Abstract
High concentrations of oxygen aggravate the severity of lung injury in patients requiring mechanical ventilation. Although mesenchymal stem cells have been shown to effectively attenuate various injured tissues, there is limited information regarding a role for amniotic fluid stem cells (AFSCs) in treating acute lung injury. We hypothesized that intravenous delivery of AFSCs would attenuate lung injury in an experimental model of hyperoxia-induced lung injury. AFSCs were isolated from EGFP transgenic mice. The in vitro differentiation, surface markers, and migration of the AFSCs were assessed by specific staining, flow cytometry, and a co-culture system, respectively. The in vivo therapeutic potential of AFSCs was evaluated in a model of acute hyperoxia-induced lung injury in mice. The administration of AFSCs significantly reduced the hyperoxia-induced pulmonary inflammation, as reflected by significant reductions in lung wet/dry ratio, neutrophil counts, and the level of apoptosis, as well as reducing the levels of inflammatory cytokine (IL-1β, IL-6, and TNF-α) and early-stage fibrosis in lung tissues. Moreover, EGFP-expressing AFSCs were detected and engrafted into a peripheral lung epithelial cell lineage by fluorescence microscopy and DAPI stain. Intravenous administration of AFSCs may offer a new therapeutic strategy for acute lung injury (ALI), for which efficient treatments are currently unavailable.
Introduction
Acute respiratory distress syndrome (ARDS) is a condition characterized by acute onset, bilateral lung infiltrates, refractory hypoxemia, and the absence of cardiogenic pulmonary edema. ARDS and acute lung injury (ALI) are major causes of mortality and morbidity in critically ill patients [1,2]. A cohort study in 21 hospitals in the United States revealed the age-adjusted incidence of ALI to be 86.2 per 100,000 person-years with 40% in-hospital mortality [3]. Clinically, ALI may be the end result of several disorders, such as pneumonia and pulmonary contusion, that directly injure the lung or those that indirectly injure the lung, including sepsis, severe trauma, and transfusions of blood products [4,5]. Histologically, the acute exudative phase (the first 24-72 h) of ALI is characterized by infiltration of inflammatory cells and disruption of the alveolar–capillary barrier, leading to a proteinaceous exudate that floods the alveolar spaces and then impairs gas exchange and precipitates respiratory failure [6,7].
Patients with ALI may require mechanical ventilation support with a high concentration of inspired oxygen. However, supplemental oxygen can exacerbate the pathogenic processes within the lung [8,9], and this may result in hyperoxia-induced ALI [10,11], the pathological changes of which resemble ARDS in animal models [12]. Thus, therapeutic strategies for hyperoxia-induced ALI may be the same as for clinical ARDS. Despite decades of efforts to identify pharmacologic agents to treat ALI, there are still no effective therapeutic agents for this condition [13].
Bone marrow-derived mesenchymal stem cells (BM-MSCs) can migrate to, or participate in the development of, lung tissue [14,15], and have been shown to have anti-inflammatory effects [16]. Several recent studies have demonstrated that stem/progenitor cells, including mesenchymal stem cells (MSCs), embryonic stem cells (ESCs), genetically engineered stem cells, and umbilical cord MSCs, have the potential to be used as cellular therapies that contribute to lung repair mechanisms after ALI [17–22]. Human amniotic fluid stem cells (AFSCs) have shown the ability to differentiate into lineages belonging to all three germ layers, and appear to have many of the key therapeutic benefits of ESCs while avoiding their ethical drawbacks [23]. Human AFSCs have intermediate characteristics between embryonic and adult stem cells and are able to differentiate into lineages representative of all three germ layers but unlike embryonic stem cells they do not have tumorigenic effect in vivo [24]. Furthermore, AFSCs represent an accessible source of cells that can be reprogrammed into pluripotent stem cells with two Yamanaka factors. These characteristics, together with absence of ethical issues concerning their employment, have made stem cells from amniotic fluid a promising candidate for cell therapy and tissue engineering [25].
However, information regarding the use of AFSCs for treating acute lung injury is limited [26]. In this study, we investigated whether murine AFSCs from enhanced green fluorescent protein (EGFP) transgenic mice have beneficial effects on lung function and animal survival in a model of hyperoxia-induced ALI.
Materials and Methods
Isolation and culture of murine amniotic fluid stem cells (AFSCs)
The animal use protocol was reviewed and approved by the Institutional Animal Care and Use Committee of the National Chung Hsing University (IACUC Approval No. 101-21). The samples of amniotic fluid (AF) were obtained from pregnant 6-8 week-old, female, EGFP-expressing transgenic mice [27]. Maternal uterine tissue was removed and placed in α-minimal essential medium (α-MEM) supplemented with 10% fetal bovine serum (FBS), then collected AF samples using a sterile insulin syringe with a 29-G x 1/2” needle. The AF samples were centrifuged for 5 min at 1,500 rpm. Cells thus obtained were plated in α-MEM supplemented with 10% FBS and incubated for 48 h at 37 oC containing 95% air and 5% CO2. The medium was changed every 72 h, and the cell colonies increased gradually over the next 7 to 10 days [28].
Colony-forming unit-fibroblast (CFU-F) assay and pluripotency markers detection
The CFU-F potential of suspension-derived cells was determined by plating aliquots (1,000 cells/cm2) of cells in complete MesenCultTM medium for 14 days and analyzing the colonies as previously described [29]. Two pluripotency markers, Oct-4 and Nanog, were detected by RT-PCR in original isolates (F-0) and fifth passages (F-5) of mouse AFSCs. The primer sets were designed (forward and reverse, respectively) as follows: Oct-4, 5’-GTACTTGTTTAGGGTTAGAG-3’ and 5’-GTGGAAAGACGGCT CACCT-3’; Nanog, 5’-AGTGCTCCTTCCAAACCCCA-3’ and 5’-CTGTGCTCC ACGCTGATG-3’. A pair of β-action primer (see below) was used as an internal control.
Flow cytometry analysis
Cultured cells (passages 5-8) were detached with trypsin/EDTA and counted. Approximately 1 × 106 cells were divided into aliquots in 5-mL centrifuge tubes. The cells were then stained with fluorescent phenyleneethylene (PE)-conjugated rat anti-mouse antibodies: CD44-PE (20 µg/mL, IM7, BD Biosciences, Oxford, UK), or CD105-PE (10 µg/mL, MJ7/18, Abcam, San Francisco, CA, USA), at 25 oC for 30 min. The cells were pelleted, washed twice with PBS and fixed with 1% paraformaldehyde. After fixation, analysis was performed by using a Cytomics FC500 (Beckman Coulter Inc., Fullerton, CA, USA) as described [30].
In vitro differentiation
Purified cell populations were cultured to 100% confluency and then differentiated by the appropriate inducing media for 21 days. For adipogenesis, the cultured cells were incubated in medium containing α-MEM, 10% FBS, 1 µM dexamethasone, 0.5 mM isobutylmethylxanthine, 10 µg/mL insulin, and 100 µM indomethacin. The cultured cells were then fixed and stained with 0.5% Oil Red O (Sigma-Aldrich, St. Louis, MO, USA). For osteogenesis, the cultured cells were incubated in inducing medium containing α-MEM, 10% FBS, 1 µM dexamethasone, 10 mM glycerol 2-phosphate, and 50 μM ascorbic acid 2-phosphate. The cultured cells were fixed and stained with 2% Alizarin red S (A5533, Sigma-Aldrich) for microscopic examination.
