Abstract
Background
The purpose of this study was to quantify the walking time and frequency of postural changes in daily life in patients with chronic obstructive pulmonary disease (COPD) using a new triaxial accelerometer system.
Methods
Twenty-six elderly patients with stable COPD (age 76.8 ± 6.2 years; percent forced expiratory volume in one second [%FEV1] 52.9% ± 26.3%) and 20 age-matched elderly subjects (age 73.0 ± 4.2 years; %FEV1 124.0% ± 22.3%) participated in the study. The subjects’ time spent walking (slow, fast), standing, sitting, and lying down and the frequency of their postural changes (getting up, standing up) were assessed for 7 consecutive days using an Activity Monitoring And Evaluation System (A-MES™). We analyzed the relationships among walking times, frequency of postural changes, and physiologic factors in both COPD patients and controls.
Results
The COPD patients’ total walking time, including slow (<2 km/hour) and fast (≥2 km/hour) walking, and their frequency of standing up were significantly lower than those of the age-matched controls (P < 0.01). The fast walking time in daily life was significantly correlated with the 6-minute walking distance, quadriceps femoris muscle force, and dyspnea (P < 0.01).
Conclusion
These results suggest that both slow (<2 km/hour) and fast (≥2 km/hour) walking time and frequency of postural changes is significantly decreased in COPD patients compared with healthy elderly subjects. The data also suggest that the COPD patients’ different walking times in daily life are significantly correlated with exercise capacity and dyspnea. The 6-minute walking distance had the strongest correlation with fast walking time.
Keywords: slow walking, fast walking, frequency of postural changes, chronic obstructive pulmonary disease, triaxial accelerometer
Introduction
Physical activity is an important clinical parameter related to morbidity and mortality in many chronic diseases, including chronic obstructive pulmonary disease (COPD).1 Physical activity is defined as any bodily movement produced by skeletal muscles that results in energy expenditure beyond resting energy expenditure.1 In COPD, the level of physical activity reported by patients is related to lung function, hospitalizations, and mortality.2–4 Physical activity can be quantified using self-report questionnaires5,6 as well as by motion sensors such as pedometers and accelerometers.4,7–9 A self-report questionnaire is often subject to recall bias, correlates only poorly with objectively qualified physical activity, and does not provide an accurate estimate of free-living energy expenditure.10–12 In contrast, pedometers and accelerometers generate objective data in terms of quantifying steps or body movements performed over a period of time.10
Physical activity in terms of walking time and standing time has been shown to be reduced in patients with COPD.9,13 However, to our knowledge, the average walking speed of COPD patients in daily life and the frequency of their postural changes have not been quantified. Therefore, the objective of the present study was to quantify the different walking times and frequency of postural changes in daily life in elderly patients with COPD using a newly developed triaxial accelerometer system (A-MES™ [Activity Monitoring and Evaluation System], Solid Brains Inc, Kumamoto, Japan).14
Materials and methods
Twenty-six patients with stable mild to very severe COPD according to Global initiative for chronic Obstructive Lung Disease (GOLD) criteria15 and 20 healthy age-matched elderly subjects living in public facilities were enrolled (Table 1). The patients with COPD and the healthy age-matched subjects were all retired. The inclusion criteria for the study were as follows: the subject was in a stable condition with no infection or exacerbation of COPD for at least the previous 3 months; the subject was able to walk unassisted and operate the A-MES; and the subject had no severe and/or unstable cardiac disease, orthopedic disease, or mental disorder that could impair physical activity in daily life. A detailed overview of the comorbidities in both groups is shown in Table 2.
Table 1.
Baseline characteristics in COPD subjects and controls
| COPD (n = 26) | Healthy elderly subjects (n = 20) | |
|---|---|---|
| Age, years | 77 ± 6 | 73 ± 4 |
| Sex, male/female | 26/0** | 8/12 |
| BMI, kg/m2 | 21.2 ± 3.5* | 24.2 ± 2.7 |
| GOLD stage I, II, III, IV | 4/7/11/4 | – |
| TLC, L | 6.5 ± 1.0 | – |
| DLCO, mL/min/mmHg | 9.8 ± 5.2 | – |
| PaO2, mmHg | 67.8 ± 20.3 | – |
| FVC, L | 2.9 ± 0.7 | 2.9 ± 0.5 |
| FEV1, L | 1.3 ± 0.7** | 2.2 ± 0.4 |
| FEV1/FVC, % | 43.0 ± 16.1** | 77.7 ± 9.1 |
| FEV1, %pred | 52.9 ± 26.3** | 124.0 ± 22.3 |
| PImax, cmH2O | 63.3 ± 23.7* | 78.0 ± 24.8 |
| PEmax, cmH2O | 102.6 ± 29.4 | 86.8 ± 17.0 |
| 6MWD, m | 381.1 ± 172.9** | 536.3 ± 50.7 |
| Borg (dyspnea) | 4.3 ± 3.2* | 2.6 ± 1.6 |
| Borg (leg fatigue) | 2.2 ± 2.4 | 1.7 ± 1.7 |
Notes: All data are shown as the mean ± standard deviation.
