Abstract
Withdrawal Seizure-Prone (WSP) and Withdrawal Seizure-Resistant (WSR) mouse lines were bidirectionally selectively bred, respectively, to have severe or mild ethanol withdrawal handling-induced convulsions (HICs) after cessation of 3 days of ethanol vapor inhalation. Murine genotypes with severe withdrawal have been found to show low ethanol consumption, and high consumers show low withdrawal. An early drinking study with WSP and WSR mice showed modest evidence consistent with this genetic correlation, but there were several limitations to that experiment. We therefore conducted a thorough assessment of two-bottle ethanol preference drinking in both replicate pairs of WSP/WSR selected lines in mice of both sexes. Greater preference drinking of WSR-2 than WSP-2 female mice confirmed the earlier report. However, in the parallel set of selected lines, the WSP-1 mice drank more than the WSR-1s. Naive mice tested for preference for sucrose, saccharin and quinine did not differ markedly for any tastant. Finally, in a test of binge-like drinking, Drinking in the Dark (DID), WSP mice drank more than WSR mice and attained significantly higher (but still modest) blood ethanol concentrations. Tests of acute withdrawal showed a mild, but significant elevation in handling induced convulsions in the WSP line. These results provide further evidence that 2-bottle ethanol preference and DID are genetically distinguishable traits.
Keywords: ethanol withdrawal, selective breeding, ethanol preference, drinking in the dark, mouse, genetics
Introduction
Mice show substantial genetic differences in ethanol withdrawal severity as indicated by handling-induced convulsions (HICs) after ethanol administration ceases. For example, inbred strains differ markedly in HIC severity after exposure to ethanol vapor for 72 hr (Metten and Crabbe, 2005) or after periods of intermittent ethanol vapor exposure (Metten et al., 2010). The strain differences cannot be explained by differences in alcohol metabolism, because each strain was exposed to ethanol vapor concentrations designed to result in equivalent blood ethanol concentrations (BECs). Strains also differ in HIC severity following an acute, anesthetic dose of ethanol (Metten and Crabbe, 1994), and recombinant inbred strains derived from the intercross of the high-withdrawal DBA/2J and low-withdrawal C57BL/6J inbred strains show a range of acute (Buck et al., 1997) and chronic (Crabbe, 1998) withdrawal HIC scores.
Murine genotypes also show pronounced differences in willingness to ingest ethanol solutions offered under a variety of conditions. C57BL/6J (B6) mice are well-established high drinkers (Wahlsten et al., 2006) but show modest withdrawal HICs, while DBA/2J (D2) mice are abstainers, but show severe withdrawal HICs (Metten and Crabbe, 2005;Wahlsten et al., 2006). A review of several studies with populations of mice derived from B6 and D2 intercrosses reported a consistent, substantial negative genetic correlation between g/kg intake of ethanol in a two-bottle preference test for 10% ethanol vs. water and severity of acute or chronic withdrawal HICs (Metten et al., 1998). Pooled across 6 experiments using B6/D2-derived populations, the mean genetic correlation was r = −0.39 [two-tailed p = 3 × 10−5; (Metten et al., 1998)]. However, the negative correlation was less striking in populations derived from multiple genotypes, including other inbred strains. This finding suggested an important role of alleles from the B6 and D2 lineages, but left open the possibility that some other genotypes might also show the inverse relationship between drinking and withdrawal.
In 1985, we initiated a long-term, replicated selective breeding project to create mouse lines bred for severe (Withdrawal Seizure-Prone; WSP) or mild (Withdrawal Seizure-Resistant; WSR) withdrawal, based on HIC scores following 3 days of ethanol vapor inhalation (Crabbe et al., 1985). These lines were selected from HS/Ibg, a genetically segregating heterogeneous stock derived from systematic intercrosses of 8 inbred strains, including B6 and D2 (McClearn et al., 1970). Female mice from the 17th and 19th selected generation of WSP-1, WSP-2, WSR-1, and WSR-2 and both unselected control lines (WSC-1 and WSC-2) were tested in two different ethanol preference drinking paradigms where water was always offered as an alternative. We found that WSR mice generally drank more ethanol than WSP, consistent with the negative genetic correlation in B6/D2 populations, although the pattern of drinking depended upon the paradigm employed and varied somewhat over time (Kosobud et al., 1988). However, there were several features of these early drinking studies that were not optimal: the relatively short duration of the test; no examination of male mice; the use of mice for one experiment that had previous experience with an ethanol solution; and the use of an unusual drinking protocol in the other experiment. Furthermore, WSP/WSR mice were subsequently directionally selected through generation 26 (S26), and many more generations have since ensued under relaxed selection, allowing for the possible effects of genetic drift to accumulate, which could have changed the pattern of correlated responses to selection in these lines (Falconer and Mackay, 1996). Therefore, in the current experiments, we systematically examined two bottle ethanol preference drinking across a range of concentrations using what has become our standard preference drinking protocol (Phillips et al., 1994). We tested additional, naive mice for their taste sensitivity and preference for sucrose, saccharin and quinine solutions. Finally, we tested naive mice using a relatively new form of binge-like ethanol intake called drinking in the dark [DID: (Rhodes et al., 2005)].
Experimental Procedures
We have previously published a detailed description of our animal husbandry and colony procedures, as well as of the ethanol preference and tastant drinking protocols we employed for Experiments 1 and 2 (Crabbe et al., 2011). The reader is referred to that paper for details, and a summary of the methods is presented below.
Animals and Husbandry
Mice from the Withdrawal Seizure-Prone (WSP-1 and -2) and -Resistant (WSR-1 and -2) selected lines were bred in our colonies in the Portland VA Veterinary Medical Unit. All mice were naive at the beginning of each experiment and were from the 26th selected generation and filial generations ranged from 98 - 127 (e.g., S26G98). These two pairs of replicate lines have been maintained without selection pressure using a rotational, within-family mating scheme with 9-27 breeding pairs/generation of animals since selection ceased at S26. The lines differed at least 10-fold in chronic ethanol withdrawal severity after 11 selected generations (Crabbe et al., 1985), and periodic comparisons have shown no decline in the magnitude of withdrawal differences between WSP and WSR lines [(Phillips et al., 1989) and unpublished data]. All mice were between 50 and 98 days old at the start of testing.
Mice were maintained in standard plastic cages on Bed-o-cob bedding (Andersons, Maumee, OH, USA) with stainless steel wire bar tops with a recess for chow. Rodent chow 5001 (PMI Nutrition International, Brentwood, MO, USA) and tap water were available ad libitum and colonies and testing rooms were maintained on a 12 hr:12 hr light:dark schedule at a temperature of 21+/−1°C. Two weeks before the start of an experiment, mice were transferred to a procedure room with the same environmental conditions and were individually housed. Animals in Experiment 3 were acclimated during this time to a reversed light:dark schedule of 21:30 lights on: 09:30 lights out. All procedures were approved by the Portland VA Medical Center Institutional Animal Care and Use Committee and were performed according to NIH Guidelines for the Care and Use of Laboratory Animals.
Experiment 1: Two bottle ethanol consumption and preference
Sixty-nine mice were tested (n = 7-10 per selected line, replication, and sex), using our standard method (Phillips et al., 1994).The water bottle was replaced with two 25ml graduated cylinders with stainless steel drinking spouts, both containing tap water, for two days. During the next 16 days, one cylinder contained tap water and the other an ethanol solution. The preference test commenced with 3% ethanol (v/v) in tap water on the left side vs water on the right side. Twenty-four hrs later, intake was recorded, and the bottles were left in place until 48 hrs. Ethanol (Decon Laboratories, Inc., King of Prussia, PA) and water cylinder positions were then switched. Mice were exposed to ethanol vs water for 16 days, 4 days each at 3%, 6%, 10%, and 20%, with daily readings and positions switched each 48 hrs. Body weights were taken the day the experiment started, and at every concentration switch. Two spillage control cages with fluids (but without mice) were used, one at each end of the rack.
Each day’s data were first corrected by subtracting the average loss of each fluid from the two control cages. We computed consumption (g ethanol / kg body weight) and preference ratio (volume from the ethanol tube / total fluid volume consumed from ethanol + water). We also report water (or total fluid) consumption and body weight. Data from the occasional leaking tubes were treated as missing, as described in detail elsewhere (Crabbe et al., 2011).
Experiment 2: Tastant preference
Sixty-four naive mice were tested (n = 6 - 10 per selected line, replication, and sex) using the same procedures described for Experiment 1. Mice were serially offered three tastants (dissolved in tap water) versus tap water for 24 days. Each tastant was offered for 8 days, first at a low and then at a higher concentration. Tastants and concentrations, in the order of presentation, were: quinine hemisulfate salt monohydrate (Sigma-Aldrich, St. Louis, MO), at 0.1 mM (0.004%) and then 0.8mM (0.032%); saccharin sodium salt hydrate (Sigma-Aldrich, St. Louis, MO) at 3.2 mM (0.066%) and then 10 mM (0.21%); and sucrose (Fisher Scientific, Pittsburgh, PA) at 49.7 mM (1.7%) and then 124 mM (4.25%). Six days of water only drinking were given between each tastant. All procedures were performed as in Experiment 1, and data were treated as described for Experiment 1. Only preference ratios were analyzed (see Results).
