Abstract
Purpose
To determine prevalence and risk factors for renal scar in children referred for urologic assessment of febrile UTI and/or VUR.
Methods
Pre-determined risk factors for renal scar were prospectively recorded in consecutive patients referred for UTI/VUR. Age, gender, VUR grade, and reported number of febrile and non-febrile UTIs were analyzed with logistic regression to determine risk for focal cortical defects on non-acute DMSA.
Results
Of 565 consecutive children, 24 (4%) had congenital renal dysplasia and 84 (15.5%) had focal defect(s). VUR, especially grades IV–V, recurrent febrile UTI, and older age increased risk. For any age child with the same number of UTIs, VUR increased odds of renal defect 5.4-fold (OR = 5.4, 95% CI = 2.7–10.6, AUC = 0.759).
Conclusions
Focal DMSA defects were present in 15.5% of 565 consecutive children referred for febrile UTI and/or VUR; 4% had presumed congenital reflux nephropathy without cortical defect. All VUR grades increased risk for these defects, as did recurrent febrile UTIs and older age. However, 43% with grades IV–V VUR and 76% with recurrent UTI had normal DMSA.
Keywords: Focal renal cortical defect, Renal scar, Vesicoureteral reflux, Urinary tract infection, DMSA, Technetium-99m dimercaptosuccinic acid renography
Introduction
One justification for the detection and management of vesicoureteral reflux (VUR) after urinary tract infection (UTI) is increased risk for renal scarring when VUR is present. However, few studies report likelihood for renal scar after UTI, with sometimes conflicting results in patient populations that may not be representative of those referred to pediatric urologists. For example, some prospective studies using dimercapto-succinic acid (DMSA) scintigraphy to diagnose renal scarring found increased risk in patients with VUR [1–3] while others found no increased risk [4–6]. Of the populations evaluated in these studies, 4 were limited to children with their first febrile UTI [1,3,5,6], 2 were limited to children less than 1 or 2 years of age [1,5], and 1 was limited to patients with unilateral VUR [3].
Previously reported meta-analyses [7–9] demonstrate an overall increased risk for “abnormal DMSA” in children with UTI and VUR ranging from 2.6 to 2.8 times. Yet the meta-analysis performed by the American Urological Association (AUA) VUR Guideline Update Committee comprised only 4 articles, 3 limited to patients with their first known febrile UTI and 2 reporting median ages younger than 9 months [7]. A subsequent meta-analysis by Shaikh et al. was also limited to children after their first UTI, did not define abnormal DMSA (focal defects, decreased function, or both), and only 2.5% of the patients had grades IV–V VUR [9]. The meta-analysis by Faust et al. was limited to patients with acute DMSA lesions to determine subsequent renal scarring, and so was not designed to evaluate scar rates among patients with and without VUR [8]. The fact that each meta-analysis reported approximately the same odds for renal scarring with VUR is not surprising since there was considerable overlap in the reviewed articles.
Given the small numbers of patients with grade-stratified VUR evaluated for renal scarring, narrow inclusion criteria, and heterogeneity between study populations in published reports, the risk for renal scarring posed by VUR remains incompletely defined. This is especially true among patients referred to pediatric urologists, who present at various ages, often after more than 1 febrile UTI, and may have proportionately higher grades of VUR than encountered in studies by primary care providers. Consequently, we obtained non-acute DMSA scintigraphy in consecutive children referred with VUR and/or febrile UTI, and now report likelihood for presumed acquired renal scarring and associated baseline risk factors in this cross sectional study of consecutive patients.
Materials and methods
Patients
Datasheets were created to capture data for pre-determined factors potentially related to acquired renal scar – defined as focal cortical defect(s) on non-acute DMSA scintigraphy - including patient age, gender, VUR grade, febrile versus non-febrile UTI, the number of UTIs, and voiding habits for toilet trained children. These datasheets were then used by pediatric urology providers following a standard protocol in evaluating consecutive patients referred for febrile UTI and/or VUR between October 2008 and April 2011, with the goal of determining the percentage of referred patients with abnormal DMSA.
DMSA scintigraphy was scheduled at or beyond 3 months after the latest febrile UTI, when present. Data was prospectively entered into a database and analyzed for this report following institutional review board approval. Exclusion criteria included solitary kidney, ureteropelvic or ureterovesical junction obstruction, duplication anomalies with ectopic ureter or ureterocele, neurogenic bladder, posterior urethral valves and/or prune belly syndrome. Patients with presumed congenital reflux nephropathy (CRN), defined as ≤44% reduced ipsilateral function without focal cortical defects, were excluded from analysis of acquired renal defects.
Caregivers of toilet-trained children were questioned regarding bowel and bladder dysfunction (BBD), defined as presence of any of the following: infrequent voiding ≤3 times per day; urinary frequency ≥8 times per day; diurnal incontinence; and/or infrequent stooling <3 times per week, hard or painful stools, scybalous or excessively large stools, or bowel incontinence. BBD as a separate potential risk factor for abnormal DMSA was not assessed in our primary model since there are no standardized criteria for diagnosis and we lacked data in our large percentage of patients who were not toilet trained. Instead, we performed univariate and multivariate analysis among a subset of toilet-trained children to evaluate the risk of BBD.
