Abstract
NFAT upregulation has been linked to cellular transformation intrinsically, but it is unclear whether and how tissue cells with NFAT activation change the local environment for tumor initiation and progression. Direct evidence showing NFAT activation initiates primary tumor formation in vivo is also lacking. Using inducible transgenic mouse systems, we show that tumors form in a subset of, but not all, tissues with NFATc1 activation, indicating that NFAT oncogenic effects depend on cell types and tissue contexts. In NFATc1-induced skin and ovarian tumors, both cells with NFATc1 activation and neighboring cells without NFATc1 activation have significant upregulation of c-Myc and activation of Stat3. Besides known and suspected NFATc1 targets, such as Spp1 and Osm, we have revealed the early upregulation of a number of cytokines and cytokine receptors, as key molecular components of an inflammatory microenvironment that promotes both NFATc1+ and NFATc1− cells to participate in tumor formation. Cultured cells derived from NFATc1-induced tumors were able to establish a tumorigenic microenvironment, similar to that of the primary tumors, in an NFATc1-dependent manner in nude mice with T cell deficiency, revealing an addiction of these tumors to NFATc1 activation and downplaying a role for T cells in the NFATc1-induced tumorigenic microenvironment. These findings collectively suggest that beyond the cell autonomous effects on the upregulation of oncogenic proteins, NFATc1 activation has non-cell autonomous effects through the establishment of a promitogenic microenvironment for tumor growth. This study provides direct evidence for the ability of NFATc1 in inducing primary tumor formation in vivo and supports targeting NFAT signaling in anti-tumor therapy.
Keywords: NFAT, oncogene, tumorigenesis, tumor, microenvironment
Introduction
The NFAT (the nuclear factor of activated T cells) family of transcription factors is important for organ development and cellular homeostasis. The four NFATc (c1-c4) proteins reside in the cytoplasm in quiescent cells. When intracellular Ca2+ increases, the serine/threonine phosphatase calcineurin dephosphorylates the NFATc proteins and exposes the concealed nuclear localization signals, leading to their cytoplasm to nuclear translocation. In the nucleus, NFATc proteins complex with their partners to control the transcription of target genes. Nuclear import and activation of NFATc are opposed by phosphorylation of NFATc by GSK3 and other NFAT kinases (1). However, when the serines targeted for phosphorylation by NFATc kinases and for dephosphorylation by calcineurin are changed to alanines, the modified NFAT proteins become constitutively nuclear and active (2).
Constitutively active NFATc1 was shown to induce transformation in preadipocytes (3). NFATc1 activation, as evidenced by its nuclear presence and/or dephosphorylation state, has been observed in multiple types of human lymphomas (4-6). The observation of ectopic activation of NFATc1 in pancreatic cancer and other cancer types suggests that NFAT activation may be used in a much wider set of tumors originated from cells with no normal expression of these proteins (7-9). Although NFAT genes have been linked to multiple aspects of various tumor types, direct evidence showing NFAT activation initiates primary tumor formation in situ is lacking. In addition, the cellular function of NFAT signaling appears to be multifaceted and context-dependent (10). Thus, the biological consequences of NFAT activation in different tissues may be very different and the mechanism by which NFAT affects tumorigenesis needs to be further investigated.
In this study, we generated a transgenic system in which NFATc1 activation can be controlled by the administration of Doxycycline (Dox) in targeted tissues. We have discovered that NFATc1 activation induces tumor formation in situ by promoting local cytokine production to create an inflammatory microenvironment for cells with NFAT activation and their neighbors without NFAT activation to participate in tumor formation. Between two models with overlapping NFATc1 activation domains in the skin, only the one with NFATc1 expression in follicle stem/progenitor cells produced skin tumors, suggesting progenitor cell involvement. These and other findings reported here provide mechanistic insights into the tumorigenic effects of NFAT activation beyond its reported and suspected roles in direct transcriptional regulation of oncogenes.
Results
Conditional activation of NFAT signaling results in tumors in specific sites
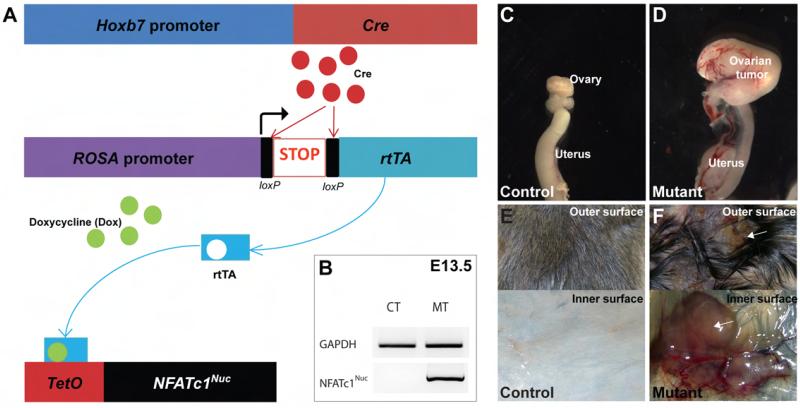
To study the role of NFAT signaling in urogenital organs, we created a transgenic model for inducible NFATc1 activation in cells targeted by the Hoxb7-Cre transgene (11, 12) that has known expression in the Wolffian duct, an embryonic structure providing progenitors for multiple urogenital organs (Fig. 1). In this system, Cre expression induces the removal of the transcriptional stop cassette in a ROSArtTA allele and the production of rtTA (reverse tetracycline-controlled transactivator). When doxycycline (Dox) is present, the Dox-rtTA complex binds to TetO of the TetO-NFATc1Nuc transgene (2) to induce the transcription of NFATc1Nuc (an activated form of NFATc1) (Fig. 1A). We refer to mice carrying all three alleles (Hoxb7-Cre, ROSArtTA, TetO-NFATc1Nuc) as mutants. Their littermates without the full set of three alleles cannot have NFATc1 activation even when treated with Dox and thus are regarded as controls. NFATc1Nuc transcripts were detected in Dox-treated mutants, but not in controls (Fig. 1B, E13.5 embryos, E: embryonic day). Dox-induced NFATc1 activation in Wolffian duct derivatives during embryogenesis results in congenital renal defects and reduced viability with incomplete penetrance in mutants (the renal defects will be described separately). We examined mutants that survived past weaning and found tumors in the urogenital systems of both genders and in the skin. In females, the tumors were in the ovary (Fig. 1C-D) while the male tumors were in the epididymis (data not shown). Since epididymal tumors are very rare in humans, we chose to perform most of the subsequent experiments in ovarian and skin tumors. The ovarian tumors can be noticed as early as at 3 weeks of age. 100% of the female mutants (n=8) with Dox treatment since E0 (Embryonic day 0) developed tumors in the ovary. In addition to urogenital tumors, Dox-treated mutants developed occasional skin tumors among numerous precancerous lesions (Fig. 1E-F). As early as 1 week after Dox treatment at P21, small lumps appeared randomly in skin throughout the body in ~98% of mutants (n=150). While most lumps stayed small, some of these apparent precancerous lesions continued to grow into tumors of substantial size under continuous Dox treatment. No control mice developed tumor at any sites.
Fig. 1. The Hoxb7-Cre transgene-mediated inducible activation of NFATc1 causes tumor formation.