Experiment 3: Drinking in the Dark (DID)
Eighty-five naive mice were tested (n = 10 - 13 per selected line, replication, and sex). We used the 4 day DID test originally described in (Rhodes et al., 2005). Details of the apparatus and procedure are available (http://www.scripps.edu/cnad/inia/modelmousedrinkingindark.pdf). Mice were individually housed and placed on a reversed light-dark cycle for two weeks. All subsequent procedures were performed under red light. Mice were weighed and scored for baseline handling induced convulsions (see below) 4hrs before the start of the drinking test. Starting 3 hrs after lights off, each water bottle was replaced with a single 10 ml stoppered Falcon disposable clear polystyrene serological pipet (Fisher Scientific) filled with a 20% (v/v) ethanol solution fit with a sipper tube containing a ball bearing. The ethanol tube was left in place for 2 hr and was then replaced with the water bottle. This procedure was repeated for 3 consecutive days. On the 4th day, after reading the level at 2 hr, tubes were left in place for an additional 2 hr. At the end of the 4 hr session on Day 4, tubes were read and each animal was scored for HIC immediately and then had a 20ul blood sample drawn from the periorbital sinus with a capillary tube. These samples were subsequently processed to determine blood ethanol concentration (BEC) using a published gas chromatographic method (Rustay and Crabbe, 2004). Drinking data were converted to g/kg intake for analysis.
To assess whether mice had ingested sufficient ethanol to display a mild withdrawal reaction at the end of the session, mice were scored for the handling-induced convulsion. This behavioral convulsion is elicited by picking the mouse up by the tail and, if necessary, twirling it through a 180° arc. Scores range from 0 (no convulsion) or 1 (facial grimace) through 7 (lethal tonic hind limb extensor seizure elicited by cage disturbance) as detailed elsewhere (Crabbe et al., 1991). HICs were scored each hour between hrs 0 and 6 after the post-DID blood ethanol sample, for a total of 7 HIC scores.
Statistical analyses
Data were analyzed with ANOVAs using Systat (version 13). For Experiment 1, initial analyses of the alcohol drinking data included the factors selected line, replicate, and sex with alcohol concentration as a repeated measure. We then pursued significant interactions with follow-up ANOVAs as appropriate, ultimately employing the Tukey HSD procedure for post hoc comparisons. Water drinking was evaluated separately. For Experiment 2, separate ANOVAs were performed for each tastant using the factors selected line, replicate and sex, with concentration as a repeated measure. For Experiment 3, we first analyzed the data for hrs 1-2 and 3-4 on Day 4 on the factors selected line, replicate and sex, with time block as a repeated measure; we also analyzed total intake on Day 4. We then analyzed the data across all 4 drinking days by including only the first 2-hr block of Day 4. Baseline HIC scores and withdrawal scores were analyzed similarly. Withdrawal scores were calculated as post-DID sum of scores from hours 2-6 minus 5 × Baseline. If the withdrawal score for an animal was negative, it was corrected to zero prior to analyses. We report all significant main effects and interactions; those not mentioned in the text were found to be not statistically significant (P > 0.05). These experiments were not designed to enable a robust test of 3- or 4-way interactions (ns = 6-13 per cell). The principal hypotheses of interest were the selected line differences and their potential interactions with replicate line. Thus, some marginally-significant effects reported (and some approaching but not reaching significance) might emerge in a higher-powered experiment.
Results
Experiment 1: Two bottle ethanol consumption and preference
The ethanol consumption data for each day are shown in Figures 1 and 2. The 4-way ANOVA (Line, Replicate, Sex, and Concentration) of ethanol consumption data did not yield any significant main effects other than Concentration [F(3,183) = 3.1, P < 0.05] (all other main effect Fs < 2.8). Of the two-way interactions, Line × Replicate [F(1,61) = 29.0, P < 0.0001] and Line × Concentration [F(3,183) = 4.4, P < 0.01] were significant, as was the 3-way interaction of Line × Replicate × Concentration [F(3,183) = 5.2, P < 0.01]. No interactions involving Sex were significant. We therefore analyzed data for each replicate pair of selected lines separately.
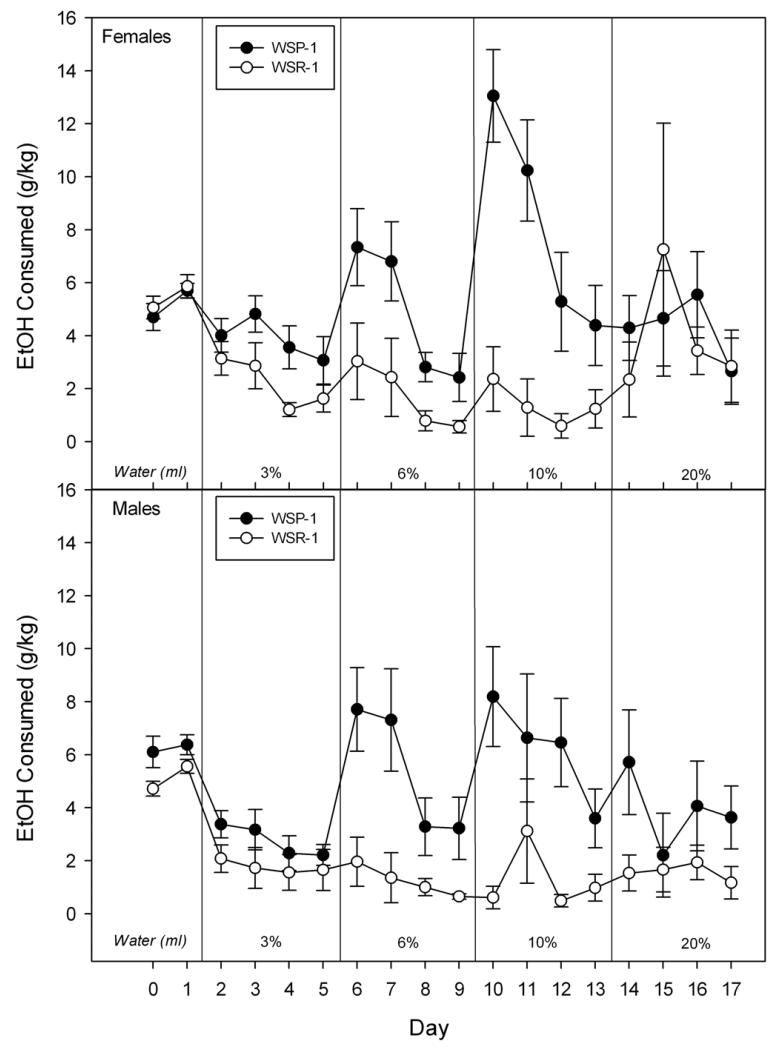
Figure 1.
Consumption of female (upper panel) and male (lower panel) mice from WSP-1 and WSR-1 selected lines. Vertical lines divide water consumption (in ml: first 2 days) and g/kg consumption of increasing concentrations of ethanol in water (3%, 6%, 10% and 20%) during successive 4 day periods. Mean +/−SE is shown. Sexes are presented separately to facilitate comparison with data from early generations (Kosobud et al., 1988).
