Abstract
Purpose
Difference in the quality of care may contribute to the less optimal prostate cancer treatment outcomes among Blacks compared with Whites. Our objective was to determine whether a racial quality of care gap exists in surgical care for prostate cancer, as evidenced by racial variation in the utilization of high-volume surgeons and facilities, and in certain outcome measures of care quality.
Materials and Methods
We performed cross-sectional and cohort analyses of administrative data from the Healthcare Cost and Utilization Project's all-payer State Inpatient Databases, encompassing all non-Federal hospitals in Florida, Maryland and New York State (1996-2007). Included were men 18 or older with a diagnosis of prostate cancer who underwent radical prostatectomy. We compared use of surgeons and/or hospitals in the top quartile of annual volume for this procedure, inpatient blood transfusion, complications, mortality and length of stay (LOS) between Black and White patients.
Results
Among 105,972 cases, 81,112 (76.5%) were White, 14,006 (13.2%) were Black, 6,999 (6.6%) were Hispanic and 3,855 (3.6%) were All Other. In mixed effects multivariate models, Blacks had markedly lower use of high-volume hospitals (Odds Ratio [OR] = 0.73, 95% Confidence interval [0.70, 0.76]), and surgeons (0.67 [0.64, 0.70]) compared to Whites. Blacks also had a higher odds of receiving a blood transfusion (1.08 [1.01, 1.14]), of longer LOS (1.07 [1.06, 1.07]) and of inpatient mortality (1.73 [1.02, 2.92]).
Conclusions
Using an all-payer dataset, we identified concerning potential quality of care gaps between Blacks and Whites undergoing radical prostatectomy for prostate cancer.
Keywords: Health disparities, quality of care, prostate cancer, surgery
Introduction
Prostate cancer (PCa) is the most common non-cutaneous solid malignancy among men in the United States, and is the second leading cause of cancer death1. Blacks bear a disproportionate burden of PCa, with an age-adjusted relative risk of 1.59 for incidence and 2.41 for PCa-specific death relative to Whites2. Racial differences in PCa outcomes persist even when controlling for clinical characteristics such as stage at presentation3, and currently proposed biological mechanisms do not fully explain these differences. Racial variation in the quality of care received may contribute to differences in PCa outcomes4, 5. Quality variation can be studied using quality indicators,6 such as surgeon and hospital volume, which are associated with downstream clinical outcomes after radical prostatectomy (RP)7, 8.
The goal of this study was to determine whether a racial quality of care gap exists in surgical care for PCa, as evidenced by racial variation in the utilization of high-volume surgeons (HVHs) and hospitals (HVHs), and in certain outcome measures (transfusion, complications, length of stay [LOS], and in-hospital mortality). Our hypothesis was that Black patients undergoing RP would be less likely to use HVSs and HVHs than would Whites. We also hypothesized that Black patients would experience more adverse events, perhaps in part explained by differential use of HVSs and HVHs.
Methods
Prior to initiating the study, we obtained a review exemption from the local Institutional Review Board and signed AHRQ's data-use agreement.
Dataset
We obtained encounter-level administrative data from specific State Inpatient Databases (SIDs) compiled through the Agency for Healthcare Research and Quality (AHRQ)'s Healthcare Cost and Utilization Project (HCUP). As described in detail elsewhere9, the SIDs contain information on all patients discharged from non-Federal hospitals in HCUP-participating States. Over 100 data elements are uniformly recorded for each SID discharge, including principal and secondary discharge diagnoses and procedures, patient demographics, expected payment source, and LOS. Some States also include elements such as race and surgeon identifier.
In order to facilitate the collection of valid data, we restricted our analysis to States meeting all of the following criteria: 1) race and Hispanic ethnicity coding was considered sufficiently complete for the State to be included in HCUP's disparities analytic file10; 2) surgeon identifier was uniform across hospitals within the State; 3) data were available through HCUP's Central Distributor. Within the only three States (Florida , New York , and Maryland) meeting these criteria, we excluded data from entire hospitals with potentially suspect coding of race, using the following criteria which reflect methods used in AHRQ's disparities analytic file10, to: 1) >30% of discharges had race reported as “other”; 2) race was missing in >50% of discharges ; 3) all discharges had race coded as White, other, or missing; or 4) 100% of discharges had race coded as White.
