Abstract
Decline in the apparent quality of rice (Oryza sativa L.) grain due to high temperatures during ripening recently became a major concern in many areas in Japan. The occurrence of white-back kernels (WBK) is one of the main problems of heat-induced quality decline. We identified QTLs associated with the occurrence of WBK using recombinant inbred lines (RILs) and verified their effects using near-isogenic lines (NILs). The QTL analysis used F7 and F8 RILs derived from ‘Hana-echizen’ (HE), which is tolerant to high temperature, × ‘Niigata-wase’ (NW), which is sensitive to high temperature. Four QTLs were identified on chromosomes 3, 4, 6, and 9 (qWB3, qWB4, qWB6 and qWB9). To verify the effects of qWB6 and qWB9, we developed two NILs in which qWB6 or both were introduced from HE into the NW background. The HE allele at qWB6 significantly decreased WBK under multiple environments. The combination of qWB6 and qWB9 in an F2 population derived from a cross between a NIL and NW showed that the NW allele at qWB9 significantly decreased WBK if the qWB6 allele was HE. These results will be of value in marker-assisted selection for the breeding of rice with tolerance to heat-induced quality decline.
Keywords: white-back kernels, rice breeding, QTL, high temperature, heat-induced quality decline
Introduction
High temperatures have seriously harmed rice (Oryza sativa L.) production in many areas in Japan in recent years (Kawatsu et al. 2007). Temperatures in August 2010 were the hottest on record in Japan; the average daily temperature was 1.8°C higher than usual years. The high temperatures seriously harmed grain quality, mainly in central and western Japan (http://www.maff.go.jp/j/seisan/kankyo/ondanka/index.html, Kondo et al. 2012, Nakagawa et al. 2012, Yoshida et al. 2012). Therefore, improving tolerance to heat-induced damage has become an important objective in rice breeding in Japan.
Heat induces several kinds of damage, such as sterility (Oh-e et al. 2007), decreased grain yield (Kawatsu et al. 2007), kernel cracks (Nagata et al. 2013), deterioration of eating quality (Matsue et al. 2003, Oh-e et al. 2007, Yoshida et al. 2012) and occurrence of chalky kernels (such as white-back, basal-white and milky white: Hakata et al. 2012, Morita 2000, Tabata et al. 2007, Terashima et al. 2001). All of these effects reduce the market value. Milky white kernels are due to excess spikelets (Takata et al. 2010), deficiency of sugar source (Nakagawa et al. 2006), low insolation (Kodani et al. 2006) and typhoon/foehn-induced dry wind (Wada et al. 2012) in addition to high temperature. On the other hand, white-back kernels (WBK) are mainly due to high temperatures during the ripening period (Nagato and Ebata 1965) and their occurrence is a measure of sensitivity (Iida et al. 2002). Grains contain a few layers of starch cells distributed along the vascular bundles on the dorsal side. WBK look opaque owing to incomplete starch filling in those layers (Taira 1995, Tsuyama and Tanaka 2012).
Previously we detected three QTLs associated with the occurrence of WBK using F2 and F3 populations derived from crosses between the tolerant ‘Hana-echizen’ (HE; Fig. 1A) and the susceptible ‘Niigata-wase’ (NW; Fig. 1B) (Kobayashi et al. 2007). One of the QTLs was detected on the short arm of chromosome 6 in both populations and it showed the largest LOD value and percentage of phenotypic variance. The other two QTLs were detected on chromosomes 3 and 4 in one or the other population.
Fig. 1.
Apparent quality of (A) tolerant HE and (B) susceptible NW grown in the paddy field at Fukui in 2012 and ripened under high temperature (28.1°C). Bar; 1.0 cm.
However, the reliability of QTL analysis using these F2 or F3 populations is low, and the genetic map had a few gaps. Thus, here we conducted a further QTL analysis using a population of recombinant inbred lines (RILs) to confirm these QTLs, and verified the effects of the QTLs using near-isogenic lines (NILs). In addition, we combined two QTLs in a segregating F2 population. These results would directly benefit breeding programs using marker-assisted selection for heat tolerance.
Materials and Methods
Plant materials
For QTL analysis, we developed 178 RILs using the single-seed descent method from HE × NW F3 lines used for the previous QTL analysis (Kobayashi et al. 2007). F7 and F8 lines were used to survey the occurrence of WBK in 2006 and 2008, respectively.
To confirm QTL effects, we developed two NILs, NIL1 and NIL2, in which the HE segment of the QTL region for WBK was introduced into the NW genetic background by backcrossing. Both had an HE segment for the QTL region on chromosome 6. NIL2 also had an HE segment for a QTL region on chromosome 9. The generations of NIL1 and NIL2 were BC5F4 and BC4F4, respectively, in 2011 and BC5F5 and BC4F5, respectively, in 2012.
In addition, we developed an F2 population to identify the combined effect of the QTLs. The population consisted of 416 F2 plants derived from a cross between NIL2 and NW. We selected plants homozygous for alleles at the QTL regions on chromosomes 6 and 9.
