Abstract
Root system development is an important target for improving yield in rice. Active roots that can take up nutrients more efficiently are essential for improving grain yield. In this study, we performed quantitative trait locus (QTL) analyses using 215 recombinant inbred lines derived from a cross between Xieqingzao B (XB), a maintainer line with short roots and R9308, a restorer line with long roots. Only a QTLs associated with root length were mapped on chromosomes 7. The QTL, named qRL7, was located between markers RM3859 and RM214 on chromosome 7 and explained 18.14–18.36% of the total phenotypic variance evaluated across two years. Fine mapping of qRL7 using eight BC3F3 recombinant lines mapped the QTL to between markers InDel11 and InDel17, which delimit a 657.35 kb interval in the reference cultivar Nipponbare. To determine the genotype classes for the target QTL in these BC3F3 recombinants, the root lengths of their BC3F4 progeny were investigated, and the result showed that qRL7 plays a crucial role in root length. The results of this study will increase our understanding of the genetic factors controlling root architecture, which will help rice breeders to breed varieties with deep, strong and vigorous root systems.
Keywords: rice (Oryza sativa L.), root length, QTL, qRL7, hydroponic conditions
Introduction
The root system performs essential functions in plant development, including anchoring the plant, and the uptake of water and nutrient (Dorlodot et al. 2007, Fujii 1961, Gewin 2010, Liao et al. 2004). For example, a deep, thick and branched root system results in better survival under adverse conditions, such as water or nutrient deficits (Price et al. 2002, Yano et al. 2005, Zheng et al. 2000). The high adaptive plasticity of roots complicates the genetic dissection of genes controlling variation in root structure, representing a bottleneck for the efficient selection of specific root ideotypes (Kamoshita et al. 2002a). The genetic basis of root structural variation has been studied mainly through QTL analysis. Many QTLs for root-releated traits have been identified in rice under normal or abiotic stress conditions (Kamoshita et al. 2002a, 2002b, Li et al. 2005, Qu et al. 2008, Rebouillat et al. 2009, Steele et al. 2006, 2013). Most researchers were interested in QTLs affecting rice root traits under specific environments. However, for rice production, the climate is usually normal, with few adverse conditions; therefore, mapping QTLs for rice roots under normal conditions becomes more important.
The fibrous rice root system contains many roots from each plant at the mature stage, making them difficult to study; consequently, there have been few studies of rice root traits at the reproductive stage (Courtois et al. 2009). Yield is mostly determined during the ripening stage; therefore, studies on rice roots during later stages would be more useful. The super hybrid rice, ‘Xieyou 9308’, is a ‘late-stage vigor’ rice variety with vigorous leaf and root growth during the late growth period (Cheng et al. 2005). During the late growth stage, its leaves remain green until the mature panicle stage (Cheng et al. 2005, 2007a).
In this study, we performed QTL analyses using 215 recombinant inbred lines (RILs) derived from a cross between Xieqingzao B (XB), a maintainer line with short roots and R9308, a restorer line with long roots. A QTL associated with root length was mapped on chromosome 7. The QTL, named qRL7, was located between markers RM3859 and RM214 on chromosome 7 and explained 18.14–18.36% of the total phenotypic variance evaluated over 2 years. Furthermore, by using advanced-backcross progeny, we validated the genetic effect of qRL7 for root length on chromosome 7 and delimited its candidate genomic region. The results of this study will increase our understanding of the genetic factors controlling root architecture, which will help rice breeders to breed varieties with deep, strong and vigorous root systems.
Materials and Methods
Experimental materials
For QTL analyses, 215 RIL lines (F16) were developed by the single-seed-descent method. XB is the maintainer line of ‘Xieyou 9308’ and has short roots. R9308 is the restorer line of ‘Xieyou 9308’ and has long roots.
To perform fine mapping of the QTL for root length on chromosome 7, we developed six BC3F3 lines in which recombination had occurred within the region containing the target QTL. MAS was used to select these lines from advanced backcross progeny derived from a cross between R9308 (the recurrent parent) and one RIL line (S4) in which the target QTL region was homozygous for the XB (donor parent) allele. Six BC3F2 recombinant plants (BC3F2-1-1, BC3F2-7-6, BC3F2-4-3, BC3F2-5-1, BC3F2-8-4 and BC3F2-9-2) were obtained and self-pollinated to produce homozygous recombinant BC3F3 lines. Two NILs (qRL7-NIL1 and qRL7-NIL2) were obtained in the BC3F3 using a MAS strategy. qRL7-NIL1 harbored the XB allele at qRL7, while qRL7-NIL2 carried the R9308 allele. These eight lines were genotyped with DNA markers distributed across all chromosome regions, as described in “DNA marker screening and QTL analysis”. To determine the genotype classes for the target QTL in the six BC3F3 recombinants, the root lengths of their BC3F4 progeny were investigated.