Murine model of hyperoxia-induced acute lung injury
Eight-week-old female CD-1 (ICR) wild type mice were obtained from the National Laboratory Animal Center (Taipei, Taiwan). The mice were provided with food and water ad libitum throughout this study and maintained at a temperature of 25 oC and humidity of 50-70% [30]. The mice were randomly assigned to one of the following four groups (n=10): (1) hyperoxia-exposed mice were treated with AFSCs; (2) hyperoxia-exposed mice were treated with PBS; (3) normoxia-exposed mice were treated with AFSCs; (4) normoxia-exposed mice were treated with PBS. The hyperoxia-exposed mice were housed in humidified 99% oxygen in a hyperoxia chamber with normobaric pressure for 60 h. After 60-h exposure of 99% oxygen, 5 × 105 AFSCs suspended in 100 µL PBS were injected via the tail vein using a sterile insulin syringe with a 29-G x 1/2” needle. The mice were sacrificed at days 1, 3, and 7 after the end of the exposure to the oxygen. For survival rate evaluation, additional two experimental groups received the same dose (5 x 105) of mouse embryonic fibroblast cells (MEFCs) or mouse bone marrow-mesenchymal stem cells (BM-MSCs) were added as the cell therapy controls. All the animal trials were repeated triplicate.
Histology and immunohistochemistry (IHC)
Lung tissues were fixed in 4% paraformaldehyde overnight, embedded in paraffin, and cut into sections for hematoxylin and eosin (H&E) staining. For IHC analysis, the sections were incubated with primary antibody of goat anti-eGFP (1:200; GeneTex Inc., San Antonio, TX, USA) at 4oC for 16 h. After wash with PBS, the sections were double stain with Alexa Fluor® 546 conjugated donkey anti-goat IgG for 1 h. After wash with PBS, the slides were mounted with DAPI-Fluoromount-GTM (SouthernBiotech, Birmingham, AL, USA) and analyzed by confocal microscopy (LSM5l0, Carl Zeiss, Germany) [31].
Lung wet-to-dry weight ratio analysis
The lung tissues were placed into previously weighed microcentrifuge tubes, and the initial (wet) weight of these lung tissues were measured. After these lung tissues were dried in an oven at 70 oC overnight, the (dry) weight was measured again. The wet/dry ratio was defined as the wet lung mass divided by the dry lung mass.
Analysis of airway inflammation in bronchoalveolar lavage fluid (BALF)
Bronchoalveolar lavage fluid (BALF) was collected using 500 µL of sterile endotoxin-free saline to wash the lungs. BALF cells were collected by centrifugation at 500 x g at 4°C. The number of BALF cells was determined using a hemocytometer [30].
TUNEL assay
Apoptotic cells in lung tissue sections were identified as terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL)-positive cells using a commercial kit (Chemicon, Temecula, CA, USA), according to the manufacturer’s instructions [32].
Masson trichrome stain for lung fibrosis analysis
The quantity of the collagen fibers in lung tissue sections was evaluated by Masson stain solution as described previously [33].
RNA isolation and quantitative real-time RT-PCR
Total RNA from the lung tissues from normoxic and hyperoxic mice was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and treated with RNase-free DNase I (MBI Fermentas Inc., Lithuania, Germany) to remove any genomic DNA contamination [34]. One microgram of total RNA was reverse-transcribed with MuLV reverse transcriptase and the cDNA was analyzed by real-time PCR using intron-spanning primers. For the quantification of mouse IL-1β, IL-6, and TNF-α mRNA levels, PCR was performed using Brilliant SYBR Green Q-PCR Master Mix (Stratagene, La Jolla, CA, USA) [35]. The results were normalized to β-actin mRNA levels. The primer sequences used (forward and reverse, respectively) were as follows: IL-1β: 5’-GCCCATCCTCTGTGACTCAT-3’ and 5’-AGGCCACAGGTATTTTGTCG-3’; IL-6: 5’-GTTGCCTTCTTGGGACTGAT-3’ and 5’-TGTACTCCAGGTAGCTATGG-3’; TNF-α: 5’-GCCCCCAGTCTGTATCCTTC-3’ and 5’-AGGCAACCTGACCACTC TCC-3; MCP-3: 5'-TCTGCCACGCTTCTGTGCCT-3' and 5'-GCTCTTGAGATTCC TCTTGGGGAT-3'; and β-actin: 5’-ACACCCGCCACCAGTTCGC-3’ and 5’-ACCCAT TCCCACCATCACAC-3’.
Co-culture experiments
The co-culture method was used to evaluate the impact of AFSCs on the hyperoxia-injured or normal lung tissues in vitro. The entire lung was placed in 1 mL α-MEM and then mechanically dispersed to create a suspension. The supernatants from the tissues from hyperoxia-exposed mice or normoxia-exposed mice were placed in the wells of 24-well plate and a 6-µm-pore size membrane trans-well containing 5 × 103 EGFP-expressing AFSCs was inserted into the supernatant-containing wells.
Statistical analysis
The data are presented as the means ± SD. A Mann–Whitney U-test and Student’s t-test were applied for data analyses. Differences with p<0.05 were considered to be statistically significant.
Results
Isolation of AFSCs from EGFP-transgenic mice
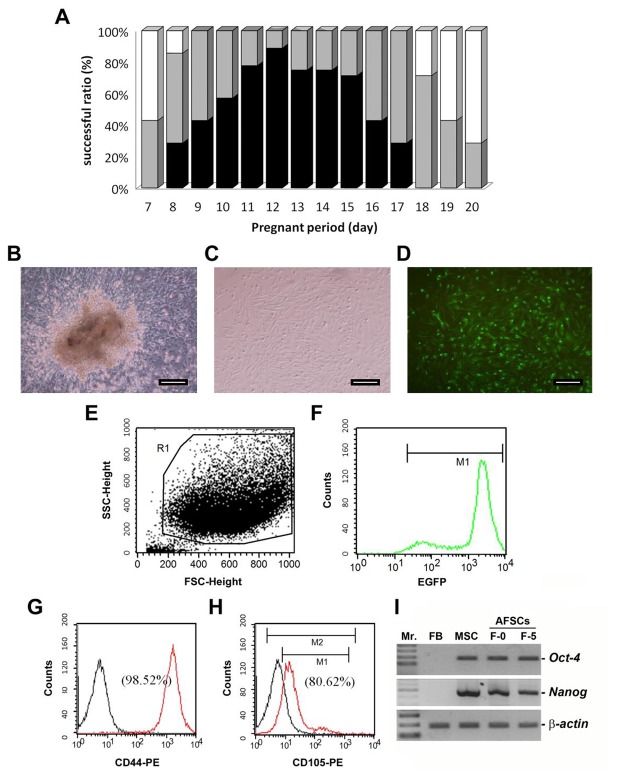
After isolation of the cells from amniotic fluid from mice at gestation stages earlier than Day 7, a few adherent cells were observed, but these cells failed to proliferate. Successful isolation of amniotic fluid stem cells (AFSCs) was achieved from pregnant females between Day 7 and Day 20 of gestation (Figure 1A). Furthermore, the CFU-F test indicated 70-90% higher success rates for the culture of fibroblast-like adherent AFSCs during the middle gestation stages of Days 11 and 12 (Figure 1A).
Figure 1. Isolation and characterization of AFSCs from EGFP-expressing transgenic mice.
(A) The influence of the different pregnancy periods on the success rate of obtaining the murine stem cells from amniotic fluid. The black columns represent the AFSC cells, easily identified after amniotic fluid cell culture and confirmed by the colony forming assay. Grey columns represented that fibroblast-like adherent cells were identified after amniotic fluid cell culture but failed to pass the colony forming test. White columns represented a few cells obtained that were not confirmed to be AFSC cells. n=7 in each examined pregnancy period. (B, C) The morphologies of identified mouse AFSCs in single layer under bright and fluorescence fields, respectively. (D) EGFP-AFSCs expand from the colony after 10 days in culture (100x magnification). Scale bar = 50 µm. (E, F) Flow cytometric identification of the EGFP-expressing AFSCs. Regions R1 and M1 represent the corresponding fluorescence profile of the EGFP-expressing AFSCs, respectively. (G, H) Immunophenotypes of EGFP-AFSCs by flow cytometric analysis of the cell surface antigens CD44 and CD105, respectively. Black line represents the control group (unstained cell). Red line represents the cells stained with fluorescent phenyleneethylene (PE)-conjugated rat anti-mouse antibodies with CD44 or CD105. (I) Two pluripotency markers of Oct-4 and Nanog in the original isolates (F-0) and fifth passages (F-5) of mouse AFSCs were detected by RT-PCR. Mr.: 100-bp ladder DNA marker. FB: mouse fibroblast cells; MSC: mesenchymal stem cells.