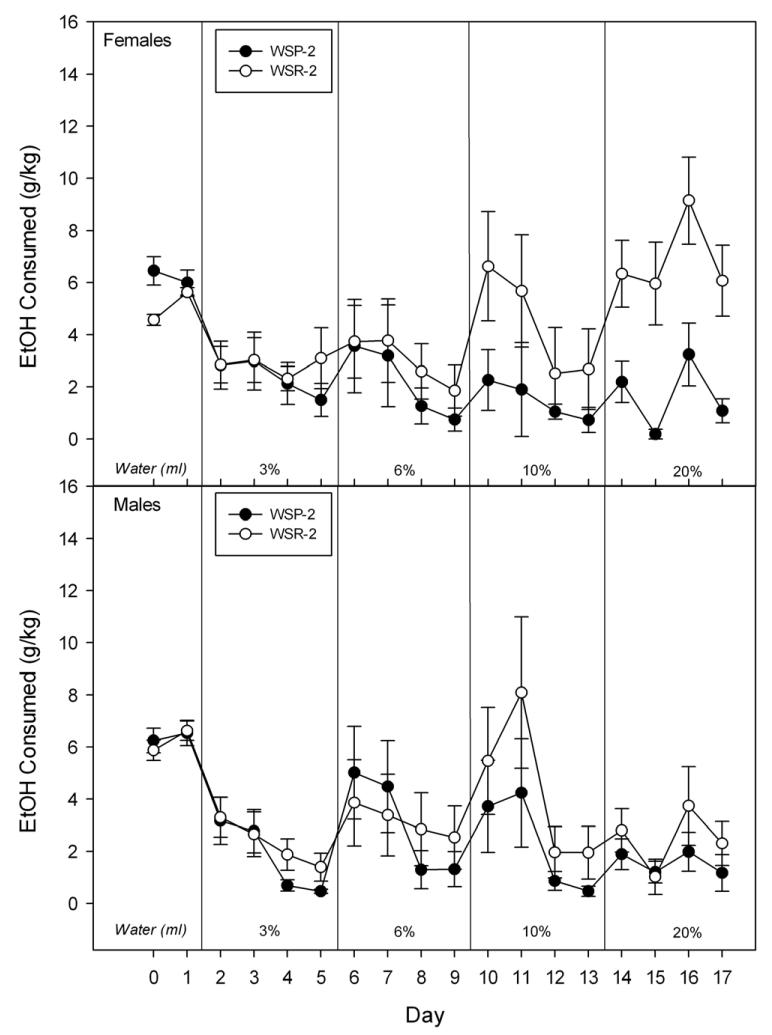
Figure 2.
Consumption of female (upper panel) and male (lower panel) mice from WSP-2 and WSR-2 selected lines. See caption to Figure 1.
WSP-1 mice drank more ethanol than WSR-1 mice [F1,32) = 31.1, P < 0.0001], and the difference depended on concentration [F(3,96) = 7.0, P < 0.001]. The lines differed significantly at the three lower concentrations [Fs ≥ 7.8, Ps ≤ 0.01], but not at the 20% concentration (F = 1.1, see Figure 1). There were no significant main or interactive effects involving Sex (all Fs ≤ 1.5).
For the second replicate, the main effect of Selected Line [F(1,33) = 6.0, P < 0.05] and the interaction of Concentration × Line [F(3,99) = 3.0, P < 0.05] were significant. Although it appeared that the greater consumption of WSR-2 than WSP-2 mice was more pronounced at the 10% and 20% concentrations (see Figure 2), this interaction only tended to reach significance [F(3,93) = 2.3, P = .08] and Lines only differed significantly at the 20% concentration [F(1,22) = 13.1, P < 0.001]. There were no significant main or interactive effects involving Sex (all Fs ≤ 2.1).
Water consumption (data not shown) on the first day showed a main effect of line where WSP mice drank more water than did WSR mice (5.86 +/− 0.28 vs 5.05 +/− 0.19 ml/day) but they did not differ in water consumption by the second day for any factor (all Fs ≤ 3.0). Total fluid intake across days (data not shown) showed main effects of Line [F(1,60) = 4.2, P < 0.05] and Sex [F(1,60) = 10.9, P < 0.01], as well as interactions of Line × Replicate [F(1,60) = 6.6, P < 0.05] and Line × Sex [F(1,60) = 8.3, P < 0.01], but no other significant effects, including across the days of the study (all Fs ≤ 2.3).
Preference data are shown in Table 1 for reference. When we analyzed these data, the pattern of statistical outcomes was virtually identical to those reported above for the intake data.
Table 1. Ethanol Preference Ratios by Concentration Offered in Experiment 1.
| Females | ||||
|---|---|---|---|---|
| Line-Replicate | 3% | 6% | 10% | 20% |
| WSP-1 | 63.6 +/− 9.8% | 38.5 +/− 8.7% | 47.3 +/− 11.1% | 8.3 +/− 3.0% |
| WSR-1 | 35.3 +/− 11.0% | 13.0 +/− 6.6% | 7.7 +/− 5.0% | 9.4 +/− 4.2% |
| WSP-2 | 32.9 +/− 10.4% | 15.4 +/− 7.7% | 8.2 +/− 6.5% | 1.3 +/− 0.5% |
| WSR-2 | 37.5 +/− 11.0% | 22.5 +/− 10.2% | 25.5 +/− 10.2% | 12.1 +/− 2.4% |
| all WSP | 49.2 +/− 7.9% | 27.6 +/− 6.4% | 28.9 +/− 8.1% | 5.0 +/− 0.2% |
| all WSR | 36.4 +/− 7.6% | 17.5 +/− 5.9% | 16.2 +/− 5.7% | 10.7 +/− 2.4% |
| all females | 42.4 +/− 5.5% | 22.3 +/− 4.3% | 22.2 +/− 4.9% | 8.0 +/− 1.6% |
| Males | ||||
| Line-Replicate | 3% | 6% | 10% | 20% |
| WSP-1 | 42.1 +/− 7.5% | 49.6 +/− 11.2% | 32.4 +/− 10.0% | 7.0 +/− 2.1% |
| WSR-1 | 34.5 +/− 11.7% | 11.7 +/− 6.5% | 7.4 +/− 4.3% | 3.3 +/− 1.3% |
| WSP-2 | 27.9 +/− 7.8% | 27.3 +/− 10.8% | 8.4 +/− 5.1% | 2.6 +/− 0.8% |
| WSR-2 | 36.5 +/− 9.9% | 27.2 +/− 12.4% | 33.2 +/− 8.5% | 4.3 +/− 1.7% |
| all WSP | 34.2 +/− 5.6% | 37.2 +/− 8.0% | 19.1 +/− 5.9% | 4.5 +/− 1.1% |
| all WSR | 35.6 +/− 7.3% | 20.0 +/− 7.3% | 21.2 +/− 5.9% | 3.8 +/− 1.1% |
| all males | 34.8 +/− 4.4% | 29.4 +/− 5.6% | 20.0 +/− 4.1% | 4.2 +/− 0.8% |
Body weights are shown in Table 2 for each experiment. We analyzed the two sexes separately for body weight on Day 1 and for percent change in body weight using between groups factors Line and Replicate. Analysis of initial body weights for female mice showed there were no significant main effects of Line or Replicate [Fs(1,32) < 1]. A significant Line × Replicate interaction [F(1,32) = 5.2, P < 0.05] was observed. It appeared that for Replicate 1, WSR-1 mice may have weighed more than WSP-1, and that for Replicate 2, the opposite seemed to be true (WSP-2 > WSR-2); however, post hoc testing did not confirm either of these inferences. We next analyzed the percent body weight gain over the course of the experiment. Due to a significant main effect of Replicate and a Line × Replicate interaction [Fs(1,32) > 4.8, Ps < 0.05] in the absence of a main effect of Line (F < 1) in female mice, we next analyzed the replicates separately. For Replicate 1 females, the lines did not differ [F(1,17) =1.8]; however WSP-2 females gained more weight than did WSR-2 females.
Table 2. Body weights (g) by Sex for Each Experiment.