A, The Hoxb7-Cre transgene directs the production of rtTA, which in turn, induces the transcription of NFATc1Nuc in the presence of Dox. B, RT-PCR detected transcripts of the NFATc1Nuc transgene only in the Dox-treated mutants. NFATc1 activation induced tumors in the ovary (C-D) and skin (E-F). Arrows in F point to the tumor mass.
NFATc1-induced tumors resemble ovarian sarcoma and skin squamous cell carcinoma in humans
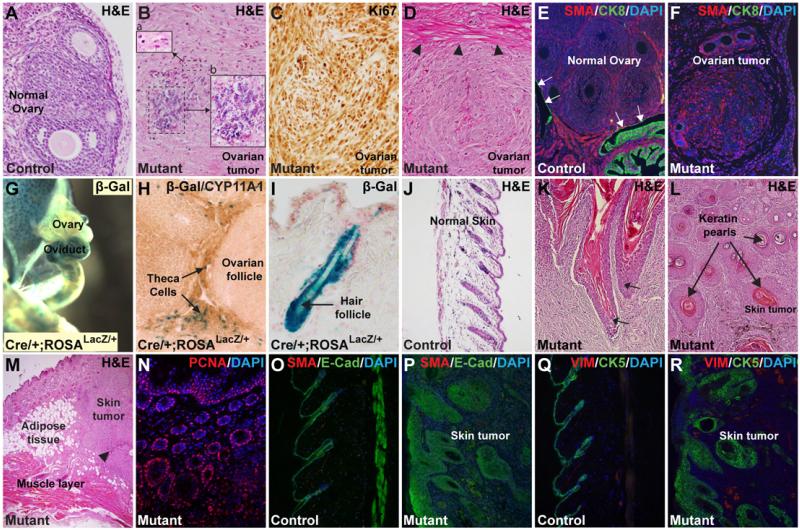
Cytologically, the ovarian tumors were comprised of spindle-shaped cells that bear morphologic resemblance to undifferentiated mesenchymal cells (Fig. 2A-F). Many tumor cells showed mild to moderate atypia. Regions of coagulative tumor cell necrosis were also present (Fig. 2B). These tumors have scattered mitotic figures on H&E stained sections and high mitotic index as revealed by Ki67 immunostaining (Fig. 2C). The tumors showed destructive stromal invasion within the ovary and are associated with desmoplastic responses (Fig. 2D). The ovarian tumor cells strongly expressed mesenchymal markers, including α-smooth muscle actin (αSMA). There was insignificant expression of epithelial markers, such as Cytokeratin 8 (CK8) (Fig. 2E-F) and E-Cadherin (Not shown) in the tumor. Taken together, the constellation of findings (mesenchymal cellular morphology and marker expression, high mitotic index, atypia, necrosis, desmoplasia, destructive stromal invasion, and others) indicate that the ovarian tumors in these mice resemble malignant ovarian stromal tumors (13), rather than benign ovarian fibroma, in humans. The ovarian tumors came as a surprise to us at first because there was no previous report of Hoxb7-Cre expression in the ovary. By crossing the Hoxb7-Cre transgene to the ROSALacZ reporter mice, we found β-Galactosidase (β-Gal) activity, reflecting Cre expression, in the ovarian stroma containing the theca cells and other SMA+ stromal cells surrounding the ovarian follicles (Fig. 2G-H and data not shown). These tumors were CD10−, unlike some of the ovarian sarcomas with an endometrial stromal origin. These findings are consistent with the hypothesis that the ovarian tumors found in the mutants originated from the ovarian stromal cells with NFATc1 activation.
Fig. 2. NFATc1-induced tumors resemble ovarian sarcoma and skin squamous cell carcinoma in humans.
Unlike the normal ovarian structures (A), NFATc1-induced ovarian tumors are filled with spindle-shaped cells (B-F). These tumors have cellular atypia (B, inset-a) and necrotic zones (B, inset-b). Rectangular area in B are enlarged in the insets a and b. These tumors have high proliferation rates as indicated by extensive Ki67 immunostaining (C). These tumors have desmoplastic zones (arrowheads in D), where adjacent tissues react to the rapid expansion of the tumor. The ovarian tumors have abundant mesenchymal cells expressing αSMA with few epithelial cells expressing Cytokeratin 8 (CK8) (E-F). Arrows in E indicate epithelial expression of CK8 in the normal ovary. As revealed by β-Galactosidase (β-Gal) assays, Hoxb7-Cre is expressed in ureter, ovary, oviduct (G-H), and hair follicles (I). Inside the ovary, Hoxb7-Cre is expressed in the stroma containing the theca cells (H).The skin tumors have keratin pearls similar to those seen in human squamous cell carcinoma (SCC) (J-N). Arrows in K point to areas where the hyperplastic hair follicles invading the dermis layer of the skin (K). Arrows in L indicate presence of keratin pearls. These tumors can invade the subcutaneous adipose layer beneath the dermis and sometimes reach the muscle layer (M). Arrowhead in M indicates an area of necrosis. High proliferation rate in the skin tumor is evident by the extensive presence of PCNA+ cells (N). Immunostaining with epithelial markers E-Cadherin (E-Cad) and Cytokeratin 5 (CK5) reveals the expansion and dermal invasion of the skin tumor cells (O-R). Unlike the ovarian tumors, most cells in the skin tumors have epithelial characteristics with only a small number of cells expressing mesenchymal markers SMA and Vimentin (VIM) (O-R).
Hoxb7-Cre expression, as reflected by β-Gal activity, was also detected in the skin, primarily in hair follicle cells (Fig. 2I). Histopathological analyses showed that the skin tumors diffusely invaded the dermis, the subcutaneous fat, and superficially into the subadjacent connective tissues and skeletal muscle (Fig. 2K-M). Geographic necrosis was present (arrowhead in Fig. 2M). These skin tumors have keratin pearls typical of human cutaneous squamous cell carcinoma (SCC), though vague rudimentary structures resembling skin adnexal structures were present (Fig. 2L). Similar to the ovarian tumors, these skin tumors were highly proliferative, as revealed by PCNA staining (Fig. 2N). Many cells in the skin tumors expressed the epithelial marker E-Cadherin (E-Cad, Fig. 2O-P). Immunostaining with Cytokeratin-5, a diagnostic marker for human SCC, further confirms the extensive presence of cells of epithelial origin in the tumor proper and its resemblance to human SCC (Fig. 2Q-R). Although both the skin tumor and ovarian tumors showed histologic features of malignancy and local invasion, no metastasis was observed in the mutants we have examined. Since most mutants died from tumor burden and other comorbidities associated with NFATc1 activation in other tissues, it is currently unknown if any of the NFATc1-induced tumors will become metastatic if given sufficient time to progress in these mice. Although epididymal tumors were not extensively characterized in this study, Hoxb7-Cre expression in the epididymal epithelia, a Wolffian duct derivative, is well established (11, 12), making these tumors most likely primary tumors induced by NFATc1 activation but not metastasis from other sites. Besides the three sites mentioned, tumors have not been found in other places with known Hoxb7-Cre expression, including neuronal tissues, renal collecting duct, and ureteral epithelia. It appears that the oncogenic effects of NFATc1 activation are tissue/cell type-specific.