Hydroponic experiments
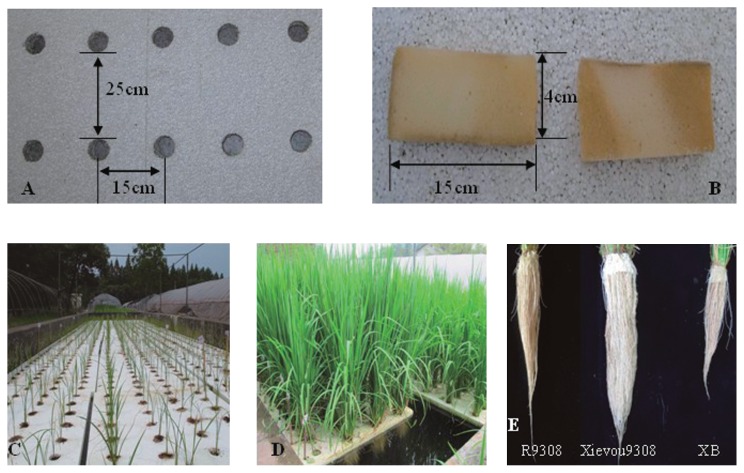
The hydroponic experiments were conducted on the experimental base of the China National Rice Research Institute (CNRRI) in 2010 and 2011 in Fuyang city, Zhejiang province, China. Each pool was 12 m × 3 m × 1.5 m (length × width × depth). Each foamed plastic was 1.5 m × 1 m, resulting in 24 foamed plastics in each pool. Forty rice plants were planted in each foamed plastic; therefore, a 25 cm × 15 cm row-column design was used (Fig. 1). Forty holes were drilled in each foamed plastic, one hole for each seedling. In May, germinated seeds were sown in the field. After one month, the seedlings were removed carefully to floated plastics in the pool. Each seedling was bundled with sponges to avoid the seedling falling away from the hole, because the hole was too large for the seedling. The foamed plastics containing the planted seedlings were placed in full water pools. Three days after transplanting, the first fertilizer was added to the plant in the sponge. Ten days after the first fertilizer, the second fertilizer was applied. The third fertilizer was applied 10 days later. The fertilizer was a Nitrogen-Phosphorus-Potassium mixed fertilizer (N : P2O5 : K2O = 15 : 15 : 15) (Sinochem Group), microelements and rapeseed cake in a quality ratio of 10 : 1 : 20. Thereafter, no fertilizer should be required during the rest of the growing season. About 6 g fertilizer was applied for each plant and insecticide (Triazophos, Sinochem Group) was sprayed every week until the heading stage. Three trials were performed for each design, and each line was grown in a randomized complete block design, with one plant of each line per hole. For each RIL, five plants were grown in a randomized complete block design, with one plant of each line per hole.
Fig. 1.
Hydroponic method and phenotypes of plants. A: Image for foamed plastics, B: Image for sponge. C: Image taken from seedling stage. D: Image of heading stage. E: Image taken after the plants were removed from the foamed plastics.
Phenotype evaluation
Plant height (PH), root length (RL), dry weight of roots (DWR) and dry weight of shoot (DWS) were measured for each plant at the heading stage (Fig. 1). After measuring the root length, the roots were cut off, placed in an envelope and dried in an oven at 80°C. The root traits were measured for three of the five plants (excluding border plants, to avoid edge effects); the average value of the three plants was used as the mean value for each RIL. Six BC3F3, BC3F4 lines and two NILs were grown on the experimental base of CNRRI in 2012 for fine mapping of qRL7.
DNA marker screening and QTL analysis
The linkage map for the RILs, which comprised 198 simple sequence repeat (SSR) markers, spanned 1814.5 cM, with an average spacing of 9.2 cM between adjacent markers. Among them, 165 were from the work of Shen et al. (2008); the other 33 SSR markers were added to fill the gaps in the map (Feng et al. 2010). Using criteria set for a rice molecular marker linkage map from a previous study (McCouch et al. 2002), the molecular map was determined to be suitable for QTL analysis. Putative QTLs were detected using the composite interval mapping (CIM) function of QTL Cartographer 2.5 (Wang et al. 2005). The CIM threshold was based on the results of 1000 permutations at a 5% significance level (Churchill and Doerge 1994). The additive effect and the percentage of phenotypic variance explained (PVE) by each QTL were estimated at the maximum logarithm of odds (LOD) score.
For genotyping the BC3F2 and BC3F3 populations, 121 SSR markers were used. PCR amplifications from the RILs were performed as previously described (Shen et al. 2008). To narrow down the candidate region of the qRL7 on chromosome 7, an additional forty-two insertion-deletion (Indel) markers in the interval between RM3859 and RM214 were selected. The genome resequencing of the two parents, XB and R9308, have been completed (unpublished); therefore, Indel marker design is very effective. Forty-two Indel markers were designed using software Primer premier 3.0 software (Chin et al. 2010), which generated 100–300 bp amplicons across the qRL7 regions. The Indel markers were tested in both parents. Among these 42 markers, nine showed a polymorphism between XB and R9308 when assayed by acrylamide gel electrophoresis, and were named as Indel1, Indel4, Indel7, Indel11, Indel13, Indel16, Indel17, Indel21 and Indel41 (Supplemental Table 1). Gene annotation within the specific genomic regions was carried out using IRGSP1.0 from the Rice Annotation Project (RAP, http://rapdb.dna.affrc.go.jp/) (Pan et al. 2013, Rice Annotation Project et al. 2008). The genotypes of each line were estimated from the results of Dunnett’s test at a significance level of 0.1%.