Characterization of AFSCs
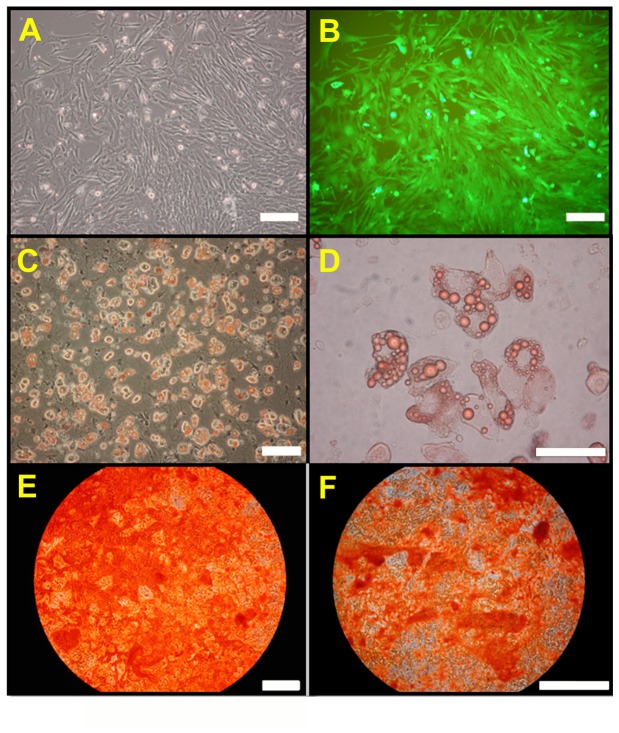
Colony forming units of AFSCs (Figure 1B) were developed from single EGFP-expressing cells (Figure 1C, 1D). Selected AFSCs by flow cytometry (region R1, Figure 1E) was counted and confirmed for EGFP fluorescent expression (Figure 1F). In Figure 1G and 1H, black line represented the control group (unstained cell). Red line represents the cells stained with fluorescent phenyleneethylene (PE)-conjugated rat anti-mouse antibodies with CD44 or CD105. Flow cytometric analysis revealed that the EGFP-positive AFSCs strongly expressed the cell interaction, adhesion, and migration surface marker CD44 (98.52%; Figure 1G) and moderately expressed CD105 (80.62%; Figure 1H). Two pluripotency markers of Oct-4 and Nanog were also expressed in the original isolates (F-0) and fifth passages (F-5) of mouse AFSCs by RT-PCR analysis (Figure 1I). These results indicated that the cells established from the amniotic fluid were stem cells. In vitro differentiation assays demonstrated that the AFSCs from EGFP transgenic mice retained their ability to form osteoblasts (Figure 2C and 2D), and adipocytes (Figure 2E and 2F) at passages 10-15 under the appropriate media conditions. Morphologically, the cells expressed EGFP (Figure 2B) and had a spindled, fibroblast-like appearance in culture (Figure 2A), consistent with the characteristics of AFSC.
Figure 2. In vitro differentiation of EGFP-AFSCs by specific inducing conditions examined by staining.
(A, B) Myofibroblast-like AFSCs expressing EGFP after 7 days in culture under bright and fluorescence fields, respectively. (C, D) Adipogenic capability of EGFP-AFSCs by Oil Red O staining after three weeks induction, shown at 100x and 200x magnification, respectively. (E, F) Osteogenic capability of EGFP-AFSCs demonstrated by Alizarin Red S staining after three weeks induction shown at 100x and 200x magnification, respectively. Scale bar = 50 µm.
Effect of AFSCs on survival and histopathology of hyperoxia-induced lung injury
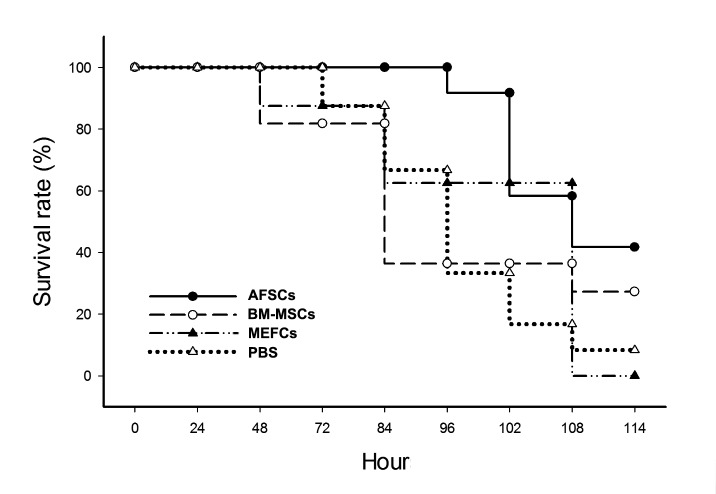
None of the mice died during the first 60 h of exposure to hyperoxia (hyperoxia-exposure period). These mice were randomly separated in four groups (n=10), one of which received the therapeutic intervention of 5 x 105 AFSCs (test group), second and third groups received the same dose of mouse bone marrow-mesenchymal stem cells (5 x 105 BM-MSCs) and mouse embryonic fibroblast cells (5 x 105 MEFCs), respectively, and fourth group received the PBS placebo as a control. During the next 48 h of hyperoxia exposure, 15% and 20% of the mice in the MEFCs- and BM-MSCs- treated groups died, respectively. After 84 h post-hyperoxia exposure, 30%, 33% and 63% of the mice died in the PBS placebo, MEFCs-treated, and BM-MSCs-treated groups, respectively, but 100% survival rate in the AFSCs-treated group. After 102 h post-hyperoxia exposure, the mice that had received intravenous AFSCs therapy had a significant higher survival rate (90%) than the mice that had received intravenous MEFCs (60%), BM-MSCs (37%), and PBS (30%) (p<0.01; Figure 3). The survival rate in the AFSCs-treated mice was stably maintained at 43% from 114 h to 14 days post-hyperoxia exposure, but only 27%, 10%, and 0% survival rates were obtained in the BM-MSCs-, PBS-, and MEFCs-treated groups, respectively, during the longer-term observation.
Figure 3. Comparison of the survival rates from the AFSCs-, BM-MSCs-, MEFCs-, and PBS-treated groups.
After hyperoxia exposure for 60 h, mice were randomly separated in four groups which received intravenously 5 x 105 AFSCs or BM-MSCs as the test groups (n=10), MEFCs as a control group (n=10), and PBS treatment as a placebo control group (n=10). The survival rates were observed and plotted at 6 h or 12 h intervals. The experiments were repeated three times.
