Abstract
Mosquitoes and other arthropods may transmit medically important pathogens, in particular viruses such as West Nile virus. The presence of suitable hosts and competent vectors for those zoonotic viruses is essential for an enzootic transmission, which is a prerequisite for epidemics. To establish reliable risk projections, it is an urgent need for an exact identification of mosquito species, which is especially challenging in the case of sibling species, such as Culex. pipiens pipiens biotypes pipiens and molestus. To facilitate detection of different Culex pipiens forms and their hybrids we established a multiplex real-time PCR. Culex pipiens samples were obtained by egg raft collection and rearing until imago stage or adult sampling using CO2 baited traps and gravid traps. In total, we tested more than 16,500 samples collected all over Germany in the years 2011 and 2012. The predominant species in Germany are Culex pipiens pipiens biotype pipiens and Culex. torrentium, but we also detected Culex pipiens pipiens biotype molestus and hybrids of the two pipiens biotypes at sites where both species occur sympatrically. This report of a potentially important bridge vector for West Nile virus might have major impact in the risk projections for West Nile virus in Germany.
Introduction
In the past decades, West Nile virus (WNV) has spread from Africa and conquered various regions of temperate climate with substantial outbreaks in North America and Europe. WNV belongs to the group of arthropod-borne viruses (arbovirus), and is transmitted by mosquitoes. Accordingly, the spread of WNV is dependent on the presence of suitable mosquito vectors, and knowledge of the local mosquito species and their distribution are prerequisites for regional risk assessments of possible arbovirus-outbreaks. In Europe and other temperate regions, members of the Culex pipiens complex are the most ubiquitous mosquito species [1], [2] which serve as principal vectors for various arboviruses including WNV [3], [4], [5], [6], [7]. Culex pipiens pipiens (Linnaeus 1758) (Cpp.) can be subdivided into two distinct biotypes, which are morphologically indistinguishable but differ greatly in physiology and behaviour. The biotype pipiens is mainly ornithophilic (i.e. bird biting host preference), anautogenous (requires blood meal for first oviposition) and eurygamous (mating in outdoor swarms only), whereas the biotype molestus (Forskål 1775) is mammophilic (i.e. prefers biting mammals incl. humans), autogenous (first oviposition without prior blood meal) and stenogamous (ability to mate in a narrow space) [1]. In addition, Culex torrentium, another Culex species of temperate regions, exhibits virtually the same bionomic and morphological characters as Cpp. biotype pipiens. Although Cpp. and Cx. torrentium can be differentiated by the morphology of the male hypopygia, wild-caught females of both species with a spoiled thoracic chaetotaxy (presence of specific setae and/or scales) are morphologically indistinguishable. Presently, there is ongoing discussion whether they belong to different subgroups within the pipiens group of the subgenus Culex [8], or whether they should be treated as sibling species of the pipiens complex [9]. Another isssue of importance for WNV risk assessment is the identification of hybrids between Cpp. pipiens and molestus biotypes. These hybrids show opportunistic feeding behaviour and may serve as important bridge vectors for the transmission of viruses from infected birds to humans as revealed during outbreaks in the United States [10]. So far, hybrids of pipiens and molestus biotypes have been occasionally observed in several parts of Europe, but have not been described for Germany [11], [12]. However, information is lacking for most European countries about the distribution and composition of the different Culex species, biotypes or biotype hybrids due to the lack of suitable protocols that allow sensitive and specific high throughput screening of large sample sizes, generated during nationwide mosquito surveys. Here we report on a newly developed multiplex real-time PCR that allows discrimination of the various morphologically indistinguishable Culex species and biotypes. The method was used to analyse more than 16,500 Culex specimens from a recent nationwide mosquito surveillance program in Germany. The results indicate an uneven distribution of Cpp. and Cx. torrentium throughout the country, sympatric occurrence of Cpp. biotype pipiens and biotype molestus and first detection of Cpp. biotype pipiens and biotype molestus hybrids at different locations in Germany.
Materials and Methods
Mosquito samples and morphological species identification
Mosquitoes were trapped with CO2 baited traps or gravid traps from May to September 2011 and 2012 (Table 1). In addition, mosquito eggs were collected from suitable breeding sites such as water ponds or rain barrels. Two strategies were used to obtain a priori identified reference samples of the different taxa: Firstly, in the case of the morphospecies Cpp. and Cx. torrentium single egg batches collected outdoors were reared and the resulting adult males hypopygia were assessed [9] to determine the identity of each egg batch. Secondly, the identity of Cpp. biotype molestus was inferred from the behaviour of wild-caught and colonised F0 females and their offspring. For instance F1 females, produced by F0 females (collected indoors), are characterized by immediate stenogamy and autogeny without adaptation or selection over several generations. Another character for distinguishing biotype molestus was the shape of the egg raft, which is smaller and more irregular than the boat-like rafts of biotype pipiens and Cx. torrentium. As a positive control for the molestus×pipiens hybrid detection we used the F1 progeny of a Cpp. biotype pipiens×Cpp. biotype molestus experimental cross. The Cx. p. quinquefasciatus laboratory culture was obtained from Bayer HealthCare (Bayer; Germany).