Imaging
Technetium-99m DMSA (40–120 MBq) was injected intravenously, with dose calculated using Clark’s rule (weight in kg/70 × standard adult dose = 5 mCi), with a minimum dose of 1 mCi and a maximum dose of 5 mCi. Imaging was performed 1.5–3 h after injection. Images were obtained using parallel hole, low energy high resolution collimators on either a Phillips Prism 1500 single head camera or a Phillips Axis head camera. Planar images were magnified 1–4 times, based on the patient’s size. Planar images in the anterior, posterior, and right and left posterior oblique images of both kidneys were obtained for 5 min each. Magnified posterior images were obtained at 4 times magnification. Differential activity of the kidneys was calculated from both the anterior and posterior images, with total differential function determined by averaging the percentages from the anterior and posterior images.
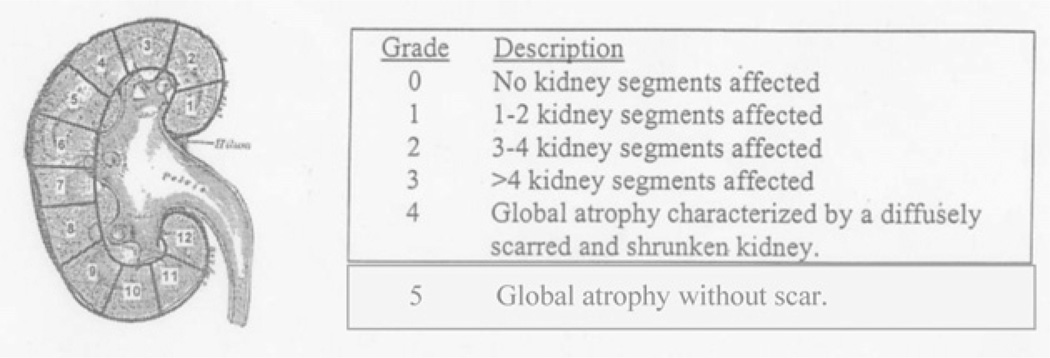
DMSA studies were reviewed by 2 pediatric radiologists blinded to VUR status, with discrepancies resolved by consensus. Results were graded using Randomized Intervention for Children with Vesicoureteral Reflux (RIVUR) criteria (Fig. 1), modified to distinguish patients with global atrophy and diffuse scarring (Category 4) versus global atrophy of ≤44% ipsilateral function without focal defects (Category 5). Presumed acquired renal scar on DMSA was defined as presence of any focal uptake defects (Categories 1–4), and presumed congenital reflux nephropathy was defined as decreased function ≤44% with a smooth renal contour without focal uptake defects (Category 5).
Figure 1.
Modified DMSA grading scale, used with permission from the RIVUR study. Abnormal DMSA was defined as presence of any focal renal defect (grades 1–4) and normal DMSA as no focal defects (grade 0). Patients with grade 5 scores had ipsilateral function ≤44% without focal.
Voiding cystourethrograms (VCUGs) were reviewed by 2 pediatric urologists to confirm reported grade using International Reflux Study criteria, with discrepancies resolved by consensus. Cases with bilateral VUR had the highest grade of reflux recorded. For the 3 patients with nuclear cystography, reflux was categorized as “mild” (grade II), “moderate” (III), and “severe” (IV).
Timing
Age in months was recorded at the time of DMSA scan. Time in months was recorded from the last documented febrile or non-febrile UTI (when present) until date of DMSA scan. In any patient who underwent DMSA before the recommended 3 months, we did not repeat DMSA when the renogram was normal. In 2 patients with focal defects on DMSA obtained <3 months after UTI, scans were repeated at 8 and 12 months, respectively, without change. Therefore, all patients with focal defects on DMSA had DMSA scans obtained ≥3 months after UTI.
UTI
UTI was defined as a symptomatic child with more than 50,000 colony-forming units (CFU)/ml in catheterized specimens, or more than 100,000 CFU/ml of a single bacterial species in voided specimens. Febrile UTI was fever >38.3 °C, and the number of UTIs was classified as none, one, and ≥2 separately for febrile and non-febrile UTI. Data regarding UTIs was obtained from hospital and primary care provider records, in addition to history provided by caregivers, with discrepancies resolved by medical records. Because prior American Academy of Pediatrics (AAP) Guidelines did not mandate abnormal urinalysis in the diagnosis of UTI, this information was not available for all patients [10]. Accordingly, we separately analyzed those with “confirmed febrile UTI” according to the most recent AAP Guidelines, with colony counts described above and pyuria on urinalysis (>5WBC/HPF in centrifuged urine) or positive leukocyte esterase on dipstick [11]. Those with febrile UTI not meeting these criteria, usually lacking urinalysis, were analyzed as “non-confirmed febrile UTI”.
Statistical analysis
The primary outcome was focal cortical defect on DMSA scintigraphy. DMSA was a binomial variable operationally defined as normal (no focal renal defects, grade 0) or abnormal (focal renal defects, grades 1–4). We chose to analyze normal versus abnormal when interim analysis revealed good agreement between radiology reviewers using a dichotomous outcome, with less reliable agreement regarding specific grade of DMSA abnormality using the RIVUR classification. We also analyzed DMSA data combining focal uptake defects and congenital reflux nephropathy in order to compare our results to meta-analyses and randomized controlled trials which typically merge these categories. Multiple logistic regression was used to estimate the odds of abnormal DMSA, modeling the probability of abnormal DMSA (focal renal defects) with stepwise regression of the following pre-determined potential risk factors: age (continuously measured in months, reported by year), grade of VUR (I–V compared to no VUR as the reference group), number of febrile and non-febrile UTIs (ranked 1 and ≥2, compared to no UTI as the reference group), and gender (binary indicator). Subset analyses were performed as listed below, including the subset of children in whom DMSA was done ≥6 months after last febrile UTI and the subset of toilet-trained children evaluating the impact of BBD. The 95% profile likelihood ratio confidence intervals (95% CI) were calculated for the adjusted Odds Ratios (OR), and the likelihood ratio Chi-square statistic was used to test for significant association between each risk factor and abnormal DMSA. The receiver operating characteristic area under the curve (AUC) for the multiple logistic regression model was reported. Variance Inflation Factor was measured for each independent covariate, all ≤2.5, suggesting multicollinearity was not present in the model. Kappa statistic was used to evaluate agreement between normal and abnormal DMSA as graded by 2 blinded radiologists. All analyses were performed using SAS software, Version 9.2 (SAS Institute, Inc., Cary, NC). The level of significance for all tests was set at α = 0.05 (two-tailed).