Results
Phenotypic variation of four traits among RILs
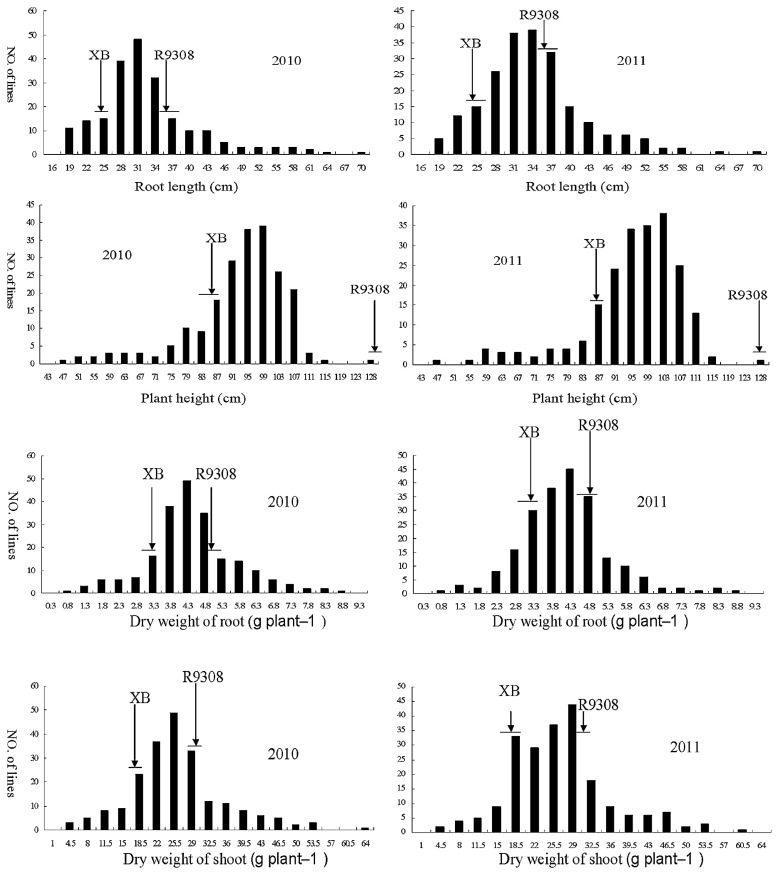
As shown in Table 1, significant phenotypic differences (P < 0.01) were found between the parents, XB and R9308. Transgressive segregations for the four traits were observed over 2 years (Fig. 2). Most of the RILs had RLs that fell between the values of the two parental lines and ranged from 25.26 cm to 35.2 cm (Fig. 2). The phenotypic correlation coefficients (PCC) among the four traits in the 215 RILs over 2 years were shown in Table 2. Highly significant correlations were found for each pair of traits (RL, PH, DWR and DWS). Every PCC was greater than or equal to 0.80 and the highest significant correlations were found between DWR and DWS. This indicated that the root length, plant height, the biomass of roots and shoots are probably regulated by same main factors.
Table 1.
Statistical analysis of the four traits of the RIL population
| Years | Item | RLa (cm) | PHb (cm) | DWRc (g plant−1) | DWSd (g plant−1) |
|---|---|---|---|---|---|
| 2010 | Mean ± SDe | 29.6 ± 5.23 | 90.73 ± 10.41 | 4.37 ± 0.39 | 19.99 ± 3.52 |
| Range | 16.3–68.3 | 43.1–121.4 | 0.32–8.35 | 1.25–61.84 | |
| Kurtosis | 0.42 | 0.49 | 1.28 | 0.47 | |
| Skewness | 0.35 | −0.13 | 0.91 | 0.88 | |
| 2011 | Mean ± SDe | 30.43 ± 5.75 | 92.03 ± 9.43 | 4.35 ± 0.28 | 28.82 ± 3.23 |
| Range | 14.3–47.7 | 35.9–124.5 | 0.44–8.28 | 1.89–60.71 | |
| Kurtosis | 0.56 | 0.43 | −0.01 | −0.08 | |
| Skewness | −0.73 | −0.4 | −0.11 | 0.13 | |
| XB | Mean ± SDe | 25.26 ± 3.59 | 87.64 ± 5.31 | 3.31 ± 0.36 | 18.26 ± 2.89 |
| R9308 | Mean ± SDe | 35.2 ± 5.62 | 128.7 ± 7.78 | 4.95 ± 0.56 | 29.51 ± 3.9 |
| Significance level | ** | ** | ** | ** |
RLa, root length; PHb, plant height; DWRc, dry weight of roots.
DWSd, dry weight of shoot; SDe, standard deviation.
significance at P = 0.01 between two parents.
Fig. 2.
Frequency distribution of four traits (RL, PH, DWR, DWS) in RILs. Arrowheads and horizontal lines indicate the mean values and standard deviation for the two parents. XB Xieqingzao B.
Table 2.
Correlation coefficients among the traits measured in the RIL population
| Traits | 2010 | 2011 | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| RL | PH | DWR | DWS | RL | PH | DWR | DWS | |
| RL | ||||||||
| PH | 0.92** | 0.90** | ||||||
| DWR | 0.93** | 0.95** | 0.92** | 0.93** | ||||
| DWS | 0.82** | 0.94** | 0.99** | 0.85** | 0.93** | 0.99** | ||
P < 0.01. For definitions of RL, PH, DWR and DWS, see the footnote to Table 1.