Cohort Definition
We included all patients ≥ 18 years old with a diagnosis of PCa (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] diagnosis code 185 or 198.82) who underwent RP (ICD-9-CM procedure code 60.5) from 1996 through 2007 by open, laparoscopic or robotic approaches. In order to avoid including patients with incidental PCa, we excluded all patients with cystectomies (ICD-9-CM procedure code 57.6-57.7) performed during the index admission, and those in whom RP was not coded as the principal procedure.
Independent Variables
Using the uniformly coded ‘race’ variable from the SID, patients were classified into race/ethnicity groups of ‘White,’ ‘Black,’ ‘Hispanic,’ ‘Asian/Pacific Islander,’ ‘Native American,’ or ‘Other.’ Numbers of patients identified as ‘Asian/Pacific Islander’ or ‘Native American’ were too small to permit meaningful separate analyses, so we categorized patients as White, Black, Hispanic or All Other (which includes all remaining groups).
Other independent variables included patient age; expected payer (Private Insurance, Medicare, Medicaid, Self-pay, No Charge or Other); number of comorbidities (0, 1, 2, 3, or 4+) calculated from secondary diagnostic codes using the Elixhauser method11; State in which the procedure was performed; patient location (large metropolitan, small metropolitan, “micropolitan” or rural area); median household income (expressed in quartiles of US household incomes, based on the ZIP code of patient residence); and marital status (married, single, divorced, or widowed).
Outcome Definition
Annual procedure volume for each surgeon was calculated as the number of cases performed in all hospitals in the State in each calendar year of the analysis. Hospital volume was calculated similarly. HVSs and HVHs were defined as surgeons and hospitals in the top 25th percentile for that State in that year.
In-hospital mortality and LOS were reported for all discharges in the SID. The administration of homologous packed red blood cells was identified by ICD-9-CM procedure code (99.04), and/or Uniform Billing (UB-92) codes. Based on previous studies using administrative data to investigate complications after RP12, 13 we used the presence or absence of specific secondary discharge diagnoses (Supplemental Table 1) to compose a binary variable indicating whether or not the patient had any complications.
Statistical Considerations
Descriptive statistics were computed for patient demographics, and each of the outcome variables: use of an HVH, use of an HVS, in-hospital mortality, administration of a blood transfusion, any complications and LOS. Statistical significance testing was unnecessary for these univariate comparisons, because we analyzed the entire population. Missing data were considered missing at random.
Generalized linear mixed-effects regression models were used to model the outcomes of interest. A logit link function was used for all outcomes except LOS, which was modeled with a log link function. Random effects (REs) were specified for each hospital and for some models the hospital RE was nested within State in order to account for similarity of usage patterns (i.e., correlation) within hospital and State. Exponentiated coefficients from a model with a logit link were interpreted as odds ratios (ORs), while those from the model with a log link were interpreted as a ratio of median durations of LOS. Univariate models were used to characterize the impact of a single covariate, while multivariate models were used to characterize the adjusted impact of a covariate after accounting for other factors. Each multivariate model was fit twice - once with both HVH and HVS and once with neither - in order to evaluate differences in each covariate that might be mediated by HVH and HVS utilization. Categorical predictors were modeled using indicator functions. Restricted cubic splines with 3 and 4 knots were used for LOS and age to capture potential non-linear relationships14. Each model was adjusted for age, expected payer, number of comorbidities, and year of surgery, and included State as an RE. HVH, HVS and LOS were also used as predictors for other outcomes when appropriate. Predicted probabilities of in-hospital mortality were computed for all possible combinations of HVS, HVH, expected payer and number of comorbidities, while fixing age, LOS and year at reference values of 60 years, 3 days and 2007, respectively. All analyses were performed and all figures were generated using R version 2.11.1 (R Development Core Team, Vienna, Austria).
