Abstract
The importance of the upper airway (nose, pharynx, and larynx) in health and in the pathogenesis of sleep apnea, asthma, and other airway diseases, discussed elsewhere in the Comprehensive Physiology series, prompts this review of the biomechanical properties and functional aspects of the upper airway. There is a literature based on anatomic or structural descriptions in static circumstances, albeit studied in limited numbers of individuals in both health and disease. As for dynamic features, the literature is limited to studies of pressure and flow through all or parts of the upper airway and to the effects of muscle activation on such features; however, the links between structure and function through airway size, shape, and compliance remain a topic that is completely open for investigation, particularly through analyses using concepts of fluid and structural mechanics. Throughout are included both historically seminal references, as well as those serving as signposts or updated reviews. This article should be considered a resource for concepts needed for the application of biomechanical models of upper airway physiology, applicable to understanding the pathophysiology of disease and anticipated results of treatment interventions.
Introduction
The upper airway is that part of the respiratory system between the nostrils or lips and the trachea (106), and is an important contributor to overall respiratory resistance and to conditioning of inspired air (9, 113, 117, 118). Particular functions of the upper airway include air warming and humidification, pathways for olfaction, coordination of ventilation with swallowing and protection from aspiration of food, primary defense of infection, and especially for humans, speech. Evolution of speech in man has required laryngeal motility, leaving the human upper airway reliant on surrounding soft tissues for support and thus vulnerable to collapse (1). All of these functions are controlled by highly evolved neuromuscular systems under both voluntary and involuntary control (8). These systems work efficiently in health but can come into conflict in diseases of the lungs and chest wall, and the upper airway in particular (22, 70, 114). In health and during wakefulness, common coordinated activities of this complex neuromuscular control system include coughs, hiccups, aspiration recovery, vomiting, and sneezing (90). Vocal Cord Dysfunction, which can be a mimic of asthma, appears to be a function of the neural coordination of breathing phase and vocal cord movements (72).
During sleep, however, maintenance of upper airway patency is a primary physiologic objective, failure of which can lead to obstructive sleep apnea (OSA) and its sequelae. Sleep is a time of particular vulnerability when protective upper airway reflexes are attenuated (37, 38, 68, 132, 156, 157), leaving the upper airway susceptible to collapse (102). Recent evidence suggests that the pathogenesis of sleep apnea involves a complex interplay of upper airway anatomy (121), pharyngeal dilator motor control (69, 132), ventilatory control instability (loop gain) (17, 50, 154, 160), alone and/or in combination. The contribution of each of these factors to OSA in a given individual is quite variable (115), emphasizing the importance of defining the underlying mechanism of OSA in afflicted patients to achieve the goal of individualized, targeted therapy (115). This article focuses on the mechanics of the upper airway, understanding that other factors are thoroughly discussed elsewhere in the Handbook and in the literature (40).
Structural and Anatomic Features of the Upper Airway
Tissues
There are narrow regions that functionally limit airway caliber. These include the nostrils, the lips, the palate, and the larynx (Fig. 1). The walls of the upper airway are covered with mucosa; in the nose, the submucosal vascular network has characteristics of erectile tissue and is capable of influencing airway caliber (26, 126). The pharyngeal wall and, hence, its lumen are highly deformable while the nose, larynx, and trachea are surrounded by a more rigid framework of cartilage (134). The impact of craniofacial form is a relatively static feature; however, the size of the cranial base, the length of the mandible, and the length of each airway segment change throughout childhood and to a lesser degree with aging (19, 138). These features set the surrounding cage for the structures around the airway lumen (67). In addition, there are a variety of slowly changing physiological mechanisms, including vascular and muscular tone, which also influence regional airway geometry (112).
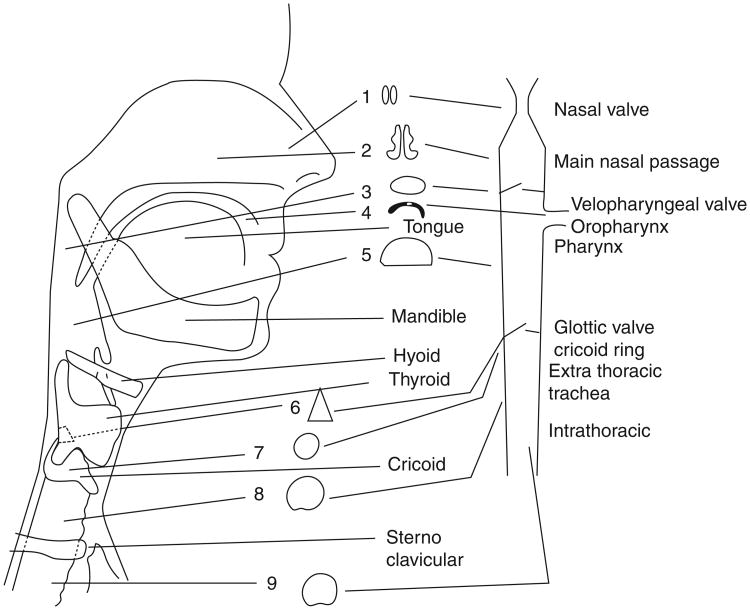
Figure 1.
A drawing in the sagittal plane of the shape changes of the airway lumen and size and the location of valves (nasal, velopharyngeal, and glottic or laryngeal) encountered as air travels from the external nares to the trachea on inspiration or from the lungs in expiration. (Reprinted, with permission, from Proctor ([105])
Figures 2 and 3 illustrate the structural features of the upper airway in sagittal and axial magnetic resonance images (MRIs). The figures give a qualitative indication of the relative cross-sectional area of this airway channel under static conditions during wakefulness (120), and the anatomy surrounding an airway through which ventilation of the lungs is effected by serial convection of air movement. Axial imaging at the level of the velopharynx (behind the soft palate) reveals a pharyngeal lumen surrounded by lateral pharyngeal walls, parapharyngeal fat pads, and other soft tissues within a bony enclosure (153). The importance of the bony enclosure has been debated given the lack of a “floor” in the mandible (140). In theory, accumulation of fat and other soft tissues around the airway would raise tissue pressure and contribute to a more negative, less positive pharyngeal airway transmural pressure, that is, an airway more prone to collapse (45, 154). Midsagittal imaging reveals the relevant structures along the upper airway from the nose to the trachea. During inspiration, air would normally travel through the nasopharynx, past the velopharyngeal (behind the soft palate) through the retroglossal airway (oropharynx) to the level of the larynx before entering the trachea (7). The pharyngeal airway is typically narrowest at the level of the velopharynx, although pharyngeal collapse commonly occurs during sleep when both the tongue and soft palate move posteriorly together (43, 44). Imaging during wakefulness can be helpful to define various anatomical structures, although the exact relevance of these findings to pharyngeal collapse during sleep has been debated (44). Endoscopic studies during sleep and during anesthesia have been helpful in defining the importance of velopharyngeal narrowing (7), although the extent to which these experimental models predict actual pharyngeal mechanics is also controversial (49).
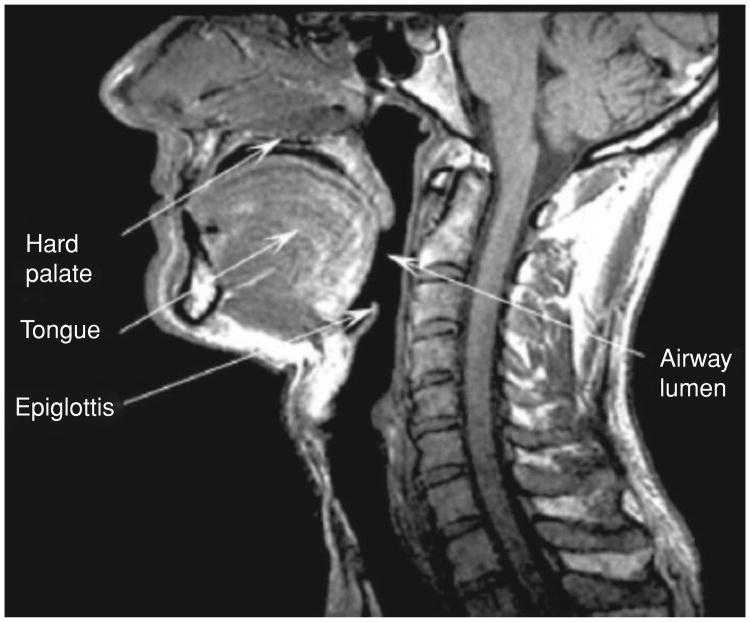
Figure 2.
A midsagittal MRI plane view of the upper airways, showing the anatomy and position of important structural tissue features.
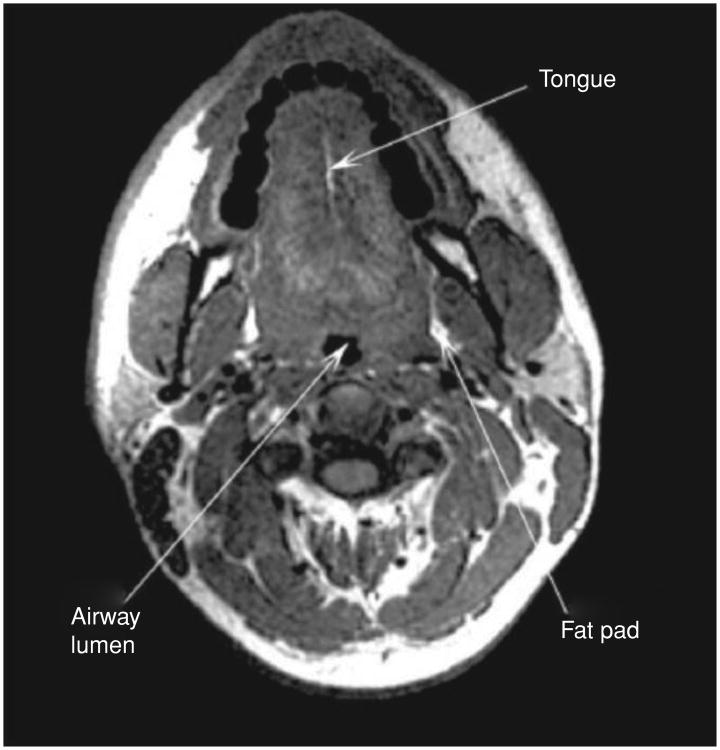
Figure 3.
An axial MRI image at the level of the velopharynx, showing the relative positions of the tongue and airway lumen.
Mucosal function in the upper airway
The upper airway has an epithelial mucosal lining that provides protection and humidification. These functions are influenced by both external environment in terms of the character of the inspired air and internal humoral, autonomic, behavioral, and emotional factors.
The most notable function is that of a barrier. Mucus production occurs not only through specialized cells but also submucosal glands. The mucous layer consists not only of proteoglycans and polysaccharides but also of immunoglobulins like IgA attached covalently to proteoglycans. In an acute response to infection or antigen, immunoglobulins appear at the same time as they occur in the bloodstream. Salivary glands secrete short-chain proteoglycans as well as other proteins like lysozymes that can split proteins and neutralize viruses. The mucus blanket is a dynamic structure not only in its water, salt, and solutes, but also stirred and moved by ciliary cells that beat in a coordinated manner to move the mucus blanket. Specialized dendritic cells act as antigen-presenting cells, and nonmyelinated nerve endings respond to changes in peptides, proteins, as well as humidification. Reflex arcs affect not only blood vessels but also mucus production and mucosal volume, in response to cold dry air and even cold exposure localized to the skin. Withdrawal of cholinergic tone results in vasoconstriction and decreased secretions, and of sympathetic tone, vasodilatation and increased secretions.
Hormonal modulation of mucosal function is exemplified by the changes in vascularity during the menstrual cycle and the chronic congestion and increased viscosity of mucus in hypothyroidism. Emotional stress, anxiety, and erotic stimulation each can evoke an initial inflammatory response followed by mucus secretion and vascular engorgement. This response is more apparent in some individuals, but is present to some degree in all subjects.
Atopic and immunologic responses in the nasal airway in many ways mimic those seen the lower respiratory tract. Acute responses to allergen include reflex effects on the mucosa as well as reflex sneezing and vasodilation. Delayed responses also occur with a similar time frame (6-12 h) as those in the lower airways. Immunologic responses are also similar; however, given the accessibility of the nasal passage and the ability of inoculation to produce a general and longstanding mucosal response, vaccines can be given intranasally.
Pharmacologic agents either applied locally or given systemically affect mucus production, mucus viscosity, vascular size, or ciliary beat frequency. A detailed discussion is beyond the scope of this article, but a drug effect intended to alter one function can affect others. For instance, vasoconstriction while increasing airway size will reduce available surface area and impair temperature and water conditioning of inspired air, as well as reduce mucus flow and increase viscosity by impairing blood flow to the glands. Morphine, atropine, and scopolamine slow mucus movement. The impact of these various influences on airway surface tension and pharyngeal mechanical hysteresis has received minimal attention.
The upper respiratory tract acts in defense against infection in two “specialized” ways. First, bacteria and viruses become trapped in the mucus blanket, swept away by ciliary action, and swallowed, disposed of in the stomach. Second, the upper airway is surrounded by a rich network of capillaries and lymphoid tissue. One anatomic feature is called Waldeyer's tonsillar ring (or the pharyngeal lymphoid ring) and it describes a ring of submucosal lymphoid tissue in the pharynx and the oral cavity. There are pharyngeal, palatine, lingual, and the tubal or Eustachian tonsils.
To some extent the effectiveness of the upper airway in the prevention of infection depends upon deposition of the agent, the integrity of the mucosal barrier, and the effectiveness of ciliary removal, in addition to the factors of cellular attachment, penetration, and responses. The hydration of droplets entering and conditioned by the upper airway is an important factor that predisposes to health or disease. That the onset of respiratory disease is often heralded by upper respiratory tract symptoms is suggestive that the origin of illness involves upper airway defenses. Given the potential role of upper airway inflammation in the pathogenesis of OSA, further work into potential causal pathways is recommended (21, 111).
Pressures Acting on the Upper Airways
A “pressure walk” is instructive (159). Pressure at the junction of the intrathoracic and extrathoracic Pjunc is determined by alveolar pressure (Palv) less the pressure drop along the intrathoracic airways during flow ΔPaw = Pjunc − Palv (here ΔPaw is positive or negative during inspiration or expiration, respectively [23, 35, 46, 148]). Alveolar pressure is determined by the intrinsic elastic recoil of the lung (Pel) and the action of the chest wall on the lungs, via pleural pressure (Ppl). Pressure before the lips and nose (Pao) is atmospheric pressure (Pb, usually taken as zero for a reference pressure insofar as absolute values of pressures are largely irrelevant; pressure differences alone drive the mechanics of tissue deformation and gas flow) (59, 69). In the absence of energy dissipation, the total head (or energy density) Ptot is constant, given by the Bernoulli equation Ptot = P + ½ρu2, where ρ is gas density and u is gas velocity along any streamline. These two terms respectively reflect the contribution of potential energy density (pressure) and kinetic energy density. There are a wide variety of descriptors of these different terms: the pressure P is sometimes referred to as “lateral pressure,” historically secondary to its method of measurement via a side hole in a closed catheter. Similarly Ptot is sometimes called “total pressure head,” or from its measurement method, “end hole” or “stagnation” pressure; it is measured by a catheter whose end is open to, and facing into, a flowing gas stream. Finally, ½ρu2 is often called the Bernoulli or convective accelera-tive (Pca) term. The transmural pressure Ptm is the difference between P (or Plat) and Pb (or pressure outside the airway). Ptm is a major factor determining the area of the lumen in its interactions with the static elastic property of the airway wall. Tissue pressure (Ptis) is the extraluminal pressure immediately adjacent to this hypothetical airway (45, 124, 125, 154). Ptis lies in the extrathoracic airway wall and is systematically different from both Ppl and Pb(110). [See later for a discussion of Ptis in the context of transmural pressures]. Finally, while only indirectly dependent on intrathoracic pressures, there is some evidence that the extrathoracic airway can be influenced by pleural pressure (12, 142, 143, 146).
