Abstract
During the past decade, the molecular mechanisms underlying the mammalian circadian clock have been defined. A core set of circadian clock genes common to most cells throughout the body code for proteins that feed back to regulate not only their own expression, but also that of clock output genes and pathways throughout the genome. The circadian system represents a complex multioscillatory temporal network in which an ensemble of coupled neurons comprising the principal circadian pacemaker in the suprachiasmatic nucleus of the hypothalamus is entrained to the daily light/dark cycle and subsequently transmits synchronizing signals to local circadian oscillators in peripheral tissues. Only recently has the importance of this system to the regulation of such fundamental biological processes as the cell cycle and metabolism become apparent. A convergence of data from microarray studies, quantitative trait locus analysis, and mutagenesis screens demonstrates the pervasiveness of circadian regulation in biological systems. The importance of maintaining the internal temporal homeostasis conferred by the circadian system is revealed by animal models in which mutations in genes coding for core components of the clock result in disease, including cancer and disturbances to the sleep/wake cycle.
Keywords: circadian clock genes, suprachiasmatic nucleus, complex traits, ENU mutagenesis, sleep-wake cycle
INTRODUCTION
For circadian biologists, the past decade has been an extremely exciting and fast-paced period. The basic molecular mechanisms of the biological clock have been defined not only in vertebrate species, but also in Drosophila, plants, fungi, and even cyanobacteria (75). Indeed, one of the major themes to emerge from the field is that circadian rhythms are ubiquitous: they are represented in two of the three domains of life, Eukarya and Bacteria, and there is circumstantial evidence to suggest that even among some members of the so-called “extremeophile” domain, the Archaea, circadian rhythms may be found. Furthermore, it can be argued that if, as the geological record suggests, non-heterocystous cyanobacteria represent one of the most ancient cellular life forms on Earth, first appearing perhaps more than 3.8 billion years ago, then the presence of circadian clocks in extant non-heterocystous species (47) is indicative of a truly ancient biological timing system. What were the selective pressures so pervasive since the origin of life on Earth to the present day that circadian clock systems could have arisen perhaps multiple times in different phylogenetic lineages via convergent evolution? For the cyanobacteria, the answer seems straightforward: the incompatible processes of oxygenic photosynthesis and nitrogen fixation must be separated either temporally as is the case in many non-heterocystous species, or spatially as occurs in the heterocystous species (22). For most organisms, the direct effects of ultraviolet radiation and photooxidative damage are prime suspects and have given rise to what has been called the “escape from UV” hypothesis where restriction of S phase (DNA synthesis) of the cell cycle to night would have strong selective value (148, 166). Stated in a more general way, regardless of the particular recurring environmental pressures, a timing system that can create an internal biological day synchronized to the external world enables an organism to predict and prepare for daily environmental fluctuations (148).
The investigation of the molecular genetic basis of circadian rhythms in higher eukaryotes began with the pioneering forward genetic screens of Ronald Konopka and Seymour Benzer which led to the discovery of the period (per) locus in Drosophila melanogaster (16, 108, 229). Through analysis of per circadian mutants, as well as mutations in additional Drosophila clock genes identified subsequently, the demonstration that the underlying generative molecular mechanism of the circadian clock consists of a set of core clock genes and their protein products which together participate in positive and negative autoregulatory feedback loops of transcription and translation, proved also to be generally true in other organisms (67, 115, 182). Indeed, in Drosophila and mice, organisms which diverged from a common ancestor some 600–700 million years ago, many of the genes comprising the circadian clock are orthologous (227).
In the following review, we present a general overview of the mammalian circadian system and highlight recent findings from several sources including microarray, mutagenesis, and quantitative trait locus studies, all of which emphasize a growing theme emerging from circadian research: the presence of complex, genome-wide levels of temporal organization. For more comprehensive treatments of the molecular mechanisms and physiological aspects of circadian rhythms in mammals, we refer the reader to several recent reviews (77, 162, 194).
OVERVIEW OF THE MAMMALIAN CIRCADIAN SYSTEM
In all circadian systems identified to date, regardless of phylogenetic origin, three major components are present: 1) a light input pathway to a self-sustained master circadian pacemaker, 2) the circadian pacemaker itself, and 3) and output pathways by which the circadian pacemaker regulates overt rhythms in biochemistry, physiology, and behavior throughout the organism (77, 194). In humans and other mammals, entrainment of the circadian system by light relies on retinal photoreceptors including not only the rods and cones, but also a recently-discovered distinct subset of intrinsically-photosensitive retinal ganglion cells containing the novel photopigment, melanopsin (23, 165). Together, the rod-cone system and melanopsin-containing ganglion cells account for all accessory visual functions, including those related to photic entrainment of the circadian system (78, 145). Photic information received by the retina is projected to the hypothalamus via the retinohypothalamic tract (RHT). The retina, together with the RHT, represent the sole light input pathway in mammals despite an erroneous report of extraretinal photoreception in humans (33) which has since been convincingly refuted (54, 80, 119, 121, 218). Neural projections from the RHT terminate in the bilaterally-paired suprachiasmatic nuclei of the anterior hypothalamus, the location of the master circadian pacemaker in mammals (131, 186).
Each suprachiasmatic nucleus (SCN) contains approximately 8–10,000 neurons (203), and each SCN neuron is capable of independently generating self-sustained circadian rhythms when dissociated from SCN tissue (81, 89, 120, 212) or when grown as immortalized cells (53). Neurons within the intact SCN, however, are coupled to form an ensemble expressing synchronized circadian rhythms of spontaneous electrical activity (155), calcium oscillations (43, 92, 146, 204), humoral output (8, 40, 98, 109), metabolic activity (178), and gene expression (161), with distinct spatial and temporal properties (44, 93, 123, 135, 173, 221, 225). Conclusive evidence that the SCN comprises the master circadian pacemaker came from lesioning studies and from experiments showing that transplantation of SCN tissue from donor animals harboring circadian clock gene mutations into SCN-lesioned wild-type hosts conferred upon the host the mutant circadian phenotype (157, 188).
Light information entering the SCN is transduced into neural and humoral output signals that influence various rhythms in the body including, for example, temperature and levels of activity and hormone secretion (30). Of particular relevance is the ability of the SCN to regulate the sleep-wake cycle via intrahypothalamic projections (3, 12). Signals from the SCN also impinge upon the autonomic nervous system (202). In rodents, daily bouts of locomotor activity are dependent on diffusible signals released by the SCN (184). One of these output signals may be TGFα which is synthesized rhythmically by the SCN and which acts to suppress locomotor activity (109). Another molecule implicated in SCN output and which is also synthesized rhythmically by the SCN is prokineticin 2 (40). When injected into the cerebral ventricles at night, prokineticin 2 inhibits locomotor activity.
Circadian Phenotypes: Wheel-running Locomotor Activity
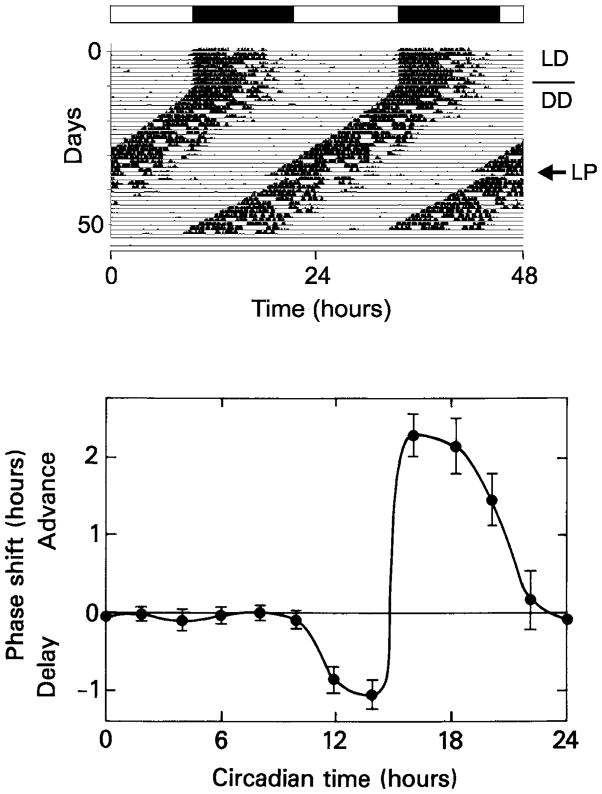
In circadian biology, the term Zeitgeber (literally, “time giver” in German) is used to describe any daily environmental cue to which the circadian system can synchronize or entrain. In general, light is the most important Zeitgeber owing to the daily rotation of the Earth, but other ~24-h recurring fluctuations also qualify as Zeitgebers including temperature (18) and social cues (11). Under laboratory conditions, experimental animals are housed such that it is possible to control artificially the daily period of light and dark. Thus, we define Zeitgeber time (ZT) relative to the experimental light/dark (LD) cycle. In an LD cycle of 12 hours light and 12 hours darkness (LD 12:12), by definition ZT0 = lights on, and ZT12 = lights off. When animals are experimentally isolated from all external time cues, as for example either in constant darkness (DD) or constant light (LL), it is possible to measure the endogenous or “free-running” period of the circadian clock. For rodents, this is most conveniently done by assaying the circadian period of the free-running locomotor activity rhythm using running wheels connected to computerized data acquisition systems (149, 192). Animals are first entrained to an LD cycle for a few weeks, after which they are exposed to constant conditions (e.g., DD) for several additional weeks during which the endogenous circadian period is measured by analyzing daily locomotor activity records (Figure 1A). Each day of the free-running circadian rhythm measured in constant conditions is divided into 24 hours of circadian time (CT). CT0 and CT12 are defined as the endogenous phases which coincide with dawn and dusk, respectively, in the entrained steady state established by the preceding LD 12:12 entrainment regimen. The first half of the cycle (CT0–CT12) is termed subjective day, while the second half of the cycle (CT12–CT24) is termed subjective night. In nocturnal rodents then, the period of locomotor activity in DD is mainly restricted to the animal’s subjective night.
Figure 1.
(A) A representative wheel-running locomotor activity record from a wild-type mouse. The record is double-plotted so that 48 h are shown for each horizontal trace. Dark regions represent locomotor activity. For the first seven days the animal was housed in an LD 12:12 cycle, denoted by the bar above the record. The animal was transferred to DD conditions on day 8, as indicated by the horizontal line to the right of the record. After approximately 3 weeks of DD exposure, a light pulse was administered (arrow). A phase shift (delay) in locomotor activity is observed following the light pulse.
(B) A phase response curve (PRC) to light. Rodents were first housed in DD conditions for several weeks. Light pulses were then administered to animals at different circadian times, every two hours, for one complete cycle. Phase advances and delays are plotted above and below the horizontal line, respectively. Light pulses cause phase delays during the early subjective night (CT 13–15) and phase advances during the late subjective night (CT 18–20).
In the natural environment, an organism’s circadian system synchronizes the sleep-wake cycle, behavior (e.g., periodic activity bouts), and internal biological processes to the prevailing light/dark cycle by phase advancing or phase delaying these daily rhythms. For example, nocturnal mammals that begin their daily activity bouts at the onset of darkness must advance or delay the phase of their activity depending on the season of the year, becoming active slightly earlier each day during the short days of winter, and active slightly later each day during the long days of summer. The resetting effects of light on the circadian system can be studied in the laboratory by administering brief light pulses at specific circadian times to animals maintained in DD conditions. Light pulses during the early subjective night (CT13–15) cause maximum phase delays, light pulses delivered during the late subjective night (CT18–20) produce maximum phase advances, and light pulses during the subjective day elicit either small phase shifts or none at all. The differential responsiveness to light stimuli, which is described as a phase response curve (Figure 1B), is functionally conserved in all circadian systems and is the basis for both period and phase control (entrainment) of circadian oscillators to rhythmic inputs (147).
THE MAMMALIAN CORE OSCILLATORY MECHANISM
Core circadian clock genes are defined as genes whose protein products are necessary components for the generation and regulation of circadian rhythms; that is, proteins which form the primary molecular circadian oscillatory mechanism within individual cells throughout the organism. As mentioned earlier, a common feature among the circadian systems of phylogenetically diverse organisms such as Neurospora, Drosophila, and mammals, is the participation of core clock genes in interconnected positive and negative autoregulatory feedback loops of transcription and translation (67, 115, 182). Indeed, in some instances, the genes involved are evolutionarily conserved, particularly among the metazoans (52). The daily rhythms generated by the circadian clock are thus genetically based and arise from complex cycles of transcription, translation, protein-protein interaction, phosphorylation, nuclear translocation, and protein degradation, all of which impose delays at various times each day to the create a coordinated molecular cycle that approximates the ~24-h environmental light/dark period. Several core clock genes in mammals have now been identified and characterized through studies of experimental animals harboring naturally-occurring, chemically-induced, and targeted (knockout) mutations, and through various comparative genomic approaches. The current list of mammalian core clock genes is shown in Table 1, along with the function of each in either the positive or negative loops of the molecular clock, and the approximate range of peak times of mRNA and protein expression for each in the SCN and in peripheral tissues. Figure 2 presents a diagram of the mammalian core circadian clock.
TABLE 1.