Results
Application of our inclusion/exclusion criteria identified 108,331 cases (Supplemental Figure). Excluding 2,331 cases (2.2%) where race was missing and 28 cases (0.03%) missing other key variables left 105,972 cases for analysis. Some 81,112 (76.5%) were White; 14,006 (13.2%) were Black; 6,999 (6.6%) were Hispanic; and 3,855 (3.6%) were All Other (Table 1). At least 19% of the patients in each State were non-White.
Table 1. Patient Characteristics.
| Characteristic | Analytic Set | White | Black | Hispanic | All Others |
|---|---|---|---|---|---|
| N = 105,972 | N = 81,112 | N = 14,006 | N = 6,999 | N = 3,855 | |
| Age in years | 60.8 (7.1) | 61.1 (7.0) | 59.2 (7.2) | 60.8 (7.0) | 60.1 (6.8) |
| Mean (SD) | |||||
| Payer | |||||
| Medicare | 30,152 (28.5) | 24,500 (30.2) | 3,014 (21.5) | 1,873 (26.8) | 765 (19.8) |
| Medicaid | 2,199 (2.1) | 739 (0.9) | 696 (5.0) | 601 (8.6) | 163 (4.2) |
| Private Ins | 68,950 (65.1) | 53,043 (65.4) | 9,442 (67.4) | 3,859 (55.1) | 2,606 (67.6) |
| Self-pay | 2,550 (2.4) | 1,511 (1.9) | 440 (3.1) | 375 (5.4) | 224 (5.8) |
| No charge | 343 (0.3) | 88 (0.1) | 95 (0.7) | 149 (2.1) | 11 (0.3) |
| Other | 1,778 (1.7) | 1,231 (1.5) | 319 (2.3) | 142 (2.0) | 86 (2.2) |
| Comorbidity Count | |||||
| 0 | 43,244 (40.8) | 34,088 (42.0) | 4,477 (32.0) | 2,999 (42.8) | 1,680 (43.6) |
| 1 | 39,030 (36.8) | 29,656 (36.6) | 5,457 (39.0) | 2,523 (36.0) | 1,394 (36.2) |
| 2 | 17,474 (16.5) | 12,815 (15.8) | 2,939 (21.0) | 1,096 (15.7) | 624 (16.2) |
| 3 | 4,890 (4.6) | 3,571 (4.4) | 894 (6.4) | 301 (4.3) | 124 (3.2) |
| 4+ | 1,334 (1.3) | 982 (1.2) | 239 (1.7) | 80 (1.1) | 33 (0.9) |
| Patient Location* | |||||
| Large Metro | 28,534 (63.5) | 19,508 (57.9) | 4,786 (80.1) | 2,760 (82.8) | 1,480 (76.3) |
| Small Metro | 12,133 (27.0) | 10,397 (30.9) | 957 (16.0) | 431 (12.9) | 348 (17.9) |
| Micro | 3,168 (7.1) | 2,821 (8.4) | 161 (2.7) | 106 (3.2) | 80 (4.1) |
| Rural | 1,091 (2.4) | 953 (2.8) | 70 (1.2) | 36 (1.1) | 32 (1.6) |
| Median household income quartile** | |||||
| 1 | 3,587 (17.9) | 2,070 (13.9) | 893 (35.7) | 468 (29.1) | 156 (15.2) |
| 2 | 4,386 (21.9) | 3,284 (22.1) | 526 (21.0) | 356 (22.1) | 220 (21.4) |
| 3 | 4,845 (24.2) | 3,646 (24.5) | 510 (20.4) | 461 (28.6) | 228 (22.2) |
| 4 | 7,200 (36.0) | 5,882 (39.5) | 570 (22.8) | 326 (20.2) | 422 (41.1) |
| Marital Status*** | |||||
| Married | 5,853 (84.6) | 4,839 (86.1) | 768 (75.9) | 71 (84.5) | 175 (86.2) |
| Single | 632 (9.1) | 454 (8.1) | 153 (15.1) | <10 (<10) | 18 (8.9) |
| Divorced | 324 (4.7) | 243 (4.3) | 70 (6.9) | <10 (<10) | <10 (<5) |
| Widowed | 110 (1.6) | 84 (1.5) | 21 (2.1) | <10 (<10) | <10 (<5) |
Cells represent number (percent). Percentages may not sum to 100% due to rounding. AHRQ's data use agreement prohibits publication of cell values smaller than 10.