Table 1. Trapping-sites of the nationwide surveillance program selected for the study.
| Trapping-site | Coordinates | Trap type | Number of individuals (number of pools) |
| Upper Rhine valley/Baden-Württemberg | |||
| Neckargerach | N 49°23′ | GT | 93 (8) |
| E 9°4′ | |||
| Heidelberg | N 49°24′ | GT | 3800 (162) |
| E 08°39′ | |||
| Heidelberg | N 49°24′ | GT | 977 (85) |
| E 08°39′ | |||
| Karlsruhe - Island Rott | N 49°09′ | EVS | 43 (2) |
| E 08°23′ | |||
| Karlsruhe - Russheim | N 49°11′ | EVS | 203 (11) |
| E 08°25′ | |||
| Mümling-Grumbach | N 49°46′ | GT | 106 (9) |
| E 8°59′ | |||
| Weinheim 2011 | N 49°31′ | GT | 414 (19) |
| E 08°38′ | |||
| Weinheim 2012 | N 49°31′ | GT | 2002 (112) |
| E 08°38′ | |||
| Beerfelden | N 49°33′ | GT | 33 (5) |
| E 8°57′ | |||
| Eberstadt | N 49°48′ | GT | 194 (11) |
| E 8°38′ | |||
| Großsachsen | N 49°31′ | GT | 418 (18) |
| 2011 | E 08°40′ | ||
| Großsachsen | N 49°31′ | GT | 558 (31) |
| E 08°40′ | |||
| Hemsbach | N 49°35′ | GT | 77 (9) |
| E 8°39′ | |||
| Waghäusel | N 49°15′ | EVS | 139 (8) |
| E 08°31′ | |||
| Sandhausen | N 49°20′ | GT | 265 (13) |
| E 8°39′ | |||
| Lower-Rhine Valley/Palatine | |||
| Bad Dürkheim | N 49°22′ | GT | 52 (28) |
| E 08°08′ | |||
| Bobenheim-Roxheim | N 49°34′ | GT | 222 (26) |
| E 8°21′ | |||
| Dirmstein | N 49°34′ | GT | 549 (134) |
| E 08°14′ | |||
| Kühkopf 2012 | N 49°49′ | EVS | 36 (2) |
| E 08°24′ | |||
| Kühkopf 2011 | N 49°49′ | EVS | 197 (25) |
| E 08°24′ | |||
| Neustadt a. d. Weinstraße | N 49°22′ | GT | 238 (74) |
| E 08°08′ | |||
| Alsheim | N 49°45′ | GT | 1 |
| E 08°18′ | |||
| Mettenheim | N 49°44′ | GT | 182 (10) |
| E 8°19′ | |||
| Römerberg | N 49°17′ | GT | 1721 (84) |
| E 8°24′ | |||
| Lake Constance/Bavaria West | |||
| Radolfzell (Lake Constance) | N 47°43′ | EVS | 144 (8) |
| E 08°59′ | |||
| Lake Chiemsee/Bavaria East | |||
| Chieming (Lake Chiemsee) | N 47°53′ | EVS | 89 (6) |
| E 12°31′ | |||
| Hirschauer Bucht (Lake Chiemsee) | N 47°51′ | EVS | 13 (2) |
| E 12°31′ | |||
| Chieming (Lake Chiemsee) | N 47°53′ | EVS | 6 (2) |
| E 12°31′ | |||
| Plattling (Isar) | N 48°47′ | EVS | 83 (5) |
| E 12°55′ | |||
| Stöttham (Lake Chiemsee) | N 47°45′ | EVS | 20 (2) |
| E 12°31′ | |||
| Iffeldorf (Easter Lakes) | N 47°46′ | EVS | 1 |
| E 11°18′ | |||
| Upper Elbe Valley/Saxonia | |||
| Coswig (Elbe) | N 51°51′ | EVS | 74 (11) |
| E 12°26′ | |||
| Oder Valley/Brandenburg | |||
| Oderaue (Oder) | N 52°47′ | EVS | 272 (52) |
| E 14°14′ | |||
| Baltic Sea/Mecklenburg | |||
| Greifswald - Eldena | N 54°05′ | EVS | 1960 (78) |
| E 13°27′ | |||
| Greifswald - Loissin | N 54°07′ | EVS | 668 (27) |
| E 13°30′ | |||
| Metropolitan Region Hamburg | |||
| Langenlehsten | N 53°30′ | Egg | 349 |
| E 10°44 | Collection | ||
| Wulksfelde | N53°43′ | BT | 55 |
| E10°6′ | GT | ||
| HH/Ohlsdorf | N53°37′ | BT | 23 |
| E10°2′ | |||
| HH/Poppenbüttel | N53°39′ | EVS | 4 |
| E10°05′ | BT | ||
| HH/Cranz | N53°32′ | ex- La. | 8 |
| E09°46′ | |||
| HH/Hummelsbüttel | N53°38′ | ex- La. | 38 |
| E10°02′ | |||
| HH/Airport | N53°37′ | BT | 3 |
| E 10°00′ | |||
| HH/Barmbek | N53°35′ | ex- La. | 10 |
| E10°2′ | |||
| HH/Fuhlsbüttel | N53°37′ | Swarm | 12 |
| E10°01′ | |||
| HH/Ohlstedt | N53°41′ | EVS | 10 |
| E10°8′ | |||
| Höhbeck/Vietze 3 | N53°03′ | ex- Pu. | 14 |
| E11°24′ | |||
| Stelle-Ashauen | N53°21′ | BT | 17 |
| E10°6′ | |||
| Schleswig-Holstein | |||
| Sepel | 54°07′ | BT | 4 |
| 10°22′ | |||
| Gammendorf | N54°29′ | BT | 22 |
| E11°08′ | |||
| Warwerort | N54°08′ | BT | 67 |
| E08°55 | |||
| Wyk auf Föhr | N54°41′ | indoors | 10 |
| E8°33′ | |||
| Hesse | |||
| Heubach1 | N50°22′ | ex- La. | 24 |
| E 09°42′ | |||
| Heubach2 | N50°22′ | ex- La. | 39 |
| E 09°42′ | |||
| Heubach3 | N50°22′ | ex- La. | 7 |
| E 09°42′ | |||
| total individuals | 16566 |
Trap types: BT: Biogents Sentinel; GT: Gravid trap; EVS: Enceph. Vector Surveillance Trap (Bioquip); ex-La.: reared from larvae ; ex-Pu.: reared from pupae.
