; Sheraidah et al., 1993; Sunness et al., 1988, 1985). The deposition of lipids and cholesterol may also contribute to the formation of basal laminar and basal linear deposits. Additionally, increased consumption of cholesterol, monounsaturated, polyunsaturated, and saturated fats have been linked to the development of AMD (Mares-Perlman et al., 1995; Seddon et al., 2003). Genetic polymorphisms in apolipoproteins, which mediate lipid transport have also been implicated in the risk of AMD (Klaver et al., 1998; Simonelli et al., 2001). As will be detailed below, a number of animal models have been created to explore the relationship between lipid metabolism and AMD.
2.4.1. Aging mice+/− high fat diet+/− light treatment
To study the relationship of lipid metabolism in the development of basal laminar deposits beneath the RPE, Cousins and coworkers analyzed young (2 months) and old (16 months) C57BL/6 mice fed normal and high fat diets (Cousins et al., 2002). They found only older mice fed high fat diets developed basal laminar deposits. Additional exposure to blue-green light and estrogen depletion increased the number and severity of deposits (Cousins et al., 2002, 2003). Dithmar and colleagues performed a similar study where they analyzed five groups of animals: 2-month-old mice on a normal diet, and 8-month-old mice on a normal or high fat diet, with or without phototoxic treatment (Dithmar et al., 2001). They found thickening of Bruch's membrane in 8-month-old mice on both normal and high fat diets. 8-month-old mice on high fat diets that also received laser treatment demonstrated the development of basal linear deposits.
2.4.2. High glycemic index diet
Consumption of low glycemic index (GI) diets has been associated with a decreased risk for the onset and progression of AMD (Chiu et al., 2006; Chiu et al., 2011, 2007a,b; Chiu and Taylor, 2011). Advanced glycation end products (AGEs) have been found as a component of drusen. Uchiki and coworkers sought to determine if a diet with an elevated glycemic index could lead to the development of AMD-like lesions in an animal model (Uchiki et al., 2011). Senescent animals on a low GI diet showed fewer and smaller basal laminar deposits and more preservation of RPE basal infoldings compared to those on a high glycemic diet. Additionally, low GI diet animals had lower levels of AGEs in the retina. Animals on the high GI diet demonstrated increased AGEs in the RPE and Bruch's membrane correlating with previous studies that have shown these byproducts to be present in human drusen (Crabb et al., 2002). The incidence and severity of retinal changes progressed as the animals aged with 23.5-month-old animals showing more thickening of Bruch's membrane, more basal laminar deposits, disorganization of RPE basal infolding, and increased photoreceptor atrophy compared to 17-month-old mice (Weikel et al., 2011).
2.4.3. ApoE−/− mice
Mice lacking ApoE develop very high levels of circulating cholesterol (Plump et al., 1992; Zhang et al., 1992). Histological examination of these animals at 8 months of age, revealed thickening of Bruch's membrane and the presence of electrolucent particles and membrane-bounded material (Dithmar et al., 2000).
2.4.4. APOEe2/e4 transgenic mice
To emulate and study the various APOE alleles seen in humans, transgenic animals were made where the native mouse ApoE was replaced by the various human APOE alleles (i.e. e 2, e 3, e 4) (Malek et al., 2005, 2006; Sullivan et al., 1997). When placed on a high fat diet, APOE e 2 and APOε4 mice over 16 months of age, showed thickening of Bruch's membrane, vacuolization of the RPE, and RPE pigmentary changes. These changes were worse in the APOEe4 mice than in the APOEe2 mice. APOEe4 mice additionally demonstrated areas of CNV and photoreceptor atrophy. Transgenic APOEe4 mice fed high fat cholesterol enriched diets have also been shown to accumulate amyloid β (Aβ), a component of human drusen (Mullins et al., 2000). Systemic administration of antibodies to Aβ40 and Aβ42 provide visual protection in these animals suggesting a role for Aβ in the pathogenesis of AMD (Ding et al., 2011). In humans, the APOEe4 allele is correlated with relative protection from the development of AMD compared with the APOEe2, while in the mice the opposite appears to be true (Baird et al., 2004; Klaver et al., 1998; Schmidt et al., 2002; Souied et al., 1998). It is unclear why this discrepancy exists between species.
2.4.5. APO*E3-Leiden transgenic mice
APO*E3-Leiden is a dysfunctional form of APO-E3 that was first discovered in a Dutch family with hyperlipoproteinemia and early onset atherosclerosis (Havekes et al., 1986). Mice constructed to express this mutant protein develop basal laminar drusen, which are worsened when the mice are placed on a high fat diet (Kliffen et al., 2000; van den Maagdenberg et al., 1993).
2.4.6. APOB100 transgenic mice + high fat diet
Apolipoprotein B100 (APOB100) is the major apolipoprotein in low-density lipoprotein (LDL) cholesterol. Lipoprotein particles in Bruch's membrane have been shown to contain APOB100. To study how APOB100 might contribute to the formation of lipoproteinacious deposits in AMD, several groups have examined mice that express the human form of APOB100. Young APOB100 mice when fed a high fat diet and exposed to oxidative blue-green light developed basal laminar deposits (Espinosa-Heidmann et al., 2004). At older ages, APOB100 mice fed a normal diet demonstrated increased thickness of Bruch's membrane, loss of the basal infoldings of the RPE, and development of basal laminar deposits (Fujihara et al., 2009; Sallo et al., 2009). When these animals were additionally fed high fat diets, basal linear deposits were observed.
2.4.7. Ldl receptor−/− mice + high fat diet
Ldl receptor−/− mice are not able to import cholesterol, resulting in high increased plasma cholesterol levels and membrane translucent particles in Bruch's membrane (Rudolf et al., 2004, 2005). Feeding these animals high fat diets leads to further thickening of Bruch's membrane and was correlated with increased expression of the pro-angiogenic factor VEGF in the RPE and the outer retina.
2.4.8. Vldl receptor−/− mice
Mouse homozygous for mutations in the gene encoding the very low-density lipoprotein (VLDL) receptor do not develop dyslipidemia, but have been shown to develop retinal neovascularization starting at 2 weeks of age (Frykman et al., 1995; Heckenlively et al., 2003; Tiebel et al., 1999). These vessels grow towards the subretinal space and between 1 and 2 months can form choroidal anastomosis or cause retinal hemorrhages. Further examination of this model revealed the development of CNV, elevated levels of VEGF, and dysregulation of the Wnt pathway (Chen et al., 2007). These findings suggest that the VLDL receptor functions as a negative regulator of CNV (Chen et al., 2007). Thus, modulation of the VLDL receptor may offer a new pathway for treatment in AMD.
2.4.9. CD36−/− mice
Polymorphisms in CD36 have been shown to be protective in AMD (Kondo et al., 2009). CD36 is a scavenger receptor for oxidized low density lipoproteins (OxLDL) and is expressed in the apical and basolateral membrane of the RPE (Gordiyenko et al., 2004; Hayes et al., 1989; Ryeom et al., 1996). Mice deficient for CD36 accumulate subretinal OxLDL even when fed a regular diet (Picard et al., 2010). These animals demonstrate thickening of Bruch's membrane, which is worsened when these animals were crossed with ApoE−/− mice to create ApoE−/−.
Cd36−/− double knockout mice. Interestingly, treatment of ApoE−/− mice with a ligand of the CD36 receptor, EP80317, decreases thickness of Bruch's membrane and prevents photoreceptor atrophy (Picard et al., 2010).
CD36−/− mice have also been reported to develop age dependent choroidal involution thought to be secondary to down regulation of COX2, an upstream activator of VEGF (Houssier et al., 2008).
2.4.10. Mcd/mcd mice (transgenic mutant cathepsin D)
Cathepsin D is an aspartic protease that is highly expressed in the RPE and plays an important role in processing photoreceptor outer segments (Bosch et al., 1993; Regan et al., 1980). Cathepsin D is secreted as a proenzyme, which requires further post translational processing for activity (Davies, 1990). The accumulation of inactive forms of procathepsin D have been linked to RPE dysfunction (Rakoczy et al., 1996). To study the effects of inactive cathepsin accumulation on photoreceptors, transgenic mice with a mutant form of cathepsin (mcd) have been created (Zhang et al., 2002). Mice homozygous for the transgene (mcd/mcd) demonstrate areas of RPE atrophy starting at 9 months of age (Rakoczy et al., 2002). Mice between 10 and 18 months of age develop areas of RPE hypertrophy, photoreceptor atrophy and reduced ERGs. Notably, these animals develop small yellowish spots in the fundus, which on histology resemble basal laminar and basal linear spots. Autofluoresent imaging reveals hyperautofluoresent areas resembling accumulation lipofuscin. The mcd/2/mcd2 mouse, which expresses a mutant form of procathepsin D, but has higher levels of inactive proteins, demonstrates similar findings as the mcd1/mcd1 mice, but they manifest earlier at 3 months of age (Zhang et al., 2005).
2.5. Other rodent models of AMD
2.5.1. Senescence accelerated mice
The senescence accelerated mouse (SAM) model is comprised of mice originally derived from AKR/J mice that have been selectively bred for advanced aging (Takeda et al., 1994). The original progenitor lines were subsequently expanded to nine senescence prone lines (SAMP) and four senescence resistant lines (SAMR). The phenotypic characteristics vary from line to line, but animals from the SAMP lines collectively demonstrate early onset development of senile amyloidosis, osteoporosis, degenerative joint disease, cataracts, hearing impairment and brain atrophy with deficits in learning and memory (Takeda et al., 1997, 1994). SAMP1 mice have been shown to exhibit thickening of Bruch's membrane and RPE changes including swelling of basal infoldings and accumulation of lipofuscin granules (Ogata et al., 1992; Takada et al., 1993, 1994). At 10 months, SAMP8 mice also demonstrate thickening of Bruch's membrane, deposition of sub-RPE basal laminar deposits, basal linear-like deposits, and small areas of intra-Bruch's membrane neovascularization (Majji et al., 2000).
2.5.2. Single gene mutations causing macular dystrophies
A number of animal models of inherited macular dystrophies caused by single genetic mutations have been constructed and used to as models of AMD. These include the Timp3−/− mice (model for Sorsby Fundus Dystrophy) (Weber et al., 2002), the Abcr−/− mice (model for autosomal recessive Stargardt disease) (Mata et al., 2000; Weng et al., 1999), the ELOVL4 transgenic mouse (model for autosomal dominant Stargardt disease) (Karan et al., 2005), and fibulin-3 transgenic mice (model for human EFEMP1 mutations) (Marmorstein et al., 2007). These models will not be covered in this review and the reader is referred to several excellent reviews on these animals (Edwards and Malek, 2007; Rakoczy et al., 2006; Ramkumar et al., 2010; Zeiss, 2010).
3. Non-human primate models of dry AMD
3.1. Nonhuman primate retinal anatomy
Nonhuman primates are the only animals with a retinal structure closely resembling that found in humans, most notably the presence of a macula. In the center of the macula, the central fovea has multiple features designed to optimize spatial resolution. These include a predominance of cone photoreceptors, their unique morphology and high packing density, as well as displacement of other retinal cell types to create the foveal pit and allow an unobstructed path of light to the cone photoreceptors. In addition, blood vessels are absent from the inner retina and a central capillary-free zone. The macula is also characterized by the presence of the yellow macular pigment (hence the full name “macula lutea” meaning yellow spot), which consists of the plant pigments lutein and zeaxanthin. The macula is exposed to high levels of focused light, has an unusually high metabolic rate and oxygen tension, and has only the underlying choroidal circulation to provide oxygen and nutrients and to remove waste products. All of these features may contribute to the macula's special vulnerability to age-related disease in human and nonhuman primates. Furthermore, several nonhuman primate species develop the early to intermediate stages of AMD.
3.2. Age-related maculopathy in rhesus and cynomolgus macaques
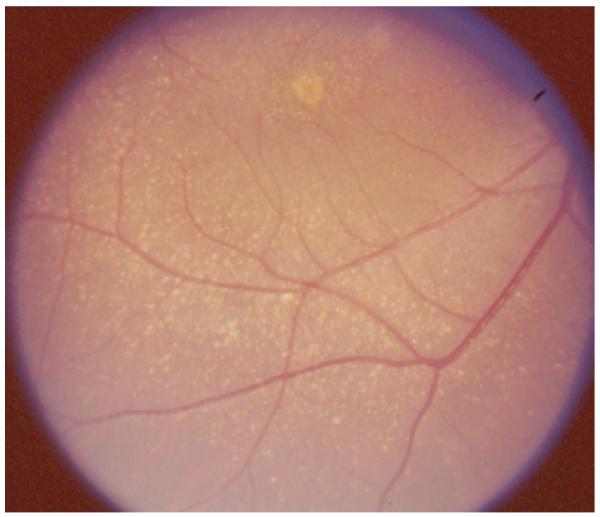
Monkeys rarely show spontaneous development of either the wet or dry form of advanced AMD. However, many studies have documented the signs of early to intermediate AMD, including drusen and pigmentary changes, in older rhesus macaque monkeys (Macaca mulatta) (Bellhorn et al., 1981; Dawson et al., 1989; El-Mofty et al., 1980; Francis et al., 2008; Hope et al., 1992; Jonas et al., 2003; Monaco and Wormington, 1990; Nicolas et al., 1996; Olin et al., 1995; Stafford et al., 1984; Ulshafer et al., 1987); see Fig. 1. The prevalence of drusen in these reports ranges from 6% in populations that included young and middle-aged adults (Bellhorn et al., 1981; Stafford et al., 1984) to 50% or more in older animals. A particularly high prevalence was found in a free-ranging colony established in the 1938 on the Caribbean island of Cayo Santiago (Dawson et al., 1989; El-Mofty et al., 1980; Hope et al., 1992). A similar late-onset drusen syndrome was found in another macaque species, the cynomolgus macaque (Macaca fascicularis) (Umeda et al., 2005a).
Fig. 1.
Macular drusen in a 25-year-old aged rhesus monkey.
The structural similarity to human drusen has been confirmed by histopathology in both species (Gouras et al., 2008a,b, 2010; Ishibashi et al., 1986; Olin et al., 1995; Ulshafer et al., 1987; Umeda et al., 2005b). Electron microscopy of affected monkey retinas provided evidence for several steps in drusen formation, including budding and disconnection of segments of basal epithelial cytoplasm and thickened basal lamina, and for the consistent presence of macrophages in Bruch's membrane near drusen and in the segments of disconnected cytoplasm (Gouras et al., 2008b). In some cases RPE cells overlying drusen were atrophic. In cynomolgus macaques, drusen were shown by immunohistochemistry and by proteomic analysis of isolated drusen to contain many of the compounds present in human drusen, including apolipoprotein E, amyloid P component, complement component C5, the terminal C5b-9 complement complex, vitronectin, membrane cofactor protein, annexins, crystallins and immunoglobulins (Umeda et al., 2005b).
In some cases, ophthalmoscopic spots appearing as drusen were found to correspond not with sub-RPE deposits but with RPE cells filled with vacuoles and lipid droplets, a pattern termed lipoidal degeneration (Anderson et al., 2006; Feeney-Burns et al., 1981; Fine and Kwapien, 1978; Umeda et al., 2005b). Feeney-Burns et al. (1981) found that these degenerating RPE cells generally corresponded to punctate fluorescein angiographic window defects, while patches of RPE cells with high lipofuscin and low melanin content corresponded to areas of more diffuse hyperfluorescence.
Genetic factors have been implicated in age-related monkey macular disease. In the Cayo Santiago colony, certain matri-lines were found to have a particularly high prevalence of macular disease (Dawson et al., 1989). More recently, two of the major genes involved in human AMD were found to be linked to maculopathy in rhesus monkeys. Specifically, one polymorphism in ARMS2 (LOC387715) and one in the HTRA1 promoter region were significantly associated with the presence of drusen (Francis et al., 2008). In functional analyses, the disease-associated HTRA1 polymorphism resulted in a 2-fold increase in gene expression, supporting a role in pathogenesis. Among several primate species, orthologous exons for the human ARMS2 gene were present only in Old World monkeys and apes. Furthermore, the predicted gene was transcribed in rhesus and human retinas. Associations with polymorphisms in these two genes were confirmed in a second population, although significant association with drusen was found for a different HTRA1 variant (Singh et al., 2009). These findings represent the first case of genetic factors for a prevalent, complex disease that are shared between humans and macaques, and they support the assumption that pathogenetic mechanisms are also shared.
Monkeys and humans also appear to share key nutritional risk factors. Rhesus monkeys fed diets throughout life that lack the macular pigment carotenoids lutein and zeaxanthin have no macular pigment, show angiographic window defects, (Malinow et al., 1980) and develop drusen at younger ages than those fed standard laboratory diets (Neuringer et al., 2010). In middle age (14–16 years), some of those receiving diets lacking both carotenoids and omega-3 fatty acids develop patches of RPE atrophy as seen ophthalmoscopically (Neuringer et al., 2010), which represent the first documented nonhuman primate cases of more advanced macular disease. Thus, the major limitation of the rhesus model of AMD, that monkeys fail to develop the most advanced forms of the disease, may be due in part to their intake of standard laboratory diets that are extremely low in fat and rich in protective nutrients.