All States, 2003-2007
All States, 2006-2007
Maryland 2004-2007
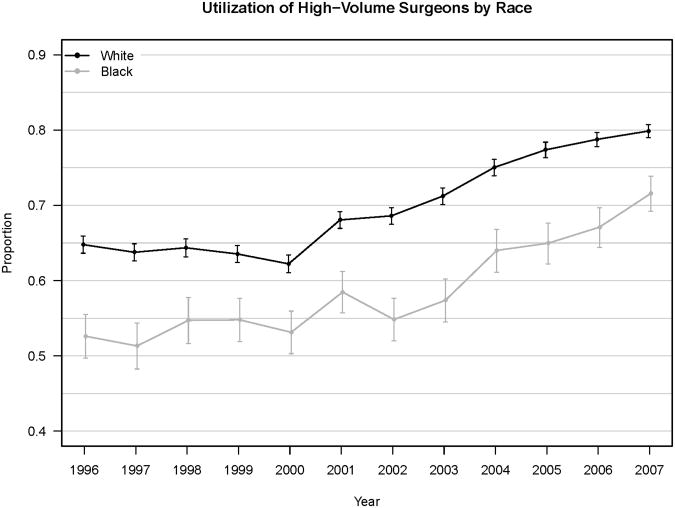
For surgeons, the 75th percentile of annual case volume ranged between 7 and 12 cases, depending on the year and State. Overall, 68.0% of procedures were performed by HVSs (70.0% for Whites, 59.1% for Blacks, 63.1% for Hispanics, 68.7% for All Others, (p<0.001) (Table 2). Although use of HVSs increased for all groups over the study period, Whites' utilization of HVSs exceeded that of Blacks in each year by absolute values of 8.3-13.9% (Figure 1A).
Table 2. Outcome Measures by Racial Group.
| Outcome | Analytic Set | White | Black | Hispanic | All Others |
|---|---|---|---|---|---|
| N = 105,972 | N = 81,112 | N = 14,006 | N = 6,999 | N = 3,855 | |
| Surgeon Volume Quartile | |||||
| 1 | 4,768 (4.5) | 3,228 (4.0) | 899 (6.4) | 416 (5.9) | 225 (5.8) |
| 2 | 9,212 (8.7) | 6,531 (8.1) | 1,637 (11.7) | 706 (10.1) | 338 (8.8) |
| 3 | 19,905 (18.8) | 14,614 (18.0) | 3,189 (22.8) | 1,459 (20.8) | 643 (16.7) |
| 4 | 72,087 (68.0) | 56,739 (70.0) | 8,281 (59.1) | 4,418 (63.1) | 2,649 (68.7) |
| Hospital Volume Quartile | |||||
| 1 | 3,165 (3.0) | 2072 (2.6) | 677 (4.8) | 308 (4.4) | 108 (2.8) |
| 2 | 7,453 (7.0) | 5,318 (6.6) | 1,250 (8.9) | 635 (9.1) | 250 (6.5) |
| 3 | 17,906 (16.9) | 13,416 (16.5) | 2,823 (20.2) | 1,124 (16.1) | 543 (14.1) |
| 4 | 77,448 (73.1) | 60,306 (74.3) | 9,256 (66.1) | 4,932 (70.5) | 2,954 (76.6) |
| Transfusion | |||||
| 11,077 (10.5) | 7,893 (9.7) | 1,853 (13.2) | 797 (11.4) | 534 (13.9) | |
| Any Complication | |||||
| 10,943 (10.3) | 8,083 (10.0) | 1,770 (12.6) | 745 (10.6) | 345 (8.9) | |
| LOS (days) | |||||
| Mean | 3.3 | 3.2 | 3.7 | 3.4 | 3.1 |
| Median | 3 | 3 | 3 | 3 | 3 |
| Range | 0-124 | 0-122 | 0-124 | 0-43 | 0-25 |
| In-Hospital Mortality | |||||
| 88 (8.3 per 10,000) | 59 (7.3 per 10,000) | 22 (15.7 per 10,000) | <10 (<10 per 10,000) | <10 (<10 per 10,000) | |
Cells represent number (percent), except for LOS, which is in days. Percentages may not sum to 100% due to rounding. AHRQ's data use agreement prohibits publication of cell values smaller than 10. Statistical significance of comparisons across race was not assessed since we analyzed the entire population and there is no larger group about which we would be making statistical inferences.