With the exception of Hamburg Airport and Ohlsdorf all sampling sites within the various cluster areas are located either on public grounds for which specific permissions was not required, or on privately owned land. All owners of the private grounds gave permission to perform this study.
Permissions for Hamburg Airport and Ohlsdorf cemetery in Hamburg were given and are attached with manuscript submission.
The traps used in this study were specific for mosquitoes and did not catch any endangered or protected species.
DNA extraction and multiplex real-time PCR assay
Mosquitoes collected at the various study sites were frozen at −70°C and transported to the laboratory where they were first identified morphologically at the genus level [13]. Subsequently, Culex ssp. from individual collections were pooled to up to 25 specimens per pool. All pooled samples were placed in sterile 2-mL cryovials, and subsequently maintained at −70°C until being assayed. Each mosquito pool was triturated in 500 µL of cell culture medium (high glucose Dulbecco's modified Eagle's medium (Sigma-Aldrich) with 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin, 100 µg/mL streptomycin, and 2.5 µg/mL amphotericin B using two stainless steel beads (5 mm; Qiagen) in a TissueLyser (Qiagen) for 2 min at 50 oscillation/s. The suspensions were clarified by centrifugation (5,000× g for 1 min), and the supernatant was used for DNA extraction with AquaGenomic™-Solution (protocol for Drosophila samples, MultiTarget Pharmaceuticals) or QIAamp viral RNA mini kit according to the manufacturer's instructions.
The extracted DNA was analyzed by a newly designed multiplex real-time PCR using the primers for Culex pipiens F (5′- GCGGCCAAATATTGAGACTT -3′; nucleotide [nt] position 3 to 22 [the nt positions are given according to the numbering in the Culex reference strain 258c, GenBank accession number gb/DQ470148.1) and Cx. pipiens R (5′- CGTCCTCAAACATCCAGACA -3′; nt position 146 to 165) and probes Cx. pipiens all (5′- Cy55- GGAACATGTTGAGCTTCGGK -BBQ-1 -3′; nt position 77 to 95), Cx. pipiens pipiens biotype pipiens (5′- JOE GCTTCGGTGAAGGTTTGTGT-BHQ1 –3′) nt position 89 to 108 and Cx. pipiens pipiens biotype molestus (5′- Rox- TGAACCCTCCAGTAAGGTATCAACTAC- BHQ2 -3′; nt position 41 to 67; Reference strain 284b, GenBank accession number gb/470150.1) of the microsatelite locus CQ11. Cx. torrentium DNA was detected using the primers Cx. torrentium F (5′ -GACACAGGACGACAGAAA -3′; nt position 86 to 103), Cx. torrentium R (5′- GCCTACGCAACTACTAAA -3′; nt position 363 to 380) and the probe Cx. torrentium (5′- FAM- CGATGATGCCTGTGCTACCA-BHQ1 -3′; nt position 112 to 131) of the ace2 gene (Cx. torrentium reference strain, GenBank accession number gb/AY497525.1). Multiplex real-time PCR was performed in a 20 µL reaction volume using HotStarTaq® Master Mix Kit according to the manufacturer's protocol (Qiagen). The specific primer molarities and sequence alignments of the loci used for primer design are shown in Figure S1.