3.3. Early onset drusen in Japanese and cynomolgus macaques
In a large pedigree of cynomolgus macaques (M. fascicularis), a group of Japanese investigators identified an early-onset macular degeneration syndrome (Nicolas et al., 1996; Suzuki et al., 2003; Umeda et al., 2005b). Widespread drusen are present in both the macula and periphery starting at 1–2 years of age and show a dominant inheritance pattern, thus closely resembling a cluster of human diseases called dominant drusen that include Malattia Leventinese and Doyne's Honeycomb Dystrophy. Drusen from these animals have been confirmed by histopathology to closely resemble human drusen and, like rhesus drusen, have been shown by immunohistochemistry and proteomic analysis to contain markers of human drusen, including apolipoprotein E, amyloid P component, complement component C5, the terminal C5b-9 complement complex, vitronectin, membrane cofactor protein, annexins, crystallins and immunoglobulins (Umeda et al., 2005b). The pedigree was screened for mutations in 13 genes that are known to underlie early macular degeneration syndromes in humans, and all were excluded (Umeda et al., 2005a). A similar syndrome has been identified in the Japanese macaque (M. fuscata) (Neuringer et al., 2010) (Fig. 2). The dominant inheritance and early onset of this disease could facilitate production of animals for preclinical therapy development.
Fig. 2.
Panretinal drusen in a 15-year-old Japanese macaque.
4. Rodent models of wet macular degeneration
CNV is the hallmark lesion of exudative AMD and is a significant cause of severe vision loss in the elderly population (Klein et al., 1995). Before the era of anti-vascular endothelial growth factor (VEGF) therapy, treatment options for CNV were limited, consisting mainly of ablative modalities (1991; 1999). In the following decades, animal models served to elucidate some of the molecular mechanisms involved in the pathogenesis of CNV while providing reproducible, short time-course models for identifying and testing novel treatments. As a result, anti-VEGF therapy has dramatically altered the prognosis for patients afflicted with exudative AMD and shifted the therapeutic paradigm to increasingly targeted pharmacotherapy (Martin et al., 2011).
Despite the recent improvements in treatments for CNV, the exact underlying pathogenesis of CNV remains elusive; similarly, current animal models of CNV are limited in their ability to recapitulate the complex chain of events leading to the development of CNV in patients with AMD. Ideally, an animal model of exudative AMD would not only include the neovascular component of the disease, but also the underlying senescent degeneration of the macula that predisposes to the growth of new blood vessels from the choroid. The majority of present models rely on either laser or direct mechanical injury to the RPE/Bruch's membrane complex, or alteration of the RPE and surrounding environment by external interventions (such as exogenous compounds injected in the subretinal space) or internally (such as genetic knock-out models).
4.1. Laser induced CNV
While the laser trauma model was first developed in non-human primates by Ryan more than three decades ago (Ryan, 1979b) rodent adaptations of the model are perhaps the most employed. In the laser trauma model, high-powered, focused laser energy is used to induce a break in Bruch's membrane. In 1989 Dobi et al. first described the model in rats using a 647 nm krypton laser (100 μm, 50–160 mW, 0.1 s) (Dobi et al., 1989). Their initial observations regarding the power necessary to reliably produce CNV remain salient: burns that were insufficient to create a break in Bruch's membrane (60 mW) did not result in CNV; burns that were too powerful (130 mW and higher) resulted in extensive choroidal hemorrhage and eventual avascular scarring without CNV; burns that created a white spot with central bubble formation, with or without associated hemorrhage, were found to reliably induce breaks in Bruch's membrane and produce CNV (120 mW in their study) (Dobi et al., 1989). In 1998 the first mouse model was produced by Tobe et al. also using a krypton laser (50 μm, 350–400 mW, 0.05 s) to make three burns in the posterior pole, with CNV formation in 87% of burns at 2 weeks (Tobe et al., 1998). The authors noted a higher rate of CNV formation in burns with bubble formation. Variations using diode, Nd:YAG, or argon lasers have also been commonly employed (Campos et al., 2006; Giani et al., 2011; Olson et al., 2007).
Numerous methods of analyzing the experimental CNV have been described. Fluorescein angiography may be used to visualize leakage from membranes, though leakage is difficult to quantify and mature CNV complexes may not leak, possibly due to surrounding RPE cells (Tobe et al., 1998). Histology is adept at identifying CNV, particularly when aided by immunohistochemical stains such as alkaline phosphatase, or antibodies to CD31 or CD102, among others (Campa et al., 2008; Semkova et al., 2003). However, it is labor intensive, difficult to quantify, and limited by the technical difficulty required to achieve adequate sampling. Sclera-choroid-RPE flatmounts may be created and combined with fluoresceinated high molecular weight dextran angiography to allow computer assisted, quantitative imaging (Edelman and Castro, 2000). It has been questioned whether the vascular tissue imaged in this technique is choroidal in origin, (Semkova et al., 2003) and further, non-perfused vessels are unlikely to be imaged (Campos et al., 2006). Fluorescent labeling of individual cell populations of RPE-choroid flatmounts followed by 3D reconstruction and volume analysis using confocal microscopy may provide more robust quantification of CNV membrane development (Campos et al., 2006). With the recent development of hand-held spectral-domain optical coherence tomography, advanced in vivo imaging modalities with sophisticated post-acquisition processing algorithms may represent the future method of choice to study CNV membrane formation after laser injury (Giani et al., 2011).
The rodent laser trauma model is appealing for its lower cost, ability to efficiently generate high numbers of CNV lesions for statistical analysis, and its short time course. Similar to the CNV that occurs in human ocular disease, laser-induced CNV follows predictable stages of development: early membrane formation, establishment of a mature fibrovascular network, and involution (Miller et al., 1990a). The time course of CNV development after laser is similar in mice and rats, with the early phase occurring over the first week, and mature membranes developing by 10 to 14 days in most studies (Edelman and Castro, 2000; Tobe et al., 1998). It is not clear at what point involutional changes begin, some investigators have found a slight increase in neovascular area during the first 30 days while others have found involutional changes beginning at 5 days post laser (Edelman and Castro, 2000; Giani et al., 2011). The primary drawback of the rodent laser trauma model is its inability to recapitulate the complex sequence of events that leads to CNV in AMD. It is a model of acute injury and inflammation, rather than a result of long standing senescent degeneration and chronic inflammation. Anatomic discrepancies exist as well, since the laser coagulation causes significant damage to the overlying neural retina to a greater degree than is typical of human AMD. It is unknown to what extent this may alter the local environment surrounding the experimental CNV.
Either through direct pathologic study of cell types and their mediators of angiogenesis, or through the use of transgenic models, the rodent laser trauma model has contributed greatly towards our present understanding of the pathogenesis of CNV. Numerous components of the angiogenic cascade have been studied in murine laser models, including secreted factors such as fibroblast growth factor 2 (FGF-2), (Ogata et al., 1996; Yamada et al., 2000) Ang-2, (Oshima et al., 2004) VEGF, (Shen et al., 1998; Wada et al., 1999; Yi et al., 1997) nitric oxide, (Ando et al., 2002) and matrix metalloproteinases (Berglin et al., 2003; Lambert et al., 2002). Intracellular kinase signaling of growth factors has also been studied, (Seo et al., 1999; Zhu et al., 2009) as have the various cell types involved in CNV formation, including macrophages, (Sakurai et al., 2003; Yi et al., 1997) endothelial cells, (Dobi et al., 1989) fibroblasts, (Ogata et al., 1996) and RPE (Zhang et al., 2011). Laser induced CNV in rodents has also been used to evaluate the role of the complement cascade in experimental CNV (Bora et al., 2006; Lyzogubov et al., 2010).
In addition to improving our understanding of the pathogenesis of CNV at a molecular level, the laser trauma model has supported early work in novel treatments of CNV, and helped to establish proof of concept for pharmacotherapy of CNV. In 1999, Seo and colleagues demonstrated a dramatic reduction in laser-induced CNV in a rodent model with an orally administered protein kinase inhibitor, and in doing so, anticipated the era of pharmacologic therapy of CNV (Seo et al., 1999). Since that time, pharmacologic inhibition of laser CNV has been an active area of research. Intravitreal injections of steroid preparations, (Ciulla et al., 2001) various anti-angiogenic and anti-inflammatory compounds, (Kim and Toma, 2010; Lima e Silva et al., 2005; Olson et al., 2009; Zou et al., 2006) antibodies (Olson et al., 2007; Rennel et al., 2011; Xie et al., 2009) and sustained release preparations have all been studied (Fu et al., 2007; Pan et al., 2011). Inhibition of VEGF beyond binding antibodies and other molecules has also been explored, including small-interfering RNA mediated techniques (Reich et al., 2003) and receptor blockade (Huang et al., 2011; Takahashi et al., 2006). The most recent anti-VEGF treatment to gain FDA approval for use in patients with AMD, aflibercept ophthalmic solution, has also been studied in the rodent laser model (Saishin et al., 2003). Gene therapy (Balaggan et al., 2006; Lai et al., 2001; Mori et al., 2002) initially evaluated in rodent laser models has recently resulted in investigation in human trials and shows great promise for adjuvant therapy to anti-VEGF modalities. Despite these promising results, it remains critical to maintain strict standards in the evaluation of toxicity and safety when transitioning successful compounds from the rodent laser trauma model to human studies, as illustrated by recent experience with infliximab (Giganti et al., 2010; Olson et al., 2007; Theodossiadis et al., 2009). Furthermore, it is currently unclear whether rodent models are a logical choice for the study of humanized antibodies as a recent investigation in rats found no significant inhibition of CNV formation with intravitreal bevacizumab or ranibizumab, a result attributed to species difference (Lu and Adelman, 2009b).
4.2. Subretinal injection induced CNV
Injection of material between the neural retina and RPE holds promise as an additional mechanism of producing experimental CNV. In this approach, angiogenic substances can be placed in near proximity to the choroidal vasculature, and perturbation of the RPE during injection, when combined with angiogenic signals, appears to be sufficient insult to allow invasion of the subretinal space by CNV (Oshima et al., 2004).
4.2.1. Subretinal matrigel injection
Matrigel is a mixture of extracellular matrix proteins secreted from Engelbreth-Holm Swarm mouse sarcoma cells, primarily containing laminin, collagen IV, entactin/nidogen, perlecan, certain matrix metalloproteinases, and various growth factors, including FGF-2 (Kleinman and Martin, 2005). The mixture is a liquid at 4 °C and solidifies at 24–37 °C, such as when in injected in vivo; in this solid form is thought to allow slow release of angiogenic factors and recruit host cells (Qiu et al., 2006). Its utility in angiogenesis research was demonstrated in early experiments, which became known as the matrigel plug assay (Passaniti et al., 1992). CNV was induced in ccl2-deficient mice and wild type mice by subretinal injection of matrigel in the peripheral mouse fundus. Also observed were RPE and photoreceptor degeneration, RPE migration, and various degrees of CNV (Shen et al., 2006). A similar model in the rabbit eye, using subretinal injection of matrigel alone or matrigel with VEGF, achieved CNV in 100% of the lesions (Qiu et al., 2006). Further studies on matrigel in rodents demonstrated the necessary relationship between the RPE and Bruch's membrane to prevent CNV. In a model in which matrigel is injected between the RPE and neurosensory retina, RPE cells migrate across the deposit in order to reestablish contact with photoreceptors, creating a sub-RPE deposit; new vessels from the choroid are then allowed to penetrate Bruch's membrane, resulting in CNV (Zhao et al., 2007). Subsequently, the model was further evaluated in the context of VEGF inhibition with VEGF Trap, demonstrating not only inhibition of CNV in treated rats, but also profiling the cellular constituents of the neovascular response and highlighting similarities with human exudative AMD lesions (Cao et al., 2010). In light of its ability to mirror the subretinal deposit component of exudative AMD and its more lasting capacity to promote angiogenesis—either through sustained elution of angiogenic factors or recruitment of host cells—subretinal matrigel is an appealing model (Cao et al., 2010; Qiu et al., 2006).
4.2.2. Subretinal VEGF gene therapy
The central role of VEGF in ocular angiogenesis has been well established; (Miller et al., 1994a) the RPE is recognized as a principle secretor of VEGF and dysfunction of this system has been suggested to play a critical role in the pathogenesis of CNV (Blaauwgeers et al., 1999). As such, numerous groups have sought to create animal models of CNV by inducing overexpression of VEGF in the RPE. In 2000, Baffi et al. and Spilsbury et al. each described models of CNV in rats given subretinal injections of adenovirus vectors expressing VEGF (Baffi et al., 2000; Spilsbury et al., 2000). Both groups found robust evidence of CNV, as measured by angiography and histology, at 4 weeks following injection despite the reportedly transient infection seen in adenoviral models; in Spilsbury's study, organized neovascular complexes were still seen at 80 days when evaluated by histology. In 2003, Wang's group demonstrated a 95% rate of CNV formation in rats given subretinal injections of adeno-associated virus vectors expressing VEGF, with duration of membrane complexes as late as 20 months post injection (Wang et al., 2003).
The importance of the subretinal injection in these models is elucidated by transgenic mice. In rho/VEGF mice, which display increased VEGF expression by photoreceptors, florid neovascularization arising from the retinal circulation occurs without CNV (Ohno-Matsui et al., 2002). When VEGF expression is induced in the RPE, such as in VMD2/VEGF mice with a tetracycline-inducible promoter system, either no CNV occurs, (Oshima et al., 2004) or intrachoroidal neovascularization is seen without penetration of Bruch's membrane (Schwesinger et al., 2001). However, with additional insult to RPE integrity such as subretinal injection of a gutless adenovirus vector, pronounced CNV develops (Oshima et al., 2004). Moreover, Schmack and colleagues found that subretinal injection of RPE cells produced CNV, which they attributed to VEGF production by the injected cells coupled with mechanical disturbance of the RPE during the injection (Schmack et al., 2009). Notably, they also found CNV, albeit to a lesser degree, in eyes undergoing a subretinal injection of control substance.
4.2.3. Subretinal injection of macrophages, lipid hydroperoxide, and polyethylene glycol
Additional attempts to recreate the unique pathology of exudative AMD have been made by injecting other components or surrogate components of CNV lesions in the subretinal space. For example, macrophages are believed to be important cellular participants in CNV formation, secrete a variety of growth factors relative to angiogenesis and have been isolated from CNV membranes (Grossniklaus et al., 2002; Oh et al., 1999). Jo and colleagues attempted to recapitulate the fibrotic aspect of CNV by performing subretinal injection of macrophages on C57BL/6 or Ccl2 knockout mice (Jo et al., 2011). They found that CNV co-existed with fibrosis in the experimental model, however it is worth noting that part of the experimental protocol included rupture of Bruch's membrane with a laser at the site of injection.
Lipid deposition in Bruch's membrane is a characteristic component of the pathogenesis of AMD (Wang et al., 2009) and oxidized lipids have been detected in aged Bruch's membrane (Spaide et al., 1999). Subretinal injection of one such oxidized lipid (13(S)-Hydroperoxy-9Z,11E-octadecadienoic acid (HpODE) led to CNV in a rabbit model (Tamai et al., 2002). Baba et al. extended subretinal injection of HpODE to a rat model, finding photoreceptor degeneration, lipid laden macrophages and RPE cells, and resultant CNV (Baba et al., 2010). While they made efforts to exclude animals in which a break in Bruch's membrane occurred during the subretinal injection, they postulated that HpODE induced RPE, endothelial, or infiltrating inflammatory cells to secrete proteases, compromising Bruch's membrane. Their observations also highlight the necessity of both a disturbance of Bruch's membrane and a continued stimulus for neovascularization.
The role of the complement cascade in the formation of CNV has been demonstrated in the laser trauma model (Bora et al., 2005). Drusen are known to contain components of the complement cascade, (Nozaki et al., 2006b) and the largest genetic risk factor known for AMD rests on complement factor H, a gene tied to regulation of the complement system (Haines et al., 2005). Lyzogubov et al. employed subretinal PEG-8 to create a new model of CNV (Lyzogubov et al., 2011). In this model, PEG-8 injection induced activation of the complement cascade and dose-dependent CNV production, which remained present at the last study time point of 42 days.
5. Rabbit and pig models of wet macular degeneration
While rodent models of wet AMD have proven indispensable, it is sometimes desirable to study pathology and particularly pharmacology in larger eyes. The rabbit eye is substantially larger than the rat eye and more akin in size to the human eye. However, it is unique in that the retina is supplied by a superior central ray of vessels and there is no distinct macula. The pig eye is, of the non-primate models, perhaps the most similar to the human, with an area of increased cone density arranged in a central horizontal band considered analogous to the human macula (Sanchez et al., 2011). Due to their anatomical qualities, numerous models of CNV have been developed in rabbit and pig eyes, some with considerable overlap with their rodent counterparts, some with entirely novel features.