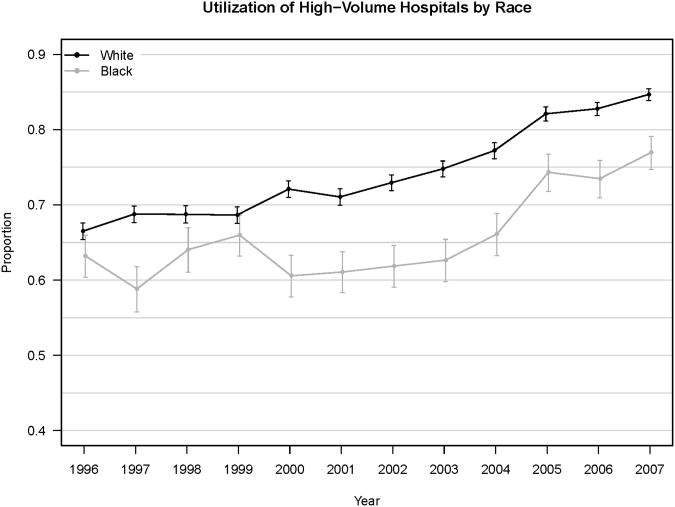
Figure 1.
Percent of Black vs. White Patients in NY, FL and MD Utilizing A) High-Volume Surgeons and B) High-Volume Hospitals.
Error bars indicate 95% confidence interval.
For hospitals, the 75th percentile of annual case volume ranged between 21 and 35 cases per year. Some 73.1% of cases were performed at HVHs (74.3% for Whites, 66.1% for Blacks, 70.5% for Hispanics, 76.6% for All Others, p<0.001) (Table 2). Blacks utilized HVHs less frequently than did Whites (2.7% vs. 12.2%), despite an overall increase in the proportion of cases performed at HVHs over time (Figure 1B).
We performed several univariate analyses to assess the association between race/ethnicity and inpatient outcomes (Table 2). Blacks had higher use of blood transfusion (13.2%) than did Whites (9.7%) or Hispanics (11.4%). Blacks also had higher complication rates, longer LOS and higher in-hospital mortality than other groups. Other univariate analyses examined the associations between surgeon and hospital volumes and these clinical outcomes (Table 3). Use of higher-volume surgeons and hospitals was associated with improvements in transfusion rate, complication rate, LOS and mortality.
Table 3. Outcomes by Surgeon Volume Quartile (SVQ) and Hospital Volume Quartile (HVQ).