Tissue stem cells/progenitor cells participate in NFATc1-induced tumorigenesis
In the skin, stem cells are known to reside in the hair follicle bulge and express CD34 and NFATc1 [(Fig. 3A) and (14)]. Although NFATc1 expression appeared stronger in the CD34+ bulge stem cells in the Dox-treated mutants, the NFATc1 antibody could not distinguish between the endogenous NFATc1 and the transgene-derived NFATc1Nuc proteins (Fig. 3B). We thus examined the presence of the HA tag fused to the NFATc1Nuc protein that specifically recognizes the transgene product. HA signal was absent in the control without transgene expression (Fig. 3C) but present in the CD34+ bulge stem cells in the Dox-treated mutant (Fig. 3D), further confirming that the Cre expression targets CD34+ stem cell populations in the hair follicles as shown by β-Gal activity in Fig. 2I. Co-immunostaining of mutant hair follicles with NFATc1 and HA antibodies showed that most NFATc1+ cells were also HA+ (Fig. 3E-F). In addition, the skin tumors contained a large number of cells that co-express NFATc1 and CD34, indicating a potential abnormal expansion of the skin stem cell population within the tumor (Fig. 3G-I), likely as a direct effect of transgene-induced NFATc1 activation in the CD34+ hair follicle stem cells. Interestingly, while a high percentage of cells within the tumor expressed NFATc1, the cells surrounding the keratin pearls were exclusively devoid of NFATc1 and CD34 (Fig. 3H). Keratin-14 (K14), a known diagnostic marker for SCC, had extensive expression in NFATc1-induced SCC-like tumors, especially in cells surrounding the keratin pearls (Fig. 3J). A potential explanation for these data is that cells with NFATc1 activation did not participate in, but created the environment for, the formation of the keratin pearls and that multiple lineages of cells participated in the development of the SCC-like tumor with NFATc1+, CD34+ cells expanding and acting as tumor inducing cells for NFATc1− cells at the same time.
Fig. 3. Tissue stem cells/progenitor cells may participate in NFATc1-induced tumorigenesis.
The CD34+ hair follicle stem cells in the bulge expressed NFATc1 in both the control and mutant (A and B). Although the expression of NFATc1 appeared more intense in some mutant cells, definitive identification of transgene-produced NFATc1Nuc relies on the detection of the transgene-specific HA tag (C-F). There was no HA staining in the controls (C). In the mutants, most CD34+ bulge stem cells were HA+ (D) and most NFATc1+ cells in the hair follicles were HA+ (E-F). NFATc1 and CD34 had extensive co-expression in the skin tumor but were both absent in cells surrounding the keratin pearls (G-I). Keratin 14 (K14) had extensive expression in the skin tumor and was present in the NFATc1−, CD34− cells surrounding the keratin pearls (J). CD44+, NFATc1+ cells were present in hair follicle bulge (K). Many CD44+, NFATc1+ cells were present in the skin tumor (L-N). Keratin pearls were exclusively CD44+, K14+ (O). The Msx2-rtTA transgene was expressed in hair follicle matrix cells and hair shaft excluding the bulge where stem cells reside (P).The Msx2-rtTA-driven NFATc1 activation did not elicit tumorigenesis in the skin of mutants treated for 3 months since weaning (Q, R).Immunostaining of human SCC sections shows the upregulation of NFATc1, compared to normal human skin sections (S-T). Inset in T shows the NFATc1 immunostaining in higher magnification.
CD44 is expressed in multiple areas in normal skin, including bulge cells with NFATc1 expression (Fig. 3K). Co-expression of CD44 and NFATc1 was widely seen in mutant hair follicles and NFATc1-induced skin tumors (Fig. 3L-M), likely due to the expansion of the NFATc1+, CD44+ hair follicle cells. However, CD44 was also present in cells within the keratin pearls that are NFATc1− (Fig. 3N-O). Since CD44 has expression in other skin cells, such as keratinocytes ((15) and data not shown), NFATc1 is probably acting through cell autonomous effects on CD44+, NFATc1+ cells for their expansion and through non-cell autonomous effects on CD44+, NFATc1− cells (likely including keratinocytes) for their participation in the keratin pearls. In a separate model (Msx2-rtTA; TetO-NFATc1Nuc) where NFATc1Nuc expression was Dox-induced in hair follicle matrix cells and hair shaft excluding the bulge, no skin tumor was observed (Fig. 3P-R and (16)). Since the key difference in the targeted cell population between the two models was the presence of NFATc1Nuc in bulge stem cells in the Hoxb7-Cre; ROSArtTA; TetO-NFATc1Nuc model, these results suggest that the abnormal activation of bulge stem cells could play an important role in the tumorigenesis of the SCC-like skin tumor.
To assess the prevalence of NFATc1 expression in the human SCC, we performed NFATc1 immunostaining on human SCC tissue arrays that consisted of human SCC specimens histopathologically confirmed by board certified pathologists. Among 42 human SCC specimens investigated, we observed NFATc1-expressing cells in 24 (57% of the total) specimens (Fig. 3S-T). 12 control samples analyzed show no NFATc1 expression beyond that in the hair follicle bulge (Fig. 3S-T). Thus, NFATc1 expression appears to be associated with a substantial percentage of the human SCCs we examined.
Activation of oncogenic proteins in NFATc1− cells appears to result from non-cell autonomous mechanisms
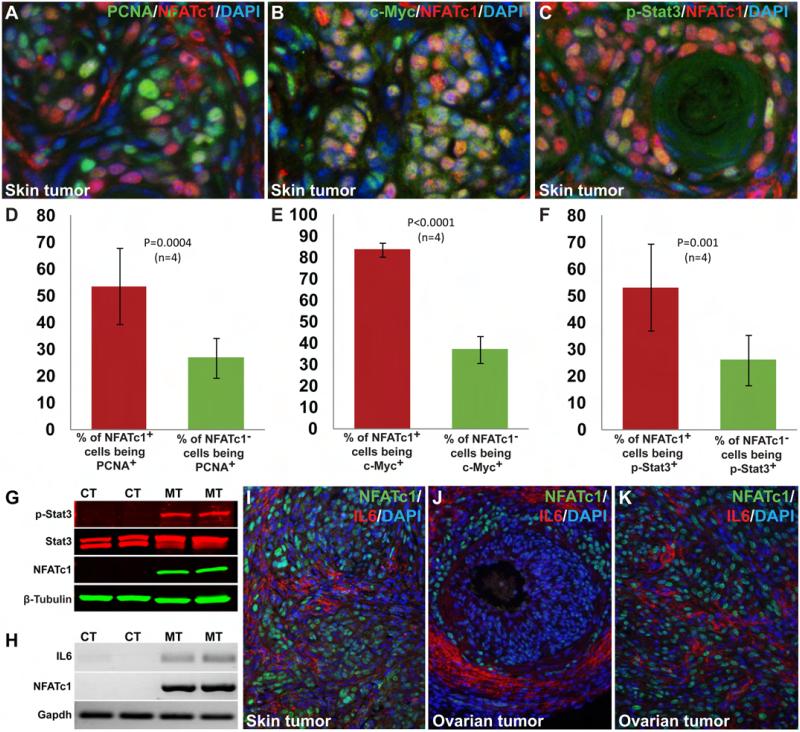
Although NFATc1+ cells were extensively present in the NFATc1-induced tumors, not all NFATc1+ cells were proliferating while many neighboring NFATc1− cells were undergoing active proliferation. In the example of a skin tumor given in Fig. 4A and D, 53.67±14.22% of NFATc1+ cells in the tumor were proliferating (PCNA+). Within the same tumor, a smaller but still substantial percentage (26.92±7.43%) of the NFATc1− cells were also proliferating (Fig. 4D). NFATc1 activation in these tumors had induced extensive c-Myc expression (Fig. 4B). While about 83.74±3.17% NFATc1+ cells were expressing c-Myc, 37.07±6.42% NFATc1− cells also had c-Myc expression (Fig. 4E). Although earlier reports have suggested that NFATc1 can directly regulate c-Myc expression (7), our finding of c-Myc activation in a significant percentage of NFATc1− cells raised the possibility that NFATc1 activation may also regulate c-Myc expression in a non-cell autonomous fashion through paracrine actions. By using an antibody targeting phospho-Stat3 (Tyr705), we found that 53.14±16.23% NFATc1+ cells and 26.18±9.42% NFATc1− cells had nuclear phosphorylated Stat3 in NFATc1-induced tumors (Fig. 4C, F). Immunoblotting of lysates from normal skin and NFATc1-induced skin tumors showed that the level of phospho-Stat3 was greatly increased in NFATc1-induced skin tumors, though total Stat3 level was unchanged (Fig. 4G). Since NFATc1 is a transcription factor, the observed upregulation of c-Myc and Stat3 activation in NFATc1− cells reflects indirect non-cell autonomous effects of NFATc1 activation, likely initiated by the secretion of factors that have paracrine functions.