Results and Discussion
Multiplex real-time PCR for simultaneous detection and differentiation of Cpp. biotypes and Cx. torrentium
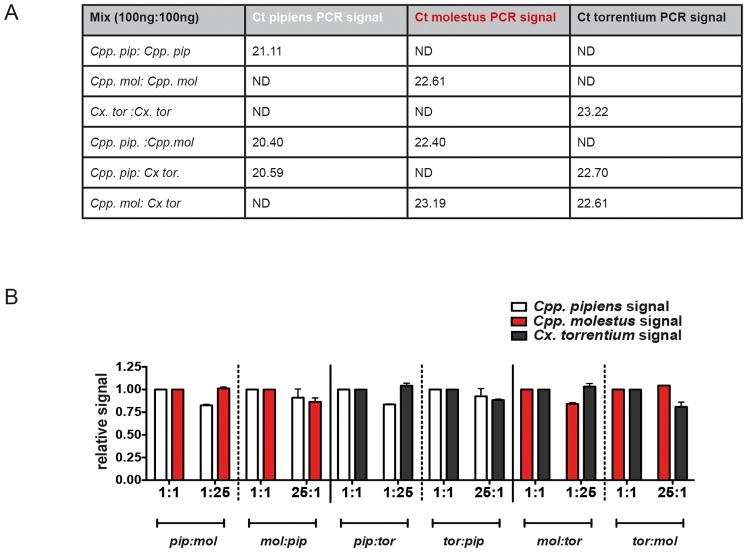
Molecular assays to differentiate Cpp. and Cx. torrentium or to distinguish between the Cpp. biotypes reported so far are based on gel electrophoretic analyses of particular DNA fragments amplified by PCR [14], [15], [16]. These assays are time-consuming and prone to laboratory contamination, which are major drawbacks for the analysis of large sample sizes. To circumvent those problems we have developed a multiplex real-time PCR that allows differentiation of otherwise indistinguishable Culex mosquitoes in a single PCR reaction in a closed tube format. The assay targets the gene locus for acetylcholinesterase 2 (ace2) to discriminate between Cx. torrentium and Cpp. and the CQ11 microsatellite locus for discrimination between Cpp. biotypes [14], [16], [17]. Using a large collection of about 350 well-defined mosquito specimens (consisting of 227 Cpp. pipiens, 3 Cpp. molestus and 119 Cx. torrentium samples), the assay was evaluated and revealed 100% specificity for the respective Culex species or biotypes. Moreover, the PCR clearly identified hybrids generated by laboratory crosses between Cpp. biotype pipiens and biotype molestus. There was no or only little signal reduction when the assay was run with mixed DNA samples from the two Cpp. biotypes and Cx. torrentium as indicated by the similarity of Ct-values for mixed versus single DNA preparations (Figure 1A). As samples from surveillance programs are often analysed as pools of morphological identical mosquitoes, the multiplex real-time PCR was assessed using various numbers of Culex species or biotypes in mixed pools of up to 25 individuals. Although signal intensity was dependent on the number of insects to be detected, PCR specifically identified as little as single individuals in a mix of 25 mosquitoes (Figure 1B). Taken together, by combining simultaneous analyses of the two loci CQ11 and ace2, the multiplex real-time PCR reported here shows all characteristics required for large-scale analyses and differentiation of Cpp. biotypes and Cx. torrentium, respectively, in a single assay format.
Figure 1. Establishment of a multiplex qPCR to differentiate Cpp. biotypes, biotype hybrids and Cx. Torrentium.
A) Signal intensities for single species and mixed species DNA samples. The DNA of single individuals of the three taxa was extracted and quantified. For the reaction mixtures, either 200 ng of single species DNA templates or a 1∶1 mix of two species DNA templates (100 ng DNA species 1 and 100 ng species 2) were subjected to PCR testing. The reaction mix was prepared including the sets of primers and probes specific for Cpp. pipiens, Cpp. molestus and Cx. torrentium in a total reaction volume of 20 µL (for individual concentration please refer to Figure S1C). The signal intensities of each species-specific probe were measured for single taxon samples and taxon mixtures and are expressed as crossing points (Ct-values). A sample that is not targeted by the respective species-specific probe, is indicated as non detected (ND). B) As pools of up to 25 mosquitoes were analysed, the detection of individual DNA from Cpp. biotype pipiens, Cpp. biotype molestus or Cx. torrentium in mixed samples were analysed by the multiplex qPCR test. DNA of single individuals from the three taxa was extracted and quantified. The reaction mixtures were prepared with either 1∶1 mixed DNA template of two species (100 ng of each single species DNA sample) or 1∶25 diluted DNA sample (8 ng DNA of species 1 and 192 ng DNA of species 2). Subsequently, the DNA templates were subjected to a reaction mix containing species-specific sets of primer/probe for all three taxa and tested for amplification signals. The signal intensity for the 1∶1 mixture was set to 1 and signal intensities of 1∶25 mixed samples are expressed as relative values. The signal intensities are color-coded as followed: Cpp. pipiens in white, Cpp. molestus in red and Cx. torrentium in black. In the graph, relative signal intensities for 1∶25 DNA mixes of Cpp. biotype pipiens and Cpp. biotype molestus are named pip∶mol when Cpp. pipiens DNA was diluted in Cpp. molestus DNA, or mol∶pip when Cpp. molestus DNA was diluted in Cpp. pipiens DNA. Accordingly 1∶25 DNA mixes of Cpp. pipiens with Cx. torrentium were named pip∶tor if Cpp. pipiens DNA was diluted in Cx. torrentium DNA and tor∶pip if Cx. torrentium DNA was diluted in Cpp. pipiens DNA. The same nomenclature was used for 1∶25 mixes of Cpp. biotype molestus DNA with Cx. torrentium DNA. Values presented are the mean and standard deviation of two independent experiments.