Experimental design and conduct
For QTL analysis in 2006 and 2008, we grew the RILs and the parents in paddy fields at Fukui Agricultural Experiment Station (Fukui, Japan, 35°59′N). Plant materials were sowed and transplanted on April 19 and May 11 in 2006 and April 23 and May 15 in 2008. The fields received 7.5 g m−2 of basal nitrogen in 2006 and 2.4 g m−2 in 2008. Ten plants were raised per line with one replicate.
We grew the two NILs and the parents in paddy fields as control plots in 2011 and 2012, and in a greenhouse built on a paddy field as a high-temperature plot in 2012, at Fukui, without nitrogen application. Plant materials were sowed and transplanted on April 14 and May 6 in 2011 and April 20 and May 14 in 2012. The greenhouse was closed after heading, and it was ventilated when the temperature exceeded 35°C. We grew them also in paddy fields at Kagoshima Prefectural Institute for Agricultural Development (Kagoshima, Japan, 31°48′N) in 2012, with 4.0 g m−2 of basal application of nitrogen. Plant materials were sowed and transplanted on April 23 and May 17. Five to ten plants were raised per line, with three to six replicates in the fields and one replicate in the greenhouse.
The F2 population was sowed and transplanted on April 20 and May 14 in 2012 and grown in a paddy field at Fukui.
The temperature during the ripening period was measured by a thermometer (TR-77Ui, T&D Corporation, Japan) placed in the center of the paddy field or the greenhouse in Fukui, and by a weather station (Yokogawa Denshikiki Co. Ltd., Japan) at Kagoshima Prefectural Institute for Agricultural Development. Days-to-heading (DTH) was calculated as days from transplanting to heading.
Evaluation of heat-induced quality decline
For the QTL analysis, 500 seeds from self-pollination of each RIL were harvested as a bulk at maturity, air-dried, hulled and sieved at 1.6 mm. When the opaque portion of the dorsal side of a kernel was clearly longer than half of the kernel and was clearly recognized from directly perpendicular, the kernel was classified as white-back. The number of WBK was counted by eyes. The percentage of WBK was arc-sine transformed to normalize the variance and used for QTL analysis. The 1,000 kernel weight (KW) was also measured after placed at room temperature for two weeks to adjust the moisture content.
For the QTL verification, seeds from self-pollination in the two largest panicles from each of three plants with moderate growth from each NIL were harvested at maturity in order to evaluate the QTL effects more accurately based on the result of our previous study (Kobayashi et al. 2012). And then the kernels were prepared and classified as above. The average percentages of WBK were analyzed by the Tukey-Kramer method.
DNA marker and statistical analysis
Total DNA was extracted from the leaves by the CTAB method (Murray and Thompson 1980). PCR was performed in a 5-μL reaction mixture containing 20 ng of genomic DNA, 2.0 μM forward and reverse primers and 2.5 μL of GoTaq Green Master Mix (Promega, USA). The amplification profile was 5 min at 94°C; 35 cycles of 45 s at 94°C and 1.5 min at 55°C and 7 min at 55°C for the final extension. We used an iCycler thermal cycler (Bio-Rad Laboratories, USA). PCR products were fractionated in 2.5% agarose TBE gel. When the predicted PCR product sizes of the parental alleles differed by less than 5 bases, we used the GoTaq Colorless Master Mix (Promega) and a QIAxcel capillary electrophoresis system (Qiagen, USA).
A linkage map was constructed using 175 SSR markers distributed among the 12 chromosomes (IRGSP 2005, McCouch et al. 2002, Temnykh et al. 2001) in the MAPMAKER/EXP 3.0 software (Lander et al. 1987). The same 175 SSR markers were used to evaluate the isogenic status of the NILs. We performed QTL analysis for WBK and DTH by composite interval mapping in Windows QTL Cartographer 2.5 (Wang et al. 2007) as described in Kobayashi et al. (2007).
To confirm detection of the QTLs, we analyzed homozygous plants from the 416 F2 population, which was derived from a cross between NIL2 and NW, for eight segregating SSR markers (RM1369 and RM8125 on chromosome 6; RM6971, RM2915, RM2482 and RM2255 on chromosome 9; and RM5352 and RM5494 on chromosome 10) as above. In addition, we used two-way analysis of variance (ANOVA) of the occurrence of WBK in the F2 population to identify interactions between QTLs.
Results
Phenotypic variation in RILs
The occurrence of WBK, KW and DTH of the RILs showed transgressive segregation relative to the parents in both 2006 and 2008 (Table 1).
Table 1.
Phenotypic variations in RILs and the parents
| Traits | 2006 | 2008 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| HE | NW | RILs (F7) | HE | NW | RILs (F8) | |||||
|
|
|
|||||||||
| Ave. | Max. | Min. | Ave. | Max. | Min. | |||||
| WBK (%) | 4.4 | 20.2 | 16.8 | 56.6 | 0.0 | 21.7 | 45.1 | 31.0 | 79.5 | 0.0 |
| KW (g) | 22.4 | 21.1 | 21.3 | 23.3 | 19.5 | 22.1 | 21.2 | 21.4 | 23.5 | 19.6 |
| DTH (days) | 72 | 73 | 74 | 80 | 66 | 67 | 67 | 68 | 72 | 62 |
| Temperaturea (°C) | 26.3 | 26.3 | 26.5 | 27.6 | 25.8 | 27.6 | 27.6 | 27.6 | 27.7 | 27.5 |
WBK, white-back kernels; KW, 1,000 kernel weight; DTH, days-to-heading.