P < 0.05;
P < 0.01 versus controls.
Abbreviations: COPD, chronic obstructive pulmonary disease; BMI, body mass index; GOLD, Global initiative for chronic Obstructive Lung Disease; TLC, total lung capacity; DLCO, carbon monoxide diffusing capacity; PaO2, arterial oxygen partial pressure; FVC, forced vital capacity; FEV1, forced expiratory volume in one second; PImax, maximum inspiratory mouth pressure; PEmax, maximum expiratory mouth pressure; 6MWD, six-minute walking distance; Borg (dyspnea, leg fatigue), Borg scale after 6MWT.
Table 2.
Comorbidities in COPD patients and healthy age-matched subjects
| Comorbidities | COPD (n = 26) | Healthy elderly subjects (n = 20) |
|---|---|---|
| Osteoporosis | 9 | 0 |
| Diabetes | 4 | 2 |
| Chronic heart failure | 3 | 0 |
| Hypertension | 7 | 7 |
| Low back pain | 6 | 0 |
| Arthritis | 4 | 3 |
Abbreviation: COPD, chronic obstructive pulmonary disease.
We first informed the subject about how to use the A-MES and confirmed that they understood how to operate the device. Each subject was given an A-MES, an instruction book, and the appropriate clothing; they were then instructed to measure their own physical activity in daily life. Given that physical activity varies according to the cycle of the seasons,16 we measured physical activity in all subjects from April 2010 until October 2010. The assessment was done for a maximum of 7 consecutive days, including weekdays and weekends, and physical activity was measured with the A-MES from waking time until 12 hours after waking time.13 In the end, breakdown of the number of measurement days showed a mean (standard deviation) 3 ± 2 days in the COPD patients. However, we used the data from all subjects who provided at least 2 days of valid assessment to study the variability in a portion of the study population. The protocols and results are described in detail in the Supplementary materials. We also considered the minimum number of days necessary for a reliable assessment of physical activity in daily life.10
The study was reviewed and approved by the ethics committees at our hospital and at the Akita University Graduate School of Medicine, and was carried out in conformity with the Declaration of Helsinki.17 The objective and content of the study were explained verbally to the participants, with additional documents provided. Written consent was obtained after the subjects were informed that they could decide whether or not to participate based on their own free will and that their privacy would be sufficiently considered.
Assessment of physical activity in daily living
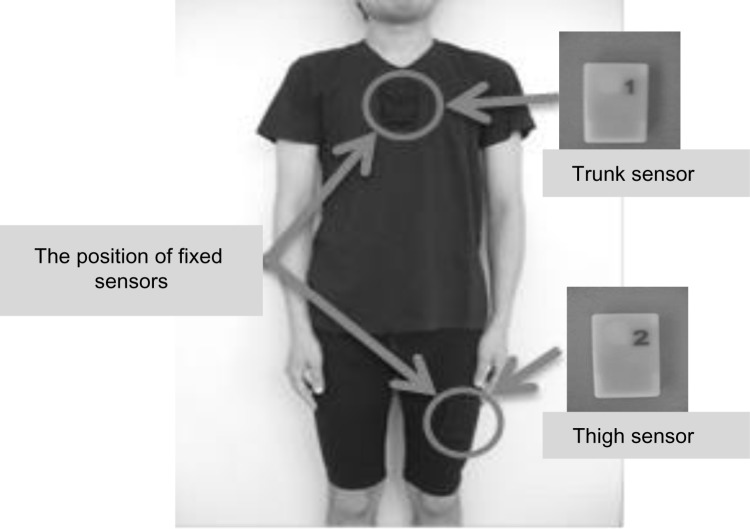
Assessment of physical activity in daily living was done using the A-MES, a new triaxial accelerometer system.14 The A-MES consists of two sensors (69 height × 44 width × 11.5 depth mm each, weight 28 g each), a station, and analytical software used with a personal computer. These sensors are so small and lightweight that they can be attached to the thigh and chest of the subject wearing clothing with two pockets (Figure 1). The physical activity data recorded by the two sensors is sent to the A-MES station and analyzed by the A-MES software.