| Experiment 1 | ||||||
|---|---|---|---|---|---|---|
| Females | ||||||
| Line-Replicate | Day 1 | Day 5 | Day 9 | Day 13 | Average | % change |
| WSP-1 | 20.2 +/− 0.7 | 20.4 +/− 0.8 | 20.8 +/− 0.7 | 20.8 +/− 0.7 | 20.5 +/− 0.7 | 2.8 +/− 1.2 |
| WSR-1 | 22.1 +/− 0.7 | 22.2 +/− 0.6 | 22.5 +/− 0.6 | 23.2 +/− 0.8 | 22.5 +/− 0.7 | 5.3 +/− 1.4 |
| WSP-2 | 20.8 +/− 0.4 | 21.9 +/− 0.4 | 23.0 +/− 1.0 | 22.5 +/− 0.4 | 22.0 +/− 0.5 | 8.0 +/− 1.2 |
| WSR-2 | 20.0 +/− 0.4 | 20.5 +/− 0.5 | 20.9 +/− 0.3 | 21.0 +/− 0.5 | 20.6 +/− 0.4 | 5.2 +/− 0.6 |
| all WSP | 20.5 +/− 0.4 | 21.1 +/− 0.5 | 21.9 +/− 0.6 | 21.6 +/− 0.5 | 21.2 +/− 0.5 | 5.2 +/− 1.1 |
| all WSR | 21.1 +/− 0.5 | 21.4 +/− 0.4 | 21.8 +/− 0.4 | 22.2 +/− 0.5 | 21.6 +/− 0.4 | 5.3 +/− 0.8 |
| all females | 20.8 +/− 0.3 | 21.3 +/− 0.3 | 21.8 +/− 0.4 | 21.9 +/− 0.4 | 21.4 +/− 0.3 | 5.2 +/− 0.6 |
| Males | ||||||
| Line-Replicate | Day 1 | Day 5 | Day 9 | Day 13 | Average | % change |
| WSP-1 | 25.0 +/− 1.0 | 25.2 +/− 1.1 | 25.0 +/− 1.0 | 25.7 +/− 1.2 | 25.2 +/− 1.0 | 2.3 +/− 1.2 |
| WSP-2 | 24.9 +/− 0.7 | 25.9 +/− 0.8 | 26.1 +/− 0.8 | 26.2 +/− 0.8 | 25.8 +/− 0.8 | 5.0 +/− 1.1 |
| WSR-1 | 26.0 +/− 0.3 | 26.3 +/− 0.4 | 26.6 +/− 0.4 | 26.8 +/− 0.3 | 26.4 +/− 0.3 | 3.0 +/− 0.9 |
| WSR-2 | 27.8 +/− 0.6 | 28.0 +/− 0.6 | 28.3 +/− 0.6 | 28.3 +/− 0.5 | 28.1 +/− 0.5 | 1.9 +/− 1.5 |
| all WSP | 25.0 +/− 0.6 | 25.6 +/− 0.6 | 25.6 +/− 0.6 | 26.0 +/− 0.7 | 25.5 +/− 0.6 | 3.8 +/− 0.9 |
| all WSR | 27.0 +/− 0.4 | 27.2 +/− 0.4 | 27.5 +/− 0.4 | 27.6 +/− 0.4 | 27.3 +/− 0.4 | 2.5 +/− 0.9 |
| all males | 25.9 +/− 0.4 | 26.3 +/− 0.4 | 26.5 +/− 0.4 | 26.7 +/− 0.4 | 26.3 +/− 0.4 | 3.2 +/− 0.6 |
| Experiment 2 | ||||||
| Females | ||||||
| Line-Replicate | Day 1 | Day 15 | Day 29 | Average | % change | |
| WSP-1 | 22.4 +/− 0.5 | 22.9 +/− 0.5 | 23.2 +/− 0.6 | 22.8 +/− 0.5 | 3.9 +/− 0.9 | |
| WSR-1 | 25.0 +/− 0.4 | 25.6 +/− 0.5 | 25.8 +/− 0.6 | 25.5 +/− 0.5 | 3.5 +/− 1.4 | |
| WSP-2 | 22.7 +/− 0.8 | 23.7 +/− 0.9 | 24.3 +/− 0.9 | 23.5 +/− 0.9 | 7.0 +/− 0.8 | |
| WSR-2 | 24.7 +/− 0.5 | 26.1 +/− 0.5 | 26.4 +/− 0.4 | 25.7 +/− 0.5 | 7.2 +/− 0.6 | |
| all WSP | 22.5 +/− 0.5 | 23.3 +/− 0.5 | 23.8 +/− 0.6 | 23.2 +/− 0.5 | 5.6 +/− 0.7 | |
| all WSR | 24.8 +/− 0.3 | 25.8 +/− 0.3 | 26.1 +/− 0.4 | 25.6 +/− 0.3 | 5.1 +/− 1.0 | |
| all females | 23.5 +/− 0.4 | 24.4 +/− 0.4 | 24.8 +/− 0.4 | 24.3 +/− 0.4 | 5.4 +/− 0.6 | |
| Males | ||||||
| Line-Replicate | Day 1 | Day 15 | Day 29 | Average | % change | |
| WSP-1 | 24.9 +/− 1.1 | 26.7 +/− 0.5 | 26.9 +/− 0.5 | 26.5 +/− 0.5 | 2.1 +/− 1.0 | |
| WSR-1 | 27.8 +/− 0.5 | 28.6 +/− 0.5 | 29.2 +/− 0.4 | 28.6 +/− 0.5 | 5.0 +/− 1.1 | |
| WSP-2 | 30.3 +/− 0.3 | 30.4 +/− 0.2 | 30.7 +/− 0.2 | 30.4 +/− 0.2 | 1.4 +/− 0.5 | |
| WSR-2 | 30.4 +/− 0.8 | 30.9 +/− 0.8 | 30.7 +/− 0.8 | 30.7 +/− 0.8 | 1.3 +/− 0.8 | |
| all WSP | 28.2 +/− 0.6 | 28.5 +/− 0.6 | 28.9 +/− 0.6 | 28.4 +/− 0.6 | 1.7 +/− 0.5 | |
| all WSR | 29.1 +/− 0.5 | 29.8 +/− 0.5 | 30.0 +/− 0.5 | 29.6 +/− 0.5 | 3.1 +/− 0.8 | |
| all males | 28.6 +/− 0.4 | 29.2 +/− 0.4 | 29.5 +/− 0.4 | 29.0 +/− 0.4 | 2.5 +/− 0.5 | |
| Experiment 3 | ||||||
| Females | ||||||
| Line-Replicate | Day 1 | Day 3 | % change | |||
| WSP-1 | 21.9 +/− 0.4 | 22.0 +/− 0.3 | 0.3 +/− 0.2 | |||
| WSR-1 | 22.9 +/− 0.3 | 23.1 +/− 0.3 | 0.0 +/− 0.0 | |||
| WSP-2 | 21.9 +/− 0.4 | 22.2 +/− 0.3 | 1.3 +/− 0.7 | |||
| WSR-2 | 24.0 +/− 0.3 | 23.8 +/− 0.3 | 0.0 +/− 0.0 | |||
| all WSP | 21.9 +/− 0.3 | 22.1 +/− 0.2 | 0.9 +/− 0.7 | |||
| all WSR | 23.5 +/− 0.2 | 23.5 +/− 0.2 | -0.0 +/− 0.5 | |||
| all females | 22.7 +/− 0.2 | 22.8 +/− 0.2 | 0.4 +/− 0.4 | |||
| Males | ||||||
| Line-Replicate | Day 1 | Day 3 | % change | |||
| WSP-1 | 25.5 +/− 0.7 | 26.0 +/− 0.6 | 0.4 +/− 0.4 | |||
| WSR-1 | 28.3 +/− 0.5 | 28.7 +/− 0.5 | 0.0 +/− 0.0 | |||
| WSP-2 | 27.2 +/− 0.9 | 27.8 +/− 0.8 | 0.6 +/− 0.6 | |||
| WSR-2 | 28.0 +/− 0.9 | 28.3 +/− 0.8 | 0.2 +/− 0.2 | |||
| all WSP | 26.3 +/− 0.6 | 26.9 +/− 0.5 | 2.2 +/− 0.6 | |||
| all WSR | 28.1 +/− 0.5 | 28.5 +/− 0.5 | 1.2 +/− 0.4 | |||
| all males | 27.3 +/− 0.4 | 27.7 +/− 0.4 | 1.7 +/− 0.3 |
Analysis of the data for male mice showed a different pattern. Initial body weights differed by Line [WSR > WSP; F(1,29) = 7.7, P < 0.01] but no other effects were significant (Fs ≤ 1.9). There were no significant effects for percent change in body weight (Fs < 2.5).
Experiment 2: Tastant preference
The tastant preference data (average of 2nd and 4th day at each tastant concentration, as described) are shown in Figures 3 and 4. Body weights were analyzed as described in Experiment 1 and are shown in Table 2.
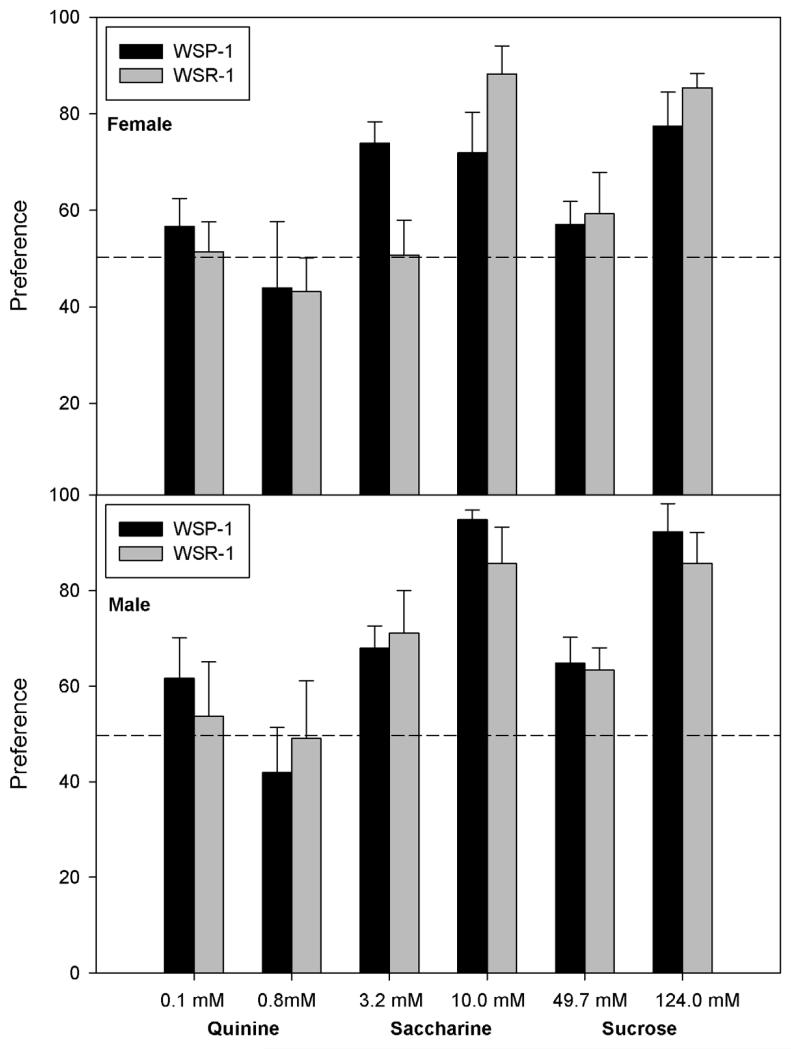
Figure 3.