Mammalian circadian clock genes
| Genea | Chromosome (mouse/human) | Classification | Function | Mutation phenotype | Peak CT expression (RNA/protein)
|
|
|---|---|---|---|---|---|---|
| SCN | Periphery | |||||
| Clock | 5/4 | bHLH-PAS | Transcription factor | 4.0 h longer period; arrhythmicity in DD | Constitutive | 21–03/constitutive |
| Bmal1/Mop3 (Arntl) | 7/11 | bHLH-PAS | Transcription factor | Arrhythmicity in DD | 15–18/0–8 | 21–03/15–03 |
| Per1 | 11/17 | PAS domain | PER/CRY interaction; CLOCK:BMAL1 inhibitor | 0–1.1 h shorter period | 4–6/10–14 | 10/15–18 |
| Per2 | 1/2 | PAS domain | PER/CRY interaction; CLOCK:BMAL1 inhibitor | 1.5 h shorter period; arrhythmicity in DD | 6–12/10–14 | 15/18 |
| Per3 | 4/1 | PAS domain | PER/CRY interaction | 0–0.5 h shorter period | 4–9/10 | 12–15/NDb |
| Cry1 | 10/12 | Flavoprotein | Interaction with PERs; inhibition of CLOCK:BMAL1 | 1.0 h shorter period | 8–12/12–18 | 14–17/21–24 |
| Cry2 | 2/11 | Flavoprotein | Interaction with PERs; inhibition of CLOCK:BMAL1 | 1.0 h longer period | 8–16/12–16 | 8–10/15–21 |
| Rev-erbα (Nr1d1) | 11/17 | Orphan nuclear receptor | Inhibitor of Bmal1; links negative and positive feedback loops | 0.4 h shorter period | 2–6/ND | 2–6/6–8 |
| CKIε (Csnk1e) | 15/22 | Casein kinase | Phosphorylation of PERs, CRYs, and BMAL1 | 4.0 h shorter period (tau mutant hamster) | Constitutive | Constitutive |
| Timeless | 10/12 | PER-interacting | May dimerize with PER proteins | Embryonic lethal in homozygotes | Constitutive/12 | ND |
| Npas2/Mop4 | 1/2 | bHLH-PAS | Transcription factor; Clock paralog | 0.2 h shorter period | Not expressed in SCN | 0–5/ND |
| Bmal2/Mop9 (Arntl2) | 6/12 | bHLH-PAS | Transcription factor; Bmal1 paralog | ND | ND | ND |
Standard human gene symbols for each of the clock genes are as follows (in parentheses): Clock (CLOCK), Bmal1 (ARNTL), Per1 (PER1), Per2 (PER2), Per3 (PER3), Cry1 (CRY1), Cry2 (CRY2), Rev-erbα (NR1D1), CKIε (CSNK1E), Timeless (TIMELESS), Npas2 (NPAS2), Bmal2 (ARNTL2).
ND = not determined.
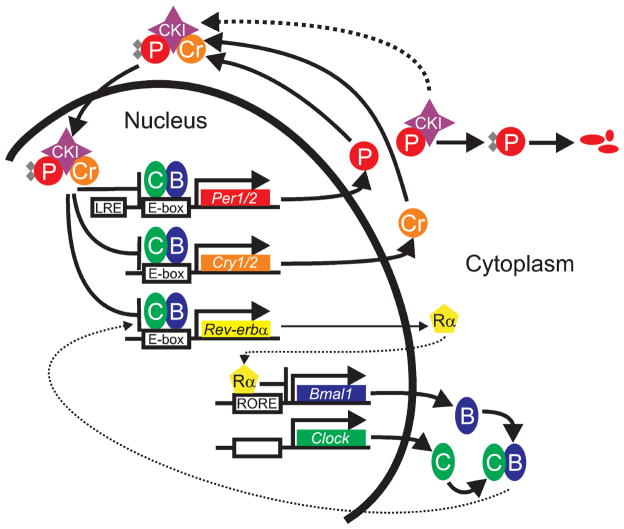
Figure 2.
A diagram of the mammalian core circadian clock as described in the text. Abbreviations: PER (P), CRY (Cr), BMAL1 (B), CLOCK (C), CKIε (CKI), REV-ERBα (Rα), light-responsive elements in the Per promoters (LRE). Phosphorylation is represented by gray diamonds.
In mammals, two core clock genes, Clock and Bmal1 (Mop3), encode proteins that are members of the basic helix-loop-helix (bHLH)-PAS (Period-Arnt-Single-minded) transcription factor family (31, 64, 86, 103). While Clock mRNA and protein are constitutively expressed in the SCN, Bmal1 transcript peaks in the middle of the circadian night (128). Through their PAS protein-protein interaction domains, CLOCK and BMAL1 heterodimerize in the cytoplasm to form a complex that, following translocation to the nucleus, activates transcription from target genes containing E-box cis-regulatory enhancer sequences (64, 86). The CLOCK:BMAL1 cis element known as “M34”, is an imperfect palindrome with a core E-box element and has the consensus sequence 5′-G/TGA/GACACGTGACCC-3′ (86). Particularly important targets subject to CLOCK:BMAL1-mediated activation are themselves bone fide core clock genes. These include the Period genes (Per1, Per2, and Per3), paralogous members of the PAS protein family, and two Cryptochrome genes (Cry1 and Cry2), members of the vitamin B2-based blue-light photoreceptor/photolyase family (64, 86, 94, 111, 114), transcript levels from all of which peak during the mid to late circadian day antiphase to the Bmal1 peak. After delays imposed by transcription, translation, and posttranslational modification, the PERs, CRYs, and other proteins form a heteromultimeric complex that translocates to the nucleus and directly abrogates the transcriptional activity of the CLOCK:BMAL1 complex, thereby lowering Per and Cry RNA levels (70, 94, 111, 114). This inhibition appears mainly to be effected by the CRY proteins, perhaps through repression of histone acetyl transferase (HAT) activity (59), while one or more of the PER proteins may function in mediating nuclear translocation of the complex (70, 111). Thus, it is the daily transcriptional activity of the CLOCK:BMAL1 heterodimer that forms the positive feedback loop of the mammalian molecular clock, and as such, it is both extremely critical in establishing the rhythm and it represents an important point for regulation by CRY-PER complexes. The cessation of CLOCK:BMAL1-mediated transcription by the CRYs and associated proteins to directly inhibit their own transcription establishes the negative feedback loop.
The positive and negative feedback loops are connected by the product of yet another core clock gene, Rev-erbα, which codes for an orphan nuclear receptor (65). Interestingly, Reverbα, like the Per and Cry genes, is activated by CLOCK:BMAL1 heterodimers acting through E-box enhancers in its promoter (154, 200). The REV-ERBα protein inhibits transcription from the Bmal1 gene, and perhaps also from the Clock and Cry1 genes, by binding retinoic acid-related orphan receptor response elements (ROREs) in their promoters (4, 59, 73, 142, 154, 200). Hence, by participating in the transcriptional activation of Rev-erbα, BMAL1 acts to attenuate its own transcription. Unlike CRY-PER complexes which repress their own expression by acting directly on CLOCK:BMAL1 heterodimers, REV-ERBα inhibits its own transcription in an indirect manner by repressing transcription of at least one of its activators, Bmal1. Furthermore, as a target of CLOCK:BMAL1-mediated activation, Rev-erbα transcription is also repressed by the inhibitory action of CRY-PER complexes on CLOCK:BMAL1 (154, 228). Together these processes have the effect of annulling REV-ERBα-mediated inhibition of Bmal1 expression such that BMAL1 accumulates at the proper time to heterodimerize with CLOCK, translocate to the nucleus, and initiate a new round of transcription as CRY-PER levels decline. Indeed, appropriately timed circadian nuclear accumulation of CLOCK:BMAL1 is mainly BMAL1-dependent (107), and activation of Bmal1 transcription appears to be mPER2-dependent, perhaps via coactivation or by nuclear shuttling of an activator (182). Taken together, these results help to reconcile previous findings that mPER2 enhances (182), and the CLOCK:BMAL1 complex inhibits (228), Bmal1 transcription.
Several significant posttranslational modifications play a role in generating the 24-h rhythms of RNA and protein levels just described. Phosphorylation of all of the core circadian proteins at various times seems to contribute ultimately to their degradation, most likely through ubiquitin-mediated proteasomal degradation pathways (5, 107, 114, 220). At least one member of the casein kinase I family, casein kinase I epsilon (CKIε), functions in this process. In vitro evidence suggests that casein kinase I delta (CKIε) may also participate in the clock mechanism (5, 114). A role for CKIε in PER protein phosphorylation, nuclear entry, and turnover has been clearly demonstrated (5, 32, 101, 114, 124, 207). In addition, BMAL1 and the CRYs are substrates for CKIε in vitro, but apparently only after they form a complex with CKIε and the PERs (58).
Mutations in Mammalian Core Clock Genes
Mutation of the mammalian core clock genes has been essential in elucidating the functional role of each in the molecular clock mechanism. Whether from naturally-occurring or chemically-induced lesions, or through gene targeting approaches, mutation of all core clock genes has been achieved (Table 1). Indeed, compound mutants have also been bred in which animals carry mutations in at least two of the core clock genes. In the early 1990s, our laboratory initiated a forward genetics approach in mice very similar to that used with success 20 years earlier in Drosophila by Seymour Benzer (108). Male mice exposed to the chemical mutagen, N-ethyl-N-nitrosourea (ENU), were bred and the resulting progeny screened for circadian mutations (208). We identified a single-gene, semidominant mutation, Clock, which lengthened circadian period both in heterozygous and homozygous mice, and in constant conditions, led to a loss of circadian rhythmicity in homozygous Clock mutants. The Clock gene was mapped to mouse chromosome 5 and was ultimately identified using a combination of positional cloning and transgenic rescue of the mutant phenotype with wild-type bacterial artificial chromosomes (BACs) (9, 103, 216). The Clock mutation is caused by an A→T transversion in a splice donor site which causes exon skipping and disruption of the transactivation domain of the resulting mutant bHLH-PAS CLOCK protein. Genetic analysis of the Clock mutant allele over a deletion showed that Clock is an antimorph, a type of dominant negative mutation (102). Several additional ENU circadian screens are currently underway and will be discussed later.
Gene-targeting of the partner of Clock, Bmal1/Mop3, causes an immediate and complete loss of circadian rhythmicity in constant darkness and is the only targeted allele to cause such a profound effect (31). The central role of BMAL1 in the mammalian circadian mechanism is underscored by the observed abolition of Per1 and Per2 rhythms in the SCN of Bmal1 null animals, and the alteration of behavioral entrainment.
Targeted mutations have also been generated in all three of the mouse Per paralogs. Only null mutants of the Per2 locus have substantial circadian phenotypes: the period length is shortened by about 1.5 h and the majority of mice become arrhythmic in DD (231). Three groups independently generated Per1 null mutations, and mutant progeny from these three lines exhibit subtle and slightly different circadian phenotypes. Weaver and colleagues generated Per1ldc mice which, in the homozygous condition, have slightly short free-running periods in constant conditions, but experience a loss of rhythmicity following at least two weeks in constant conditions (13). Lee and colleagues generated Per1Brdm1 mice which, as homozygotes, exhibit persistent free-running periods that are approximately 1 h shorter than wild-type animals, a result similar to that reported by Cermakian and coworkers (37). Differences in targeting approaches and genetic background may also contribute to the observed phenotypic disparities among the studies. Importantly, double mutants of Per1 and Per2 have very strong circadian phenotypes: locomotor activity rhythms are completely arrhythmic in DD (13, 230). Null mutations in the Per3 locus cause a slightly shortened circadian period (181). Because the absence of PER3 protein had no effect on circadian behavior either in Per1ldc/Per3 or Per2ldc/Per3 double mutant mice, Per3 is not an essential component of the circadian core oscillatory mechanism; however, it may play a role in clock output pathways (13, 181).
Targeted mutations in the two Cry genes result in opposite effects. Animals homozygous for a Cry1 null mutation show a free-running period approximately 1 h shorter than wild-type mice, while the Cry2 null mice express free-running periods that are about 1 h longer than wild-type animals (196, 205, 209). Cry double knockout animals experience complete behavioral arrythmicity upon transfer to constant conditions (196, 205, 209) and show constant, noncycling expression of Per1 and Per2 in the SCN and in peripheral tissues (141).
Knockout of the Rev-erbα gene results in slightly shorter free-running behavioral rhythms when mice are exposed to constant conditions (154). In addition, the distribution of period lengths is scattered in Rev-erbα homozygous null mutant animals. There is also a drastic reduction in the circadian rhythm of transcription of Clock and Bmal1 in Rev-erbα−/− animals, such that CLOCK and BMAL1 protein levels are elevated at all times of day in liver tissue of these animals. Although Cry1 does not cycle in mutant animals, CRY1 protein still oscillates in mutants.
The naturally-occurring Syrian hamster tau mutation, serendipitously discovered more than 15 years ago, represents the first mammalian single-gene circadian mutation identified (158). Animals carrying this semidominant mutation express altered endogenous circadian periods; ~22 h in heterozygotes and ~20 h in homozygotes. Unfortunately, it was not immediately possible to elucidate the gene involved as there are few genetic resources in hamster. The tau mutation, however, was particularly important in physiological experiments demonstrating that the SCN harbors the master circadian pacemaker (157), that a diffusible signal can drive circadian rhythms in the animal (184), and that SCN-independent circadian oscillators exist in the mammalian retina (199). Our laboratory cloned the mutation using a comparative genomics approach that we refer to as positional syntenic cloning, and showed that the tau mutation results from a C→T transition in the gene encoding hamster casein kinase I epsilon. The transition mutation causes an arginine to cysteine amino acid substitution at one of three conserved residues forming a phosphate binding site on the enzyme (124). As a result, the mutant CKIε enzyme exhibits decreased maximal velocity and is deficient in its ability to phosphorylate the PER proteins. Among the mammalian core clock genes, CKIε occupies the unique position as the only enzyme identified thus far. As such, it represents a potential target for pharmacological intervention in circadian rhythm disorders.
Time Now for Timeless?
Included in Table 1 is the gene Timeless, previously reported to be a mammalian ortholog of Drosophila tim (105, 170, 195, 197, 232). In flies, TIM and PER heterodimerize, translocate to the nucleus, and inhibit CLOCK:CYCLE-mediated transcription, much like the CRY-PER complexes just described in mammals (7, 215). Also, tim RNA and protein levels oscillate in DD in Drosophila, and light exposure degrades TIM. In the mammalian circadian system the role of Tim, if any, has remained ambiguous. Conflicting reports exist regarding cycling of Tim RNA in the SCN of mice, and studies reveal no oscillation or light-responsiveness of TIM protein in the SCN (61, 76). Although TIM has been reported to interact with PER1 in cell culture (195), it does not influence the cellular localization of the PER proteins in immunofluorescence studies (111, 207). It does, however, interact with the CRY proteins both in cell culture and in native SCN extracts (61, 111). Moreover, a Drosophila gene named timeout has been discovered which has greater sequence similarity to mammalian Tim, yet its function is not known (20, 69). These results, among others, have led some to discount the role of TIM in the mammalian core oscillator. Complicating analysis of mammalian Tim is the fact that targeted disruption of the gene results in early embryonic lethality in the homozygous state, while heterozygotes are indistinguishable from wild-type animals in circadian-relevant measures (69). This result, however, highlights the unequivocal role of mammalian TIM in development (116, 219). An intriguing result in C. elegans involving the timeout ortholog, tim-1, points to an important role for the TIM-1 protein in chromosome cohesion (38). Indeed, the authors suggest that Drosophila timeless represents a duplication and specialization of the ancient timeout gene, which in C. elegans and perhaps in other metazoans, retains its original function in sister chromosome cohesion.
