Abstract
Forward genetic approaches (phenotype to gene) are powerful methods to identify mouse circadian clock components. The success of these approaches, however, is highly dependent on the quality of the phenotype— specifically, the ability to measure circadian rhythms in individual mice. This article outlines the factors necessary to measure mouse circadian rhythms, including choice of mouse strain, facilities and equipment design and construction, experimental design, high-throughput methods, and finally methods for data analysis.
Introduction
We have undertaken a forward genetics approach to identify the components governing circadian behavior in the mouse. Benzer and colleagues used forward genetics to identify Drosophila genes involved in such behaviors as learning and memory (Dudai et al., 1976), courtship, and circadian rhythms (Konopka and Benzer, 1971). The success of their screens depended on their abilities to design and build equipment to perform high-throughput screens for these robust and quantitative behaviors and to utilize the tools of Drosophila genetics.
In Drosophila, the screen for circadian rhythm mutants was performed on populations of flies rather than individuals. Daan and Pittendrigh (1976a–e) questioned whether these same methods used to measure circadian rhythms in populations of flies could be applied to individual rodents, designed the equipment to do so, and ultimately published a series of papers in which they describe their methods and detailed descriptions of rodent running-wheel behavior. In the late 1980s the first mammalian circadian rhythm mutant tau was identified (Ralph and Menaker, 1988), confirming a genetic basis for circadian behavior in mammals. By the early 1980s, methods were developed to produce highly mutagenized mice (Russell et al., 1979). A highly automated assay, the identification of a genetic basis for the circadian behavior in mammals, and identification of a powerful mutagen for mice coupled with the tools of mouse genetics made a forward genetics screen for circadian rhythms mutants in mice possible. The result was identification of the first mouse circadian mutant, Clock (Vitaterna et al., 1994). We have initiated a large-scale recessive screen to identify even more mouse circadian rhythm mutants. The goal is to screen 10,000 mutant mice per year to identify both new genes and mutant alleles of known genes that control circadian behavior. This article describes our refinement of the running wheel equipment designs and the methods we used to record circadian rhythm wheel running activity in mice.
Strain Choice
The choice of mouse strain is the most important consideration for any mouse circadian rhythm screen and ultimately dictates the ability to identify mutants. Poor runners or variable wheel running activity will muddle the interpretation of results and increase the number of false-positive mutants. For example, BALB/cJ mice would be a poor choice of strain for a mutant screen. These mice are poor runners and have a highly variable circadian free running period (Shimomura et al., 2001). Similarly, mice with mixed genetic backgrounds or outbred strains would also have a highly variable free running period and would complicate mutant identification. To minimize the number of false positives in our screen, we have elected to use inbred strains with robust and relatively invariant wheel running activity for our mutagenesis program. The two strains that we use, BTBR/J and C57BL/ 6J, were also chosen for their N-nitroso-N-ethylurea (ENU) mutation rate and their favorable reproductive behavior.
Facilities and Equipment Needed for a Circadian Rhythm Screen
The basic equipment required to conduct a circadian activity assay are as follows: a dedicated room for the assay, one or more light-tight chambers, running wheel cages, and a computer to collect data.
Our rooms are approximately 12×15 ft in size. All windows are permanently sealed and the doors are fitted with gaskets and sweeps to keep the room light tight. One of our rooms has a locked anteroom to further minimize light leaks from the door into the room. A dead bolt with an interior latch is installed in the room door to prevent accidental entries while performing procedures in complete darkness. Alternatively, a darkroom revolving door can be used in place of a regular door. Darkroom doors, however, are harder to secure and are too small to allow for carts of wheel cages and mice into the room.
In addition to the regular fluorescent light fixtures in the room, we have installed ceiling light fixtures (Kodak Darkroom lamp #152 1178) fitted with light filters (Kodak #11) to illuminate the room when we use infrared night vision goggles (http://www.nightvisionweb.com).
All cables leading from the light-tight boxes to the data collection computer are housed in 3-in PVC conduit mounted 8 ft above ground to protect the wires from damage during room cleaning and sanitization.
Although we are constantly refining and improving our cabinet designs, all have the same basic features and dimensions—horizontal light-tight wooden cabinets (approximately 6 ft long, 2.5 ft high, and 2.5 ft deep) constructed out of 1/2×3/4-in plywood. The exterior is finished with clear varnish and the interior with flat black epoxy paint. The cabinets are stacked four high on rolling platforms and are arranged around the perimeter of the room. Each cabinet contains an interior shelf. Ten to 12 wheel cages are arranged on each shelf for a total of 20–24 cages per box. The doors to the cabinets hinge on the bottom of the door for easy access. The door for the top box of each stack, however, hinges on the top and is held open by automotive hatchback hydraulic pumps.