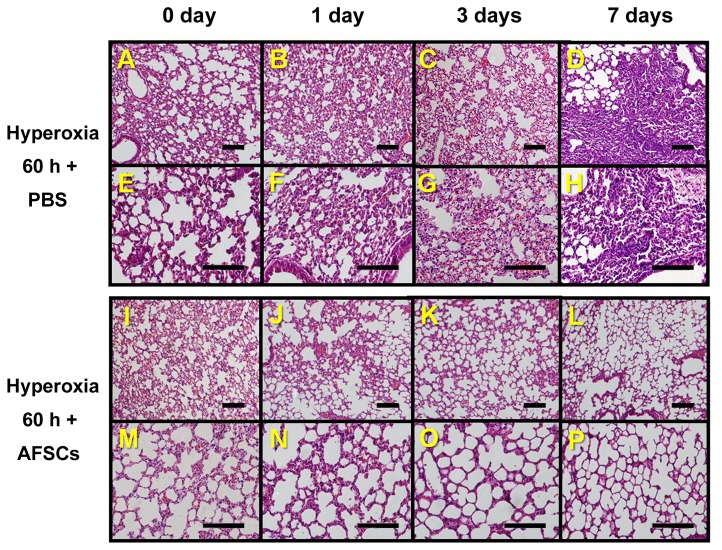
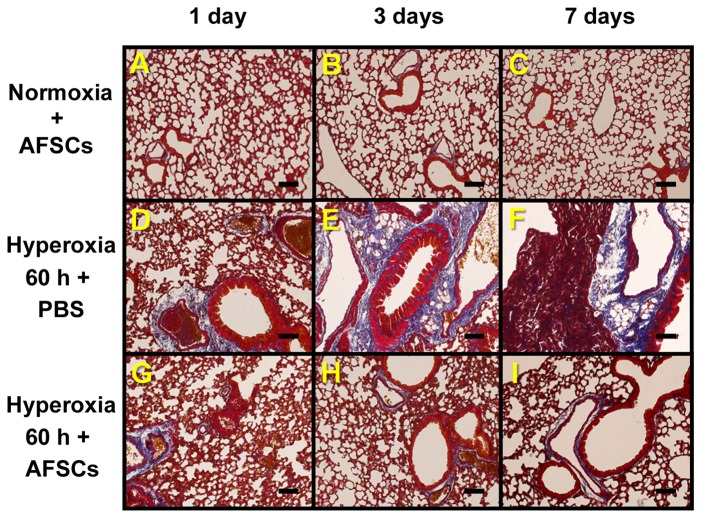
Lung tissue was harvested on days 1, 3, and 7 after 60-hour hyperoxia. The obvious pathological differences between the control and treated groups were began at the day 1 post-hyperoxia, at which point the H&E staining of lung sections from AFSCs-treated mice had significant less lung injury (inflammatory cell infiltration and alveolar wall destruction, Figure 4I–P) than the control mice given PBS (Figure 4A–H).
Figure 4. Histological comparisons of lung tissues from the hyperoxia-exposed control mice and the hyperoxia-exposed AFSCs-treated mice.
(A, B, C, D) Lung histology (H&E staining, 200x magnification) of mice exposed to hyperoxia for 60 h, shown at 0, 1, 3, and 7 days, respectively, after treatment with PBS. (E, F, G, H) The same groups as above shown at 400x magnification. (I, J, K, L) Histology (H&E staining, 200x magnification) of lung sections from animals exposed to hyperoxia for 60 h, shown at 0, 1, 3, and 7 days, respectively, after treatment with AFSCs. (M, N, O, P) The same groups as shown in I, J, K, and L shown at 400x magnification. Scale bar =100 µm.
Effect of AFSCs on lung edema, inflammation, apoptosis, and fibrosis induced by hyperoxia
As expected, the mice exposed to 60 h hyperoxia had a significantly higher wet/dry ratio than the control mice (6.7 vs. 4.7, p<0.01). Furthermore, one day after hyperoxia exposure, the mice that received the AFSCs treatment had a significantly lower wet/dry ratio (5.2 vs. 6.3, p<0.05) than the mice without AFSCs treatment (Figure 5A). To determine the effect of AFSCs on the inflammatory response, BALF was analyzed for neutrophil content. The mice exposed to hyperoxia had significantly higher levels of neutrophil infiltration as measured in the cytospin slide than the control mice (16% vs. 2%, p<0.01). Neutrophil infiltration was significantly less in the mice that received the AFSCs treatment than in the mice that received PBS (p<0.01), at all three post-hyperoxia periods (days 1, 3, or 7 post-hyperoxia) (Figure 5B).
Figure 5. Comparison of the wet/dry ratio, neutrophil infiltration, and TUNEL assay in lungs from the hyperoxia-exposed PBS-treated control mice and the AFSCs-treated mice.
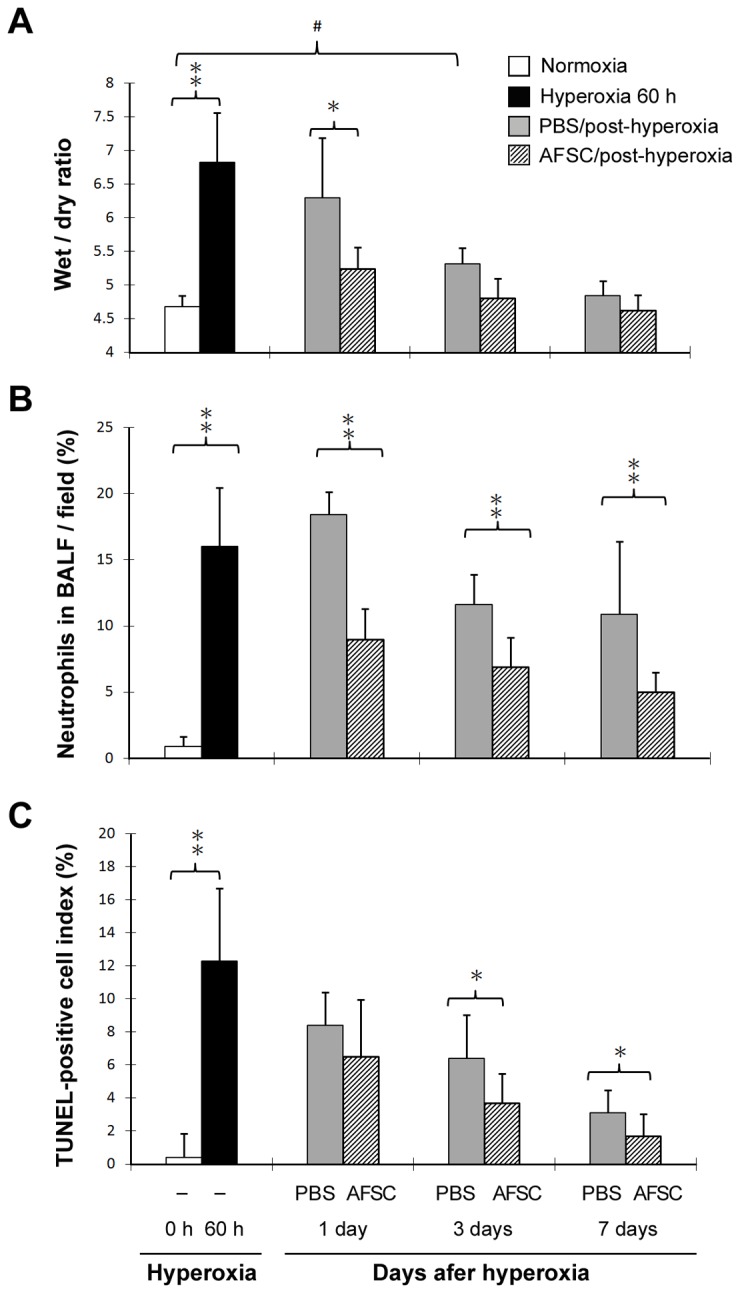
(A) Pulmonary edema was measured as the wet/dry weight ratio of the lungs (n=10 in each group). (B) Pulmonary inflammation measured as the leukocyte counts of the BALF (n=10 in each group). (C) Pulmonary cell apoptosis measured as the TUNEL assay of the lungs (n=10 in each group). **p<0.01; *p<0.05; # p<0.05.