In mammals, two transcripts exist for Tim; one encodes a large protein, while a shorter transcript codes for only the last 475 residues of the large isoform (116). Using rat brain slices Barnes and coworkers recently showed that full-length Tim antisense oligonucleotides abolished the circadian rhythm of spontaneous neuronal firing, compared to control oligonucleotides which had no effect (17). Antisera raised against the full-length and truncated TIM isoforms revealed a robust oscillation in the SCN for the full-length isoform only. The antisera also coimmunoprecipitated all three PER proteins from SCN cell line extracts, indicating that TIM can interact with the PERs similar to what is observed in flies. The authors suggest that the inability of others (61, 76) to discern TIM protein oscillations in the SCN is accounted for by the abundance of the short TIM isoform, and that the full-length mammalian TIM isoform is a functional homolog of Drosophila TIM and that it is part of the negative autoregulatory feedback loop where it may act in concert with PER2 (17). Indeed, earlier work in cell culture showed that TIM can inhibit CLOCK:BMAL1-mediated transcription (94, 170). Further studies should clarify the role of TIM in the mammalian clock system.
CIRCADIAN CLOCKS IN PERIPHERAL TISSUES
As each of the genes comprising the core circadian clock was identified, it became clear that their expression was not limited to the SCN. In neural tissues outside of the SCN, as well as in tissues throughout the body, what circadian biologists refer to as “peripheral tissues”, core circadian clock genes are expressed and most exhibit a circadian oscillation of expression. Indeed, perhaps most striking was the demonstration that even in cultures of Rat-1 fibroblasts, a serum shock could elicit circadian gene expression lasting for at least three cycles (15). The peripheral oscillators, however, differ from the SCN pacemaker in several ways. First, the phase of the peripheral clock oscillation is delayed by 3–9 h relative to the SCN (233). This suggests that the peripheral clocks receive synchronizing signals from the pacemaker in the SCN, not an unreasonable inference given that mammalian peripheral tissues, unlike those in Drosophila (150) and zebrafish (213), are not directly light responsive. Second, ex vivo cultures of peripheral tissues from transgenic rats in which the mouse Per1 promoter drives a luciferase reporter gene reveal circadian rhythms in luminescence that damp out after 2–7 days (2, 224). Damping in peripheral oscillations has also been reported after lesioning the SCN (6, 169). Recent work, however, using the Per2 locus shows that peripheral oscillators are not damped and can sustain circadian rhythms in isolation for more than 20 cycles (226). By knocking luciferase into the C-terminal exon of Per2, a PER2::LUCIFERASE (PER2::LUC) fusion protein was created to replace the endogenous gene. Interestingly, the PER2::LUC fusion protein is completely functional and rescues circadian rhythms in vivo. Because both transcriptional and post-transcriptional regulation of Per2 are likely to be critical for normal circadian rhythms and because the PER2::LUC reporter can faithfully reflect these levels of regulation, this circadian reporter system appears to be superior to previous conventional Per1::luciferase transgenic constructs (110, 217, 222, 224). In addition, to the persistence of circadian rhythms in peripheral tissues, the PER2::LUC mice also reveal that the SCN are not essential for driving peripheral oscillations, but rather appear to act as synchronizers of peripheral oscillators (226).
While the core oscillator genes are expressed in most peripheral tissues, it is clear that tissue-specific oscillator genes also play an important role in mediating peripheral circadian rhythms. For example, in Clock homozygous mutants, Bmal1 RNA rhythms are absent in the SCN, but persist at a low level in the periphery (139). The SCN versus peripheral differences in clock function may in part be mediated by members of the bHLH-PAS family other than CLOCK and BMAL1 that can dimerize to form active transcriptional complexes. For example, in the forebrain the product of the Clock gene paralog, Npas2 (Mop4) (see Table 1), heterodimerizes with BMAL1 to drive circadian expression of Per2 through E-boxes in this gene’s promoter (159). A similar mechanism occurs in the vasculature where BMAL1 associates with CLOCK and/or NPAS2 to form transcriptionally active complexes (130). Bmal2 (Mop9) (Table 1), a paralog of Bmal1, may also associate with CLOCK and/or other bHLH-PAS proteins to form circadian-relevant, tissue-specific transcriptional activators in the periphery (87, 177). Thus, various members of the bHLH-PAS family may be differentially expressed from one peripheral tissue to another and act through E-box enhancers to allow local, tissue-specific circadian alterations in gene expression in response to daily environmental fluctuations.
MAMMALIAN CLOCK-CONTROLLED GENES
The above model of the molecular components of the mammalian circadian system also provides an explanation for observations that RNA and protein of genes which are not part of the core oscillatory mechanism, cycle with a period close to 24 h. Many of these genes, which are often tissue-specific, harbor E-boxes, and are thus dependent on activation by CLOCK:BMAL1 complexes. This highlights the direct role of core clock components in controlling the expression of downstream genes. The expression of other genes may also be controlled by the clock in a more indirect manner. For example, by directly regulating the expression of a specific transcription factor, the clock indirectly regulates the expression of that transcription factor’s target genes. Whether the control is direct or indirect, these downstream genes are referred to as clock-controlled genes (CCGs), and they are often important in regulating local processes within a specific tissue.
An example of a gene shown to be under direct transcriptional control by the CLOCK:BMAL1 heterodimer is vasopressin (AVP). The CLOCK:BMAL1 heterodimer activates rhythmic AVP expression in the SCN via an E-box in the gene’s promoter (94). In Clock mutant mice, discussed later, the amplitude of AVP transcript is severely reduced. Another CCG directly controlled by CLOCK:BMAL1 is the PAR-leucine zipper transcription factor, albumin D-element binding protein (DBP). DBP is also rhythmic in the SCN, yet unlike AVP, its expression is controlled through E-boxes located in intronic sequences (163, 223). Interestingly, DBP appears to influence the clock system, perhaps indirectly through its role as a transcription factor, as DBP-deficient mice express shortened free-running periods of locomotor activity (122).
Upon discovery of the role of REV-ERBα in the core clock mechanism, it became clear that this protein too, may act to mediate expression of downstream genes via its ability to bind to and repress transcription from genes harboring RORE sequences. Indeed, Ueda and colleagues used microarrays to identify ten transcripts cycling in the SCN and which harbor RORE response elements (200). Among these cycling transcripts are Bmal1, and that for E4bp4 which codes for a basic leucine zipper transcription factor closely related to DBP. As discussed in the next section, the global analysis of gene expression offers a means by which to identify additional CCGs and the biochemical pathways they influence.
GENOME-WIDE APPROACHES REVEAL CIRCADIAN ORGANIZATION
And “Array” We Go…
As just described, much is known regarding how the mammalian core clock genes and their protein products interact to form the autoregulatory positive and negative feedback loops that comprise the circadian oscillatory mechanism at the cellular level. In addition, the list of clock-controlled genes continues to grow, owing in large part to studies utilizing high-density oligonucleotide arrays (HDAs), commonly referred to as microarrays, to assay for circadian expression profiles among thousands of genes simultaneously at different time points (45, 50, 171). Furthermore, via microarray studies, circadian patterns of gene expression can be compared among different tissues in the same animal to elucidate clock control of tissue-specific processes. This global approach to circadian expression analysis has been used with success not only in mammals (6, 51, 71, 82, 91, 104, 140, 144, 187, 200), but also in other circadian experimental models including Drosophila (36, 42, 117, 129, 201), Neurospora (138), and Arabidopsis (74, 174). As a result, microarray analyses offer the potential to make cross-species comparisons to identify among organisms, genes and biological pathways in common upon which the circadian system exerts regulation.
Using Affymetrix GeneChip HDA arrays which assay approximately one third of the mouse genome (6000 genes and an additional 6000 expressed sequence tags), two studies recently compared the circadian expression of genes in the SCN and liver of mice (144, 200). Panda and coworkers identified 337 genes showing circadian expression in the SCN compared to 101 cycling genes found by Ueda and coworkers. These cycling transcripts represented about 8–10% of the transcriptome detectable in the SCN. A similar proportion of cycling transcripts were found in the liver by at least four different groups (6, 144, 187, 200). When comparing the sets of cycling transcripts between the SCN and the liver, only about 10% are in common. This was also seen in other pairwise comparisons of different tissues (187). Interestingly, more than half of the remaining 90% of the tissue-specific cycling transcripts were expressed in both tissues; however, cycling was seen in only one of the tissues. Thus, circadian control of transcription appears to be tissue-specific rather than locus-specific. In the SCN, Panda and colleagues found that the largest categories of cycling transcripts were involved in protein synthesis (ribosomal proteins, translational components), protein folding, proteosome-mediated degradation, and several components of vesicle trafficking. In addition, there was a striking circadian regulation of mitochondrial transcripts involved in Complexes I, II, III, and IV (oxidative phosphorylation). In the liver, a large number of fundamental metabolic pathways were under circadian control including glycolysis, gluconeogenesis, fatty acid metabolism, and cholesterol metabolism. Importantly, transcripts encoding rate-limiting enzymes in most of these pathways were under circadian regulation suggesting that the circadian clock can exert its influence directly on metabolism (168).
Thus, several important themes emerge regarding circadian control of gene expression. First, these results add to a growing list of genes under circadian control in various mammalian tissues. The circadian expression of some of these genes is mediated directly by CLOCK:BMAL1, while others are regulated indirectly by the clock via the circadian expression of relevant transcription factors. Second, tissue-specific circadian control of gene expression is pervasive and reflects tissue- rather than locus-specific circadian regulation. Third, within each tissue, clock-controlled genes associated with particular pathways (e.g., glucose metabolism in the liver) often exhibit phase coordination, presumably such that genes coding for proteins regulating that pathway are coexpressed. Finally, approximately 8–10% of all transcripts expressed in any tissue are regulated by the circadian clock, including key rate-limiting enzymes, such that throughout the organism the clock impinges upon perhaps every major pathway.
Complex Trait Analysis of Circadian Rhythms
Although single-gene mutations can profoundly affect the mammalian circadian system, it is a complex system that is polygenic. As such, genes other than those identified as core clock genes influence circadian behavior in mammals, although the individual effect of such loci may be small, contributing perhaps 10% or less to differences in circadian behavior from animal to animal. In addition, alleles at two or more loci may interact to modify or alter circadian behavior sometimes in an epistastic manner. Such genes define quantitative trait loci (QTL); that is, loci segregating alleles that detectably, yet minimally affect the overall phenotype of a trait that is under complex genetic control (48). In contrast to phenotypes controlled by single genes, so-called Mendelian traits, identifying genes underlying QTLs is fraught with difficultly. Despite the many complex traits analyzed in mice via genome-wide linkage studies, only about eight genes have been identified at the molecular level following intense analysis of regions containing QTLs (66). The prospects for greater success in the near future, however, are heightened with the completion of the human and mouse genome sequencing projects (113, 206, 211), the development of dense genetic maps in mouse based on rapidly-typed single-nucleotide polymorphisms (SNPs) (118), and the availability of powerful analytical algorithms for complex trait analysis (34, 41, 49, 134, 180). In addition, a collection of well-established mouse inbred strains provides a starting point for QTL studies, as any two animals from a single inbred strain are genetically identical. Hence, two inbred strains divergent for a particular trait of interest can be used to localize QTLs associated with that trait to particular chromosome regions.
Fortunately for the circadian researcher, inbred strains of mice exhibit well-defined strain-specific differences in circadian behavior (55, 151, 179). For example, the free-running period of locomotor activity in the BALB/c strain is short (< 23 h), in the C57BL/6 strain it is about 24 h, and in the CS strain, it exceeds 24 h (1, 179). Given these differences, several groups have used inbred strains to identify potential QTLs underlying circadian behavior in mice. Typically, these studies begin by producing an F2 intercross using two parental inbred strains disparate for a particular circadian trait (e.g., free-running period of locomotor activity); alternatively, recombinant inbred strains can be used (164). While F1 animals tend to be heterozygous for all markers and QTL, each animal from the F2 generation is genetically unique and, when sufficient numbers of F2 animals are phenotyped for a trait, results reveal continuous variation for that trait. Chromosomal regions are defined for QTLs influencing the trait by testing for association of the trait and markers spaced at specific intervals throughout the genome. In most circumstances, the resulting genome-wide scan identifies chromosomal intervals that are between 10–30 centimorgans (cM) in length for each QTL, which corresponds to 10–30 megabases of DNA or about 100–300 genes per interval (66). Indeed, although the number of genes mapping to a QTL interval is daunting, genome-wide significance thresholds have been established to evaluate QTL results such that false positive and false negative results are minimized (66, 112).
The first attempts to identify circadian rhythm QTLs in mice relied on recombinant inbred (RI) strains phenotyped for the free-running period of locomotor activity (83–85, 127). Unfortunately, the results of these studies did not achieve the currently accepted significance thresholds (112). Recent work by Ebihara and coworkers led to the identification of several circadian QTLs influencing the free-running period of locomotor activity, phase angle of entrainment, and circadian activity level (189, 190). Using recombinant inbred strains derived from SM/J and A/J inbred strains, the genome-wide suggestive level was surpassed for two QTL associated with free-running period (chromosomes 7 and 18), one for phase angle of entrainment (chromosome 7), and two for circadian activity (chromosomes 1 and 17) (189). When the free-running period of locomotor activity was tested in two F2 intercrosses (C57BL/6J x CS and CS x MSM), the genome-wide significance level was surpassed for a QTL on the distal region of chromosome 19, and three suggestive QTLs were mapped to chromosomes 12 and 19 (190). None of the QTLs identified in these studies map to regions containing known core clock genes.
Our laboratory recently completed a comprehensive screen for circadian-related QTLs. We evaluated 196 (C57BL/6J X BALB/cJ)F2 hybrid mice for five circadian traits: free-running circadian period, phase angle of entrainment, amplitude of circadian rhythm, circadian activity level, and dissociation of rhythmicity (183). We chose the C57BL/6J and BALB/cJ strains owing to the previously reported differences in their circadian periods and stability of free-running activity rhythms (152). We performed a genome-wide scan of the F2 population using markers spaced at intervals of approximately 20 cM throughout the genome. After calculating the probability of linkage of each marker to differences in the five circadian traits studied, those loci attaining suggestive levels of significance (LOD ≥ 2.8) were evaluated further with multiple flanking markers within a 30 cM interval to test for stronger linkage. With the combined data we performed genome-wide single marker and pairwise scans and applied a nonparametric resampling method based on permutation testing (41).