In the absence of dynamic effects secondary to wall motion, inertia, and hysteresis, the local luminal cross-sectional area of the airway is a single-valued function of the transmural pressure. This pressure/area characteristic defines the “tube law” of the pharyngeal airway. To the extent that energy dissipation is negligible, pressure and velocity are related via the Bernoulli equation earlier, velocity and area are related by conservation of mass, u = V̇/A, and finally, area and pressure are linked through the tube law. Thus, flow through the extrathoracic airway will depend upon the relative magnitude of driving pressures and the deformability of the airway walls. Besides the assumption here of negligible energy dissipation, another important factor is that this simple description is spatially “local,” in the sense that the airway luminal area at any given axial point along the airway is assumed to be a single function of only the transmural pressure at that same point. In particular, the whole concept of a “tube law” depends on this oversimplification as a one-dimensional model. In some circumstances, the assumptions underlying a tube law may be reasonable, but in others, axial coupling may be important, particularly with massive deformable structures as the tongue or soft palate. That is, as will be discussed, mechanical forces in one region of the pharynx (e.g., soft palate) may influence the collapsibility of “the tube” at another region (e.g., tongue), making the assumptions underlying a single pressure/area characteristic overly simplistic. This concept of axial coupling has been minimally discussed in the upper airway literature or in the flow limitation literature, but may be critical to understanding commonly observed phenomena including reduced airflow with increased inspiratory driving pressure (negative effort dependence).
With inspiration, the diaphragm and external intercostal muscles contract, decreasing pleural pressure that decreases alveolar pressures, thus drawing air into the lungs through the extrathoracic airway (Palv is then less than Pb.). With expiration, air flows out of the lungs when Palv exceeds Pb. During quiet breathing, this process is largely passive deflation of the lungs due to lung recoil, but with active exhalation, expiratory muscles including abdominal and internal intercostal muscles also contribute. Local pressures in the upper airways are thus determined by the interaction between alveolar pressure and pressure drops in the intrathoracic airways, and the pressure drops in the upper airways. These pressure drops are those associated with both convective acceleration (associated with going from many airways into a single trachea) and dissipative or resistive losses (pressure drop lost as heat). As noted earlier, these local pressures in turn affect upper airway luminal area, and hence both convective acceleration and energy dissipation. Thus, as pressures rise and fall during expiration and inspiration, the upper airway geometry will change, with concomitant changes in its resistance. In steady flow (e.g., in the absence of snoring during sleep), flows are taken to be quasi-steady, in which case the dynamic characteristics of the airway walls (and in particular, their effective mass) are negligible. This assumption is likely valid in healthy humans, who have a respiratory frequency significantly lower than any dynamical characteristic frequencies, but this issue becomes problematic in small animal models (e.g., neonatal humans) where frequencies are an order of magnitude higher, and a number of lung and airway mechanical features are dynamically determined (e.g., end-expiratory lung volumes are significantly higher than relaxation volumes.)
While the driving pressure at the level of Ppl is largely determined by intrathoracic events, the resulting flow may be importantly influenced by extrathoracic airway properties (102). These important factors include airway geometry and airway compliance, both of which are considerably different along the path from nares or lips to the thoracic inlet. Moreover, additional geometric changes associated with, for example, nasal obstruction, can markedly increase inspiratory resistance and decrease flow (14). This problem is in turn exacerbated by large negative pressures distal to the obstruction, with further increases in resistance to the extent that the pharyngeal airway in the critical region collapses under negative pressure (based on local compliance and upper airway muscle responsiveness (42, 68, 69, 73, 74, 76, 161).
Pressure-Volume Characteristics
General behavior of elastic tubes
One conceptual framework for describing the pressure/volume relationships in an airway is that of a tube with internal radius a and external radius b, composed of an elastic material with Young's modulus E, as shown in Figure 4. For simplicity, here we describe results only when the tissue is taken as essentially incompressible. This is equivalent to taking Poisson's ratio to be 0.5. This simplifies analytic approaches, but somewhat paradoxically, makes numerical simulations through finite element methods (FEM) more difficult. For this reason, most FEM studies take Poisson's ratio to be, say, 0.49; importantly, however, this is an issue of numerical analysis. The physics and physiology display no singularities in the limit of incompressibility.
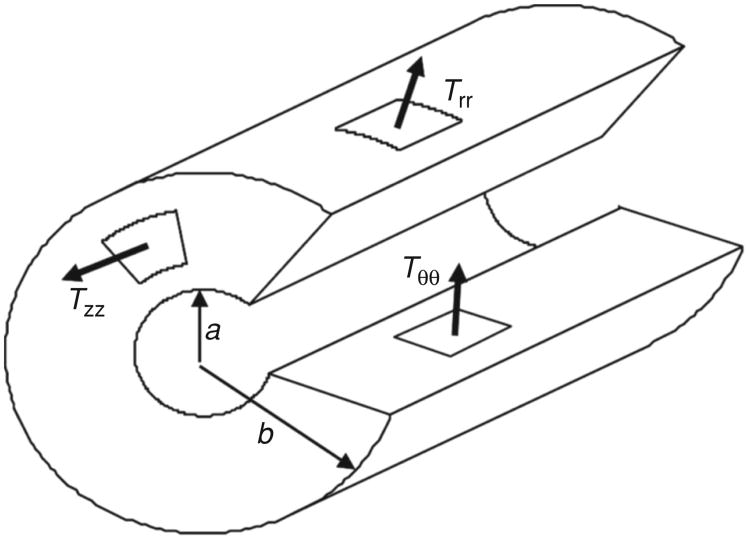
Figure 4.
A cut-away cartoon of a thick-walled airway, showing the geometric arrangement in cylindrical coordinates of the tissue stresses in respectively the axial direction (Tzz), the radial direction (Trr), and the circumferential direction (Tϑϑ). Shear stresses are not depicted. The internal and external radii are shown as a and b, respectively.
Four subsets of the general incompressible problem are useful to examine. First, two cases can be considered depending on the relative thickness of the tube. If a ≈ b, then the tube is said to thin-walled, if a ≪ b, then the tube is thought of as a “hole” in a large annular medium (which may be infinite medium if b → ∞). Second, for each of these situations, there is another set of conditions, perhaps underappreciated, that depends on the axial tension or strain in the tube. Let z denote the axial coordinate. The condition of zero axial tension is given by Tzz = 0, and the tube in cross-section is said to be in plane stress, whereas if the tube is held at constant length, then the longitudinal or axial strain ezz = 0, and the tube is in a state of plane strain. The pressure/area characteristics are different for each of these four subsets. In general, for interior pressure p0 and exterior pressure p1, the radial and circumferential stresses in the tube are functions of the radial coordinate r, given by
The axial stress is independent of r, but depends on the magnitude of the axial strain ezz:
This gives the axial stress directly when the airway is isolength (ezz = 0); it also gives the axial strain ezz when the axial stress vanishes, simply by setting Tzz = 0.
In the special case of a thin-walled tube of thickness h = b − a, the formulae simplify. Referencing all pressures to the exterior of the tube (i.e., taking p1 = 0), we have the approximate formulae: the radial stress is linear in r, from −p0 at the interior boundary, and zero at the outer boundary. The average radial stress is simply Trr = −p0/2. The circumferential tension is uniform in r, given by Tϑϑ = p0a/h, which we recognize as a form of the Laplace relation connecting stress across a surface p0 to the product of effective surface tension, here given by Tϑϑh, and the curvature of the surface 1/a.
Similarly, for the airway modeled as a hole in an infinite elastic medium (outer radius b → ∞), we have the elementary result Trr = −Tϑϑ= −p0a2/r2.
Finally, in the general incompressible case with circumferential symmetry, the radial displacements are given by
where A = −ezz/2 and B = (3/2E)(p0 − p1)a2b2/(b2 − a2). From these displacements evaluated at r = a, we can deduce the change in area δA = 2πau for any given internal and external pressures. Writing δP = P0 − P1 as the change in transmural pressure, the area compliance is δA/δP, and the tube's specific compliance is (1/A)δA/δP.
Specializing this solution to the four cases earlier, we have
Note the presence of an additional term in the infinite medium, zero axial stress case. Here the displacements are not functions solely of the change in transmural pressure. This is counterintuitive when the tissue is incompressible, but arises from the specification of zero axial stress—this introduces an anisotropy such that adding uniform pressure (P1 = P2) does indeed induce axial (and hence radial) displacements.
It is important to recognize that the “tube law” approach outlined earlier does not take into account the degrees of freedom associated with mechanical linkage of the various structural elements within the upper airway. Thus, for example, axial caudal displacement of the tongue during inspiration is necessarily coupled to posterior displacement into the oropharyngeal lumen, and this cannot be described by simple tube law ideas, even by extending them to include possible variations in axial length or tension. In consequence, while tube laws may be helpful in qualitatively describing pressure/flow characteristics (and inspiratory flow limitation in particular), they cannot be expected to account for the complex geometry and coupled displacements that occur in vivo. Indeed, it is likely that phenomena such as the coupled axial and posterior movement of the tongue play a role in the so-called “negative effort dependence” of inspiratory flows with increasing effort (increasingly negative pleural pressures and intrathoracic airway pressures leading to reduced airflow or even complete cessation of flow with complete airway collapse). With this concept in mind, it is the pressure dependence of both area and integrated volumes secondary to tissue coupling that are important for pressure/flow relationships, and to which we now turn.
Satisfactory measurements of the pressure/volume behavior of the upper airway are limited (38). The deficiencies of existing estimates of upper airway compliance are that only partial pressure/volume plots are made and no estimate of minimal airway volume performed. In addition, ongoing neuromuscular activity can be altered by pharyngeal pressure such that the effective elastance of the airway may be a more realistic assessment of its mechanical behavior (66). Measurement of specific compliance demands some estimate of absolute airway volume, or local area if specific compliance is used in a tube law approach. Without this measurement of volume or area, it is difficult to determine whether a change in apparent pressure-volume behavior represents a change in the true pressure-volume characteristic of the airway or represents a calculation made from different positions on two identical pressure-volume curves.
Thus, the very concept of a well-defined transmural pressure is problematic. In the radial direction, and in the absence of a stress jump at the gas/tissue interface of the airway, the radial stress varies from the luminal gas pressure to atmospheric pressure. This variation in simple models of homogeneous tissue properties is described earlier, but there are important inhomogeneities in tissue that call this assumption into question. Furthermore, axial variations in tissue elastic properties are necessarily coupled to radial stresses. Thus, for example, it is only in the lumped sense that variations in volume with quasistatic changes in intraluminal pressures can be interpreted as compliance, here with transmural pressures given by the difference of luminal gas pressure and atmospheric pressure. The independent role of tissue pressures acts through their contributions secondary to inhomogeneities, both radial and axial, throughout the tissues of the upper airways.
The concept of “tissue pressure” raises a separate set of issues. First, we note that the very concept of pressure is not always straightforward. The definition of pressure is the average value of normal stresses in three orthogonal planes within the medium in question. For a static gas or liquid, the normal stresses are isotropic, and so measurement in any orientation of the probe suffices. In the extraluminal tissues of the upper airways, this situation (of isotropic normal stresses) is not likely to be present. From the measurement perspective, fluid-filled catheters may mimic the local pressure, but some catheters may show an orientation dependence, which in turn may depend on the size of the space introduced and its filling with endogenous serous fluid. Second, the upper airway tissues are prestressed by muscle tone. These conditions imply departures, which may be of considerable magnitudes, between those stresses that would otherwise vary smoothly from the airway lumen to the body surface. These prestresses, to be in mechanical equilibrium locally, require a substantial anisotropy and inhomogeneity. At any local point, stresses may be compressive along one axis and tensile along a perpendicular axis. Similarly, from region to region, different places may show compression and others tension. These variations in Ptis will of course contribute to the transmural pressure/area or pressure/volume characteristics, but they do so through changing the state of the tissue primarily through changes in prestress. This concept may be particularly important in cases where deposits of fatty tissue cause the resting luminal areas to decrease, even to points where Pcrit may be positive. Note that this does not imply a Ptis that is positive, only that the material properties have changed to such an extent that in the absence of a positive airway pressure, the lumen may approach or even reach full closure.
Airway collapse and Pcrit
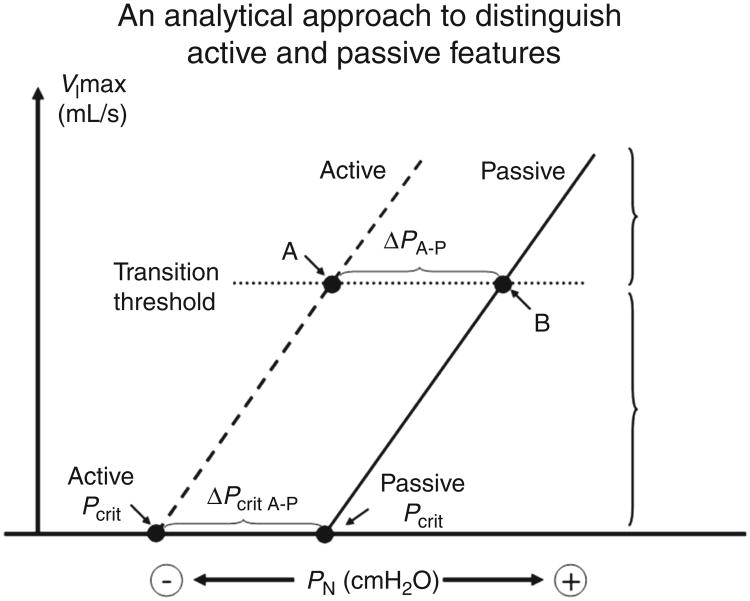
Closing pressure is a term used to indicate the fact that a negative intraluminal pressures may collapse the airway (111, 135). The Pcrit (critical closing pressure) is commonly measured in experimental settings as a quantitative metric of airway mechanics ((27; see Fig. 5). The method has both active and passive versions (92). The passive technique is said to be without neuromuscular activity whereas the active technique assumes active neuromuscular reflex activity in the upper airway. The passive Pcrit has utility in separating OSA patients from controls since its values define a spectrum from very negative values (−15 cmH2O, stable airway) to very positive values (+10 cmH2O, unstable airway) (155). Patients with OSA generally have values at or above atmospheric pressure, whereas patients with upper airway resistance or simple snoring have more negative values. The passive Pcrit is determined using brief drops in the airway opening pressure to induce inspiratory flow limitation (122). The data from flow-limited breaths are plotted on a pressure/flow curve with the x-intercept defining the Pcrit for the pharyngeal airway. The Pcrit is extrapolated to the pressure intercept (i.e., at what would be the point of zero flow). In contrast to the passive Pcrit in which intermittent pressure drops are performed (i.e., typically 3-5 breaths), the active Pcrit is a progressive pressure drop (i.e., over several minutes in which ongoing neuromuscular activity is allowed to accumulate leading to a measure of pharyngeal collapsibility reflecting both anatomy and neuromuscular responsiveness (93, 130).
Figure 5.