Distribution of Cpp. biotypes and Cx. torrentium in Germany
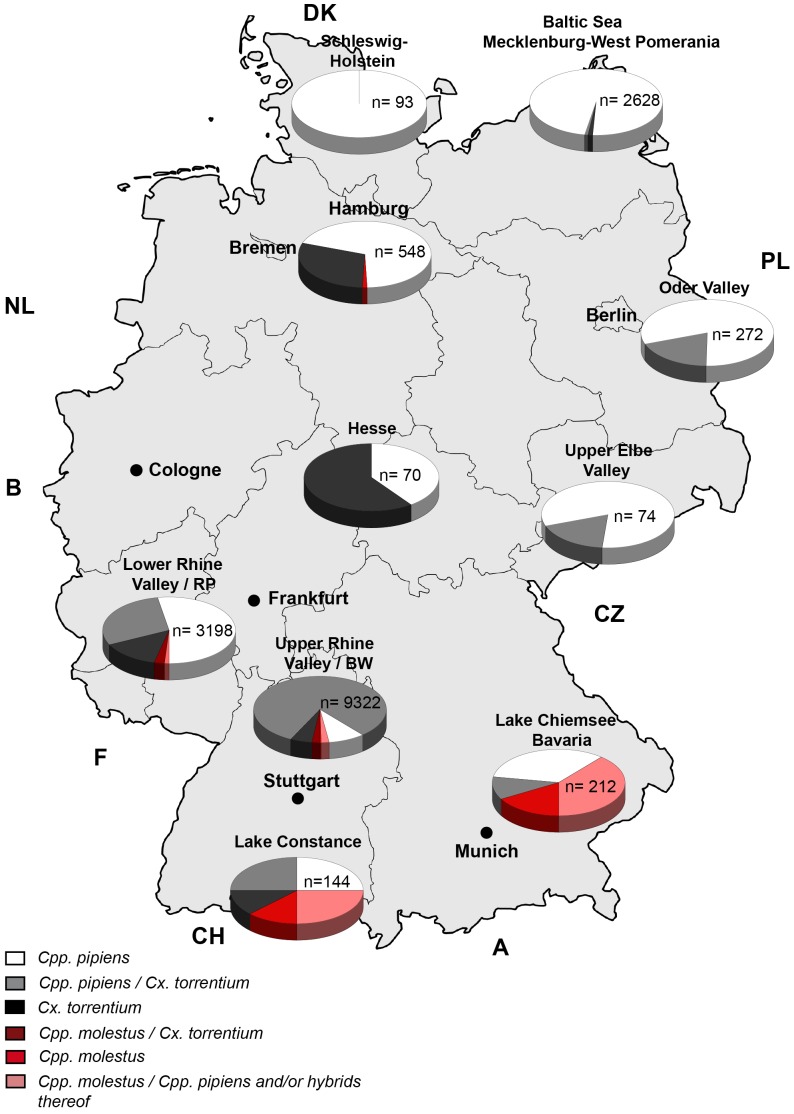
Morphologically indistinguishable Cpp./Cx. torrentium samples, collected in 2011 and 2012, during a nationwide mosquito surveillance programme were subjected to the newly developed multiplex real-time PCR in order to determine the distribution of Cpp. biotypes and Cx. torrentium across Germany. The 48 collection sites were selected according to the following criteria: known mosquito habitats e.g. extensive wetlands and presence of migratory birds (risk of imported WNV) and represent 10 major cluster areas, namely the Lower Rhine Valley and further sites in Palatine, the Upper Rhine Valley and further sites in Baden-Württemberg, Lake Constance, Lake Chiemsee and other sites in Bavaria, Hesse, the Upper Elbe Valley in Saxonia, the Oder Valley in Brandenburg, the Baltic Sea coast in Mecklenburg-West Pomerania, Metropolitan Region Hamburg and various sites in Schleswig-Holstein (Table 1). A total of more than 16,500 Culex ssp. were analysed out of which 716 represent individual specimens and 1,081 represent pooled samples. A pool usually contained between 5 and 25 mosquitoes from the same trap of a given time-point. Overall the predominant Culex species in Germany was found to be Cpp, with a mean abundance of 58% at the 48 selected sampling sites. However, considerable regional differences in species abundance and species composition were observed (Figure 2). Populations from coastal habitats in the North comprise almost exclusively Cpp. whereas Cx. torrentium is apparently absent in this region. In contrast, Cx. torrentium comprises up to 60% of the Cpp./Cx. torrentium populations and shows substantial overlaps with Cpp at all other sampling sites. Moreover, biotype analyses indicated absence of Cpp. molestus in all samples from North and East Germany, except Hamburg metropolitan area where a small proportion of the Culex population of around 1.3% consisted of Cpp. biotype molestus. On the other hand, up to 50% of the Culex populations at sampling sites in South and Southwest Germany were found to consist of Cpp. biotype molestus.
Figure 2. Classification of Culex samples from the German mosquito surveillance program.
Graphical representation of the Culex species composition in Germany. 48 different trapping sites in Germany were combined according to their geographical relatedness to form 10 cluster areas shown representing Lower Rhine Valley and further sites in Palatine, Upper Rhine Valley and further sites in Baden-Württemberg, Lake Constance, Lake Chiemsee and other sites in Bavaria, Hesse, Upper Elbe Valley in Saxonia, Oder Valley in Brandenburg, Baltic Sea in Mecklenburg-West Pomerania, Metropolitan Region Hamburg and various sites in Schleswig-Holstein (for more details see also Table 1)). White (Cpp. pipiens), black (Cx. torrentium) and red (Cpp. molestus) quarters indicate pools that were composed of a single species. Grey (Cpp. pipiens+Cx. torrentium) and dark-red (Cpp. molestus+Cx. torrentium) quarters indicate pools composed of two species. With the current set-up (i.e.using pooled samples) the composition of pink quarters could be either two biotypes Cpp. pipiens and Cpp. molestus or hybrids of both biotypes. The n-numbers given in the graphs notify total numbers of individuals analysed in each cluster.