We identified a total of 14 loci that affected the five circadian traits studied; three for free-running period, five for phase angle of entrainment, two for amplitude, two for activity level, and two for dissociation. Five of the loci exhibited significant main effects on three traits: phase, period, and amplitude. More than half of the 14 QTLs (9 total), however, were identified primarily or solely from strong interactions between specific allelic combinations at different loci. We compared the map positions of each of the 14 QTLs to chromosomal locations of the nine core clock genes. Only one core clock gene, CKIε, maps to within 10 cM of one of the QTLs on chromosome 15. Thus, the majority of allelic variants contributing to interstrain differences in circadian phenotypes appear to arise from loci other than those involved in the core circadian mechanism. Perhaps subtle variations that shape quantitative features of circadian phenotypes operate predominantly in secondary interacting genetic pathways of the system.
Forward Genetic Screens
In contrast to reverse genetics (gene to phenotype) in which the effects of deliberate mutations in cloned genes are analyzed, forward genetic (phenotype to gene) methods begin not with a specific gene, but rather with a phenotype (193). Pioneering work by early geneticists used the forward genetic approach with great success. With respect to behavior, it was Seymour Benzer who first championed the use of random mutagenesis to screen for novel behavior phenotypes (21). Saturating the genome with mutations through this method offers an attractive means to identify novel genes involved in various biological pathways, as long as appropriate phenotypic screens can be devised to detect them. The search for chemicals that mutagenize mice with high efficiency led ultimately to the discovery of N-ethyl-N-nitrosourea (ENU) as the most potent mutagen in mice (167). ENU is particularly attractive as a mutagen for several reasons. First, as just stated, it is extremely efficient: under appropriate conditions, it can induce 1 mutation per gene per every 700 gametes in male mice. If the mouse genome contains 25–40,000 genes, then with a specific locus mutation frequency of approximately 1 in 1000, a single mouse treated with ENU should have between 25 and 40 different mutagenized genes, each potentially producing a recessive phenotype (14). Second, ENU is an alkylating agent that primarily induces point mutations as opposed to large chromosomal rearrangements, deletions, or inversions typically produced by other mutagens such as X rays or chlorambucil. Empirical results show that approximately 64% of ENU-induced mutations are missense, 10% are nonsense, and 26% are splicing error mutations (97). Finally, because the highest mutation rates with ENU are achieved in pre-meiotic spermatogonial stem cells of male mice, a single mutagenized male mouse can be bred to multiple female animals such that subsequent generations can be produced rapidly (14). For these reasons, ENU has become the mutagen of choice for large-scale phenotype-driven screens.
ENU projects have employed one of two approaches to identify mutants: dominant screens or recessive screens. Of the two, dominant screens are relatively straightforward and have been used with success by several groups. With this method male mice are first mutagenized with ENU and then bred to several females to produce an F1 (technically G1) generation. The G1 offspring are then screened for phenotypes of interest. Those mice exhibiting aberrant phenotypes are then test bred to confirm transmission of the mutation. Subsequently, heterozygous mutants can be backcrossed to the mutagenized father or intercrossed with each other to produce homozygous mutants. In two published large-scale dominant screens, approximately 2% of all F1 mice exhibited a heritable phenotypic trait (90, 137). Recessive screens, unfortunately, are more time consuming and expensive compared to dominant screens. This approach is necessary, however, because dominant mutations may not be recoverable for all genes. Similar to the dominant screen, the recessive screen begins by mating mutagenized male mice to several females to produce a G1 generation. The cross is then expanded by mating G1 mice to wild-type animals to produce a G2 generation. Female G2 animals are then backcrossed to G1 males. If the G2 animal carries a mutation, then approximately one quarter of the backcross progeny will be homozygous for that mutation (14). Currently, several large-scale recessive screens are underway (100).
With respect to circadian biology, ENU mutagenesis screens provide another means by which to isolate genes involved in the biological timing system. Our discovery of the Clock mutation through a dominant ENU screen for mice exhibiting abnormal circadian locomotor activity rhythms, described earlier, provided proof-of-principle for this approach (208). A second clock-relevant gene has also been identified through a dominant ENU screen (99, 136). Kapfhamer and colleagues reported the discovery of a semidominant mutation called earlybird (Ebd) that shortens the circadian period of locomotor activity in mice and maps to chromosome 8 (99). Of five candidate genes analyzed for ENU-induced mutations, only Rab3a, a gene encoding a GTP-binding protein involved in synaptic transmission, harbored a point mutation in the coding region resulting in the substitution of a glycine residue for aspartic acid at a conserved motif common to all GTP-binding proteins. The mutation causes reduced levels of Rab3a protein and also alters sleep homeostasis in affected animals. The authors propose that Rab3a-mediated synaptic transmission is involved in these behaviors.
It is expected that the centers currently initiating recessive screens will have even more success in isolating circadian-relevant genes. Indeed, at least three groups, including our own at the Center for Functional Genomics at Northwestern University (http://genome.northwestern.edu), are screening for circadian phenotypes. Another group at the Medical Research Council in the United Kingdom (http://www.mgu.har.mrc.ac.uk) reports the identification of 68 abnormal circadian phenotypes, of which about 14 show robust inheritance and are being analyzed further (100). The Genomics Institute of the Novartis Research Foundation (http://www.gnf.org) is also undertaking a recessive screen for circadian phenotypes. Given the existence of circadian regulation at essentially every level of biological organization, large-scale mutagenesis screens should yield a wide array interesting new mutations.
EMERGING RELATIONSHIPS BETWEEN THE CIRCADIAN SYSTEM AND CELL CYCLE CONTROL
Within most cells, regardless of phylogenetic origin, there exist two endogenous cyclic systems. One, the circadian clock and the major subject of this review, acts to provide the cell with information that it can use to anticipate daily environmental changes. The second devoted to the control of cell division, mediates the entry into and exit from, the cell cycle. Among many unicellular organisms such as cyanobacteria (106), Chlamydomonas (68), Euglena (35), and Gonyaulax (88), evidence exists that the circadian clock controls cell division (132). In higher eukaryotes, however, a similar link between the two cellular clocks has not been widely reported, although there are some examples in the literature. Diurnal cycles of cell division have been observed in mammalian tongue and oral epithelia (24, 63, 153), gastrointestinal cells (24, 29, 125), epidermis (28), liver (79), bone marrow (185), and cornea (176). The molecular mechanisms by which the circadian clock may exert control over division in these various cell types have not been elucidated. It is clear, however, that in mammals the circadian clock is not dependent upon the cell cycle clock, for although adult neurons in the SCN do not divide, they nevertheless exhibit robust circadian rhythms of gene expression. Moreover, when cell division in cultured rat fibroblasts is blocked, rhythmic gene expression continues (15).
Recent work by Matsuo and colleagues provides evidence for a molecular link through which the circadian clock can control cell division in mouse hepatocytes (126). Under normal circumstances most liver cells in mammals do not actively divide, yet following partial removal of the liver (hepatectomy), this organ exhibits a remarkable capacity to regenerate (60). In addition, during regeneration hepatocytes enter the mitotic cycle synchronously. Following partial hepatectomy in mice, Matsuo and colleagues studied the hepatocyte cell division cycle and observed that cells entered the G2 phase around the same time each day regardless of the time of hepatectomy (ZT0 or ZT8). Such strictly regulated control over the G2/M transition suggests that the circadian clock gates entry into this phase of the cell cycle. After examining several cell cycle genes for circadian expression profiles, a strong candidate was identified that contributes specifically to control of the G2/M transition: wee1. The transcript for this gene exhibits a robust circadian oscillation in the liver and codes for WEE1 kinase which inactivates the CDC2-cyclin B1 complex through phosphorylation of CDC2 on a conserved tyrosine residue. It is the CDC2-cyclin B1 complex that directly regulates the G2/M transition, thus cells can proceed to this phase only when WEE1 kinase levels are low, during which time phosphatase CDC25 removes the inhibitory phosphate group, thereby activating the complex. Interestingly, the promoter of the wee1 gene contains three E-boxes and in transfection experiments, wee1 is activated by CLOCK:BMAL1 and repressed by CRY proteins. These results have been confirmed in Clock mutant mice in which wee1 levels are constitutively low. Conversely, in Cry1/Cry2 double mutant mice (205), WEE1 kinase levels are constitutively elevated resulting in sustained inactivation of the CDC2-cyclin B1 complex and hindered cell division cycles in regenerating liver (126). The eventual completion of liver regeneration in Cry1/Cry2-deficient mice and the lack of reported cell division defects in mice harboring mutations in other core clock genes suggest that circadian control of cell division is modulatory rather than absolute.
Another recent report linking the circadian system to cell proliferation reveals an important role for Per2 in tumor suppression and DNA damage responses. Fu and colleagues show that compared to wild-type controls, homozygous Per2 mutant mice (231) are more prone to salivary gland hyperplasia, and spontaneous lymphomas (62). The Per2 mutant animals also develop tumors more rapidly than wild-type mice in response to γ radiation, at least partially owing to decreased apoptosis in damaged cells. In normal mice, γ radiation elicits upregulation of several of the core clock genes including Clock, Bmal1, Per1, Per2, and Cry1. In homozygous Per2 mutants, however, expression of these genes was not significantly altered following radiation exposure. Particularly interesting is the finding that expression of the protooncogene c-myc, which codes for a bHLH-containing transcription factor that binds to E-box enhancers in target genes (25), is rhythmic in wild-type liver, but in Per2 mutants c-myc expression is shifted and its transcript levels are elevated. Because c-myc contains E-box sequences in its promoter (19), Fu and colleagues reasoned that it might be a direct target for control by the circadian clock. Indeed, in cell culture experiments NPAS2:BMAL1 heterodimers suppress c-myc expression, presumably by binding to E-boxes. This repression is relieved by CRY1. Furthermore, Cyclin D1 and Gadd45α, both of which participate in cell proliferation and are themselves targets of c-myc activation, oscillate in wild-type mice, but exhibit altered rhythms in Per2 mutants. Taken together, these results suggest that PER2 normally acts to suppress c-myc, albeit indirectly, via its stimulatory effect on Bmal1 transcription described as earlier. In Per2 mutants, the decreased levels of BMAL1 prevent formation of NPAS2:BMAL1 or CLOCK:BMAL1 heterodimers which, in turn, leads to derepression of c-myc. Overexpression of c-myc subsequently causes DNA damage and tumor formation owing to loss of cell cycle control (62).
The mechanisms by which the circadian system can control cell division in mammals are slowly being resolved. It should be mentioned here that circumstantial evidence for a circadian role in human cancer has been reported. Women who work night shifts for several years exhibit an increased risk of breast cancer (72, 175). The emerging field of chronotherapy (133), in which treatments for cancer and other diseases are administered at times of the day most likely to yield the greatest efficacy, will rely on further understanding the links between the circadian clock and the cell cycle clock.
HUMAN CIRCADIAN RHYTHMS AND DISORDERS
In many ways, sleep and the need for it remains an enigma, yet even subtle alterations in the sleep-wake cycle can affect human health (143, 156, 191). It is known that the timing of sleep and wakefulness is mediated by two interconnected processes: a homeostatic regulatory process (also called process S) that increases during waking and decreases during sleep, and a circadian clock-dependent mechanism (26, 27, 46, 57). Four intrinsic sleep disorders are associated with circadian system function: advanced sleep phase syndrome, delayed sleep phase syndrome, non-24-hour sleep-wake syndrome, and irregular sleep-wake pattern (210). Given the recent discovery of the underlying molecular clock mechanism, the search is on for mutations in mammalian core clock genes that may give rise to circadian sleep disorders (214). To date, the greatest progress has been made in understanding how mutations or polymorphisms in the Per genes may influence human sleep.
Advanced Sleep Phase Syndrome
Advanced sleep phase syndrome (ASPS) is a rare disorder in which affected individuals sleep (~19:00 h) and wake (~04:00 h) earlier than desired (210). Recently, a familial form of ASPS has been identified in which patients from three families have a very short circadian period with a 4-h advance of the daily sleep-wake cycle (96). Transmission of the trait is consistent with an autosomal dominant mode of inheritance. This familial form of ASPS was recently associated with a missense mutation that replaces a serine for a glycine in the human Per2 gene at a site normally phosphorylated by CKIε (198). Toh and coworkers confirmed that, as expected, the mutant form of PER2 is hypophosphorylated. As mentioned earlier, mutations in mouse Per2 produce a short circadian phenotype reminiscent of ASPS (231). In addition, the hamster tau mutation, a missense mutation in CKIε, also shortens the endogenous circadian period and results in behavior that resembles ASPS (124). Thus, mutations that alter the phosphorylation state of PER2 can disrupt sleep-wake patterns by altering the circadian timing of sleep. Another study also identified a family in which ASPS segregates with an autosomal dominant mode of inheritance (160), yet affected family members possess no mutations in any of the three Per genes (39). Similarly, results from two Japanese familial ASPS pedigrees found no linkage between affected individuals and Per2 (172). These latter two studies support the idea of genetic heterogeneity of familial ASPS in humans.
Delayed Sleep Phase Syndrome
Delayed sleep phase syndrome (DSPS) results in a phenotype opposite that of ASPS: individuals sleep (03:00 – 06:00 h) and wake (10:00 – 14:00 h) later than desired (210). DSPS represents the most common circadian sleep disorder and is more frequently reported among young adults. One human study of 48 DSPS patients reported an amino acid polymorphism in PER3 that occurred with a higher frequency in the DSPS patients compared to control individuals (56). The polymorphism occurs in a region near a putative CKIε phosphorylation site. Interestingly, another study reports the association of the same Per3 polymorphism with self-reported diurnal preference in a different group of patients (95). Finally, a recent study identified a length polymorphism in Per3 that is associated both with extreme diurnal preference and DSPS (10). The length polymorphism (4 or 5 repeating units) occurs in a region of human PER3 containing several putative CKIε phosphorylation sites. The longer allele was associated with morningness while the shorter allele was associated with eveningness. Moreover, the shorter allele was strongly associated with DSPS subjects, 75% of whom were homozygous for the shorter allele.
CONCLUSION
As the most important environmental time cue, the daily cycle of light and dark has been such a dominant selective force that organisms from all major phyla have evolved circadian timing mechanisms with which to perceive light and synchronize their biochemistry, physiology and behavior to fluctuations in this cycle. In mammals, the circadian system exists as a complex multioscillatory network composed of a central pacemaker located in a specialized retinorecipient region of the hypothalamus termed the suprachiasmatic nucleus (SCN). Tightly-coupled cells of the SCN expressing self-sustained circadian oscillations entrain to the light/dark cycle and subsequently transduce photic information into neural and humoral signals that are then imparted to circadian oscillators in tissues throughout the body. Local oscillators in peripheral tissues share with cells of the SCN a common set of core circadian clock genes. The products of these clock genes feed back to repress their own expression and act to influence transcription of downstream tissue-specific output genes and pathways. In this way, each tissue responds appropriately, based on local conditions, to photic information relayed by the SCN. Recent results reveal the complex and polygenic properties of the mammalian circadian system. Surprisingly, the circadian clock impinges upon biological pathways critical to cell survival, metabolism, and growth.