Illustrations of the determination of Pcrit. (extrapolation, flow/pressure plots). The pressure-flow plots including data from flowlimited breaths that occur following drops in mask pressure (Pn is nasal pressure, analogous to Pao, which is airway opening pressure). V̇max is the maximum flow occurring during flow-limited breathing. The active curve is acquired from data gathered during gradual progressive drops in Pn whereas the passive curve is from breaths following brief drops in Pn from a holding pressure. The passive curve is said to reflect the passive mechanics of the airway, whereas the active curve includes neuromuscular compensation and other factors. (Adapted, with permission, from Patil et al. [94])
Pcrit is one estimate of airway stability, but should not be interchangeably used with terms relating to compliance of the airway. For instance, the closing pressure does not represent that pressure when total airway volume is zero, since only a portion of the upper airway may be closed (38). On the other hand, there is ample evidence that Pcrit is an important estimator of airway stability—Pcrit is known to be correlated with clinically important events, such as airway closure during sleep (27, 44). Other measures of airway mechanics complement the Pcrit metric, for example, airway resistance, presence or absence of flow limitation, and inspiratory V̇ max provide useful metrics to quantify airway properties during sleep. Assessment of airway compliance, like the active Pcrit, is also complicated by neuromuscular reflexes since the pressure/volume characteristics of the pharyngeal airway are influenced by such mechanisms (54). Some authors prefer to use the term “effective elastance” to describe the volume/pressure characteristic to acknowledge the contributions of these reflexes (42). As a point of emphasis, these assessments provide independent and complementary information about the dynamical properties of the upper airway. For example, a large airway may have minimal airflow resistance but could still be highly collapsible depending on the characteristics of the airway walls (38). Indeed Pcrit explains only a fraction of the variance in apnea occurrence, emphasizing the importance of other physiological traits.
The total elastic modulus of the airway wall is affected by the elasticity of several structural elements, including muscular, nonmuscular, and mucosal elements discussed specifically in the forthcoming few sections. A potentially important architectural feature of the airway is the mechanical linkage between the conducting airway and the surrounding, extra-luminal structures (142, 143, 146). In spite of its potential importance in determining how the airway changes shape and size, this topic has received little attention. For instance, in the pharynx, the posterior constrictors insert directly onto the pharyngeal mucosa, but the rest of the upper airway muscles act on the airway indirectly, through their attachments to the tongue or hyoid (111, 145). As these muscles contract, they change length and tension. The nature of the mechanical events linking hyoid movement to airway mechanics is unclear, although a caudal traction force yields an improvement in airway collapsibility (18, 33, 34, 131). Of note, anterior movement of the airway wall can be produced by anterior movement of the hyoid (133). This coupling between the muscles and the airway wall is thought to arise, phylogenetically, as the result of increased specialization of upper airway muscles for vocalization and for deglutition (16, 30, 139), and may be more functional for nonrespiratory than for respiratory tasks.
Structural contributions to stability
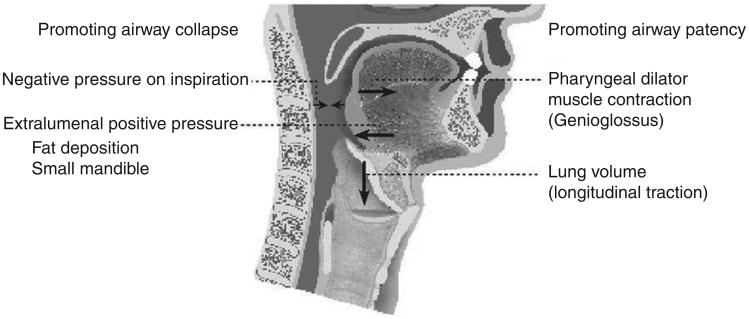
There are additional factors that maintain airway stability other than the direct activation of muscles (see Fig. 6). In the apneic, anesthetized rabbit abolition of muscle activity with gallamine will produce little change in the airway closing pressure, but subsequent death results in the airways being significantly easier to close (48, 85, 86). This latter observation suggests that muscle activity alone does not have predominant effects on upper airway stability. In disease, muscle activity may be more important, as Isono et al. have shown major effects of muscle paralysis on the human upper airway such that people with sleep apnea experience pharyngeal airway occlusion under general anesthesia with muscle paralysis (44). In these individuals, positive pharyngeal pressure is required to maintain luminal patency. Most rodent models, even in obese models, show relatively stable upper airways, in contrast to the findings in humans (83). Thus, there is a role for pharyngeal muscles in the maintenance of pharyngeal patency in the resting state, the potential for species differences in their relative importance, and the need for activation when the mechanical properties of the upper airway create an increased closing pressure.
Figure 6.
A schematic of the pressures and structural features promoting airway collapse and patency, respectively. Negative pharyngeal pressure during inspiration and various craniofacial abnormalities can promote pharyngeal collapse, whereas pharyngeal dilator muscle activation and caudal traction from increased end-expiratory lung volume can promote pharyngeal patency. (Adapted, with permission, from Malhotra and White [71])
Flexion and extension of the neck affect the mechanics of the upper airway (104, 129, 147), because the axis of rotation for extension and flexion is behind the airway. Because the jaw is more anterior than the hyoid, extension results in a lengthening of the muscles that elevate and anteriorly draw the hyoid and a shortening of those that draw it posteriorly. Flexion has the opposite effect, and causes the whole tissue mass behind and below the jaw to be displaced inferiorly and posteriorly, into the airway.
Another element independent of muscle activation or posture is the vascular supply to the mucosa and tissues surrounding the airway. Muscle stiffness can vary with perfusion pressure, as demonstrated by a direct relationship of the relaxed stiffness of the cardiac papillary muscle to the perfusion pressure in its vascular bed (109). The mucosal vascular bed of the airway is perfused at 20 to 30 cmH2O; presumably, this level of pressure, even partially transmitted to the airway wall, could also help resist distortion in a way that would contribute to collapse resistance. Thus, changes in blood pressure and/or the vascularity of pharyngeal muscles could affect airway stability and influence airway patency. Mucosal blood flow may either help resist distortion or contribute to narrowing if engorged.
Effects of lung volume
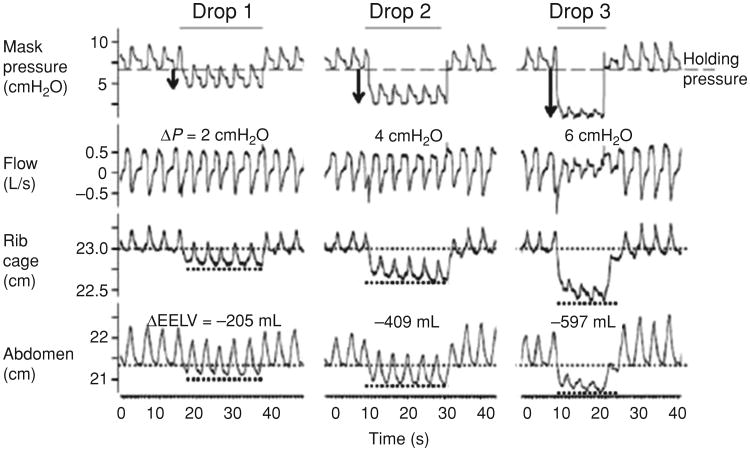
Considerable experimental and human data support the idea that end-expiratory lung volume has a significant effect on pharyngeal mechanics (see Fig. 7). In animal models that increasing end-expiratory lung volume results in reduced pharyngeal collapsibility (142, 143). A number of mechanisms were proposed including direct caudal traction, reflex activation of the pharyngeal dilator muscles, lowering of tissue pressure yielding a reduced transmural airway pressure and unfolding of the pharyngeal airway walls changing their associated tube law. Investigators subsequently assessed upper airway caliber at various lung volumes during wake-fulness (36). Although the authors did observe increased pharyngeal size with lung inflation during wakefulness, such studies are confounded by behavioral influences on the upper airway (13). For instance, the conscious act of performing a total lung capacity (TLC) maneuver would activate upper airway dilator muscles and thus, any observed airway dilation may not relate to lung inflation per se. Begle et al. quantified the effect of end-expiratory lung volume (EELV) on pharyngeal resistance during NREM sleep (12). A number of subsequent investigators have examined these relationships, with the preponderance of evidence suggesting that direct caudal traction forces can serve to stabilize the upper airway, independent of pharyngeal dilator muscle activity (123, 131).
Figure 7.
Effects of lung volume on upper airway mechanics. Caudal traction promotes stiffening of the lower portion of the upper airways, contributing to changes in airflow. Reduced lung volume leads to diminished airflow ostensibly via reductions in caudal traction forces. (Adapted, with permission, from Owens et al. [91])
Several points deserve emphasis. First, elevation of EELV can reduce pharyngeal resistance, whereas reduction of EELV will raise pharyngeal resistance (131). Second, pharyngeal collapsibility is also altered with changes in lung volume, such that EELV elevation leads to a lower airway collapsibility (91, 127). In addition, the continuous positive airway pressure (CPAP) level required to eliminate inspiratory flow limitation is reduced with EELV elevation and the apnea hypopnea index (a measure of sleep apnea severity) is improved concomitantly. Third, genioglossal activity (as an assessment of phasic pharyngeal dilator muscle activity) appears to respond to the improvement in pharyngeal patency with a fall in activity, strongly suggesting that the mechanism underlying improvement in airway mechanics is independent of neuromuscular reflexes involving the genioglossus muscle (131).
Mucosal factors specific to stability
Secretions within the airway lumen are formed from the mucosa and submucosal glands. Near the onset of airway closure pooling of airway secretions occurs, compromising intraluminal size (104). Under these conditions, airway surface factors could affect airway stability independently of airway size changes. Perhaps more importantly, once the airway closes the influence of airway secretions may become a dominant factor determining the pressures needed to reopen the airway (48). For example, a simple application of the Laplace relationship implies that hemispherical air/liquid surfaces with radii of curvatures on the order of, say, 100 μ, together with surface tensions on the order of 25 dyne/cm, will support a pressure difference of around 5 cmH2O. These pressures scale linearly with surface tension and inversely with the harmonic mean radius of curvature. Thus, for example, surfaces with curvature radius of 1 mm will support pressure differences of 0.5 cmH2O; surfaces with curvature radii in the tens of micron range will support (and require in excess of to expand or break open) many tens of cmH2O. That is, mucosal factors could influence pharyngeal mechanics considerably, particularly with regard to hysteresis, for example, differences between airway opening and closing pressures.
Stability is also affected as a result of a change in shape. For instance, as a patent upper airway closes, its shape may change, and the mechanics of further shape change are commensurately altered. As a corollary, the process of airway reopening is mechanically different from the process of closing. In addition to surface forces, additional influences on the airway during reopening would be the following: altered mechanical coupling among dilator muscles, extrinsic to the airway, and airway lumen.
In essence, the features of the airway wall that influence luminal patency and stability include nonmuscular as well as muscular structures (85). Measurement of global structural stability will depend upon an ability to determine specific compliance, including reliable estimates of transluminal pressure. The interpretation of pressure-volume behavior will depend on whether it is appropriate to consider the airway as a tube or as a space surrounded by an elastic medium. Inherent in the consideration of this problem is the mechanical attachments to and relative elastic moduli of structures surrounding the airway. Reasoned extrapolation from other systems and experimental evidence suggest that nonmuscular factors, such as vascular perfusion pressure or properties of the airway mucosa, may influence channel stability directly or alter the importance of muscle action on the airway itself. Finally, airway reopening is not simply a reversal of the events that led to its closing.
Skeletal Muscle Contribution to Upper Airway Characteristics
General features
In the absence of airflow, at least in anesthetized animals, the airway enlarges with each neural inspiration and this enlargement is due to contraction of the upper airway muscles. In this preparation muscles act to make the airway bigger, and work to do so either alone or in combination (29, 144). In fact, multiple muscles have a similar functional outcome, so that a focus on one or another “predominant” muscle is usually based on its perceived importance rather than its precise mechanical orientation to the airway. For example, one can argue that the genioglossal muscle is the major player in the maintenance of upper airway function, but from the literature on electrical stimulation (84), stimulation of muscles that attach to the hyoid may be equally able to reduce the closing pressure (make the airway harder to close) as is the genioglossus. Thus, multiple muscles contribute to patency in regard to breathing while anesthetized and presumably while asleep. However, the genioglossus is accessible for study and likely reasonably representative of phasic respiratory muscles in the upper airway.
Activation patterns and control
The upper airway muscles are controlled at the level of the brainstem through complex mechanisms: a schematic of the neural pathways is given in Figure 8. There are 23 pairs of muscles being controlled primarily through hypoglossal (cranial nerve XII) and through trigeminal motor output (V3) (55-57, 107, 108). These muscles are classified in various different ways, for example, phasic versus tonic (i.e., those that burst with each inspiration vs. those with constant activity through the respiratory cycle), protruders versus retractors, dilators versus constrictors, or those with or without state dependence (i.e., degree of change in activity at sleep onset) (115, 116). Numerous factors can influence activity at these brainstem motor nuclei, as reviewed elsewhere in the Handbook.
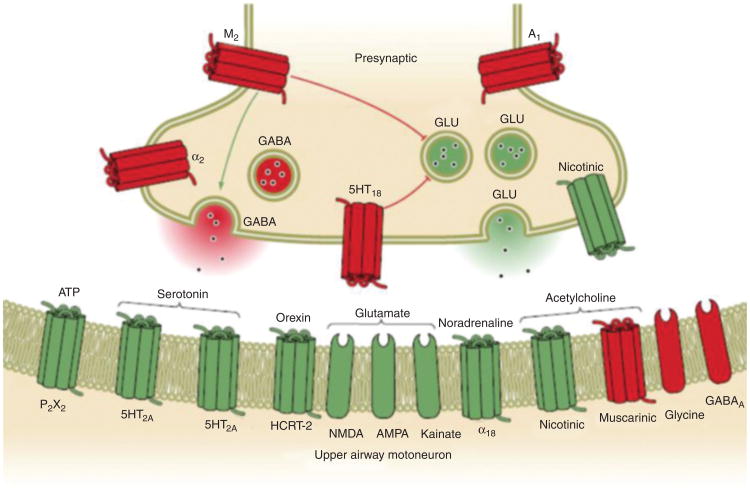
Figure 8.
A box diagram of the neural pathways for activation of muscles associated with upper airway motor output. Multiple different receptors are present at the hypoglossal motor nucleus yielding the possibility of pharmacological targets to increase upper airway tone. (Adapted, with permission, from Dempsey et al. [20])
The number of factors influencing the hypoglossal motor nucleus is too numerous to list. However, some of the more important ones are reviewed here and more extensively elsewhere (38-40):
The central pattern generator provides rhythmic output to various respiratory centers. The PreBoetzinger complex (PBC) influences the hypoglossal motor nucleus through interneurons, which have recently been characterized (24, 106). The PBC provides rhythmic output to the diaphragm to other centers with respiratory modulation.
Mechanoreceptive inputs also influence hypoglossal output (41, 42, 66, 68, 69). The negative pressure reflex describes the robust activation of the pharyngeal dilator muscles in response to pharyngeal negative pressure (i.e., subatmospheric pressure) (75, 76). The reflex is thought to be a protective reflex that preserves pharyngeal patency in the face of a collapsing perturbation. Recent studies have used pseudo-rabies virus vectors to map the various pathways followed by pharmacological blockade to define the important brainstem regions (32). The periobex region near the base of the fourth ventricle in the medulla appears crucial to mediating the negative pressure reflex in rats. Subsequent studies have shown a potential role for mediating these reflexes through cholinergic mechanisms, suggesting possible therapeutic targets (150).
Chemoreceptive mechanisms are also important in influencing the output from the hypoglossal motor nucleus (62, 88, 103, 130). Elevation of CO2 yields an increase in genioglossal activity both during wakefulness and during sleep. The mechanisms underlying these chemoreceptive mechanisms are complex, but may involve the retrotrapezoid nucleus. The majority of neurons in isolation will show some sensitivity to pH, emphasizing the need for careful studies in intact preparation to define these mechanisms.