The box doors are light sealed with heavy-duty piano felt. Three or four equally spaced heavy-duty brass window latches are used to secure the doors closed. The doors are kept closed and latches are locked at all times to prevent the wood doors from warping due to seasonal humidity changes. All holes drilled through the cabinets to accommodate wires are sealed with black caulk.
Each cabinet door is fitted with a baffle to allow air to flow into the cabinet. Baffled air exhaust holes with fans are at the rear of the cabinet and are linked directly to the HVAC system of the room to eliminate odors and minimize particulate material in the room. The exhaust system generates a considerable amount of “ white noise,” which masks the noises we generate (carts moving, cages banging, human voices, etc.) when we work in the room.
We control the light cycle in each box with panels of LED lights mounted above each shelf. Previously, we used fluorescent light fixtures mounted on the rear wall of the box. We found, however, that the fluorescent fixtures generated so much heat that it was difficult to design an airflow system to control the temperature adequately inside the cabinets. We now use a panel of green LEDs (Newark Electronics Cat. No. 88C0836, Agilent Technologies, Part No. HLMP-AM01-Q0000) arranged in a two-dimensional matrix spaced every 8–10 cm for uniform illumination. Green LEDs burn cooler, use less energy, are longer lived than fluorescent lights, are brighter than white LEDs (100 lux 25 cm from the bottom of the wheel cage), and, most importantly, emit light in the proper part of the spectrum to entrain mice to a light cycle (Takahashi et al., 1984).
Each box is also equipped with light sensors, door sensors (to detect door openings), and temperature and humidity probes (VWR Cat No. 61161-378). All wires within the boxes are contained in metal conduit to prevent escaped mice from damaging the equipment.
After the boxes are assembled, we test for light leaks with photosensitive paper. In darkness, the paper is placed in each box, the boxes are closed, and the room lights are left on for 24 h. The paper is removed in darkness the next day and is developed to detect light leaks in the cabinets. We also test for light leaks by eye. An investigator sits in the dark room for 30 min to adapt to the darkness. The lights in the boxes are turned on in each light-tight box (with the doors latched) and the investigator looks for light leaks by eye.
We construct running wheel cages using polycarbonate cages (Fischer Scientific, Cat. Nos. 01-288-1B and 01-288-21) fitted with 4.75-in stainless-steel diameter running wheels (custom built for us by Lab Products, Inc., Seaford, DE). All metal hardware used in cage assembly is made of stainless steel to prevent rusting. Two holes are drilled into the side of the cage and are fitted with banana plug receptacles (Newark Electronics, Cat. No. 35F713) to mount the microswitches to the side of each wheel cage. Because this equipment must be durable enough to collect data for 1 month and to survive cage washing, we use locking nuts and lock-tight whenever possible to keep the equipment intact.
Wheel revolutions activate microswitches (Newark Electronics, Cat. No. 84F514) modified with two single banana plugs (Newark Electronics, Cat. No. 39F889) to mount the switch to the side of the wheel cage. Heavy-duty lamp wire (Newark Cat. No. 19122-250-1) is soldered to the switch and a double banana plug (Newark Electronics, Cat. No. 34F857) is attached at the end of the wire. The microswitch assembly plugs into a panel of banana plug receptacles. This panel feeds microswitch counts to the data collection computer through a National Instruments data acquisition board (Model PCI-6023e). With time, the physical switch wears down, but it can be easily removed and replaced with a new switch. All other parts in the switch assembly are reusable, and we keep a stock of assembled switches on hand to rapidly replace and repair bad switches. Detailed instructions for cage and microswitch assembly are available by contacting the authors.
Running wheel revolutions are counted using ClockLab software (Actometrics, Evanston, IL) by computers housed in a protective enclosure (http://www.itsenclosures.com). The computer both controls the lights within the light-tight box and collects data from up to three stacks of light control cabinets (or the equivalent of 256 data collection channels). The collection computers are kept on a UPS battery backup in case of power failure and are backed up nightly to an off-site server. The computer clocks are synchronized nightly. Additionally, the computer clocks are adjusted for daylight savings time, but the collection software clock does not change. Time in the collection computer, therefore, is maintained as experimental time and not real time. During daylight savings time, we simply adjust our schedules to account for the 1-h time difference. Data files are copied from the collection computers weekly for analysis.