Acknowledgments
We thank Jason L. Chong for assistance with the information in Table 1. Joseph S. Takahashi is an Investigator at the Howard Hughes Medical Institute.
Contributor Information
Phillip L. Lowrey, Email: p-lowrey@northwestern.edu.
Joseph S. Takahashi, Email: j-takahashi@northwestern.edu.
LITERATURE CITED
- 1.Abe H, Honma S, Honma K, Suzuki T, Ebihara S. Functional diversities of two activity components of circadian rhythm in genetical splitting mice (CS strain) J Comp Physiol [A] 1999;184:243–51. doi: 10.1007/s003590050322. [DOI] [PubMed] [Google Scholar]
- 2.Abe M, Herzog ED, Yamazaki S, Straume M, Tei H, et al. Circadian rhythms in isolated brain regions. J Neurosci. 2002;22:350–6. doi: 10.1523/JNEUROSCI.22-01-00350.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abrahamson EE, Moore RY. Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Res. 2001;916:172–91. doi: 10.1016/s0006-8993(01)02890-6. [DOI] [PubMed] [Google Scholar]
- 4.Adelmant G, Begue A, Stehelin D, Laudet V. A functional Rev-erbα responsive element located in the human Rev-erbα promoter mediates a repressing activity. Proc Natl Acad Sci USA. 1996;93:3553–8. doi: 10.1073/pnas.93.8.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akashi M, Tsuchiya Y, Yoshino T, Nishida E. Control of intracellular dynamics of mammalian period proteins by casein kinase I ε (CKIε) and CKIε in cultured cells. Mol Cell Biol. 2002;22:1693–703. doi: 10.1128/MCB.22.6.1693-1703.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akhtar RA, Reddy AB, Maywood ES, Clayton JD, King VM, et al. Circadian cycling of the mouse liver transcriptome, as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus. Curr Biol. 2002;12:540–50. doi: 10.1016/s0960-9822(02)00759-5. [DOI] [PubMed] [Google Scholar]
- 7.Allada R, Emery P, Takahashi JS, Rosbash M. Stopping time: the genetics of fly and mouse circadian clocks. Annu Rev Neurosci. 2001;24:1091–119. doi: 10.1146/annurev.neuro.24.1.1091. [DOI] [PubMed] [Google Scholar]
- 8.Allen G, Rappe J, Earnest DJ, Cassone VM. Oscillating on borrowed time: diffusible signals from immortalized suprachiasmatic nucleus cells regulate circadian rhythmicity in cultured fibroblasts. J Neurosci. 2001;21:7937–43. doi: 10.1523/JNEUROSCI.21-20-07937.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antoch MP, Song EJ, Chang AM, Vitaterna MH, Zhao Y, et al. Functional identification of the mouse circadian Clock gene by transgenic BAC rescue. Cell. 1997;89:655–67. doi: 10.1016/s0092-8674(00)80246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Archer SN, Robilliard DL, Skene DJ, Smits M, Williams A, et al. A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep. 2003;26:413–5. doi: 10.1093/sleep/26.4.413. [DOI] [PubMed] [Google Scholar]
- 11.Aschoff J, Fatranska M, Giedke H, Doerr P, Stamm D, Wisser H. Human circadian rhythms in continuous darkness: entrainment by social cues. Science. 1971;171:213–5. doi: 10.1126/science.171.3967.213. [DOI] [PubMed] [Google Scholar]
- 12.Aston-Jones G, Chen S, Zhu Y, Oshinsky ML. A neural circuit for circadian regulation of arousal. Nat Neurosci. 2001;4:732–8. doi: 10.1038/89522. [DOI] [PubMed] [Google Scholar]
- 13.Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron. 2001;30:525–36. doi: 10.1016/s0896-6273(01)00302-6. [DOI] [PubMed] [Google Scholar]
- 14.Balling R. ENU mutagenesis: analyzing gene function in mice. Annu Rev Genomics Hum Genet. 2001;2:463–92. doi: 10.1146/annurev.genom.2.1.463. [DOI] [PubMed] [Google Scholar]
- 15.Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–37. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- 16.Bargiello TA, Jackson FR, Young MW. Restoration of circadian behavioural rhythms by gene transfer in Drosophila. Nature. 1984;312:752–4. doi: 10.1038/312752a0. [DOI] [PubMed] [Google Scholar]
- 17.Barnes JW, Tischkau SA, Barnes JA, Mitchell JW, Burgoon PW, et al. Requirement of mammalian Timeless for circadian rhythmicity. Science. 2003;302:439–42. doi: 10.1126/science.1086593. [DOI] [PubMed] [Google Scholar]
- 18.Barrett RK, Takahashi JS. Temperature compensation and temperature entrainment of the chick pineal cell circadian clock. J Neurosci. 1995;15:5681–92. doi: 10.1523/JNEUROSCI.15-08-05681.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Battey J, Moulding C, Taub R, Murphy W, Stewart T, et al. The human c-myc oncogene: structural consequences of translocation into the IgH locus in Burkitt lymphoma. Cell. 1983;34:779–87. doi: 10.1016/0092-8674(83)90534-2. [DOI] [PubMed] [Google Scholar]
- 20.Benna C, Scannapieco P, Piccin A, Sandrelli F, Zordan M, et al. A second timeless gene in Drosophila shares greater sequence similarity with mammalian tim. Curr Biol. 2000;10:R512–3. doi: 10.1016/s0960-9822(00)00594-7. [DOI] [PubMed] [Google Scholar]
- 21.Benzer S. From the gene to behavior. JAMA. 1971;218:1015–22. [PubMed] [Google Scholar]
- 22.Bergman B, Gallon JR, Rai AN, Stal LJ. N2 fixation by non-heterocystous cyanobacteria. FEMS Microbiol Rev. 1997;19:139–85. [Google Scholar]
- 23.Berson DM. Strange vision: ganglion cells as circadian photoreceptors. Trends Neurosci. 2003;26:314–20. doi: 10.1016/S0166-2236(03)00130-9. [DOI] [PubMed] [Google Scholar]
- 24.Bjarnason GA, Jordan R. Rhythms in human gastrointestinal mucosa and skin. Chronobiol Int. 2002;19:129–40. doi: 10.1081/cbi-120002595. [DOI] [PubMed] [Google Scholar]
- 25.Blackwell TK, Huang J, Ma A, Kretzner L, Alt FW, et al. Binding of myc proteins to canonical and noncanonical DNA sequences. Mol Cell Biol. 1993;13:5216–24. doi: 10.1128/mcb.13.9.5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 27.Borbely AA, Achermann P. Homeostasis of human sleep and models of sleep regulation. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Philadelphia: W. B. Saunders Co; 2000. pp. 377–90. [Google Scholar]
- 28.Brown WR. A review and mathematical analysis of circadian rhythms in cell proliferation in mouse, rat, and human epidermis. J Invest Dermatol. 1991;97:273–80. doi: 10.1111/1523-1747.ep12480379. [DOI] [PubMed] [Google Scholar]
- 29.Buchi KN, Moore JG, Hrushesky WJ, Sothern RB, Rubin NH. Circadian rhythm of cellular proliferation in the human rectal mucosa. Gastroenterology. 1991;101:410–5. doi: 10.1016/0016-5085(91)90019-h. [DOI] [PubMed] [Google Scholar]
- 30.Buijs RM, Kalsbeek A. Hypothalamic integration of central and peripheral clocks. Nat Rev Neurosci. 2001;2:521–6. doi: 10.1038/35081582. [DOI] [PubMed] [Google Scholar]
- 31.Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–17. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Camacho F, Cilio M, Guo Y, Virshup DM, Patel K, et al. Human casein kinase Iδ phosphorylation of human circadian clock proteins period 1 and 2. FEBS Lett. 2001;489:159–65. doi: 10.1016/s0014-5793(00)02434-0. [DOI] [PubMed] [Google Scholar]
- 33.Campbell SS, Murphy PJ. Extraocular circadian phototransduction in humans. Science. 1998;279:396–9. doi: 10.1126/science.279.5349.396. [DOI] [PubMed] [Google Scholar]
- 34.Carlborg O, Andersson L, Kinghorn B. The use of a genetic algorithm for simultaneous mapping of multiple interacting quantitative trait loci. Genetics. 2000;155:2003–10. doi: 10.1093/genetics/155.4.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carre IA, Edmunds LN., Jr Oscillator control of cell division in Euglena: cyclic AMP oscillations mediate the phasing of the cell division cycle by the circadian clock. J Cell Sci. 1993;104 ( Pt 4):1163–73. doi: 10.1242/jcs.104.4.1163. [DOI] [PubMed] [Google Scholar]
- 36.Ceriani MF, Hogenesch JB, Yanovsky M, Panda S, Straume M, Kay SA. Genome-wide expression analysis in Drosophila reveals genes controlling circadian behavior. J Neurosci. 2002;22:9305–19. doi: 10.1523/JNEUROSCI.22-21-09305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cermakian N, Monaco L, Pando MP, Dierich A, Sassone-Corsi P. Altered behavioral rhythms and clock gene expression in mice with a targeted mutation in the Period1 gene. EMBO J. 2001;20:3967–74. doi: 10.1093/emboj/20.15.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chan RC, Chan A, Jeon M, Wu TF, Pasqualone D, et al. Chromosome cohesion is regulated by a clock gene paralogue TIM-1. Nature. 2003;424:1002–9. doi: 10.1038/nature01697. [DOI] [PubMed] [Google Scholar]
- 39.Chang AM, Reid KJ, Turek FW, Siddique T, Takahashi JS, Zee PC. Genetic characterization of familial advanced sleep phase syndrome. Presented at Eighth meeting of the Society for Research on Biological Rhythms; Jacksonville, FL. 2002. [Google Scholar]
- 40.Cheng MY, Bullock CM, Li C, Lee AG, Bermak JC, et al. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature. 2002;417:405–10. doi: 10.1038/417405a. [DOI] [PubMed] [Google Scholar]
- 41.Churchill GA, Doerge RW. Empirical threshold values for quantitative trait mapping. Genetics. 1994;138:963–71. doi: 10.1093/genetics/138.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Claridge-Chang A, Wijnen H, Naef F, Boothroyd C, Rajewsky N, Young MW. Circadian regulation of gene expression systems in the Drosophila head. Neuron. 2001;32:657–71. doi: 10.1016/s0896-6273(01)00515-3. [DOI] [PubMed] [Google Scholar]
- 43.Colwell CS. Circadian modulation of calcium levels in cells in the suprachiasmatic nucleus. Eur J Neurosci. 2000;12:571–6. doi: 10.1046/j.1460-9568.2000.00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daan S, Albrecht U, van der Horst GT, Illnerova H, Roenneberg T, et al. Assembling a clock for all seasons: are there M and E oscillators in the genes? J Biol Rhythms. 2001;16:105–16. doi: 10.1177/074873001129001809. [DOI] [PubMed] [Google Scholar]
- 45.Delaunay F, Laudet V. Circadian clock and microarrays: mammalian genome gets rhythm. Trends Genet. 2002;18:595–7. doi: 10.1016/s0168-9525(02)02794-4. [DOI] [PubMed] [Google Scholar]
- 46.Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15:3526–38. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ditty JL, Williams SB, Golden SS. A cyanobacterial circadian timing mechanism. Annu Rev Genet. 2003;37:513–43. doi: 10.1146/annurev.genet.37.110801.142716. [DOI] [PubMed] [Google Scholar]
- 48.Doerge RW. Mapping and analysis of quantitative trait loci in experimental populations. Nat Rev Genet. 2002;3:43–52. doi: 10.1038/nrg703. [DOI] [PubMed] [Google Scholar]
- 49.Doerge RW, Churchill GA. Permutation tests for multiple loci affecting a quantitative character. Genetics. 1996;142:285–94. doi: 10.1093/genetics/142.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duffield GE. DNA microarray analyses of circadian timing: the genomic basis of biological time. J Neuroendocrinol. 2003;15:991–1002. doi: 10.1046/j.1365-2826.2003.01082.x. [DOI] [PubMed] [Google Scholar]
- 51.Duffield GE, Best JD, Meurers BH, Bittner A, Loros JJ, Dunlap JC. Circadian programs of transcriptional activation, signaling, and protein turnover revealed by microarray analysis of mammalian cells. Curr Biol. 2002;12:551–7. doi: 10.1016/s0960-9822(02)00765-0. [DOI] [PubMed] [Google Scholar]
- 52.Dunlap JC. Molecular bases for circadian clocks. Cell. 1999;96:271–90. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- 53.Earnest DJ, Liang FQ, DiGiorgio S, Gallagher M, Harvey B, et al. Establishment and characterization of adenoviral E1A immortalized cell lines derived from the rat suprachiasmatic nucleus. J Neurobiol. 1999;39:1–13. doi: 10.1002/(sici)1097-4695(199904)39:1<1::aid-neu1>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 54.Eastman CI, Martin SK, Hebert M. Failure of extraocular light to facilitate circadian rhythm reentrainment in humans. Chronobiol Int. 2000;17:807–26. doi: 10.1081/cbi-100102116. [DOI] [PubMed] [Google Scholar]
- 55.Ebihara S, Tsuji K, Kondo K. Strain differences of the mouse’s free-running circadian rhythm in continuous darkness. Physiol Behav. 1978;20:795–9. doi: 10.1016/0031-9384(78)90308-6. [DOI] [PubMed] [Google Scholar]
- 56.Ebisawa T, Uchiyama M, Kajimura N, Mishima K, Kamei Y, et al. Association of structural polymorphisms in the human period3 gene with delayed sleep phase syndrome. EMBO Rep. 2001;2:342–6. doi: 10.1093/embo-reports/kve070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Edgar DM, Dement WC, Fuller CA. Effect of SCN lesions on sleep in squirrel monkeys: evidence for opponent processes in sleep-wake regulation. J Neurosci. 1993;13:1065–79. doi: 10.1523/JNEUROSCI.13-03-01065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eide EJ, Vielhaber EL, Hinz WA, Virshup DM. The circadian regulatory proteins BMAL1 and cryptochromes are substrates of casein kinase I ε. J Biol Chem. 2002;277:17248–54. doi: 10.1074/jbc.M111466200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Etchegaray JP, Lee C, Wade PA, Reppert SM. Rhythmic histone acetylation underlies transcription in the mammalian circadian clock. Nature. 2003;421:177–82. doi: 10.1038/nature01314. [DOI] [PubMed] [Google Scholar]