5.1. Subretinal injection induced CNV
A number of subretinal injection strategies have also been employed in the rabbit. Subretinal matrigel and oxidized lipid have each been described with similar results to rodent models of the same (Qiu et al., 2006; Tamai et al., 2002). Likewise, subretinal injection of adenovirus vectors expressing VEGF have been shown to induce CNV related leakage in 83% of rabbits (Julien et al., 2008). Ni and colleagues performed one of the longest conducted experiments, following rabbits given subretinal injections of lipopolysaccharide and FGF-2 incorporated in heparin-Sepharose beads for as long as 3 years (Ni et al., 2005). They found CNV in 100% of eyes at 2 weeks with some lesions demonstrating clinical leakage to the end of the study period.
5.2. VEGF/bFGF eluting scleral pellets
The suprachoroidal space is another potential space for the delivery of angiogenic substances and gene therapy vectors (Peden et al., 2011). Zahn et al. used a scleral cut down and cyclodialysis spatula to access the suprachoroidal space in rabbits and implanted pellets impregnated with VEGF and FGF-2 or a control solution (Zahn et al., 2009a). In eyes treated with the growth factor releasing pellets, florid CNV and leakage developed at 2 weeks, while eyes with control pellets did not demonstrate any leakage on angiography. The study also demonstrated a dose-dependent reduction in CNV in eyes treated with a small-molecule inhibitor of integrin α5β1. The exact mechanism by which angiogenic compounds originating in the suprachoroidal space result in changes in Bruch's membrane in relation to CNV formation remains to be described.
5.3. Surgical rupture of Bruch's membrane
The barrier role of Bruch's membrane has long been recognized. Traumatic mechanical rupture of this membrane, such as that occurring in choroidal rupture and chorioretinitis sclopetaria is known to cause CNV (Goldberg, 1976). Additionally, surgical repair of retinal detachment may result in CNV at sites of iatrogenic breaks in Bruch's membrane (Goldbaum et al., 1983; Wilson and Green, 1987). Initial studies of a surgical model of CNV were undertaken by Kiilgaard et al. in 2004, comparing xenon lamp and diode laser induced CNV with mechanical debridement of RPE cells and surgical perforation of Bruch's membrane (Kiilgaard et al., 2005). The surgical technique was associated with a higher success rate of CNV induction, and histology results led the authors to conclude that the surgically induced CNV more accurately emulated human CNV. In comparison, the laser or xenon burns demonstrated substantial components of gliosis and neuroretinal damage and were significantly less efficient in producing CNV. Further study sought to refine the surgical technique and found that perforation of Bruch's membrane without prior RPE debridement resulted in the most reliable method of inducing CNV development (Lassota et al., 2007). This early research in the model was later complemented by angiographic and immunohistochemical evaluation showing persistently vascularized CNV at 42 days but with decreasing leakage (Lassota et al., 2008). Injection of bevacizumab in the porcine surgical model resulted in a significant reduction in endothelial cell presence in membranes and a trend towards decreased leakage on angiography but did not show any significant difference in CNV size (Lassota et al., 2010). While the surgical model has benefits of reduced neuroretinal damage, the technique is limited by higher cost and the necessity of a three port vitrectomy.
6. Non-human primate models of wet macular degeneration
6.1. Laser Induced CNV
As noted above, the use of laser injury to disrupt Bruch's membrane and induce CNV was first developed in a nonhuman primate, specifically the stumptailed macaque (Macaca speciosa) (Ryan, 1979a). It has since has been implemented in cynomolgus macaques (M. fascicularis), rhesus macaques (Macaca mulatta) and African green monkeys (Chlorocebus sabaeus) to investigate the pathogenesis of CNV (Miller et al., 1990b, 1994b), to document the strong selective vulnerability of the macular region to laser-induced CNV compared with more peripheral retina (Shen et al., 2004), and to test a variety of anti-angiogenic treatments. Most notably, the method was used to demonstrate the safety and efficacy of the treatment that is the current standard of care for neovascular AMD, the anti-VEGF antibody ranibizumab (Husain et al., 2005; Krzystolik et al., 2002). Rodent models proved to be inappropriate to test the effects of this humanized anti-VEGF antibody and showed no effect of treatment (Lu and Adelman, 2009a), presumably due to differences between the rodent and human forms of VEGF, whereas the studies in macaques showed strong evidence of safety and efficacy and helped to provide the basis for human clinical trials. A recent study in cynomolgus monkeys similarly demonstrated effectiveness of bevaciumab (Lichtlen et al., 2010). Other inhibitors of the neovascular process tested with this model include steroids (Ishibashi et al., 1985) and Ryan, 1985**), a phosphorothioate oligonucleotide targeting the VEGF gene (Shen et al., 2002), an inhibitor of the urokinase plasminogen activator system (Koh et al., 2006), and an integrin α5β1 inhibitor (Zahn et al., 2009b), and TNF-α inhibitors, including a topically-applied antibody fragment (Lichtlen et al., 2010). The model also has been used to demonstrate safety and efficacy of an adeno-associated virus (AAV) gene therapy delivering the sFlt-1 gene, a soluble form of the Flt-1 VEGF receptor (Lai et al., 2005). Given the variable effectiveness of current anti-VEGF treatment, and the need for repeated monthly injections that are burdensome for many elderly patients and increase the cumulative risk of side effects, the need continues for a preclinical model that best predicts effectiveness in human patients.
Typical argon laser exposure parameters for the induction of CNV have included a 50 μm spot size, 100 ms duration and powers ranging from 300 to 700 mW but in some studies as high as 1500 mW. Photocoagulation is sometimes repeated to produce a break in Bruch's membrane and bubble formation. Outcome measures of efficacy include the extent of growth of laser-induced new vessels, the total area of blood vessel leakage and the percentage of laser lesions resulting in clinically significant (Grade III and IV) vessel leakage, as visualized by fluorescein angiography and in some cases confirmed by histopathology. A limitation of the model is that not all eyes respond with new vessel formation. In some earlier studies, the incidence of CNV in eyes without experimental anti-CNV treatment was as low as 30% (Ryan, 1982; Shen et al., 2002). More recent studies have optimized laser parameters to produce Grade III lesions in approximately 70% of laser exposures and Grade IV lesions in 20–40% (Goody et al., 2011). A typical result is that 40% of laser lesions in control eyes result in vessel growth and Grade IV leakage, whereas this percentage is reduced to 15–30% in eyes receiving anti-angiogenic treatments (Zahn et al., 2009b). Group sizes of 8–10 monkeys are typically required to document significant effects, and in some studies smaller group sizes have generated inconclusive results (Tolentino et al., 2004a,b; Zahn et al., 2009b).
7. Summary and conclusions
Developing new treatments for a complex disease, such as age related macular degeneration, remains challenging. Animal models of this disease have provided new insights into the underlying pathological mechanisms. Rodent models have offered the advantages of relative cost-effectiveness, accelerated time scale, and ease of genetic manipulation. Mouse models have been able to recreate many of the histological features of AMD such as the thickening of Bruch's membrane, the development of drusen-like subretinal deposits, and immune dysregulation resulting in complement activation and the accumulation of macrophages or microglia. Additionally, laser-induced CNV in rodents has served as the backbone for studying novel treatments for wet AMD. Together, these models have helped unlock the contributions of different pathological mechanisms such as genetic polymorphisms oxidative damage, lipid and carbohydrate metabolism, and complement dysregulation. Rodent models of AMD are still limited by the anatomical lack of a macula, which might explain whey none of these models has yet been able to capture the complexity of the evolution from early to late AMD. However, as the new insights into the genetic and environmental underpinnings of AMD emerge, there is no doubt that rodent models will remain on the front lines for modeling AMD.
Emerging models in non-human primates offer the hope of recreating some of the features of AMD not easily modeled in lower species as well as providing a platform for testing pre-clinical therapeutics. However, the development of primate models is not without many obstacles. Although, non-human primates offer the closest anatomy to humans, they are costly to maintain, have a slow time course for the development of disease, and are difficult to manipulate genetically. Currently their primary contribution is in the laser-induced CNV model of wet AMD, and in providing preclinical assessment of the safety of experimental treatments.
In spite of the challenges, the number of animal models for age related macular degeneration continues to grow. Increased understanding of the underlying pathological mechanisms will allow the creation models that more accurately recreate the histological and functional changes of the disease and serve as more optimal platforms for testing new therapies. The limitations of the current treatments for wet AMD, and the nearly complete lack of effective therapeutic options for dry AMD, drives a continuing need for such models.
Acknowledgements
We would like to thank Laura Erker for critical reading of this manuscript. Funding: Foundation Fighting Blindness (CDA to M.E.P. and grant to M.N.), Research to Prevent Blindness (Unrestricted, CEI), K08 Career Development Award: 1 K08 EY021186-01 and NIH grant RR-00163 (M.N.).
References
- Ambati J, Anand A, Fernandez S, Sakurai E, Lynn BC, Kuziel WA, Rollins BJ, Ambati BK. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat. Med. 2003;9(11):1390–1397. doi: 10.1038/nm950. [DOI] [PubMed] [Google Scholar]
- Anderson M, Dawson WW, Gonzalez-Martinez J, Curcio CA. Drusen and lipid-filled retinal pigment epithelium cells in a rhesus macula. Vet. Ophthalmol. 2006;9(3):201–207. doi: 10.1111/j.1463-5224.2006.00463.x. [DOI] [PubMed] [Google Scholar]
- Ando A, Yang A, Mori K, Yamada H, Yamada E, Takahashi K, Saikia J, Kim M, Melia M, Fishman M, Huang P, Campochiaro PA. Nitric oxide is proangiogenic in the retina and choroid. J. Cell. Physiol. 2002;191(1):116–124. doi: 10.1002/jcp.10083. [DOI] [PubMed] [Google Scholar]
- Baba T, Bhutto IA, Merges C, Grebe R, Emmert D, McLeod DS, Armstrong D, Lutty GA. A rat model for choroidal neovascularization using subretinal lipid hydroperoxide injection. Am. J. Pathol. 2010;176(6):3085–3097. doi: 10.2353/ajpath.2010.090989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baffi J, Byrnes G, Chan CC, Csaky KG. Choroidal neovascularization in the rat induced by adenovirus mediated expression of vascular endothelial growth factor. Invest. Ophthalmol. Vis. Sci. 2000;41(11):3582–3589. [PubMed] [Google Scholar]
- Baird PN, Guida E, Chu DT, Vu HT, Guymer RH. The epsilon2 and epsilon4 alleles of the apolipoprotein gene are associated with age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2004;45(5):1311–1315. doi: 10.1167/iovs.03-1121. [DOI] [PubMed] [Google Scholar]
- Balaggan KS, Binley K, Esapa M, MacLaren RE, Iqball S, Duran Y, Pearson RA, Kan O, Barker SE, Smith AJ, Bainbridge JW, Naylor S, Ali RR. EIAV vector-mediated delivery of endostatin or angiostatin inhibits angiogenesis and vascular hyperpermeability in experimental CNV. Gene Ther. 2006;13(15):1153–1165. doi: 10.1038/sj.gt.3302769. [DOI] [PubMed] [Google Scholar]
- Behndig A, Svensson B, Marklund SL, Karlsson K. Superoxide dismutase isoenzymes in the human eye. Invest. Ophthalmol. Vis. Sci. 1998;39(3):471–475. [PubMed] [Google Scholar]
- Bellhorn RW, King CD, Aguirre GD, Ripps H, Siegel IM, Tsai HC. Pigmentary abnormalities of the macula in rhesus monkeys: clinical observations. Invest. Ophthalmol. Vis. Sci. 1981;21(6):771–781. [PubMed] [Google Scholar]
- Berglin L, Sarman S, van der Ploeg I, Steen B, Ming Y, Itohara S, Seregard S, Kvanta A. Reduced choroidal neovascular membrane formation in matrix metalloproteinase-2-deficient mice. Invest. Ophthalmol. Vis. Sci. 2003;44(1):403–408. doi: 10.1167/iovs.02-0180. [DOI] [PubMed] [Google Scholar]
- Blaauwgeers HG, Holtkamp GM, Rutten H, Witmer AN, Koolwijk P, Partanen TA, Alitalo K, Kroon ME, Kijlstra A, van Hinsbergh VW, Schlingemann RO. Polarized vascular endothelial growth factor secretion by human retinal pigment epithelium and localization of vascular endothelial growth factor receptors on the inner choriocapillaris. Evidence for a trophic paracrine relation. Am. J. Pathol. 1999;155(2):421–428. doi: 10.1016/S0002-9440(10)65138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora NS, Kaliappan S, Jha P, Xu Q, Sohn JH, Dhaulakhandi DB, Kaplan HJ, Bora PS. Complement activation via alternative pathway is critical in the development of laser-induced choroidal neovascularization: role of factor B and factor H. J. Immunol. 2006;177(3):1872–1878. doi: 10.4049/jimmunol.177.3.1872. [DOI] [PubMed] [Google Scholar]
- Bora PS, Sohn JH, Cruz JM, Jha P, Nishihori H, Wang Y, Kaliappan S, Kaplan HJ, Bora NS. Role of complement and complement membrane attack complex in laser-induced choroidal neovascularization. J. Immunol. 2005;174(1):491–497. doi: 10.4049/jimmunol.174.1.491. [DOI] [PubMed] [Google Scholar]
- Bosch E, Horwitz J, Bok D. Phagocytosis of outer segments by retinal pigment epithelium: phagosome-lysosome interaction. J. Histochem. Cytochem. 1993;41(2):253–263. doi: 10.1177/41.2.8419462. [DOI] [PubMed] [Google Scholar]
- Bressler NM, Bressler SB, Fine SL. Age-related macular degeneration. Surv. Ophthalmol. 1988;32(6):375–413. doi: 10.1016/0039-6257(88)90052-5. [DOI] [PubMed] [Google Scholar]
- Cai J, Nelson KC, Wu M, Sternberg P, Jr., Jones DP. Oxidative damage and protection of the RPE. Progr. Retin. Eye Res. 2000;19(2):205–221. doi: 10.1016/s1350-9462(99)00009-9. [DOI] [PubMed] [Google Scholar]
- Campa C, Kasman I, Ye W, Lee WP, Fuh G, Ferrara N. Effects of an anti-VEGF-A monoclonal antibody on laser-induced choroidal neovascularization in mice: optimizing methods to quantify vascular changes. Invest. Ophthalmol. Vis. Sci. 2008;49(3):1178–1183. doi: 10.1167/iovs.07-1194. [DOI] [PubMed] [Google Scholar]
- Campos M, Amaral J, Becerra SP, Fariss RN. A novel imaging technique for experimental choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 2006;47(12):5163–5170. doi: 10.1167/iovs.06-0156. [DOI] [PubMed] [Google Scholar]