Fig. 4. High level of heterogeneity in the tumors and the non-cell autonomous promitogenic effects of NFATc1 activation.
Immunostaining using NFATc1 and PCNA as well as the subsequent statistical analyses (two-tailed t-test) indicated that a high percentage of NFATc1+ cells were actively proliferating (53.67±14.22%). The percentage of proliferating NFATc1− cells (26.92±7.43%, P=0.0004, n=4), albeit smaller, was very substantial (A, D). NFATc1 activation led to significant upregulation of c-Myc in the tumor tissue (B, E). 83.74±3.17% of the NFATc1+ cells expressed c-Myc, whereas only 37.07±6.42% NFATc1− cells were c-Myc+ (P<0.0001, n=4) (E). Double immunostaining with NFATc1 and phospho-Stat3 antibodies showed higher percentage of NFATc1+ cells with nuclear Stat3 (53.14±16.23%) than NFATc1− cells with nuclear Stat3 (26.18±9.42%, P=0.001, n=4) (C, F). Western blot analysis of normal skin lysates from control mice and skin tumor from mutant mice was done by using an antibody against Stat3 (revealing the level of Stat3 proteins regardless of phosphorylation status) and an antibody against phospho-Stat3 (Tyr705, revealing the level of activated Stat3). The results showed activation of Stat3 specifically in NFATc1-induced skin tumors while the level of total Stat3 was unchanged (G). NFATc1 activation specifically up-regulated IL6 in the tumors as revealed by RT-PCR (H). IL6 upregulation was also detected by immunostaining in skin (I) and ovarian tumors (J-K). CT; control, MT; mutant.
Since NFATc proteins are known regulators of a number of cytokines and IL6, in particular, has been shown to regulate Stat activation in tumors (17), we sought to investigate the potential role of IL6 and other cytokines in mediating the effects of NFATc1 activation. By RT-PCR, we showed that NFATc1 activation was accompanied by an increased IL6 transcription in skin tumors (Fig. 4H). Areas of high levels of IL6 are found in both skin and ovarian tumors (Fig. 4I-J). However, many areas with high IL6 levels were populated primarily by NFATc1− cells (Fig. 4I-J), suggesting that NFATc1 did not directly upregulate IL6 transcription in these cells. Although some IL6 may be secreted by infiltrating immune cells, high IL6 levels can be seen in local resident non-immune cells (Fig. 4I-K and data not shown). Thus, NFATc1+ cells appeared to signal their neighboring cells, likely through a paracrine action, to secrete IL6. The upregulation of IL6, and possibly other cytokines, provides an explanation to the observed wide spread nuclear presence of Stat3 in these tumors (Fig. 4C, F, G). These findings suggest that a major effect of NFATc1 activation is the creation of a promitogenic local environment for both the tumor initiating cells and their neighbors to contribute in tumor formation.
Allografts of NFATc1-induced tumors showed continuous dependency on NFATc1 for tumor progression and survival but not on T cell functions
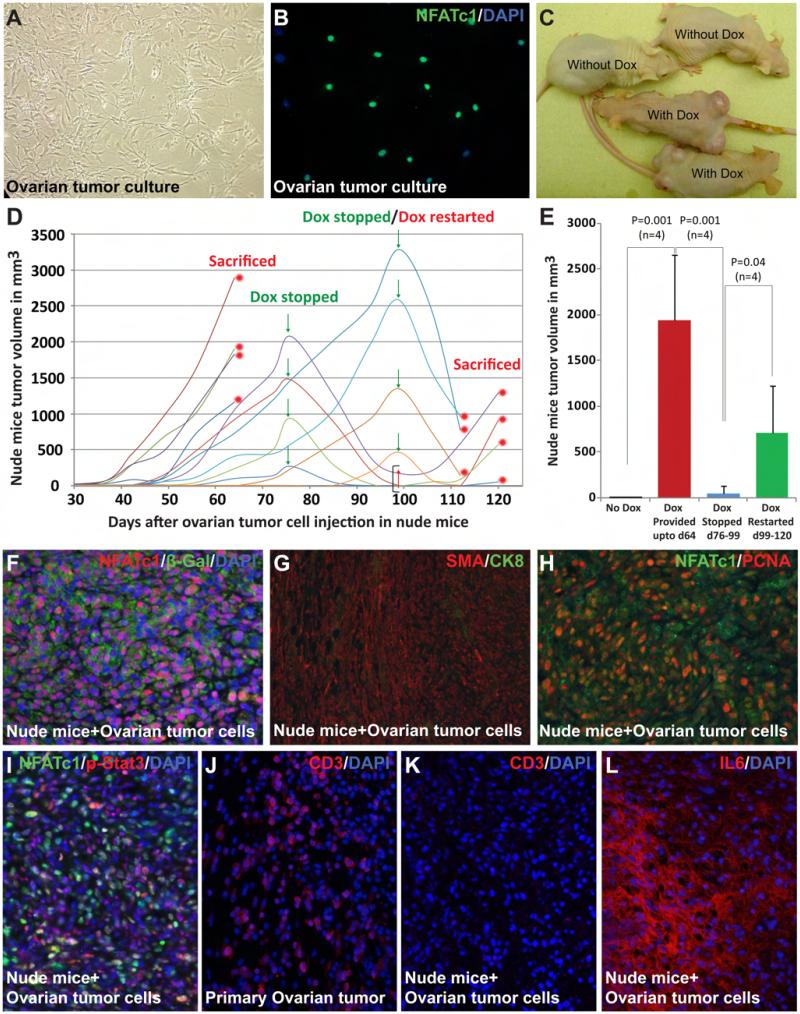
To directly test the essential role of NFATc1 activation in tumorigenesis and to examine the involvement of T cells in establishing the tumorigenic microenvironment, we studied the ability of the cells with NFATc1 activation to initiate tumorigenesis in athymic nude mice with severe T cell deficiency. We first cultured the cells from an NFATc1-induced ovarian tumor (Fig. 5A-B). These cells were then injected to the rear flanks of nude mice. Tumor growth was detected at about six weeks after the injection in the Dox-treated recipient mice, but not in untreated ones (Fig. 5C). To further test the dependency of tumor growth and progression on NFATc1 activation, we stopped Dox treatment in a subgroup of these mice. Existing tumors started to shrink within days after Dox withdrawal (Fig. 5D). Such trend was reversed when NFATc1 activation was restored (Fig. 5D-E), indicating a continuous dependency of the tumors on NFATc1 activation, similar to that seen in cases of oncogene addiction (18, 19). The tumor allografts retain some key characteristics of the primary tumors in many aspects, including the presence of a large number of NFATc1+ cells (Fig. 5F). These allografts were also primarily composed of αSMA+ mesenchymal cells (Fig. 5G). Similar to the primary tumor, NFATc1+ and NFATc1− cells intermingled and were both actively proliferating (Fig. 5H). NFATc1 activation also stimulated Stat3 activation in both NFATc1+ and NFATc1− cells (Fig. 5I). While T cells are seen in the primary tumor, the grafts grown in nude mice are completely void of T cells (Fig. 5J-K). Since inflammatory cytokines, such as IL6, are similarly upregulated in the grafts, the establishment of the inflammatory tumorigenic microenvironment is thus independent of T cells (Fig. 4J-K, 5L, and data not shown) but likely dependent on local tissue resident cells and other immune cells in this model.