Figure 1.
Activity Monitoring and Evaluation System (A-MES™). Size: 69 height × 44 width × 11.5 depth mm. Weight 28 g.
The A-MES system measures movement (lying down, sitting, standing, and walking) and the maximum continuously active time using three-dimensional analysis of acceleration (Figure 2). The system can also measure the frequency of postural changes, ie, getting up (bodily change from a recumbent to an upright position) and standing up (bodily change from a sitting to an upright position), as shown in Figure 2. Moreover, the A-MES can measure walking time and divide this into slow (<2 km/hour) and fast (≥2 km/hour) walking time by changing the sensor threshold.18 We studied validation of the A-MES in part of the population included in this study. Details regarding the protocols and results are provided in the Supplementary materials.
Figure 2.
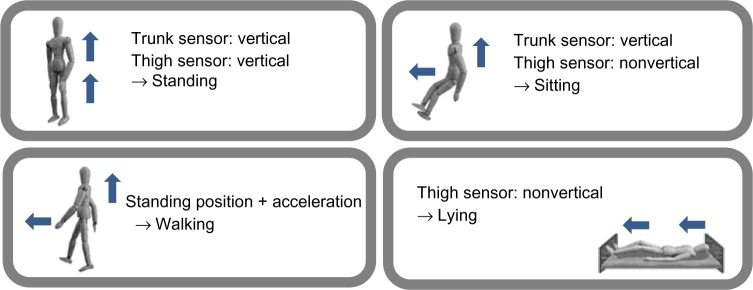
Measurement of a subject’s position, movement, and postural changes by the Activity Monitoring and Evaluation System (A-MES™) sensors.
Other measurements
Pulmonary function was assessed as forced vital capacity (FVC), forced expiratory volume in one second (FEV1), FEV1/FVC, and %FEV1, measured using a multifunctional spirometer (HI-701, Chest M.I., Inc., Tokyo, Japan). The mouth pressure was measured as respiratory muscle strength using a respiratory dynamometer (Vitalopower KH-101, Chest M.I., Inc.) following the method recommended by the American Thoracic Society (ATS)/European Respiratory Society (ERS).19
For quadriceps femoris muscle force, the maximum isometric extension and contraction of this muscle was measured at 0 degrees per second and 80 degree knee flexion using the Hydromusculator GT-160 (OG Giken Co, Tokyo, Japan).20 For measurement of exercise performance, the subject performed a corridor walk for 6 minutes according to the ATS guidelines.21 The distance walked in 6 minutes (6MWD) and the modified Borg scale22 were used in the analysis. The subjects’ dyspnea was assessed using the Medical Research Council (MRC) dyspnea scale.23 Disease-specific health-related quality of life was measured using the Japanese version of the Chronic Respiratory Disease Questionnaire (CRQ).24 The questionnaire scores four domains, ie, dyspnea, fatigue, emotion, and mastery.
Statistical analysis
Statistical analyses were performed using Statistical Package for the Social Sciences software version 12.0 J for Windows (SPSS Inc, Chicago, IL, USA). The level of significance was set at P < 0.05. Comparisons of baseline characteristics, time spent performing different activities, and frequency of postural changes between the COPD and control groups were done using unpaired t-tests, except in the case of sex and the maximum continuously active time for each position and movement, for which the equivalent nonparametric test (chi-square test, Mann–Whitney U test) was used. In addition, comparison of time spent walking fast and slow in both groups was carried out using paired t-tests.
The Pearson’s product–moment correlation coefficient was used for single correlations between walking (total, active, and passive) time with other physiologic measurements, and Spearman’s rank correlation coefficient was used for the ordinal scales (ie, Borg scale and MRC dyspnea scale) in the COPD group. A stepwise multiple regression analysis was performed to assess independent contributors to total time of walking and movement in daily life in the COPD group. We adopted a series of independent factors (age, body mass index, FVC, FEV1/FVC, %FEV1, maximum inspiratory mouth pressure, 6MWD, MRC dyspnea scale, quadriceps femoris muscle force, and CRQ [total score]), excluding factors that were mutually related and the items that gave a correlation coefficient >0.90 in order to remove the influence of multicollinearity.
Results
With regard to baseline characteristics, the proportions of males and females were significantly different, and patients with COPD were characterized by airflow obstruction as well as reduced body mass index and inspiratory muscle and maximum exercise capacity compared with healthy elderly subjects (Table 1).