Preference of female (upper panel) and male (lower panel) mice from WSP-1 and WSR-1 selected lines for various tastants during successive 4 day periods. Preference (% of total fluid from the tastant tube) for quinine, saccharine and sucrose is indicated on the ordinate. Concentrations of each tastant are given on the abscissa. Mean +/− SE is shown. Sexes are presented separately to facilitate comparison with ethanol consumption and preference data. Dashed line indicates 50% (equal preference for water and tastant).
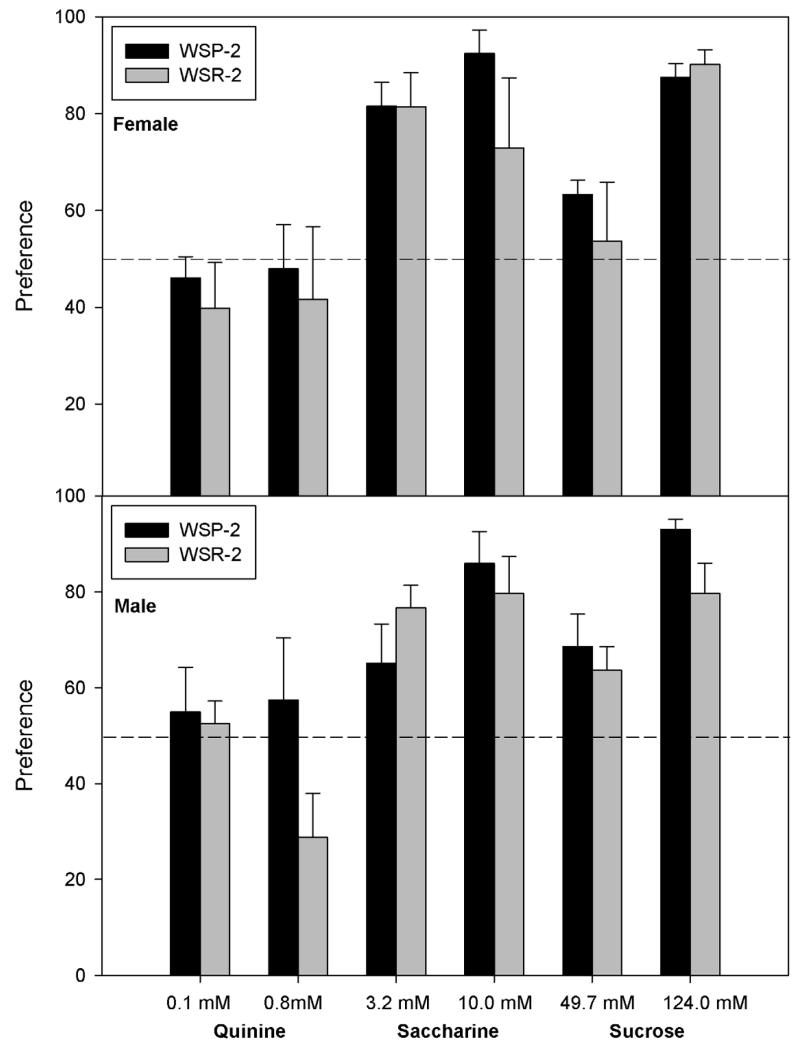
Figure 4.
Preference of female (upper panel) and male (lower panel) mice from WSP-2 and WSR-2 selected lines for various tastants during successive 4 day periods. See caption to Figure 3.
Quinine
The 4-way ANOVA of quinine preference data yielded no significant main effects or interactions [all Fs(1,56) ≤ 2.5, P > 0.10]. Overall, mice were indifferent to the low concentration of quinine (mean preference = 52.2%), but tended to avoid the higher concentration (mean preference = 44.3%).
Saccharine
There was a significant main effect of Concentration [F(1,55) = 15.3, P < 0.001], as animals preferred the higher concentration. There were significant interactions of Replicate × Sex [F(1,55) = 5.0, P < 0.05], Replicate × Concentration [F(1,55) = 4.1, P < 0.05], and Line × Replicate × Concentration [F(1,55) = 6.3, P < 0.05]. We therefore ran separate analyses for each replicate. For Replicate 2, no effects were significant [all Fs(1,28) ≤ 3.6, P ≥ 0.07]. Replicate 1 showed significant main effects of Sex and Concentration as well as a significant interaction of Line × Sex × Concentration [all Fs(1,27) ≥ 5.8, P < 0.05]. Therefore, we next ran separate ANOVAs for each Sex. Female mice showed a main effect of Concentration and a Line × Concentration interaction [Fs(1,14) > 7.2, Ps < 0.05]. WSR-1 females showed no preference for the low concentration of saccharine, but the WSP-1 females did (50% vs 74%, respectively). Both lines showed preference for the higher saccharine concentration, but this did not differ significantly between WSP and WSR [F(1,14) = 2.6]. Male mice of both lines had a greater preference for the higher concentration of saccharine [main effect of Concentration, F(1,13) = 11.0, P < 0.01], but no other effects were significant [Fs(1,13) < 1].
Sucrose
Only the main effect of Concentration was significant [F(1,54) = 74.2, P < 0.0001], although there was a trend toward greater preference in females than males [F(1,54) = 2.8, P = 0.10]. For all other main effects and interactions, F ≤ 2.0, P > 0.10.
Water Consumption (data not shown)
During quinine testing, water consumption did not differ among lines, replicates, sexes, or across quinine concentrations (data not shown: all Fs ≤ 2.8, NS; overall mean 4.2 ml). During saccharine testing, water consumption differed across the different saccharine concentrations, with water consumption decreasing by about half when the more preferred, higher saccharine concentration was offered (F(1,52) = 16.5, P < 0.001). There were also significant interactions of Replicate × Sex [F(1,52) = 4.2, P < 0.05], Concentration × Line × Sex [F(1,52) = 6.7, P < 0.05], and the 4-way interaction of Concentration × Line × Replicate × Sex [F(1,52) = 11.0, P < 0.01]. Therefore, original global analysis was followed by a series of ANOVAs eliminating one factor at a time, and then by performance of one- or two-way ANOVAs for one value of a factor independently (e.g., sexes in separate ANOVAs), followed by Tukey’s HSD where appropriate. Ultimately, these showed that for female mice, there were significant effects of Concentration and Line × Concentration [Fs(1,14) > 6.3, P < 0.05]. WSP-1 female mice consumed significantly less water (2.0 ml/day) than did WSR-1 female mice (4.0 ml/day) while the lower saccharine concentration was offered (Tukey’s HSD, P < 0.05). At the higher concentration, there were no significant effects. For male mice, only a significant effect of Concentration was observed [F(1,11) = 7.6, P < 0.05; males drank only 0.6 ml water/day when the higher concentration was offered, while they drank 2.0 ml water/day during the lower concentration offering].
For water consumed during the sucrose testing, main effects of Sex [F(1,55) = 5.2, P < 0.05] and Concentration [F(1,55) = 79.5, P < 0.0001] were found; all other effects were NS. Both sexes consumed more water when the lower sucrose concentration was offered.
Body Weight
Analyses were performed as for Experiment 1 (see Table 2). For female mice, there was a significant effect of Line on initial body weight [WSR > WSP; F(1,28) = 12.1, P < 0.01] but no other significant effects (Fs < 1). In contrast, for percent weight gain, there was a main effect of Replicate [2>1; F(1,28) = 11.6, P < 0.01] but no other significant effects (Fs < 1). For male mice, there were main effects of Replicate [Fs(1,28) > 6.8, P < 0.05] on both initial body weight (2>1) and percent weight gain (1>2), but no other significant main effects or interactions.