Evidently, the use of different traps needs to be taken into account when evaluating regional species differences. Gravid traps were used more frequently within the clusters Lower and Upper Rhine valley as well as in the Hamburg metropolitan area compared to other clusters. Therefore, additional analyses were performed restricted to Culex specimens collected by CO2 baited traps (EVS and BG) but excluding mosquitoes obtained by gravid traps or from reared egg batches. The results indicate some changes in the relative abundances of Cpp and Cx. torrentium at some areas, in particular in the cluster area Upper Rhine Valley. However, the general distribution of Cx. torrentium and Cpp across Germany was unaffected. Moreover, trapping rates of Cpp. biotype molestus were not altered at all using the alternative dataset (see Figure S2).
However, this study did not aim at a systematic analysis of Cpp. habitats and therefore, we can neither confirm nor reject previous data on the occurrence of Cpp. biotype molestus, such as underground breeding or their preference for urban habitats [9]. Further detailed studies are needed to highlight this topic.
Regarding Cx. torrentium, it appears that this species has spread considerably within Europe and Germany during the last 60 years. Until the 1950s Cx. torrentium was considered rare in Central and Western Europe, primarily colonizing higher altitudes [18], [19], [20]. In a monograph from 1969, presence of Cx. torrentium was reported only at four localities in central and southern Germany [13]. Careful analyses of previous and recent data have suggested that the apparent increase in abundance and distribution of Cx. torrentium during recent years was probably due to ecological adaptation and anthropogenic spread, rather than the result of an increased awareness of taxonomists who may have previously misidentified Cx. torrentium as Cx. pipiens. Nowadays, Cx. torrentium is widespread in Europe though the exact distribution limits remain to be determined [9].
Screen for Cpp. biotype pipiens and biotype molestus hybrids in selected areas
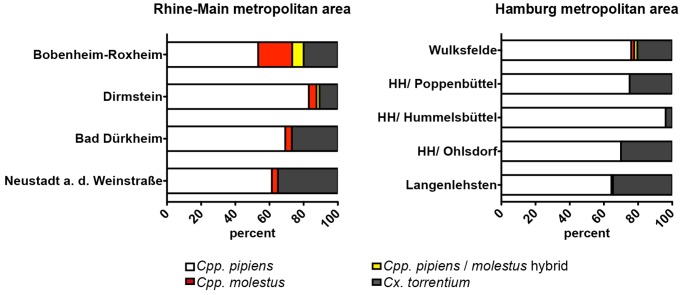
In order to determine whether Cpp. biotype hybrids do exist in Germany a total of four sites within the Rhine-Main metropolitan region were selected where Cpp. biotype pipiens and biotype molestus were found to occur sympatrically. As pooled samples are not suitable for the analyses of possible hybrids, a number of individual mosquito specimens from the region were subjected to multiplex real-time PCR. Hybrids were detected at two of the four sites with a frequency of 1.7% and 6.6% respectively (Figure 3). Moreover, analysis of individual specimens from 5 sites of the Hamburg metropolitan area revealed another site positive for Cpp. biotype hybrids with a frequency of 1.8% (Figure 3). Given the relatively low prevalence of biotype molestus at the selected study sites (7.9% in average), the results suggest that crossbreeding between the two Cpp. biotypes is a frequent event in Germany. In fact, the incidence of biotype hybrids might be even higher than established by this limited analysis of individual mosquitoes, as overlapping occurrence of both biotypes was detected in the pooled samples of four southern cluster areas. Therefore, it is likely that at least some of the pools that revealed a signal for Cpp. biotype pipiens and Cpp. biotype molestus may contain biotype hybrids. Certainly, more comprehensive analyses are required, to determine the importance of this finding for arbovirus risk assessment in Germany. In particular, more individual mosquito samples need to be analysed to evaluate realistic hybrid frequencies and the vector competence of those hybrids for the transmission of WNV and other viruses. Nevertheless, the findings presented here are of concern in light of epidemic spread of WNV in the United States, where crossbreads of biotype molestus and pipiens frequently occurred and facilitated transmission of WNV to humans [10], [21].
Figure 3. Identification of Cpp. biotype hybrids in two German metropolitan areas.
Detailed species composition at the 4 sampling sites of the Rhine-Main metropolitan area (right graph) and 5 sampling sites at Hamburg metropolitan area (left graph). DNA samples from single individuals collected at these sampling sites were subjected to the multiplex real-time PCR and analysed for the presence of biotype hybrids. Bars represent species distribution in percent at each site. White indicates Cpp. biotype pipiens; red Cpp. biotype molestus; yellow hybrids of biotypes pipiens and molestus. Biotype hybrids were found at Dirmstein and Bobenheim-Roxheim trapping sites in Southwest Germany and at the Wulksfelde trapping site in northern Germany. Additionally, the presence of Cx. torrentium was assed and is indicated by the grey bars in both graphs.