- 60.Fausto N. Liver regeneration. J Hepatol. 2000;32:19–31. doi: 10.1016/s0168-8278(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 61.Field MD, Maywood ES, O’Brien JA, Weaver DR, Reppert SM, Hastings MH. Analysis of clock proteins in mouse SCN demonstrates phylogenetic divergence of the circadian clockwork and resetting mechanisms. Neuron. 2000;25:437–47. doi: 10.1016/s0896-6273(00)80906-x. [DOI] [PubMed] [Google Scholar]
- 62.Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111:41–50. doi: 10.1016/s0092-8674(02)00961-3. [DOI] [PubMed] [Google Scholar]
- 63.Garcia MN, Barbeito CG, Andrini LA, Badran AF. Circadian rhythm of DNA synthesis and mitotic activity in tongue keratinocytes. Cell Biol Int. 2001;25:179–83. doi: 10.1006/cbir.2000.0585. [DOI] [PubMed] [Google Scholar]
- 64.Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, et al. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564–9. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- 65.Giguere V. Orphan nuclear receptors: from gene to function. Endocr Rev. 1999;20:689–725. doi: 10.1210/edrv.20.5.0378. [DOI] [PubMed] [Google Scholar]
- 66.Glazier AM, Nadeau JH, Aitman TJ. Finding genes that underlie complex traits. Science. 2002;298:2345–9. doi: 10.1126/science.1076641. [DOI] [PubMed] [Google Scholar]
- 67.Glossop NR, Lyons LC, Hardin PE. Interlocked feedback loops within the Drosophila circadian oscillator. Science. 1999;286:766–8. doi: 10.1126/science.286.5440.766. [DOI] [PubMed] [Google Scholar]
- 68.Goto K, Johnson CH. Is the cell division cycle gated by a circadian clock? The case of Chlamydomonas reinhardtii. J Cell Biol. 1995;129:1061–9. doi: 10.1083/jcb.129.4.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gotter AL, Manganaro T, Weaver DR, Kolakowski LFJ, Possidente B, et al. A time-less function for mouse Timeless. Nat Neurosci. 2000;3:755–6. doi: 10.1038/77653. [DOI] [PubMed] [Google Scholar]
- 70.Griffin EA, Jr, Staknis D, Weitz CJ. Light-independent role of CRY1 and CRY2 in the mammalian circadian clock. Science. 1999;286:768–71. doi: 10.1126/science.286.5440.768. [DOI] [PubMed] [Google Scholar]
- 71.Grundschober C, Delaunay F, Puhlhofer A, Triqueneaux G, Laudet V, et al. Circadian regulation of diverse gene products revealed by mRNA expression profiling of synchronized fibroblasts. J Biol Chem. 2001;276:46751–8. doi: 10.1074/jbc.M107499200. [DOI] [PubMed] [Google Scholar]
- 72.Hansen J. Increased breast cancer risk among women who work predominantly at night. Epidemiology. 2001;12:74–7. doi: 10.1097/00001648-200101000-00013. [DOI] [PubMed] [Google Scholar]
- 73.Harding HP, Lazar MA. The monomer-binding orphan receptor Rev-Erb represses transcription as a dimer on a novel direct repeat. Mol Cell Biol. 1995;15:4791–802. doi: 10.1128/mcb.15.9.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harmer SL, Hogenesch JB, Straume M, Chang HS, Han B, et al. Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science. 2000;290:2110–3. doi: 10.1126/science.290.5499.2110. [DOI] [PubMed] [Google Scholar]
- 75.Harmer SL, Panda S, Kay SA. Molecular bases of circadian rhythms. Annu Rev Cell Dev Biol. 2001;17:215–53. doi: 10.1146/annurev.cellbio.17.1.215. [DOI] [PubMed] [Google Scholar]
- 76.Hastings MH, Field MD, Maywood ES, Weaver DR, Reppert SM. Differential regulation of mPER1 and mTIM proteins in the mouse suprachiasmatic nuclei: new insights into a core clock mechanism. J Neurosci. 1999;19:RC11, 1–7. doi: 10.1523/JNEUROSCI.19-12-j0001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci. 2003;4:649–61. doi: 10.1038/nrn1177. [DOI] [PubMed] [Google Scholar]
- 78.Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:75–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Haus E, Halberg F. Persisting circadian rhythm in hepatic glycogen of mice during inanition and dehydration. Experientia. 1966;22:113–4. doi: 10.1007/BF01900185. [DOI] [PubMed] [Google Scholar]
- 80.Hebert M, Martin SK, Eastman CI. Nocturnal melatonin secretion is not suppressed by light exposure behind the knee in humans. Neurosci Lett. 1999;274:127–30. doi: 10.1016/s0304-3940(99)00685-0. [DOI] [PubMed] [Google Scholar]
- 81.Herzog ED, Takahashi JS, Block GD. Clock controls circadian period in isolated suprachiasmatic nucleus neurons. Nat Neurosci. 1998;1:708–13. doi: 10.1038/3708. [DOI] [PubMed] [Google Scholar]
- 82.Hirota T, Okano T, Kokame K, Shirotani-Ikejima H, Miyata T, Fukada Y. Glucose down-regulates Per1 and Per2 mRNA levels and induces circadian gene expression in cultured rat-1 fibroblasts. J Biol Chem. 2002;277:44244–51. doi: 10.1074/jbc.M206233200. [DOI] [PubMed] [Google Scholar]
- 83.Hofstetter JR, Mayeda AR. Provisional quantitative trait loci (QTL) for the Aschoff effect in RI mice. Physiol Behav. 1998;64:97–101. doi: 10.1016/s0031-9384(98)00031-6. [DOI] [PubMed] [Google Scholar]
- 84.Hofstetter JR, Mayeda AR, Possidente B, Nurnberger JI., Jr Quantitative trait loci (QTL) for circadian rhythms of locomotor activity in mice. Behav Genet. 1995;25:545–56. doi: 10.1007/BF02327578. [DOI] [PubMed] [Google Scholar]
- 85.Hofstetter JR, Possidente B, Mayeda AR. Provisional QTL for circadian period of wheel running in laboratory mice: quantitative genetics of period in RI mice. Chronobiol Int. 1999;16:269–79. doi: 10.3109/07420529909116857. [DOI] [PubMed] [Google Scholar]
- 86.Hogenesch JB, Gu YZ, Jain S, Bradfield CA. The basic-helix-loop-helix-PAS orphan MOP3 forms transcriptionally active complexes with circadian and hypoxia factors. Proc Natl Acad Sci USA. 1998;95:5474–9. doi: 10.1073/pnas.95.10.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hogenesch JB, Gu YZ, Moran SM, Shimomura K, Radcliffe LA, et al. The basic helix-loop-helix-PAS protein MOP9 is a brain-specific heterodimeric partner of circadian and hypoxia factors. J Neurosci. 2000;20:RC83. doi: 10.1523/JNEUROSCI.20-13-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Homma K, Hastings JW. Cell growth kinetics, division asymmetry and volume control at division in the marine dinoflagellate Gonyaulax polyedra: a model of circadian clock control of the cell cycle. J Cell Sci. 1989;92 ( Pt 2):303–18. doi: 10.1242/jcs.92.2.303. [DOI] [PubMed] [Google Scholar]
- 89.Honma S, Shirakawa T, Katsuno Y, Namihira M, Honma K. Circadian periods of single suprachiasmatic neurons in rats. Neurosci Lett. 1998;250:157–60. doi: 10.1016/s0304-3940(98)00464-9. [DOI] [PubMed] [Google Scholar]
- 90.Hrabe de Angelis MH, Flaswinkel H, Fuchs H, Rathkolb B, Soewarto D, et al. Genome-wide, large-scale production of mutant mice by ENU mutagenesis. Nat Genet. 2000;25:444–7. doi: 10.1038/78146. [DOI] [PubMed] [Google Scholar]
- 91.Humphries A, Klein D, Baler R, Carter DA. cDNA array analysis of pineal gene expression reveals circadian rhythmicity of the dominant negative helix-loop-helix protein-encoding gene, Id-1. J Neuroendocrinol. 2002;14:101–8. doi: 10.1046/j.0007-1331.2001.00738.x. [DOI] [PubMed] [Google Scholar]
- 92.Ikeda M, Sugiyama T, Wallace CS, Gompf HS, Yoshioka T, et al. Circadian dynamics of cytosolic and nuclear Ca2+ in single suprachiasmatic nucleus neurons. Neuron. 2003;38:253–63. doi: 10.1016/s0896-6273(03)00164-8. [DOI] [PubMed] [Google Scholar]
- 93.Jagota A, de La Iglesia HO, Schwartz WJ. Morning and evening circadian oscillations in the suprachiasmatic nucleus in vitro. Nat Neurosci. 2000;3:372–6. doi: 10.1038/73943. [DOI] [PubMed] [Google Scholar]
- 94.Jin X, Shearman LP, Weaver DR, Zylka MJ, de Vries GJ, Reppert SM. A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell. 1999;96:57–68. doi: 10.1016/s0092-8674(00)80959-9. [DOI] [PubMed] [Google Scholar]
- 95.Johansson C, Willeit M, Smedh C, Ekholm J, Paunio T, et al. Circadian clock-related polymorphisms in seasonal affective disorder and their relevance to diurnal preference. Neuropsychopharmacology. 2003;28:734–9. doi: 10.1038/sj.npp.1300121. [DOI] [PubMed] [Google Scholar]
- 96.Jones CR, Campbell SS, Zone SE, Cooper F, DeSano A, et al. Familial advanced sleep-phase syndrome: A short-period circadian rhythm variant in humans. Nat Med. 1999;5:1062–5. doi: 10.1038/12502. [DOI] [PubMed] [Google Scholar]
- 97.Justice MJ, Noveroske JK, Weber JS, Zheng B, Bradley A. Mouse ENU mutagenesis. Hum Mol Genet. 1999;8:1955–63. doi: 10.1093/hmg/8.10.1955. [DOI] [PubMed] [Google Scholar]
- 98.Kalsbeek A, Buijs RM. Output pathways of the mammalian suprachiasmatic nucleus: coding circadian time by transmitter selection and specific targeting. Cell Tissue Res. 2002;309:109–18. doi: 10.1007/s00441-002-0577-0. [DOI] [PubMed] [Google Scholar]
- 99.Kapfhamer D, Valladares O, Sun Y, Nolan PM, Rux JJ, et al. Mutations in Rab3a alter circadian period and homeostatic response to sleep loss in the mouse. Nat Genet. 2002;32:290–5. doi: 10.1038/ng991. [DOI] [PubMed] [Google Scholar]
- 100.Keays DA, Nolan PM. N-ethyl-N-nitrosourea mouse mutants in the dissection of behavioural and psychiatric disorders. Eur J Pharmacol. 2003;480:205–17. doi: 10.1016/j.ejphar.2003.08.107. [DOI] [PubMed] [Google Scholar]
- 101.Keesler GA, Camacho F, Guo Y, Virshup D, Mondadori C, Yao Z. Phosphorylation and destabilization of human period I clock protein by human casein kinase I epsilon. Neuroreport. 2000;11:951–5. doi: 10.1097/00001756-200004070-00011. [DOI] [PubMed] [Google Scholar]
- 102.King DP, Vitaterna MH, Chang AM, Dove WF, Pinto LH, et al. The mouse Clock mutation behaves as an antimorph and maps within the W19H deletion, distal of Kit. Genetics. 1997;146:1049–60. doi: 10.1093/genetics/146.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, et al. Positional cloning of the mouse circadian Clock gene. Cell. 1997;89:641–53. doi: 10.1016/s0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kita Y, Shiozawa M, Jin W, Majewski RR, Besharse JC, et al. Implications of circadian gene expression in kidney, liver and the effects of fasting on pharmacogenomic studies. Pharmacogenetics. 2002;12:55–65. doi: 10.1097/00008571-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 105.Koike N, Hida A, Numano R, Hirose M, Sakaki Y, Tei H. Identification of the mammalian homologues of the Drosophila timeless gene, Timeless1. FEBS Lett. 1998;441:427–31. doi: 10.1016/s0014-5793(98)01597-x. [DOI] [PubMed] [Google Scholar]
- 106.Kondo T, Ishiura M. The circadian clock of cyanobacteria. Bioessays. 2000;22:10–5. doi: 10.1002/(SICI)1521-1878(200001)22:1<10::AID-BIES4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 107.Kondratov RV, Chernov MV, Kondratova AA, Gorbacheva VY, Gudkov AV, Antoch MP. BMAL1-dependent circadian oscillation of nuclear CLOCK: posttranslational events induced by dimerization of transcriptional activators of the mammalian clock system. Genes Dev. 2003;17:1921–32. doi: 10.1101/gad.1099503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Konopka RJ, Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci USA. 1971;68:2112–6. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kramer A, Yang FC, Snodgrass P, Li X, Scammell TE, et al. Regulation of daily locomotor activity and sleep by hypothalamic EGF receptor signaling. Science. 2001;294:2511–5. doi: 10.1126/science.1067716. [DOI] [PubMed] [Google Scholar]
- 110.Kuhlman SJ, Quintero JE, McMahon DG. GFP fluorescence reports Period 1 circadian gene regulation in the mammalian biological clock. Neuroreport. 2000;11:1479–82. [PubMed] [Google Scholar]
- 111.Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, et al. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 1999;98:193–205. doi: 10.1016/s0092-8674(00)81014-4. [DOI] [PubMed] [Google Scholar]
- 112.Lander E, Kruglyak L. Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet. 1995;11:241–7. doi: 10.1038/ng1195-241. [DOI] [PubMed] [Google Scholar]
- 113.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 114.Lee C, Etchegaray JP, Cagampang FR, Loudon AS, Reppert SM. Posttranslational mechanisms regulate the mammalian circadian clock. Cell. 2001;107:855–67. doi: 10.1016/s0092-8674(01)00610-9. [DOI] [PubMed] [Google Scholar]
- 115.Lee K, Loros JJ, Dunlap JC. Interconnected feedback loops in the Neurospora circadian system. Science. 2000;289:107–10. doi: 10.1126/science.289.5476.107. [DOI] [PubMed] [Google Scholar]