The TUNEL assay was used to determine whether AFSCs influence the apoptosis of the lung cells in the hyperoxia model. The mice exposed to hyperoxia had significantly higher percentages of TUNEL-positive cells than the mice exposed to room air (12% vs. 0.5%, p<0.01). The mice that received AFSCs had significantly fewer TUNEL-positive cells than the mice that received PBS days 3 and 7 after the 60 h period of hyperoxia (Figure 5C). Masson trichrome staining indicated that the normal lung images were shown in Figure 6A–C and the mice that received PBS at days 3 and 7 post-hyperoxia exposure (Figure 6D–F) had significantly severer fibrosis than the mice that received the AFSCs treatment (Figure 6G–I).
Figure 6. Comparison of fibrosis status using Masson trichrome stain in lungs from the hyperoxia-exposed PBS- or AFSCs-treated mice.
(A, B, C) The normoxia-exposed mice treated with AFSCs at 1, 3, and 7 days, respectively. (D, E, F) The hyperoxia-exposed mice treated with PBS at 1, 3, and 7 days, respectively. (G, H, I) The hyperoxia-exposed mice treated with AFSCs at 1, 3, and 7 days, respectively. Scale bar =100 µm.
AFSCs reduced inflammatory cytokines in mice lung tissues
To further evaluate the anti-inflammatory actions by AFSCs, quantitative real-time RT-PCR was used to measure the levels of proinflammatory cytokines expressions in the lung tissues of the mice (Figure 7). Inflammatory cytokines (IL-1β, IL-6, and TNF-α) were all elevated in lung tissues in response to hyperoxia exposure (8.9 ± 0.6, 5.6 ± 1.7, and 6.4 ± 0.9 folds, respectively) compared with normoxia (p<0.05). The treatment with AFSCs significantly decreased the levels of proinflammatory cytokine expression (5.5 ± 1.2, 2.7 ± 0.4, and 1.9 ± 0.5 folds, respectively) compared with PBS treatment (p<0.05).
Figure 7. Comparison of inflammatory cytokine mRNA expression levels in the lungs of the hyperoxia-exposed PBS-treated control mice and the AFSCs-treated mice.
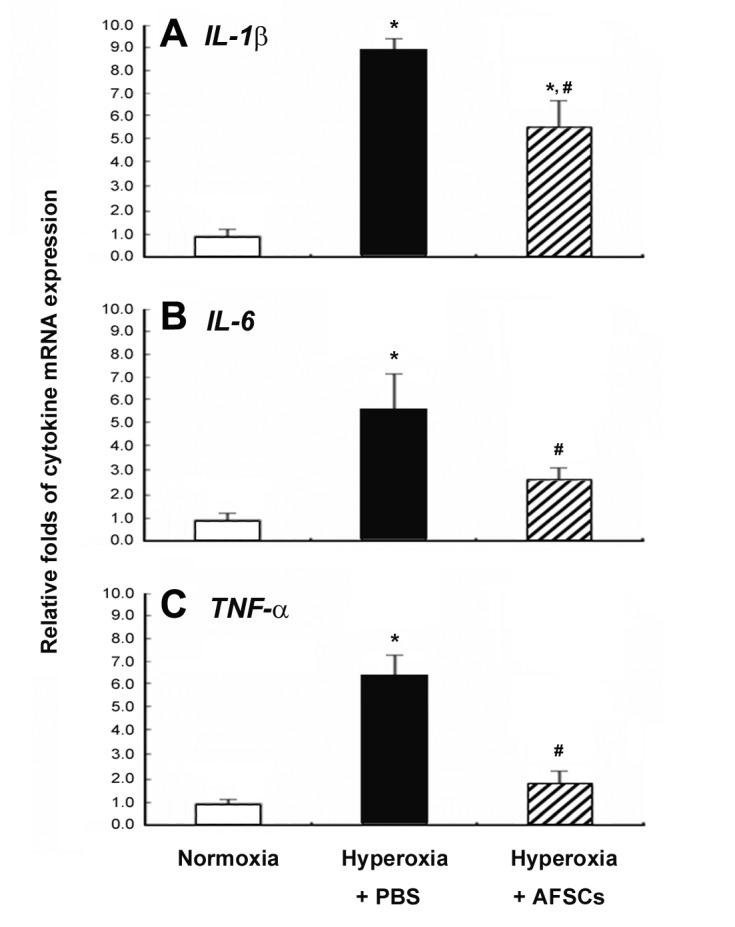
IL-1β (A), IL-6 (B), and TNF-α (C) mRNA expression levels were measured using quantitative RT-PCR from the following three groups: normoxia mice, hyperoxia-exposed mice treated by PBS, and hyperoxia-exposed mice treated by AFSCs at day 7. *p<0.05 vs. normoxia group; # p<0.05 vs. hyperoxia-exposed with PBS group.
Accumulation of AFSCs within the inflammatory site of the injured lung
To determine whether EGFP-expressing AFSCs could accumulate within the lung tissue, we analyzed the EGFP-expressing cells in the lung sections obtained on days 1, 3, and 7 after hyperoxia-exposure. Fluorescence microscopy revealed that the numbers of EGFP-expressing cells were greatest on day 1 after hyperoxia-exposure (Figure 8A). As shown in Figure 8, the numbers of EGFP-expressing cells gradually decreased on the following days (Figure 8A, 8B, and 8C) relative to the DAPI-stained cell populations (Figure 8D, 8E, and 8F).
Figure 8. Migration of the intravenously injected EGFP-expressing AFSCs to lung tissue after hyperoxia exposure for 60 h.
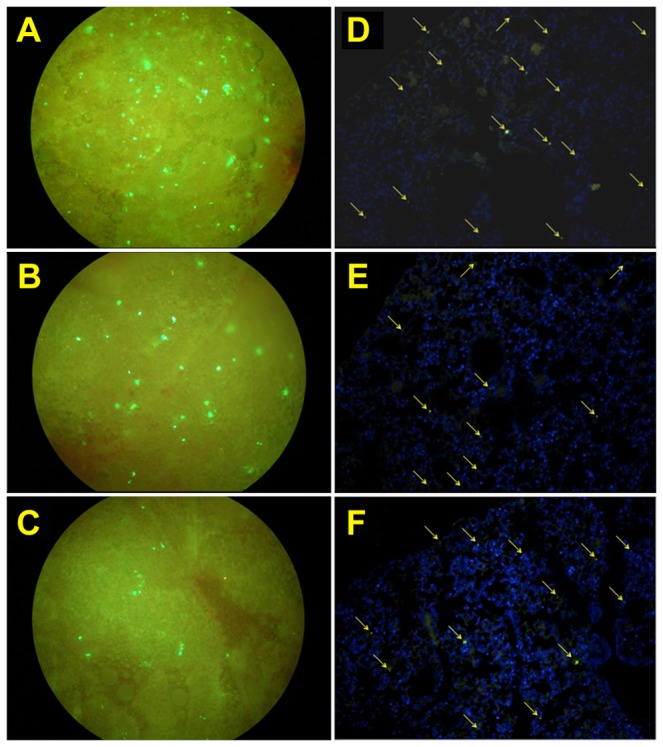
(A, B, C) The lung tissues were minced and examined by fluorescence microscopy at 200x magnification 1, 3, and 7 days, respectively, after AFSCs were injected. (D, E, F) The paraffin-embedded lung sections were stained with DAPI and immunostained for EGFP-positive AFSCs 1, 3, and 7 days, respectively, after injection of the AFSCs, shown at 200x magnification. Scale bar = 100 µm.
Conditioned medium from hyperoxia-exposed lung tissues increased AFSCs migration
To confirm whether AFSCs has the ability to migrate to the inflammatory sites in vitro, we performed co-culture of hyperoxia-exposed lung tissues and AFSCs (Figure 9A). In this experiment, the medium from hyperoxia-exposed lung tissues significantly increased the numbers of migrating AFSCs more than 5-fold relative to the numbers that migrated in response to the control lung-conditioned medium (p<0.01; Figure 9B).