Compared with the healthy elderly subjects, the COPD patients had significantly reduced maximum continuously active walking time and significantly reduced total times of both slow walking (<2 km/hour) and fast walking (≥2 km/hour) in daily life. The COPD patients also had significantly reduced frequencies of standing up (Table 3). Moreover, fast walking time was significantly reduced compared with slow walking time in both groups.
Table 3.
Physical activities of COPD patients and healthy elderly subjects by daily measurements
| COPD (n = 26) | Healthy elderly subjects (n = 20) | P-value | |
|---|---|---|---|
| Days of measurement | 4 ± 2 | 6 ± 1 | P = 0.140 |
| Measurement time, minutes per day | 705 ± 117 | 764 ± 44 | P = 0.097 |
| Total time, minutes per day | |||
| Total walking | 118 ± 72 | 210 ± 57 | P < 0.001 |
| Fast (≥2 km/hour) | 36 ± 35* | 82 ± 42* | P < 0.001 |
| Slow (<2 km/hour) | 69 ± 30 | 141 ± 40 | P < 0.001 |
| Standing | 79 ± 48 | 80 ± 23 | P = 0.920 |
| Sitting | 417 ± 116 | 316 ± 67 | P = 0.001 |
| Lying | 107 ± 105 | 101 ± 65 | P = 0.818 |
| Maximum continuously active time, minutes per day | |||
| Walking | 7 ± 7 | 10 ± 6 | P = 0.027 |
| Standing | 6 ± 4 | 4 ± 3 | P = 0.355 |
| Sitting | 65 ± 32 | 40 ± 11 | P = 0.011 |
| Lying | 32 ± 27 | 41 ± 20 | P = 0.250 |
| Frequency of postural change, times per day | |||
| Total | 88 ± 35 | 126 ± 42 | P = 0.002 |
| Getting up | 37 ± 28 | 39 ± 25 | P = 0.816 |
| Standing up | 49 ± 22 | 84 ± 24 | P < 0.001 |
Notes: All data are shown as the mean ± standard deviation;
P < 0.05 compared with slow walking time in each group.
Abbreviation: COPD, chronic obstructive pulmonary disease.
In Table 4, the total time spent walking in daily life in the COPD patients was positively correlated with their maximum inspiratory mouth pressure, maximum expiratory mouth pressure, 6MWD, and quadriceps femoris muscle force (0.40< r <0.52, P < 0.05), and was negatively correlated with age, and the MRC and Borg dyspnea scales (−0.61< r <−0.41). Moreover, total fast walking time was positively correlated with FVC and FEV1. In contrast, total time spent lying down in daily life was positively correlated with the MRC dyspnea scale (r = 0.43, P < 0.05), and was negatively correlated with the total score, dyspnea, and mastery of CRQ (−0.51< r <−0.40, P < 0.05). The factors of age and 6MWD were found to be independent contributors to total walking time in patients with COPD (R2 = 0.71, P < 0.001). No other factors contributed significantly to the variance of total time spent standing, sitting, and lying down by the stepwise multiple regression analysis (Table 5).
Table 4.
Single correlation coefficients between physical activity and other physiologic measurements
| Total walking | Fast walking (≥2 km/hour) | Slow walking (<2 km/hour) | Standing | Sitting | Lying | |
|---|---|---|---|---|---|---|
| Age | −0.47* | −0.65* | −0.27 | −0.09 | 0.15 | 0.13 |
| BMI | 0.25 | 0.28 | 0.17 | 0.18 | −0.26 | 0.27 |
| FVC | 0.26 | 0.41* | 0.07 | −0.21 | −0.26 | 0.17 |
| FEV1 | 0.40 | 0.42* | 0.28 | −0.24 | −0.29 | 0.07 |
| FEV1/FVC | 0.38 | 0.29 | 0.37 | −0.23 | −0.19 | 0.02 |
| %FEV1 | 0.33 | 0.34 | 0.26 | −0.23 | −0.24 | 0.07 |
| PImax | 0.47* | 0.45* | 0.39 | 0.14 | −0.29 | −0.10 |
| PEmax | 0.40* | 0.42* | 0.31 | 0.11 | −0.30 | −0.02 |
| 6MWD | 0.52** | 0.51* | 0.44* | −0.09 | −0.34 | 0.05 |
| Borg (dyspnea) | −0.41* | −0.36 | −0.32 | −0.05 | 0.08 | 0.24 |
| Borg (leg fatigue) | −0.08 | 0.07 | −0.04 | −0.11 | 0.06 | 0.11 |
| QF | 0.46* | 0.60* | 0.33 | 0.26 | −0.24 | −0.17 |
| MRC dyspnea scale | −0.61** | −0.66* | −0.54* | −0.12 | 0.18 | 0.43* |
| CRQ (total) | 0.28 | 0.29 | 0.20 | 0.23 | 0.06 | −0.51** |
| Dyspnea | 0.31 | 0.25 | 0.30 | 0.11 | 0.18 | −0.40** |
| Fatigue | 0.18 | 0.19 | 0.14 | 0.04 | 0.05 | −0.29 |
| Emotional function | 0.07 | 0.17 | 0.02 | 0.27 | −0.08 | −0.12 |
| Mastery | 0.26 | 0.29 | 0.19 | 0.26 | −0.03 | −0.44* |
Notes:
P < 0.05;
P < 0.01.