The wakefulness stimulus (or so-called “wakefulness drive” to breathe) also has influences on hypoglossal output (61, 89). The transition from wakefulness to sleep (alpha-theta transition) provides insight into the wakeful-ness stimulus. A major reduction in genioglossus activity is seen at the alpha-theta transition independent of changes in chemoreceptive or mechanoreceptive influences.
Other factors also contribute greatly to hypoglossal control. For example, various neurochemical systems provide input to the hypoglossal motor nucleus including serotonergic systems (through raphe neurons), cholinergic inputs (through the lateral dorsal tegmentum and pediculopontine tegmental LDT/PPT), adrenergic (locus ceruleus specifically A7 region), histaminergic (through the tuberomammilary nucleus), and orexinergic (through hypothalamic centers) (40, 87). Thus, the control of upper airway muscles is complex but is gradually being elucidated by various investigative groups. Insights from these basic studies may well yield new therapeutic targets to modulate output to the upper airway muscles pharmacologically. Such efforts are likely to be fruitful in those patients whose underlying pathogenesis involves dysfunctional upper airway motor control.
Pressure-Flow Characteristics
Dimensional measures
The basic tool used in the description of upper airway patency has been the measurement of an axial pressure drop along the airway or airway segment and the bulk flow between the points at which pressure was measured. The relationship between pressure and flow is linear under conditions of laminar flow, which obtains at low volume flows during quiet breathing (6, 78, 98). At higher flow rates this linearity is not true (Fig. 5). Recognition of the curvilinear nature of the relationship between pressure change and resultant flow is over 100 years old. Several events contribute to this nonlinearity. One is the transition from laminar to turbulent flow regimes as flow increases. The highest flow at which flow remains laminar is a characteristic of the fluid density and viscosity, and importantly, the geometry of the tube. A second event that can contribute to the alinearity of pressure-flow relationships is the influence of wall deformation as the driving pressure for airflow is increased. This changes the effective size of the airway. On inspiration, this phenomenon occurs as the result of the sum of Bernoulli and frictional pressure drops associated with negative intraluminal pressure. (This additive effect is only true during inspiration; on expiration, the Bernoulli and dissipative losses are of opposite sign and tend to cancel.) A third contribution could be the development of orifice-like flow in some segment of the airway. In this instance, while at low flow rates laminar flow may still be obtained, while at a higher flow rate, orifice flow may predominate. Distinguishing such a mechanism from turbulent losses is problematic, insofar as both pressure drops scale directly with the gas density and quadratically with gas velocity or flow rate.
Flow regimes, laminar, and turbulent
The major variables related to flow through a rigid tube are shown in Figure 6. Size of the passage can be defined by length parameters, while shape of the passage is expressed by a set of parameters relating to angles and length ratios. Fluid properties include density and viscosity (69). To the extent that the airways are not rigid, the elastic properties of the surrounding tissue are important, whether expressed as a local elastic modulus or as an effective specific compliance. This situation is due to the fact that airflow patterns are determined through interaction of local gas velocity with airway geometry, and these are coupled through pressure changes, either of dissipative or Bernoulli origin. The surface tension at the air/tissue interface is likely to be negligible, except for the issue of reopening mechanics, where high curvatures may exist between open and closed segments, and across which commensurately larger pressures are needed to establish flow. Computation of actual volumetric flows is a difficult task insofar as volume flow is given by the local axial fluid velocity, integrated over the airway lumen (43). Except in the most elementary cases, the details of the velocity profile are largely unknown on both theoretical and experimental grounds. The profiles themselves are strong functions of the airway's internal shape and surface roughness. In the upper airway, shape is often not explicitly known and assumptions are often made as to a specific geometry.
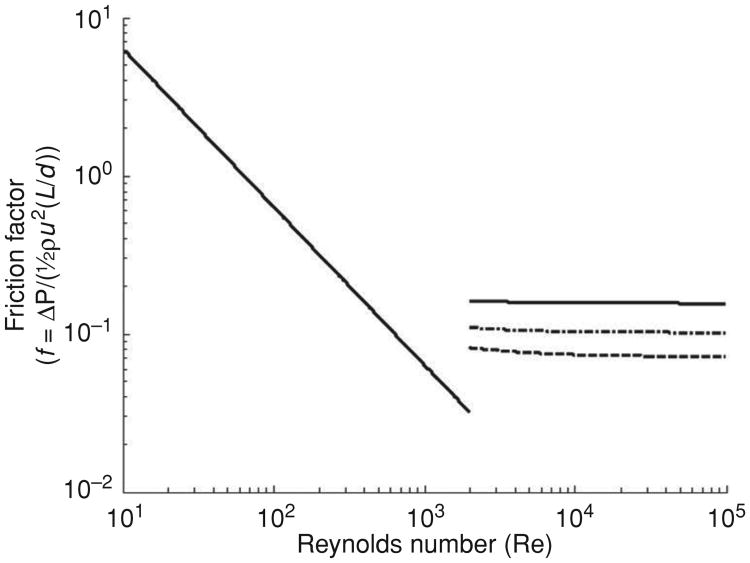
The pressure drops associated with flow through a pipe or channel are often displayed in a Moody diagram. Examples for steady flow through a long pipe are shown in Figure 9. On the horizontal axis is plotted Reynolds' number, Re = ρud/μ, where ρ is fluid density, u is a characteristic gas velocity, d is airway diameter, and μ is the gas viscosity. Re is dimension-less and characterizes the ratio of inertial stresses (or dynamic pressure) ρu2 to viscous stresses μu/d. On the vertical axis is plotted friction factor f, defined as the ratio of the pressure drop δP to dynamic pressure ½ρu2, and scaled by the aspect ratio of the tube d/L, where L is the airway segment length. Thus, the pressure drop is given by . The friction factor is also dimensionless, and thus covers a wide range of possible parameters. Logarithmic coordinates are convenient both in covering several orders of magnitude of independent and dependent variables, but more importantly, in showing the power law behavior in different regimes. For example, at low Reynolds' numbers and where laminar flow conditions prevail, ΔP is linear in flow, and hence in velocity u. This relationship implies f scales simply as 1/u, seen as the double logarithmic slope of − 1 in the low Re regime of the Moody diagram, and in the case of fully developed Poiseuille flow f = 64/Re, shown in the solid line in Figure 7. This equation is valid up to Reynolds' numbers on the order of 1000 to 2000. As the driving pressure increases, secondary flows begin to develop, and steadiness in flow gives way to unsteady behavior, culminating in turbulence (which can often usefully be described in quasi-steady flow terms). In the case of fully turbulent flow, ΔP is quadratic in velocity, leading to a plateau in ΔP/(½ρu2), equivalent to a plateau in friction factor; this situation is depicted in the high Re portion of the Moody diagram, where f, given by the Colebrook-White analytic approximation, is plotted for tubes of three different roughnesses, defined as the ratio of the scale of axial unevenness in airway diameter to the diameter itself. Thus, for example, axial undulations in airway caliber on the order of 1 mm in a 1 cm diameter airway corresponds to a roughness parameter of 0.1. The transition between laminar and turbulent regimes is not well defined; it occurs roughly in the neighborhood of Re ≈ 2000, but its precise value is difficult to determine for several reasons. First, the transition itself is gradual, and passes through a number of states of varying degree of secondary flows and unsteady behavior. Second, even in fully steady-state conditions, the transition is significantly affected by lumen roughness, angulation, and bends. In the examples shown in Figure 9, there is a break in the friction factor behavior at a Reynolds' number of approximately 1000, representing the transition from laminar to nonlaminar flow. At the higher Re numbers, three curves are shown, respectively representing the behavior of airways with roughnesses of 0.05, 0.1, and 0.2.
Figure 9.
A Moody diagram showing the relationship between friction factor f , defined as the ratio of the pressure drop (ΔP) to the Bernoulli term (½ρu2) and scaled by the length to diameter ratio (L /d). Here, ρ is gas density and u is characteristic velocity. The Reynolds number is given by Re = ρud/μ, where μ is gas viscosity. Both f and Re are dimensionless. Two characteristic regimes are displayed. For low Re, flow is laminar, pressure drops are linearly proportional to flow and gas viscosity. Beyond a transition region of Re ≈ 1000 to 2000, the flow becomes turbulent, and pressure drops become quadratic in flow, and proportional to gas density. The three curves above the transition correspond to different roughnesses of the airway, defined as the ratio of the magnitude of radial undulations in the lumen to the airway diameter. The dotted, dot-dash, and dashed curves are for roughnesses of 0.05, 0.1, and 0.2, respectively. The detailed behavior in the transition region is difficult to define with any accuracy, as it is associated with fine details of airway geometry.
Some numerical examples may help in appreciating the various flow regimes. For example, a tidal volume of 500 mL and a respiratory rate of 12 per min corresponds to an instantaneous maximal flow rate of about 300 mL/s. For an airway luminal diameter of 1 cm, this flow rate implies a velocity of 400 cm/s. The kinematic viscosity of air is about 0.2 cm2/s, so if lateral characteristic dimensions of the airway are on the order of 1 cm, then the Reynolds' number is about 2000. This value is precisely at the transition point region between laminar and turbulent flow in the Moody diagram. For flow rates substantially less than this value, the flow is likely to be adequately characterized by a simple linear dependence of pressure drop on flow, whereas for flows greater than this, or importantly, when airway luminal areas are diminished such as during incipient airway collapse in obstructive conditions, the flow is likely to be dominated by turbulence, scaling quadratically with the flow rate.
The actual pressure drops behave differently in laminar and turbulent flow. In laminar flow, ΔP is linear in flow and inversely proportional to the fourth power of tube radius, specifically, ΔP = 8μLV/πr4. This equation in turn implies that even modest narrowing of the airway, say by 50%, is associated with a 16-fold increase in the pressure drop. This marked sensitivity to tube radius is even more striking in turbulent flow. For the higher Re regimes shown in Figure 9, the friction factor is essentially constant for a given airway roughness. This implies that ΔP ∝ u2L/r ∝ V2L/r5, that is, the pressure drop is inversely proportional to the fifth power of the tube radius. For the same numerical example given earlier, with a roughness of 0.1, the friction factor is approximately constant at 0.1. Numerically, for a tube of length L of 20 cm, and for gas density of 0.0013 gm/cm3, the pressure drop is ΔP ≈ 0.1 cmH2O, a very modest drop. But with luminal narrowing of 50%, as earlier, the drop increases by a factor of 32, to 6.4 cmH2O. This is in the range of Pcrit, where instability of the airway is important, and the risk of airway collapse increases. A further decreases in diameter to, for example, 25% resting levels will increase the pressure drop by a factor of 1000, to 100 cmH2O, a level at which patency cannot be sustained. In summary, for modest breathing rates and for normally patent airways, the pressure drops are quite small and physiologically unimportant. But with increased ventilation, and more importantly, with even modest changes in airway caliber, the pressure drops can reach levels that are important, and that can lead to inspiratory obstruction. Forced oscillation methods have been used to quantify these changes during sleep in a moment-to-moment manner with identification of temporal changes in elastance during the act of airway closing and reopening (79, 80); the advantages of forced oscillation is its noninvasiveness and ease of application, but details of site anatomy are not available. Although requiring more technology and expense, imaging techniques can resolve anatomic details and with improvements are likely to be helpful in defining the anatomic features of dynamic phenomena in selected subjects (15).
It is important to appreciate that none of the limiting cases of classical tube flow ever prevail in the upper airways. In particular, at low Re, laminar flow may indeed occur, but it is not Poiseuille flow insofar as the flow is never fully developed with parabolic profiles—this requires tube lengths that are many times the tube diameters, which is not the case for any air passages in the respiratory tract. Second, turbulence is also never fully developed, although the essential flatness of the curves for high Re in Figure 9 suggest that assuming fully developed turbulent flow does not incur serious error. Some efforts have been made to vary gas density and viscosity as means to assess upper airway flow patterns (128), although more work is clearly needed in this area (69, 141)(119). We also emphasize that the principles we are discussing are largely limited to steady flows but recognize that the more complex unsteady flows (as would occur with snoring) can also occur. Unsteady flows occur, depending on both the physical properties of the gas and the inertia and elastance of the tissues, but are poorly understood and beyond the scope of this review (2, 3).
Wall compliance
Another factor influencing flow behavior through a tube is the elasticity of the walls (97, 101). Regardless of the flow regime, the existence of pressure drops due to both convective acceleration and to dissipative losses means that the local geometry of compliant structures will change in response to these distributed transmural pressures. This phenomenon is encountered in the extrathoracic airway and is particularly important during inspiration when intraluminal pressures fall and cause a decrease in cross-sectional area. It is possible that an individual might have a low inspiratory airway resistance at low flows (because the airway is large to begin with) but higher resistance at higher flows (because the airway is compliant and subject to collapse with negative intra-luminal pressure). As these pressure drops are flow regime dependent, it remains a challenge to separate their respective contributions to the overall pressure/flow behavior of the upper airway. Indeed, its nonlinear characteristic is a function not only of transitions between laminar and turbulent flow, but also an independent function of airway geometry changes secondary to the pressure distribution (thus nonlinear behavior is completely compatible with fully laminar flow, and does not necessarily imply turbulence).
The upper airway can be viewed in steady state as a group of resistors arranged in series, each with its individual pressure/flow properties. As such, individual regions may dominate the pressure/flow behavior, especially from regions approximating orifice flow. Depending on the flow rate, this situation can result in an abrupt transition from laminar to turbulent flow, at least locally, and thus be a factor contributing to curvilinear pressure-flow measurements. The pressure/flow behavior of a given element is dependent on its geometry, and thus along the airway the pressure drop at any given flow may be larger in one region and smaller in another. In converging geometries, convective acceleration leads to pressure losses, and these generally become dominant at higher flow rates, but other geometries play a role as well. In particular, orifice flow generally displays pressure drops associated with inertial forces (and hence gas density) over a wider range of flows than simple converging or diverging channel flow. The idea of an orifice is typically taken to mean a constriction whose length is negligible and whose diameter is less than half the tube diameter. Even at moderate flow rates, flow separation downstream of the orifice leads to pressure drops that are density dependent, although at very low flows, this effect is negligible. To the extent that inertial effects are important, pressure drops scale quadratically with the flow, and these may be highly localized in areas approximating orifice behavior.
For instance, if the larynx were in a conformation that permits orifice flow to develop, the pressure drop across the pharynx and mouth would be negligible as a proportion of the total pressure drop from the distal lung airway to atmosphere on both inspiration and expiration. This does not imply that these regions would be irrelevant to the mechanics of the upper airway, insofar as being compliant, they necessarily respond to the local pressures, whether determined locally or at remote orifice sites. It follows that a small change in the size of the laryngeal opening, associated with small changes in the level of laryngeal muscle activity, could have far more impact on upper airway resistance than large changes in pharyngeal muscle activity.
Empirical descriptors of pressure/flow relations
Here, we discuss current methods to describe upper airway flow behavior. The majority of experimental work on the upper airway has involved examining only the pressure drop along and the bulk flow through either one section of the airway or the entire upper airway. Most studies have been performed on pressure/flow relationships through the nose, and we will concentrate on this body of data. Efforts to reduce or illustrate pressure/flow data to understand mechanical properties of the airway may be grouped as follows:
Resistance is conventionally defined as the ratio of pressure difference to flow, independent of whether the pressure/flow relationship is linear or not. This is equivalent to a “chord” resistance, by analogy to chord compliances reported in the pulmonary literature. To the extent that pressure is nonlinearly related to flow, this concept requires the identification of a target flow or pressure at which to calculate the resistance (81, 100). Different laboratories have chosen different flow rates at which to calculate flow resistance, usually using a flow value in the range of 0.2 to 0.5 L/s, but this variation makes the results between laboratories or between interventions in the same subject difficult to interpret. A variation on the chord resistance model is to assume that flow is turbulent, in which case the ratio of pressure drop to the square of flow is constant and can be meaningfully interpreted (82). It is important when using this approach, however, to demonstrate that pressure is adequately represented as a quadratic function of flow, by repeating measurements over a range of flows. It is an irony of upper airway physiology that the flow rates habitually chosen for these calculations (0.2-0.5 L/s) are in the region of transition between laminar to nonlaminar flow, so that neither the laminar nor the turbulent condition is fulfilled.