Results
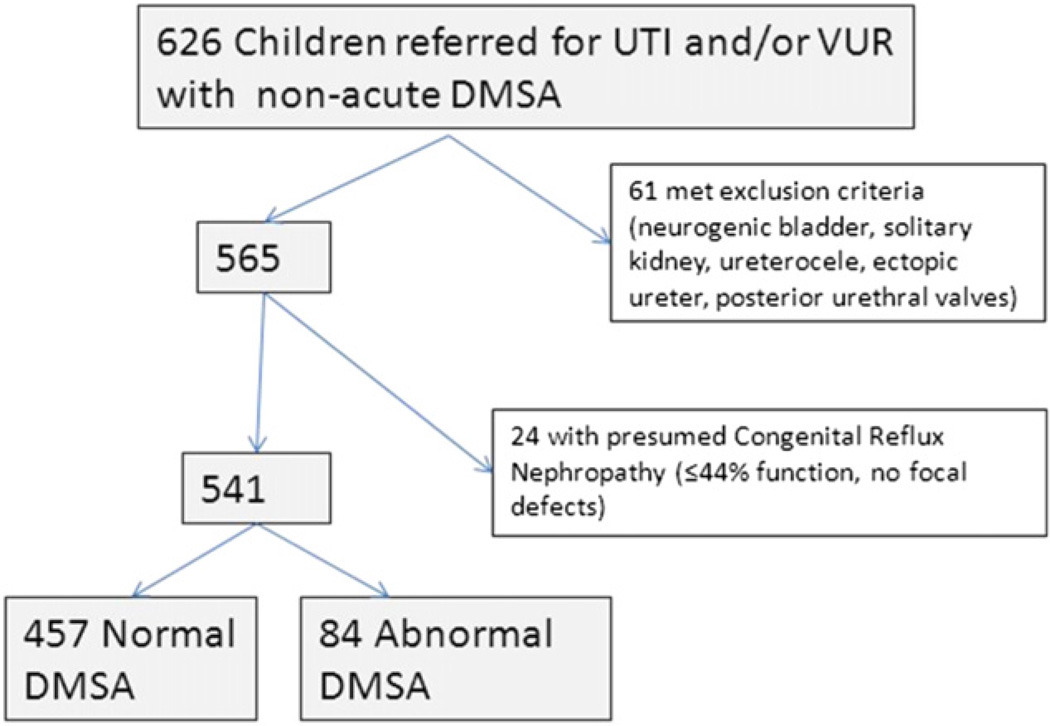
Of 626 consecutive children with DMSA scintigraphy for VUR and/or febrile UTI, 565 met inclusion criteria (Fig. 2). Among these, 24 (4%) had presumed CRN and were excluded from analysis of focal defects, while 84 (15.5%) of the remaining 541 patients had focal cortical defect(s) which we considered presumed acquired renal damage. Of the 84 patients with focal defects on DMSA, 45 (54%) had ipsilateral function ≤44%, with RIVUR-graded defects 1–4 in 46, 11, 4, and 23, respectively. Twenty-two percent of patients with RIVUR grade 1 defects had ipsilateral function ≤44%. Demographics are summarized in Table 1: mean age was 53 months (SD 44, range 3–218), with 443 (82%) female, and 115 (21%) patients less than 1 year of age. As expected, the mean age for males was younger than females (26 versus 60 months, p < 0.0001). Similarly, males were less likely than females to have experienced recurrent febrile UTIs (15.5% versus 48%, p < 0.0001). There was no history of UTI (febrile or non-febrile) in 21 patients with VUR diagnosed by prenatal hydronephrosis or sibling screening. Of these, only 1 patient with grade V VUR had focal cortical defects and 1 patient with grade IV VUR had function <45% without focal defects.
Figure 2.
Patient population.
Table 1.
Demographics, separated by those with confirmed febrile urinary tract infection (pyuria and single species growth on voided or catheterized specimen) plus those without a history of febrile urinary tract infection, and those in whom febrile UTI could not be confirmed (records unavailable, growth on urine culture without pyuria, or positive urinalysis without confirmatory urine culture).