- 116.Li Z, Stuart RO, Qiao J, Pavlova A, Bush KT, et al. A role for Timeless in epithelial morphogenesis during kidney development. Proc Natl Acad Sci USA. 2000;97:10038–43. doi: 10.1073/pnas.97.18.10038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lin Y, Han M, Shimada B, Wang L, Gibler TM, et al. Influence of the period-dependent circadian clock on diurnal, circadian, and aperiodic gene expression in Drosophila melanogaster. Proc Natl Acad Sci USA. 2002;99:9562–7. doi: 10.1073/pnas.132269699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lindblad-Toh K, Winchester E, Daly MJ, Wang DG, Hirschhorn JN, et al. Large-scale discovery and genotyping of single-nucleotide polymorphisms in the mouse. Nat Genet. 2000;24:381–6. doi: 10.1038/74215. [DOI] [PubMed] [Google Scholar]
- 119.Lindblom N, Heiskala H, Hatonen T, Mustanoja S, Alfthan H, et al. No evidence for extraocular light induced phase shifting of human melatonin, cortisol and thyrotropin rhythms. Neuroreport. 2000;11:713–7. doi: 10.1097/00001756-200003200-00012. [DOI] [PubMed] [Google Scholar]
- 120.Liu C, Weaver DR, Strogatz SH, Reppert SM. Cellular construction of a circadian clock: period determination in the suprachiasmatic nuclei. Cell. 1997;91:855–60. doi: 10.1016/s0092-8674(00)80473-0. [DOI] [PubMed] [Google Scholar]
- 121.Lockley SW, Skene DJ, Thapan K, English J, Ribeiro D, et al. Extraocular light exposure does not suppress plasma melatonin in humans. J Clin Endocrinol Metab. 1998;83:3369–72. doi: 10.1210/jcem.83.9.5244. [DOI] [PubMed] [Google Scholar]
- 122.Lopez-Molina L, Conquet F, Dubois-Dauphin M, Schibler U. The DBP gene is expressed according to a circadian rhythm in the suprachiasmatic nucleus and influences circadian behavior. EMBO J. 1997;16:6762–71. doi: 10.1093/emboj/16.22.6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Low-Zeddies SS, Takahashi JS. Chimera analysis of the Clock mutation in mice shows that complex cellular integration determines circadian behavior. Cell. 2001;105:25–42. doi: 10.1016/s0092-8674(01)00294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lowrey PL, Shimomura K, Antoch MP, Yamazaki S, Zemenides PD, et al. Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science. 2000;288:483–91. doi: 10.1126/science.288.5465.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Marra G, Anti M, Percesepe A, Armelao F, Ficarelli R, et al. Circadian variations of epithelial cell proliferation in human rectal crypts. Gastroenterology. 1994;106:982–7. doi: 10.1016/0016-5085(94)90757-9. [DOI] [PubMed] [Google Scholar]
- 126.Matsuo T, Yamaguchi S, Mitsui S, Emi A, Shimoda F, Okamura H. Control mechanism of the circadian clock for timing of cell division in vivo. Science. 2003;302:255–9. doi: 10.1126/science.1086271. [DOI] [PubMed] [Google Scholar]
- 127.Mayeda AR, Hofstetter JR, Belknap JK, Nurnberger JI., Jr Hypothetical quantitative trait loci (QTL) for circadian period of locomotor activity in CXB recombinant inbred strains of mice. Behav Genet. 1996;26:505–11. doi: 10.1007/BF02359755. [DOI] [PubMed] [Google Scholar]
- 128.Maywood ES, O’Brien JA, Hastings MH. Expression of mCLOCK and other circadian clock-relevant proteins in the mouse suprachiasmatic nuclei. J Neuroendocrinol. 2003;15:329–34. doi: 10.1046/j.1365-2826.2003.00971.x. [DOI] [PubMed] [Google Scholar]
- 129.McDonald MJ, Rosbash M. Microarray analysis and organization of circadian gene expression in Drosophila. Cell. 2001;107:567–78. doi: 10.1016/s0092-8674(01)00545-1. [DOI] [PubMed] [Google Scholar]
- 130.McNamara P, Seo SP, Rudic RD, Sehgal A, Chakravarti D, FitzGerald GA. Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: a humoral mechanism to reset a peripheral clock. Cell. 2001;105:877–89. doi: 10.1016/s0092-8674(01)00401-9. [DOI] [PubMed] [Google Scholar]
- 131.Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–6. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- 132.Mori T, Johnson CH. Circadian control of cell division in unicellular organisms. Prog Cell Cycle Res. 2000;4:185–92. doi: 10.1007/978-1-4615-4253-7_16. [DOI] [PubMed] [Google Scholar]
- 133.Mormont MC, Levi F. Cancer chronotherapy: principles, applications, and perspectives. Cancer. 2003;97:155–69. doi: 10.1002/cncr.11040. [DOI] [PubMed] [Google Scholar]
- 134.Nakamichi R, Ukai Y, Kishino H. Detection of closely linked multiple quantitative trait loci using a genetic algorithm. Genetics. 2001;158:463–75. doi: 10.1093/genetics/158.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nakamura W, Honma S, Shirakawa T, Honma K. Regional pacemakers composed of multiple oscillator neurons in the rat suprachiasmatic nucleus. Eur J Neurosci. 2001;14:666–74. doi: 10.1046/j.0953-816x.2001.01684.x. [DOI] [PubMed] [Google Scholar]
- 136.Nolan PM, Kapfhamer D, Bucan M. Random mutagenesis screen for dominant behavioral mutations in mice. Methods. 1997;13:379–95. doi: 10.1006/meth.1997.0545. [DOI] [PubMed] [Google Scholar]
- 137.Nolan PM, Peters J, Strivens M, Rogers D, Hagan J, et al. A systematic, genome-wide, phenotype-driven mutagenesis programme for gene function studies in the mouse. Nat Genet. 2000;25:440–3. doi: 10.1038/78140. [DOI] [PubMed] [Google Scholar]
- 138.Nowrousian M, Duffield GE, Loros JJ, Dunlap JC. The frequency gene is required for temperature-dependent regulation of many clock-controlled genes in Neurospora crassa. Genetics. 2003;164:923–33. doi: 10.1093/genetics/164.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Oishi K, Fukui H, Ishida N. Rhythmic expression of BMAL1 mRNA is altered in Clock mutant mice: differential regulation in the suprachiasmatic nucleus and peripheral tissues. Biochem Biophys Res Commun. 2000;268:164–71. doi: 10.1006/bbrc.1999.2054. [DOI] [PubMed] [Google Scholar]
- 140.Oishi K, Miyazaki K, Kadota K, Kikuno R, Nagase T, et al. Genome-wide expression analysis of mouse liver reveals CLOCK-regulated circadian output genes. J Biol Chem. 2003;278:41519–27. doi: 10.1074/jbc.M304564200. [DOI] [PubMed] [Google Scholar]
- 141.Okamura H, Miyake S, Sumi Y, Yamaguchi S, Yasui A, et al. Photic induction of mPer1 and mPer2 in Cry-deficient mice lacking a biological clock. Science. 1999;286:2531–4. doi: 10.1126/science.286.5449.2531. [DOI] [PubMed] [Google Scholar]
- 142.Onishi H, Yamaguchi S, Yagita K, Ishida Y, Dong X, et al. Rev-erbα gene expression in the mouse brain with special emphasis on its circadian profiles in the suprachiasmatic nucleus. J Neurosci Res. 2002;68:551–7. doi: 10.1002/jnr.10226. [DOI] [PubMed] [Google Scholar]
- 143.Pace-Schott EF, Hobson JA. The neurobiology of sleep: genetics, cellular physiology and subcortical networks. Nat Rev Neurosci. 2002;3:591–605. doi: 10.1038/nrn895. [DOI] [PubMed] [Google Scholar]
- 144.Panda S, Antoch MP, Miller BH, Su AI, Schook AB, et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–20. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 145.Panda S, Provencio I, Tu DC, Pires SS, Rollag MD, et al. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301:525–7. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- 146.Pennartz CM, de Jeu MT, Bos NP, Schaap J, Geurtsen AM. Diurnal modulation of pacemaker potentials and calcium current in the mammalian circadian clock. Nature. 2002;416:286–90. doi: 10.1038/nature728. [DOI] [PubMed] [Google Scholar]
- 147.Pittendrigh CS. Circadian systems: entrainment. In: Aschoff J, editor. Handbook of Behavioral Neurobiology: Biological Rhythms. New York: Plenum Press; 1981. pp. 95–124. [Google Scholar]
- 148.Pittendrigh CS. Temporal organization: reflections of a Darwinian clock-watcher. Annu Rev Physiol. 1993;55:16–54. doi: 10.1146/annurev.ph.55.030193.000313. [DOI] [PubMed] [Google Scholar]
- 149.Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. I. The stability and lability of spontaneous frequency. J Comp Physiol. 1976;106:223–52. [Google Scholar]
- 150.Plautz JD, Kaneko M, Hall JC, Kay SA. Independent photoreceptive circadian clocks throughout Drosophila. Science. 1997;278:1632–5. doi: 10.1126/science.278.5343.1632. [DOI] [PubMed] [Google Scholar]
- 151.Possidente B, Hegmann JP. Gene differences modify Aschoff’s rule in mice. Physiol Behav. 1982;28:199–200. doi: 10.1016/0031-9384(82)90126-3. [DOI] [PubMed] [Google Scholar]
- 152.Possidente B, Stephan FK. Circadian period in mice: analysis of genetic and maternal contributions to inbred strain differences. Behav Genet. 1988;18:109–17. doi: 10.1007/BF01067080. [DOI] [PubMed] [Google Scholar]
- 153.Potten CS, Booth D, Cragg NJ, Tudor GL, O’Shea JA, et al. Cell kinetic studies in the murine ventral tongue epithelium: thymidine metabolism studies and circadian rhythm determination. Cell Prolif. 2002;35(Suppl 1):1–15. doi: 10.1046/j.1365-2184.35.s1.1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, et al. The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–60. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 155.Prosser RA. In vitro circadian rhythms of the mammalian suprachiasmatic nuclei: comparison of multi-unit and single-unit neuronal activity recordings. J Biol Rhythms. 1998;13:30–8. doi: 10.1177/074873098128999899. [DOI] [PubMed] [Google Scholar]
- 156.Rajaratnam SM, Arendt J. Health in a 24-h society. Lancet. 2001;358:999–1005. doi: 10.1016/S0140-6736(01)06108-6. [DOI] [PubMed] [Google Scholar]
- 157.Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–8. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- 158.Ralph MR, Menaker M. A mutation of the circadian system in golden hamsters. Science. 1988;241:1225–7. doi: 10.1126/science.3413487. [DOI] [PubMed] [Google Scholar]
- 159.Reick M, Garcia JA, Dudley C, McKnight SL. NPAS2: an analog of clock operative in the mammalian forebrain. Science. 2001;293:506–9. doi: 10.1126/science.1060699. [DOI] [PubMed] [Google Scholar]
- 160.Reid KJ, Chang AM, Dubocovich ML, Turek FW, Takahashi JS, Zee PC. Familial advanced sleep phase syndrome. Arch Neurol. 2001;58:1089–94. doi: 10.1001/archneur.58.7.1089. [DOI] [PubMed] [Google Scholar]
- 161.Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol. 2001;63:647–76. doi: 10.1146/annurev.physiol.63.1.647. [DOI] [PubMed] [Google Scholar]
- 162.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–41. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 163.Ripperger JA, Shearman LP, Reppert SM, Schibler U. CLOCK, an essential pacemaker component, controls expression of the circadian transcription factor DBP. Genes Dev. 2000;14:679–89. [PMC free article] [PubMed] [Google Scholar]
- 164.Rogner UC, Avner P. Congenic mice: cutting tools for complex immune disorders. Nat Rev Immunol. 2003;3:243–52. doi: 10.1038/nri1031. [DOI] [PubMed] [Google Scholar]
- 165.Rollag MD, Berson DM, Provencio I. Melanopsin, ganglion-cell photoreceptors, and mammalian photoentrainment. J Biol Rhythms. 2003;18:227–34. doi: 10.1177/0748730403018003005. [DOI] [PubMed] [Google Scholar]
- 166.Rosato E, Kyriacou CP. Origins of circadian rhythmicity. J Biol Rhythms. 2002;17:506–11. doi: 10.1177/0748730402238232. [DOI] [PubMed] [Google Scholar]
- 167.Russell WL, Kelly EM, Hunsicker PR, Bangham JW, Maddux SC, Phipps EL. Specific-locus test shows ethylnitrosourea to be the most potent mutagen in the mouse. Proc Natl Acad Sci USA. 1979;76:5818–9. doi: 10.1073/pnas.76.11.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rutter J, Reick M, McKnight SL. Metabolism and the control of circadian rhythms. Annu Rev Biochem. 2002;71:307–31. doi: 10.1146/annurev.biochem.71.090501.142857. [DOI] [PubMed] [Google Scholar]
- 169.Sakamoto K, Nagase T, Fukui H, Horikawa K, Okada T, et al. Multitissue circadian expression of rat period homolog (rPer2) mRNA is governed by the mammalian circadian clock, the suprachiasmatic nucleus in the brain. J Biol Chem. 1998;273:27039–42. doi: 10.1074/jbc.273.42.27039. [DOI] [PubMed] [Google Scholar]
- 170.Sangoram AM, Saez L, Antoch MP, Gekakis N, Staknis D, et al. Mammalian circadian autoregulatory loop: a Timeless ortholog and mPer1 interact and negatively regulate CLOCK-BMAL1-induced transcription. Neuron. 1998;21:1101–13. doi: 10.1016/s0896-6273(00)80627-3. [DOI] [PubMed] [Google Scholar]
- 171.Sato TK, Panda S, Kay SA, Hogenesch JB. DNA arrays: applications and implications for circadian biology. J Biol Rhythms. 2003;18:96–105. doi: 10.1177/0748730403252245. [DOI] [PubMed] [Google Scholar]
- 172.Satoh K, Mishima K, Inoue Y, Ebisawa T, Shimizu T. Two pedigrees of familial advanced sleep phase syndrome in Japan. Sleep. 2003;26:416–7. doi: 10.1093/sleep/26.4.416. [DOI] [PubMed] [Google Scholar]
- 173.Schaap J, Albus H, Eilers PH, Detari L, Meijer JH. Phase differences in electrical discharge rhythms between neuronal populations of the left and right suprachiasmatic nuclei. Neuroscience. 2001;108:359–63. doi: 10.1016/s0306-4522(01)00529-2. [DOI] [PubMed] [Google Scholar]
- 174.Schaffer R, Landgraf J, Accerbi M, Simon V, Larson M, Wisman E. Microarray analysis of diurnal and circadian-regulated genes in Arabidopsis. Plant Cell. 2001;13:113–23. doi: 10.1105/tpc.13.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, et al. Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. J Natl Cancer Inst. 2001;93:1563–8. doi: 10.1093/jnci/93.20.1563. [DOI] [PubMed] [Google Scholar]