An empirical approach to the transition regime was proposed more than a century ago. Rohrer chose to approximate the relationship between pressure and flow as additive contributions of terms respectively linear and quadratic in flow:
This equation is fit to pressure-flow measurements by finding optimal values of k1 and k2. By inspection, at low flows the first term dominates, and k1 is said to represent resistance in the laminar flow domain, whereas at high flows, the second term dominates, and k2 is a measure of resistance in turbulent flow (81, 100). (Note, however, that while k1 has the dimensions of resistance, k2 does not; it nevertheless is a useful metric for characterizing convective accelerative and dissipative pressure drops in inertia dominated flow regimes.) If this is done with pressure/flow data from nasal measurements is found that k2V̇2 is much larger than k1V̇, implying that the pressure/flow relationship in the nose is dominated by turbulent flow (96, 99). As an aside, the fact that nasal pressure/flow data can be fitted to Rohrer's equation with high correlation coefficients is not sufficient to a mechanistic interpretation of laminar versus turbulent regimes, as the geometric changes associated with upper airway compliance can mimic the nonlinear characteristic of turbulent flow, at least over some range of flow. Thus, while the Rohrer constants are certainly suggestive of mechanism, they cannot be used to distinguish among more specific phenomena such as boundary layer development and flow separation
There are several variants in the choice of method by which to display pressure and flow data. A double logarithmic plot of raw data is equivalent to a dimensionalized Moody diagram, with recognizable regions of interest. Richerson and Seebohm presented their nasal pressure-flow data in this manner. Using logarithmic coordinates, the laminar region of the relationship is relatively easy to detect. Parallel shifts of the logarithmic pressure/flow plot occur when the structure becomes effectively larger. A second advantage of this method is that pressure/flow ratios (i.e., resistances) can be calculated at flow rates where a laminar flow regime can be seen to occur. If resistance is calculated at 25 L/min (log flow 1.39) would be found to have roughly half the effect that it would seem to have if resistance were calculated at 50 L/min (log flow 1.69). Thus, a subject with allergic rhinitis and a small nose, whose pressure/flow plot would show a parallel (logarithmic) shift to the left, could therefore easily appear to have a response to exercise greater than a normal subject, when the response was in fact identical.
In the double logarithmic display, regimes are readily apparent. From the form of Rohrer's equation, a log/log slope of 1 indicates ΔP is linear in V̇, implying laminar flow. Similarly, a log/log slope of 2 indicates ΔP is quadratic in V̇, implying a regime dominated by turbulence or Bernoulli pressure changes. It also follows that the intercept of the line of the slope of 1 can be used as an index of size.
Negative effort dependence
A number of points about negative effort dependence should be made before consideration of underlying mechanism. Negative effort dependence refers to decreases in airflow that occur in the setting of increased effort. In classic flow limitation, airflow is independent of driving pressure such that flow remains relatively constant over a range of driving pressures (60, 64, 77, 149). During forced exhalation, negative effort dependence is commonly observed during routine spirometry (63, 136, 137). Reasoning from classical mechanics led some to consider whether there might occur an underestimation of expiratory volume and hence a misrepresentation of lung recoil at the lung volume of interest. Subsequently, estimates of thoracic volume using plethysmography were performed to account for the influence of gas compression (47, 53, 65), with a consensus finding relatively constant flow over a range of driving pressure, consistent with the classical view of flow limitation (25, 52). However, the magnitude of this artifact is a function of the amount of gas compression (which is on the order of 5%-10% based on alveolar pressure of 50-100 cmH2O during forced exhalation at sea level) and occasionally this is insufficient to explain marked reductions in airflow that are observed.
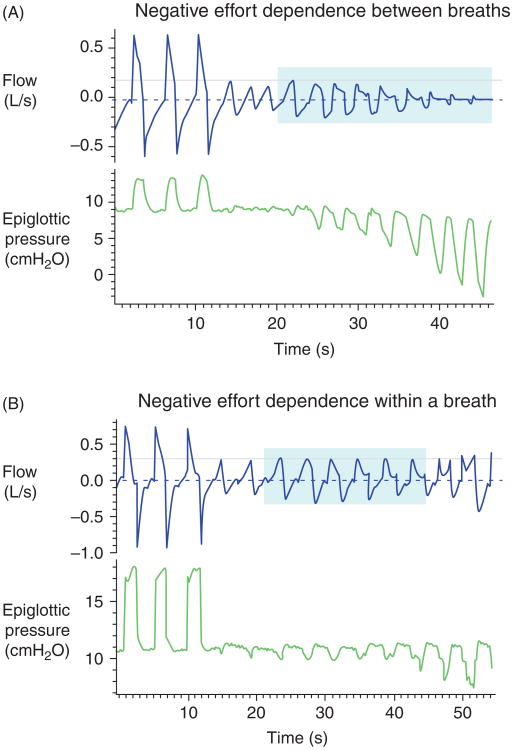
Negative effort dependence during inspiration was first observed in patients with obstructive hypopneas; by definition this finding is a reduction in bulk flow through a tube with increasing driving pressure. In this instance, the driving pressure is produced by the inspiratory action of the chest wall muscles (i.e., diaphragm and intercostal muscles), in which neural activation and force increases throughout an inspiratory effort. During inspiratory flow limitation, reductions in airflow with increasing effort can be quite marked and are clearly not a function of gas compression given that the source of inspiratory flow is ambient air. Figure 10 shows an example of the striking decrease in inspiratory flow during increasing effort as evidenced by increasingly negative pleural pressure. This phenomenon of inspiratory “negative effort dependence” can occur both within breaths and between breaths and in some cases can be quite pronounced (e.g., greater than 50% reduction in airflow [151]), but may also be mild, as evidenced by a gentle downslope in the flow versus driving pressure at fixed target volume or recoil pressure. Pronounced inspiratory flow limitation is associated upper airway collapse during sleep, with reductions in airflow leading to obstructive hypopneas, impairment in gas exchange, arousals from sleep, and swings in blood pressure and cerebral blood flow.
Figure 10.
(A and B). Illustration of flow tracings demonstrating the phenomenon of negative effort dependence. Shown are plots of flow and epiglottic pressure as a function of time. Panel A shows flows significantly decreasing with substantial drops in epiglottic pressure. This finding is in contrast to the classic Starling resistor in which flow is constant across a range of driving pressures. Panel B shows that this type of behavior can also occur even within a breath whereby flow peaks in early inspiration and decreases despite increasing driving pressure later in inspiration. The magnitude of these effects is considerable as compared with the relatively modest reductions in airflow previously described secondary to gas compression during forced expiration.
Intrathoracic mechanisms contributing to negative effort dependence during expiration have been well studied, and typically relate to artifacts associated with gas compression. But as this cannot be invoked during inspiratory flow limitation, other cmH2O potential mechanisms are required. These include:
A so-called Knudson transient whereby collapse of a central airway can contribute to a brief “blip” in volume during abrupt maneuvers (observed in expiration after a cough or other sudden increases in driving pressure). However, this volume is only observed downstream of the collapsing segment and is thus not able to explain the phenomena observed during inspiration (152).
Nonhomogeneous distribution of mechanical units. In expiration, certain lung units may empty more quickly during forced exhalation maneuvers as compared with units with higher resistance and compliance. This phenomenon can lead to observed negative effort dependence since fast lung units may not importantly contribute to exhaled volume depending on local airway mechanics. Even at a fixed initial lung volume, fast and slow compartments can have variable relative contributions to exhaled volume depending on the magnitude of the exhaled effort (51). This is unlikely to contribute to inspiratory flow limitation based on the atmospheric source of inspiratory airflow.
Changes in tube law. The pressure-area characteristic of a collapsible tube is dependent on a number of factors including axial tension. Changes in resistance within upstream segments may change axial tension at the choke point, and thus could contribute to altered airway collapsibility (60). This is much more relevant to the observations, but relatively complex given the interdependence of elements at any given point in the upper airway, and complex flow regimes through the various parts of the upper airway.
Volume and pressure history. The relative contributions of the “fast” and “slow” compartments are highly variable depending on the initial lung volume from which forced exhalation is initiated. For example, an exhalation that begins at half-TLC may not have a contribution from certain lung units, which would have emptied substantially between TLC and 0.5 TLC. This phenomenon can alter the shape of the flow-volume curve but its influence on negative effort dependence per se is not clear in the lung, but could be relevant given the properties of the upper airway wall.
Maximal expiratory flow is affected by neck extension or flexion. Neck extension can alter axial and circumferential stresses and thus the tube law in the vicinity of the choke point. Thus, increasing expiratory efforts may be associated with subtle changes in head posture, which could influence the tube law at the choke point through local mechanical forces (147).
Inspiratory flow limitation (and snoring) are the hallmarks of upper airway obstruction and sleep apnea (102), suggesting a major physiological role with regards to maintaining normal ventilation during sleep. Knudson transients are not seen at the nares during inspiration since the direction of any volume/flow transients would be observed at the level of the trachea rather than the airway opening. Parallel inhomogeneity in flow pathways is similarly unlikely to explain any observed phenomenon regarding inspiratory flow through the upper airway where “emptying” per se is not relevant. Although neck flexion and extension have large effects on the critical closing pressure of the upper airway (Pcrit), changes in head position are unlikely to manifest during sleep over the course of a single inspiration (147). Thus, many of the traditional explanations for negative effort dependence do not pertain to the upper airway.
On the other hand, tube law changes in the upper airway as a result of mechanical influences from the upstream segment are likely important in upper airway physiology. That is, the portion of the pharyngeal airway from the nares to the velopharynx through axial and circumferential tension may influence the airflow characteristics. Classic Starling resistor models and traditional flow limitation theory are not easily applicable to the upper airway. For example, physical displacement of large upper airway structures such as the tongue may exhibit major differences in behavior beyond those predicted for a simple floppy tube. Floppy tube models may provide reasonable approximations of airway behavior by describing local phenomena if axial coupling is unimportant and can be neglected. However, these influences are important, particularly in upper airway mechanics. The distinction between floppy tube models and the reality of the upper airway encompasses two existing conceptual models. First, in steady state, one must consider the axially interconnected nature of the various upper airway structures such that a single local tube law may not be applicable. Second, mass effects of large structures (such as the tongue and soft palate) may yield transient phenomena due to interactions with local airway compliances, which in turn can manifest as snoring or other nonsteady state phenomena.
Consequences of Differences in Regional Airway Mechanics
There are regional differences in upper airway mechanics. Generally speaking, the nose has a high and relatively fixed resistance, with little effect of compliance once inside the alae of the external nares; the mouth has a high but very variable resistance and a low but variable compliance; the pharynx generally has lower resistance than the nose and mouth, but greater compliance, and the larynx has very variable resistance and very low compliance. Some of these observations require comment. The mouth is commonly thought of as having a low resistance. This is a result of the practice of measuring oral resistance with a mouthpiece; when the mouth is not abnormally widely open its resistance is similar to that of the nose. The compliance of the upper airway is significant, but it is a key point that in normal individuals during wakefulness inspiratory flow limitation in the upper airway is very difficult or impossible to achieve. This is not the case in subjects with sleep apnea, nor in snorers during sleep, in whom inspiratory flow limitation is routinely observed.
The nose
The nose is an irregular tube; its lower surface is flat in sagittal section, and the upper surface is domed. There are large projections into the lumen (turbinates), which make the lumen essentially a vertical slit. The first difficulty in fluid dynamical analysis of the nose is that the airstream does not occupy the whole cross-section of the nose; the airstream is curved and convoluted, with turbinates and expansions leading to wide variations in streamlines (158). Any analysis of nasal pressure/flow properties is complicated as precise knowledge is lacking the area of the nasal passage that can conduct the airstream (i.e., neither Reynolds' number nor friction factor are well established in the nose). The nasal streamlines are influenced by the entry velocity and direction of the air, but this has not been systematically investigated. The downstream resistance, for instance the resistance at the soft palate, is presumably under active muscular control (68), and would affect the dynamics of nasal flow, but this concept also has not been studied in detail. The anterior end of the nose is under voluntary control (during wakefulness), and the mechanical arrangement of the alar cartilages and the associated muscles vary dramatically among individuals (10).
The flow regime in the nose during quite breathing appears to be largely laminar. Turbulent flow does not appear until flow rates on the order of 30 L/min in normal subjects. It may be significant that the transition from nose to mouth breathing during exercise frequently occurs at this range of flow rates. Nasal resistance is regulated both by the alar muscles and by the nasal mucosa. Exercise lowers the nasal resistance presumably by causing vasoconstriction in the mucosa, a response mediated by the sympathetic nervous system (95). Hypercapnia results in alar muscle activity and reduction in nasal resistance. This effect could result from dilation of the airway through autonomic control of the nasal vasculature or by the action of muscles in stiffening the airway wall. As an example of the effect of central autonomic control of the nasal passage, there is the curious phenomenon of out-of-phase cycling of resistance by the two nasal passages, a phenomenon apparently due to synchronous changes in the balance of autonomic neural outflow to the vasculature. Its physiologic benefit/influence is uncertain.
Moving from the upright to the supine posture affects nasal patency and generally decreases flow resistance. The mechanism for the change in flow resistance with posture is mediated in part by reflexes triggered by stimulation of pressure receptors at the body surface. These reflexes alter central autonomic neural output to the nasal vascular bed (11). Part of the response could result from passive congestion of the nasal vasculature, since there are apparently no valves in the great veins of the neck. An increase in nasal resistance with posture was greater in patients with OSA than in normal subjects (4, 5). Whether this finding is explained anatomically, as suggested by roentgenographic studies (31, 134), by differences in central reflexes (see earlier), or by the problem of measurement of resistance in different flow regimes cannot be ascertained currently.
The pharynx
The pressure/volume properties of the pharynx are still incompletely understood. Measurement of pharyngeal cross-sectional with acoustic imaging suggests that during inspiration the pharyngeal area is smaller than during expiration. Paradoxically, it is during inspiration that the pharyngeal dilator muscles show phasic activation (66, 118). Whether the respiratory changes in pharyngeal size represent a very compliant structure or result from changes in pharyngeal muscle activation remains to be determined. This effect implies that here, perhaps more than in the nose, one must be careful not to measure the pressure drop across the pharynx at some arbitrary flow and assume that the ratio represents a static “resistance.” Unless the entire pressure/flow curve is collected, and analyzed so as to separate size and compliance, there will be difficulty in comparing different subjects or different conditions.
Likewise, pharyngeal pressure/flow properties and the contributions to it of the size and compliance of the airway have not been studied in detail. There are individual differences in pharyngeal airway size that may be based on familial or gender/age effects. There are anatomic bases for differences in airway size between healthy subjects and patients with OSA (67, 121). These studies have often considered size to be a static phenomenon.
The pressure/flow properties of the pharyngeal segment need to be considered in the context of both sides of the segment. This concept is especially important in the pharynx, where the flow entering the pharynx will be radically different depending on its origin, that is, the mouth, the nose, or the larynx. Lavie and Rubin (58) demonstrated that nasal obstruction resulted in pharyngeal obstruction during sleep. Gleeson et al. (28) discovered that inspiratory pharyngeal resistance (measured at a fixed flow) increased with added external resistances, but the origin of this effect is not known.