Abbreviations: BMI, body mass index; QF, quadriceps femoris muscle force; MRC, Medical Research Council; CRQ, chronic respiratory disease questionnaire; FVC, forced vital capacity; FEV1, forced expiratory volume in one second; PImax, maximum inspiratory mouth pressure; PEmax, maximum expiratory mouth pressure; 6MWD, six-minute walking distance; Borg (dyspnea, leg fatigue), Borg scale after 6MWT.
Table 5.
Assessment of independent contributors to total walking time in patients with COPD
| B | β | P-value | 95% CI | |
|---|---|---|---|---|
| 594.19 | P < 0.001 | 311.38–877.01 | ||
| Age | −7.18 | −0.58 | P = 0.001 | −10.75 to −3.61 |
| 6MWD | 0.23 | 0.56 | P = 0.001 | 0.11–0.35 |
Notes: R2 = 0.71, analysis of variance, P < 0.001; B, partial regression coefficient; β, standardized partial regression coefficient.
Abbreviations: 6MWD, six-minute walking distance; CI, confidence interval; COPD, chronic obstructive pulmonary disease.
Discussion
Inactivity in COPD is not a surprising finding in itself. Schonhofer et al4 and Singh et al25 have reported that patients with COPD have lower daily movement counts than the average in subjects matched for age and sex. However, the devices used in these previous studies were unable to provide detailed information from the subjects’ daily lives and are likely less sensitive than the triaxial accelerometer.26 The present finding that patients with COPD spend significantly less time walking compared with healthy control subjects is in line with a 2005 study by Pitta et al.13
However, our study is the first to show that COPD patients spend significantly less time doing both slow (<2 km/hour) and fast (≥2 km/hour) walking and change their posture less frequently in daily life than do healthy elderly subjects. Moreover, the proportion of fast walking time to total time was approximately 28% lower than that of slow walking in the COPD patients, and we found that the COPD patients’ frequency of standing up was approximately 40% lower than that of the healthy elderly subjects. In addition, their maximum continuously active time of walking was approximately 30% lower than that in the healthy subjects. This sedentary behavior in COPD patients was further illustrated by the finding that they walked more slowly and changed their body position (eg, getting up, standing up) less frequently. Watz et al27 reported that the intensity of physical activity in COPD patients is significantly less than that in healthy subjects. A-MES, the triaxial accelerometer system used in this study, has been developed to detect the movement of slow walking in relatively inactive elderly individuals, so the detection threshold for walking speed is set at 2 km/hour, which is slower than the usual walking speed in daily life.14 It can be assumed that walking speed is almost always lower than 2 km/hour in daily living, and that it is more than 2 km/hour for movement in general.28,29 We found that fast walking time was significantly less than slow walking time in COPD patients, and that almost all walking time was accounted for by slow walking in these patients. These data suggest that patients with COPD might reduce their energy expenditure by walking at slower walking speeds.27
In the COPD patients, time spent walking per day was significantly correlated with muscle power in the lower extremities and exercise capacity (determined by the relationship between physical activity and physiologic factors). Because their fast walking time was correlated with more physiologic factors than their slow walking time, assessment of fast walking time in COPD patients may be useful for predicting physiologic function, which is one of the new topics addressed in this study. The 6MWD is thought to be the most important index for evaluating physical activity in patients with COPD.13 Our study also shows that the 6MWD could be selected as a variability coefficient in the stepwise multiple regression analysis of walking time. Watz et al27 have also reported that many COPD patients are forced to change their lifestyle and become more sedentary due to dyspnea upon exertion. Decreased pulmonary function in COPD patients has been shown to exacerbate their downward spiral of symptom-induced inactivity, leading to deconditioning and muscle weakness.4,7 The results of the present study suggest that a reduction in the time that COPD patients spend walking at different speeds per day as well as their decline in limb muscle and exercise capacity may influence their inactivity in daily life. Moreover, in COPD patients, the total time spent lying down was found to be significantly correlated with both dyspnea and total CRQ score. Another study has shown that inactivity in daily life may cause decreased health-related quality of life in COPD patients.6 Therefore, time spent walking and lying down in daily life might be objective indices of dyspnea, exercise capacity, and health-related quality of life in COPD patients.