Fig. 5. Allografts of NFATc1-induced tumors showed continuous dependency on NFATc1 for tumor progression and survival but not on T cell functions.
Ovarian tumors from mutants were isolated and cultured (A). Most of the cultured cells expressed NFATc1 (B). Cultured tumor cells were injected subcutaneously into the lower flanks of nude mice. 100% of the Dox-treated recipients developed tumors, whereas none of the untreated mice did (C). Termination of Dox treatment resulted in significant decrease of tumor size. Such decrease was reverted again if Dox treatment was restarted (D). Statistical analysis (two-tailed t-test) showed a significant increase in tumor volume when Dox was continuously provided up to 64 days (1940.54±714.34 mm3, P=0.001, n=4). Conversely, a significant decrease in tumor volume (40.80±81.60 mm3) was observed when Dox was interrupted from day 76 to day 99 (P=0.001, n=4). After Dox was restarted on day 99, tumor volume increased again and reached 702.34±523.30 mm3 (P=0.04, n=4) on day 120 (E). Tumor size was measured at the end of each interval. Immunostaining of tumors from nude mice indicated that the majority of tumor cells were β-Gal+ (F). The allografts in the nude mice resembled the original tumors in overall morphology, cellular composition (G), and the substantial levels of proliferation and Stat3 activation in NFATc1− cells (H-I). By immunostaining with an anti CD3 antibody, T cells were seen in primary ovarian tumor but not in the grafts grown in nude mice (J-K). Extensive IL6 expression was noticed in the grafts in the nude mice (L).
Key molecular components of a proinflammatory microenvironment induced by NFATc1 activation
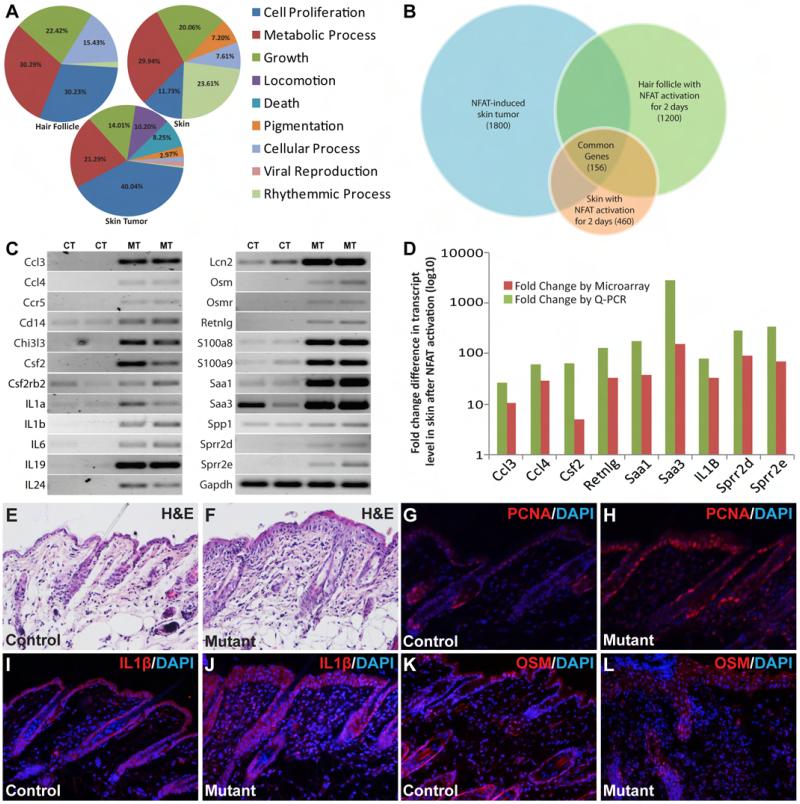
To capture the broader picture of molecular changes related to the oncogenic effects of NFATc1 activation and to further characterize the molecular components in the microenvironment of NFATc1-induced tumors, we performed whole genome transcript profiling on three sets of samples: hair follicles and skin collected from mutant and control mice treated with Dox for 2 days as well as NFATc1-induced skin tumors and normal skin from similarly treated littermate controls. In each sample sets, GO (gene ontology) analysis for biological process indicated that most of the significant changes (fold change > 2, P < 0.05) were in genes related to cell proliferation, growth, and metabolic processes, all of which are important for tumor initiation and progression (Fig. 6A). 156 significantly changed genes were present across all three data sets (Fig. 6B). Many chemokines/cytokines-related genes were among the most significantly changed genes. These and other selected genes with high fold changes were subjected to RT-PCR analysis (Fig. 6C) with a subset further confirmed by Q-PCR (Fig. 6D). All genes examined by Q-PCR showed increased expression at levels similar to those seen in microarray analyses.
Fig. 6. Transcript profiling revealed that NFATc1 activation creates a proinflammatory microenvironment for tumor development.
Gene ontology analysis revealed that the top categories for genes with significant transcriptional changes include cell proliferation, growth, and metabolic processes (A). Venn diagram showed 156 genes commonly changed across all three sets (B). A number of the secretory factors were among the genes with the most significant changes. These and other significantly changed genes with potential links to NFAT signaling were subjected to further RT-PCR using skin from mutant and control mice treated with Dox for 48 hours (C). CT; skin from control mice, MT; skin from mutant mice. Some of these genes were further validated by Q-PCR, which shows similar trend of expression change in mutant skin as suggested by microarray (D). H&E and PCNA staining showed hyper-proliferative mutant skin epidermis after 2 days of NFATc1 activation compared to control samples (E-H). Immunostaining showed more extensive expression of IL1β and OSM in skin from mutant mice compared to their littermate controls (I-L).