QTLs for four traits in the RILs
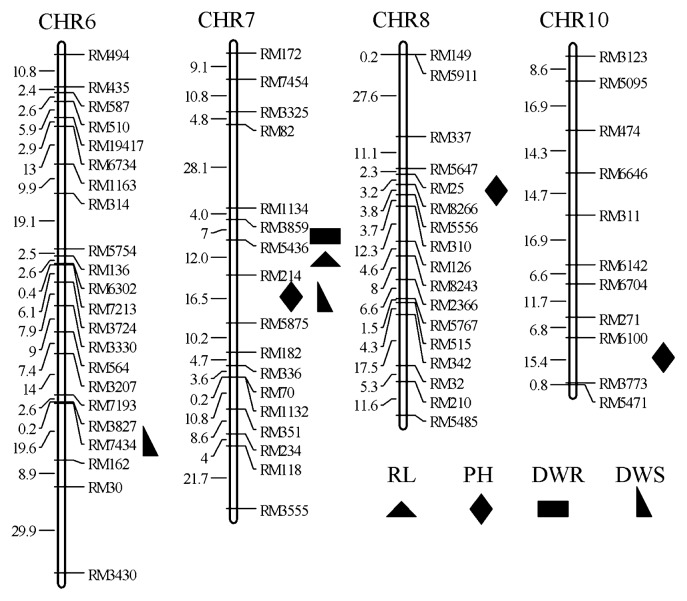
Seven QTLs for these four traits were detected on chromosomes 6, 7, 8 and 10 in during the 2 years. The LOD thresholds ranged from 4.23 to 8.89, with PVEs of 12.56% to 23.68%, respectively. In particular, four QTLs were detected on chromosome 7 (Table 3 and Fig. 3). The QTL, qRL7, which affected RL, was detected in the interval RM3859-RM214 with PVEs of 18.14% and 18.36% over 2 years. Three QTLs (qPH7, qPH8 and qPH10), one (qDWR7) and two QTLs (qDWS6 and qDWS7) affecting PH, DWR and DWS, respectively, were found in the RIL population. Among these, qPH10 was only detected in 2010. The additive effects of R9308 at all QTLs increased RL, PH, DWR and DWS by 0.29% to 9.43% over the value for XB.
Table 3.
Identification of QTLs associated with four traits in the RILs over 2 years
| QTL | Chromosome | Interval | 2010 | 2011 | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| LOD | PVEa (%) | AEb | LOD | PVEa (%) | AEb | |||
| qRL7 | 7 | RM3859-RM214 | 6.73 | 18.14 | 4.93 | 6.85 | 18.36 | 5.36 |
| qPH7 | 7 | RM214-RM5875 | 7.25 | 20.32 | 6.37 | 8.89 | 23.68 | 9.43 |
| qPH10 | 10 | RM6100-RM3773 | 4.23 | 12.56 | 1.79 | – | – | – |
| qPH8 | 8 | RM5647-RM8266 | 7.97 | 21.49 | 8.91 | 7.18 | 19.75 | 8.35 |
| qDWR7 | 7 | RM3859-RM214 | 6.45 | 17.56 | 0.38 | 6.25 | 16.26 | 0.29 |
| qDWS7 | 7 | RM214-RM5875 | 5.89 | 15.84 | 2.09 | 5.18 | 14.86 | 1.42 |
| qDWS6 | 6 | RM7434-RM162 | 7.29 | 18.87 | 2.55 | 7.05 | 19.58 | 2.63 |
Percentage of phenotypic variance explained by each QTL.
Additive effect of each QTL.
‘–’ means not detected.
Fig. 3.
Chromosome locations of QTLs for four traits (RL, PH, DWR, DWS) in RILs over two years.
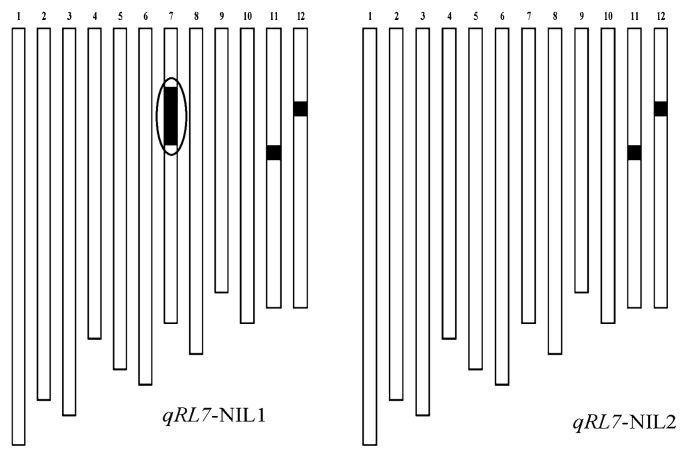
Development of NILs harboring qRL7
qRL7 for RL on chromosome 7 was responsible for high phenotypic variance over 2 years. To verify the genetic effect of this QTL at the heading stage, two NILs differing only at the qRL7 region were developed using a MAS strategy (Fig. 4). We then investigated the RLs of two NILs (BC3F3) lines for the candidate region of chromosome 7 from either XB (qRL7-NIL1) or R9308 (qRL7-NIL2) (Fig. 4). None of the qRL7-NIL1 plants had roots that were as long as those of R9308, although some plants roots were slightly elongated. qRL7-NIL2 showed a mean root length and standard deviation (SD) of 33.1 ± 2.9 cm, similar to R9308 (35.69 ± 4.3 cm), whereas the mean root length and SD of qRL7-NIL1 was 26.3 ± 2.1 cm. These results confirmed that the XB allele of the RL QTL on chromosome 7 conferred short root length at the heading stage.