- 176.Scheving LE, Pauly JE. Circadian phase relationships of thymidine-3H uptake, labeled nuclei, grain counts, and cell division rate in rat corneal epithelium. J Cell Biol. 1967;32:677–83. doi: 10.1083/jcb.32.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Schoenhard JA, Eren M, Johnson CH, Vaughan DE. Alternative splicing yields novel BMAL2 variants: tissue distribution and functional characterization. Am J Physiol Cell Physiol. 2002;283:C103–14. doi: 10.1152/ajpcell.00541.2001. [DOI] [PubMed] [Google Scholar]
- 178.Schwartz WJ. SCN metabolic activity in vivo. In: Klein DC, Moore RY, Reppert SM, editors. Suprachiasmatic Nucleus: The Mind’s Clock. New York: Oxford Univ Press; 1991. pp. 144–56. [Google Scholar]
- 179.Schwartz WJ, Zimmerman P. Circadian timekeeping in BALB/c and C57BL/6 inbred mouse strains. J Neurosci. 1990;10:3685–94. doi: 10.1523/JNEUROSCI.10-11-03685.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sen S, Churchill GA. A statistical framework for quantitative trait mapping. Genetics. 2001;159:371–87. doi: 10.1093/genetics/159.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Shearman LP, Jin X, Lee C, Reppert SM, Weaver DR. Targeted disruption of the mPer3 gene: subtle effects on circadian clock function. Mol Cell Biol. 2000;20:6269–75. doi: 10.1128/mcb.20.17.6269-6275.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Shearman LP, Sriram S, Weaver DR, Maywood ES, Chaves I, et al. Interacting molecular loops in the mammalian circadian clock. Science. 2000;288:1013–9. doi: 10.1126/science.288.5468.1013. [DOI] [PubMed] [Google Scholar]
- 183.Shimomura K, Low-Zeddies SS, King DP, Steeves TD, Whiteley A, et al. Genome-wide epistatic interaction analysis reveals complex genetic determinants of circadian behavior in mice. Genome Res. 2001;11:959–80. doi: 10.1101/gr.171601. [DOI] [PubMed] [Google Scholar]
- 184.Silver R, LeSauter J, Tresco PA, Lehman MN. A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature. 1996;382:810–3. doi: 10.1038/382810a0. [DOI] [PubMed] [Google Scholar]
- 185.Smaaland R, Sothern RB, Laerum OD, Abrahamsen JF. Rhythms in human bone marrow and blood cells. Chronobiol Int. 2002;19:101–27. doi: 10.1081/cbi-120002594. [DOI] [PubMed] [Google Scholar]
- 186.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA. 1972;69:1583–6. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, et al. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417:78–83. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- 188.Sujino M, Masumoto K, Yamaguchi S, van der Horst GT, Okamura H, Inouye SI. Suprachiasmatic nucleus grafts restore circadian behavioral rhythms of genetically arrhythmic mice. Curr Biol. 2003;13:664–8. doi: 10.1016/s0960-9822(03)00222-7. [DOI] [PubMed] [Google Scholar]
- 189.Suzuki T, Ishikawa A, Nishimura M, Yoshimura T, Namikawa T, Ebihara S. Mapping quantitative trait loci for circadian behavioral rhythms in SMXA recombinant inbred strains. Behav Genet. 2000;30:447–53. doi: 10.1023/a:1010298701251. [DOI] [PubMed] [Google Scholar]
- 190.Suzuki T, Ishikawa A, Yoshimura T, Namikawa T, Abe H, et al. Quantitative trait locus analysis of abnormal circadian period in CS mice. Mamm Genome. 2001;12:272–7. doi: 10.1007/s003350010280. [DOI] [PubMed] [Google Scholar]
- 191.Taheri S, Mignot E. The genetics of sleep disorders. Lancet Neurol. 2002;1:242–50. doi: 10.1016/s1474-4422(02)00103-5. [DOI] [PubMed] [Google Scholar]
- 192.Takahashi JS, DeCoursey PJ, Bauman L, Menaker M. Spectral sensitivity of a novel photoreceptive system mediating entrainment of mammalian circadian rhythms. Nature. 1984;308:186–8. doi: 10.1038/308186a0. [DOI] [PubMed] [Google Scholar]
- 193.Takahashi JS, Pinto LH, Vitaterna MH. Forward and reverse genetic approaches to behavior in the mouse. Science. 1994;264:1724–33. doi: 10.1126/science.8209253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Takahashi JS, Turek FW, Moore RY, editors. Handbook of Behavioral Neurobiology: Circadian Clocks. Vol. 12. New York: Kluwer Academic/Plenum Publishers; 2001. [Google Scholar]
- 195.Takumi T, Nagamine Y, Miyake S, Matsubara C, Taguchi K, et al. A mammalian ortholog of Drosophila timeless, highly expressed in SCN and retina, forms a complex with mPER1. Genes Cells. 1999;4:67–75. doi: 10.1046/j.1365-2443.1999.00238.x. [DOI] [PubMed] [Google Scholar]
- 196.Thresher RJ, Vitaterna MH, Miyamoto Y, Kazantsev A, Hsu DS, et al. Role of mouse cryptochrome blue-light photoreceptor in circadian photoresponses. Science. 1998;282:1490–4. doi: 10.1126/science.282.5393.1490. [DOI] [PubMed] [Google Scholar]
- 197.Tischkau SA, Barnes JA, Lin F, Myers EM, Soucy JW, et al. Oscillation and light induction of timeless mRNA in the mammalian circadian clock. J Neurosci. 1999;19:RC15, 1–6. doi: 10.1523/JNEUROSCI.19-12-j0005.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Toh KL, Jones CR, He Y, Eide EJ, Hinz WA, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291:1040–3. doi: 10.1126/science.1057499. [DOI] [PubMed] [Google Scholar]
- 199.Tosini G, Menaker M. Circadian rhythms in cultured mammalian retina. Science. 1996;272:419–21. doi: 10.1126/science.272.5260.419. [DOI] [PubMed] [Google Scholar]
- 200.Ueda HR, Chen W, Adachi A, Wakamatsu H, Hayashi S, et al. A transcription factor response element for gene expression during circadian night. Nature. 2002;418:534–9. doi: 10.1038/nature00906. [DOI] [PubMed] [Google Scholar]
- 201.Ueda HR, Matsumoto A, Kawamura M, Iino M, Tanimura T, Hashimoto S. Genome-wide transcriptional orchestration of circadian rhythms in Drosophila. J Biol Chem. 2002;277:14048–52. doi: 10.1074/jbc.C100765200. [DOI] [PubMed] [Google Scholar]
- 202.Ueyama T, Krout KE, Nguyen XV, Karpitskiy V, Kollert A, et al. Suprachiasmatic nucleus: a central autonomic clock. Nat Neurosci. 1999;2:1051–3. doi: 10.1038/15973. [DOI] [PubMed] [Google Scholar]
- 203.van den Pol AN. The hypothalamic suprachiasmatic nucleus of rat: intrinsic anatomy. J Comp Neurol. 1980;191:661–702. doi: 10.1002/cne.901910410. [DOI] [PubMed] [Google Scholar]
- 204.van den Pol AN, Finkbeiner SM, Cornell-Bell AH. Calcium excitability and oscillations in suprachiasmatic nucleus neurons and glia in vitro. J Neurosci. 1992;12:2648–64. doi: 10.1523/JNEUROSCI.12-07-02648.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.van der Horst GT, Muijtjens M, Kobayashi K, Takano R, Kanno S, et al. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature. 1999;398:627–30. doi: 10.1038/19323. [DOI] [PubMed] [Google Scholar]
- 206.Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, et al. The sequence of the human genome. Science. 2001;291:1304–51. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 207.Vielhaber E, Eide E, Rivers A, Gao ZH, Virshup DM. Nuclear entry of the circadian regulator mPER1 is controlled by mammalian casein kinase I ε. Mol Cell Biol. 2000;20:4888–99. doi: 10.1128/mcb.20.13.4888-4899.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowrey PL, et al. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science. 1994;264:719–25. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Vitaterna MH, Selby CP, Todo T, Niwa H, Thompson C, et al. Differential regulation of mammalian Period genes and circadian rhythmicity by cryptochromes 1 and 2. Proc Natl Acad Sci USA. 1999;96:12114–9. doi: 10.1073/pnas.96.21.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wagner DR. Disorders of the circadian sleep-wake cycle. Neurol Clin. 1996;14:651–70. doi: 10.1016/s0733-8619(05)70278-4. [DOI] [PubMed] [Google Scholar]
- 211.Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Abril JF, et al. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420:520–62. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- 212.Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- 213.Whitmore D, Foulkes NS, Sassone-Corsi P. Light acts directly on organs and cells in culture to set the vertebrate circadian clock. Nature. 2000;404:87–91. doi: 10.1038/35003589. [DOI] [PubMed] [Google Scholar]
- 214.Wijnen H, Boothroyd C, Young MW, Claridge-Chang A. Molecular genetics of timing in intrinsic circadian rhythm sleep disorders. Ann Med. 2002;34:386–93. doi: 10.1080/078538902320772133. [DOI] [PubMed] [Google Scholar]
- 215.Williams JA, Sehgal A. Molecular components of the circadian system in Drosophila. Annu Rev Physiol. 2001;63:729–55. doi: 10.1146/annurev.physiol.63.1.729. [DOI] [PubMed] [Google Scholar]
- 216.Wilsbacher LD, Sangoram AM, Antoch MP, Takahashi JS. The mouse Clock locus: sequence and comparative analysis of 204 kb from mouse chromosome 5. Genome Res. 2000;10:1928–40. doi: 10.1101/gr.10.12.1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wilsbacher LD, Yamazaki S, Herzog ED, Song EJ, Radcliffe LA, et al. Photic and circadian expression of luciferase in mPeriod1-luc transgenic mice in vivo. Proc Natl Acad Sci USA. 2002;99:489–94. doi: 10.1073/pnas.012248599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wright KP, Jr, Czeisler CA. Absence of circadian phase resetting in response to bright light behind the knees. Science. 2002;297:571. doi: 10.1126/science.1071697. [DOI] [PubMed] [Google Scholar]
- 219.Xiao J, Li C, Zhu NL, Borok Z, Minoo P. Timeless in lung morphogenesis. Dev Dyn. 2003;228:82–94. doi: 10.1002/dvdy.10346. [DOI] [PubMed] [Google Scholar]
- 220.Yagita K, Tamanini F, Yasuda M, Hoeijmakers JH, van der Horst GT, Okamura H. Nucleocytoplasmic shuttling and mCRY-dependent inhibition of ubiquitylation of the mPER2 clock protein. EMBO J. 2002;21:1301–14. doi: 10.1093/emboj/21.6.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Yamaguchi S, Isejima H, Matsuo T, Okura R, Yagita K, et al. Synchronization of cellular clocks in the suprachiasmatic nucleus. Science. 2003;302:1408–12. doi: 10.1126/science.1089287. [DOI] [PubMed] [Google Scholar]
- 222.Yamaguchi S, Mitsui S, Miyake S, Yan L, Onishi H, et al. The 5′ upstream region of mPer1 gene contains two promoters and is responsible for circadian oscillation. Curr Biol. 2000;10:873–6. doi: 10.1016/s0960-9822(00)00602-3. [DOI] [PubMed] [Google Scholar]
- 223.Yamaguchi S, Mitsui S, Yan L, Yagita K, Miyake S, Okamura H. Role of DBP in the circadian oscillatory mechanism. Mol Cell Biol. 2000;20:4773–81. doi: 10.1128/mcb.20.13.4773-4781.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, et al. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–5. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 225.Yan L, Okamura H. Gradients in the circadian expression of Per1 and Per2 genes in the rat suprachiasmatic nucleus. Eur J Neurosci. 2002;15:1153–62. doi: 10.1046/j.1460-9568.2002.01955.x. [DOI] [PubMed] [Google Scholar]
- 226.Yoo S-H, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, et al. PERIOD2::LUCIFERASE Real-Time Reporting of Circadian Dynamics Reveals Persistent Circadian Oscillations in Mouse Peripheral Tissues. Proc Natl Acad Sci U S A. 2004 doi: 10.1073/pnas.0308709101. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Young MW, Kay SA. Time zones: a comparative genetics of circadian clocks. Nat Rev Genet. 2001;2:702–15. doi: 10.1038/35088576. [DOI] [PubMed] [Google Scholar]
- 228.Yu W, Nomura M, Ikeda M. Interactivating feedback loops within the mammalian clock: BMAL1 is negatively autoregulated and upregulated by CRY1, CRY2, and PER2. Biochem Biophys Res Commun. 2002;290:933–41. doi: 10.1006/bbrc.2001.6300. [DOI] [PubMed] [Google Scholar]
- 229.Zehring WA, Wheeler DA, Reddy P, Konopka RJ, Kyriacou CP, et al. P-element transformation with period locus DNA restores rhythmicity to mutant, arrhythmic Drosophila melanogaster. Cell. 1984;39:369–76. doi: 10.1016/0092-8674(84)90015-1. [DOI] [PubMed] [Google Scholar]
- 230.Zheng B, Albrecht U, Kaasik K, Sage M, Lu W, et al. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell. 2001;105:683–94. doi: 10.1016/s0092-8674(01)00380-4. [DOI] [PubMed] [Google Scholar]
- 231.Zheng B, Larkin DW, Albrecht U, Sun ZS, Sage M, et al. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature. 1999;400:169–73. doi: 10.1038/22118. [DOI] [PubMed] [Google Scholar]
- 232.Zylka MJ, Shearman LP, Levine JD, Jin X, Weaver DR, Reppert SM. Molecular analysis of mammalian timeless. Neuron. 1998;21:1115–22. doi: 10.1016/s0896-6273(00)80628-5. [DOI] [PubMed] [Google Scholar]
- 233.Zylka MJ, Shearman LP, Weaver DR, Reppert SM. Three period homologs in mammals: differential light responses in the suprachiasmatic circadian clock and oscillating transcripts outside of brain. Neuron. 1998;20:1103–10. doi: 10.1016/s0896-6273(00)80492-4. [DOI] [PubMed] [Google Scholar]