It has been reported that pulmonary rehabilitation increases physical activity in COPD patients.30,31 Pitta et al have reported that walking time in COPD patients was improved by pulmonary rehabilitation using an accelerometer for 6 months, suggesting that a decrease in dyspnea may increase physical activity in these patients.31 One of the strategies for increasing the amount of time spent standing and walking in COPD patients might be to improve the ability to stand up. As such, it may be important to improve the function of the lower extremities by exercise. In addition, assessing physical activity using an accelerometer is an objective and useful method for tracking progress and maintaining physical activity levels. Our device is very small and light, making it very unobtrusive during measurements, which is a major benefit.
There are several limitations to the present study that must be addressed. First, the number of patients recruited was small. As such, additional research in a larger number of patients is required to clarify the significance of different walking times and the number of postural changes in patients with COPD. Second, the present study used inclusion criteria for subjects that excluded patients with severe and/or unstable heart disease, orthopedic disease, or mental disorders that could impair physical activity in daily life. Nevertheless, patients with COPD tend to have more comorbidities than controls. In addition, the healthy elderly subjects in this study were exercising of their own accord at public facilities, and the percentage of females was significant higher than that in the COPD group, so we must allow for the possibility that the results of this study may have been affected by these factors. A further study comparing groups with the same comorbidities is required to verify the reliability and validity of the findings of the present study.
Conclusion
These data suggest that both slow (<2 km/hour) and fast (≥2 km/hour) walking speeds and the frequency of postural changes are significantly decreased in COPD patients compared with healthy elderly subjects. Moreover, almost all walking time in daily life in COPD patients is slow walking, and fast walking times are significantly correlated with more physiologic factors than slow walking times. The 6MWD had the strongest correlation with fast walking time, and it was found that COPD patients with severely impaired 6MWD are likely to have very low activity levels in daily life.
Supplementary materials
Validation of A-MES
Validation of the A-MES was investigated in part of the population used in this study. Ten healthy elderly male subjects (mean age 79 ± 5 years, forced expiratory volume in one second 123.8% ± 27.6%) underwent a one-hour standardized protocol in which they performed walking and staying in different positions (standing, sitting, lying down) during a given time which was not disclosed to the subjects. Each subject wore an A-MES during the protocol, and a video recording was made of the entire protocol. The time spent performing the various activities and postures was obtained by chronometer analysis of the video recording and used as the criterion standard.1 The Pearson’s product–moment correlation coefficient was used for correlations between total time and maximum continuously active time measured by A-MES and video recording. The results of this study showed that the total time and maximum continuously active time spent walking, standing, sitting, and lying down measured by the A-MES were well correlated with the values measured by the video recording (Table S1).
Analysis of variability in physical activity during daily Japanese life
Variability was studied in part of the population included in the study. Ten COPD patients (age 75 ± 5 years, forced expiratory volume in one second 57.4% ± 28.1%) and 12 healthy elderly subjects (age 73 ± 4 years, forced expiratory volume in one second 126.9% ± 24.4%) were assessed for their physical activity in daily life on 7 consecutive days using the same methods described in the main methods section of this report. Analysis of the intraclass reliability coefficient showed that assessments on 2 consecutive days were enough to assess reliably the time spent in walking, standing, sitting, and lying in a day (Table S2).
Single correlation coefficients between total time and maximum continuously active time measured by A-MES™ and video recording in ten healthy elderly subjects (Pearson’s product–moment correlation coefficient)
| Total time | Maximum continuously active time | |
|---|---|---|
| Walking | 0.986** | 0.995** |
| Standing | 0.940** | 0.949** |
| Sitting | 0.987** | 0.982** |
| Lying | 0.992** | 0.994** |
Notes:
P < 0.01. Pearson’s product-moment correlation coefficient = r.
Abbreviation: A-MES, Activity Monitoring and Evaluation System.