Average temperature during the ripening period (20 days after heading).
The apparent quality of brown rice begins to deteriorate when the average temperature after heading exceeds 27°C (Morita 2008, Wakamatsu et al. 2008). In 2006, more than half of the plants might have escaped high temperature stress because the average temperature during ripening period was below 27°C. In 2008, all lines and the parents ripened at above 27°C. Although more than half of RILs ripened under 27°C in 2006, the correlation coefficient of the occurrence of WBK between 2006 and 2008 was highly significant (r = 0.80, P < 0.001). This finding supports the reliability of the 2006 experiment. Thus, we provided all the data of these two experiments for the following QTL analysis.
QTL identification
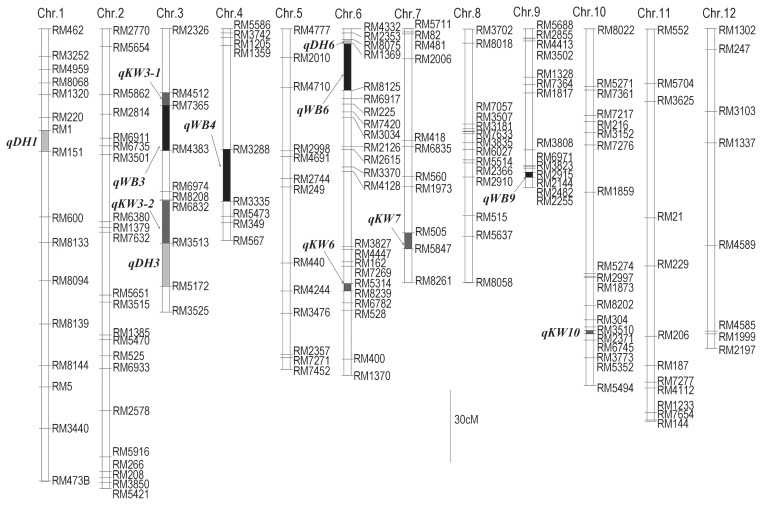
QTL analysis revealed the presence of putative QTLs associated with WBK, KW and DTH (Table 2 and Fig. 2). Four QTLs for WBK were identified on chromosomes 3, 4, 6 and 9 (qWB3, qWB4, qWB6 and qWB9). The HE allele decreased the occurrence of WBK at qWB3, qWB4 and qWB6, but increased it at qWB9. qWB6 was detected in both years and was mapped near marker RM8125 on the short arm of chromosome 6. The percentages of total phenotypic variance explained by the QTL were 31.5% in 2006 and 36.8% in 2008. In our previous study, qWB6 was detected in both F2 and F3 populations (Kobayashi et al. 2007). We detected qWB6 again in RILs in both F7 and F8 populations. qWB3 and qWB4 were detected only in 2006 and qWB9 only in 2008. In the previous study, qWB3 and qWB4 were detected in either the F2 or the F3 population but not both and qWB9 was not detected because of the lack of a genetic map for chromosome 9. We also identified five QTLs for KW on chromosomes 3 (two QTLs), 6, 7 and 10, and three QTLs for DTH on chromosomes 1, 3 and 6 (Table 2 and Fig. 2).
Table 2.
Putative QTLs for WBK and DTH in RILs population
| Traits | QTL | Chr | Nearest marker | 2006 (F7) | 2008 (F8) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| LOD | AEa | r2 b | Thresholdc | LOD | AE | r2 | Threshold | ||||
| WBK | qWB3 | 3 | RM4383 | 5.18 | −3.11 | 10.6 | 3.26 | 3.34 | |||
| qWB4 | 4 | RM3288 | 4.30 | −2.42 | 6.5 | ||||||
| qWB6 | 6 | RM8125 | 18.11 | −5.40 | 31.5 | 21.70 | −8.13 | 36.8 | |||
| qWB9 | 9 | RM2482 | 7.63 | 4.35 | 10.7 | ||||||
| KW | qKW3-1 | 3 | RM7365 | 4.56 | −0.26 | 8.9 | 3.54 | 3.82 | −0.23 | 7.4 | 3.37 |
| qKW3-2 | 3 | RM3513 | 4.53 | −0.21 | 5.9 | ||||||
| qKW6 | 6 | RM5314 | 3.76 | 0.20 | 6.0 | ||||||
| qKW7 | 7 | RM505 | 3.53 | 0.21 | 6.6 | ||||||
| qKW10 | 10 | RM2371 | 5.09 | 0.23 | 7.9 | ||||||
| DTH | qDH1 | 1 | RM151 | 4.54 | 5.08 | −0.22 | 19.1 | 3.76 | |||
| qDH3 | 3 | RM5172 | 4.98 | −0.53 | 11.7 | ||||||
| qDH6 | 6 | RM1369 | 5.30 | 0.68 | 8.4 | 8.22 | 0.54 | 12.1 | |||
WBK, white-back kernels; KW, 1,000 kernel weight; DTH, days-to-heading.