| Confirmed febrile UTI (or no febrile UTI), n = 374 |
Unconfirmed febrile UTI, n = 191 |
Total, n = 565 | ||
|---|---|---|---|---|
| Male gender | 71 (19.0%) | 35 (18.3%) | 106 (18.8%) | |
| Age in months (SD) | 51.8 (42.4) | 55.8 (47.5) | 53.1 (44.2) | |
| <12 mo | 89 (23.8%) | 35 (18.2%) | 124 (21.9%) | |
| ≥12 mo | 285 (76.2%) | 157 (81.8%) | 442 (78.1%) | |
| Normal DMSA | 302 (80.8%) | 155 (81.2%) | 457 (80.9%) | |
| Focal defect on DMSA | 58 (15.5%) | 26 (13.6%) | 84 (14.9%) | |
| DMSA function < 45%, no focal defect | 14 (3.7%) | 10 (5.2%) | 24 (4.3%) | |
| VUR grade, n = 452 | 0 | 78 (24.9%) | 38 (23.9%) | 116 (24.6%) |
| 1 | 13 (4.2%) | 11 (6.9%) | 24 (5.1%) | |
| 2 | 91 (29.1%) | 44 (27.7%) | 135 (28.6%) | |
| 3 | 94 (30.0%) | 41 (25.7%) | 135 (28.6%) | |
| 4 | 31 (10.0%) | 18 (11.3%) | 49 (10.4%) | |
| 5 | 6 (1.9%) | 7 (4.4%) | 13 (2.8%) | |
| # Febrile UTI, n = 529 | 0 | 83 (22.6%) | N/A | 84 (15.2%) |
| 1 | 139 (37.9%) | 104 (55.9%) | 243 (43.9%) | |
| ≥2 | 145 (39.5%) | 81 (43.5%) | 226 (40.9%) | |
| # Non-febrile UTI, n = 513 | 0 | 209 (59.2%) | 125 (70.6%) | 334 (63.0%) |
| 1 | 37 (10.5%) | 7 (4.0%) | 44 (8.3%) | |
| ≥2 | 107 (30.3%) | 45 (25.4%) | 152 (28.7%) | |
Reported as mean and standard deviation (SD), or number of patients and percentage (%).
At least 1 febrile UTI was reported by caregivers and/or referring physicians in 469 children, but could not be confirmed by both pyuria and culture in 191 (39%), as shown in Table 1. However, there were no significant differences in demographics or results of those with confirmed versus non-confirmed febrile UTI. Abnormal focal DMSA defects were present in 58/374 (15%) children with confirmed febrile infection(s) versus 26/191 (14%) in those not confirmed, and results of the logistic regression demonstrated nearly identical results when limited to patients with confirmed febrile UTIs (AUC = 0.804).
DMSA was performed a median of 4.2 months (intraquartile range [IQR] 3.2–6.7; mean 7.4 months) after the last known febrile or non-febrile UTI, which was similar in patients with and without focal DMSA defects [3.8 months (IQR 3.1–5.6) versus 4.3 (IQR 3.3–6.7), respectively, p = 0.47]. All patients with positive DMSA had imaging ≥3 months after febrile UTI, including 2 patients with confirmed defects on repeat DMSA at 8 and 12 months when initial DMSA at <3 months demonstrated focal defects. Subset analysis of 110 patients with DMSA ≥6 months after febrile UTI was similar (Appendix); normal DMSA was seen in 87 (79%), focal defects in 16 (15%), and CRN in 7 (6%). Agreement between radiologists in grading DMSA scans was achieved in 96% (Kappa = 0.87, 95% CI 0.81–0.93).
Table 2 lists analysis of risk factors for focal DMSA cortical defects. Multiple logistic regression (AUC = 0.810) demonstrated increased risk with all VUR grades, but especially grades IV and V (OR = 28.2, 95% CI = 10.5–75.6; and OR = 84.9, 95% CI = 15.0–480.3), and with ≥2 febrile UTIs (OR = 3.9, 95% CI = 1.4–6.1). Focal defects occurred more often in children 1 year or older than in infants less than 1 year of age (18% versus 6%, p = 0.001), with increasing year of age an independent risk factor for focal defects in multiple logistic regression analysis (OR = 1.2, 95% CI = 1.1–1.3). Gender, non-febrile UTIs, and a single febrile UTI did not increase risk. Separate analysis of males and females (Appendix) demonstrated VUR grades IV and V were the only risk factors for cortical defects in males (OR = 6.2 and 160.7, 95% CI = 1.2–31.6 and 11.1–>999.9, AUC = 0.815).
Table 2.
Multiple logistic regression modeling for focal renal uptake defects on DSMA among 541 patients, which excludes 24 patients with presumed congenital reflux nephropathy (renal function < 45%).
| Effect | Total subjects |
N with abnormal DMSA |
OR estimate |
95% CI | P-value (χ2) | |
|---|---|---|---|---|---|---|
| Age in Years | 541 | 84 | 1.22 | 1.13–1.33 | <0.0001 | |
| Female | 443 | 71 | 0.93 | 0.34–2.32 | 0.859 | |
| VUR Grade | 0 | 112 | 17 | 1.00 | – | – |
| 1 | 23 | 4 | 3.99 | 1.07–14.84 | 0.0392 | |
| 2 | 133 | 15 | 3.08 | 1.33–7.13 | 0.0085 | |
| 3 | 130 | 19 | 3.91 | 1.67–9.15 | 0.0017 | |
| 4 | 44 | 21 | 28.19 | 10.51–75.64 | <0.0001 | |
| 5 | 10 | 6 | 84.92 | 15.01–480.32 | <0.0001 | |
| No. of Febrile UTIs | 0 | 81 | 9 | 1.00 | – | – |
| 1 | 226 | 18 | 0.62 | 0.26–1.43 | 0.259 | |
| ≥2 | 222 | 53 | 3.41 | 1.89–6.15 | 0.0218 | |
| No. of non-febrile UTIs | 0 | 321 | 46 | 1.00 | – | – |
| 1 | 45 | 9 | 1.90 | 0.73–5.00 | 0.190 | |
| ≥2 | 147 | 24 | 0.07 | 0.01–1.63 | 0.874 |
Receiver operating characteristic area under the curve for the model = 0.810.