Fig. 4.
Graphical representation of the genotypes of two NILs for each of the two qRL7 alleles. Chromosome numbers are indicated above each linkage map. White and black boxes represent regions that are homozygous for marker alleles from R9308 or homozygous for marker alleles from XB, respectively. The circle was the position of qRL7.
Substitution mapping of qRL7
Six recombinant lines were used to map the qRL7 as a single locus (Table 4). Progeny testing classified the six BC3F3 lines into two groups, those with short roots and those with long roots. Three lines (BC3F3-1-1, BC3F3-7-6 and BC3F3-4-3) had relatively short roots, ranging from 26.1 cm to 26.3 cm, whereas the other three lines (BC3F3-5-1, BC3F3-8-4 and BC3F3-9-2) had root lengths of 33.1 cm to 33.2 cm, which were similar to that of qRL7-NIL2 (Table 4). These phenotypic groups were predicted to be associated with genotype classes that were homozygous for the XB allele and for the R9308 allele. These results clearly showed that the RL QTL is located between markers InDel11 and InDel17 on chromosome 7 (Fig. 5). The candidate genomic region of qRL7 between InDel11 and InDel17 spans 657.35 kb in the Nipponbare genome (Fig. 5).
Table 4.
Genotypes of nine DNA markers on chromosome 7 in the BC3F3 lines and root lengths in the BC3F4 progeny
| lines | Genotype of marker in BC3F3 linesa | Root length (cm) in BC3F4 lines | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| InDel1 | InDel4 | InDel7 | InDel11 | InDel13 | InDel16 | InDel17 | InDel21 | InDel41 | mean ± SD | Pb | Predicted genotype of qRL7c | |
| XB | A | A | A | A | A | A | A | A | A | 25.26 ± 3.59 | 0.95 | XB |
| R9308 | B | B | B | B | B | B | B | B | B | 35.2 ± 5.62 | <1 × 10−6* | R9308 |
| qRL7-NIL1 | A | A | A | A | A | A | A | A | A | 26.3 ± 2.3 | – | qRL7-NIL1 |
| qRL7-NIL2 | B | B | B | B | B | B | B | B | B | 35.1 ± 1.9 | <1 × 10−6* | qRL7-NIL2 |
| BC3F3-5-1 | A | A | B | B | B | B | B | B | B | 33.1 ± 1.4 | <1 × 10−6* | R9308 |
| BC3F3-8-4 | B | B | B | B | B | B | A | A | A | 33.2 ± 1.9 | <1 × 10−6* | R9309 |
| BC3F3-9-2 | B | B | B | B | B | B | A | A | A | 33.1 ± 1.5 | <1 × 10−6* | R9308 |
| BC3F3-1-1 | B | B | B | B | A | A | A | A | A | 26.1 ± 1.1 | 0.87 | XB |
| BC3F3-7-6 | A | A | A | A | A | A | A | B | B | 26.3 ± 1.5 | 0.91 | XB |
| BC3F3-4-3 | A | A | A | A | A | A | B | B | B | 26.2 ± 0.9 | 0.89 | XB |
Genotypes of DNA markers are represented by A (white) for XB homozygous and B (black) for R9308 homozygotes.
P, probability of no significant difference between control line (qRL7-NIL1) and recombinant BC3F3 line in Dunnett’s test.
Indicates significance at the 0.1% level.
Genotypes of qRL7 were predicted from the results of Dunnett’s test using a 0.1% level of significance.
Fig. 5.
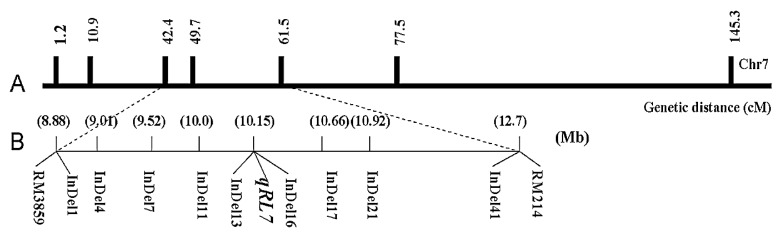
Location of qRL7 on rice chromosome 7. A: Linkage map of the RILs derived from XB × R9308. B: Linkage map constructed from the six BC3F3 recombinants. Numbers in parentheses beside the DNA markers indicate their physical map position (Mb) on chromosome 7 of Nipponbare.
Discussion
The materials and DNA markers
Progress in rice genome sequencing has provided valuable resources and tools for rice molecular genetics (Mergemann et al. 2000). In particular, large numbers of genetic markers, such as SSRs, have helped the genetic dissection of complex traits in rice using QTL analysis (Yamamoto et al. 2009). The genome of the two parents of XB and R9308 were resequenced in 2010 to design Indel markers. Xieyou9308 is a commercial super hybrid rice released in 1996 in China and is a ‘late-stage vigor’ rice variety: its leaves remain green until the rice spike matures, and its roots in the reproductive stage remain vigorous and a white color, they do not turn brown (Cheng et al. 2005, 2007b). When the gene representing qRL7 is molecularly identified in Xieyou 9308, which will help us to improve the root lengths of these rice varieties and gain higher yields.