| Outcome | Analytic Set | SVQ 1 | SVQ 2 | SVQ 3 | SVQ 4 |
|---|---|---|---|---|---|
| N = 105,972 | N = 4,768 | N = 9,212 | N = 19,905 | N = 72,087 | |
| Transfusion | |||||
| 11,077 (10.5) | 872 (18.3) | 1,341 (14.6) | 2,518 (12.7) | 6,346 (8.8) | |
| Any Complication | |||||
| 10,943 (10.3) | 709 (14.9) | 1,347 (14.6) | 2,594 (13.0) | 6,293 (8.7) | |
| LOS (days) | |||||
| Mean | 3.3 | 4.4 | 4.2 | 3.9 | 3.0 |
| Median | 3.0 | 4.0 | 4.0 | 3.0 | 3.0 |
| Range | 0-124 | 0-77 | 1-40 | 0-79 | 0-124 |
| In-Hospital Mortality | |||||
| 88 (8.3 per 10,000) | <10 (∼20 per 10,000) | 16 (17.4 per 10,000) | 23 (11.6 per 10,000) | 40 (5.5 per 10,000) | |
|
| |||||
| Outcome | Analytic Set | HVQ 1 | HVQ 2 | HVQ 3 | HVQ 4 |
|
| |||||
| N = 105,972 | N = 3,165 | N = 7,453 | N = 17,906 | N = 77,448 | |
| Transfusion | |||||
| 11,077 (10.5) | 631 (19.9) | 1,159 (15.6) | 2,302 (12.9) | 6,985 (9.0) | |
| Any Complication | |||||
| 10,943 (10.3) | 511 (16.1) | 1,043 (14.0) | 2,387 (13.3) | 7,002 (9.0) | |
| LOS (days) | |||||
| Mean | 3.3 | 4.6 | 4.2 | 3.9 | 3.0 |
| Median | 3.0 | 4.0 | 4.0 | 4.0 | 3.0 |
| Range | 0-124 | 0-79 | 0-40 | 1-124 | 0-122 |
| In-Hospital Mortality | |||||
| 88 (8.3 per 10,000) | <10 (∼25 per 10,000) | 15 (20.1 per 10,000) | 19 (10.6 per 10,000) | 46 (5.9 per 10,000) | |
Cells represent number (percent), except for LOS, which is in days. Percentages may not sum to 100% due to rounding. AHRQ's data use agreement prohibits publication of cell values smaller than 10. Statistical significance of comparisons across race was not assessed since we analyzed the entire population and there is no larger group about which we would be making statistical inferences.
In multivariate analyses, Blacks had markedly lower use of HVHs (OR 0.73, 95% CI [0.70, 0.76], p<0.001) and HVSs (OR 0.67, 95% CI [0.64, 0.70], p<0.001) than Whites (Table 4). Black race was also associated with higher use of blood transfusion, longer LOS, and higher risk of inpatient mortality compared with White race. Hispanics had lower use of HVSs, higher use of blood transfusion and longer LOS than Whites, but also had slightly lower likelihood of complications. Including HVS or HVH in the model somewhat attenuated the effect of race/ethnicity on these outcomes, although race remained statistically significant. Patient location, income quartile and marital status were collected only in certain States during certain years; there were too many missing values to permit their use in multivariate analyses.
Table 4. Generalized Linear Mixed Effects Models.
| Quality Indicator | Univariate Models | P-value | Multivariable Models | P-value | Multivariable Models w/ HVH, HVS | P-value | |||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | ||||
| Use of HVH | |||||||||
| Black vs White | 0.65 | 0.63 – 0.68 | <0.01 | 0.73 | 0.70 – 0.76 | <0.01 | --- | --- | --- |