Experiment 3: Ethanol Drinking in the Dark
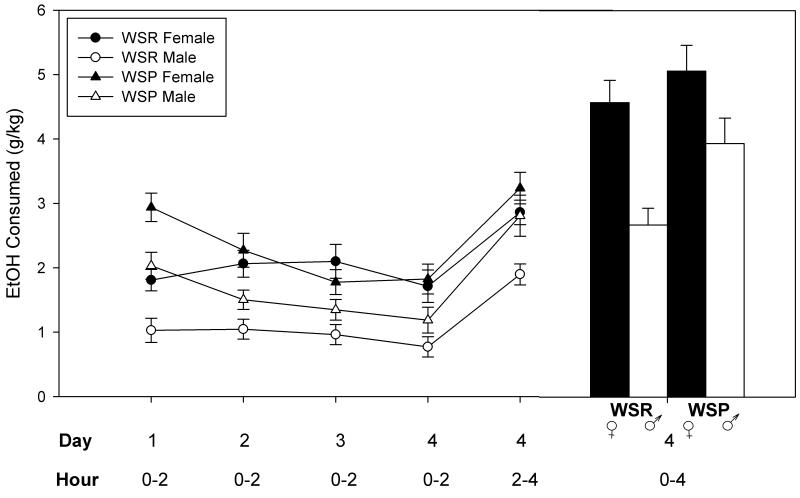
Data are shown in Figure 5. The two, 2-hr blocks of drinking on day 4 were analyzed as described. The main effects of Line, 2-hr Block, and Sex were all significant [Fs(1,76) of 6.63, P <0.05; 88.8, P < 0.0001; and 16.4, P = 0.0001, respectively]. Neither the main effect of Replicate nor any interactions were significant (all Fs < 2). WSP mice consumed more than WSR, and consumption was greater in females and during the second 2-hr block. Analysis of total consumption on Day 4 also showed that WSP mice consumed more ethanol on that day than did WSR mice [F(1,77) = 6.30, P < 0.05] and females consumed more than males [F(1,77) = 17.5, P = 0.0001]. Neither the interactions nor the main effect of Replicate were significant (Fs < 1).
Figure 5.
Consumption of WSP and WSR selected lines during the 4-day drinking in the dark test. Access to 20% ethanol was for 2 h on Days 1-3 and for 4 h (hours 0-2 and 2-4) on Day 4. Total consumption on Day 4 in WSP mice was significantly greater than WSR mice, and greater in females than in males.
Analyses of the first 2 hr of drinking on days 1-4 showed similar patterns of significance, with main effects of Line, Sex [Fs(1,75) ≥ 7.7, Ps ≤ 0.01] and Day [F(3, 225) = 9.7, P < 0.0001] except that there was also a significant interaction of Day × Line [F(3,225) = 7.4, P = 0.0001]. WSP mice consumed more ethanol on Day 1 than did WSR mice [F(1,83) = 24.5, P < 0.0001], but the line difference did not persist beyond Day 1 (Fs ≤ 2.06).
Body weights were analyzed with the sexes separately, as before (see Table 2). Both male and female WSR mice had greater initial body weights compared with their same-sex WSP mice [Fs(1,37-40) ≥ 5.6, Ps < 0.05]. Neither sex showed any significant effects of Line or Replicate (Fs ≤ 2.3) on percent body weight change.
The WSP lines are well known to have higher baseline HIC scores than the WSR lines (Crabbe et al. 1991) and this experiment proved no exception. Baseline HIC scores before the DID test were analyzed by ANOVA. Main effects of Line [F(1,77) = 330, P < 0.0001] and Replicate [F(1,77) = 16.7, P = 0.0001] were found as well as a Line × Replicate interaction [F(1,77) = 16.7, P = 0.0001]. No significant effects or interactions of Sex with other factors were found. Post hoc analyses showed that baseline HICs were greater in WSP-2 than in WSP-1, in both WSP replicates than in their respective WSR replicates (all Tukey’s HSD tests, P < 0.0001), and that WSR replicates did not differ from each other. Because there were no main effects of Sex or interactions of Sex with either Line or Replicate for the Baseline scores or in a preliminary repeated-measure analysis of post-DID HIC scores, we collapsed on Sex and Replicate for further analyses.
Alcohol inhibits HIC scores, so to assess withdrawal, it is necessary to wait until blood alcohol has been mostly eliminated. Post-DID HIC scores overall still averaged less than baseline at Hr 0 and Hr 1, so we summed the HIC scores from hrs 2 - 6 to provide an estimate of the area under the withdrawal curve (HICSUM). Withdrawal under the curve corrected for baseline for each animal (CORWDR) was then computed by the formula, CORWDR = HICSUM - (5 × BASELINE) as we generally do to index acute withdrawal severity (Metten and Crabbe, 1994). We then subjected CORWDR to ANOVA on Line. WSP showed greater scores than WSR [F(1,77) = 5.5, P < 0.05]. WSP mice displayed CORWDR scores of 0.7 +/− 0.3: this differed significantly from zero [t(40) = 2.6, P < 0.01], indicating a mild withdrawal reaction. WSR scores averaged 0.1 +/− 0.1 (See Table 3).
Table 3. Blood ethanol concentrations (mg/ml) and handling induced convulsion scores.
| Line | Blood ethanol concentration |
Handling induced convulsion scores |
||
|---|---|---|---|---|
| (mg/ml)(N) | Baseline | CORWDR | N | |
| WSP | 0.66 +/− 0.13(24) | 2.7 +/− 0.2 | 0.7 +/− 0.3 | 41 |
| WSR | 0.09 +/− 0.06(18) | 0.0 +/− 0.0 | 0.1 +/− 0.1 | 44 |
Mean +/− SE are shown. CORWDR is the sum of those withdrawal HIC scores exceeding baseline (hrs 2-6) minus baseline scores for each animal.
The time delay between the end of the 4-hr DID test on Day 4 and blood sampling became unavoidably longer as time progressed. Therefore, BEC data from the first 42 animals’ blood samples, which were collected within 30 minutes, were used to estimate BECs in this experiment (see Table 3). Animals were sampled in the order in which tubes were read, which was randomly assigned across Lines, replicates and sexes. Consistent with their significantly greater intake, WSP mice showed significantly greater blood ethanol concentrations [F(1,40) = 13.1, P < 0.001].
Discussion
The ethanol preference drinking data showed clear genetic differences across both pairs of selected lines. However, the direction of those differences was different in the two replicates of the selection. WSR-2 mice were found to drink more than WSP-2 mice, but this was only seen in females. When preference was assessed in the 17th and 19th selected generations, greater preference drinking was also seen in WSR-2 females than WSP-2 females. The difference was significant at the 2.2% and 10% concentrations, but only tended to be so at the 4.6% concentration (Kosobud et al., 1988). In the 1988 data, there was a significant main effect of Line on drinking, and no main effect of Replicate or interaction at 2.2% or 4.6%. A significant Line × Replication difference at 10% was shown to result from greater intake in WSR-2 than WSP-2 females, but not in WSR-1 vs WSP-1. Following our (subsequently-published) guidelines for interpreting genetic differences in replicated pairs of selected lines (Crabbe et al., 1990), we concluded that this suggested a negative genetic relationship between drinking and withdrawal severity. However, the current study found that WSP-1 females drank significantly more than WSR-1 females. Nonetheless, these results with the first replicate females bear some similarities with data from the earlier generations. In 1988, WSP-1 and WSR-1 females did not differ in intake of 2.2, 4.6 or 10% ethanol vs. water during 8-day exposures to each concentration successively. This differs from the current results, although exposures to each concentration at present were half the earlier duration (see Figure 1). A second experiment in 1988 with all 4 lines offered them ethanol concentrations in two-day blocks using an up-and-down procedure. Mice were first offered 1% ethanol vs. water, and if they showed a preference ratio of 0.2 or greater, they were next offered a higher concentration. At each choice, they were either offered greater or lesser concentrations during the next 2 day block, contingent upon the 0.2 preference ratio criterion. Testing continued for 10 blocks and concentrations ranged between 1% and 14.5%. WSR-1 females showed a fairly linear increase in g/kg consumption as concentrations increased. WSP-1 females, however, showed a rapid escalation of intake and drank twice as much ethanol as WSR-1 through blocks 3-5. Thereafter, they began to avoid ethanol, and by blocks 8-10, they were drinking nearly no ethanol, while WSR-1 females were drinking 2 g/kg/day. In contrast, WSR-2 females consistently drank twice as much as WSP-2 females across the last 7 two-day blocks. Thus, the WSP-1/WSR-1 comparison in the earlier generations yielded a more complicated picture than revealed in the current experiments, depending on the drinking protocol used, and there was evidence from selected generations S17 and S19 for both greater and lesser preference in WSP-1 vs WSR-1. No males were tested in the early experiment (Kosobud et al., 1988).
We conclude from Experiment 1 that the preference for ethanol solutions during 24 hr access cannot be considered to be a strongly correlated response to selection of WSP/WSR for withdrawal HIC severity. While there are clear genetic differences in preference in both pairs of withdrawal-selected lines, their direction is not consistent. This does not rule out the possibility that there might be some alcohol drinking regimen that would reveal a clear and consistent difference between WSP and WSR lines of both replicates and sexes. As discussed above, evidence for the negative genetic correlation is almost entirely based on ethanol preference for specifically 10% solutions vs. withdrawal HIC, and is strongest in populations derived from B6 and D2 inbred strains. The current data with WSP-2 and WSR-2 lines suggest that the relationship obtains across both 10% and 20% concentrations, at least in females.