Advantages and defects of the hydroponic method
Generally, it is difficult to evaluate the whole root system when rice is planted in soil. Using hydroponics, the whole rice root system can be obtained for detailed evaluation. Adverse environments affect genetic mechanisms of root architecture. Some studies found that certain genes controlling rice root traits were expressed in soil culture conditions, but not in hydroponic conditions. For example, the major QTL, Phosphorus uptake 1 (Pup1), which confers tolerance to low phosphorus treatment, was expressed in field conditions, but not in hydroponic conditions (Joong et al. 2011). Therefore, some scientists doubt the results of studies of rice roots in hydroponic conditions. However, these QTLs or genes were all related to specific environment conditions: the environment stimulated the expression of genes or QTLs controlling rice root traits. Thus, they should be named conditional QTLs or conditional genes, and may not represent the intrinsic genetic program of rice root traits. Therefore, stable environmental control is needed to investigate rice root architecture (Uga et al. 2012). The hydroponic method provides homogeneous growth conditions for rice roots and QTLs detected in this environment reflect the intrinsic genetic program of rice root traits.
A major QTL for root length in rice
“Thick with leaves and deep-rooted” is a term used to mean that if you want a rice plant to grow luxuriantly, the roots must be advanced. Rice root traits have been extensively studied, for example, qSOR1, a major rice QTL involved in soil-surface rooting in paddy fields was studied (Uga et al. 2012) and QTLs for root morphology of a rice population adapted to rainy lowland conditions were analyzed (Kamoshita et al. 2002a). Some QTLs associated with root traits that increase yield in upland rice have been investigated (Steele et al. 2013). A major QTL, Dro1, involved in deep rooting of rice under upland field conditions, was detected on chromosome 9 (Uga et al. 2011). A distinct QTL-qREP-6 involved in root elongation induced by phosphorus deficiency was detected on the long arm of chromosome 6 (Shimizu et al. 2008). Brt4, a major QTL conferring basal root thickness, was located to chromosome 4 (Liu et al. 2008). Other QTLs associated with rice roots have been mapped (Adnan et al. 2002, Kondo et al. 2001, Zheng et al. 2003, 2006). However, there has been no research on rice root traits at the heading stage, because the roots at this stage are difficult to investigate if the rice plants are planted in the field. The present study, using hydroponic, seven QTLs were detected for four traits and a major QTL, qRL7, was identified for root length at the heading stage. Further study using eight BC3F3 recombinant lines, allowed qRL7 to be mapped to a 657.35 kb region between markers InDel11 and InDel17 (Table 4 and Fig. 5). The IRGSP1.0 from the Rice Annotation Project (RAP, http://rapdb.dna.affrc.go.jp/) predicts 96 genes in the candidate region for qRL7. To clone the gene(s) represented by qRL7, a large population derived from the cross of qRL7-NIL1 and qRL7-NIL2 would be useful.
Potential applications of QTLs for root length in rice
In rice, water deficiency is one of the dominant abiotic stresses limiting productivity under upland and rainy lowland conditions (Kirk et al. 1998). Almost 50% of the soils in rice cultivation areas of the world are currently water deficient (Ismail et al. 2007); therefore, enhancement of water acquisition efficiency is a very important breeding target in rice. QTLs for root traits of deep root ratio and deep root mass were detected on chromosomes 2, 3, 4, 9 and 11 (Kamoshita et al. 2002b). A major QTL, qRL6.1, for root length of rice seedlings grown under a wide range of NH4+ concentrations in hydroponic conditions was fine-mapped (Mitsuhiro et al. 2010). qRL6.1 could explain 13.5% to 21.1% of the phenotypic variation and enhance rice yield. However, these studies for architectural features of the root system were not performed at the heading stage. Root traits have a major effect on rice yield from the heading stage to the ripening stage. The major QTL qRL7 can improve root length significantly at the heading stage and probably has an important effect on yield. Further fine mapping and map-based cloning of qRL7 will be helpful for understanding the ‘late-stage vigor’ mechanism of Xieyou9308 and accelerate molecular breeding using qRL7.
Acknowledgements
This work was supported by grants from the Chinese Natural Science Foundation (31071398 and 31101203) and a grant from the National Program on Super Rice Breeding, the Ministry of Agriculture.