Besides cytokines, a number of genes with the largest fold changes are related to acute inflammation, innate immune response, and recruitment of lymphocytes (SAA3, SAA1, LCN2, and RETNLG), as well as to keratinization of the skin (SPRRs, EG433016, CHI3Ls, KRTs) (Supplementary Tables 1-3). These results are consistent with the histological findings of keratin pearls and the extensive presence of cells with K14 expression in skin tumors. We found that the skin epidermis became hyper-proliferative after 2 days of NFATc1 activation in mutants (Fig. 6E-F). Increased levels of proliferation, as evidenced by PCNA staining, was observed in the basal layer of the epidermis and in the outer root sheath cells of the hair follicle in the mutants 2 days after NFATc1 activation (Fig. 6G-H). Since transgene expression was not detected in some of these cells, the hyperproliferation in these NFATc1− cells again pointed to non-cell autonomous effects of NFATc1 activation, most likely through alterations in microenvironment. By immunostaining, we have identified more extensive expression of IL1β in skin from mutant mice compared to controls 2 days after NFATc1 activation (Fig. 6I-J). Similar increases in Oncostatin M (OSM) levels were observed in mutant skin (Fig. 6K-L). The extensive presence of proinflammatory cytokines at very early stage of NFATc1 activation suggests that tissue resident cells are capable of initiating the establishment of a promitogenic environment before significant infiltration of immune cells. The cytokine cascades within these local cells and subsequently in infiltrating immune cells may support the continuous evolution of the tumorigenic microenvironment and the progression of these tumors.
Discussion
We have previously shown that Calcineurin-NFAT signaling is involved in the development of multiple organ systems and in the cellular responses to extracellular environment (1, 20-23). As we continue these investigations, we serendipitously discovered that the activation of NFAT signaling in vivo can cause tumor formation in a subset of tissues, including the ovary and skin. Our studies on the primary and the transplanted tumors provide direct in vivo evidence that NFATc1 activation is sufficient for initiating tumorigenesis and is necessary for the progression of the tumors formed. At least in the systems we tested, NFATc1 can behave as a pleiotropic oncogene and tumors caused by its activation remain addicted to it.
Although the effects of NFATc1 activation may differ in different tissues, it is common for the NFATc1-induced tumors we studied, as well as the tumor allografts in the nude mice, that NFATc1 activation has effects beyond the cells where the activation occurs. Although c-Myc upregulation and Stat3 activation have been observed in some, but not all, NFATc1+ cells in NFATc1-induced tumors, many NFATc1− cells within the tumor also show upregulation of c-Myc and activation of Stat3 (Fig. 4B-C, E-F). These findings suggest that the role of NFAT goes beyond cell autonomous effects in ways of direct regulation of c-Myc transcription and Stat3 activation. Combined with our analyses of the molecular and cellular components of the microenvironment in these tumors, a picture emerges that factors secreted by the NFATc1+ cells initiate the establishment of an inflammatory and promitogenic tissue environment for both NFATc1+ and NFATc1− cells to proliferate and become part of the tumor. From our comparison of two models with or without hair follicle stem cell involvement, it appears that both the cell autonomous effects of NFATc1 activation and the alterations in tissue microenvironment may affect the stem cells/progenitor cells and their niches, leading to their participation in tumor formation.
One intriguing question is the nature of this secreted factor(s) that has the autocrine/paracrine function. This secreted factor is most likely a major initiating factor for, and a component of, the proinflammatory and promitogenic microenvironment. Our finding of elevated IL6 transcript level in the tumors led to the suspicion of IL6 fulfilling the role of such a secreted factor (Fig. 4H). However, further immunostaining of the tumor tissues, especially tissues from early stage tumors, revealed distinct populations of cells expressing NFATc1 and IL6, respectively (Fig. 4I-K). Thus, it appears that the upregulation of IL6 is itself induced by this secreted factor(s). Transcript profiling suggested the presence of proinflammatory microenvironment in the very early stages after NFATc1 activation (Fig. 6). There is growing evidence supporting the role of proinflammatory cytokines in tumor progression (24). Upregulation of a number of proinflammatory cytokines (Figures 4 and 6), as a result of NFATc1 activation, can provide the mitogenic signals for both NFATc1+ and NFATc1− cells to proliferate. The ability of the NFATc1+ cells to recruit neighboring NFATc1− cells into the formation of tumors (beyond the vasculature) also makes the NFATc1+ cells behave like tumor-initiating cells. Whether the cytokine genes are direct or indirect targets of NFATc1 is still under investigation. It is also possible that one of the cytokine genes is directly regulated by NFATc1 and its upregulation initiates a cascade of cytokine production, leading to the establishment of the proinflammatory and promitogenic environment.
NFATc1 is normally expressed in the CD34+ hair follicle bulge stem cells. Inhibition of calcineurin/NFATc1 signaling has been shown to relieve the NFATc1-mediated repression of CDK4 and leads to stem cell activation and hair growth (14). In the Hoxb7-Cre, ROSArtTA, TetO-NFATc1Nuc mutants, NFATc1 activation in hair follicle stem cells led to apparent expansion of the CD34+ stem cells and the development of SCC-like skin tumor (Fig. 3H-I). The involvement of the stem cells in this model is further supported by the absence of skin tumors in the Msx2-rtTA, TetO-NFATc1Nuc mutants where NFATc1 activation is in an overlapping set of skin cells excluding the bulge stem cells. These results seem contradictory to the finding by Horsley et al. on surface. However, it is well known that the calcineurin/NFAT pathway operates through establishing a balanced signaling with additional inputs from other pathways in different cellular contexts (25, 26). CDK2 and CDK4 have been found to be dispensable for cell cycle progression, likely due to redundancy (27, 28). In the tumor model, the key for stem cell expansion may be in the alteration of the stem cell microenvironment/niche. This is consistent with the finding that escape of hair follicle stem cells from growth restrictive niches was a key process in wounding-induced tumorigenesis in skin (29). The expansion of the CD34+ stem cells can only partially explain the NFATc1-induced skin tumor formation that may involve a complex set of sequential or parallel processes, including the induction of other cell types to build structures like the keratin pearls and the possible differentiation of the CD34+ stem cells into other cell types.
Upregulation of NFATc1 expression has been reported previously in a number of human malignancies (10). Our finding in human SCC specimens further expands the tumor types in which significant NFATc1 expression can be found in tumor cells and may contribute to the initiation and progression of these tumors. Since our study indicates that NFATc1 activation has pleiotropic effects on the cells expressing it and on the neighboring cells in the context of alterations in a number of signaling pathways and in the microenvironment, inhibiting NFATc1 activation could be more effective than targeting one or more of the downstream pathways and factors in treating tumors with NFATc1 activation. The oncogene addiction revealed in the tumor allograft study further suggests that NFAT signaling may be an attractive target for anti-tumor therapy.
Materials and Methods
Mouse (Mus musculus) strains and Doxycycline (Dox) treatment
All animal studies were approved by the Washington University Animal Studies Committee and have been conducted according to relevant NIH guidelines. The Hoxb7-Cre, ROSArtTA, TetO-NFATc1Nuc strains and the relevant genotyping methods were described previously (2, 11, 23, 30). Nude mice (Foxn1nu, Harlan) were used for tumor grafting at 6-8 weeks old. Dox was given through drinking water provided ad libitum at 2 mg/ml. Dox treatment was started at conception and at birth through water intake by the mother or at 21 days postnatally by the targeted mice.