Experimental Design and Throughput
The goal of our phenotyping facility is to assay 10,000 mice for wheel running behavior per year. Because of the high-throughput nature of the screen, we keep the experimental design fairly straightforward. Circadian behavior is measured in 200 mice each week by continuously monitoring running wheel activity for a period of 1 month. The assay is divided into two distinct data collection phases. During the first phase of the assay, mice are placed in individual running wheel cages and are maintained under the same 12:12 LD cycle to which they entrained since birth. After collecting running wheel activity data for 1 week, the mice are released into constant darkness (DD) and activity data are collected for an additional 21 days. The screen can also be altered to identify mice that have abnormal behaviors under constant light conditions or have altered responses to light pulses. To collect data for either of these conditions, however, requires extended time on the running wheel or, in the case of the light pulse screen, direct intervention of the investigator. Because our goal is to assay as many mice per year using an automated system, we have not included an LL phase or any light pulses as part of our screen.
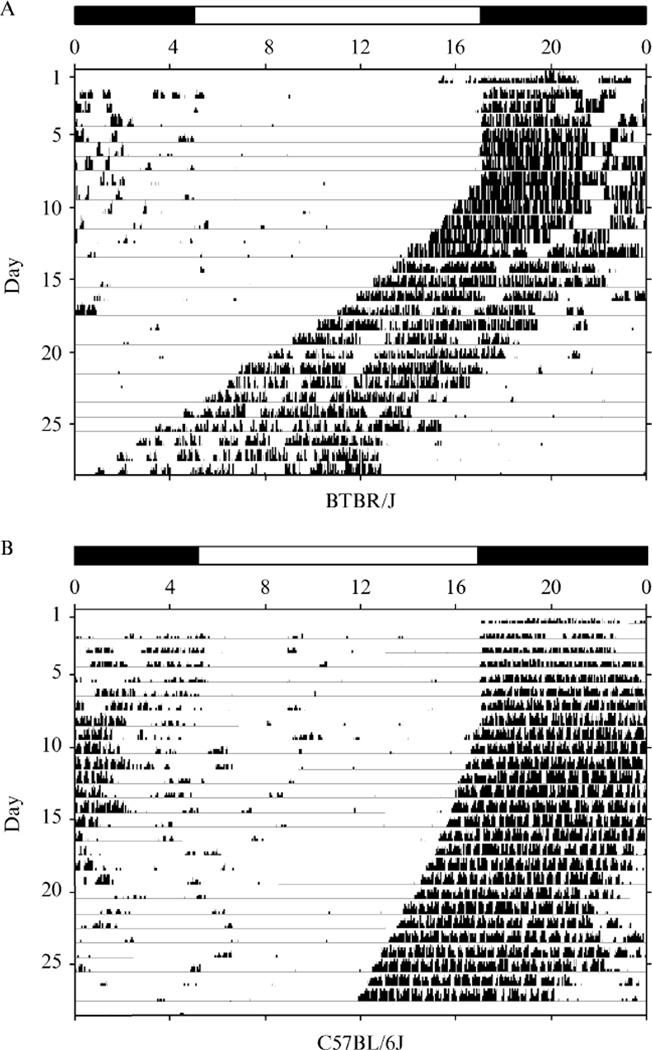
Circadian rhythm mutants are identified as mice that display abnormal measurements in one or more of the following parameters: free running period, phase angle of entrainment to the LD cycle, period amplitude, and daytime running activity. Each of these measurements is extracted from the wheel running records (or actograms) (Fig. 1) using ClockLab software (http://www.actimetrics.com). In general, mice with circadian measurements more than 3 SD from the mean for any of these parameters are selected as putants (or putative mutants) and mated to determine trait heritability.
Figure 1.
Mouse actograms. (A) BTBR/J mouse. (B) C57BL/6J mouse. The light cycle for the LD portion of the experiment is represented by a bar at the top of the actogram where lights are on at 5:00 h and off at 17:00 h. Activity bouts are represented as histogram bars (the sum of wheel revolutions per discrete time interval) across a 24-h period. Each horizontal line represents one 24-h period of the experiment. Mice were maintained on a LD cycle for the first 7 days of the experiment and DD for the last 21 days. The difference in free running periods between these two mice is solely due to background strain differences.