Subset analysis of 242 toilet-trained children including BBD as a covariate (Appendix) demonstrated BBD was not an independent risk factor for focal defects with multivariate analysis (OR = 0.484, 95% CI = 0.222–1.053, AUC = 0.808). Symptoms of BBD were present in 156 (65%) of these children, with normal and abnormal DMSA present in 132 (85%) and 24 (15%), respectively. Of the 86 (35%) children without BBD, normal and abnormal DMSA were present in 64 (74%) and 22 (26%), with no difference in renal defects between groups on univariate analysis (p = 0.06).
By design we excluded patients with presumed CRN from analysis of focal cortical defects, including 8 (33%) boys and 16 (67%) girls, mean age 41 months (3–125). These patients had VUR grades I–V in 1, 2, 5, 5, and 3 patients, respectively, with no VUR and no VCUG at time of DMSA in 4 patients each. Inclusion of these 24 patients would minimally change logistic regression results (Appendix). VUR grades IV and V would still confer greatest risk for abnormal DMSA with age, febrile UTI, and VUR grades II–III also increasing risk. However, in this model no risk was associated with VUR grade I, likely because a higher proportion of these children with CRN had high grade VUR (AUC = 0.811).
In order to compare our data to other studies evaluating focal DMSA defects based solely upon VUR not stratified by grade, we performed logistic regression evaluating only the presence or absence of any grade of VUR, without controlling for the other pre-determined risk factors. This demonstrated a 2.3-fold risk of focal defects with VUR (OR = 2.3, 95% CI 1.3–4.0), similar to published meta-analyses, but the model was less robust (AUC = 0.586). However, when we analyzed the data controlling for the 3 risk factors identified with logistic regression, for any given age and same number of UTIs, a child with any grade of VUR had a 5.4-fold chance of renal scarring compared to a child without (OR = 5.4, 95% CI = 2.7–10.6, AUC = 0.759).
Discussion
Our study of 565 consecutive children referred for urologic assessment after diagnosis of febrile UTI and/or VUR demonstrates the majority (80%) had no evidence of renal damage determined by DMSA scintigraphy. Diminished ipsilateral function without cortical defects was considered evidence of congenital reflux nephropathy and occurred in 4% of patients. Focal cortical renal defects on DMSA obtained at least 3 months after febrile UTI were present in 15.5% of children, presumed to represent acquired renal damage.
All grades of VUR increased risk for such focal cortical defects versus no VUR. However, analysis showed grades I and II conferred similar risk as did grade III, while grades IV and V had much greater likelihood for DMSA cortical defects. Swerkersson et al. similarly reported likelihood for abnormal late DMSA by individual VUR grade (combining IV and V) in 80 refluxing children and also noted grades II and III to have equivalent risk, which was significantly less than grades IV–V [12]. These observations indicate the term “dilating reflux” does not accurately represent patients at highest risk for acquired renal damage when it combines grade III with higher grades. Rather, these data suggest the use of “low grade” (I–III) versus “high grade” (IV–V) VUR better conveys the relative risks posed for renal damage.
Although it is commonly stated infants have a higher risk for renal scar, our data show less likelihood for focal DMSA cortical defects in children younger than 1 year versus those older than 1 year of age, with each year of life increasing odds of having focal DMSA defect(s) after UTI. Our finding of cortical defects in 6% of 115 children less than 1 year of age is similar to the 7% reported in a prospective observational study of 72 term neonates with febrile UTI [13]. Others also found renal scars less often [6,14] or with similar frequency [15,16] in infants versus older children. Therefore, infants presenting for urologic evaluation after febrile UTI should not be assumed to have a greater risk, and older children should not be considered at lesser risk, for renal scarring.
We found that 2 or more febrile UTIs increased risk for DMSA cortical defects, whereas a single febrile UTI and non-febrile UTIs did not. Others have similarly noted increased renal scarring on DMSA imaging with recurrent infection [14]. While this might indicate recurrent febrile UTIs increase likelihood for damage over time, potentially explaining increased scarring in older children, it is note-worthy that 76% of our patients with recurrent febrile UTIs had normal DMSA renograms, even among a referred patient population that likely represents patients with more numerous UTIs than those typically encountered in the primary care setting. Furthermore, since those with focal cortical defects did not have prior DMSA scintigraphy for comparison, it is possible that the defects found after 2 or more infections developed following the index UTI. This conclusion is supported by 2 longitudinal studies indicating the risk for renal scarring is less than 5% with recurrent UTI when the index DMSA renogram is normal [17,18].
Taken together, these observations suggest that children with febrile UTI can be separated into 2 groups based upon DMSA: the majority (80%) without cortical defects who are unlikely to develop new renal damage despite recurrent infection [17,18], and a smaller group (20%) with focal defects and/or ipsilateral renal function ≤44%. The recent AUA Guideline Committee recommended VUR therapy be tailored by perceived patient risk [7]. DMSA scintigraphy ≥3 months after febrile UTI may help guide management, but longitudinal data for patients with normal and abnormal DMSA are needed.