Additive effect of the HE allele.
Percentage of total phenotypic variance explained by the QTL.
Significant threshold LOD value (P 0.05) determined from 1000 permutations.
Fig. 2.
HE × NW linkage map and putative QTLs for occurrence of WBK, KW and DTH detected in RIL population. Blocks represent chromosomes. Black, dark gray and light gray blocks represent marker intervals where LOD peaks were detected for WBK, KW and DTH, respectively. SSR markers mapped to the same position are omitted.
Verification of allelic differences in qWB6 and qWB9
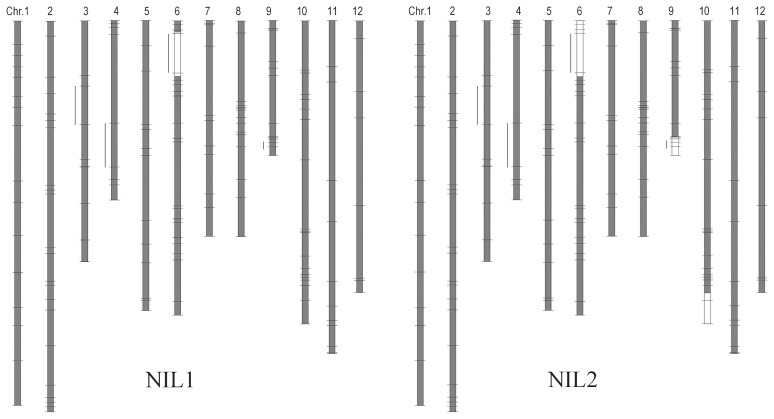
qWB6 was detected in all four generations (F2, F3, F7 and F8), and the HE allele decreased the occurrence of WBK. qWB9 was detected only in the F8 RILs, and the NW allele decreased the occurrence. We developed two NILs in which the HE segments of either qWB6 (NIL1) or of both QTLs (NIL2) were introduced into the NW genetic background (Fig. 3). NIL2 had a longer HE segment than NIL1 in the qWB6 region, and also included a segment of HE on chromosome 10. The average temperature during the ripening period in every plot was above 27°C (Table 3). All differences in the occurrence of WBK between HE and NW were significant (P < 0.05). The occurrences of WBK did not differ significantly between NIL1 and HE in any plot. This result clearly shows that the effect of qWB6 on the occurrence of WBK was reproducible. In contrast, the occurrence of WBK in NIL2 (with HE segments of both qWB6 and qWB9) differed by plot. In the greenhouse, the occurrence of WBK was not significantly different between NIL2 and HE. In contrast, in the Fukui paddy field in 2011, it was not significantly different between NIL2 and NW. In the paddy fields at Fukui and Kagoshima in 2012, the occurrence of WBK in NIL2 was intermediate between HE and NW.
Fig. 3.
Graphical genotypes of NILs and QTL regions for WBK. Blocks represent chromosomes. Horizontal lines show the position of SSR markers. Recombination points were arbitrarily determined as the midpoint between markers of different genotypes. White and gray blocks denote the homozygous HE allele and NW allele, respectively. Bars next to chromosomes denote putative QTLs for WBK.
Table 3.
WBK, DTH and average temperature during the ripeing period of NILs and parents
| Cultivars or Lines | Fukui paddy field | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| 2011 | 2012 | |||||
|
|
|
|||||
| DTH days | Tempa °C | WBKb % | DTH days | Temp °C | WBK % | |
| HE | 66 | 27.3 | 14.1 ± 5.4 a | 66 | 28.1 | 37.2 ± 16.1 ac |
| NW | 67 | 27.3 | 50.8 ± 21.6 b | 68 | 28.1 | 82.5 ± 18.3 bd |
| NIL1c | 68 | 27.3 | 12.9 ± 9.4 a | 69 | 28.2 | 15.1 ± 2.6 a |
| NIL2d | 68 | 27.3 | 51.3 ± 20.9 b | 70 | 28.2 | 57.0 ± 13.1 cd |
|
| ||||||
| Cultivars or Lines | Fukui greenhouse | Kagoshima | ||||
|
|
|
|||||
| 2012 | 2012 | |||||
|
|
|
|||||
| DTH days | Temp °C | WBK % | DTH days | Temp °C | WBK % | |
|
| ||||||
| HE | 62 | 31.1 | 46.9 ± 4.0 a | 63 | 27.9 | 3.5 ± 3.3 a |
| NW | 64 | 31.0 | 89.2 ± 16.9 b | 63 | 27.9 | 55.4 ± 14.5 b |
| NIL1 | 65 | 31.1 | 57.0 ± 9.4 a | 63 | 27.9 | 13.4 ± 7.5 a |
| NIL2 | 65 | 31.1 | 62.1 ± 10.4 a | 64 | 27.9 | 34.5 ± 6.7 c |
WBK, white-back kernels; DTH, days-to-heading.
Average temperature during the ripening period (20 days after heading).