Literature Cited
- Adnan, K., Shashidhar, H.E. and Shailaja, H (2002) Mapping of QTL associated with root and related traits in DH population of rice (Oryza sativa L.). Indian J. Genet. Plant Breed. 62: 287–290 [Google Scholar]
- Cheng, S.H., Cao, L.Y., Chen, S.G., Zhu, D.F., Wang, X, Min, S.K. and Zhai, H.Q. (2005) Conception of late-stage vigor hybrid rice and its biological significance. Chinese J. Rice Sci. 19: 280–284 [Google Scholar]
- Cheng, S.H., Cao, L.Y., Zhuang, J.Y., Chen, S.G., Zhan, X.D., Fan, Y.Y., Zhu, D.F. and Min, S.K. (2007a) Super hybrid rice breeding in China: achievements and prospects. J. Integrative Plant Biol. 49: 805–810 [Google Scholar]
- Cheng, S.H., Zhuang, J.Y., Fan, Y.Y., Du, J.H. and Cao, L.Y. (2007b) Progress in research and development on hybrid rice: a super-domesticate in China. Annals. Bot. 100: 959–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin, J.H., Lu, X.C., Haefele, S.M., Gamuyao, R, Ismail, A, Wissuwa, M and Heuer, S (2010) Development and application of gene-based markers for the major rice QTL phosphorus uptake 1. Theor. Appl. Genet. 120: 1073–1086 [DOI] [PubMed] [Google Scholar]
- Churchill, G.A. and Doerge, R.W. (1994) Empirical threshold values for quantitative trait mapping. Genetics 138: 963–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtois, B., Ahmadi, N., Khowaja, F., Price, A.H., Rami, J.F., Frouin, J., Hamelin, C. and Ruiz, M. (2009) Rice root genetic architecture: metaanalysis from a drought QTL database. Rice 2: 115–128 [Google Scholar]
- Dorlodot, S., Forster, B, Pages, L, Price, A, Tuberosa, R and Draye, X (2007) Root system architecture: opportunities and constraints for genetic improvement of crops. Trends Plant Sci. 12: 474–481 [DOI] [PubMed] [Google Scholar]
- Feng, Y., Cao, L.Y., Wu, W.M., Shen, X.H., Zhan, X.D., Zhai, R.R., Wang, R.C., Chen, D.B. and Cheng, S.H. (2010) Mapping QTLs for nitrogen-deficiency tolerance at seedling stage in rice (Oryza sativa L.) Plant Breed. 129: 652–656 [Google Scholar]
- Fujii, Y. (1961) Studies on the regularity of root growth in rice and wheat plants. Agri. Bull. Saga Univ. 12: 1–117 [Google Scholar]
- Gewin, V. (2010) Food: An underground revolution. Nature 466: 552–553 [DOI] [PubMed] [Google Scholar]
- Ismail, A.M., Heuer, S, Thomson, M.J. and Wissuwa, M (2007) Genetic and genomic approaches to develop rice germplasm for problem soils. Plant Mol. Biol. 65: 547–570 [DOI] [PubMed] [Google Scholar]
- Joong, H., Rico, G.Y., Cheryl, D, Masdiar, B, Joko, P, Sugiono, M, Matthias, W and Sigrid, H (2011) Developing rice with high yield under phosphorus deficiency: Pup1 sequence to application. Plant Physiol. 156: 1202–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamoshita, A., Wade, J, Ali, M.L., Pathan, M.S., Zhang, J, Sarkarung, S and Nguyen, H.T. (2002a) Mapping QTLs for root morphology of a rice population adapted to rainfed lowland conditions. Theor. Appl. Genet. 104: 880–893 [DOI] [PubMed] [Google Scholar]
- Kamoshita, A., Zhang, J, Siopongco, J, Sarkarung, S, Nguyen, H.T. and Wade, J (2002b) Effects of phenotyping environment on identification of quantitative trait loci for rice root morphology under anaerobic conditions. Crop Sci. 42: 255–265 [DOI] [PubMed] [Google Scholar]
- Kirk, G.J.D., George, T, Courtois, B and Senadhira, D (1998) Opportunities to improve phosphorus efficiency and soil fertility in rainfed lowland and upland rice ecosystems. Field Crop Res. 56: 73–92 [Google Scholar]
- Kondo, M., Courtois, B., Aguilar, A., Abe, J. and Morita, S. (2001) Morphology of root system as affected by N supply conditions and QTL analysis in a doubled-haploid rice population. Nippon Sakumotsu Gakkai Koenkai Yoshi, Shiryoshu 212: 229–230 [Google Scholar]
- Li, Z., Mu, P, Li, C, Zhang, H, Gao, Y and Wang, X (2005) QTL mapping of root traits in a doubled haploid population from a cross between upland and lowland japonica rice in three environments. Theor. Appl. Genet. 110: 1244–1252 [DOI] [PubMed] [Google Scholar]
- Liao, H., Yan, X, Rubio, G, Beebe, S.E., Blair, M.W. and Lynch, J.P. (2004) Genetic mapping of basal root gravitropism and phosphorus acquisition efficiency in common bean. Funct. Plant Biol. 31: 959–970 [DOI] [PubMed] [Google Scholar]
- Liu, L.F., Mu, P., Li, X.Q., Qu, Y.Y., Wang, Y. and Li, Z.C. (2008) Localization of QTL for basal root thickness in japonica rice and effect of marker-assisted selection for a major QTL. Euphytica 164: 729–737 [Google Scholar]
- McCouch, S.R., Teytelman, L, Xu, Y, Lobos, K.B., Clare, K, Walton, M, Fu, B, Maghirang, R, Li, Z and Xing, Y.Z. (2002) Development and mapping of 2240 new SSR markers for rice (Oryza sativa L.). DNA Res. 9: 199–207 [DOI] [PubMed] [Google Scholar]