We recommended renograms at least 3 months after the last known febrile UTI to increase clinical applicability of the findings (i.e. to decrease concern for additional UTI if testing is postponed to 6 months), hoping to decrease detection of acute pyelonephritis while still completing testing before recurrent infection, knowing that normal studies would be accurate. Based on the longitudinal studies previously mentioned, a negative DMSA scan despite VUR and recurrent febrile UTI appears to indicate risk <5% of new renal scar with subsequent infections [17,18]. However, focal cortical defects on a 3-month scan might represent incompletely resolved inflammation from acute pyelonephritis rather than renal scar. Although our mean time for scintigraphy at 7.4 months was similar to the 6 months commonly used to detect permanent renal damage, and was similar between patients with and without focal DMSA defects, 2 reports found resolution of defects originally noted at 6 months with subsequent scans at ≥1 year in 9% of 32 patients and 46% of 13 patients [19,20]. Therefore our reported prevalence of focal cortical defects may overestimate true renal scar risk. Nonetheless, we saw similar rates of normal DMSA scans in patients undergoing testing at ≥3 months (80%) compared to the subset of 110 children whose scans occurred ≥6 months after febrile UTI (79%), and the timing of DMSA did not change the overall independent risk factors for focal defects (Appendix).
Some cortical defects may represent congenital rather than acquired renal damage, particularly among those with high grade VUR. Febrile UTIs were present in 83/84 (99%) patients with focal DMSA defects; only 1 patient (1%) with bilateral grade V VUR had focal defects despite no known UTI. Without DMSA scintigraphy before UTI, the distinction between congenital and acquired damage is impossible to make with absolute certainty, and so our finding of focal defects in 50% of patients with grades IV and V VUR may overstate acquired damage. While detected focal defects might be congenital in some cases and acquired in others, a negative scan still has potential clinical usefulness for individualized patient management, while a positive scan can serve as baseline for comparison should recurrent febrile UTI occur.
A possible limitation of our report is inclusion of patients with non-confirmed febrile UTI. We report results from a referral population and urologists must make therapeutic decisions in children with VUR and a history of “UTI” that cannot always be verified. As such, we analyzed patients with confirmed UTI versus those not meeting current AAP Guideline standards and found comparable results for focal DMSA defects and logistic regression. Similarly, Shaikh et al. found no difference in renal scar with subgroup analysis including and excluding patients with UTI diagnosed via bagged specimen, which does not meet current AAP criteria [9,10]. Because our patients were often referred 6 weeks or more after the index infection and we were not primary caregivers during the acute pyelonephritis episode, studies such as acute DMSA scintigraphy, C-reactive protein, and sedimentation rate were rarely ordered and thus could not be evaluated as independent risk factors.
Finally, we did not include bowel and bladder dysfunction as an independent risk factor in our full model since standardized definitions are lacking, particularly among non-toilet trained children, and we felt multiple imputation (assigning statistically-derived BBD habits to the infants to compensate for this unknown data) was inappropriate. Among older children who were toilet trained we diagnosed BBD in 65%. Using similar criteria in a large pediatric referral population, Chen et al. ([21]) reported BBD in 44% of patients with UTI, while Koff et al. ([22]) diagnosed BBD in 46% with primary VUR. Although BBD is associated with increased UTI, our subset analysis in toilet trained children did not find it an independent risk factor for renal defects on univariate or multivariate analysis.
Conclusions
The overall rate of focal DMSA cortical defects in 565 consecutive children referred for urologic evaluation after febrile UTI and/or VUR diagnosis was 15.5%, with an additional 4% having presumed congenital reflux nephropathy without focal defects. In addition to stratifying risk by each grade of VUR, which demonstrates grades IV and V confer higher risk than I–III, we show that recurrent febrile UTIs and older age are independent risk factors for DMSA cortical defects. However, 43% of patients with grades IV–V VUR and 76% with recurrent febrile UTIs still had negative DMSA scintigraphy.
Acknowledgements
We would like to thank Patricio Gargollo, Karen Pritzker, William Smith, and Janelle Traylor for data contributions, and Tisha Franklin and Janet Parker for manuscript preparation. This project was supported by Grant KL2RR024983.
Conflict of interest
Dr. Bush is supported by a research grant from Coloplast Corporation.
Appendix A
Odds Ratio Estimates: Subset results for stepwise multiple logistic regression including potential risk factors for renal defects on DMSA. Each subset contains the labeled covariate (risk factor), odds ratio (OR), 95% confidence intervals (95% CI), and area under the curve for the logistic model (AUC). Covariates include VUR grades I–V, Febrile UTI (FUTI), Non-febrile UTI (UTINF), age in years (Ageyr), and bowel and bladder dysfunction for subsets of toilet trained patients (BBD).