All values are means ± SD. Means with the same letter do not differ among NILs and the parents (P 0.05).
Contains the qWB6 allele from HE in the NW genetic background.
Contains the qWB6 and qWB9 alleles from HE in the NW genetic background.
Occurrence of WBK in F2 population from a cross between NIL2 and NW
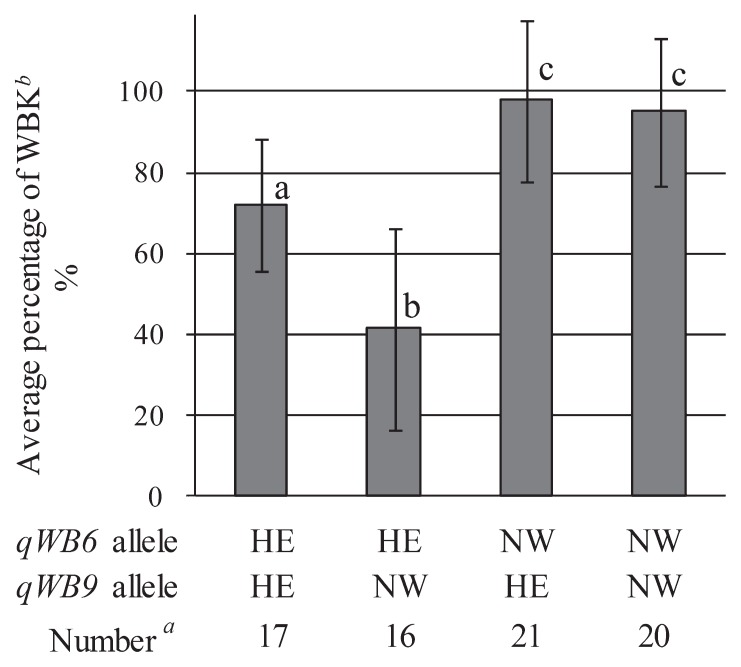
qWB6 and qWB9 were detected again in the F2 population (Table 4), but no QTL was detected on chromosome 10. Two-way ANOVA showed that the alleles at qWB6 contributed most to the occurrence of WBK and there was a significant (P = 0.00054) interaction effect between qWB6 and qWB9. Plants with NW allele at qWB6 had a significantly higher occurrence of WBK than those with HE allele (Fig. 4). This result confirms that the occurrence of WBK in the F2 population was determined mainly by different alleles at qWB6. In addition, in the presence of the HE allele at qWB6 in the NW background, the NW allele at qWB9 significantly decreased WBK and the HE allele significantly increased it. But in the presence of the NW allele at qWB6 in the NW background, the occurrence of WBK was high regardless of the qWB9 allele.
Table 4.
QTLs for WBK detected in F2 population
F2 population was derived from a cross between NIL2 and NW.
Additive effect of the HE allele.
Percentage of total phenotypic variance explained by the QTL.
Fig. 4.
Occurrence of WBK in plants that had homozygous alleles at both qWB6 and qWB9 in the F2 population derived from a cross between NIL2 and NW. a Number of plants. b Mean values with the same letter do not differ significantly (P 0.05) by the Tukey-Kramer method.
Discussion
The difference in DTH affected the occurrence of WBK by causing changes in temperatures during the ripening period (Hori et al. 2012). However, we could exclude the effect of heading date on WBK based on three reasons: the correlation between the occurrence of WBK and DTH in the RILs was not significant (r = 0.03 in 2006, r = 0.15 in 2008, P < 0.05); QTLs for DTH were detected in different regions from QTLs for WBK (Fig. 2) and DTH of NILs and the parents had no influence on the temperature during the ripening period (Table 3).
The occurrence of WBK and KW was positively correlated in the previous study (Kobayashi et al. 2007). In this study, the occurrence of WBK and KW in the RILs was positively and significantly correlated (r = 0.22, P < 0.01) in F7 population, but there was not significant correlation (r = 0.05, P < 0.05) in F8 population. In addition, QTLs for KW were detected in different regions from QTLs for WBK (Fig. 2). Tabata et al. (2005) also showed that there was no correlation between the ratio of WBK and that of KW using F2 and F3 populations derived from a cross between ‘Chiyonishiki’ and ‘Koshijiwase’. Thus we conclude that the effect of KW on occurrence of WBK was limited.
We identified four QTLs associated with the occurrence of WBK. We had already identified qWB3, qWB4 and qWB6 in our previous study using F2 and F3 populations derived from a cross between the same parents (Kobayashi et al. 2007). In the previous study, we could not construct a complete genetic map. In particular, we succeeded in mapping only one SSR marker on chromosome 9 and two on chromosome 12, for which we could not construct genetic map covering chromosomes 9 and 12. Here, however, we constructed a complete genetic map using 175 SSR markers, and could therefore map qWB3, qWB4 and qWB6 with more accuracy. The qWB6 was detected in both F2 and F3 populations and also detected in both F7 and F8 populations in this study. These results show that a gene with a major effect on the short arm of chromosome 6 controls the occurrence of WBK and plays an important role in the suppression of heat-induced quality decline.