- Mergemann, H and Sauter, M (2000) Ethylene induces epidermal cell death at the site of adventitious root emergence in rice. Plant Physiol. 124: 609–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhiro, O., Tamura, W, Ebitani, T, Yano, M, Sato, T and Yamaya, T (2010) Fine-mapping of qRL6.1, a major QTL for root length of rice seedlings grown under a wide range of NH4+concentrations in hydroponic conditions. Theor. Appl. Genet. 121: 535–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, B.H., Sheng, J, Sun, W.N., Zhao, Y.H., Hao, P and Li, X (2013) OrysPSSP: a comparative platform for small secreted proteins from rice and other plants. Nucleic Acids Res. 41: 1192–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, A.H., Cairns, J.E., Horton, P, Jones, H.G. and Griffiths, H (2002) Linking drought-resistance mechanisms to drought avoidance in upland rice using a QTL approach: progress and new opportunities to integrate stomatal and mesophyll responses. J. Exp. Bot. 53: 989–1004 [DOI] [PubMed] [Google Scholar]
- Qu, Y., Mu, P., Zhang, H., Chen, C.Y., Gao, Y., Tian, Y., Wen, F. and Li, Z. (2008) Mapping QTLs of root morphological traits at different growth stages in rice. Genetica 133: 187–200 [DOI] [PubMed] [Google Scholar]
- Rebouillat, J., Dievart, A., Verdeil, J.L., Escoute, J., Giese, G., Breitler, J.C., Gantet, P., Espeout, S., Guiderdoni, E. and Périn, C. (2009) Molecular genetics of rice root development. Rice 2: 15–34 [Google Scholar]
- Rice Annotation Project, Tanaka, T., Antonio, B.A., Kikuchi, S., Matsumoto, T., Nagamura, Y., Numa, H., Sakai, H., Wu, J. and Itoh, T.et al. (2008) The Rice Annotation Project Database (RAP-DB): 2008 update. Nucleic Acids Res. 36: 1028–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, X.H., Zhao, X.D., Chen, S.G., Cheng, D.B., Cao, L.Y. and Cheng, S.H. (2008) Construction of genetic linkage map based on a RIL population derived from super hybrid rice XY9308. Molec. Plant Breed. 6: 861–866 [Google Scholar]
- Shimizu, A., Kato, K.J., Komatsu, A, Motomura, K.J. and Ikehashi, H.S. (2008) Genetic analysis of root elongation induced by phosphorus deficiency in rice (Oryza sativa L.): fine QTL mapping and multivariate analysis of related traits. Theor. Appl. Genet. 117:987–996 [DOI] [PubMed] [Google Scholar]
- Steele, K.A., Price, A.H., Shashidhar, H.E. and Witcombe, J.R. (2006) Marker-assisted selection to introgress rice QTLs controlling root traits into an Indian upland rice variety. Theor. Appl. Genet. 112: 208–215 [DOI] [PubMed] [Google Scholar]
- Steele, K.A., Price, A.H., Witcombe, J.R., Shrestha, R, Singh, B.N., Gibbons, J.M. and Virk, D.S. (2013) QTLs associated with root traits increase yield in upland rice when transferred through marker-assisted selection. Theor. Appl. Genet. 126: 101–108 [DOI] [PubMed] [Google Scholar]
- Uga, Y., Okuno, K and Yano, M (2011) Dro1, a major QTL involved in deep rooting of rice under upland field conditions. J. Exp. Bot. 62: 2485–2494 [DOI] [PubMed] [Google Scholar]
- Uga, Y., Hanzawa, E, Nagai, S, Sasaki, K, Yano, M and Sato, T (2012) Identification of qSOR1, a major rice QTL involved in soil-surface rooting in paddy fields. Theor. Appl. Genet. 124: 75–86 [DOI] [PubMed] [Google Scholar]
- Wang, S., Basten, C.J. and Zeng, Z.B. (2005) Windows QTL cartographer 2.5. department of statistics, North Carolina State University, Raleigh [Google Scholar]
- Yamamoto, T., Yonemaru, J and Yano, M (2009) Towards the understanding of complex traits in rice: substantially or superficially? DNA Res. 16: 141–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano, M., Muraki, M., Fujimori, M., Takamizo, T. and Kindiger, B. (2005) Identification of QTL controlling adventitious root formation during flooding conditions in teosinte (Zea mays ssp. huehuetenangensis) seedlings. Euphytica 142: 33–42 [Google Scholar]
- Zheng, H.G., Babu, R.C., Pathan, M.S., Ali, L., Huang, N., Courtois, B. and Nguyen, H.T. (2000) Quantitative trait loci for root-penetration ability and root thickness in rice: comparison of genetic backgrounds. Genome 43: 53–61 [PubMed] [Google Scholar]
- Zheng, B.S., Yang, L, Zhang, W.P., Mao, C.Z., Wu, Y.R., Yi, K.K., Yi, F.Y. and Wu, P (2003) Mapping QTLs and candidate genes for rice root traits under different water-supply conditions and comparative analysis across three populations. Theor. Appl. Genet. 107: 1505–1513 [DOI] [PubMed] [Google Scholar]
- Zheng, B.S., Yang, L., Mao, C.Z., Zhang, W.P. and Wu, P. (2006) QTLs and candidate genes for rice root growth under flooding and upland conditions. Acta Genetica Sinica 33: 141–151 [DOI] [PubMed] [Google Scholar]