Supporting Information
Sequence alignment of Ace-2 and CQ11 loci and detailed qPCR reaction mixture. A) Sequence alignment for microsatellite locus CQ11 of Cpp. biotype pipiens (reference strain 258c; accession number gb/DQ470148.1) and Cpp. biotype molestus (reference strain 284b, accession number gb/470150.1). Primer binding sites for Cpp. pipiens/molestus forward and reverse primer are indicated in red, the control probe for Cpp. is indicated yellow, the species specific probes for Cpp. biotype pipiens and Cpp. biotype molestus are indicated in green and blue respectively. B) Sequence alignment for the Ace-2 locus of Cpp. biotype pipiens (reference strain isolate 41; accession number gb|JF430595.1) and Cx. torrentium (reference strain, accession number AY497525.1). The primer binding sites of Cx. torrentium forward and reverse are indicated in dark blue, the binding site of Cx. torrentium is indicated in orange. C) Detailed composition of the multiplex reaction mix used for all experiments presented in this publication. All primer and probes are colour-coded according to figure A) and B) and specific molarities in the 20 µL multiplex reaction are given.
(TIF)
Classification of Culex samples from the German Surveillance program without gravid trap data. Graphical representation of the species composition in Germany using the same dataset as figure 2 excluding all data derived from gravid traps. The 48 trapping sites in Germany were combined according to their geographical relatedness to form 10 cluster areas shown in the figure (Lower Rhine Valley and further sites in Palatine, Upper Rhine Valley and further sites in Baden-Württemberg, Lake Constance, Lake Chiemsee and other sites in Bavaria, Hesse, Upper Elbe Valley in Saxonia, Oder Valley in Brandenburg, Baltic Sea in Mecklenburg-West Pomerania, Metropolitan Region Hamburg and various sites in Schleswig-Holstein (see also table.1)). White (Cpp. pipiens), black (Cx. torrentium) and red (Cpp. molestus) quarters indicate pools that were composed of a single species. Grey (Cpp. pipiens+Cx. torrentium) and dark-red (Cpp. molestus+Cx. torrentium) quarters indicate pools composed of two species. With the current set-up (i.e. using pooled samples) the composition of pink quarters could be either two biotypes Cpp. pipiens and Cpp. molestus or hybrids of both biotypes. The n-numbers given in the graphs notify total numbers of individuals analysed in each cluster.
(TIF)
Acknowledgments
We are grateful to Rolf Garms, Bernd Noack, Iris Bruchhaus, Christian Timmann, Monic. Hagedorn, Tobias Spielmann and Thomas Kruppa for their contribution to mosquito sampling. We would also like to thank the Hamburg Airport GmbH and the Hamburg Ohlsdorf cemetery for permissions to carry out mosquito collections.
Funding Statement
This work was financially supported by the Leibniz Association, grant number SAW-2011-BNI-3-29, and the German Centre for Infection Research (DZIF). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Harbach RE, Harrison BA, Gad AM (1984) Culex (Culex) molestus Forskål (Diptera: Culicidae): Neotype designation, description, variation, and taxonomic status. Proc Entomol Soc Wash 86: 521–542. [Google Scholar]
- 2. Harbach RE, Dahl C, White GB (1985) Culex (Culex) pipiens Linnaeus (Diptera: Culicidae): Concepts, type designations, and description. Proc Entomol Soc Wash 87: 1–24. [Google Scholar]
- 3. Amraoui F, Krida G, Bouattour A, Rhim A, Daaboub J, et al. (2012) Culex pipiens, an Experimental Efficient Vector of West Nile and Rift Valley Fever Viruses in the Maghreb Region. PLoS ONE 7 (5) e36757 doi:10.1371/journal.pone.0036757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andreadis TG, Anderson JF, Vossbrinck CR, Main AJ (2004) Epidemiology of West Nile virus in Connecticut: a five-year analysis of mosquito data 1999–2003. Vector Borne Zoonotic Dis 4: 360–378. [DOI] [PubMed] [Google Scholar]
- 5. Farajollahi A, Fonseca DM, Kramer LD, Marm Kilpatrick A (2011) “Bird biting” mosquitoes and human disease: a review of the role of Culex pipiens complex mosquitoes in epidemiology. Infect Genet Evol 11: 1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Meegan JM, Khalil GM, Hoogstraal H, Adham FK (1980) Experimental transmission and field isolation studies implicating Culex pipiens as a vector of Rift Valley fever virus in Egypt. Am J Trop Med Hyg 29: 1405–1410. [DOI] [PubMed] [Google Scholar]
- 7. Moutailler S, Krida G, Schaffner F, Vazeille M, Failloux AB (2008) Potential vectors of Rift Valley fever virus in the Mediterranean region. Vector Borne Zoonotic Dis 8: 749–753. [DOI] [PubMed] [Google Scholar]
- 8. Harbach RE (2011) Classification within the cosmopolitan genus Culex (Diptera: Culicidae): the foundation for molecular systematics and phylogenetic research. Acta Trop 120: 1–14. [DOI] [PubMed] [Google Scholar]
- 9.Becker N, Petrić D, Zgomba M, Boase C, Madon M, et al.. (2010) Mosquitoes and their control. Berlin, Heidelberg: Springer. 577 p.