Figure 9. Migration of AFSCs is induced by hyperoxia-exposed lung tissues.
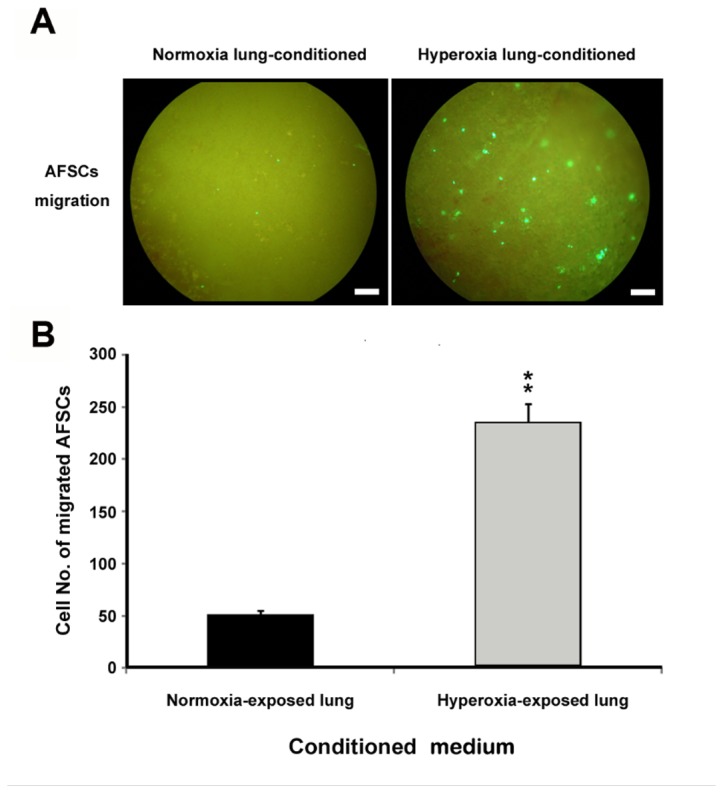
(A) AFSCs migration assay using the transwell system. A total of 5x103 AFSCs were seeded in the inner chamber and minced lung tissues from mice exposed to normoxia (left) or 60 h of hyperoxia (right) were placed in outer chambers. (B) The EGFP-expressing AFSCs that had migrated to the outer chambers were quantified. **p<0.01. Scale bar = 50 µm.
Discussion
The results of this study are the first to demonstrate that AFSCs from EGFP transgenic mice attenuates lung injury in mice with hyperoxia-induced ALI. Engraftment of the AFSCs reduced the lung water content, neutrophil infiltration, apoptosis and pulmonary fibrosis, and levels of inflammatory cytokines, suggesting that the therapeutic benefits of AFSCs in ALI are likely to be the result of their anti-inflammatory and immune-modulating effects.
Oxygen supplementation is frequently used in critically ill patients, especially those with ALI [36] or cardiopulmonary diseases. Short-term exposure to hyperoxia (48-72 h) has been shown to induce ALI, whereas prolonged exposure (>96–120 h) causes death in rodents [8]. Thus, in the present study, ALI produced by a relatively short (60 h) period of hyperoxia exposure was used as a model to investigate the mechanisms that control lung injury, repair, and inflammation. Previous studies have shown that exposure to hyperoxia for 60–72 h causes damage to lung alveolar epithelial and endothelial cells, resulting in increased vascular and alveolar permeability and inflammatory cellular infiltration [10,37]. Therefore, it is crucial to focus the therapeutic goals toward regeneration of these cell types after lung injury [38].
Regarding regeneration of the injured lung cells, exogenous stem cells have shown potential as cellular therapeutic agents to enhance lung repair mechanisms. However, embryonic stem cell research has been hampered by moral objections to the destruction of embryos required to harvest the cells. In contrast, amniotic fluid stem cells appear to have many of the key therapeutic benefits of embryonic stem cells while avoiding their significant ethical, medical, and logistical drawbacks. In the present study, we isolated AFSCs with basic mesenchymal stem cell (MSC) characteristics from EGFP transgenic mice. The isolated cell population exhibited typical characteristics: fibroblastic morphology, clonogenic capacity, multipotential differentiation capability and expression of a typical set of surface antigens [39–41]. Although no single specific marker is known for murine MSC, previous studies have demonstrated the strong expression of CD44 by murine AFSC [42–44]. To determine the ability of AFSC to migrate to mice lung, we injected EGFP-expressing AFSCs from EGFP transgenic mice into non-transgenic animals and used fluorescence microscopy to identify the injected cells in the lung at different time points. We confirmed that AFSCs were present in the hyperoxia-injured lungs 24 h after injection. During the following days, the AFSCs circulated throughout the body (data not shown) and EGFP-positive cells in the lung gradually diminished (Figure 8). To further evaluate whether lung damage plays a crucial role in attracting AFSCs to injured lungs, we performed a co-culture experiment to expose the AFSCs to conditioned medium from control or hyperoxia-exposed lung tissue. We found that medium from damaged lungs promoted the migration of significantly greater numbers of AFSCs than that of normal lung. This result is consistent with previous findings [45], that showed that accumulation of inflammatory mediators could attract stem cells [26].
Notably, intravenously injected AFSCs reduced the edema, neutrophil infiltration, apoptosis in the lungs and early fibrosis at 7 days post-hyperoxia (Figure 5). These results suggest that the early modulation of inflammation by AFSCs leads to an attenuation of the downstream events that cause collagen deposition and fibrosis. The reduction of inflammatory cytokine expression at 7 days after AFSCs injection further indicates the immunomodulatory effects of AFSCs in the hyperoxia-injured lung. The immunomodulatory effects of BM-MSCs have been studied in a variety of inflammatory conditions including graft-versus-host disease and autoimmune diseases [46,47]. In this study, we found that AFSCs is the best repair cells to improve mice survival rate after hyperoxia-induced ALI compared with BM-MSCs- and MEFCs-treated groups (Figure 3). For the safety test of AFSCs, mice monitored up to 3 months from the intravenous injection did not show any neoplasia arising from the EGFP-expressing AFSCs. Also, intramuscular injection of AFSCs in the recipient mice did not produce any tumor formation during 3 months observation (data not shown). The same results have been shown previously that fetal mesenchymal stromal cells can be extensively expanded from amniotic fluid, showing no karyotypic abnormalities or transformation potential in vitro and no tumorigenic effect in vivo [48,49]. The utilization of AFSCs for tissue repair and regeneration offers advantages over the use of ESCs or adult BM-MSCs: (1) AF represents a convenient and non-argued source for obtaining stem cells; (2) their derivation is relatively simple and rapid; (3) no feeder layers are required for their cultivation; and (4) their stem cell phenotype is not affected by long-term storage [24]. Therefore, the application of AFSCs for tissue repair or replacement therapies is a great potential for clinical use.
Several studies have shown that AFSCs are an effective viable source in regenerative medicine. In experimental studies, AFSCs have be successfully used in the treatment of a variety of diseases, such as large-scale skin wounds and burns [50], injured urethral sphincter [51], chronic allograft vasculopathy [52], and neural tube defects [53]. A recent study also showed that the intravenous grafts of AFSCs induced endogenous cell proliferation in ischemic stroke rats [54]. In clinical application, AFSCs used in the treatment of acute stroke by intravenous route of transplantation has been developing.
In conclusion, this study demonstrates that intravenous injection of AFSCs in an experimental model of hyperoxia-induced ALI provides a significant survival advantage that is associated with a reduction of lung edema, neutrophil infiltration, apoptosis, cytokine expression, and lung fibrosis. AFSCs may offer a new therapeutic strategy for acute lung injury (ALI), for which efficient treatments are currently unavailable.