Since the 1988 report (Kosobud et al., 1988) and the 1998 meta-analysis (Metten et al., 1998), other data have been generated relating withdrawal and drinking. The strongest evidence for the negative genetic correlation, reported in 1998, was derived from two pairs of selected lines of mice, the High (HAW) and Low (LAW) Alcohol Withdrawal lines (selected for withdrawal HIC severity following acute high-dose ethanol injections) and the STRDRHI/STDRLO lines (selected for continuous access, two bottle preference for 10% ethanol solutions). Both selections started with an F2 intercross of the C57BL/6J and DBA/2J inbred strains. Naive mice from the 4th selected generation of each pair of lines were tested for the other trait, and showed significant differences supporting the correlation (preference: LAW>HAW, and acute withdrawal: STDRLO>STDRHI, respectively; see Metten et al, 1998). Both these short-term selections have since been repeated, with nearly identical results (Metten, Belknap, Phillips & Crabbe, unpublished data).
Other studies employed related traits. Mice from two pairs of lines selectively bred for preference for 10% ethanol have also been tested for withdrawal after an acute injection of 4.0 g/kg ethanol. Males from both lines selected for Low Alcohol Preference (LAP-1 and LAP-2), and female LAP-1 mice, showed reduced acoustic startle responses during withdrawal as compared with their pre-injection baseline sensitivity, while the HAP lines tended to show exacerbated startle responses. These results seem to be in the opposite direction from the HIC data in other mouse genotypes: the HAP and LAP mice were not tested for HICs (Chester and Barrenha, 2007). A similar question was asked in rat lines selected for high (P, HAD-1 and HAD-2) versus low preference (NP, LAD-1 and LAD-2). Animals were infused intragastrically with 4 g/kg ethanol and startle responses during withdrawal were compared with pre-infusion baseline scores. Startle responses were elevated during withdrawal in NP and LAD-1, but not LAD-2 rats, which seems more consistent with a negative genetic relationship between preference and withdrawal severity (Chester et al., 2003). In another experiment P/NP and HAD-1/LAD-1 rats were given 10 daily intragastric infusions of 4 g/kg ethanol. During periodic withdrawal tests, LAD-1 showed behavioral signs of withdrawal (tail stiffness, body posture) after 1 but not after 5 or 10 infusions. NP rats showed withdrawal signs after 5 infusions, and tended to show such signs after 10 infusions, but not after 1 infusion. Neither P nor HAD-1 rats expressed withdrawal signs (Chester et al., 2002). Finally, HAP and LAP mice of both sexes were exposed to ethanol vapor using a chronic-intermittent design. Mice were exposed for 16 hr to vapor and then tested for HICs for 8 hrs, apparently for 4 days in succession. Overall, it appears that LAP-1 and -2 mice showed greater HIC scores than HAP-1 and -2 mice, which would support the negative genetic correlation. However, these studies also included C3H/HeNcr mice in the statistical analyses, and there were differences among the HAP and LAP genotypes in HICs after air exposure. Thus, it is not entirely clear to us from the statistical analyses reported that withdrawal was greater in LAP mice than HAP mice, or whether this was true only for some replicate-sex combinations (Lopez et al, 2011). Given the possible species differences, the different withdrawal phenotypes, and the somewhat contradictory results from these four studies, they do not appear to offer either strong confirmation or refutation of the putative negative genetic correlation.
The genetic differences between pairs of WSP and WSR lines in ethanol preference cannot easily be attributed to differences in taste sensitivity. There were no consistent differences between pairs of selected lines of either replicate in preference for saccharin or sucrose, or in avoidance of quinine solutions. The similarity of results for saccharine and sucrose also argues that WSP and WSR mice are electing to ingest ethanol for reasons other than taste; in this they resemble many other genotypes that show selective interest in ethanol solutions. It is also unlikely that differences in ethanol absorption, distribution or metabolism were responsible for the preference drinking differences in Experiment 1 and the difference in DID intake in Experiment 3. Published studies have failed to find differences between WSP and WSR mice in blood ethanol concentrations or pharmacokinetics [(Crabbe and Kosobud, 1986; Terdal and Crabbe, 1994) and unpublished data].
WSP mice drank more ethanol than WSR in a relatively new test of binge-like drinking, drinking in the dark. Neither selected line drank a great deal in this test, as compared with standard inbred strains (Crabbe et al., 2012b). WSP mice, however, drank enough to achieve measurable blood alcohol levels. Of the 24 WSP mice from which we obtained a usable blood sample, 16 registered non-zero values ranging as high as 1.93 mg/ml. Of the 18 WSR mice samples, only 2 registered measurable blood alcohol levels (maximum value = 0.83 mg/ml). Thus, significantly more WSP than WSR mice reached non-zero blood alcohol levels (χ2 = 13.0, P < 0.001), and those levels led WSP mice to experience mildly exacerbated HICs after drinking terminated. The WSP mice drank 4.3 g/kg, and the WSR mice drank 3.8 g/kg on Day 4 of their DID test. BECs were 0.66 and 0.09 mg/ml, respectively. Data from inbred strain means suggests that g/kg intake and BEC are correlated about 0.73 by Pearson’s r. Thus, intake predicts about 53% of the variance in BECs (Rhodes et al, 2007). Given the relative dearth of specific information about this relationship for any given genotype, and the relatively weak linearity (there is a significant, scalloping upward second-degree relationship as well), we were not surprised to find a difference of 0.5 mg/ml between the genotypes in BEC; for the WSR mice, 0.09 mg/ml is a BEC that is apparently too low to yield significant withdrawal HICs.
We have selectively bred two lines of mice for high blood ethanol concentrations after a 2-day DID test (Crabbe et al., 2009). When we recently tested the High Drinking in the Dark (HDID-1) mice after a 4-day DID test, they achieved blood ethanol concentrations averaging 1.0 mg/ml and showed withdrawal HICs that were significantly elevated (unpublished data). Although there is a substantial genetic correlation across inbred strains between intake during preference drinking and during DID tests, there are certainly strains whose data do not adhere to this pattern (Crabbe et al., 2012b), so it is not surprising to find that the DID and preference drinking outcomes for WSP and WSR were different. When the lines selectively bred for high DID (HDID-1 and -2) were tested for chronic withdrawal severity after forced ethanol vapor inhalation using methods substantially similar to those that served as the selection phenotype for WSP and WSR, we found that the HDID lines did not differ in withdrawal severity from non-selected controls (Crabbe et al., 2012a).
Water and total fluid consumption in Experiments 1 and 2 showed complicated and inconsistent effects. In particular, intake of water was obviously affected by the different tastant alternatives, but we could glean no pattern from the outcomes that enlightened us about selected line differences in ethanol intake. Body weights differed initially in all 3 experiments. There was an overall tendency for WSR mice to weigh more than WSP (males only in Experiment 1, females only in Experiment 2, and both sexes in Experiment 3). The percent change in body weight across days in Experiments 1 and 2 appeared idiosyncratic with respect to genotype, replicate and sex combination: there was very little weight gain and there were no significant changes across the 4 days of Experiment 3.
Thus, recent generations of WSP and WSR mice confirm that WSR-2 mice drink more ethanol in 2-bottle preference tests than WSP-2. However, the difference between WSR-1 and WSP-1 is in the opposite direction, with WSP-1 generally showing stronger preference. We do not believe that differences in preference for sweet taste or avoidance of bitter taste account for the differences between selected lines; neither do differences in absorption, distribution and elimination of ethanol. Because all four lines show roughly equivalent preference for sucrose and saccharine solutions, we do not believe that the differences in alcohol preference can be attributed to calorie seeking. However, the opposite direction of the preference findings in the two replicates of WSP vs WSR do not offer additional support for the negative genetic correlation between withdrawal severity and drinking. The differences between the 1988 data and the current data may have arisen due to genetic drift; that is, some genes segregating at the time the 1988 studies were conducted (Kosobud et al., 1988) may now have fixed alleles. Response to selection for withdrawal severity was possibly already complete by the 11th selected generation (Crabbe et al., 1985), and was definitely complete when selection was terminated after 25 generations (Crabbe and Phillips, 1993). Because we took care to minimize inbreeding, many genes not related to withdrawal severity remained polymorphic for more generations than those actively selected for or against. However, given the necessarily limited sample sizes of the breeding populations, with time inbreeding at other genes is inevitable, and some of those genes could, for example, have become fixed in either the WSP-1, WSR-1, or both lines, leading to the significant difference in preference (WSP-1 > WSR-1) in the current generations. The DID test did not exist until recently, so no data are available from earlier generations. However, the significant difference in DID favoring WSP mice of both replicates is fairly strong evidence of a correlated response to selection (Crabbe et al., 1990). The current data cannot distinguish whether differential sensitivity to pre-absorptive (e.g., taste or smell) or post-absorptive (seeking positive hedonic effects of drinking, avoidance of negative consequences of drinking, or both) factors account for the differences between the pairs of selected lines for either DID or preference drinking.