Primary tumor cell culture
For primary tumor cell culture, ovarian tumors from 5-week-old Hoxb7-Cre; ROSArtTA; TetO-NFATc1Nuc mice were harvested and cut into small pieces of <1mm and cultured in Dulbecco’s Modified Eagle’s Medium-F12 (10% FBS, 5% penicillin/streptomycin and 2μg/ml Dox). Cells grew out of the tumor tissue chunks were fed with fresh media every 2-3 days, and subcultured before confluence. Cultured cells were tested for nuclear NFATc1 expression in the presence of Dox.
Tumor growth in nude mice
For allografts, aforementioned cultured tumor cells (<15 passages) were grown to 70-80% confluence, trypsinized, and re-suspended in phosphate-buffered saline. 3×106 cells were injected subcutaneously into lower flanks of 8 nude mice. Group 1 mice were untreated. Groups 2-4 were Dox-treated from the day of injection. Group 2 was sacrificed on the 64th day. Dox was stopped for group 3 on the 76th day and restarted on the 99th day. Group 3 mice were finally sacrificed on the 127th day. Dox was stopped for group 4 on the 99th day and the mice were sacrificed on the 113th day. Tumor volume was determined using a previously described formula (31).
Histology, Immunostaining, and Western blotting
Tissues were fixed with 4% paraformaldehyde and embedded in paraffin. Seven μm sections were collected and stained with H&E. Human tissue microarrays were obtained from Biomax (Biomax Inc, Rockville, MD). X-Gal staining and Immunostaining on cryostat sections were performed as described (21, 32). Antibodies used were: rabbit anti-CYP11a1 (Chemicon AB1294, 1:200), mouse monoclonal anti-αSMA (Sigma A2547, 1:200), rat anti-Cytokeratin 8 (TROMA-1, Developmental Studies Hybridoma Bank, 1:200), mouse monoclonal anti-NFATc1 (Pharmingen 556602, 1:100), mouse monoclonal anti-CD3 (DAKO A0452, 1:200), rat monoclonal anti-CD34 (Abcam ab8158, 1:50), rat monoclonal anti-CD44 (BD Pharmingen 558739, 1:50), rabbit monoclonal anti E-Cadherin (Cell Signaling 3195, 1:200), rabbit polyclonal anti-IL6 (Abcam ab6672, 1:600), rabbit polyclonal anti-PCNA (Bethyl Labs, IHC-00012, 1:200), rabbit polyclonal anti-cMyc (Abcam ab39688, 1:200), rabbit monoclonal anti-Stat3 (Cell Signaling 4904, 1:50), rabbit polyclonal anti-Phospho Stat3 (Cell Signaling 9131, 1:100), chicken polyclonal anti-β-Galactosidase (Abcam 9361, 1:100), rabbit anti-β-Catenin (Sigma C2206, 1:200), rat monoclonal anti-PECAM (BD Pharmingen 550274, 1:100), rabbit polyclonal anti-IL1β (Santa Cruz Biotechnology sc7884, 1:50), goat polyclonal anti-OSM (R&D Systems af495na, 1:50). Appropriate Alexa Flour488 or 555-conjugated secondary antibodies (Molecular Probes, 1:1000) were used for detection. For Western blotting, protein extracts were separated by SDS-PAGE, transferred to Immobilon-P membranes (Millipore), and probed by the appropriate primary antibodies. After incubation with Alexa680-conjugated goat anti-rabbit IgG (Molecular Probes) and IRDye800CW-conjugated goat anti-mouse IgG (Rockland) secondary antibodies, antibody complexes were visualized by an Odyssey Infrared Imaging System (LI-COR) (32).
RT-PCR, Microarray, and Q-PCR
Total RNA was isolated using Trizol reagent (Invitrogen) and purified with RNeasy Mini Kit (Qiagen). cDNA was prepared from RNA using Invitrogen ThermoScript™ RT-PCR System (Invitrogen). The detailed information about the primer sequences and PCR conditions are provided as Supplemental Table-4. For transcript profiling, three pairs of control and mutant mice were treated with Dox for 48 hours before hair follicles and dorsal skin patches were harvested. In addition, three skin tumors from mutants treated with Dox from P21 for 3-4 weeks and three normal skin samples from similarly treated control mice were also collected. Microarray was done on MouseWG-6 v2.0 Expression BeadChips (Illumina) at the Genome Institute at Washington University in St Louis. Results were analyzed using the Partek Genomics Suite (Partek). Genes with significant changes (≥2 fold and P<0.05) were further validated by quantitative PCR (Q-PCR). cDNA was generated with oligo-dT primers using the Superscript III Kit (Invitrogen). Real-time quantification was done using a SYBRGreen PCR kit (Life Technologies). Relative gene expression was deduced using the ΔΔCt method.
Supplementary Material
Acknowledgements
We thank Dr. Crabtree for providing the TetO-NFATc1Nuc mice, Dr. Yuan Zhu for helpful discussion of the study. F.C. is supported in part by institutional funds from the Department of Medicine at Washington University School of Medicine and NIH grants (DK81592 and DK67386). We also thank the Georg M. O’Brien Center for Kidney Disease Research at Washington University (P30DK079333) for core services. We thank Michael Heinz from the Genome Technology Access Center (P30 CA91842, UL1RR024992) in the Department of Genetics at Washington University School of Medicine for help with gene expression analysis.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Oncogene website
References
- 1.Graef IA, Chen F, Crabtree GR. NFAT signaling in vertebrate development. Curr Opin Genet Dev. 2001;11(5):505–12. doi: 10.1016/s0959-437x(00)00225-2. [DOI] [PubMed] [Google Scholar]
- 2.Pan M, Winslow MM, Chen L, Kuo A, Felsher D, Crabtree GR. Enhanced NFATc1 Nuclear Occupancy Causes T Cell Activation Independent of CD28 Costimulation. J Immunol. 2007;178(7):4315–21. doi: 10.4049/jimmunol.178.7.4315. [DOI] [PubMed] [Google Scholar]
- 3.Neal JW, Clipstone NA. A constitutively active NFATc1 mutant induces a transformed phenotype in 3T3-L1 fibroblasts. J Biol Chem. 2003;278(19):17246–54. doi: 10.1074/jbc.M300528200. Epub 2003/02/25. [DOI] [PubMed] [Google Scholar]
- 4.Marafioti T, Pozzobon M, Hansmann ML, Ventura R, Pileri SA, Roberton H, et al. The NFATc1 transcription factor is widely expressed in white cells and translocates from the cytoplasm to the nucleus in a subset of human lymphomas. Br J Haematol. 2005;128(3):333–42. doi: 10.1111/j.1365-2141.2004.05313.x. Epub 2005/01/26. [DOI] [PubMed] [Google Scholar]
- 5.Medyouf H, Alcalde H, Berthier C, Guillemin MC, dos Santos NR, Janin A, et al. Targeting calcineurin activation as a therapeutic strategy for T-cell acute lymphoblastic leukemia. Nat Med. 2007;13(6):736–41. doi: 10.1038/nm1588. Epub 2007/05/23. [DOI] [PubMed] [Google Scholar]