Acknowledgments
The authors would like to thank Prof. Jiung-Wang Liao for his help with the pathology analysis and our colleagues (Drs. Tung-Chou Tsai, Yu-Tang Tung, and Zi-Lun Lai) in the Molecular Embryology & DNA Methylation Laboratory for their help with discussions and technical issues.
Funding Statement
This research was supported by grant NSC-98-2313-B-005-012 from the National Science Council and was partly supported by the Ministry of Education, Taiwan, Republic of China, under the Aiming Top University plan (ATU-101-S0508). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Brower RG, Ware LB, Berthiaume Y, Matthay MA (2001) Treatment of ARDS. Chest 120: 1347-1367. doi:10.1378/chest.120.4.1347. PubMed: 11591581. [DOI] [PubMed] [Google Scholar]
- 2. Ware LB, Matthay MA (2000) The acute respiratory distress syndrome. N Engl J Med 342: 1334-1349. doi:10.1056/NEJM200005043421806. PubMed: 10793167. [DOI] [PubMed] [Google Scholar]
- 3. Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP et al. (2005) Incidence and outcomes of acute lung injury. N Engl J Med 353: 1685-1693. doi:10.1056/NEJMoa050333. PubMed: 16236739. [DOI] [PubMed] [Google Scholar]
- 4. Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K et al. (1994) The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 149: 818-824. doi:10.1164/ajrccm.149.3.7509706. PubMed: 7509706. [DOI] [PubMed] [Google Scholar]
- 5. Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K et al. (1994) Report of the American-European consensus conference on ARDS: definitions, mechanisms, relevant outcomes and clinical trial coordination. Consensus Committee Intensive Care Med 20: 225-232. doi:10.1007/BF01704707. [DOI] [PubMed] [Google Scholar]
- 6. Guidot DM, Folkesson HG, Jain L, Sznajder JI, Pittet JF et al. (2006) Integrating acute lung injury and regulation of alveolar fluid clearance. Am J Physiol Lung Cell Mol Physiol 291: L301-L306. doi:10.1152/ajplung.00153.2006. PubMed: 16698856. [DOI] [PubMed] [Google Scholar]
- 7. Orfanos SE, Mavrommati I, Korovesi I, Roussos C (2004) Pulmonary endothelium in acute lung injury: from basic science to the critically ill. Intensive Care Med 30: 1702-1714. PubMed: 15258728. [DOI] [PubMed] [Google Scholar]
- 8. Crapo JD (1986) Morphologic changes in pulmonary oxygen toxicity. Annu Rev Physiol 48: 721-731. doi:10.1146/annurev.ph.48.030186.003445. PubMed: 3518622. [DOI] [PubMed] [Google Scholar]
- 9. Smith LJ (1985) Hyperoxic lung injury: biochemical, cellular, and morphologic characterization in the mouse. J Lab Clin Med 106: 269-278. PubMed: 2993457. [PubMed] [Google Scholar]
- 10. Bhandari V (2008) Molecular mechanisms of hyperoxia-induced acute lung injury. Front Biosci 13: 6653-6661. PubMed: 18508685. [DOI] [PubMed] [Google Scholar]
- 11. Altemeier WA, Sinclair SE (2007) Hyperoxia in the intensive care unit: why more is not always better. Curr Opin Crit Care 13: 73-78. doi:10.1097/MCC.0b013e32801162cb. PubMed: 17198052. [DOI] [PubMed] [Google Scholar]
- 12. Matute-Bello G, Frevert CW, Martin TR (2008) Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 295: L379-L399. doi:10.1152/ajplung.00010.2008. PubMed: 18621912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Calfee CS, Matthay MA (2007) Nonventilatory treatments for acute lung injury and ARDS. Chest 131: 913-920. doi:10.1378/chest.06-1743. PubMed: 17356114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD et al. (2002) Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418: 41-49. doi:10.1038/nature00870. PubMed: 12077603. [DOI] [PubMed] [Google Scholar]
- 15. Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S et al. (2001) Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 105: 369-377. doi:10.1016/S0092-8674(01)00328-2. PubMed: 11348593. [DOI] [PubMed] [Google Scholar]
- 16. Iyer SS, Rojas M (2008) Anti-inflammatory effects of mesenchymal stem cells: novel concept for future therapies. Expert Opin Biol Ther 8: 569-581. doi:10.1517/14712598.8.5.569. PubMed: 18407762. [DOI] [PubMed] [Google Scholar]
- 17. Yamada M, Kubo H, Kobayashi S, Ishizawa K, Numasaki M et al. (2004) Bone marrow-derived progenitor cells are important for lung repair after lipopolysaccharide-induced lung injury. J Immunol 172: 1266-1272. PubMed: 14707105. [DOI] [PubMed] [Google Scholar]
- 18. Kähler CM, Wechselberger J, Hilbe W, Gschwendtner A, Colleselli D et al. (2007) Peripheral infusion of rat bone marrow derived endothelial progenitor cells leads to homing in acute lung injury. Respir Res; 8: 50. doi:10.1186/1465-9921-8-50. PubMed: 17620112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gupta N, Su X, Popov B, Lee JW, Serikov V et al. (2007) Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol 179: 1855-1863. PubMed: 17641052. [DOI] [PubMed] [Google Scholar]
- 20. Moodley Y, Atienza D, Manuelpillai U, Samuel CS, Tchongue J et al. (2009) Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. Am J Pathol 175: 303-313. doi:10.2353/ajpath.2009.080629. PubMed: 19497992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kumamoto M, Nishiwaki T, Matsuo N, Kimura H, Matsushima K (2009) Minimally-cultured Bone Marrow mesenchymal stem cells ameliorate fibrotic lung injury. Eur Respir J 34: 740-748. doi:10.1183/09031936.00128508. PubMed: 19324956. [DOI] [PubMed] [Google Scholar]
- 22. Leblond AL, Naud P, Forest V, Gourden C, Sagan C et al. (2009) Developing cell therapy techniques for respiratory disease: Intratracheal delivery of genetically engineered stem cells in a murine model of airway injury. Hum Gene Ther 20: 1329-1343. doi:10.1089/hum.2009.035. PubMed: 19606934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. De Coppi P, Bartsch G Jr, Siddiqui MM, Xu T, Santos CC et al. (2007) Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol 25: 100-106. doi:10.1038/nbt1274. PubMed: 17206138. [DOI] [PubMed] [Google Scholar]
- 24. Klemmt PA, Vafaizadeh V, Groner B (2011) The potential of amniotic fluid stem cells for cellular therapy and tissue engineering. Expert Opin Biol Ther 11: 1297-1314. doi:10.1517/14712598.2011.587800. PubMed: 21623704. [DOI] [PubMed] [Google Scholar]
- 25. Abdulrazzak H, De Coppi P, Guillot PV (2013) Therapeutic potential of amniotic fluid stem cells. Curr Stem Cell Res Ther 8: 117-124. doi:10.2174/1574888X11308020002. PubMed: 23157178. [DOI] [PubMed] [Google Scholar]
- 26. Carraro G, Perin L, Sedrakyan S, Giuliani S, Tiozzo C et al. (2008) Human amniotic fluid stem cells can integrate and differentiate into epithelial lung lineages. Stem Cells 26: 2902-2911. doi:10.1634/stemcells.2008-0090. PubMed: 18719226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y (1997) 'Green mice' as a source of ubiquitous green cells. FEBS Lett 407: 313-319. doi:10.1016/S0014-5793(97)00313-X. PubMed: 9175875. [DOI] [PubMed] [Google Scholar]