- Cao J, Zhao L, Li Y, Liu Y, Xiao W, Song Y, Luo L, Huang D, Yancopoulos GD, Wiegand SJ, Wen R. A subretinal matrigel rat choroidal neovascularization (CNV) model and inhibition of CNV and associated inflammation and fibrosis by VEGF trap. Invest. Ophthalmol. Vis. Sci. 2010;51(11):6009–6017. doi: 10.1167/iovs.09-4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci. 2006;9(7):917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- Cashman SM, Desai A, Ramo K, Kumar-Singh R. Expression of complement component 3 (C3) from an adenovirus leads to pathology in the murine retina. Invest. Ophthalmol. Vis. Sci. 2011;52(6):3436–3445. doi: 10.1167/iovs.10-6002. [DOI] [PubMed] [Google Scholar]
- Chan CC, Ross RJ, Shen D, Ding X, Majumdar Z, Bojanowski CM, Zhou M, Salem N, Jr., Bonner R, Tuo J. Ccl2/Cx3cr1-deficient mice. An animal model for age-related macular degeneration. Ophthalmic Res. 2008;40(3–4):124–128. doi: 10.1159/000119862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CC, Tuo J, Bojanowski CM, Csaky KG, Green WR. Detection of CX3CR1 single nucleotide polymorphism and expression on archived eyes with age-related macular degeneration. Histol. Histopathol. 2005;20(3):857–863. doi: 10.14670/hh-20.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Hu Y, Lu K, Flannery JG, Ma JX. Very low density lipoprotein receptor, a negative regulator of the wnt signaling pathway and choroidal neovascularization. J. Biol. Chem. 2007;282(47):34420–34428. doi: 10.1074/jbc.M611289200. [DOI] [PubMed] [Google Scholar]
- Chiu CJ, Hubbard LD, Armstrong J, Rogers G, Jacques PF, Chylack LT, Jr., Hankinson SE, Willett WC, Taylor A. Dietary glycemic index and carbohydrate in relation to early age-related macular degeneration. Am. J. Clin. Nutr. 2006;83(4):880–886. doi: 10.1093/ajcn/83.4.880. [DOI] [PubMed] [Google Scholar]
- Chiu CJ, Liu S, Willett WC, Wolever TM, Brand-Miller JC, Barclay AW, Taylor A. Informing food choices and health outcomes by use of the dietary glycemic index. Nutr. Rev. 2011;69(4):231–242. doi: 10.1111/j.1753-4887.2011.00382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CJ, Milton RC, Gensler G, Taylor A. Association between dietary glycemic index and age-related macular degeneration in nondiabetic participants in the Age-Related Eye Disease Study. Am. J. Clin. Nutr. 2007a;86(1):180–188. doi: 10.1093/ajcn/86.1.180. [DOI] [PubMed] [Google Scholar]
- Chiu CJ, Milton RC, Klein R, Gensler G, Taylor A. Dietary carbohydrate and the progression of age-related macular degeneration: a prospective study from the Age-Related Eye Disease Study. Am. J. Clin. Nutr. 2007b;86(4):1210–1218. doi: 10.1093/ajcn/86.4.1210. [DOI] [PubMed] [Google Scholar]
- Chiu CJ, Taylor A. Dietary hyperglycemia, glycemic index and metabolic retinal diseases. Prog. Retin. Eye Res. 2011;30(1):18–53. doi: 10.1016/j.preteyeres.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciulla TA, Criswell MH, Danis RP, Hill TE. Intravitreal triamcinolone acetonide inhibits choroidal neovascularization in a laser-treated rat model. Arch. Ophthalmol. 2001;119(3):399–404. doi: 10.1001/archopht.119.3.399. [DOI] [PubMed] [Google Scholar]
- Coffey PJ, Gias C, McDermott CJ, Lundh P, Pickering MC, Sethi C, Bird A, Fitzke FW, Maass A, Chen LL, Holder GE, Luthert PJ, Salt TE, Moss SE, Greenwood J. Complement factor H deficiency in aged mice causes retinal abnormalities and visual dysfunction. Proc. Nat. Acad. Sci. USA. 2007;104(42):16651–16656. doi: 10.1073/pnas.0705079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combadiere C, Feumi C, Raoul W, Keller N, Rodero M, Pezard A, Lavalette S, Houssier M, Jonet L, Picard E, Debre P, Sirinyan M, Deterre P, Ferroukhi T, Cohen SY, Chauvaud D, Jeanny JC, Chemtob S, Behar-Cohen F, Sennlaub F. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J. Clin. Investig. 2007;117(10):2920–2928. doi: 10.1172/JCI31692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combadiere C, Potteaux S, Gao JL, Esposito B, Casanova S, Lee EJ, Debre P, Tedgui A, Murphy PM, Mallat Z. Decreased atherosclerotic lesion formation in CX3CR1/apolipoprotein E double knockout mice. Circulation. 2003;107(7):1009–1016. doi: 10.1161/01.cir.0000057548.68243.42. [DOI] [PubMed] [Google Scholar]
- Cousins SW, Espinosa-Heidmann DG, Alexandridou A, Sall J, Dubovy S, Csaky K. The role of aging, high fat diet and blue light exposure in an experimental mouse model for basal laminar deposit formation. Exp. Eye Res. 2002;75(5):543–553. doi: 10.1006/exer.2002.2047. [DOI] [PubMed] [Google Scholar]
- Cousins SW, Marin-Castano ME, Espinosa-Heidmann DG, Alexandridou A, Striker L, Elliot S. Female gender, estrogen loss, and Sub-RPE deposit formation in aged mice. Invest. Ophthalmol. Vis. Sci. 2003;44(3):1221–1229. doi: 10.1167/iovs.02-0285. [DOI] [PubMed] [Google Scholar]
- Crabb JW, Miyagi M, Gu X, Shadrach K, West KA, Sakaguchi H, Kamei M, Hasan A, Yan L, Rayborn ME, Salomon RG, Hollyfield JG. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc. Nat. Acad. Sci. USA. 2002;99(23):14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruickshanks KJ, Hamman RF, Klein R, Nondahl DM, Shetterly SM. The prevalence of age-related maculopathy by geographic region and ethnicity. The Colorado-Wisconsin Study of Age-Related Maculopathy. Arch. Ophthalmol. 1997;115(2):242–250. doi: 10.1001/archopht.1997.01100150244015. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Millican CL, Bailey T, Kruth HS. Accumulation of cholesterol with age in human Bruch's membrane. Invest. Ophthalmol. Vis. Sci. 2001;42(1):265–274. [PubMed] [Google Scholar]
- Davies DR. The structure and function of the aspartic proteinases. Annu. Rev. Biophys. Biophys. Chem. 1990;19:189–215. doi: 10.1146/annurev.bb.19.060190.001201. [DOI] [PubMed] [Google Scholar]
- Dawson WW, Ulshafer RJ, Engel HM, Hope GM, Kessler MJ. Macular disease in related rhesus monkeys. Doc. Ophthalmol. Adv. Ophthalmol. 1989;71(3):253–263. doi: 10.1007/BF00170974. [DOI] [PubMed] [Google Scholar]
- Despriet DD, Bergen AA, Merriam JE, Zernant J, Barile GR, Smith RT, Barbazetto IA, van Soest S, Bakker A, de Jong PT, Allikmets R, Klaver CC. Comprehensive analysis of the candidate genes CCL2, CCR2, and TLR4 in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2008;49(1):364–371. doi: 10.1167/iovs.07-0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding JD, Johnson LV, Herrmann R, Farsiu S, Smith SG, Groelle M, Mace BE, Sullivan P, Jamison JA, Kelly U, Harrabi O, Bollini SS, Dilley J, Kobayashi D, Kuang B, Li W, Pons J, Lin JC, Bowes Rickman C. Anti-amyloid therapy protects against retinal pigmented epithelium damage and vision loss in a model of age-related macular degeneration. Proc. Nat. Acad. Sci. USA. 2011;108(28):E279–287. doi: 10.1073/pnas.1100901108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dithmar S, Curcio CA, Le NA, Brown S, Grossniklaus HE. Ultrastructural changes in Bruch's membrane of apolipoprotein E-deficient mice. Invest. Ophthalmol. Vis. Sci. 2000;41(8):2035–2042. [PubMed] [Google Scholar]
- Dithmar S, Sharara NA, Curcio CA, Le NA, Zhang Y, Brown S, Grossniklaus HE. Murine high-fat diet and laser photochemical model of basal deposits in Bruch membrane. Arch. Ophthalmol. 2001;119(11):1643–1649. doi: 10.1001/archopht.119.11.1643. [DOI] [PubMed] [Google Scholar]
- Dobi ET, Puliafito CA, Destro M. A new model of experimental choroidal neovascularization in the rat. Arch. Ophthalmol. 1989;107(2):264–269. doi: 10.1001/archopht.1989.01070010270035. [DOI] [PubMed] [Google Scholar]
- Dunaief JL. Iron induced oxidative damage as a potential factor in age-related macular degeneration: the Cogan Lecture. Invest. Ophthalmol. Vis. Sci. 2006;47(11):4660–4664. doi: 10.1167/iovs.06-0568. [DOI] [PubMed] [Google Scholar]
- Duvall-Young J, MacDonald MK, McKechnie NM. Fundus changes in (type II) mesangiocapillary glomerulonephritis simulating drusen: a histopathological report. Br. J. Ophthalmol. 1989a;73(4):297–302. doi: 10.1136/bjo.73.4.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvall-Young J, Short CD, Raines MF, Gokal R, Lawler W. Fundus changes in mesangiocapillary glomerulonephritis type II: clinical and fluorescein angiographic findings. Br. J. Ophthalmol. 1989b;73(11):900–906. doi: 10.1136/bjo.73.11.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman JL, Castro MR. Quantitative image analysis of laser-induced choroidal neovascularization in rat. Exp. Eye Res. 2000;71(5):523–533. doi: 10.1006/exer.2000.0907. [DOI] [PubMed] [Google Scholar]
- Edwards AO, Malek G. Molecular genetics of AMD and current animal models. Angiogenesis. 2007;10(2):119–132. doi: 10.1007/s10456-007-9064-2. [DOI] [PubMed] [Google Scholar]
- Edwards AO, Ritter R, 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308(5720):421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- El-Mofty AA, Eisner G, Balazs EA, Denlinger JL, Gouras P. Retinal degeneration in rhesus monkeys, Macaca mulatta. Survey of three seminatural free-breeding colonies. Exp. Eye Res. 1980;31(2):147–166. doi: 10.1016/0014-4835(80)90075-5. [DOI] [PubMed] [Google Scholar]
- Espinosa-Heidmann DG, Sall J, Hernandez EP, Cousins SW. Basal laminar deposit formation in APO B100 transgenic mice: complex interactions between dietary fat, blue light, and vitamin E. Invest. Ophthalmol. Vis. Sci. 2004;45(1):260–266. doi: 10.1167/iovs.03-0910. [DOI] [PubMed] [Google Scholar]
- Espinosa-Heidmann DG, Suner IJ, Catanuto P, Hernandez EP, Marin-Castano ME, Cousins SW. Cigarette smoke-related oxidants and the development of sub-RPE deposits in an experimental animal model of dry AMD. Invest. Ophthalmol. Vis. Sci. 2006;47(2):729–737. doi: 10.1167/iovs.05-0719. [DOI] [PubMed] [Google Scholar]
- Evans JR. Risk factors for age-related macular degeneration. Prog. Retin. Eye Res. 2001;20(2):227–253. doi: 10.1016/s1350-9462(00)00023-9. [DOI] [PubMed] [Google Scholar]
- Feeney-Burns L, Malinow R, Klein ML, Neuringer M. Maculopathy in cynomolgus monkeys. A correlated fluorescein angiographic and ultrastructural study. Arch. Ophthalmol. 1981;99(4):664–672. doi: 10.1001/archopht.1981.03930010664013. [DOI] [PubMed] [Google Scholar]
- Fine BS, Kwapien RP. Pigment epithelial windows and drusen: an animal model. Invest. Ophthalmol. Vis. Sci. 1978;17(11):1059–1068. [PubMed] [Google Scholar]
- Francis PJ, Appukuttan B, Simmons E, Landauer N, Stoddard J, Hamon S, Ott J, Ferguson B, Klein M, Stout JT, Neuringer M. Rhesus monkeys and humans share common susceptibility genes for age-related macular disease. Hum. Mol. Genet. 2008;17(17):2673–2680. doi: 10.1093/hmg/ddn167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frykman PK, Brown MS, Yamamoto T, Goldstein JL, Herz J. Normal plasma lipoproteins and fertility in gene-targeted mice homozygous for a disruption in the gene encoding very low density lipoprotein receptor. Proc. Nat. Acad. Sci. USA. 1995;92(18):8453–8457. doi: 10.1073/pnas.92.18.8453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Ponce ML, Thill M, Yuan P, Wang NS, Csaky KG. Angiogenesis inhibition and choroidal neovascularization suppression by sustained delivery of an integrin antagonist, EMD478761. Invest. Ophthalmol. Vis. Sci. 2007;48(11):5184–5190. doi: 10.1167/iovs.07-0469. [DOI] [PubMed] [Google Scholar]
- Fujihara M, Bartels E, Nielsen LB, Handa JT. A human apoB100 transgenic mouse expresses human apoB100 in the RPE and develops features of early AMD. Exp. Eye Res. 2009;88(6):1115–1123. doi: 10.1016/j.exer.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerth C. The role of the ERG in the diagnosis and treatment of age-related macular degeneration. Doc. Ophthalmol. Adv. Ophthalmol. 2009;118(1):63–68. doi: 10.1007/s10633-008-9133-x. [DOI] [PubMed] [Google Scholar]
- Giani A, Thanos A, Roh MI, Connolly E, Trichonas G, Kim I, Gragoudas E, Vavvas D, Miller JW. In vivo evaluation of laser-induced choroidal neovascularization using spectral-domain optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 2011;52(6):3880–3887. doi: 10.1167/iovs.10-6266. [DOI] [PubMed] [Google Scholar]
- Giganti M, Beer PM, Lemanski N, Hartman C, Schartman J, Falk N. Adverse events after intravitreal infliximab (Remicade) Retina. 2010;30(1):71–80. doi: 10.1097/IAE.0b013e3181bcef3b. [DOI] [PubMed] [Google Scholar]
- Gold B, Merriam JE, Zernant J, Hancox LS, Taiber AJ, Gehrs K, Cramer K, Neel J, Bergeron J, Barile GR, Smith RT, Hageman GS, Dean M, Allikmets R. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat. Genet. 2006;38(4):458–462. doi: 10.1038/ng1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbaum MH, Weidenthal DT, Krug S, Rosen R. Subretinal neovascularization as a complication of drainage of subretinal fluid. Retina. 1983;3(2):114–117. doi: 10.1097/00006982-198300320-00008. [DOI] [PubMed] [Google Scholar]
- Goldberg MF. Editorial: Bruch's membrane and vascular growth. Invest. Ophthalmol. 1976;15(6):443–446. [PubMed] [Google Scholar]
- Goody RJ, Hu W, Shafiee A, Struharik M, Bartels S, Lopez FJ, Lawrence MS. Optimization of laser-induced choroidal neovascularization in African green monkeys. Exp. Eye Res. 2011;92(6):464–472. doi: 10.1016/j.exer.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Gordiyenko N, Campos M, Lee JW, Fariss RN, Sztein J, Rodriguez IR. RPE cells internalize low-density lipoprotein (LDL) and oxidized LDL (oxLDL) in large quantities in vitro and in vivo. Invest. Ophthalmol. Vis. Sci. 2004;45(8):2822–2829. doi: 10.1167/iovs.04-0074. [DOI] [PubMed] [Google Scholar]
- Gouras P, Ivert L, Landauer N, Mattison JA, Ingram DK, Neuringer M. Drusenoid maculopathy in rhesus monkeys (Macaca mulatta): effects of age and gender. Graefes Arch. Clin. Exp. Ophthalmol. 2008a;246(10):1395–1402. doi: 10.1007/s00417-008-0910-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras P, Ivert L, Mattison JA, Ingram DK, Neuringer M. Drusenoid maculopathy in rhesus monkeys: autofluorescence, lipofuscin and drusen pathogenesis. Graefes Arch. Clin. Exp. Ophthalmol. 2008b;246(10):1403–1411. doi: 10.1007/s00417-008-0911-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras P, Ivert L, Neuringer M, Mattison JA. Topographic and age-related changes of the retinal epithelium and Bruch's membrane of rhesus monkeys. Graefes Arch. Clin. Exp. Ophthalmol. 2010;248(7):973–984. doi: 10.1007/s00417-010-1325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves DT, Jiang Y. Chemokines, a family of chemotactic cytokines. Crit. Rev. Oral Biol. Med. 1995;6(2):109–118. doi: 10.1177/10454411950060020101. [DOI] [PubMed] [Google Scholar]
- Green WR. Histopathology of age-related macular degeneration. Mol. Vis. 1999;5:27. [PubMed] [Google Scholar]
- Green WR, Enger C. Age-related macular degeneration histopathologic studies. The 1992 Lorenz E. Zimmerman Lecture. Ophthalmology. 1993;100(10):1519–1535. doi: 10.1016/s0161-6420(93)31466-1. [DOI] [PubMed] [Google Scholar]
- Grossniklaus HE, Kang SJ, Berglin L. Animal models of choroidal and retinal neovascularization. Prog. Retin. Eye Res. 2010;29(6):500–519. doi: 10.1016/j.preteyeres.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossniklaus HE, Ling JX, Wallace TM, Dithmar S, Lawson DH, Cohen C, Elner VM, Elner SG, Sternberg P., Jr. Macrophage and retinal pigment epithelium expression of angiogenic cytokines in choroidal neovascularization. Mol. Vis. 2002;8:119–126. [PubMed] [Google Scholar]