| Hispanic vs White | 0.94 | 0.89 – 0.99 | 0.017 | 0.98 | 0.93 – 1.04 | 0.513 | --- | --- | --- |
| All Others vs White | 1.07 | 0.99 – 1.16 | 0.069 | 1.01 | 0.93 – 1.10 | 0.806 | --- | --- | --- |
| Use of HVS | --- | --- | --- | ||||||
| Black vs White | 0.60 | 0.58 – 0.62 | <0.01 | 0.67 | 0.64 – 0.70 | <0.01 | --- | --- | --- |
| Hispanic vs White | 0.83 | 0.79 – 0.88 | <0.01 | 0.84 | 0.80 – 0.89 | <0.01 | --- | --- | --- |
| All Others vs White | 0.90 | 0.84 – 0.97 | 0.004 | 0.82 | 0.76 – 0.89 | <0.01 | --- | --- | --- |
| In-hospital Mortality | |||||||||
| Black vs White | 2.16 | 1.32 – 3.53 | 0.002 | 1.93 | 1.15 – 3.26 | 0.013 | 1.73 | 1.02 – 2.92 | 0.042 |
| Hispanic vs White | 0.79 | 0.29 – 2.16 | 0.641 | 0.83 | 0.30 – 2.32 | 0.720 | 0.82 | 0.29 – 2.28 | 0.697 |
| All Others vs White | 1.07 | 0.34 – 3.42 | 0.909 | 1.31 | 0.41 – 4.23 | 0.650 | 1.28 | 0.40 – 4.13 | 0.684 |
| HVH: Yes vs No | 0.40 | 0.27 – 0.61 | <0.01 | --- | --- | --- | 0.62 | 0.39 – 1.00 | 0.049 |
| HVS: Yes vs No | 0.39 | 0.26 – 0.60 | <0.01 | --- | --- | --- | 0.44 | 0.27 – 0.72 | 0.001 |
| Transfusion | |||||||||
| Black vs White | 1.38 | 1.30 – 1.45 | <0.01 | 1.11 | 1.05 – 1.18 | <0.01 | 1.08 | 1.01 – 1.14 | 0.016 |
| Hispanic vs White | 1.40 | 1.29 – 1.51 | <0.01 | 1.17 | 1.07 – 1.28 | <0.01 | 1.16 | 1.07 – 1.27 | 0.01 |
| All Others vs White | 1.29 | 1.17 – 1.42 | <0.01 | 1.27 | 1.15 – 1.40 | <0.01 | 1.25 | 1.13 – 1.38 | <0.01 |
| HVH: Yes vs No | 0.50 | 0.48 – 0.52 | <0.01 | --- | --- | --- | 0.76 | 0.72 – 0.80 | <0.01 |
| HVS: Yes vs No | 0.51 | 0.49 – 0.53 | <0.01 | --- | --- | --- | 0.78 | 0.74 – 0.81 | <0.01 |
| Any Complications | |||||||||
| Black vs White | 1.33 | 1.25 – 1.40 | <0.01 | 0.95 | 0.90 – 1.01 | 0.125 | 0.96 | 0.90 – 1.02 | 0.223 |
| Hispanic vs White | 1.04 | 0.96 – 1.13 | 0.354 | 0.89 | 0.81 – 0.97 | 0.010 | 0.90 | 0.82 – 0.98 | 0.019 |
| All Others vs White | 0.96 | 0.85 – 1.07 | 0.429 | 0.95 | 0.84 – 1.08 | 0.449 | 0.94 | 0.83 – 1.06 | 0.291 |
| HVH: Yes vs No | 0.64 | 0.62 – 0.67 | <0.01 | --- | --- | --- | 1.08 | 1.03 – 1.14 | 0.003 |
| HVS: Yes vs No | 0.62 | 0.59 – 0.64 | <0.01 | --- | --- | --- | 1.04 | 0.99 – 1.09 | 0.117 |
| LOS* | |||||||||
| Black vs White | 1.10 | 1.09 – 1.11 | <0.01 | 1.09 | 1.09 – 1.10 | <0.01 | 1.07 | 1.06 – 1.07 | <0.01 |
| Hispanic vs White | 1.03 | 1.02 – 1.04 | <0.01 | 1.06 | 1.05 – 1.07 | <0.01 | 1.05 | 1.04 – 1.06 | <0.01 |
| All Others vs White | 0.96 | 0.95 – 0.98 | <0.01 | 1.01 | 1.00 – 1.02 | 0.014 | 1.00 | 1.00 – 1.02 | 0.170 |
| HVH: Yes vs No | 0.79 | 0.79 – 0.79 | <0.01 | --- | --- | --- | 0.90 | 0.89 – 0.90 | <0.01 |
| HVS: Yes vs No | 0.78 | 0.78 – 0.79 | <0.01 | --- | --- | --- | 0.87 | 0.87 – 0.88 | <0.01 |
Instead of odds ratios, estimates for LOS denote the ratio of geometric means. These may also be interpreted as the ratio of medians.