- 28. Shen CJ, Cheng WTK, Wu SC, Chen HL, Tsai TC et al. (2012) Differential differences in methylation status of putative imprinted genes among cloned swine genomes. PLOS ONE 7: e32812. doi:10.1371/journal.pone.0032812. PubMed: 22393450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baksh D, Davies JE, Zandstra PW (2003) Adult human bone marrow-derived mesenchymal progenitor cells are capable of adhesion-independent survival and expansion. Exp Hematol 31: 723-732. doi:10.1016/S0301-472X(03)00106-1. PubMed: 12901978. [DOI] [PubMed] [Google Scholar]
- 30. Yen CC, Lin CY, Chong KY, Tsai TC, Shen CJ et al. (2009) Lactoferrin as a natural regimen for selective decontamination of the digestive tract: recombinant porcine lactoferrin expressed in the milk of transgenic mice protects neonates from pathogenic challenge in the gastrointestinal tract. J Infect Dis 199: 590-598. doi:10.1086/596212. PubMed: 19125673. [DOI] [PubMed] [Google Scholar]
- 31. Tsai TC, Lin W, Yang SH, Cheng WT, Cheng EH et al. (2010) Granzyme G is expressed in the two-cell stage mouse embryo and is required for the maternal-zygotic transition . BMC Dev Biol 10: 88. doi:10.1186/1471-213X-10-88. PubMed: 20704734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen JY, Chen HL, Cheng JC, Lin HJ, Tung YT et al. (2012) A Chinese herbal medicine, Gexia-Zhuyu Tang (GZT), prevents dimethylnitrosamine-induced liver fibrosis through inhibition of hepatic stellate cells proliferation. J Ethnopharmacol 142: 811-818. [DOI] [PubMed] [Google Scholar]
- 33. Tung YT, Chen HL, Lai CW, Shen CJ, Lai YW et al. (2011) Curcumin reduces pulmonary tumorigenesis in vascular endothelial growth factor (VEGF) - overexpressing transgenic mice. Mol Nutr Food Res 55: 1036-1043. [DOI] [PubMed] [Google Scholar]
- 34. Chen HL, Wang LC, Chang CH, Yen CC, Cheng WT et al. (2008) Recombinant porcine lactoferrin expressed in the milk of transgenic mice protects neonatal mice from a lethal challenge with enterovirus type 71. Vaccine 26: 891-898. doi:10.1016/j.vaccine.2007.12.013. PubMed: 18207613. [DOI] [PubMed] [Google Scholar]
- 35. Roubelakis MG, Trohatou O, Anagnou NP (2012) Amniotic fluid and amniotic membrane stem cells: marker discovery. Stem Cells Int, 2012: 107836 PubMed: 22701492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rodrigues MT, Lee BK, Lee SJ, Gomes ME, Reis RL et al. (2012) The effect of differentiation stage of amniotic fluid stem cells on bone regeneration. Biomaterials 33: 6069-6078. doi:10.1016/j.biomaterials.2012.05.016. PubMed: 22672834. [DOI] [PubMed] [Google Scholar]
- 37. Fernandes RA, Wenceslau CV, Reginato AL, Kerkis I, Miglino MA (2012) Derivation and characterization of progenitor stem cells from canine allantois and amniotic fluids at the third trimester of gestation. Placenta 33: 640-644. doi:10.1016/j.placenta.2012.03.009. PubMed: 22560723. [DOI] [PubMed] [Google Scholar]
- 38. Yen CC, Lai YW, Chen HL, Lai CW, Lin CY et al. (2011) Aerosolized human extracellular superoxide dismutase prevents hyperoxia-induced lung injury . PLOS ONE 6: e26870. doi:10.1371/journal.pone.0026870. PubMed: 22046389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M et al. (2002) Role of NRF2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol 26: 175-182. doi:10.1165/ajrcmb.26.2.4501. PubMed: 11804867. [DOI] [PubMed] [Google Scholar]
- 40. Tang PS, Mura M, Seth R, Liu M (2008) Acute lung injury and cell death: how many ways can cells die? Am J Physiol Lung Cell Mol Physiol 294: L632-L641. doi:10.1152/ajplung.00262.2007. PubMed: 18203816. [DOI] [PubMed] [Google Scholar]
- 41. Minamino T, Komuro I (2006) Regeneration of the endothelium as a novel therapeutic strategy for acute lung injury. J Clin Invest 116: 2316-2319. doi:10.1172/JCI29637. PubMed: 16955131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nadri S, Soleimani M (2007) Comparative analysis of mesenchymal stromal cells from murine bone marrow and amniotic fluid. Cytotherapy 9: 729-737. doi:10.1080/14653240701656061. PubMed: 17917881. [DOI] [PubMed] [Google Scholar]
- 43. Yoon BS, Moon JH, Jun EK, Kim J, Maeng I et al. (2010) Secretory profiles and wound healing effects of human amniotic fluid-derived mesenchymal stem cells. Stem Cells Dev 19: 887-902. doi:10.1089/scd.2009.0138. PubMed: 19686050. [DOI] [PubMed] [Google Scholar]
- 44. Barria E, Mikels A, Haas M (2004) Maintenance and self-renewal of long-term reconstituting hematopoietic stem cells supported by amniotic fluid. Stem Cells Dev 13: 548-562. doi:10.1089/scd.2004.13.548. PubMed: 15588512. [DOI] [PubMed] [Google Scholar]
- 45. Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45. doi:10.1093/nar/29.9.e45. PubMed: 11328886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M et al. (2004) Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 363: 1439-1441. doi:10.1016/S0140-6736(04)16104-7. PubMed: 15121408. [DOI] [PubMed] [Google Scholar]
- 47. Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I et al. (2005) Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood 106: 1755-1761. doi:10.1182/blood-2005-04-1496. PubMed: 15905186. [DOI] [PubMed] [Google Scholar]
- 48. Sessarego N, Parodi A, Podestà M, Benvenuto F, Mogni M et al. (2008) Multipotent mesenchymal stromal cells from amniotic fluid: solid perspectives for clinical application. Haematologica 93: 339-346. doi:10.3324/haematol.11869. PubMed: 18268281. [DOI] [PubMed] [Google Scholar]
- 49. Mei SH, McCarter SD, Deng Y, Parker CH, Liles WC et al. (2007) Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLOS Med 4: e269. doi:10.1371/journal.pmed.0040269. PubMed: 17803352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Skardal A, Mack D, Kapetanovic E, Atala A, Jackson JD et al. (2012) Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cells. J Transl Med 1: 792-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chun SY, Cho DH, Chae SY, Choi KH, Lim HJ et al. (2012) Human amniotic fluid stem cell-derived muscle progenitor cell therapy for stress urinary incontinence. J Korean Med Sci 27: 1300-1307. doi:10.3346/jkms.2012.27.11.1300. PubMed: 23166409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Santana AC, Dellê H, Cavaglieri RC, Lopes MA, Francisco RP et al. (2012) Protective effects of human amniotic fluid stem cells in a model of aorta allograft vasculopathy in rats. Transplant Proc 44: 2490-2494. doi:10.1016/j.transproceed.2012.07.022. PubMed: 23026627. [DOI] [PubMed] [Google Scholar]
- 53. Turner CG, Klein JD, Wang J, Thakor D, Benedict D et al. (2013) The amniotic fluid as a source of neural stem cells in the setting of experimental neural tube defects. Stem Cells Dev 22: 548-553. doi:10.1089/scd.2012.0215. PubMed: 22957979. [DOI] [PubMed] [Google Scholar]
- 54. Tajiri N, Acosta S, Glover LE, Bickford PC, Jacotte Simancas A et al. (2012) Intravenous grafts of amniotic fluid-derived stem cells induce endogenous cell proliferation and attenuate behavioral deficits in ischemic stroke rats. PLOS ONE 7: e43779. doi:10.1371/journal.pone.0043779. PubMed: 22912905. [DOI] [PMC free article] [PubMed] [Google Scholar]