Results of logistic regression for girls only, AUC = 0.775
| Covariate | OR | 95% | CI |
|---|---|---|---|
| VUR1 | 1.217 | 0.318 | 4.657 |
| VUR2 | 2.782 | 1.371 | 5.646 |
| VUR3 | 2.810 | 1.330 | 5.938 |
| VUR4 | 14.969 | 5.853 | 38.283 |
| VUR5 | 88.217 | 14.546 | 535.021 |
| FUTI | 1.578 | 1.232 | 2.021 |
| UTINF | 1.047 | 0.845 | 1.298 |
| Ageyr | 1.212 | 1.119 | 1.312 |
Results of logistic regression for boys only, AUC = 0.815
| Covariate | OR | 95% | CI |
|---|---|---|---|
| VUR1 | 9.164 | 0.559 | 150.240 |
| VUR2 | 2.128 | 0.367 | 12.332 |
| VUR3 | 1.760 | 0.251 | 12.334 |
| VUR4 | 6.169 | 1.206 | 31.557 |
| VUR5 | 160.735 | 11.124 | >999.999 |
| FUTI | 2.312 | 0.909 | 5.880 |
| UTINF | 1.096 | 0.372 | 3.228 |
| Ageyr | 1.145 | 0.961 | 1.365 |
Results of logistic regression for toilet trained patients, with BBD defined as presence of any of the following: infrequent voiding ≤ 3 times per day; urinary frequency ≥ 10 times per day; diurnal incontinence; and/or medical therapy for constipation (polyethylene glycol and/or fiber supplementation), AUC = 0.808
| Covariate | OR | 95% | CI |
|---|---|---|---|
| VUR1 | 2.419 | 0.404 | 14.483 |
| VUR2 | 3.740 | 1.213 | 11.530 |
| VUR3 | 3.451 | 1.180 | 10.092 |
| VUR4 | 37.676 | 8.765 | 161.945 |
| VUR5 | >999.999 | <0.001 | >999.999 |
| FUTI | 1.448 | 1.022 | 2.052 |
| BBD | 0.484 | 0.222 | 1.053 |
| Ageyr | 1.021 | 1.010 | 1.033 |
Results of logistic regression for patients with DMSA performed ≥6 months after febrile UTI, AUC = 0.938
| Covariate | OR | 95% | CI |
|---|---|---|---|
| VUR1 | <0.001 | <0.001 | >999.999 |
| VUR2 | 9.709 | 0.355 | 265.513 |
| VUR3 | 58.073 | 1.359 | >999.999 |
| VUR4 | 674.139 | 6.427 | >999.999 |
| VUR5 | >999.999 | <0.001 | >999.999 |
| FUTI | 1.302 | 0.402 | 4.217 |
| BBD | 0.820 | 0.140 | 4.811 |
| Ageyr | 1.707 | 1.107 | 2.631 |
Results of logistic regression for patients with abnormal DMSA, where abnormal DMSA is defined as both focal uptake defects and/or congenital reflux nephropathy (ipsilateral function ≤44% without focal defect) with all patients, AUC = 0.811
| Covariate | OR | 95% | CI |
|---|---|---|---|
| VUR1 | 3.711 | 0.996 | 13.829 |
| VUR2 | 2.787 | 1.192 | 6.520 |
| VUR3 | 3.863 | 1.647 | 9.061 |
| VUR4 | 28.033 | 10.483 | 74.967 |
| VUR5 | 90.396 | 15.917 | 513.396 |
| FUTI | 1.744 | 1.317 | 2.309 |
| Ageyr | 1.226 | 1.131 | 1.329 |
Results of logistic regression for patients with abnormal DMSA, where abnormal DMSA is defined as both focal uptake defects and/or congenital reflux nephropathy (ipsilateral function ≤44% without focal defect) with toilet trained patients, AUC = 0.840
| Covariate | OR | 95% | CI |
|---|---|---|---|
| VUR1 | 2.411 | 0.399 | 14.551 |
| VUR2 | 3.603 | 1.173 | 11.072 |
| VUR3 | 4.641 | 1.653 | 13.034 |
| VUR4 | 35.799 | 9.424 | 135.993 |
| VUR5 | 279.619 | 18.506 | >999.999 |
| FUTI | 1.544 | 1.096 | 2.175 |
| BBD | 0.507 | 0.276 | 0.930 |
| Ageyr | 1.303 | 1.151 | 1.474 |
References
- 1.Hoberman A, Charron M, Hickey RW, Baskin M, Kearney DH, Wald ER. Imaging studies after a first febrile urinary tract infection in young children. N Engl J Med. 2003 Jan 16;348(3):195–202. doi: 10.1056/NEJMoa021698. [DOI] [PubMed] [Google Scholar]
- 2.Polito C, Rambaldi PF, Signoriello G, Mansi L, La Manna A. Permanent renal parenchymal defects after febrile UTI are closely associated with vesicoureteric reflux. Pediatr Nephrol. 2006 Apr;21(4):521–526. doi: 10.1007/s00467-006-0036-3. [DOI] [PubMed] [Google Scholar]
- 3.Lee JH, Son CH, Lee MS, Park YS. Vesicoureteral reflux increases the risk of renal scars: a study of unilateral reflux. Pediatr Nephrol. 2006 Sep;21(9):1281–1284. doi: 10.1007/s00467-006-0147-x. [DOI] [PubMed] [Google Scholar]
- 4.Garin EH, Olavarria F, Garcia Nieto V, Valenciano B, Campos A, Young L. Clinical significance of primary vesicoureteral reflux and urinary antibiotic prophylaxis after acute pyelonephritis: a multicenter, randomized, controlled study. Pediatrics. 2006 Mar;117(3):626–632. doi: 10.1542/peds.2005-1362. [DOI] [PubMed] [Google Scholar]
- 5.Doganis D, Siafas K, Mavrikou M, Issaris G, Martirosova A, Perperidis G, et al. Does early treatment of urinary tract infection prevent renal damage? Pediatrics. 2007 Oct;120(4):e922e8. doi: 10.1542/peds.2006-2417. [DOI] [PubMed] [Google Scholar]
- 6.Pecile P, Miorin E, Romanello C, Vidal E, Contardo M, Valent F, et al. Age-related renal parenchymal lesions in children with first febrile urinary tract infections. Pediatrics. 2009 Jul;124(1):23–29. doi: 10.1542/peds.2008-1192. [DOI] [PubMed] [Google Scholar]