A further aspect of pharyngeal airway mechanics, which has received relatively little attention is the surface properties of the mucosa. When the pharynx closes, the forces favoring reopening have to overcome the adhesion of the apposed mucosal surfaces. It appears that the neutral position of the airway is open, since even in the dead animal some negative pressure is required to keep the airway closed. When the production of upper airway secretions and saliva is stimulated with methacholine, however, the airway can be made stable in the closed position, so that positive pressures are required to reopen it. The properties of respiratory mucus may be important in determining airway mechanics at points of near closure or with reopening.
The larynx
The vocal folds themselves act like a valve rather than a tube and when influencing flow resistance have a dominant orifice effect. The laryngeal valves can be influenced passively by changing transmural pressures; however, the effect on flow resistance is thought to be negligible unless there is nerve paralysis or there is activation or inhibition of the laryngeal muscles asynchronous with breathing. The walls of the larynx are relatively more rigid than the supraglottic airway and resist collapse pressures up to −50 cmH2O. This observation may explain the fact that the larynx is generally patent during obstructive apneas in sleep (49), and emphasizes the importance to consider neuromuscular control in functional obstruction triggered by aspiration reflex and associated vocal cord dysfunction.
The size and shape of the laryngeal and the supralaryngeal airway are dynamically altered by skeletal muscle activation. During inspiration the size of the laryngeal orifice is larger than during expiration. This phenomenon is exaggerated with hypercapnia and hypoxia. Furthermore, the shape of the larynx changes with hypoxia and with hypercapnia, presumably because the larynx is acting to reduce expiratory flow and/or regulate end-expiratory volume. This change in caliber is also affected by intrathoracic airway narrowing, either induced asthma or chronic airflow obstruction. A panting maneuver results in the larynx being widely open. Thus, the size and shape of the laryngeal orifice is actively controlled and coordinated stereotypically for different functions—singing and speech and swallowing—in the absence of anatomic encroachment.
Summary and Future Directions
Although considerable progress has been made regarding the pathogenesis of upper airway collapse, further work is clearly required. Unanswered questions remain for the scientist and for the clinician, some of which we summarize here. For the scientist, questions include what are the mechanisms underlying negative effort dependence and are these different for reduced flow within breaths as compared to between breaths? Why can there be such marked hysteresis in terms of upper airway opening and closing? How does upper airway collapse interact with other pathophysiological traits known to be important in sleep apnea pathogenesis? How does genetics influence the various pathophysiological traits? Advances in technology including various dynamic imaging techniques, computational power for finite element modeling and more sophisticated analytical techniques may facilitate mechanistic insights. However, advances in our understanding of pharyngeal airway mechanics might provide new therapeutic targets for diseases including OSA. Ultimately approaches which measure and target-specific features underlying structural and functional pathology in a given individual are likely to move the field forward in terms of predicting the response to current and future therapy.
References
- 1.Abu Alhaija ES, Aldaikki A, Al-Omairi MK, Al-Khateeb SN. The relationship between personality traits, pain perception and attitude toward orthodontic treatment. Angle Orthod. 2010;80:1141–1149. doi: 10.2319/012710-59.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aittokallio T, Gyllenberg M, Polo O. A model of a snorer's upper airway. Math Biosci. 2001;170:79–90. doi: 10.1016/s0025-5564(00)00062-6. [DOI] [PubMed] [Google Scholar]
- 3.Aittokallio T, Saaresranta T, Polo-Kantola P, Nevalainen O, Polo O. Analysis of inspiratory flow shapes in patients with partial upper-airway obstruction during sleep. Chest. 2001;119:37–44. doi: 10.1378/chest.119.1.37. [DOI] [PubMed] [Google Scholar]
- 4.Anch AM, Remmers JE, Bunce Hd. Supraglottic airway resistance in normal subjects and patients with occlusive sleep apnea. J Appl Physiol. 1982;53:1158–1163. doi: 10.1152/jappl.1982.53.5.1158. [DOI] [PubMed] [Google Scholar]
- 5.Anch AM, Remmers JE, Sauerland EK, Degroot WJ. Oropharyngeal patency during walking and sleep in the Pickwickian syndrome: Electromyographic activity of the tensor veli palatini. Electromyogr Clin Neurophysiol. 1981;21:317–330. [PubMed] [Google Scholar]
- 6.Andersen I, Lundqvist GR, Molhave L, Pedersen OF, Proctor DF, Vaeth M, Wyon DP. Human response to controlled levels of toluene in six-hour exposures. Scand J Work Environ Health. 1983;9:405–418. doi: 10.5271/sjweh.2393. [DOI] [PubMed] [Google Scholar]
- 7.Badr MS, Roiber F, Skatrud JB, Dempsey J. Pharyngeal narrowing/occlusion during central sleep apnea. J Appl Physiol. 1995;78:1806–1815. doi: 10.1152/jappl.1995.78.5.1806. [DOI] [PubMed] [Google Scholar]
- 8.Baker TL. Sleep apnea disorders. Introduction to sleep and sleep disorders. Med Clin North Am. 1985;69:1123–1152. doi: 10.1016/s0025-7125(16)30979-8. [DOI] [PubMed] [Google Scholar]
- 9.Banchero N, Cronin L, Nolan AC, Wood EH. Blood oxygen changes induced by forward (+Gx) acceleration. Aerosp Med. 1965;36:608–617. [PubMed] [Google Scholar]
- 10.Banchero N, Cronin L, Rutishauser WJ, Tsakiris AG, Wood EH. Effects of transverse acceleration on blood oxygen saturation. J Appl Physiol. 1967;22:731–739. doi: 10.1152/jappl.1967.22.4.731. [DOI] [PubMed] [Google Scholar]
- 11.Banchero N, Rutishauser WJ, Tsakiris AG, Wood EH. Pericardial pressure during transverse acceleration in dogs without thoracotomy. Circ Res. 1967;20:65–77. doi: 10.1161/01.res.20.1.65. [DOI] [PubMed] [Google Scholar]
- 12.Begle RL, Badr S, Skatrud JB, Dempsey JA. Effect of lung inflation on pulmonary resistance during NREM sleep. Am Rev Respir Dis. 1990;141:854–860. doi: 10.1164/ajrccm/141.4_Pt_1.854. [DOI] [PubMed] [Google Scholar]
- 13.Bradley TD, Brown IG, Zamel N, Phillipson EA, Hoffstein V. Differences in pharyngeal properties between snorers with predominantly central sleep apnea and those without sleep apnea. Am Rev Respir Dis. 1987;135:387–391. doi: 10.1164/arrd.1987.135.2.387. [DOI] [PubMed] [Google Scholar]
- 14.Brouillette RT, Manoukian JJ, Ducharme FM, Oudjhane K, Earle LG, Ladan S, Morielli A. Efficacy of fluticasone nasal spray for pediatric obstructive sleep apnea. J Pediatr. 2001;138:838–844. doi: 10.1067/mpd.2001.114474. [DOI] [PubMed] [Google Scholar]
- 15.Burger CD, Stanson AW, Sheedy PF, 2nd, Daniels BK, Shepard JW., Jr Fast-computed tomography evaluation of age-related changes in upper airway structure and function in normal men. Am Rev Respir Dis. 1992;145:846–852. doi: 10.1164/ajrccm/145.4_Pt_1.846. [DOI] [PubMed] [Google Scholar]
- 16.Chami HA, Baldwin CM, Silverman A, Zhang Y, Rapoport D, Punjabi NM, Gottlieb DJ. Sleepiness, quality of life, and sleep maintenance in REM versus non-REM sleep-disordered breathing. Am J Respir Crit Care Med. 2010;181:997–1002. doi: 10.1164/rccm.200908-1304OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cherniack NS. Respiratory dysrhythmias during sleep. N Engl J Med. 1981;305:325–330. doi: 10.1056/NEJM198108063050606. [DOI] [PubMed] [Google Scholar]
- 18.Chervin RD, Aldrich MS. Effects of esophageal pressure monitoring on sleep architecture. Am J Respir Crit Care Med. 1997;156:881–885. doi: 10.1164/ajrccm.156.3.9701021. [DOI] [PubMed] [Google Scholar]
- 19.Chervin RD, Guilleminault C. Overestimation of sleep latency by patients with suspected hypersomnolence. Sleep. 1996;19:94–100. doi: 10.1093/sleep/19.2.94. [DOI] [PubMed] [Google Scholar]
- 20.Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dinges DF. A continued commitment to excellence. Sleep. 2009;32:1249–1250. doi: 10.1093/sleep/32.10.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eckert DJ, Malhotra A, Jordan AS. Mechanisms of apnea. Prog Cardiovasc Dis. 2009;51:313–323. doi: 10.1016/j.pcad.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eikermann M, Jordan AS, Chamberlin NL, Gautam S, Wellman A, Lo YL, White DP, Malhotra A. The influence of aging on pharyngeal collapsibility during sleep. Chest. 2007;131:1702–1709. doi: 10.1378/chest.06-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feldman SC, Kastin AJ. Localization of neurons containing immunore-active delta sleep-inducing peptide in the rat brain: An immunocyto-chemical study. Neuroscience. 1984;11:303–317. doi: 10.1016/0306-4522(84)90025-3. [DOI] [PubMed] [Google Scholar]
- 25.Flemons WW, Douglas NJ, Kuna ST, Rodenstein DO, Wheatley J. Access to diagnosis and treatment of patients with suspected sleep apnea. Am J Respir Crit Care Med. 2004;169:668–672. doi: 10.1164/rccm.200308-1124PP. [DOI] [PubMed] [Google Scholar]
- 26.Fogel RB, Malhotra A, Dalagiorgou G, Robinson MK, Jakab M, Kikinis R, Pittman SD, White DP. Anatomic and physiologic predictors of apnea severity in morbidly obese subjects. Sleep. 2003;26:150–155. doi: 10.1093/sleep/26.2.150. [DOI] [PubMed] [Google Scholar]
- 27.Gleadhill I, Schwartz A, Wise R, Permutt S, Smith P. Upper airway collapsibility in snorers and in patients with obstructive hypopnea and apnea. Am Rev Respir Dis. 1991;143:1300–1303. doi: 10.1164/ajrccm/143.6.1300. [DOI] [PubMed] [Google Scholar]
- 28.Gleeson K, Zwillich CW, Bendrick TW, White DP. Effect of inspiratory nasal loading on pharyngeal resistance. J Appl Physiol. 1986;60:1882–1886. doi: 10.1152/jappl.1986.60.6.1882. [DOI] [PubMed] [Google Scholar]
- 29.Gottfried S, Strohl K, Van de Graaff W, Fouke J, DiMarco A. Effects of phrenic stimulation on upper airway resistance in anesthetized dogs. J Appl Physiol. 1983;55:419–426. doi: 10.1152/jappl.1983.55.2.419. No abstract available. [DOI] [PubMed] [Google Scholar]
- 30.Gottlieb DJ, Yenokyan G, Newman AB, O'Connor GT, Punjabi NM, Quan SF, Redline S, Resnick HE, Tong EK, Diener-West M, Shahar E. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: The sleep heart health study. Circulation. 2010;122:352–360. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haponik E, Smith P, Bohlman M, Allan R, Goldman S, Bleecker E. Computerized tomography in obstructive sleep apnea: Correlation of airway size with physiology during sleep and wakefulness. Am Rev Respir Dis. 1983;127:221–226. doi: 10.1164/arrd.1983.127.2.221. [DOI] [PubMed] [Google Scholar]
- 32.Haxhiu MA, Kc P, Balan KV, Wilson CG, Martin RJ. Modeling of sleep-induced changes in airway function: Implication for nocturnal worsening of bronchial asthma. Adv Exp Med Biol. 2008;605:469–474. doi: 10.1007/978-0-387-73693-8_82. [DOI] [PubMed] [Google Scholar]
- 33.Heinzer RC, Stanchina ML, Malhotra A, Fogel RB, Patel SR, Jordan AS, Schory K, White DP. Lung volume and continuous positive airway pressure requirements in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172:114–117. doi: 10.1164/rccm.200404-552OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heinzer RC, Stanchina ML, Malhotra A, Jordan AS, Patel SR, Lo YL, Wellman A, Schory K, Dover L, White DP. Effect of increased lung volume on sleep disordered breathing in patients with sleep apnoea. Thorax. 2006;61:435–439. doi: 10.1136/thx.2005.052084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heinzer RC, White DP, Jordan AS, Lo YL, Dover L, Stevenson K, Malhotra A. Trazodone increases arousal threshold in obstructive sleep apnoea. Eur Respir J. 2008;31:1308–1312. doi: 10.1183/09031936.00067607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoffstein V, Zamel N, Phillipson EA. Lung volume dependence of pharyngeal cross-sectional area in patients with obstructive sleep apnea. Am Rev Respir Dis. 1984;130:175–178. doi: 10.1164/arrd.1984.130.2.175. [DOI] [PubMed] [Google Scholar]
- 37.Horner R, Innes J, Morrell M, Shea S, Guz A. The effect of sleep on reflex genioglossus muscle activation by stimuli of negative airway pressure in humans. J Physiol (Lond) 1994;476:141–151. [PMC free article] [PubMed] [Google Scholar]
- 38.Horner RL. Motor control of the pharyngeal musculature and implications for the pathogenesis of obstructive sleep apnea. Sleep. 1996;19:827–853. doi: 10.1093/sleep/19.10.827. [DOI] [PubMed] [Google Scholar]
- 39.Horner RL. Impact of brainstem sleep mechanisms on pharyngeal motor control. Respir Physiol. 2000;119:113–121. doi: 10.1016/s0034-5687(99)00106-1. [DOI] [PubMed] [Google Scholar]
- 40.Horner RL. The neuropharmacology of upper airway motor control in the awake and asleep states: Implications for obstructive sleep apnoea. Respir Res. 2001;2:286–294. doi: 10.1186/rr71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horner RL, Innes JA, Guz A. Reflex pharyngeal dilator muscle activation by stimuli of negative airway pressure in awake man. Sleep. 1993;16:S85–S86. doi: 10.1093/sleep/16.suppl_8.s85. [DOI] [PubMed] [Google Scholar]