- 6.Pham LV, Tamayo AT, Yoshimura LC, Lin-Lee YC, Ford RJ. Constitutive NF-kappaB and NFAT activation in aggressive B-cell lymphomas synergistically activates the CD154 gene and maintains lymphoma cell survival. Blood. 2005;106(12):3940–7. doi: 10.1182/blood-2005-03-1167. Epub 2005/08/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchholz M, Schatz A, Wagner M, Michl P, Linhart T, Adler G, et al. Overexpression of c-myc in pancreatic cancer caused by ectopic activation of NFATc1 and the Ca2+/calcineurin signaling pathway. EMBO J. 2006;25(15):3714–24. doi: 10.1038/sj.emboj.7601246. Epub 2006/07/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jauliac S, Lopez-Rodriguez C, Shaw LM, Brown LF, Rao A, Toker A. The role of NFAT transcription factors in integrin-mediated carcinoma invasion. Nat Cell Biol. 2002;4(7):540–4. doi: 10.1038/ncb816. [DOI] [PubMed] [Google Scholar]
- 9.Muller MR, Rao A. NFAT, immunity and cancer: a transcription factor comes of age. Nat Rev Immunol. 2010;10(9):645–56. doi: 10.1038/nri2818. Epub 2010/08/21. [DOI] [PubMed] [Google Scholar]
- 10.Pan MG, Xiong Y, Chen F. NFAT Gene Family in Inflammation and Cancer. Curr Mol Med. 2012 doi: 10.2174/1566524011313040007. Epub 2012/09/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao H, Kegg H, Grady S, Truong HT, Robinson ML, Baum M, et al. Role of fibroblast growth factor receptors 1 and 2 in the ureteric bud. Dev Biol. 2004;276(2):403–15. doi: 10.1016/j.ydbio.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo Q, Tripathi P, Poyo E, Wang Y, Austin PF, Bates CM, et al. Cell death serves as a single etiological cause of a wide spectrum of congenital urinary tract defects. J Urol. 2011;185(6):2320–8. doi: 10.1016/j.juro.2011.02.044. Epub 2011/04/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson B, Turner DA, Benda J. Ovarian sarcoma. Gynecol Oncol. 1987;26(2):183–92. doi: 10.1016/0090-8258(87)90272-1. Epub 1987/02/01. [DOI] [PubMed] [Google Scholar]
- 14.Horsley V, Aliprantis AO, Polak L, Glimcher LH, Fuchs E. NFATc1 balances quiescence and proliferation of skin stem cells. Cell. 2008;132(2):299–310. doi: 10.1016/j.cell.2007.11.047. Epub 2008/02/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yasaka N, Furue M, Tamaki K. CD44 expression in normal human skin and skin tumors. The Journal of dermatology. 1995;22(2):88–94. doi: 10.1111/j.1346-8138.1995.tb03349.x. Epub 1995/02/01. [DOI] [PubMed] [Google Scholar]
- 16.Lin C, Yin Y, Chen H, Fisher AV, Chen F, Rauchman M, et al. Construction and characterization of a doxycycline-inducible transgenic system in Msx2 expressing cells. Genesis. 2009;47(5):352–9. doi: 10.1002/dvg.20506. Epub 2009/04/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lesina M, Kurkowski MU, Ludes K, Rose-John S, Treiber M, Kloppel G, et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19(4):456–69. doi: 10.1016/j.ccr.2011.03.009. Epub 2011/04/13. [DOI] [PubMed] [Google Scholar]
- 18.Torti D, Trusolino L. Oncogene addiction as a foundational rationale for targeted anti-cancer therapy: promises and perils. EMBO Mol Med. 2011;3(11):623–36. doi: 10.1002/emmm.201100176. Epub 2011/09/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCormick F. Cancer therapy based on oncogene addiction. J Surg Oncol. 2011;103(6):464–7. doi: 10.1002/jso.21749. Epub 2011/04/12. [DOI] [PubMed] [Google Scholar]
- 20.Graef IA, Chen F, Chen L, Kuo A, Crabtree GR. Signals transduced by Ca(2+)/calcineurin and NFATc3/c4 pattern the developing vasculature. Cell. 2001;105(7):863–75. doi: 10.1016/s0092-8674(01)00396-8. [DOI] [PubMed] [Google Scholar]
- 21.Chang CP, McDill BW, Neilson JR, Joist HE, Epstein JA, Crabtree GR, et al. Calcineurin is required in urinary tract mesenchyme for the development of the pyeloureteral peristaltic machinery. J Clin Invest. 2004;113(7):1051–8. doi: 10.1172/JCI20049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li SZ, McDill BW, Kovach PA, Ding L, Go WY, Ho SN, et al. Calcineurin-NFATc signaling pathway regulates AQP2 expression in response to calcium signals and osmotic stress. Am J Physiol Cell Physiol. 2007;292(5):C1606–16. doi: 10.1152/ajpcell.00588.2005. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Jarad G, Tripathi P, Pan M, Cunningham J, Martin DR, et al. Activation of NFAT signaling in podocytes causes glomerulosclerosis. J Am Soc Nephrol. 2010;21(10):1657–66. doi: 10.1681/ASN.2009121253. Epub 2010/07/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sansone P, Bromberg J. Environment, inflammation, and cancer. Curr Opin Genet Dev. 2011;21(1):80–5. doi: 10.1016/j.gde.2010.11.001. Epub 2010/12/15. [DOI] [PubMed] [Google Scholar]
- 25.Wu H, Peisley A, Graef IA, Crabtree GR. NFAT signaling and the invention of vertebrates. Trends Cell Biol. 2007;17(6):251–60. doi: 10.1016/j.tcb.2007.04.006. Epub 2007/05/12. [DOI] [PubMed] [Google Scholar]
- 26.Rao A. Signaling to gene expression: calcium, calcineurin and NFAT. Nat Immunol. 2009;10(1):3–5. doi: 10.1038/ni0109-3. Epub 2008/12/18. [DOI] [PubMed] [Google Scholar]
- 27.Barriere C, Santamaria D, Cerqueira A, Galan J, Martin A, Ortega S, et al. Mice thrive without Cdk4 and Cdk2. Mol Oncol. 2007;1(1):72–83. doi: 10.1016/j.molonc.2007.03.001. Epub 2007/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li W, Kotoshiba S, Berthet C, Hilton MB, Kaldis P. Rb/Cdk2/Cdk4 triple mutant mice elicit an alternative mechanism for regulation of the G1/S transition. Proc Natl Acad Sci U S A. 2009;106(2):486–91. doi: 10.1073/pnas.0804177106. Epub 2009/01/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong SY, Reiter JF. Wounding mobilizes hair follicle stem cells to form tumors. Proc Natl Acad Sci U S A. 2011;108(10):4093–8. doi: 10.1073/pnas.1013098108. Epub 2011/02/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belteki G, Haigh J, Kabacs N, Haigh K, Sison K, Costantini F, et al. Conditional and inducible transgene expression in mice through the combinatorial use of Cre-mediated recombination and tetracycline induction. Nucleic Acids Res. 2005;33:e51. doi: 10.1093/nar/gni051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janik P, Briand P, Hartmann NR. The effect of estrone-progesterone treatment on cell proliferation kinetics of hormone-dependent GR mouse mammary tumors. Cancer Res. 1975;35(12):3698–704. Epub 1975/12/01. [PubMed] [Google Scholar]
- 32.McDill BW, Li SZ, Kovach PA, Ding L, Chen F. Congenital progressive hydronephrosis (cph) is caused by an S256L mutation in aquaporin-2 that affects its phosphorylation and apical membrane accumulation. Proc Natl Acad Sci U S A. 2006;103(18):6952–7. doi: 10.1073/pnas.0602087103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.