Intraclass reliability coefficients for days 2–7 of A-MES™ activity monitoring in a group of 10 COPD patients and 12 healthy elderly subjects
| Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | |
|---|---|---|---|---|---|---|
| Walking time | 0.943** | 0.961** | 0.974** | 0.981** | 0.982** | 0.985** |
| Standing time | 0.754** | 0.915** | 0.941** | 0.964** | 0.968** | 0.969** |
| Sitting time | 0.865** | 0.919** | 0.934** | 0.951** | 0.957** | 0.962** |
| Lying time | 0.778* | 0.824** | 0.862** | 0.897** | 0.913** | 0.918** |
Notes:
P < 0.05;
P < 0.01.
Abbreviations: A-MES, Activity Monitoring and Evaluation System; COPD, chronic obstructive pulmonary disease.
Reference
- 1.Sato T, Kitamura N, Kawagoshi A, et al. [Adequacy of physical activity time evaluated by a triaxial accelerometer] J Soc Biomech. 2011;35:197–200. Japanese. [Google Scholar]
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Thompson PD, Buchner D, Pina IL, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity) Circulation. 2003;107:3109–3116. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- 2.Pitta F, Troosters T, Probst VS, et al. Physical activity and hospitalization for exacerbation of COPD. Chest. 2006;129:536–544. doi: 10.1378/chest.129.3.536. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Aymerich J, Lange P, Benet M, et al. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax. 2006;61:772–778. doi: 10.1136/thx.2006.060145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schonhofer B, Ardes P, Geibel M, et al. Evaluation of a movement detector to measure daily activity in patients with chronic lung disease. Eur Respir J. 1997;10:2814–2819. doi: 10.1183/09031936.97.10122814. [DOI] [PubMed] [Google Scholar]
- 5.Moy ML, Matthess K, Stolzmann K, et al. Free-living physical in COPD: assessment with accelerometer and activity checklist. J Rehabil Res. 2009;46:277–286. doi: 10.1682/jrrd.2008.07.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esteban C, Quintana JM, Aburto M, et al. Impact of changes in physical activity on health-related quality of life among patients with chronic obstructive pulmonary disease. Eur Respir J. 2010;36:292–300. doi: 10.1183/09031936.00021409. [DOI] [PubMed] [Google Scholar]
- 7.Steele BG, Holt L, Belza B, et al. Quantitating physical activity in COPD using a triaxial accelerometer. Chest. 2000;117:1359–1367. doi: 10.1378/chest.117.5.1359. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen HQ, Steele B, Benditt JO. Use of accelerometers to characterize physical activity patterns with COPD exacerbations. Int J COPD. 2006;1:455–460. doi: 10.2147/copd.2006.1.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pitta F, Takaki MY, Oliveira NH, et al. Relationship between pulmonary function and physical activity in daily life in patients with COPD. Respir Med. 2008;102:1203–1207. doi: 10.1016/j.rmed.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Benzo R. Activity monitoring in chronic obstructive pulmonary disease. J Cardiopulm Rehabil. 2009;29:341–347. doi: 10.1097/HCR.0b013e3181be7a3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shephard RJ. Limits to the measurement of habitual physical activity by questionnaires. Br J Sports Med. 2003;37:197–206. doi: 10.1136/bjsm.37.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitamura N, Sato T, Kawagoshi A, et al. Evaluation of physical activity in the daily life of healthy young subjects with special reference to the reliability and validity of IPAQ as evaluated by a triaxial accelerometer. Rigakuryouho Kagaku. 2010;25:767–771. Japanese. [Google Scholar]
- 13.Pitta F, Troosters T, Spruit MA, et al. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171:972–977. doi: 10.1164/rccm.200407-855OC. [DOI] [PubMed] [Google Scholar]
- 14.Sakata S, Nagata M, Nojiri S. [Approach to the measurement of ADL (activities of daily living)] Den-O-Ken Technical Report. 2002;12:19–25. Japanese [with English abstract] [Google Scholar]
- 15.Global Initiative for Chronic Obstructive Lung Disease Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease – Executive Summary Geneva, Switzerland: Heart, Lung, and Blood Institute, World Health Organization; 2003Available from: http://www.who.int/respiratory/copd/GOLD_WR_06.pdfAccessed July 27, 2013 [Google Scholar]
- 16.Sewell L, Singh SJ, Williams JE, et al. Seasonal variations affect physical activity and pulmonary rehabilitation outcomes. J Cardiopulm Rehabil. 2010;30:329–333. doi: 10.1097/HCR.0b013e3181e175f2. [DOI] [PubMed] [Google Scholar]