- Group AS. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch. Ophthalmol. 2001;119(10):1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Meer SG, Miyagi M, Rayborn ME, Hollyfield JG, Crabb JW, Salomon RG. Carboxyethylpyrrole protein adducts and autoantibodies, biomarkers for age-related macular degeneration. J. Biol. Chem. 2003;278(43):42027–42035. doi: 10.1074/jbc.M305460200. [DOI] [PubMed] [Google Scholar]
- Gupta N, Brown KE, Milam AH. Activated microglia in human retinitis pigmentosa, late-onset retinal degeneration, and age-related macular degeneration. Exp. Eye Res. 2003;76(4):463–471. doi: 10.1016/s0014-4835(02)00332-9. [DOI] [PubMed] [Google Scholar]
- Hadziahmetovic M, Dentchev T, Song Y, Haddad N, He X, Hahn P, Pratico D, Wen R, Harris ZL, Lambris JD, Beard J, Dunaief JL. Ceruloplasmin/hephaestin knockout mice model morphologic and molecular features of AMD. Invest. Ophthalmol. Vis. Sci. 2008;49(6):2728–2736. doi: 10.1167/iovs.07-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadziahmetovic M, Song Y, Wolkow N, Iacovelli J, Grieco S, Lee J, Lyubarsky A, Pratico D, Connelly J, Spino M, Harris ZL, Dunaief JL. The Oral Iron Chelator Deferiprone Protects against Iron Overload–Induced Retinal Degeneration. Invest. Ophthalmol. Vis. Sci. 2011;52(2):959–968. doi: 10.1167/iovs.10-6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, Hageman JL, Stockman HA, Borchardt JD, Gehrs KM, Smith RJ, Silvestri G, Russell SR, Klaver CC, Barbazetto I, Chang S, Yannuzzi LA, Barile GR, Merriam JC, Smith RT, Olsh AK, Bergeron J, Zernant J, Merriam JE, Gold B, Dean M, Allikmets R. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc. Nat. Acad. Sci. USA. 2005;102(20):7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn P, Qian Y, Dentchev T, Chen L, Beard J, Harris ZL, Dunaief JL. Disruption of ceruloplasmin and hephaestin in mice causes retinal iron overload and retinal degeneration with features of age-related macular degeneration. Proc. Nat. Acad. Sci. USA. 2004;101(38):13850–13855. doi: 10.1073/pnas.0405146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, Spencer KL, Kwan SY, Noureddine M, Gilbert JR, Schnetz-Boutaud N, Agarwal A, Postel EA, Pericak-Vance MA. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308(5720):419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- Harris ZL, Durley AP, Man TK, Gitlin JD. Targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux. Proc. Nat. Acad. Sci. USA. 1999;96(19):10812–10817. doi: 10.1073/pnas.96.19.10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashizume K, Hirasawa M, Imamura Y, Noda S, Shimizu T, Shinoda K, Kurihara T, Noda K, Ozawa Y, Ishida S, Miyake Y, Shirasawa T, Tsubota K. Retinal dysfunction and progressive retinal cell death in SOD1-deficient mice. Am. J. Pathol. 2008;172(5):1325–1331. doi: 10.2353/ajpath.2008.070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havekes L, de Wit E, Leuven JG, Klasen E, Utermann G, Weber W, Beisiegel U. Apolipoprotein E3-Leiden. A new variant of human apolipoprotein E associated with familial type III hyperlipoproteinemia. Hum. Genet. 1986;73(2):157–163. doi: 10.1007/BF00291607. [DOI] [PubMed] [Google Scholar]
- Hayes KC, Lindsey S, Stephan ZF, Brecker D. Retinal pigment epithelium possesses both LDL and scavenger receptor activity. Invest. Ophthalmol. Vis. Sci. 1989;30(2):225–232. [PubMed] [Google Scholar]
- Heckenlively JR, Hawes NL, Friedlander M, Nusinowitz S, Hurd R, Davisson M, Chang B. Mouse model of subretinal neovascularization with choroidal anastomosis. Retina. 2003;23(4):518–522. doi: 10.1097/00006982-200308000-00012. [DOI] [PubMed] [Google Scholar]
- Higgins GT, Wang JH, Dockery P, Cleary PE, Redmond HP. Induction of angiogenic cytokine expression in cultured RPE by ingestion of oxidized photoreceptor outer segments. Invest. Ophthalmol. Vis. Sci. 2003;44(4):1775–1782. doi: 10.1167/iovs.02-0742. [DOI] [PubMed] [Google Scholar]
- Hollyfield JG, Bonilha VL, Rayborn ME, Yang X, Shadrach KG, Lu L, Ufret RL, Salomon RG, Perez VL. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat. Med. 2008;14(2):194–198. doi: 10.1038/nm1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollyfield JG, Perez VL, Salomon RG. A hapten generated from an oxidation fragment of docosahexaenoic acid is sufficient to initiate age-related macular degeneration. Mol. Neurobiol. 2010;41(2–3):290–298. doi: 10.1007/s12035-010-8110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollyfield JG, Salomon RG, Crabb JW. Proteomic approaches to understanding age-related macular degeneration. Adv. Exp. Med. Biol. 2003;533:83–89. doi: 10.1007/978-1-4615-0067-4_11. [DOI] [PubMed] [Google Scholar]
- Hope GM, Dawson WW, Engel HM, Ulshafer RJ, Kessler MJ, Sherwood MB. A primate model for age related macular drusen. Br. J. Ophthalmol. 1992;76(1):11–16. doi: 10.1136/bjo.76.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houssier M, Raoul W, Lavalette S, Keller N, Guillonneau X, Baragatti B, Jonet L, Jeanny JC, Behar-Cohen F, Coceani F, Scherman D, Lachapelle P, Ong H, Chemtob S, Sennlaub F. CD36 deficiency leads to choroidal involution via COX2 down-regulation in rodents. PLoS Med. 2008;5(2):e39. doi: 10.1371/journal.pmed.0050039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Shen J, Vinores SA. Blockade of VEGFR1 and 2 suppresses pathological angiogenesis and vascular leakage in the eye. PLoS ONE. 2011;6(6):e21411. doi: 10.1371/journal.pone.0021411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain D, Kim I, Gauthier D, Lane AM, Tsilimbaris MK, Ezra E, Connolly EJ, Michaud N, Gragoudas ES, O'Neill CA, Beyer JC, Miller JW. Safety and efficacy of intravitreal injection of ranibizumab in combination with verteporfin PDT on experimental choroidal neovascularization in the monkey. Arch. Ophthalmol. 2005;123(4):509–516. doi: 10.1001/archopht.123.4.509. [DOI] [PubMed] [Google Scholar]
- Hyman L, Schachat AP, He Q, Leske MC. Hypertension, cardiovascular disease, and age-related macular degeneration. Age-Related Macular Degeneration Risk Factors Study Group. Arch. Ophthalmol. 2000;118(3):351–358. doi: 10.1001/archopht.118.3.351. [DOI] [PubMed] [Google Scholar]
- Imamura Y, Noda S, Hashizume K, Shinoda K, Yamaguchi M, Uchiyama S, Shimizu T, Mizushima Y, Shirasawa T, Tsubota K. Drusen, choroidal neovascularization, and retinal pigment epithelium dysfunction in SOD1-deficient mice: a model of age-related macular degeneration. Proc. Nat. Acad. Sci. USA. 2006;103(30):11282–11287. doi: 10.1073/pnas.0602131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi T, Miki K, Sorgente N, Patterson R, Ryan SJ. Effects of intravitreal administration of steroids on experimental subretinal neovascularization in the subhuman primate. Arch. Ophthalmol. 1985;103(5):708–711. doi: 10.1001/archopht.1985.01050050100026. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Sorgente N, Patterson R, Ryan SJ. Pathogenesis of drusen in the primate. Invest. Ophthalmol. Vis. Sci. 1986;27(2):184–193. [PubMed] [Google Scholar]
- Jo YJ, Sonoda KH, Oshima Y, Takeda A, Kohno R, Yamada J, Hamuro J, Yang Y, Notomi S, Hisatomi T, Ishibashi T. Establishment of a new animal model of focal subretinal fibrosis that resembles disciform lesion in advanced age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2011;52(9):6089–6095. doi: 10.1167/iovs.10-5189. [DOI] [PubMed] [Google Scholar]
- Johnson LV, Ozaki S, Staples MK, Erickson PA, Anderson DH. A potential role for immune complex pathogenesis in drusen formation. Exp. Eye Res. 2000;70(4):441–449. doi: 10.1006/exer.1999.0798. [DOI] [PubMed] [Google Scholar]
- Jonas JB, Hayreh SS, Martus P. Influence of arterial hypertension and diet-induced atherosclerosis on macular drusen. Graefes Arch. Clin. Exp. Ophthalmol. 2003;241(2):125–134. doi: 10.1007/s00417-002-0615-3. [DOI] [PubMed] [Google Scholar]
- Julien S, Kreppel F, Beck S, Heiduschka P, Brito V, Schnichels S, Kochanek S, Schraermeyer U. A reproducible and quantifiable model of choroidal neovascularization induced by VEGF A165 after subretinal adenoviral gene transfer in the rabbit. Mol. Vis. 2008;14:1358–1372. [PMC free article] [PubMed] [Google Scholar]
- Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 2000;20(11):4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justilien V, Pang JJ, Renganathan K, Zhan X, Crabb JW, Kim SR, Sparrow JR, Hauswirth WW, Lewin AS. SOD2 knockdown mouse model of early AMD. Invest. Ophthalmol. Vis. Sci. 2007;48(10):4407–4420. doi: 10.1167/iovs.07-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karan G, Lillo C, Yang Z, Cameron DJ, Locke KG, Zhao Y, Thirumalaichary S, Li C, Birch DG, Vollmer-Snarr HR, Williams DS, Zhang K. Lipofuscin accumulation, abnormal electrophysiology, and photoreceptor degeneration in mutant ELOVL4 transgenic mice: a model for macular degeneration. Proc. Nat. Acad. Sci. USA. 2005;102(11):4164–4169. doi: 10.1073/pnas.0407698102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiilgaard JF, Andersen MV, Wiencke AK, Scherfig E, la Cour M, Tezel TH, Prause JU. A new animal model of choroidal neovascularization. Acta Ophthalmol. Scand. 2005;83(6):697–704. doi: 10.1111/j.1600-0420.2005.00566.x. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Toma HS. Inhibition of choroidal neovascularization by intravitreal ketorolac. Arch. Ophthalmol. 2010;128(5):596–600. doi: 10.1001/archophthalmol.2010.69. [DOI] [PubMed] [Google Scholar]
- Kimura K, Isashiki Y, Sonoda S, Kakiuchi-Matsumoto T, Ohba N. Genetic association of manganese superoxide dismutase with exudative age-related macular degeneration. Am. J. Ophthalmol. 2000;130(6):769–773. doi: 10.1016/s0002-9394(00)00552-3. [DOI] [PubMed] [Google Scholar]
- Klaver CC, Kliffen M, van Duijn CM, Hofman A, Cruts M, Grobbee DE, van Broeckhoven C, de Jong PT. Genetic association of apolipoprotein E with age-related macular degeneration. Am. J. Hum. Genet. 1998;63(1):200–206. doi: 10.1086/301901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R, Klein BE, Tomany SC, Cruickshanks KJ. The association of cardiovascular disease with the long-term incidence of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 2003;110(6):1273–1280. doi: 10.1016/S0161-6420(03)00599-2. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BE, Tomany SC, Meuer SM, Huang GH. Ten-year incidence and progression of age-related maculopathy: the Beaver Dam eye study. Ophthalmology. 2002;109(10):1767–1779. doi: 10.1016/s0161-6420(02)01146-6. [DOI] [PubMed] [Google Scholar]
- Klein R, Knudtson MD, Cruickshanks KJ, Klein BE. Further observations on the association between smoking and the long-term incidence and progression of age-related macular degeneration: the Beaver Dam Eye Study. Arch. Ophthalmol. 2008;126(1):115–121. doi: 10.1001/archopht.126.1.115. [DOI] [PubMed] [Google Scholar]
- Klein R, Wang Q, Klein BE, Moss SE, Meuer SM. The relationship of age-related maculopathy, cataract, and glaucoma to visual acuity. Invest. Ophthalmol. Vis. Sci. 1995;36(1):182–191. [PubMed] [Google Scholar]
- Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308(5720):385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin. Cancer Biol. 2005;15(5):378–386. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Kliffen M, Lutgens E, Daemen MJ, de Muinck ED, Mooy CM, de Jong PT. The APO(*)E3-Leiden mouse as an animal model for basal laminar deposit. Br. J. Ophthalmol. 2000;84(12):1415–1419. doi: 10.1136/bjo.84.12.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh HJ, Freeman WR, Azen SP, Flaxel CJ, Labree LD, Cheng L, Wills M, Jones TR. Effect of a novel octapeptide urokinase fragment, A6, on experimental choroidal neovascularization in the monkey. Retina. 2006;26(2):202–209. doi: 10.1097/00006982-200602000-00014. [DOI] [PubMed] [Google Scholar]
- Kolosova NG, Lebedev PA, Aidagulova SV, Morozkova TS. OXYS rats as a model of senile cataract. Bull. Exp. Biol. Med. 2003;136(4):415–419. doi: 10.1023/b:bebm.0000010967.24302.78. [DOI] [PubMed] [Google Scholar]
- Kondo N, Honda S, Kuno S, Negi A. Positive association of common variants in CD36 with neovascular age-related macular degeneration. Aging. 2009;1(2):266–274. doi: 10.18632/aging.100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzystolik MG, Afshari MA, Adamis AP, Gaudreault J, Gragoudas ES, Michaud NA, Li W, Connolly E, O'Neill CA, Miller JW. Prevention of experimental choroidal neovascularization with intravitreal anti-vascular endothelial growth factor antibody fragment. Arch. Ophthalmol. 2002;120(3):338–346. doi: 10.1001/archopht.120.3.338. [DOI] [PubMed] [Google Scholar]
- Kuziel WA, Morgan SJ, Dawson TC, Griffin S, Smithies O, Ley K, Maeda N. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc. Nat. Acad. Sci. USA. 1997;94(22):12053–12058. doi: 10.1073/pnas.94.22.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CC, Wu WC, Chen SL, Xiao X, Tsai TC, Huan SJ, Chen TL, Tsai RJ, Tsao YP. Suppression of choroidal neovascularization by adeno-associated virus vector expressing angiostatin. Invest. Ophthalmol. Vis. Sci. 2001;42(10):2401–2407. [PubMed] [Google Scholar]
- Lai CM, Shen WY, Brankov M, Lai YK, Barnett NL, Lee SY, Yeo IY, Mathur R, Ho JE, Pineda P, Barathi A, Ang CL, Constable IJ, Rakoczy EP. Long-term evaluation of AAV-mediated sFlt-1 gene therapy for ocular neovascularization in mice and monkeys. Molecular Therapy. 2005;12(4):659–668. doi: 10.1016/j.ymthe.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Lambert V, Munaut C, Jost M, Noel A, Werb Z, Foidart JM, Rakic JM. Matrix metalloproteinase-9 contributes to choroidal neovascularization. Am. J. Pathol. 2002;161(4):1247–1253. doi: 10.1016/S0002-9440(10)64401-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassota N, Kiilgaard JF, la Cour M, Scherfig E, Prause JU. Natural history of choroidal neovascularization after surgical induction in an animal model. Acta Ophthalmol. 2008;86(5):495–503. doi: 10.1111/j.1600-0420.2007.01127.x. [DOI] [PubMed] [Google Scholar]
- Lassota N, Kiilgaard JF, Prause JU, Qvortrup K, Scherfig E, la Cour M. Surgical induction of choroidal neovascularization in a porcine model. Graefes Arch. Clin. Exp. Ophthalmol. 2007;245(8):1189–1198. doi: 10.1007/s00417-006-0518-9. [DOI] [PubMed] [Google Scholar]
- Lassota N, Prause JU, Scherfig E, Kiilgaard JF, la Cour M. Clinical and histological findings after intravitreal injection of bevacizumab (Avastin) in a porcine model of choroidal neovascularization. Acta Ophthalmol. 2010;88(3):300–308. doi: 10.1111/j.1755-3768.2008.01439.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berger C, Chan PH, Wallace DC, Epstein CJ. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat. Genet. 1995;11(4):376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- Lichtlen P, Lam TT, Nork TM, Streit T, Urech DM. Relative contribution of VEGF and TNF-alpha in the cynomolgus laser-induced CNV model: comparing the efficacy of bevacizumab, adalimumab, and ESBA105. Invest. Ophthalmol. Vis. Sci. 2010;51(9):4738–4745. doi: 10.1167/iovs.09-4890. [DOI] [PubMed] [Google Scholar]