- 42.Horner RL, Innes JA, Holden HB, Guz A. Afferent pathway(s) for pharyngeal dilator reflex to negative pressure in man: A study using upper airway anaesthesia. J Physiol (Lond) 1991;436:31–44. doi: 10.1113/jphysiol.1991.sp018537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang Y, White DP, Malhotra A. Use of computational modeling to predict responses to upper airway surgery in obstructive sleep apnea. Laryngoscope. 2007;117:648–653. doi: 10.1097/MLG.0b013e318030ca55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Isono S, Remmers JE, Tanaka A, Sho Y, Sato J, Nishino T. Anatomy of pharynx in patients with obstructive sleep apnea and in normal subjects. J Appl Physiol. 1997;82:1319–1326. doi: 10.1152/jappl.1997.82.4.1319. [DOI] [PubMed] [Google Scholar]
- 45.Jordan AS, White DP, Owens RL, Eckert DJ, Rahangdale S, Yim-Yeh S, Malhotra A. The effect of increased genioglossus activity and end-expiratory lung volume on pharyngeal collapse. J Appl Physiol. 2010;109:469–475. doi: 10.1152/japplphysiol.00373.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kairaitis K, Byth K, Parikh R, Stavrinou R, Wheatley JR, Amis TC. Tracheal traction effects on upper airway patency in rabbits: The role of tissue pressure. Sleep. 2007;30:179–186. doi: 10.1093/sleep/30.2.179. [DOI] [PubMed] [Google Scholar]
- 47.Kairaitis K, Stavrinou R, Parikh R, Wheatley JR, Amis TC. Mandibular advancement decreases pressures in the tissues surrounding the upper airway in rabbits. J Appl Physiol. 2006;100:349–356. doi: 10.1152/japplphysiol.00560.2005. [DOI] [PubMed] [Google Scholar]
- 48.Kapur VK, Resnick HE, Gottlieb DJ. Sleep disordered breathing and hypertension: Does self-reported sleepiness modify the association? Sleep. 2008;31:1127–1132. [PMC free article] [PubMed] [Google Scholar]
- 49.Kezirian EJ, White DP, Malhotra A, Ma W, McCulloch CE, Goldberg AN. Interrater reliability of drug-induced sleep endoscopy. Arch Otolaryngol Head Neck Surg. 2010;136:393–397. doi: 10.1001/archoto.2010.26. [DOI] [PubMed] [Google Scholar]
- 50.Khoo M. Determinants of ventilatory instability and variability. Respir Phsiol. 2000;122:167–182. doi: 10.1016/s0034-5687(00)00157-2. [DOI] [PubMed] [Google Scholar]
- 51.Kirkness JP, Christenson HK, Garlick SR, Parikh R, Kairaitis K, Wheat-ley JR, Amis TC. Decreased surface tension of upper airway mucosal lining liquid increases upper airway patency in anaesthetised rabbits. J Physiol. 2003;547:603–611. doi: 10.1113/jphysiol.2002.031013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kirkness JP, Madronio M, Stavrinou R, Wheatley JR, Amis TC. Relationship between surface tension of upper airway lining liquid and upper airway collapsibility during sleep in obstructive sleep apnea hypopnea syndrome. J Appl Physiol. 2003;95:1761–1766. doi: 10.1152/japplphysiol.00488.2003. [DOI] [PubMed] [Google Scholar]
- 53.Kirkness JP, Madronio M, Stavrinou R, Wheatley JR, Amis TC. Surface tension of upper airway mucosal lining liquid in obstructive sleep apnea/hypopnea syndrome. Sleep. 2005;28:457–463. doi: 10.1093/sleep/28.4.457. [DOI] [PubMed] [Google Scholar]
- 54.Kobayashi I, Perry A, Rhymer J, Wuyam B, Hughes P, Murphy K, Innes J, McIvor J, Cheesman AD, Guz A. Inspiratory coactivation of the genioglossus enlarges retroglossal space in laryngectomized humans. J Appl Physiol. 1996;80:1595–1604. doi: 10.1152/jappl.1996.80.5.1595. [DOI] [PubMed] [Google Scholar]
- 55.Kubin L, Kimura H, Tojima H, Davies RO, Pack AI. Suppression of hypoglossal motoneurons during the carbachol-induced atonia of REM sleep is not caused by fast synaptic inhibition. Brain Res. 1993;611:300–312. doi: 10.1016/0006-8993(93)90517-q. [DOI] [PubMed] [Google Scholar]
- 56.Kubin L, Reignier C, Tojima H, Taguchi O, Pack AI, Davies RO. Changes in serotonin level in the hypoglossal nucleus region during carbachol-induced atonia. Brain Res. 1994;645:291–302. doi: 10.1016/0006-8993(94)91663-2. [DOI] [PubMed] [Google Scholar]
- 57.Kubin L, Tojima H, Davies RO, Pack AI. Serotonergic excitatory drive to hypoglossal motoneuron in the decerebrate cat. Neuroscience Letters. 1992;139:243–248. doi: 10.1016/0304-3940(92)90563-m. [DOI] [PubMed] [Google Scholar]
- 58.Lavie P, Rubin AE. Effects of nasal occlusion on respiration in sleep. Evidence of inheritability of sleep apnea proneness. Acta Otolaryngol (Stockh) 1984;97:127–130. doi: 10.3109/00016488409130972. [DOI] [PubMed] [Google Scholar]
- 59.Lee WM. Acute liver failure. N Engl J Med. 1993;329:1862–1872. doi: 10.1056/NEJM199312163292508. [DOI] [PubMed] [Google Scholar]
- 60.Leith DE, Mead J. Mechanisms determining residual volume of the lungs in normal subjects. J Appl Physiol. 1967;23:221–227. doi: 10.1152/jappl.1967.23.2.221. [DOI] [PubMed] [Google Scholar]
- 61.Lo YL, Jordan AS, Malhotra A, Wellman A, Heinzer RA, Eikermann M, Schory K, Dover L, White DP. Influence of wakefulness on pharyngeal airway muscle activity. Thorax. 2007;62:799–805. doi: 10.1136/thx.2006.072488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lo YL, Jordan AS, Malhotra A, Wellman A, Heinzer RC, Schory K, Dover L, Fogel RB, White DP. Genioglossal muscle response to CO2 stimulation during NREM sleep. Sleep. 2006;29:470–477. doi: 10.1093/sleep/29.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Loring SH, Malhotra A. Inspiratory efforts during mechanical ventilation: Is there risk of barotrauma? Chest. 2007;131:646–648. doi: 10.1378/chest.06-2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Loring SH, O'Donnell CR, Behazin N, Malhotra A, Sarge T, Ritz R, Novack V, Talmor D. Esophageal pressures in acute lung injury: Do they represent artifact or useful information about transpulmonary pressure, chest wall mechanics, and lung stress? J Appl Physiol. 2010;108:515–522. doi: 10.1152/japplphysiol.00835.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Madronio MR, Di Somma E, Stavrinou R, Kirkness JP, Goldfinch E, Wheatley JR, Amis TC. Older individuals have increased oro-nasal breathing during sleep. Eur Respir J. 2004;24:71–77. doi: 10.1183/09031936.04.00004303. [DOI] [PubMed] [Google Scholar]
- 66.Malhotra A, Fogel RB, Edwards JK, Shea SA, White DP. Local mechanisms drive genioglossus activation in obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161:1746–1749. doi: 10.1164/ajrccm.161.5.9907109. [DOI] [PubMed] [Google Scholar]
- 67.Malhotra A, Huang Y, Fogel R, Pillar G, Edwards JK, Kikinis R, Shea S, White DP. The male predisposition to pharyngeal collapse: The importance of airway length. Am J Resp Crit Care Med. 2002;166:1388–1395. doi: 10.1164/rccm.2112072. [DOI] [PubMed] [Google Scholar]
- 68.Malhotra A, Pillar G, Fogel R, Beauregard J, White D. Genioglossal but not palatal muscle activity relates closely to pharyngeal pressure. Am J Respir Crit Care Med. 2000;162:1058–1062. doi: 10.1164/ajrccm.162.3.9912067. [DOI] [PubMed] [Google Scholar]
- 69.Malhotra A, Pillar G, Fogel RB, Edwards JK, Ayas N, Akahoshi T, Hess D, White DP. Pharyngeal pressure and flow effects on genioglossus activation in normal subjects. Am J Respir Crit Care Med. 2002;165:71–77. doi: 10.1164/ajrccm.165.1.2011065. [DOI] [PubMed] [Google Scholar]
- 70.Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360:237–245. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 71.Malhotra A, White DP. Sleep Apnea. Lancet. 2002;360:237–245. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 72.Martinovits G, Leventon G, Goldhammer Y, Sadeh M. Vocal cord paralysis as a presenting sign in the Shy-Drager syndrome. J Laryngol Otol. 1988;102:280–281. doi: 10.1017/s0022215100104724. [DOI] [PubMed] [Google Scholar]
- 73.Mathew OP. Upper airway negative pressure effects on respiratory activity of upper airway muscles. J Appl Physiol. 1984;56:500. doi: 10.1152/jappl.1984.56.2.500. [DOI] [PubMed] [Google Scholar]
- 74.Mathew OP. Maintenance of upper airway patency. J Pediatr. 1985;106:863–869. doi: 10.1016/s0022-3476(85)80227-4. [DOI] [PubMed] [Google Scholar]
- 75.Mathew OP, Abu-Osba YK, Thach BT. Genioglossus muscle response to upper airway pressure changes: Afferent pathways. J Appl Physiol. 1982;52:445. doi: 10.1152/jappl.1982.52.2.445. [DOI] [PubMed] [Google Scholar]
- 76.Mathew OP, Abu-Osba YK, Thach BT. Influence of upper airway pressure changes on genioglossus and muscle respiratory activity. J Appl Physiol. 1982;52:438. doi: 10.1152/jappl.1982.52.2.438. [DOI] [PubMed] [Google Scholar]
- 77.Mead J, Takishima T, Leith D. Stress distribution in lungs: A model of pulmonary elasticity. J Appl Physiol. 1970;28:596–608. doi: 10.1152/jappl.1970.28.5.596. [DOI] [PubMed] [Google Scholar]
- 78.Miller MR, Pedersen OF, Pellegrino R, Brusasco V. Debating the definition of airflow obstruction: Time to move on? Eur Respir J. 2009;34:527–528. doi: 10.1183/09031936.00103309. [DOI] [PubMed] [Google Scholar]
- 79.Navajas D, Duvivier C, Farre R, Peslin R. A simplified method for monitoring respiratory impedance during continuous positive airway pressure. Eur Respir J. 2000;15:185–191. doi: 10.1183/09031936.00.15118500. [DOI] [PubMed] [Google Scholar]
- 80.Navajas D, Farre R, Rotger M, Badia R, Puig-de-Morales M, Montserrat JM. Assessment of airflow obstruction during CPAP by means of forced. Am J Respir Crit Care Med. 1998;157:1526–1530. doi: 10.1164/ajrccm.157.5.9710026. [DOI] [PubMed] [Google Scholar]
- 81.Nielsen TM, Pedersen OF. The effect of CO2 on peripheral airways. Acta Physiol Scand. 1976;98:192–199. [PubMed] [Google Scholar]
- 82.Nielsen TM, Pedersen OF. A method to correct for the influence of gas density on maximal expiratory flow rate. Acta Physiol Scand. 1976;98:123–130. doi: 10.1111/j.1748-1716.1976.tb10311.x. [DOI] [PubMed] [Google Scholar]
- 83.O'donnell CP, Schaub CD, Haines AS, Berkowitz DE, Tankersley CG, Schwartz AR, Smith PL. Leptin prevents respiratory depression in obesity. Am J Respir Crit Care Med. 1999;159:1477–1484. doi: 10.1164/ajrccm.159.5.9809025. [DOI] [PubMed] [Google Scholar]
- 84.Oliven R, Tov N, Odeh M, Gaitini L, Steinfeld U, Schwartz AR, Oliven A. Interacting effects of genioglossus stimulation and mandibu-lar advancement in sleep apnea. J Appl Physiol. 2009;106:1668–1673. doi: 10.1152/japplphysiol.91501.2008. [DOI] [PubMed] [Google Scholar]
- 85.Olson LG, Strohl KP. Non-muscular factors in upper airway patency in the rabbit. Respir Physiol. 1988;71:147–155. doi: 10.1016/0034-5687(88)90012-6. [DOI] [PubMed] [Google Scholar]
- 86.Olson LG, Ulmer LG, Saunders NA. Pressure-volume properties of the upper airway of rabbits. J Appl Physiol. 1989;66:759–763. doi: 10.1152/jappl.1989.66.2.759. [DOI] [PubMed] [Google Scholar]
- 87.Olson LG, Strohl KP. The response of the nasal airway to exercise. Am Rev Respir Dis. 1987;135:356–359. doi: 10.1164/arrd.1987.135.2.356. [DOI] [PubMed] [Google Scholar]
- 88.Onal E, Lopata M, O' Connor TD. Diaphragmatic and genioglossal electromyogram responses to CO2 rebreathing in humans. J Appl Physiol. 1981;50:1052–1055. doi: 10.1152/jappl.1981.50.5.1052. [DOI] [PubMed] [Google Scholar]
- 89.Orem J, Netick A, Dement WC. Breathing during sleep and wakefulness in the cat. Respir Physiol. 1977;30:265–289. doi: 10.1016/0034-5687(77)90035-4. [DOI] [PubMed] [Google Scholar]
- 90.Owens RL, Eckert DJ, Yeh SY, Malhotra A. Upper airway function in the pathogenesis of obstructive sleep apnea: A review of the current literature. Curr Opin Pulm Med. 2008;14:519–524. doi: 10.1097/MCP.0b013e3283130f66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Owens RL, Malhotra A, Eckert DJ, White DP, Jordan AS. The influence of end-expiratory lung volume on measurements of pharyngeal collapsibility. J Appl Physiol. 2010;108:445–451. doi: 10.1152/japplphysiol.00755.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Patil S, Punjabi N, Schneider H, O'Donnell CP, Smith PL, Schwartz AR. A simplified method for measuring critical pressures during sleep in the clinical setting. Am J Respir Crit Care Med. 2004;170:86–93. doi: 10.1164/rccm.200309-1239OC. [DOI] [PubMed] [Google Scholar]
- 93.Patil S, Schneider H, Gladmon E, Magnuson T, Smith PL, O'Donnell CP, Schwartz AR. Obesity and upper airway mechanical control during sleep. Am J Respir Crit Care Med. 2004;169:A435. [Google Scholar]
- 94.Patil SP, Schneider H, Marx JJ, Gladmon E, Schwartz AR, Smith PL. Neuromechanical control of upper airway patency during sleep. J Appl Physiol. 2007;102:547–556. doi: 10.1152/japplphysiol.00282.2006. [DOI] [PubMed] [Google Scholar]
- 95.Pedersen OF. A new way to describe the mechanics of the expiration. Bull Physiopathol Respir (Nancy) 1972;8:569–586. [PubMed] [Google Scholar]
- 96.Pedersen OF. The mechanics of the expiration evaluated by a model. Acta Physiol Scand Suppl. 1973;386:1–26. [PubMed] [Google Scholar]
- 97.Pedersen OF, Castile RG, Drazen JM, Ingram RH., Jr Density dependence of maximum expiratory flow in the dog. J Appl Physiol. 1982;53:397–404. doi: 10.1152/jappl.1982.53.2.397. [DOI] [PubMed] [Google Scholar]
- 98.Pedersen OF, Ingram RH., Jr Configuration of maximum expiratory flow-volume curve: Model experiments with physiological implications. J Appl Physiol. 1985;58:1305–1313. doi: 10.1152/jappl.1985.58.4.1305. [DOI] [PubMed] [Google Scholar]
- 99.Pedersen OF, Nielsen TM. The critical transmural pressure of the airway. Acta Physiol Scand. 1976;97:426–446. doi: 10.1111/j.1748-1716.1976.tb10283.x. [DOI] [PubMed] [Google Scholar]
- 100.Pedersen OF, Nielsen TM. The compliance curve for the flow limiting segments of the airway. I. Model studies. Acta Physiol Scand. 1977;99:385–398. doi: 10.1111/j.1748-1716.1977.tb10392.x. [DOI] [PubMed] [Google Scholar]
- 101.Pedersen OF, Nielsen TM. The compliance curve for the flow limiting segments of the airway. II. Experiments with human subjects. Acta Physiol Scand. 1977;100:139–153. doi: 10.1111/j.1748-1716.1977.tb05931.x. [DOI] [PubMed] [Google Scholar]