We developed a NIL for qWB6 to verify its effects. We changed the surveyed seeds for the QTL verification to evaluate the QTL effects more accurately based on the results of previous studies. We have reported that the apparent quality of brown rice differed with spikelet positions on a panicle (Kobayashi et al. 2012). Dong et al. (2011) and Yoshino et al. (2006) also reported that the occurrence of immature or chalky kernels differed among spikelet positions. The occurrence of WBK in the QTL analysis was determined using bulked seeds, and did not represent the exact occurrence of WBK, because they were randomly composed of seeds at various spikelet positions. In the verification analysis using all the self-pollinated seeds of two panicles from each NIL, the occurrence of WBK did not differ significantly between NIL1 and HE in any plot (Table 3). This result shows that qWB6 could decrease WBK and increase tolerance to heat-induced quality decline to the same level as in HE.
In addition, qWB9 was newly detected on chromosome 9, where the NW allele decreased WBK even though NW itself is susceptible. If qWB9 has any effect on WBK, it would be possible to improve the grain quality of HE by introducing the NW allele at qWB9. Thus, we developed a NIL that had HE segments in both regions to confirm their genetic effects. The occurrence of WBK in NIL2 (HE segments at both qWB6 and qWB9) was significantly higher than that in HE (Table 3). It was also significantly higher than in NIL1 except in the greenhouse. Further, two-way ANOVA showed that allelic variation at qWB9 influenced the effect of qWB6 on WBK in the F2 population. In the presence of the HE allele at qWB6 in the NW background, the HE allele at qWB9 significantly increased WBK (Fig. 4). These results indicate that the HE allele at qWB9 weakened the ability of qWB6 to decrease the occurrence of WBK. Although the tolerant HE also has the HE alleles at qWB6 and qWB9 regions, the NW alleles at qWB3 and qWB4 in NIL2 might interact with qWB9. To clarify the interactions among qWB3, qWB4 and qWB9, NILs with HE segments in those regions should be developed. In addition, a NIL with an NW segment at qWB9 and its neighboring region in the HE background might improve the tolerance of HE.
qWB3 was detected only in F2 population that grown under lower temperature (23.9°C) and qWB4 was detected only in F3 population that grown under higher temperature (28.2°C) in the previous study (Kobayashi et al. 2007). However in this study, qWB3 and qWB4 were detected in F7 population (26.5°C), but they were not detected in F8 population (27.6°C). In addition, qWB9 was detected only in F8 population (27.6°C). These results indicated that qWB3 would not be effective at higher temperature than 27°C. In contrast, response to temperature of qWB4 and qWB9 was not clear from these results. Further research using NILs for these QTLs is needed to confirm their responses to temperature because different responses to temperature among QTLs may be an important factor for QTL stacking.
The effect of the HE allele at qWB6 was not enough to confer complete tolerance: the NILs and HE showed a high occurrence of WBK under very high temperature (31°C) in the greenhouse (Table 3). Mizunaga et al. (2011) suggested that qWB6 was not effective at 32°C. Thus, the QTLs identified here might not express the tolerance against temperatures above 31°C.
Rice breeders have to prepare for the predicted global warming by developing cultivars with stronger tolerance to heat. To this end, other tolerant cultivars, landraces, indica rice and wild relatives should be screened. Hori et al. (2012) identified QTLs for chalkiness on chromosomes 3, 6, 8 and 11 using ‘Nipponbare’/’Koshihikari’ backcross inbred lines. The QTL they detected for white-belly kernel ratio on the short arm of chromosome 3 might be the same as qWB3. They also detected a QTL for WBK on the short arm of chromosome 6, very close to qWB6. It is not possible to determine the allelism of these QTLs, owing to the lack of shared markers between the two studies. Tabata et al. (2007) detected four QTLs associated with WBK on chromosomes 1 (two QTLs), 2 and 8 in a population derived from japonica ‘Chiyonishiki’ × ‘Koshijiwase’. Ebitani et al. (2008) detected three QTLs for white-back and basal-white kernels on chromosomes 2, 9 and 11 in an indica ‘Kasalath’ × japonica ‘Koshihikari’ cross. Hao et al. (2009) detected QTL for chalky grain on chromosome 8, at which the indica ‘Nona Bokra’ allele increased chalky grains in the ‘Koshihikari’ background. Sonoda et al. (2011) evaluated the heat-induced quality decline in Japanese rice landraces collected by Ebana et al. (2008) and found several tolerant landraces. These intensive studies will contribute to the breeding of ‘ultra-tolerant’ rice by stacking those alleles.
Acknowledgements
We thank M. Tanoi, M. Aoki and K. Sugihara, Fukui Agricultural Experiment Station, for their technical assistance. This work was supported by grants from the Ministry of Agriculture, Forestry and Fisheries of Japan (‘Genomics for Agricultural Innovation’ QTL3002 and QTL6001 and ‘Development of mitigation and adaptation techniques to global warming in the sectors of agriculture, forestry and fisheries’ 1202 and 3003).