Because the final step to identify a mutant mouse involves a heritability test, it is important that the mice finish the assay in a timely manner. In general, both male and female mice are mature to reproduce by 5–6 weeks of age. If not mated for the first time until 12 weeks of age, however, there is a dramatic decrease in female fertility. In general, we place mice on wheels at 6 weeks of age to not compromise running wheel activity and no later than 12 weeks of age to prevent fertility problems at the end of the assay. This age range for optimal wheel running activity and reproductive behavior can and does vary by mouse strain.
One other consideration in the experimental design concerns the way in which the mice are handled before and after the screen. We frequently assay our mice for other behavioral phenotypes, and the order in which these assays are performed can have a dramatic effect on the circadian behavior. Furthermore, the circadian assay screen can be particularly stressful for the mice, as they are singly housed for 1 month during the screen. Mice that have gone through the assay tend to be jumpy, more difficult to handle, and more aggressive if group housed after the assay. For these reasons, we cannot screen postwheel mice for any stress-related phenotypes. Conversely, we try to avoid any invasive assays before placing mice on wheels. Any treatment that alters activity or affects mobility would have a tremendous negative impact on the screen.
We start an experiment by first assembling all materials needed—cages, bedding, food, and water bottles. Each wheel is first lubricated with silicon spray and spun to detect any bent wheels. A handful of bedding is placed in each cage (too much bedding prevents the running wheels from moving freely) and the cage is placed in position in the light-tight box and connected to the appropriate microswitch. The wheel is spun again to ensure that the microswitch is activated by each wheel revolution. One to 2 h before lights out, we bring the mice into the room and place them one by one into individual wheel cages. We initiate the experiment at the end of the day (lights off) for two reasons. First, mice are nocturnal. By initiating the experiment at the end of the light cycle we do not disturb the mice in the middle of their rest period. Second, the wheel cage is novel to the mouse and novel wheel running behavior causes phase shifts in circadian behavior (Janik et al., 1994; Van Reeth and Turek, 1989). To minimize phase shifts due to placement into a novel wheel cage, we initiate the experiment at the end of the light portion of the LD cycle when the mouse begins its normal activity period. Once the mice are in the wheel cages we add water bottles and food and activate the data collection channels on the computer. The mice are monitored daily to look for problems—leaky water bottles, inactivity, high activity (lack of food or water, maloclusion), broken switches, unaligned switches, etc. During the last couple of hours of the light cycle of the LD phase of the experiment we top off the food bins in each cage. As much as possible, we try to detect and resolve any problems with the equipment during the LD phase of the assay.
Data Analysis
We analyze activity data 3.5 weeks into the assay. All activity records (or actograms) are first inspected visually to remove records that will give aberrant results in the batch analysis. In particular, we look for actograms of mice with extraordinarily low activity levels. These actograms will not yield accurate circadian period values. By visual inspection we are also able to detect abnormal patterns of activity not usually detected by analysis program—long rest period, abnormal onset patterns, etc. We extract five different measurements to assess the circadian behavior of the mice—free running period (as measured two ways), phase angle of entrainment, circadian amplitude, and average daily activity levels.
The circadian period can be extracted from the constant darkness phase of the wheel running activity records by either Χ2 periodogram analysis or by linear regression analysis of activity onset. Both of these measurements can be optimized in the ClockLab analysis program to account for acto-grams with low activity levels and slightly abnormal activity onsets. To date, the single most important measurement to detect circadian rhythms mutants is the free running period. Both hamster tau and mouse Clock homozygotes’ periods differed from those of wild type mice by almost 4 h (Ralph and Menaker, 1988; Vitaterna et al., 1994).
The phase angle of entrainment reflects the difference between the onset of activity and the onset of the dark period in nocturnal animals. In addition to determining this value while the animals are exposed to the LD cycle, the value is also determined by examining the onset of daily activity after the transfer to DD to verify that the exposure to light was not masking the true onset of activity. This is accomplished by determining the linear regression of activity onset for the first 7 days in constant darkness and extrapolating this line to the last day of the LD cycle. Animals with an abnormal phase angle of entrainment could be carrying a mutation affecting a photoreceptor or some component of the input pathway to the circadian pacemaker. A mutation in the period of the circadian clock itself could also lead to a change in the phase angle of entrainment, which would be assessed directly when the animals are transferred to DD.
To assess the dominant circadian component for the activity rhythm, power spectral density of the circadian peak by fast Fourier transformation (FFT) is measured. Circadian amplitude is a measurement of the robustness of the rhythm. A low circadian amplitude could reflect an unstable circadian clock or a mouse with low activity levels. An extremely low circadian amplitude may also indicate that the animal is arrhythmic.