- Lima e Silva R, Saishin Y, Akiyama H, Kachi S, Aslam S, Rogers B, Deering T, Gong YY, Hackett SF, Lai H, Frydman BJ, Valasinas A, Marton LJ, Campochiaro PA. Suppression and regression of choroidal neovascularization by polyamine analogues. Invest. Ophthalmol. Vis. Sci. 2005;46(9):3323–3330. doi: 10.1167/iovs.04-1210. [DOI] [PubMed] [Google Scholar]
- Lu B, Rutledge BJ, Gu L, Fiorillo J, Lukacs NW, Kunkel SL, North R, Gerard C, Rollins BJ. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J. Exp. Med. 1998;187(4):601–608. doi: 10.1084/jem.187.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F, Adelman RA. Are intravitreal bevacizumab and ranibizumab effective in a rat model of choroidal neovascularization? Graefes Arch. Clin. Exp. Ophthalmol. 2009a;247(2):171–177. doi: 10.1007/s00417-008-0936-y. [DOI] [PubMed] [Google Scholar]
- Lu F, Adelman RA. Are intravitreal bevacizumab and ranibizumab effective in a rat model of choroidal neovascularization? Graefes Arch. Clin. Exp. Ophthalmol. 2009b;247(2):171–177. doi: 10.1007/s00417-008-0936-y. [DOI] [PubMed] [Google Scholar]
- Luhmann UF, Robbie S, Munro PM, Barker SE, Duran Y, Luong V, Fitzke FW, Bainbridge JW, Ali RR, MacLaren RE. The drusenlike phenotype in aging Ccl2-knockout mice is caused by an accelerated accumulation of swollen autofluorescent subretinal macrophages. Invest. Ophthalmol. Vis. Sci. 2009;50(12):5934–5943. doi: 10.1167/iovs.09-3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyzogubov VV, Tytarenko RG, Jha P, Liu J, Bora NS, Bora PS. Role of ocular complement factor H in a murine model of choroidal neovascularization. Am. J. Pathol. 2010;177(4):1870–1880. doi: 10.2353/ajpath.2010.091168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyzogubov VV, Tytarenko RG, Liu J, Bora NS, Bora PS. Polyethylene glycol (PEG)-induced mouse model of choroidal neovascularization. J. Biol. Chem. 2011;286(18):16229–16237. doi: 10.1074/jbc.M110.204701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macular Photocoagulation Study Group Argon laser photocoagulation for neovascular maculopathy. Five-year results from randomized clinical trials. Arch. Ophthalmol. 1991;109(8):1109–1114. [PubMed] [Google Scholar]
- Majji AB, Cao J, Chang KY, Hayashi A, Aggarwal S, Grebe RR, De Juan E., Jr. Age-related retinal pigment epithelium and Bruch's membrane degeneration in senescence-accelerated mouse. Invest. Ophthalmol. Vis. Sci. 2000;41(12):3936–3942. [PubMed] [Google Scholar]
- Malek G, Johnson LV, Mace BE, Saloupis P, Schmechel DE, Rickman DW, Toth CA, Sullivan PM, Bowes Rickman C. Apolipoprotein E allele-dependent pathogenesis: a model for age-related retinal degeneration. Proc. Nat. Acad. Sci. USA. 2005;102(33):11900–11905. doi: 10.1073/pnas.0503015102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek G, Mace B, Saloupis P, Schmechel D, Rickman D, Sullivan P, Rickman CB. Initial observations of key features of age-related macular degeneration in APOE targeted replacement mice. Adv. Exp. Med. Biol. 2006;572:109–117. doi: 10.1007/0-387-32442-9_17. [DOI] [PubMed] [Google Scholar]
- Malinow MR, Feeney-Burns L, Peterson LH, Klein ML, Neuringer M. Diet-related macular anomalies in monkeys. Invest. Ophthalmol. Vis. Sci. 1980;19(8):857–863. [PubMed] [Google Scholar]
- Maller JB, Fagerness JA, Reynolds RC, Neale BM, Daly MJ, Seddon JM. Variation in complement factor 3 is associated with risk of age-related macular degeneration. Nat. Genet. 2007;39(10):1200–1201. doi: 10.1038/ng2131. [DOI] [PubMed] [Google Scholar]
- Mares-Perlman JA, Brady WE, Klein R, VandenLangenberg GM, Klein BE, Palta M. Dietary fat and age-related maculopathy. Arch. Ophthalmol. 1995;113(6):743–748. doi: 10.1001/archopht.1995.01100060069034. [DOI] [PubMed] [Google Scholar]
- Markovets AM, Saprunova VB, Zhdankina AA, Fursova A, Bakeeva LE, Kolosova NG. Alterations of retinal pigment epithelium cause AMD-like retinopathy in senescence-accelerated OXYS rats. Aging. 2011;3(1):44–54. doi: 10.18632/aging.100243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmorstein AD, Marmorstein LY. The challenge of modeling macular degeneration in mice. Trends Genetics. 2007;23(5):225–231. doi: 10.1016/j.tig.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Marmorstein LY, McLaughlin PJ, Peachey NS, Sasaki T, Marmorstein AD. Formation and progression of sub-retinal pigment epithelium deposits in Efemp1 mutation knock-in mice. A model for the early pathogenic course of macular degeneration. Hum. Mol. Genet. 2007;16(20):2423–2432. doi: 10.1093/hmg/ddm199. [DOI] [PubMed] [Google Scholar]
- Marsili S, Salganik RI, Albright CD, Freel CD, Johnsen S, Peiffer RL, Costello MJ. Cataract formation in a strain of rats selected for high oxidative stress. Exp. Eye Res. 2004;79(5):595–612. doi: 10.1016/j.exer.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2011;364(20):1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matapalli MJ, Wawrousek EF, Chan CC, Zhao H, Roychoudhury J, Ferguson TA, Caspi RR. The Rd8 Mutation of the Crb1 Gene is Present in Vendor Lines of C57BL/6N Mice adn Embroyonic Stem Cells, and Confounds Ocular Induced Mutant Phenotypes. Invest. Ophthalmol. Vis. Sci. 2012;53(6):2921–2927. doi: 10.1167/iovs.12-9662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata NL, Weng J, Travis GH. Biosynthesis of a major lipofuscin fluorophore in mice and humans with ABCR-mediated retinal and macular degeneration. Proc. Nat. Acad. Sci. USA. 2000;97(13):7154–7159. doi: 10.1073/pnas.130110497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller H, Miller B, Ishibashi T, Ryan SJ. Pathogenesis of laser-induced choroidal subretinal neovascularization. Invest. Ophthalmol. Vis. Sci. 1990a;31(5):899–908. [PubMed] [Google Scholar]
- Miller H, Miller B, Ishibashi T, Ryan SJ. Pathogenesis of laser-induced choroidal subretinal neovascularization. Invest. Ophthalmol. Vis. Sci. 1990b;31(5):899–908. [PubMed] [Google Scholar]
- Miller JW, Adamis AP, Shima DT, D'Amore PA, Moulton RS, O'Reilly MS, Folkman J, Dvorak HF, Brown LF, Berse B, et al. Vascular endothelial growth factor/vascular permeability factor is temporally and spatially correlated with ocular angiogenesis in a primate model. Am. J. Pathol. 1994a;145(3):574–584. [PMC free article] [PubMed] [Google Scholar]
- Miller JW, Adamis AP, Shima DT, D'Amore PA, Moulton RS, O'Reilly MS, Folkman J, Dvorak HF, Brown LF, Berse B, et al. Vascular endothelial growth factor/vascular permeability factor is temporally and spatially correlated with ocular angiogenesis in a primate model. Am. J. Pathol. 1994b;145(3):574–584. [PMC free article] [PubMed] [Google Scholar]
- Miyajima H, Nishimura Y, Mizoguchi K, Sakamoto M, Shimizu T, Honda N. Familial apoceruloplasmin deficiency associated with blepharospasm and retinal degeneration. Neurology. 1987;37(5):761–767. doi: 10.1212/wnl.37.5.761. [DOI] [PubMed] [Google Scholar]
- Monaco WA, Wormington CM. The rhesus monkey as an animal model for age-related maculopathy. Optometry Vision Sci. 1990;67(7):532–537. doi: 10.1097/00006324-199007000-00011. [DOI] [PubMed] [Google Scholar]
- Montes T, Tortajada A, Morgan BP, Rodriguez de Cordoba S, Harris CL. Functional basis of protection against age-related macular degeneration conferred by a common polymorphism in complement factor B. Proc. Nat. Acad. Sci. USA. 2009;106(11):4366–4371. doi: 10.1073/pnas.0812584106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Gehlbach P, Yamamoto S, Duh E, Zack DJ, Li Q, Berns KI, Raisler BJ, Hauswirth WW, Campochiaro PA. AAV-mediated gene transfer of pigment epithelium-derived factor inhibits choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 2002;43(6):1994–2000. [PubMed] [Google Scholar]
- Morita H, Ikeda S, Yamamoto K, Morita S, Yoshida K, Nomoto S, Kato M, Yanagisawa N. Hereditary ceruloplasmin deficiency with hemosiderosis: a clinicopathological study of a Japanese family. Ann. Neurol. 1995;37(5):646–656. doi: 10.1002/ana.410370515. [DOI] [PubMed] [Google Scholar]
- Mullins RF, Russell SR, Anderson DH, Hageman GS. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000;14(7):835–846. [PubMed] [Google Scholar]
- Murphy PM, Baggiolini M, Charo IF, Hebert CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ, Power CA. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol. Rev. 2000;52(1):145–176. [PubMed] [Google Scholar]
- Neroev VV, Archipova MM, Bakeeva LE, Fursova A, Grigorian EN, Grishanova AY, Iomdina EN, Ivashchenko Zh N, Katargina LA, Khoroshilova-Maslova IP, Kilina OV, Kolosova NG, Kopenkin EP, Korshunov SS, Kovaleva NA, Novikova YP, Philippov PP, Pilipenko DI, Robustova OV, Saprunova VB, Senin II, Skulachev MV, Sotnikova LF, Stefanova NA, Tikhomirova NK, Tsapenko IV, Shchipanova AI, Zinovkin RA, Skulachev VP. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 4. Age-related eye disease. SkQ1 returns vision to blind animals. Biochemistry. 2008;73(12):1317–1328. doi: 10.1134/s0006297908120043. [DOI] [PubMed] [Google Scholar]
- Neuringer M, Francis PJ, Renner L, Weiss A, Jeffrey BG. Atrophic macular degeneration in rhesus monkeys deficient in xanthophylls and n-3 fatty acids. Association for Research in Vision and Ophthalmology. 2010 [Google Scholar]
- Ni M, Holland M, Jarstadmarken H, De Vries G. Time-course of experimental choroidal neovascularization in Dutch-Belted rabbit: clinical and histological evaluation. Exp. Eye Res. 2005;81(3):286–297. doi: 10.1016/j.exer.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Nicolas MG, Fujiki K, Murayama K, Suzuki MT, Mineki R, Hayakawa M, Yoshikawa Y, Cho F, Kanai A. Studies on the mechanism of early onset macular degeneration in cynomolgus (Macaca fascicularis) monkeys. I. Abnormal concentrations of two proteins in the retina. Exp. Eye Res. 1996;62(3):211–219. doi: 10.1006/exer.1996.0026. [DOI] [PubMed] [Google Scholar]
- Nowak M, Swietochowska E, Wielkoszynski T, Marek B, Karpe J, Gorski J, Glogowska-Szelag J, Kos-Kudla B, Ostrowska Z. Changes in blood antioxidants and several lipid peroxidation products in women with age-related macular degeneration. Eur. J. Ophthalmol. 2003;13(3):281–286. doi: 10.1177/112067210301300307. [DOI] [PubMed] [Google Scholar]
- Nozaki M, Raisler BJ, Sakurai E, Sarma JV, Barnum SR, Lambris JD, Chen Y, Zhang K, Ambati BK, Baffi JZ, Ambati J. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc. Nat. Acad. Sci. USA. 2006a;103(7):2328–2333. doi: 10.1073/pnas.0408835103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki M, Raisler BJ, Sakurai E, Sarma JV, Barnum SR, Lambris JD, Chen Y, Zhang K, Ambati BK, Baffi JZ, Ambati J. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc. Natl. Acad. Sci. USA. 2006b;103(7):2328–2333. doi: 10.1073/pnas.0408835103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata N, Matsushima M, Takada Y, Tobe T, Takahashi K, Yi X, Yamamoto C, Yamada H, Uyama M. Expression of basic fibroblast growth factor mRNA in developing choroidal neovascularization. Curr. Eye Res. 1996;15(10):1008–1018. doi: 10.3109/02713689609017649. [DOI] [PubMed] [Google Scholar]
- Ogata N, Ohkuma H, Kanai K, Nango K, Takada Y, Uyama M. Histological changes in the retinal pigment epithelium and Bruch's membrane in senescence accelerated mouse. Nihon Ganka Gakkai zasshi. 1992;96(2):180–189. [PubMed] [Google Scholar]
- Oh H, Takagi H, Takagi C, Suzuma K, Otani A, Ishida K, Matsumura M, Ogura Y, Honda Y. The potential angiogenic role of macrophages in the formation of choroidal neovascular membranes. Invest. Ophthalmol. Vis. Sci. 1999;40(9):1891–1898. [PubMed] [Google Scholar]
- Ohno-Matsui K, Hirose A, Yamamoto S, Saikia J, Okamoto N, Gehlbach P, Duh EJ, Hackett S, Chang M, Bok D, Zack DJ, Campochiaro PA. Inducible expression of vascular endothelial growth factor in adult mice causes severe proliferative retinopathy and retinal detachment. Am. J. Pathol. 2002;160(2):711–719. doi: 10.1016/S0002-9440(10)64891-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olin KL, Morse LS, Murphy C, Paul-Murphy J, Line S, Bellhorn RW, Hjelmeland LM, Keen CL. Trace element status and free radical defense in elderly rhesus macaques (Macaca mulatta) with macular drusen. Proc. Soc. Exp. Biol. Med. 1995;208(4):370–377. doi: 10.3181/00379727-208-43864. [DOI] [PubMed] [Google Scholar]
- Olson JL, Courtney RJ, Mandava N. Intravitreal infliximab and choroidal neovascularization in an animal model. Arch. Ophthalmol. 2007;125(9):1221–1224. doi: 10.1001/archopht.125.9.1221. [DOI] [PubMed] [Google Scholar]
- Olson JL, Courtney RJ, Rouhani B, Mandava N, Dinarello CA. Intravitreal anakinra inhibits choroidal neovascular membrane growth in a rat model. Ocul. Immunol. Inflamm. 2009;17(3):195–200. doi: 10.1080/09273940802710705. [DOI] [PubMed] [Google Scholar]
- Oshima Y, Oshima S, Nambu H, Kachi S, Hackett SF, Melia M, Kaleko M, Connelly S, Esumi N, Zack DJ, Campochiaro PA. Increased expression of VEGF in retinal pigmented epithelial cells is not sufficient to cause choroidal neovascularization. J. Cell. Physiol. 2004;201(3):393–400. doi: 10.1002/jcp.20110. [DOI] [PubMed] [Google Scholar]
- Pan CK, Durairaj C, Kompella UB, Agwu O, Oliver SC, Quiroz-Mercado H, Mandava N, Olson JL. Comparison of long-acting bevacizumab formulations in the treatment of choroidal neovascularization in a rat model. J. Ocul. Pharmacol. Ther. 2011;27(3):219–224. doi: 10.1089/jop.2010.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passaniti A, Taylor RM, Pili R, Guo Y, Long PV, Haney JA, Pauly RR, Grant DS, Martin GR. A simple, quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab. Invest. 1992;67(4):519–528. [PubMed] [Google Scholar]
- Patel BN, Dunn RJ, Jeong SY, Zhu Q, Julien JP, David S. Ceruloplasmin regulates iron levels in the CNS and prevents free radical injury. J. Neurosci. 2002;22(15):6578–6586. doi: 10.1523/JNEUROSCI.22-15-06578.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauleikhoff D, Harper CA, Marshall J, Bird AC. Aging changes in Bruch's membrane. A histochemical and morphologic study. Ophthalmology. 1990;97(2):171–178. [PubMed] [Google Scholar]
- Peden MC, Min J, Meyers C, Lukowski Z, Li Q, Boye SL, Levine M, Hauswirth WW, Ratnakaram R, Dawson W, Smith WC, Sherwood MB. Ab-externo AAV-mediated gene delivery to the suprachoroidal space using a 250 micron flexible microcatheter. PLoS ONE. 2011;6(2):e17140. doi: 10.1371/journal.pone.0017140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard E, Houssier M, Bujold K, Sapieha P, Lubell W, Dorfman A, Racine J, Hardy P, Febbraio M, Lachapelle P, Ong H, Sennlaub F, Chemtob S. CD36 plays an important role in the clearance of oxLDL and associated age-dependent sub-retinal deposits. Aging. 2010;2(12):981–989. doi: 10.18632/aging.100218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering MC, Cook HT. Translational mini-review series on complement factor H: renal diseases associated with complement factor H: novel insights from humans and animals. Clin. Exp. Immunol. 2008;151(2):210–230. doi: 10.1111/j.1365-2249.2007.03574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering MC, Cook HT, Warren J, Bygrave AE, Moss J, Walport MJ, Botto M. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat. Genet. 2002;31(4):424–428. doi: 10.1038/ng912. [DOI] [PubMed] [Google Scholar]