- 17.World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects Adapted by 59th WMA General AssemblySeoul, Korea2008Available from: http://www.wma.net/en/30publications/10policies/b3/17c.pdfAccessed July 27, 2013 [Google Scholar]
- 18.Sato T, Kitamura N, Kawagoshi A, et al. [Adequacy of physical activity time evaluated by a triaxial accelerometer] J Soc Biomech. 2011;35:197–200. Japanese. [Google Scholar]
- 19.American Thoracic Society/European Respiratory Society ATS/ERS statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166:518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- 20.Decramer M, Lacquet LM, Fagard R, et al. Corticosteroids contribute to muscle weakness in chronic airflow obstruction. Am J Respir Crit Care Med. 1994;150:11–16. doi: 10.1164/ajrccm.150.1.8025735. [DOI] [PubMed] [Google Scholar]
- 21.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 22.Borg G. Borg’s Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics; 1998. The Borg CR10 Scale. [Google Scholar]
- 23.American Thoracic Society/European Respiratory Society Definition, diagnosis and staging Standards for the Diagnosis and Management of Patients with COPD American Thoracic Society/European Respiratory Society; 2004Available from: http://www.thoracic.org/clinical/copd-guidelines/resources/copddoc.pdfAccessed July 27, 2013 [Google Scholar]
- 24.Guyatt GH, Berman L, Townsend M, et al. A measurement of quality of life for clinical trials in chronic lung disease. Thorax. 1987;42:773–778. doi: 10.1136/thx.42.10.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh S, Morgan MD. Activity monitors can detect brisk walking in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil. 2001;21:143–148. doi: 10.1097/00008483-200105000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Steele BG, Belza B, Cain K, Warms C, Coppersmith J, Howard J. Bodies in motion: monitoring daily activity and exercise with motion sensors in people with chronic pulmonary disease. J Rehabil Res Dev. 2003;40:45–58. doi: 10.1682/jrrd.2003.10.0045. [DOI] [PubMed] [Google Scholar]
- 27.Watz H, Waschki B, Meyer T, Magnussen H. Physical activity in patients with COPD. Eur Respir J. 2009;33:262–272. doi: 10.1183/09031936.00024608. [DOI] [PubMed] [Google Scholar]
- 28.Brandes M, Rosenbaum D. Correlations between the step activity monitor and the DynaPort ADL-monitor. Clin Biomech. 2004;19:91–94. doi: 10.1016/j.clinbiomech.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Takanobu S. Metabolic equivalents (METS) In: Takahashi H, Tetsuo M, Takanobu S, editors. Pulmonary Rehabilitation Understanding by Animation. 2nd ed. Tokyo, Japan: Nakayama Shoten; 2008. [Google Scholar]
- 30.Sewell L, Singh SJ, Johanna EA, et al. Can individualized rehabilitation improve functional independence in elderly patients with COPD? Chest. 2005;128:1194–1200. doi: 10.1378/chest.128.3.1194. [DOI] [PubMed] [Google Scholar]
- 31.Pitta F, Troosters T, Probst VS, et al. Are patients with COPD more active after pulmonary rehabilitation? Chest. 2008;134:273–280. doi: 10.1378/chest.07-2655. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Single correlation coefficients between total time and maximum continuously active time measured by A-MES™ and video recording in ten healthy elderly subjects (Pearson’s product–moment correlation coefficient)
| Total time | Maximum continuously active time | |
|---|---|---|
| Walking | 0.986** | 0.995** |
| Standing | 0.940** | 0.949** |
| Sitting | 0.987** | 0.982** |
| Lying | 0.992** | 0.994** |
Notes:
P < 0.01. Pearson’s product-moment correlation coefficient = r.
Abbreviation: A-MES, Activity Monitoring and Evaluation System.
Intraclass reliability coefficients for days 2–7 of A-MES™ activity monitoring in a group of 10 COPD patients and 12 healthy elderly subjects
| Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | |
|---|---|---|---|---|---|---|
| Walking time | 0.943** | 0.961** | 0.974** | 0.981** | 0.982** | 0.985** |
| Standing time | 0.754** | 0.915** | 0.941** | 0.964** | 0.968** | 0.969** |
| Sitting time | 0.865** | 0.919** | 0.934** | 0.951** | 0.957** | 0.962** |
| Lying time | 0.778* | 0.824** | 0.862** | 0.897** | 0.913** | 0.918** |
Notes:
P < 0.05;
P < 0.01.
Abbreviations: A-MES, Activity Monitoring and Evaluation System; COPD, chronic obstructive pulmonary disease.