Average daily activity is calculated by the sum of the number of wheel revolutions divided by the total numbers of days. In LD or DD, low activity levels may indicate other physical abnormalities and serve as a general assay for physical robustness. Low activity levels may also correspond with low circadian amplitude. Because circadian measurements can be interrelated, it is important to look at all measurements in addition to examining the actogram itself to characterize the circadian phenotypes of the animals.
Putants are group housed (two per cage, single sex) to resocialize and reentrain the mice to a 12:12 LD cycle. After 1 week in a 12:12 LD cycle, the putants are mated to wild-type mice, initiating the trait heritability test as described in the previous article.
In conclusion, circadian wheel running assays using automated data acquisition systems in a highly parallel fashion (>800 channels) as described here provide an extremely quantitative and sensitive phenotype for genetic screens as well as many other circadian experiments.
Acknowledgments
This work was supported by NIH grant #U01_MH61915. J. S. Takahashi is an Investigator in the Howard Hughes Medical Institute.
References
- Daan S, Pittendrigh CS. A functional analysis of circadian pacemakers in nocturnal rodents. I. The stability and lability of spontaneous frequency. J. Comp. Physiol. A. 1976a;106:223–252. [Google Scholar]
- Daan S, Pittendrigh CS. A functional analysis ofcircadianpacemakers innocturnal rodents. II. The variability of phase response curves. J. Comp. Physiol. A. 1976b;106:253–266. [Google Scholar]
- Daan S, Pittendrigh CS. A functional analysis of circadian pacemakers in nocturnal rodents. III. Heavy water and constant light: Homeostasis of frequency. J. Comp. Physiol. A. 1976c;106:267–290. [Google Scholar]
- Daan S, Pittendrigh CS. A functional analysis of circadian pacemakers in nocturnal rodents. IV. Entrainment: Pacemaker as clock. J. Comp. Physiol. A. 1976d;106:291–331. [Google Scholar]
- Daan S, Pittendrigh CS. A functional analysis ofcircadian pacemakers innocturnal rodents. V. Pacemaker structure: A clock for all seasons. J. Comp. Physiol. A. 1976e;106:333–355. [Google Scholar]
- Dudai Y, Jan YN, Byers D, Quinn WG, Benzer S. Ounce, a mutant of Drosophila deficient in learning. Proc. Natl. Acad. Sci. USA. 1976;73:1684–1688. doi: 10.1073/pnas.73.5.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janik D, Godfrey M, Mrosovsky N. Phase angle changes of photically entrained circadian rhythms following a single nonphotic stimulus. Physiol. Behav. 1994;55:103–107. doi: 10.1016/0031-9384(94)90016-7. [DOI] [PubMed] [Google Scholar]
- Konopka RJ, Benzer S. Clock mutants of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 1971;68:2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph MR, Menaker M. A mutation of the circadian system in golden hamsters. Science. 1988;241:1225–1227. doi: 10.1126/science.3413487. [DOI] [PubMed] [Google Scholar]
- Russell WL, Kelly EM, Hunsicker PR, Bangham JW, Maddux SC, Phipps EL. Specific-locus test shows ethylnitrosourea to be the most potent mutagen in the mouse. Proc. Natl. Acad. Sci. USA. 1979;76:5818–5819. doi: 10.1073/pnas.76.11.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura K, Low-Zeddies SS, King DP, Steeves TD, Whiteley A, Kushla J, Zemenides PD, Lin A, Vitaterna MH, Churchill GA, Takahashi JS. Genome-wide epistatic interaction analysis reveals complex genetic determinants of circadian behavior in mice. Genome Res. 2001;11:959–980. doi: 10.1101/gr.171601. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, DeCoursey PJ, Bauman L, Menaker M. Spectral sensitivity of a novel photoreceptive system mediating entrainment of mammalian circadian rhythms. Nature. 1984;308:186–188. doi: 10.1038/308186a0. [DOI] [PubMed] [Google Scholar]
- Van Reeth O, Turek FW. Stimulated activity mediates phase shifts in the hamster circadian clock induced by dark pulses or benzodiazepines. Nature. 1989;339:49–51. doi: 10.1038/339049a0. [DOI] [PubMed] [Google Scholar]
- Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowrey PL, McDonald JD, Dove WF, Pinto LH, Turek FW, Takahashi JS. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science. 1994;264:719–725. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]