- 102.Phillipson EA. Control of breathing during sleep. Am Rev Respir Dis. 1978;118:909–939. doi: 10.1164/arrd.1978.118.5.909. [DOI] [PubMed] [Google Scholar]
- 103.Pillar G, Malhotra A, Fogel RB, Beauregard J, Slamowitz DI, Shea SA, White DP. Upper airway muscle responsiveness to rising PCO2 during NREM sleep. J Appl Physiol. 2000;89:1275–1282. doi: 10.1152/jappl.2000.89.4.1275. [DOI] [PubMed] [Google Scholar]
- 104.Platt AB, Kuna ST, Field SH, Chen Z, Gupta R, Roche DF, Christie JD, Asch DA. Adherence to sleep apnea therapy and use of lipid-lowering drugs: A study of the healthy-user effect. Chest. 2010;137:102–108. doi: 10.1378/chest.09-0842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Proctor DF. The naso-oro-pharyngo-laryngeal airway. Eur J Respir Dis Suppl. 1983;128(Pt 1):89–96. [PubMed] [Google Scholar]
- 106.Remmers JE, Issa FG, Suratt PM. Sleep and respiration. J Appl Physiol. 1990;68:1286–1289. doi: 10.1152/jappl.1990.68.3.1286. [DOI] [PubMed] [Google Scholar]
- 107.Remmers JE, Lahiri S. Regulating the ventilatory pump: A splendid control system prone to fail during sleep. Am J Respir Crit Care Med. 1998;157:S95–S100. doi: 10.1164/ajrccm.157.4.nhldi-6. [DOI] [PubMed] [Google Scholar]
- 108.Remmers JE, Launois S, Feroah T, Whitelaw WA. Mechanics of the pharynx in patients with obstructive sleep apnea. Prog Clin Biol Res. 1990;345:261–268. discussion 269-271. [PubMed] [Google Scholar]
- 109.Remmers JE, Putnam M, Bartlett D., Jr Proceedings: Changes in respiratory pattern accompanying sleep in cats. Bull Physiopathol Respir (Nancy) 1975;11:86P–87P. [PubMed] [Google Scholar]
- 110.Rogers NL, Dinges DF, Allison KC, Maislin G, Martino N, O'Reardon JP, Stunkard AJ. Assessment of sleep in women with night eating syndrome. Sleep. 2006;29:814–819. doi: 10.1093/sleep/29.6.814. [DOI] [PubMed] [Google Scholar]
- 111.Ross RJ, Ball WA, Dinges DF, Kribbs NB, Morrison AR, Silver SM, Mulvaney FD. Rapid eye movement sleep disturbance in posttraumatic stress disorder. Biol Psychiatry. 1994;35:195–202. doi: 10.1016/0006-3223(94)91152-5. [DOI] [PubMed] [Google Scholar]
- 112.Rowley JA, Sanders CS, Zahn BR, Badr MS. Effect of REM sleep on retroglossal cross-sectional area and compliance in normal subjects. J Appl Physiol. 2001;91:239–248. doi: 10.1152/jappl.2001.91.1.239. [DOI] [PubMed] [Google Scholar]
- 113.Rutishauser W, Banchero N, Tsakiris A, Wood EH. Pleural and esophageal pressure in abdominal and dorsal position. Helv Physiol Pharmacol Acta. 1965;65:C45–C47. [PubMed] [Google Scholar]
- 114.Rutishauser WJ, Banchero N, Tsakiris AG, Sturm RE, Wood EH. End-expiratory pleural pressures in dogs in supine and prone body positions studied without thoracotomy. Amrl-Tr-64-133. AMRL TR. 1964;13:1–24. [PubMed] [Google Scholar]
- 115.Saboisky JP, Chamberlin NL, Malhotra A. Potential therapeutic targets in obstructive sleep apnoea. Expert Opin Ther Targets. 2009;13:795–809. doi: 10.1517/14728220903005608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Saboisky JP, Jordan AS, Eckert DJ, White DP, Trinder JA, Nicholas CL, Gautam S, Malhotra A. Recruitment and rate-coding strategies of the human genioglossus muscle. J Appl Physiol. 2010;109:1939–1949. doi: 10.1152/japplphysiol.00812.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sauerland EK, Harper RM. The human tongue during sleep: Electromyographic activity of the genioglossus muscle. Exp Neurol. 1976;51:160–170. doi: 10.1016/0014-4886(76)90061-3. [DOI] [PubMed] [Google Scholar]
- 118.Sauerland EK, Mitchell SP. Electromyographic activity of the human Genioglossus muscle in response to respiration and to positional changes of the head. Bull Los Angeles Neurol Soc. 1970;35:69–73. [PubMed] [Google Scholar]
- 119.Schilder DP, Roberts A, Fry DL. Effect of gas density and viscosity on the maximal expiratory flow-volume relationship. J Clin Invest. 1963;42:1705–1713. doi: 10.1172/JCI104856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schwab R, Pasirstein M, Pierson R, Mackley A, Hachadoorian R, Arens R, Maislin G, Pack A. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168:522–530. doi: 10.1164/rccm.200208-866OC. [DOI] [PubMed] [Google Scholar]
- 121.Schwab RJ. Upper airway imaging. Clin Chest Med. 1998;19:33–54. doi: 10.1016/s0272-5231(05)70430-5. [DOI] [PubMed] [Google Scholar]
- 122.Schwartz AR, Smith PL, Wise RA, Gold AR, Permutt S. Induction of upper airway occlusion in sleeping individuals with subatmospheric nasal pressure. J Appl Physiol. 1988;64:535–542. doi: 10.1152/jappl.1988.64.2.535. [DOI] [PubMed] [Google Scholar]
- 123.Series FC, Cormier Y, Desmeules M. Influence of passive changes of lung volume on upper airways. J Appl Physiol. 1990;68:2159–2164. doi: 10.1152/jappl.1990.68.5.2159. [DOI] [PubMed] [Google Scholar]
- 124.Shelton KE, Woodson H, Gay S, Suratt PM. Pharyngeal fat in obstructive sleep apnea. Am Rev Respir Dis. 1993;148:462–466. doi: 10.1164/ajrccm/148.2.462. [DOI] [PubMed] [Google Scholar]
- 125.Shelton KE, Woodson H, Gay SB, Suratt PM. Adipose tissue deposition in sleep apnea. Sleep. 1993;16:S103. discussion S103-S105. [PubMed] [Google Scholar]
- 126.Shelton RL, Jr, Bosma JF. Maintenance of the pharyngeal airway. J Appl Physiol. 1962;17:209–214. doi: 10.1152/jappl.1962.17.2.209. [DOI] [PubMed] [Google Scholar]
- 127.Simpson N, Dinges DF. Sleep and inflammation. Nutr Rev. 2007;65:S244–S252. doi: 10.1111/j.1753-4887.2007.tb00371.x. [DOI] [PubMed] [Google Scholar]
- 128.Skatrud J, Dempsey J, Badr S, Begle R. Effect of airway impedance on CO2 retention and respiratory muscle activity during NREM sleep. J Appl Physiol. 1988;65:1676–1685. doi: 10.1152/jappl.1988.65.4.1676. [DOI] [PubMed] [Google Scholar]
- 129.Stamatakis K, Sanders MH, Caffo B, Resnick HE, Gottlieb DJ, Mehra R, Punjabi NM. Fasting glycemia in sleep disordered breathing: Lowering the threshold on oxyhemoglobin desaturation. Sleep. 2008;31:1018–1024. [PMC free article] [PubMed] [Google Scholar]
- 130.Stanchina ML, Malhotra A, Fogel RB, Ayas N, Edwards JK, Schory K, White DP. Genioglossus muscle responsiveness to chemical and mechanical stimuli during non-rapid eye movement sleep. Am J Respir Crit Care Med. 2002;165:945–949. doi: 10.1164/ajrccm.165.7.2108076. [DOI] [PubMed] [Google Scholar]
- 131.Stanchina ML, Malhotra A, Fogel RB, Trinder J, Edwards JK, Schory K, White DP. The influence of lung volume on pharyngeal mechanics, collapsibility, and genioglossus muscle activation during sleep. Sleep. 2003;26:851–856. doi: 10.1093/sleep/26.7.851. [DOI] [PubMed] [Google Scholar]
- 132.Strohl K, Hensley MJ, Hallett M, Saunders NA, Ingram RH., Jr Activation of upper airway muscles before onset of inspiration in normal humans. J Appl Physiol. 1980;49:638–642. doi: 10.1152/jappl.1980.49.4.638. [DOI] [PubMed] [Google Scholar]
- 133.Strohl KP, Redline S. Nasal CPAP therapy, upper airway muscle activation, and obstructive sleep apnea. Am Rev Respir Dis. 1986;134:555–558. doi: 10.1164/arrd.1986.134.3.555. [DOI] [PubMed] [Google Scholar]
- 134.Suratt PM, Dee P, Atkinson RL, Armstrong P, Wilhoit SC. Fluoroscopic and computed tomographic features of the pharyngeal airway in obstructive sleep apnea. Am Rev Respir Dis. 1983;127:487–492. doi: 10.1164/arrd.1983.127.4.487. [DOI] [PubMed] [Google Scholar]
- 135.Suratt PM, Wilhoit SC, Cooper K. Induction of airway collapse with subatmospheric pressure in awake patients with sleep apnea. J Appl Physiol. 1984;57:140–146. doi: 10.1152/jappl.1984.57.1.140. [DOI] [PubMed] [Google Scholar]
- 136.Talmor D, Sarge T, Legedza A, O'Donnell CR, Ritz R, Loring SH, Malhotra A. Cytokine release following recruitment maneuvers. Chest. 2007;132:1434–1439. doi: 10.1378/chest.07-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Talmor D, Sarge T, Malhotra A, O'Donnell CR, Ritz R, Lisbon A, Novack V, Loring SH. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med. 2008;359:2095–2104. doi: 10.1056/NEJMoa0708638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Teodorescu M, Consens FB, Bria WF, Coffey MJ, McMorris MS, Weatherwax KJ, Durance A, Palmisano J, Senger CM, Chervin RD. Correlates of daytime sleepiness in patients with asthma. Sleep Med. 2006;7:607–613. doi: 10.1016/j.sleep.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 139.Thomas RJ, Weiss MD, Mietus JE, Peng CK, Goldberger AL, Gottlieb DJ. Prevalent hypertension and stroke in the Sleep Heart Health Study: Association with an ECG-derived spectrographic marker of cardiopul-monary coupling. Sleep. 2009;32:897–904. [PMC free article] [PubMed] [Google Scholar]
- 140.Tsakiris A, Rutishauser W, Banchero N, Donald D, Wood EH. The innervated and denervated heart in the overcoming of pressure and time volume increase. Cardiologia. 1966;48:354–356. [PubMed] [Google Scholar]
- 141.Turner MJ, MacLeod IM, Rothberg AD. Effects of temperature and composition on the viscosity of respiratory gases. J Appl Physiol. 1989;67:472–477. doi: 10.1152/jappl.1989.67.1.472. [DOI] [PubMed] [Google Scholar]
- 142.Van de Graaff WB. Thoracic influence on upper airway patency. J Appl Physiol. 1988;65:2124–2131. doi: 10.1152/jappl.1988.65.5.2124. [DOI] [PubMed] [Google Scholar]
- 143.Van de Graaff WB. Thoracic traction on the trachea: Mechanisms and magnitude. J Appl Physiol. 1991;70:1328–1363. doi: 10.1152/jappl.1991.70.3.1328. [DOI] [PubMed] [Google Scholar]
- 144.van de Graaff WB, Gottfried SB, Mitra J, van Lunteren E, Cherniack NS, Strohl KP. Respiratory functions of hyoid muscles and hyoid arch. J Appl Physiol. 1984;57:197–204. doi: 10.1152/jappl.1984.57.1.197. [DOI] [PubMed] [Google Scholar]
- 145.Van Lunteran E, Strohl KP. The muscles of the upper airways. Clin Chest Med. 1986;7:171–188. [PubMed] [Google Scholar]
- 146.Vanuxem D, Badier M, Guillot C, Delpierre S, Jahjah F, Vanuxem P. Impairment of muscle energy metabolism in patients with sleep apnoea. Respir Med. 1997;91:551–557. doi: 10.1016/s0954-6111(97)90089-5. [DOI] [PubMed] [Google Scholar]
- 147.Vazquez JC, Tsai WH, Flemons WW, Masuda A, Brant R, Hajduk E, Whitelaw WA, Remmers JE. Automated analysis of digital oximetry in the diagnosis of obstructive sleep apnoea. Thorax. 2000;55:302–307. doi: 10.1136/thorax.55.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Verma M, Seto-Poon M, Wheatley JR, Amis TC, Kirkness JP. Influence of breathing route on upper airway lining liquid surface tension in humans. J Physiol. 2006;574:859–866. doi: 10.1113/jphysiol.2005.102129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vincent NJ, Knudson R, Leith DE, Macklem PT, Mead J. Factors influencing pulmonary resistance. J Appl Physiol. 1970;29:236–243. doi: 10.1152/jappl.1970.29.2.236. [DOI] [PubMed] [Google Scholar]
- 150.Volgin DV, Rukhadze I, Kubin L. Hypoglossal premotor neurons of the intermediate medullary reticular region express cholinergic markers. J Appl Physiol. 2008;105:1576–1584. doi: 10.1152/japplphysiol.90670.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Weaver TE, Laizner AM, Evans LK, Maislin G, Chugh DK, Lyon K, Smith PL, Schwartz AR, Redline S, Pack AI, Dinges DF. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20:835–843. [PubMed] [Google Scholar]
- 152.Weaver TE, Maislin G, Dinges DF, Bloxham T, George CF, Greenberg H, Kader G, Mahowald M, Younger J, Pack AI. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30:711–719. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Welch K, Foster G, Ritter C, Wadden T, Arens R, Maislin G, Schwab R. A novel volumetric magnetic resonance imaging paradigm to study upper airway anatomy. Sleep. 2002;25:532–542. [PubMed] [Google Scholar]
- 154.Wellman A, Eckert DJ, Jordan AS, Edwards BA, Passaglia CL, Jackson AC, Gautam S, Owens RL, Malhotra A, White DP. A method for measuring and modeling the physiological traits causing obstructive sleep apnea. J Appl Physiol. 2011;110:1627–1637. doi: 10.1152/japplphysiol.00972.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wellman A, Jordan AS, Malhotra A, Fogel RB, Katz ES, Schory K, Edwards JK, White DP. Ventilatory control and airway anatomy in obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:1225–1232. doi: 10.1164/rccm.200404-510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wheatley J, Mezzanotte W, Tangel D, White D. Influence of sleep on genioglossus muscle activation by negative pressure in normal men. Am Rev Respir Dis. 1993;148:597–605. doi: 10.1164/ajrccm/148.3.597. [DOI] [PubMed] [Google Scholar]
- 157.Wheatley J, Tangel D, Mezzanotte W, White D. Influence of sleep on response to negative airway pressure of tensor palatini muscle and retropalatal airway. J Appl Physiol. 1993;75:2117–2124. doi: 10.1152/jappl.1993.75.5.2117. [DOI] [PubMed] [Google Scholar]
- 158.Wheatley JR, Tangel DJ, Mezzanotte WS, White DP. Influence of sleep on alae nasi EMG and nasal resistance in normal men. J Appl Physiol. 1993;75:626–632. doi: 10.1152/jappl.1993.75.2.626. [DOI] [PubMed] [Google Scholar]
- 159.Wilkinson V, Malhotra A, Nicholas CL, Worsnop C, Jordan AS, Butler JE, Saboisky JP, Gandevia SC, White DP, Trinder J. Discharge patterns of human genioglossus motor units during arousal from sleep. Sleep. 2010;33:379–387. doi: 10.1093/sleep/33.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Younes M, Ostrowski M, Thompson W, Leslie C, Shewchuk W. Chemical control stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163:1181–1190. doi: 10.1164/ajrccm.163.5.2007013. [DOI] [PubMed] [Google Scholar]
- 161.Zhou XS, Rowley JA, Demirovic F, Diamond MP, Badr MS. Effect of testosterone on the apneic threshold in women during NREM sleep. J Appl Physiol. 2003;94:101–107. doi: 10.1152/japplphysiol.00264.2002. [DOI] [PubMed] [Google Scholar]