Literature Cited
- Dong, M., Chen, P., Qiao, Z., Wu, X., Zhao, B., Jiang, Y. and Yang, J. (2011) Quality response of grains in different spikelet positions to temperature stress during grain filling of rice. Acta Agronomica Sinica 37: 506–513 [Google Scholar]
- Ebana, K., Kojima, Y, Fukuoka, S, Nagamine, T and Yano, M (2008) Development of mini core collection of Japanese rice landrace. Breed. Sci. 58: 281–291 [Google Scholar]
- Ebitani, T., Yamamoto, Y, Yano, M and Funane, M (2008) Identification of quantitative trait loci for grain appearance using chromosome segment substitution lines in rice. Breed. Res. 10: 91–99 [Google Scholar]
- Hakata, M., Kuroda, M, Miyashita, T, Yamaguchi, T, Kojima, M, Sakakibara, H, Mitsui, T and Yamakawa, H (2012) Suppression of α-amylase genes improves quality of rice grain ripened under high temperature. Plant Biotechnol. J. 10: 1110–1117 [DOI] [PubMed] [Google Scholar]
- Hao, W., Zhu, M.Z., Gao, J.P., Sun, S.Y. and Lin, H.X. (2009) Identification of quantitative trait loci for rice quality in a population of chromosome segment substitution lines. J. Integr. Plant Biol. 51: 500– 512. [DOI] [PubMed] [Google Scholar]
- Hori, K., Kataoka, T, Miura, K, Yamaguchi, M, Saka, N, Nakahara, T, Sunohara, Y, Ebana, K and Yano, M (2012) Variation in heading date conceals quantitative trait loci for other traits of importance in breeding selection of rice. Breed. Sci. 62: 223–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida, Y., Yokota, K, Kirihara, T and Suga, R (2002) Comparison of the occurrence of kernel damage in rice plants grown in a heated greenhouse and in a paddy field of high temperature year. Jpn. J. Crop Sci. 71: 174–177 [Google Scholar]
- IRGSP (2005) The map-based sequence of the rice genome. Nature 436: 793–800 [DOI] [PubMed] [Google Scholar]
- Kawatsu, S., Homma, K, Horie, T and Shiraiwa, T (2007) Change of weather condition and its effect on rice production during the past 40 years in Japan. Jpn. J. Crop Sci. 76: 423–432 [Google Scholar]
- Kobayashi, A., Bao, G, Ye, S and Tomita, K (2007) Detection of quantitative trait loci for white-back and basal-white kernels under high temperature stress in japonica rice varieties. Breed. Sci. 57: 107– 116. [Google Scholar]
- Kobayashi, A., Sugimoto, K, Iwasawa, N, Kondo, M and Tomita, K (2012) Apparent quality of brown rice at different spikelet positions on panicle under high temperature stress during ripening period. Breed. Res. 14 (Suppl. 2): 142 [Google Scholar]
- Kodani, T., Matsumura, Y and Kuroda, A (2006) Effects of shading treatments before and after the heading stage on grain yield and quality in rice cultivar ‘Yumemizuho’. Bull. Ishikawa Agric. Res. Cen. 27: 1–9 [Google Scholar]
- Kondo, M., Iwasawa, N, Yoshida, H, Nakagawa, H, Ohno, H, Nakazono, K, Usui, Y, Tokida, T, Hasegawa, T and Kuwagata, Tet al. (2012) Factors influencing the appearance quality in rice under high temperature in 2010. Jpn. J. Crop Sci. 81 (Extra issue 1): 120–121 [Google Scholar]
- Lander, E.S., Green, P., Abrahamson, J., Barlow, A., Daly, M.J., Lincoln, S.E. and Newburg, L. (1987) MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1: 174–181 [DOI] [PubMed] [Google Scholar]
- Matsue, Y., Ogata, T, Sato, H and Hamachi, Y (2003) Correlation of the temperature during the ripening period with the palatability and physicochemical properties of rice in Japan. Jpn. J. Crop Sci. 72 (Extra issue 1): 272–273 [Google Scholar]
- McCouch, S.R., Teytelman, L, Xu, Y, Lobos, K.B., Clare, K, Walton, M, Fu, B, Maghirang, R, Li, Z and Xing, Y (2002) Development and mapping of 2240 new SSR markers for rice (Oryza sativa L.) DNA Res. 9: 199–207 [DOI] [PubMed] [Google Scholar]
- Mizunaga, M., Kobayashi, A and Okuno, K (2011) Variation of depression of grain quality in Japanese rice varieties grown at 32 degree during filling period. Breed. Res. 13 (Suppl. 2): 223 [Google Scholar]
- Morita, S. (2000) Effects of high temperature on ripening in rice plants —analysis of ripening performance under climate conditions by changing in cropping seasons and/or transferring pots from lowland to upland. Jpn. J. Crop Sci. 69: 400–405 [Google Scholar]
- Morita, S. (2008) Prospect for developing measures to prevent hightemperature damage to rice grain ripening. Jpn. J. Crop Sci. 77: 1–12 [Google Scholar]