- Plump AS, Smith JD, Hayek T, Aalto-Setala K, Walsh A, Verstuyft JG, Rubin EM, Breslow JL. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71(2):343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- Pons M, Marin-Castano ME. Cigarette smoke-related hydroquinone dysregulates MCP-1, VEGF and PEDF expression in retinal pigment epithelium in vitro and in vivo. PLoS ONE. 2011;6(2):e16722. doi: 10.1371/journal.pone.0016722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu G, Stewart JM, Sadda S, Freda R, Lee S, Guven D, de Juan E, Varner SE., Jr. A new model of experimental subretinal neovascularization in the rabbit. Exp. Eye Res. 2006;83(1):141–152. doi: 10.1016/j.exer.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Rakoczy EP, Yu MJ, Nusinowitz S, Chang B, Heckenlively JR. Mouse models of age-related macular degeneration. Exp. Eye Res. 2006;82(5):741–752. doi: 10.1016/j.exer.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Rakoczy PE, Baines M, Kennedy CJ, Constable IJ. Correlation between autofluorescent debris accumulation and the presence of partially processed forms of cathepsin D in cultured retinal pigment epithelial cells challenged with rod outer segments. Exp. Eye Res. 1996;63(2):159–167. doi: 10.1006/exer.1996.0104. [DOI] [PubMed] [Google Scholar]
- Rakoczy PE, Zhang D, Robertson T, Barnett NL, Papadimitriou J, Constable IJ, Lai CM. Progressive age-related changes similar to age-related macular degeneration in a transgenic mouse model. Am. J. Pathol. 2002;161(4):1515–1524. doi: 10.1016/S0002-9440(10)64427-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramkumar HL, Zhang J, Chan CC. Retinal ultrastructure of murine models of dry age-related macular degeneration (AMD) Prog. Retin. Eye Res. 2010;29(3):169–190. doi: 10.1016/j.preteyeres.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raoul W, Auvynet C, Camelo S, Guillonneau X, Feumi C, Combadiere C, Sennlaub F. CCL2/CCR2 and CX3CL1/CX3CR1 chemokine axes and their possible involvement in age-related macular degeneration. J. Neuroinflamm. 2010;7:87. doi: 10.1186/1742-2094-7-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan CM, de Grip WJ, Daemen FJ, Bonting SL. Degradation of rhodopsin by a lysosomal fraction of retinal pigment epithelium: biochemical aspects of the visual process XLI. Exp. Eye Res. 1980;30(2):183–191. doi: 10.1016/0014-4835(80)90112-8. [DOI] [PubMed] [Google Scholar]
- Reich SJ, Fosnot J, Kuroki A, Tang W, Yang X, Maguire AM, Bennett J, Tolentino MJ. Small interfering RNA (siRNA) targeting VEGF effectively inhibits ocular neovascularization in a mouse model. Mol. Vis. 2003;9:210–216. [PubMed] [Google Scholar]
- Rennel ES, Regula JT, Harper SJ, Thomas M, Klein C, Bates DO. A human neutralizing antibody specific to Ang-2 inhibits ocular angiogenesis. Microcirculation. 2011;18(7):598–607. doi: 10.1111/j.1549-8719.2011.00120.x. [DOI] [PubMed] [Google Scholar]
- Reynolds R, Hartnett ME, Atkinson JP, Giclas PC, Rosner B, Seddon JM. Plasma complement components and activation fragments: associations with age-related macular degeneration genotypes and phenotypes. Invest. Ophthalmol. Vis. Sci. 2009;50(12):5818–5827. doi: 10.1167/iovs.09-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RJ, Zhou M, Shen D, Fariss RN, Ding X, Bojanowski CM, Tuo J, Chan CC. Immunological protein expression profile in Ccl2/Cx3cr1 deficient mice with lesions similar to age-related macular degeneration. Exp. Eye Res. 2008;86(4):675–683. doi: 10.1016/j.exer.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolf M, Ivandic B, Winkler J, Schmidt-Erfurth U. Accumulation of lipid particles in Bruch's membrane of LDL receptor knockout mice as a model of age-related macular degeneration. Der Ophthalmol. 2004;101(7):715–719. doi: 10.1007/s00347-003-0942-8. [DOI] [PubMed] [Google Scholar]
- Rudolf M, Winkler B, Aherrahou Z, Doehring LC, Kaczmarek P, Schmidt-Erfurth U. Increased expression of vascular endothelial growth factor associated with accumulation of lipids in Bruch's membrane of LDL receptor knockout mice. Br. J. Ophthalmol. 2005;89(12):1627–1630. doi: 10.1136/bjo.2005.071183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SJ. The development of an experimental model of subretinal neovascularization in disciform macular degeneration. Trans. Am. Ophthalmol. Soc. 1979a;77:707–745. [PMC free article] [PubMed] [Google Scholar]
- Ryan SJ. The development of an experimental model of subretinal neovascularization in disciform macular degeneration. Trans. Am. Ophthalmol. Soc. 1979b;77:707–745. [PMC free article] [PubMed] [Google Scholar]
- Ryan SJ. Subretinal neovascularization. Natural history of an experimental model. Arch. Ophthalmol. 1982;100(11):1804–1809. doi: 10.1001/archopht.1982.01030040784015. [DOI] [PubMed] [Google Scholar]
- Ryeom SW, Silverstein RL, Scotto A, Sparrow JR. Binding of anionic phospholipids to retinal pigment epithelium may be mediated by the scavenger receptor CD36. J. Biol. Chem. 1996;271(34):20536–20539. doi: 10.1074/jbc.271.34.20536. [DOI] [PubMed] [Google Scholar]
- Saishin Y, Takahashi K, Lima e Silva R, Hylton D, Rudge JS, Wiegand SJ, Campochiaro PA. VEGF-TRAP(R1R2) suppresses choroidal neovascularization and VEGF-induced breakdown of the blood-retinal barrier. J. Cell. Physiol. 2003;195(2):241–248. doi: 10.1002/jcp.10246. [DOI] [PubMed] [Google Scholar]
- Sakurai E, Anand A, Ambati BK, van Rooijen N, Ambati J. Macrophage depletion inhibits experimental choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 2003;44(8):3578–3585. doi: 10.1167/iovs.03-0097. [DOI] [PubMed] [Google Scholar]
- Salganik RI, Shabalina IG, Solovyova NA, Kolosova NG, Solovyov VN, Kolpakov AR. Impairment of respiratory functions in mitochondria of rats with an inherited hyperproduction of free radicals. Biochem. Biophys. Res. Commun. 1994a;205(1):180–185. doi: 10.1006/bbrc.1994.2647. [DOI] [PubMed] [Google Scholar]
- Salganik RI, Solovyova NA, Dikalov SI, Grishaeva ON, Semenova LA, Popovsky AV. Inherited enhancement of hydroxyl radical generation and lipid peroxidation in the S strain rats results in DNA rearrangements, degenerative diseases, and premature aging. Biochem. Biophys. Res. Commun. 1994b;199(2):726–733. doi: 10.1006/bbrc.1994.1289. [DOI] [PubMed] [Google Scholar]
- Sallo FB, Bereczki E, Csont T, Luthert PJ, Munro P, Ferdinandy P, Santha M, Lengyel I. Bruch's membrane changes in transgenic mice overexpressing the human biglycan and apolipoprotein b-100 genes. Exp. Eye Res. 2009;89(2):178–186. doi: 10.1016/j.exer.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Sanchez I, Martin R, Ussa F, Fernandez-Bueno I. The parameters of the porcine eyeball. Graefes Arch. Clin. Exp. Ophthalmol. 2011;249(4):475–482. doi: 10.1007/s00417-011-1617-9. [DOI] [PubMed] [Google Scholar]
- Sandbach JM, Coscun PE, Grossniklaus HE, Kokoszka JE, Newman NJ, Wallace DC. Ocular pathology in mitochondrial superoxide dismutase (Sod2)-deficient mice. Invest. Ophthalmol. Vis. Sci. 2001;42(10):2173–2178. [PubMed] [Google Scholar]
- SanGiovanni JP, Chew EY. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog. Retin. Eye Res. 2005;24(1):87–138. doi: 10.1016/j.preteyeres.2004.06.002. [DOI] [PubMed] [Google Scholar]
- SanGiovanni JP, Chew EY, Clemons TE, Davis MD, Ferris 3rd FL, Gensler GR, Kurinij N, Lindblad AS, Milton RC, Seddon JM, Sperduto RD. The relationship of dietary lipid intake and age-related macular degeneration in a case-control study: AREDS Report No. 20. Arch. Ophthalmol. 2007;125(5):671–679. doi: 10.1001/archopht.125.5.671. [DOI] [PubMed] [Google Scholar]
- Sarks SH. Ageing and degeneration in the macular region: a clinico-pathological study. Br. J. Ophthalmol. 1976;60(5):324–341. doi: 10.1136/bjo.60.5.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmack I, Berglin L, Nie X, Wen J, Kang SJ, Marcus AI, Yang H, Lynn MJ, Kapp JA, Grossniklaus HE. Modulation of choroidal neovascularization by subretinal injection of retinal pigment epithelium and polystyrene microbeads. Mol. Vis. 2009;15:146–161. [PMC free article] [PubMed] [Google Scholar]
- Schmidt S, Klaver C, Saunders A, Postel E, De La Paz M, Agarwal A, Small K, Udar N, Ong J, Chalukya M, Nesburn A, Kenney C, Domurath R, Hogan M, Mah T, Conley Y, Ferrell R, Weeks D, de Jong PT, van Duijn C, Haines J, Pericak-Vance M, Gorin M. A pooled case-control study of the apolipoprotein E (APOE) gene in age-related maculopathy. Ophthalmic Genet. 2002;23(4):209–223. doi: 10.1076/opge.23.4.209.13883. [DOI] [PubMed] [Google Scholar]
- Schwesinger C, Yee C, Rohan RM, Joussen AM, Fernandez A, Meyer TN, Poulaki V, Ma JJ, Redmond TM, Liu S, Adamis AP, D'Amato RJ. Intrachoroidal neovascularization in transgenic mice overexpressing vascular endothelial growth factor in the retinal pigment epithelium. Am. J. Pathol. 2001;158(3):1161–1172. doi: 10.1016/S0002-9440(10)64063-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon JM, Cote J, Rosner B. Progression of age-related macular degeneration: association with dietary fat, transunsaturated fat, nuts, and fish intake. Arch. Ophthalmol. 2003;121(12):1728–1737. doi: 10.1001/archopht.121.12.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon JM, Willett WC, Speizer FE, Hankinson SE. A prospective study of cigarette smoking and age-related macular degeneration in women. JAMA, J. Am. Med. Assoc. 1996;276(14):1141–1146. [PubMed] [Google Scholar]
- Semkova I, Peters S, Welsandt G, Janicki H, Jordan J, Schraermeyer U. Investigation of laser-induced choroidal neovascularization in the rat. Invest. Ophthalmol. Vis. Sci. 2003;44(12):5349–5354. doi: 10.1167/iovs.02-0732. [DOI] [PubMed] [Google Scholar]
- Seo MS, Kwak N, Ozaki H, Yamada H, Okamoto N, Yamada E, Fabbro D, Hofmann F, Wood JM, Campochiaro PA. Dramatic inhibition of retinal and choroidal neovascularization by oral administration of a kinase inhibitor. Am. J. Pathol. 1999;154(6):1743–1753. doi: 10.1016/S0002-9440(10)65430-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen D, Wen R, Tuo J, Bojanowski CM, Chan CC. Exacerbation of retinal degeneration and choroidal neovascularization induced by subretinal injection of Matrigel in CCL2/MCP-1-deficient mice. Ophthalmic Res. 2006;38(2):71–73. doi: 10.1159/000090266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen WY, Garrett KL, Wang CG, Zhang K, Ma ZZ, Constable IJ, Rakoczy PE. Preclinical evaluation of a phosphorothioate oligonucleotide in the retina of rhesus monkey. Lab. Invest. 2002;82(2):167–182. doi: 10.1038/labinvest.3780409. [DOI] [PubMed] [Google Scholar]
- Shen WY, Lee SY, Yeo I, Lai CM, Mathur R, Tan D, Constable IJ, Rakoczy PE. Predilection of the macular region to high incidence of choroidal neovascularization after intense laser photocoagulation in the monkey. Arch. Ophthalmol. 2004;122(3):353–360. doi: 10.1001/archopht.122.3.353. [DOI] [PubMed] [Google Scholar]
- Shen WY, Yu MJ, Barry CJ, Constable IJ, Rakoczy PE. Expression of cell adhesion molecules and vascular endothelial growth factor in experimental choroidal neovascularisation in the rat. Br. J. Ophthalmol. 1998;82(9):1063–1071. doi: 10.1136/bjo.82.9.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheraidah G, Steinmetz R, Maguire J, Pauleikhoff D, Marshall J, Bird AC. Correlation between lipids extracted from Bruch's membrane and age. Ophthalmology. 1993;100(1):47–51. doi: 10.1016/s0161-6420(13)31712-6. [DOI] [PubMed] [Google Scholar]
- Silverman MD, Zamora DO, Pan Y, Texeira PV, Baek SH, Planck SR, Rosenbaum JT. Constitutive and inflammatory mediator-regulated fractalkine expression in human ocular tissues and cultured cells. Invest. Ophthalmol. Vis. Sci. 2003;44(4):1608–1615. doi: 10.1167/iovs.02-0233. [DOI] [PubMed] [Google Scholar]
- Simonelli F, Margaglione M, Testa F, Cappucci G, Manitto MP, Brancato R, Rinaldi E. Apolipoprotein E polymorphisms in age-related macular degeneration in an Italian population. Ophthalmic Res. 2001;33(6):325–328. doi: 10.1159/000055688. [DOI] [PubMed] [Google Scholar]
- Singh KK, Krawczak M, Dawson WW, Schmidtke J. Association of HTRA1 and ARMS2 gene variation with drusen formation in rhesus macaques. Exp. Eye Res. 2009;88(3):479–482. doi: 10.1016/j.exer.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Smith CJ, Hansch C. The relative toxicity of compounds in mainstream cigarette smoke condensate. Food Chem. Toxicol. 2000;38(7):637–646. doi: 10.1016/s0278-6915(00)00051-x. [DOI] [PubMed] [Google Scholar]
- Smith W, Assink J, Klein R, Mitchell P, Klaver CC, Klein BE, Hofman A, Jensen S, Wang JJ, de Jong PT. Risk factors for age-related macular degeneration: pooled findings from three continents. Ophthalmology. 2001;108(4):697–704. doi: 10.1016/s0161-6420(00)00580-7. [DOI] [PubMed] [Google Scholar]
- Souied EH, Sales MJ, Soubrane G, Coscas G, Bigorie B, Kaplan J, Munnich A, Rotig A. Macular dystrophy, diabetes, and deafness associated with a large mitochondrial DNA deletion. Am. J. Ophthalmol. 1998;125(1):100–103. doi: 10.1016/s0002-9394(99)80243-8. [DOI] [PubMed] [Google Scholar]
- Spaide RF, Ho-Spaide WC, Browne RW, Armstrong D. Characterization of peroxidized lipids in Bruch's membrane. Retina. 1999;19(2):141–147. doi: 10.1097/00006982-199902000-00010. [DOI] [PubMed] [Google Scholar]
- Sparrow JR, Cai B, Fishkin N, Jang YP, Krane S, Vollmer HR, Zhou J, Nakanishi K. A2E, a fluorophore of RPE lipofuscin: can it cause RPE degeneration? Adv. Exp. Med. Biol. 2003;533:205–211. doi: 10.1007/978-1-4615-0067-4_26. [DOI] [PubMed] [Google Scholar]
- Spilsbury K, Garrett KL, Shen WY, Constable IJ, Rakoczy PE. Overexpression of vascular endothelial growth factor (VEGF) in the retinal pigment epithelium leads to the development of choroidal neovascularization. Am. J. Pathol. 2000;157(1):135–144. doi: 10.1016/S0002-9440(10)64525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford TJ, Anness SH, Fine BS. Spontaneous degenerative maculopathy in the monkey. Ophthalmology. 1984;91(5):513–521. doi: 10.1016/s0161-6420(84)34275-0. [DOI] [PubMed] [Google Scholar]
- Sullivan PM, Mezdour H, Aratani Y, Knouff C, Najib J, Reddick RL, Quarfordt SH, Maeda N. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J. Biol. Chem. 1997;272(29):17972–17980. doi: 10.1074/jbc.272.29.17972. [DOI] [PubMed] [Google Scholar]
- Sunness JS, Johnson MA, Massof RW, Marcus S. Retinal sensitivity over drusen and nondrusen areas. A study using fundus perimetry. Arch. Ophthalmol. 1988;106(8):1081–1084. doi: 10.1001/archopht.1988.01060140237032. [DOI] [PubMed] [Google Scholar]
- Sunness JS, Massof RW, Johnson MA, Finkelstein D, Fine SL. Peripheral retinal function in age-related macular degeneration. Arch. Ophthalmol. 1985;103(6):811–816. doi: 10.1001/archopht.1985.01050060071029. [DOI] [PubMed] [Google Scholar]
- Suzuki MT, Terao K, Yoshikawa Y. Familial early onset macular degeneration in cynomolgus monkeys (Macaca fascicularis) Primates; J. Primatol. 2003;44(3):291–294. doi: 10.1007/s10329-002-0016-6. [DOI] [PubMed] [Google Scholar]
- Treatment of age-related macular degeneration with photodynamic therapy (TAP) Study Group Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials–TAP report. Arch. Ophthalmol. 1999;117(10):1329–1345. [PubMed] [Google Scholar]