In summary, lines of mice selectively bred for severe (WSP) or mild (WSR) ethanol withdrawal convulsions show differences in preference for ethanol solutions vs. water. The differences, however, were not negatively correlated with withdrawal severity. WSP mice showed greater limited-access drinking than WSR in the drinking in the dark paradigm. They reached blood alcohol levels significantly greater than zero and showed mild handling-induced withdrawal convulsions after drinking. The differences in DID were positively correlated with withdrawal severity. These data offer further evidence of a genetic dissociation between continuous access, two bottle preference drinking and DID in mice.
Acknowledgments
These studies were supported by Grants AA10760, AA13519, and AA20245 from the NIH-NIAAA; by the Department of Veterans Affairs; and by the Department of the Army/DoD-TATRC grant 10245005.05. AMB-L was supported by NIH-NIAAA grant AA007468, the Achievement Rewards for College Scientists Foundation, and an OHSU Graduate Research Scholar fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Buck KJ, Metten P, Belknap JK, Crabbe JC. Quantitative trait loci involved in genetic predisposition to acute alcohol withdrawal in mice. J. Neurosci. 1997:3946–3955. doi: 10.1523/JNEUROSCI.17-10-03946.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester JA, Barrenha GD. Acoustic startle at baseline and during acute alcohol withdrawal in replicate mouse lines selectively bred for high or low alcohol preference. Alcohol. Clin. Exp. Res. 2007:1633–1644. doi: 10.1111/j.1530-0277.2007.00462.x. [DOI] [PubMed] [Google Scholar]
- Chester JA, Blose AM, Froehlich JC. Further evidence of an inverse genetic relationship between innate differences in alcohol preference and alcohol withdrawal magnitude in multiple selectively bred rat lines. Alcohol. Clin. Exp. Res. 2003:377–387. doi: 10.1097/01.ALC.0000056619.98553.50. [DOI] [PubMed] [Google Scholar]
- Chester JA, Price CS, Froehlich JC. Inverse genetic association between alcohol preference and severity of alcohol withdrawal in two sets of rat lines selected for the same phenotype. Alcohol. Clin. Exp. Res. 2002:19–27. [PubMed] [Google Scholar]
- Crabbe JC. Provisional mapping of quantitative trait loci for chronic ethanol withdrawal severity in BXD recombinant inbred mice. J. Pharmacol. Exp. Ther. 1998:263–271. [PubMed] [Google Scholar]
- Crabbe JC, Colville AM, Kruse LC, Cameron AJ, Spence SE, Schlumbohm JP, Huang LC, Metten P. Ethanol tolerance and withdrawal severity in high drinking in the dark selectively bred mice. Alcohol. Clin. Exp. Res. 2012a:1152–1161. doi: 10.1111/j.1530-0277.2011.01715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Kosobud A. Sensitivity and tolerance to ethanol in mice bred to be genetically prone or resistant to ethanol withdrawal seizures. J. Pharmacol. Exp. Ther. 1986:327–333. [PubMed] [Google Scholar]
- Crabbe JC, Kosobud A, Young ER, Tam BR, McSwigan JD. Bidirectional selection for susceptibility to ethanol withdrawal seizures in Mus musculus. Behav. Genet. 1985;15:521–536. doi: 10.1007/BF01065448. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Merrill CD, Belknap JK. Effects of convulsants on handling-induced convulsions in mice selected for ethanol withdrawal severity. Brain Res. 1991:1–6. doi: 10.1016/0006-8993(91)90397-e. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Metten P, Huang LC, Schlumbohm JP, Spence SE, Barkley-Levenson AM, Finn DA, Rhodes JS, Cameron AJ. Ethanol withdrawal-associated drinking and drinking in the dark: Common and discrete genetic contributions. Addiction Genet. 2012b:3–11. doi: 10.2478/addge-2012-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Metten P, Rhodes JS, Yu C-H, Brown LL, Phillips TJ, Finn DA. A line of mice selected for high blood ethanol concentrations shows drinking in the dark to intoxication. Biol. Psychiat. 2009:662–670. doi: 10.1016/j.biopsych.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ. Selective breeding for alcohol withdrawal severity. Behav. Genet. 1993:171–177. doi: 10.1007/BF01067422. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Kosobud A, Belknap JK. Estimation of genetic correlation: interpretation of experiments using selectively bred and inbred animals. Alcohol. Clin. Exp. Res. 1990:141–151. doi: 10.1111/j.1530-0277.1990.tb00461.x. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Spence SE, Brown LL, Metten P. Alcohol preference drinking in a mouse line selectively bred for high drinking in the dark. Alcohol. 2011:427–440. doi: 10.1016/j.alcohol.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer DS, Mackay TFC. Introduction to Quantitative Genetics. Longman; Harlow, England: 1996. [Google Scholar]
- Kosobud AE, Bodor AS, Crabbe JC. Voluntary consumption of ethanol in WSP, WSC and WSR selectively bred mouse lines. Pharmacol. Biochem. Behav. 1988:601–607. doi: 10.1016/0091-3057(88)90026-3. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Grahame NJ, Becker HC. Development of ethanol withdrawal-related sensitization and relapse drinking in mice selected for high- or low-ethanol preference. Alcohol. Clin. Exp. Res. 2011:953–962. doi: 10.1111/j.1530-0277.2010.01426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClearn GE, Wilson JR, Meredith W. The use of isogenic and heterogenic mouse stocks in behavioral research. In: Elliot RM, MacCorquodale K, Lindzey G, Clark KE, editors. Contributions to Behavior-Genetic Analysis: The Mouse as a Prototype. Appleton-Century-Crofts; New York: 1970. pp. 3–22. [Google Scholar]
- Metten P, Crabbe JC. Common genetic determinants of severity of acute withdrawal from ethanol, pentobarbital and diazepam in inbred mice. Behav. Pharmacol. 1994:533–547. doi: 10.1097/00008877-199408000-00014. [DOI] [PubMed] [Google Scholar]
- Metten P, Crabbe JC. Alcohol withdrawal severity in inbred mouse (Mus musculus) strains. Behav. Neurosci. 2005:911–925. doi: 10.1037/0735-7044.119.4.911. [DOI] [PubMed] [Google Scholar]
- Metten P, Phillips TJ, Crabbe JC, Tarantino LM, McClearn GE, Plomin R, Erwin VG, Belknap JK. High genetic susceptibility to ethanol withdrawal predicts low ethanol consumption. Mamm. Genome. 1998:983–990. doi: 10.1007/s003359900911. [DOI] [PubMed] [Google Scholar]
- Metten P, Sorensen ML, Cameron AJ, Yu C-H, Crabbe JC. Withdrawal severity after chronic intermittent ethanol in inbred mouse strains. Alcohol. Clin. Exp. Res. 2010:1552–1564. doi: 10.1111/j.1530-0277.2010.01240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips TJ, Crabbe JC, Metten P, Belknap JK. Localization of genes affecting alcohol drinking in mice. Alcohol. Clin. Exp. Res. 1994:931–941. doi: 10.1111/j.1530-0277.1994.tb00062.x. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Feller DJ, Crabbe JC. Selected mouse lines, alcohol and behavior. Experientia. 1989:805–827. doi: 10.1007/BF01954056. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol. Behav. 2005:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu C-H, Brown LL, Finn DA, Garland T, Jr., Crabbe JC. Mouse inbred strain differences in drinking to intoxication. Genes Brain Behav. 2007:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Rustay NR, Crabbe JC. Genetic analysis of rapid tolerance to ethanol’s incoordinating effects in mice: Inbred strains and artificial selection. Behav. Genet. 2004:441–451. doi: 10.1023/B:BEGE.0000023649.60539.dd. [DOI] [PubMed] [Google Scholar]
- Terdal ES, Crabbe JC. Indexing withdrawal in mice: matching genotypes for exposure in studies using ethanol vapor inhalation. Alcohol. Clin. Exp. Res. 1994:542–547. doi: 10.1111/j.1530-0277.1994.tb00907.x. [DOI] [PubMed] [Google Scholar]
- Wahlsten D, Bachmanov A, Finn DA, Crabbe JC. Stability of inbred mouse strain differences in behavior and brain size between laboratories and across decades. Proc. Natl. Acad. Sci. USA. 2006:16364–16369. doi: 10.1073/pnas.0605342103. [DOI] [PMC free article] [PubMed] [Google Scholar]