- Murray, M.G. and Thompson, W.F. (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 8: 4321–4325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata, K., Sasaki, R and Ohdaira, Y (2013) Cultivar differences in the grain cracking of rice under the high air temperature conditions during grain filling. Jpn. J. Crop Sci. 82: 42–48 [Google Scholar]
- Nagato, K and Ebata, M (1965) Effects of high temperature during ripening period on the development and the quality of rice kernels. Jpn. J. Crop Sci. 34: 59–66 [Google Scholar]
- Nakagawa, H., Tanaka, H, Tano, N and Nagahata, H (2006) Effects of leaf and panicle clipping on the occurrence of various types of chalky kernels in rice. Hokuriku Crop Sci. 41: 32–34 [Google Scholar]
- Nakagawa, H., Yoshida, H, Ohno, H, Nakazono, K, Kondo, M, Iwasawa, N, Usui, Y, Tokida, T, Hasegawa, T and Kuwagata, Tet al. (2012) What deteriorated the appearance of Koshihikari rice produced in and around Hokuriku region in 2010? Jpn. J. Crop Sci. 81 (Extra issue 1): 126–127 [Google Scholar]
- Oh-e, I., Matsue, Y, Saitoh, K and Kuroda, T (2007) Effects of rising temperature on grain quality, palatability and physicochemical properties of rice. Sci. Rep. Fac. Agric., Okayama Univ. 96: 13–18 [Google Scholar]
- Sonoda, J., Kondo, M and Umemoto, T (2011) Characterization and evaluation of NIAS rice core collection in grain quality with high temperature during the ripening period. Breed. Res. 13 (Suppl. 2): 125 [Google Scholar]
- Tabata, M., Iida, Y and Ohsawa, R (2005) Genetic analysis of occurrence of white-back rice and basal-white rice associated with high temperature during the ripening period of rice. Breed. Res. 7: 9–15 [Google Scholar]
- Tabata, M., Hirabayashi, H, Takeuchi, Y, Ando, I, Iida, Y and Ohsawa, R (2007) Mapping of quantitative trait loci for the occurrence of white-back kernels associated with high temperatures during the ripening period of rice (Oryza sativa L.). Breed. Sci. 57: 47–52 [Google Scholar]
- Taira, H. (1995) Physicochemical properties and quality of rice grains. In: Science of the Rice Plant, volume 2: Physiology. Rural Culture Association, Tokyo, pp. 1064–1068 [Google Scholar]
- Takata, S., Sakata, M, Kameshima, M, Yamamoto, Y and Miyazaki, A (2010) Varietal difference in the relation between the occurrence of white immature kernels caused by a high temperature during the ripening period and the amount of basal nitrogen application in rice. Jpn. J. Crop Sci. 79: 150–157 [Google Scholar]
- Temnykh, S., DeClerck, G, Lukashova, A, Lipovich, L, Cartinhour, S and McCouch, S (2001) Computational and experimental analysis of microsatellites in rice (Oryza sativa L.): frequency, length variation, transposon associations, and genetic marker potential. Genome Res. 11: 1441–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima, K., Saito, Y, Sakai, N, Watabe, T, Ogata, T and Akita, S (2001) Effects of high air temperature in summer of 1999 on ripening and grain quality of rice. Jpn. J. Crop Sci. 70: 449–458 [Google Scholar]
- Tsuyama, M and Katsube-Tanaka, T (2012) Starch granules of rice chalky grains observed by low-vacuum scanning electron microscopy. Jpn. J. Crop Sci. 81 (Extra issue 1): 210–211 [Google Scholar]
- Wada, H., Nonami, H, Yabuoshi, Y, Tanaka, F, Maruyama, A, Tanaka, A, Wakamatsu, K, Watanabe, K, Sumi, T and Wakiyama, Yet al. (2012) Mechanism for the formation of ring-shaped chalkiness in growing rice kernels under typhoon/foehn-induced dry wind condition and its practical applications. Jpn. J. Crop Sci. 81 (Extra issue 1): 190–191 [Google Scholar]
- Wakamatsu, K., Sasaki, O, Uezono, I and Tanaka, A (2008) Effect of the amount of nitrogen application on occurrence of white-back kernels during ripening of rice under high temperature conditions. Jpn. J. Crop Sci. 77: 424–433 [Google Scholar]
- Wang, S., Basten, C.J. and Zeng, Z.B. (2007) Windows QTL Cartographer 2.5. Dept. Statistics, North Carolina State Univ., Raleigh, NC, USA: http://statgen.ncsu.edu/qtlcart/WQTLCart.htm [Google Scholar]
- Yoshida, H., Nakagawa, H, Ohno, H, Nakazono, K, Kondo, M, Iwasawa, N, Usui, Y, Tokida, T, Hasegawa, T and Kuwagata, Tet al. (2012) Factors that influenced the taste of Koshihikari rice produced in Hokuriku region in 2010. Jpn. J. Crop Sci. 81 (Extra issue 1) 128–129 [Google Scholar]
- Yoshino, Y., Ota, K, Arihara, K and Koyama, Y (2006) The effect of topdressing at ear formation stage on the quality and the crude protein content of brown rice in rice cultivar “Fusaotome” and “Koshihikari”. Jpn. J. Crop Sci. 75 (Extra issue 2): 102–103 [Google Scholar]