- Takada Y, Ogata N, Ohkuma H, Uyama M. Age-related changes in Bruch's membrane of the senescence accelerated mouse. Nihon Ganka Gakkai zasshi. 1993;97(5):595–601. [PubMed] [Google Scholar]
- Takada Y, Uyama M, Ohkuma H, Ogata N, Matsushima M, Deguchi J, Sugasawa K. Immunohistological study in Bruch's membrane of senescence accelerated mouse. Nihon Ganka Gakkai zasshi. 1994;98(10):955–961. [PubMed] [Google Scholar]
- Takahashi H, Obata R, Tamaki Y. A novel vascular endothelial growth factor receptor 2 inhibitor, SU11248, suppresses choroidal neovascularization in vivo. J. Ocul. Pharmacol. Ther. 2006;22(4):213–218. doi: 10.1089/jop.2006.22.213. [DOI] [PubMed] [Google Scholar]
- Takeda T, Hosokawa M, Higuchi K. Senescence-accelerated mouse (SAM): a novel murine model of senescence. Exp. Gerontol. 1997;32(1–2):105–109. doi: 10.1016/s0531-5565(96)00036-8. [DOI] [PubMed] [Google Scholar]
- Takeda T, Hosokawa M, Higuchi K, Hosono M, Akiguchi I, Katoh H. A novel murine model of aging, Senescence-Accelerated Mouse (SAM) Arch. Gerontol. Geriatr. 1994;19(2):185–192. doi: 10.1016/0167-4943(94)90039-6. [DOI] [PubMed] [Google Scholar]
- Tamai K, Spaide RF, Ellis EA, Iwabuchi S, Ogura Y, Armstrong D. Lipid hydroperoxide stimulates subretinal choroidal neovascularization in the rabbit. Exp. Eye Res. 2002;74(2):301–308. doi: 10.1006/exer.2001.1121. [DOI] [PubMed] [Google Scholar]
- Thakkinstian A, Han P, McEvoy M, Smith W, Hoh J, Magnusson K, Zhang K, Attia J. Systematic review and meta-analysis of the association between complement factor H Y402H polymorphisms and age-related macular degeneration. Hum. Mol. Genet. 2006;15(18):2784–2790. doi: 10.1093/hmg/ddl220. [DOI] [PubMed] [Google Scholar]
- Theodossiadis PG, Liarakos VS, Sfikakis PP, Vergados IA, Theodossiadis GP. Intravitreal administration of the anti-tumor necrosis factor agent infliximab for neovascular age-related macular degeneration. Am. J. Ophthalmol. 2009;147(5):825–830. 830, e821. doi: 10.1016/j.ajo.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Thornton J, Edwards R, Mitchell P, Harrison RA, Buchan I, Kelly SP. Smoking and age-related macular degeneration: a review of association. Eye (Lond) 2005;19(9):935–944. doi: 10.1038/sj.eye.6701978. [DOI] [PubMed] [Google Scholar]
- Tiebel O, Oka K, Robinson K, Sullivan M, Martinez J, Nakamuta M, Ishimura-Oka K, Chan L. Mouse very low-density lipoprotein receptor (VLDLR): gene structure, tissue-specific expression and dietary and developmental regulation. Atherosclerosis. 1999;145(2):239–251. doi: 10.1016/s0021-9150(99)00068-4. [DOI] [PubMed] [Google Scholar]
- Tobe T, Ortega S, Luna JD, Ozaki H, Okamoto N, Derevjanik NL, Vinores SA, Basilico C, Campochiaro PA. Targeted disruption of the FGF2 gene does not prevent choroidal neovascularization in a murine model. Am. J. Pathol. 1998;153(5):1641–1646. doi: 10.1016/S0002-9440(10)65753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolentino MJ, Brucker AJ, Fosnot J, Ying GS, Wu IH, Malik G, Wan S, Reich SJ. Intravitreal injection of vascular endothelial growth factor small interfering RNA inhibits growth and leakage in a nonhuman primate, laser-induced model of choroidal neovascularization. Retina. 2004a;24(4):660. doi: 10.1097/00006982-200408000-00039. [DOI] [PubMed] [Google Scholar]
- Tolentino MJ, Brucker AJ, Wan S, Reich SJ, Gordon J, Duh YJ. Re: intravitreal injection of vascular endothelial growth factor small interfering RNA inhibits growth and leakage in a nonhuman primate, laser-induced model of choroidal neovascularization. Retina. 2004b;24(4):661. doi: 10.1097/00006982-200408000-00040. [DOI] [PubMed] [Google Scholar]
- Tomany SC, Wang JJ, Van Leeuwen R, Klein R, Mitchell P, Vingerling JR, Klein BE, Smith W, De Jong PT. Risk factors for incident age-related macular degeneration: pooled findings from 3 continents. Ophthalmology. 2004;111(7):1280–1287. doi: 10.1016/j.ophtha.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Tuo J, Bojanowski CM, Zhou M, Shen D, Ross RJ, Rosenberg KI, Cameron DJ, Yin C, Kowalak JA, Zhuang Z, Zhang K, Chan CC. Murine ccl2/cx3cr1 deficiency results in retinal lesions mimicking human age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2007;48(8):3827–3836. doi: 10.1167/iovs.07-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuo J, Smith BC, Bojanowski CM, Meleth AD, Gery I, Csaky KG, Chew EY, Chan CC. The involvement of sequence variation and expression of CX3CR1 in the pathogenesis of age-related macular degeneration. FASEB J. 2004;18(11):1297–1299. doi: 10.1096/fj.04-1862fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiki T, Weikel KA, Jiao W, Shang F, Caceres A, Pawlak D, Handa JT, Brownlee M, Nagaraj R, Taylor A. Glycation-altered proteolysis as a pathobiologic mechanism that links dietary glycemic index, aging, and age-related disease (in nondiabetics) Aging cell. 2011 doi: 10.1111/j.1474-9726.2011.00752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ufret-Vincenty RL, Aredo B, Liu X, McMahon A, Chen PW, Sun H, Niederkorn JY, Kedzierski W. Transgenic mice expressing variants of complement factor H develop AMD-like retinal findings. Invest. Ophthalmol. Vis. Sci. 2010;51(11):5878–5887. doi: 10.1167/iovs.09-4457. [DOI] [PubMed] [Google Scholar]
- Ulshafer RJ, Engel HM, Dawson WW, Allen CB, Kessler MJ. Macular degeneration in a community of rhesus monkeys. Ultrastructural observations. Retina. 1987;7(3):198–203. doi: 10.1097/00006982-198700730-00011. [DOI] [PubMed] [Google Scholar]
- Umeda S, Ayyagari R, Allikmets R, Suzuki MT, Karoukis AJ, Ambasudhan R, Zernant J, Okamoto H, Ono F, Terao K, Mizota A, Yoshikawa Y, Tanaka Y, Iwata T. Early-onset macular degeneration with drusen in a cynomolgus monkey (Macaca fascicularis) pedigree: exclusion of 13 candidate genes and loci. Invest. Ophthalmol. Vis. Sci. 2005a;46(2):683–691. doi: 10.1167/iovs.04-1031. [DOI] [PubMed] [Google Scholar]
- Umeda S, Suzuki MT, Okamoto H, Ono F, Mizota A, Terao K, Yoshikawa Y, Tanaka Y, Iwata T. Molecular composition of drusen and possible involvement of anti-retinal autoimmunity in two different forms of macular degeneration in cynomolgus monkey (Macaca fascicularis) FASEB J. 2005b;19(12):1683–1685. doi: 10.1096/fj.04-3525fje. [DOI] [PubMed] [Google Scholar]
- van den Maagdenberg AM, Hofker MH, Krimpenfort PJ, de Bruijn I, van Vlijmen B, van der Boom H, Havekes LM, Frants RR. Transgenic mice carrying the apolipoprotein E3-Leiden gene exhibit hyperlipoproteinemia. J. Biol. Chem. 1993;268(14):10540–10545. [PubMed] [Google Scholar]
- van der Schaft TL, Mooy CM, de Bruijn WC, de Jong PT. Early stages of age-related macular degeneration: an immunofluorescence and electron microscopy study. Br. J. Ophthalmol. 1993;77(10):657–661. doi: 10.1136/bjo.77.10.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen R, Ikram MK, Vingerling JR, Witteman JC, Hofman A, de Jong PT. Blood pressure, atherosclerosis, and the incidence of age-related maculopathy: the Rotterdam Study. Invest. Ophthalmol. Vis. Sci. 2003;44(9):3771–3777. doi: 10.1167/iovs.03-0121. [DOI] [PubMed] [Google Scholar]
- Vingerling JR, Hofman A, Grobbee DE, de Jong PT. Age-related macular degeneration and smoking. The Rotterdam Study. Arch. Ophthalmol. 1996;114(10):1193–1196. doi: 10.1001/archopht.1996.01100140393005. [DOI] [PubMed] [Google Scholar]
- Vulpe CD, Kuo YM, Murphy TL, Cowley L, Askwith C, Libina N, Gitschier J, Anderson GJ. Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. Nat. Genet. 1999;21(2):195–199. doi: 10.1038/5979. [DOI] [PubMed] [Google Scholar]
- Wada M, Ogata N, Otsuji T, Uyama M. Expression of vascular endothelial growth factor and its receptor (KDR/flk-1) mRNA in experimental choroidal neovascularization. Curr. Eye Res. 1999;18(3):203–213. doi: 10.1076/ceyr.18.3.203.5368. [DOI] [PubMed] [Google Scholar]
- Wang F, Rendahl KG, Manning WC, Quiroz D, Coyne M, Miller SS. AAV-mediated expression of vascular endothelial growth factor induces choroidal neovascularization in rat. Invest. Ophthalmol. Vis. Sci. 2003;44(2):781–790. doi: 10.1167/iovs.02-0281. [DOI] [PubMed] [Google Scholar]
- Wang L, Li CM, Rudolf M, Belyaeva OV, Chung BH, Messinger JD, Kedishvili NY, Curcio CA. Lipoprotein particles of intraocular origin in human Bruch membrane: an unusual lipid profile. Invest. Ophthalmol. Vis. Sci. 2009;50(2):870–877. doi: 10.1167/iovs.08-2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber BH, Lin B, White K, Kohler K, Soboleva G, Herterich S, Seeliger MW, Jaissle GB, Grimm C, Reme C, Wenzel A, Asan E, Schrewe H. A mouse model for Sorsby fundus dystrophy. Invest. Ophthalmol. Vis. Sci. 2002;43(8):2732–2740. [PubMed] [Google Scholar]
- Weikel KA, FitzGerald P, Shang F, Caceres A, Bian Q, Handa JT, Stitt AW, Taylor A. Natural History of Age-Related Retinal Lesions that Precede AMD in Mice Fed High or Low Glycemic index Diets. Investigative Ophthalmology & Visual Science. 2011 doi: 10.1167/iovs.11-8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weismann D, Hartvigsen K, Lauer N, Bennett KL, Scholl HP, Charbel Issa P, Cano M, Brandstatter H, Tsimikas S, Skerka C, Superti-Furga G, Handa JT, Zipfel PF, Witztum JL, Binder CJ. Complement factor H binds malondialdehyde epitopes and protects from oxidative stress. Nature. 2011;478(7367):76–81. doi: 10.1038/nature10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng J, Mata NL, Azarian SM, Tzekov RT, Birch DG, Travis GH. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt's disease from the phenotype in abcr knockout mice. Cell. 1999;98(1):13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]
- Wenzel A, Grimm C, Samardzija M, Reme CE. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog. Retin. Eye Res. 2005;24(2):275–306. doi: 10.1016/j.preteyeres.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Wilson DJ, Green WR. Histopathologic study of the effect of retinal detachment surgery on 49 eyes obtained post mortem. Am. J. Ophthalmol. 1987;103(2):167–179. doi: 10.1016/s0002-9394(14)74222-9. [DOI] [PubMed] [Google Scholar]
- Xie B, Shen J, Dong A, Rashid A, Stoller G, Campochiaro PA. Blockade of sphingosine-1-phosphate reduces macrophage influx and retinal and choroidal neovascularization. J. Cell. Physiol. 2009;218(1):192–198. doi: 10.1002/jcp.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Chen M, Forrester JV. Para-inflammation in the aging retina. Prog. Retin. Eye Res. 2009;28(5):348–368. doi: 10.1016/j.preteyeres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Yamada H, Yamada E, Kwak N, Ando A, Suzuki A, Esumi N, Zack DJ, Campochiaro PA. Cell injury unmasks a latent proangiogenic phenotype in mice with increased expression of FGF2 in the retina. J. Cell. Physiol. 2000;185(1):135–142. doi: 10.1002/1097-4652(200010)185:1<135::AID-JCP13>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Takahashi S, Kawanami T, Kato T, Sasaki H. Retinal degeneration in hereditary ceruloplasmin deficiency. Ophthalmologica. Journal international d'ophtalmologie. Int. J. Ophthalmol. 1998;212(1):11–14. doi: 10.1159/000027251. [DOI] [PubMed] [Google Scholar]
- Yang X, Hu J, Zhang J, Guan H. Polymorphisms in CFH, HTRA1 and CX3CR1 confer risk to exudative age-related macular degeneration in Han Chinese. Br. J. Ophthalmol. 2010;94(9):1211–1214. doi: 10.1136/bjo.2009.165811. [DOI] [PubMed] [Google Scholar]
- Yates JR, Sepp T, Matharu BK, Khan JC, Thurlby DA, Shahid H, Clayton DG, Hayward C, Morgan J, Wright AF, Armbrecht AM, Dhillon B, Deary IJ, Redmond E, Bird AC, Moore AT. Complement C3 variant and the risk of age-related macular degeneration. New Engl. J. Med. 2007;357(6):553–561. doi: 10.1056/NEJMoa072618. [DOI] [PubMed] [Google Scholar]
- Yi X, Ogata N, Komada M, Yamamoto C, Takahashi K, Omori K, Uyama M. Vascular endothelial growth factor expression in choroidal neovascularization in rats. Graefes Arch. Clin. Exp. Ophthalmol. 1997;235(5):313–319. doi: 10.1007/BF01739641. [DOI] [PubMed] [Google Scholar]
- Zahn G, Vossmeyer D, Stragies R, Wills M, Wong CG, Loffler KU, Adamis AP, Knolle J. Preclinical evaluation of the novel small-molecule integrin alpha5beta1 inhibitor JSM6427 in monkey and rabbit models of choroidal neovascularization. Arch. Ophthalmol. 2009a;127(10):1329–1335. doi: 10.1001/archophthalmol.2009.265. [DOI] [PubMed] [Google Scholar]
- Zahn G, Vossmeyer D, Stragies R, Wills M, Wong CG, Loffler KU, Adamis AP, Knolle J. Preclinical evaluation of the novel small-molecule integrin alpha5beta1 inhibitor JSM6427 in monkey and rabbit models of choroidal neovascularization. Arch. Ophthalmol. 2009b;127(10):1329–1335. doi: 10.1001/archophthalmol.2009.265. [DOI] [PubMed] [Google Scholar]
- Zareparsi S, Branham KE, Li M, Shah S, Klein RJ, Ott J, Hoh J, Abecasis GR, Swaroop A. Strong association of the Y402H variant in complement factor H at 1q32 with susceptibility to age-related macular degeneration. Am. J. Hum. Genet. 2005;77(1):149–153. doi: 10.1086/431426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiss CJ. Animals as models of age-related macular degeneration: an imperfect measure of the truth. Vet. Pathol. 2010;47(3):396–413. doi: 10.1177/0300985809359598. [DOI] [PubMed] [Google Scholar]
- Zhang D, Brankov M, Makhija MT, Robertson T, Helmerhorst E, Papadimitriou JM, Rakoczy PE. Correlation between inactive cathepsin D expression and retinal changes in mcd2/mcd2 transgenic mice. Invest. Ophthalmol. Vis. Sci. 2005;46(9):3031–3038. doi: 10.1167/iovs.04-1510. [DOI] [PubMed] [Google Scholar]
- Zhang D, Lai MC, Constable IJ, Rakoczy PE. A model for a blinding eye disease of the aged. Biogerontology. 2002;3(1–2):61–66. doi: 10.1023/a:1015259413857. [DOI] [PubMed] [Google Scholar]
- Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258(5081):468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- Zhang ZX, Wang YS, Shi YY, Hou HY, Zhang C, Cai Y, Dou GR, Yao LB, Li FY. Hypoxia specific SDF-1 expression by retinal pigment epithelium initiates bone marrow-derived cells to participate in Choroidal neovascularization in a laser-induced mouse model. Curr. Eye Res. 2011;36(9):838–849. doi: 10.3109/02713683.2011.593107. [DOI] [PubMed] [Google Scholar]
- Zhao L, Wang Z, Liu Y, Song Y, Li Y, Laties AM, Wen R. Translocation of the retinal pigment epithelium and formation of sub-retinal pigment epithelium deposit induced by subretinal deposit. Mol. Vis. 2007;13:873–880. [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Wang YS, Zhang J, Zhao W, Yang XM, Li X, Jiang TS, Yao LB. Focal adhesion kinase signaling pathway participates in the formation of choroidal neovascularization and regulates the proliferation and migration of choroidal microvascular endothelial cells by acting through HIF-1 and VEGF expression in RPE cells. Exp. Eye Res. 2009;88(5):910–918. doi: 10.1016/j.exer.2008.11.034. [DOI] [PubMed] [Google Scholar]
- Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12(2):121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- Zou Y, Xu X, Chiou GC. Effect of interleukin-1 blockers, CK112, and CK116 on rat experimental choroidal neovascularization in vivo and endothelial cell cultures in vitro. J. Ocul. Pharmacol. Ther. 2006;22(1):19–25. doi: 10.1089/jop.2006.22.19. [DOI] [PubMed] [Google Scholar]