- 10. Huang S, Hamer GL, Molaei G, Walker ED, Goldberg TL, et al. (2009) Genetic variation associated with mammalian feeding in Culex pipiens from a West Nile virus epidemic region in Chicago, Illinois. Vector Borne Zoonotic Dis 9: 637–642. [DOI] [PubMed] [Google Scholar]
- 11. Gomes B, Sousa CA, Novo MT, Freitas FB, Alves R, et al. (2009) Asymmetric introgression between sympatric molestus and pipiens forms of Culex pipiens (Diptera: Culicidae) in the Comporta region, Portugal. BMC Evolutionary Biology 2009 9: 262 doi:10.1186/1471-2148-9-262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reusken CB, de Vries A, Buijs J, Braks MA, den Hartog W, et al. (2010) First evidence for presence of Culex pipiens biotype molestus in the Netherlands, and of hybrid biotype pipiens and molestus in northern Europe. J Vector Ecol 35: 210–212. [DOI] [PubMed] [Google Scholar]
- 13. Mohrig W (1969) Die Culiciden Deutschlands. Parasitolog SchrReihe 18: 260. [Google Scholar]
- 14. Bahnck CM, Fonseca DM (2006) Rapid assay to identify the two genetic forms of Culex (Culex) pipiens L. (Diptera: Culicidae) and hybrid populations. Am J Trop Med Hyg 75: 251–255. [PubMed] [Google Scholar]
- 15. Danabalan R, Ponsonby DJ, Linton YM (2012) A critical assessment of available molecular identification tools for determining the status of Culex pipiens s.l. in the United Kingdom. J Am Mosq Control Assoc 28: 68–74. [DOI] [PubMed] [Google Scholar]
- 16. Fonseca DM, Keyghobadi N, Malcolm CA, Mehmet C, Schaffner F, et al. (2004) Emerging vectors in the Culex pipiens complex. Science 303 (5663) 1535–1538. [DOI] [PubMed] [Google Scholar]
- 17. Smith JL, Fonseca DM (2004) Rapid assays for identification of members of the Culex (Culex) pipiens complex, their hybrids, and other sibling species (Diptera: Culicidae). Am J Trop Med Hyg 70: 339–345. [PubMed] [Google Scholar]
- 18. Mattingly PF (1951) Culex (Culex) torrentium Martini, a mosquito new to Great Britain. Nature 168 (4265) 172. [DOI] [PubMed] [Google Scholar]
- 19. Service MW (1968) The taxonomy and biology of two sympatric sibling species of Culex, C. pipiens and C. torrentium (Diptera, Culicidae). J Zool, Lond 156: 313–323. [Google Scholar]
- 20. Struppe T (1989) Biologie und Ökologie von Culex torrentium Martini unter besonderer Berücksichtigung seiner Beziehungen im menschlichen Siedlungsbereich. Z f Angew Zool 76: 257–286. [Google Scholar]
- 21. Huang S, Molaei G, Andreadis TG (2011) Reexamination of Culex pipiens hybridization zone in the Eastern United States by ribosomal DNA-based single nucleotide polymorphism markers. Am J Trop Med Hyg 85: 434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequence alignment of Ace-2 and CQ11 loci and detailed qPCR reaction mixture. A) Sequence alignment for microsatellite locus CQ11 of Cpp. biotype pipiens (reference strain 258c; accession number gb/DQ470148.1) and Cpp. biotype molestus (reference strain 284b, accession number gb/470150.1). Primer binding sites for Cpp. pipiens/molestus forward and reverse primer are indicated in red, the control probe for Cpp. is indicated yellow, the species specific probes for Cpp. biotype pipiens and Cpp. biotype molestus are indicated in green and blue respectively. B) Sequence alignment for the Ace-2 locus of Cpp. biotype pipiens (reference strain isolate 41; accession number gb|JF430595.1) and Cx. torrentium (reference strain, accession number AY497525.1). The primer binding sites of Cx. torrentium forward and reverse are indicated in dark blue, the binding site of Cx. torrentium is indicated in orange. C) Detailed composition of the multiplex reaction mix used for all experiments presented in this publication. All primer and probes are colour-coded according to figure A) and B) and specific molarities in the 20 µL multiplex reaction are given.
(TIF)
Classification of Culex samples from the German Surveillance program without gravid trap data. Graphical representation of the species composition in Germany using the same dataset as figure 2 excluding all data derived from gravid traps. The 48 trapping sites in Germany were combined according to their geographical relatedness to form 10 cluster areas shown in the figure (Lower Rhine Valley and further sites in Palatine, Upper Rhine Valley and further sites in Baden-Württemberg, Lake Constance, Lake Chiemsee and other sites in Bavaria, Hesse, Upper Elbe Valley in Saxonia, Oder Valley in Brandenburg, Baltic Sea in Mecklenburg-West Pomerania, Metropolitan Region Hamburg and various sites in Schleswig-Holstein (see also table.1)). White (Cpp. pipiens), black (Cx. torrentium) and red (Cpp. molestus) quarters indicate pools that were composed of a single species. Grey (Cpp. pipiens+Cx. torrentium) and dark-red (Cpp. molestus+Cx. torrentium) quarters indicate pools composed of two species. With the current set-up (i.e. using pooled samples) the composition of pink quarters could be either two biotypes Cpp. pipiens and Cpp. molestus or hybrids of both biotypes. The n-numbers given in the graphs notify total numbers of individuals analysed in each cluster.
(TIF)