- 7.Peters CA, Skoog SJ, Arant BS, Jr, Copp HL, Elder JS, Hudson RG, et al. Summary of the AUA guideline on management of primary vesicoureteral reflux in children. J Urol. 2010 Sep;184(3):1134–1144. doi: 10.1016/j.juro.2010.05.065. [DOI] [PubMed] [Google Scholar]
- 8.Faust WC, Diaz M, Pohl HG. Incidence of post-pyelonephritic renal scarring: a meta-analysis of the dimercapto-succinic acid literature. J Urol. 2009 Jan;181(1):290–297. doi: 10.1016/j.juro.2008.09.039. [discussion 297-298] [DOI] [PubMed] [Google Scholar]
- 9.Shaikh N, Ewing AL, Bhatnagar S, Hoberman A. Risk of renal scarring in children with a first urinary tract infection: a systematic review. Pediatrics. 2010 Dec;126(6):1084–1091. doi: 10.1542/peds.2010-0685. [DOI] [PubMed] [Google Scholar]
- 10.American Academy of Pediatrics. Committee on Quality Improvement. Subcommittee on Urinary Tract Infection. Practice parameter: the diagnosis, treatment, and evaluation of the initial urinary tract infection in febrile infants and young children. Pediatrics. 1999 Apr;103(4 Pt 1):843–852. doi: 10.1542/peds.103.4.843. [DOI] [PubMed] [Google Scholar]
- 11.Roberts KB. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011 Sep;128(3):595–610. doi: 10.1542/peds.2011-1330. [DOI] [PubMed] [Google Scholar]
- 12.Swerkersson S, Jodal U, Sixt R, Stokland E, Hansson S. Relationship among vesicoureteral reflux, urinary tract infection and renal damage in children. J Urol. 2007 Aug;178(2):647–651. doi: 10.1016/j.juro.2007.04.004. [discussion 650-641] [DOI] [PubMed] [Google Scholar]
- 13.Siomou E, Giapros V, Fotopoulos A, Aasioti M, Papadopoulou F, Serbis A, et al. Implications of 99mTc-DMSA scintigraphy performed during urinary tract infection in neonates. Pediatrics. 2009 Sep;124(3):881–887. doi: 10.1542/peds.2008-1963. [DOI] [PubMed] [Google Scholar]
- 14.Orellana P, Baquedano P, Rangarajan V, Zhao JH, Eng ND, Fettich J, et al. Relationship between acute pyelonephritis, renal scarring, and vesicoureteral reflux. Results of a coordinated research project. Pediatr Nephrol. 2004 Oct;19(10):1122–1126. doi: 10.1007/s00467-004-1501-5. [DOI] [PubMed] [Google Scholar]
- 15.Patel K, Charron M, Hoberman A, Brown ML, Rogers KD. Intraand interobserver variability in interpretation of DMSA scans using a set of standardized criteria. Pediatr Radiol. 1993;23(7):506–509. doi: 10.1007/BF02012131. [DOI] [PubMed] [Google Scholar]
- 16.Hewitt IK, Zucchetta P, Rigon L, Maschio F, Molinari PP, Tomasi L, et al. Early treatment of acute pyelonephritis in children fails to reduce renal scarring: data from the Italian Renal Infection Study Trials. Pediatrics. 2008 Sep;122(3):486–490. doi: 10.1542/peds.2007-2894. [DOI] [PubMed] [Google Scholar]
- 17.Vernon SJ, Coulthard MG, Lambert HJ, Keir MJ, Matthews JN. New renal scarring in children who at age 3 and 4 years had had normal scans with dimercaptosuccinic acid: follow up study. BMJ. 1997 Oct 11;315(7113):905–908. doi: 10.1136/bmj.315.7113.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soylu A, Demir BK, Turkmen M, Bekem O, Saygi M, Cakmakci H, et al. Predictors of renal scar in children with urinary infection and vesicoureteral reflux. Pediatr Nephrol. 2008 Dec;23(12):2227–2232. doi: 10.1007/s00467-008-0907-x. [DOI] [PubMed] [Google Scholar]
- 19.Parvex P, Willi JP, Kossovsky MP, Girardin E. Longitudinal analyses of renal lesions due to acute pyelonephritis in children and their impact on renal growth. J Urol. 2008 Dec;180(6):2602–2606. doi: 10.1016/j.juro.2008.08.059. [discussion 2606] [DOI] [PubMed] [Google Scholar]
- 20.Agras K, Ortapamuk H, Naldoken S, Tuncel A, Atan A. Resolution of cortical lesions on serial renal scans in children with acute pyelonephritis. Pediatr Radiol. 2007 Feb;37(2):153–158. doi: 10.1007/s00247-006-0362-4. [DOI] [PubMed] [Google Scholar]
- 21.Chen JJ, Mao W, Homayoon K, Steinhardt GF. A multivariate analysis of dysfunctional elimination syndrome, and its relationships with gender, urinary tract infection and vesicoureteral reflux in children. J Urol. 2004 May;171(5):1907–1910. doi: 10.1097/01.ju.0000120288.82950.a2. [DOI] [PubMed] [Google Scholar]
- 22.Koff SA, Wagner TT, Jayanthi VR. The relationship among dysfunctional elimination syndromes, primary vesicoureteral reflux and urinary tract infections in children. J Urol. 1998 Sep;160(3 Pt 2):1019–1022. doi: 10.1097/00005392-199809020-00014. [DOI] [PubMed] [Google Scholar]