There were 88 inpatient deaths among 105,972 cases (0.08%), including 59 (0.07%) in Whites and 22 (0.16%) in Blacks. The OR for inpatient mortality among Blacks compared to Whites was 1.73 (95% CI [1.02, 2.92]) in a fully adjusted model. Despite the higher risk of inpatient mortality among Blacks, the model demonstrates that Blacks using HVSs or HVHs had substantially lower relative risks of in-hospital death than Blacks using lower-volume providers (Supplemental Table 2).
Discussion
In this study, we found evidence that Blacks with PCa received lower-quality surgical care than did Whites, based on racial differences in use of HVSs and HVHs and on downstream outcomes. The difference in use of HVSs and HVHs was consistent across years and persisted in multivariate analysis that adjusted for age, comorbidity, payer, year and State. Blacks had 33% lower odds of using a HVS and 27% lower odds of using a HVH than Whites. Furthermore, Blacks had higher rates of blood transfusion, longer LOS and higher risk of inpatient mortality. Although the increased risk of these adverse outcomes was independent of hospital and surgeon volume, Blacks who used HVHs and HVSs faced substantially reduced risk of adverse outcomes than did those using lower-volume providers.
Our findings of racial variation in outcomes of PCa are consistent with other population-based studies and with national cancer statistics1-5, 15-18. Some variation can be attributed to differences in stage at presentation2 and clinical management19-22, and possibly to biological explanations23, 24. However, some studies that controlled for stage and treatment type have shown that racial differences in outcomes persist3, thus implicating differences in healthcare delivery and access. This hypothesis is supported by studies demonstrating that the race-outcome association is attenuated in selected settings, such as an equal-access Department of Veterans Affairs (VA) setting25 and in specialized NCI Cancer Centers26. Furthermore, controlling tightly for education and other socioeconomic variables attenuates observed racial variation in PCa outcomes27, 28.
A volume-outcome association has been identified for many complex procedures, including RP8, 12. However, only two prior studies have evaluated the effect of surgeon and hospital volume on racial differences in outcome after RP. Gooden et al evaluated 8,349 men from the SEER-Medicare dataset from 1991-2002 and found that Blacks utilized lower-volume surgeons more than did Whites, but actually used higher-volume hospitals29. Because they identified similar differences in outcome across strata of procedure volumes, the authors concluded that hospital and surgeon volumes did not contribute to disparities in outcome. This study controlled for important potential confounders, including disease characteristics, and evaluated an important endpoint, but did not capture complications, LOS or inpatient mortality. A similar study using SEER-Medicare data showed racial variation in complication rates and costs that differed across hospital and surgeon volume strata.30
In both studies, use of SEER-Medicare data restricted the cohort to men ≥ 65 years old. This fails to consider the large population of younger RP patients, such as the 67.0% of patients in our study who were below 65 years old. Indeed, Medicare patients may have more equal access to care than younger patients, with potentially lower financial barriers to choices of physician and facility. In all-payer populations such as those reflected in SID data, differences in socio-economic status and payer may increase variability in access to high-quality care. Taken together, these studies suggest that racial differences in PCa outcomes may be partially explained by differences in access to high-quality care, which, in turn, may reflect differences in patient resources. Our findings that Blacks with PCa received lower-quality surgical care than Whites is consistent with prior studies demonstrating racial differences in outcomes when access to high-quality care is variable.
Our study has important limitations. The SIDs do not capture discharges from Federal (military and VA) institutions. However, since such hospitals are generally considered equal-access settings, not having included them may have enhanced our efforts to highlight the impact of differential access to high-quality resources. The “race” variable provided in the SIDs reflects variable coding conventions regarding provider-versus self-identification in participating hospitals, and it conflates race and Hispanic ethnicity. It is likely that unmeasured factors account for some or all of the observed differences between racial groups. For example, patient-level socioeconomic status may have a significant impact on healthcare access, and is known to be associated with race. Clinical disease characteristics and severity of comorbidities are also unavailable in this administrative dataset and the ability to adjust for these variables could have attenuated the observed racial variation. We could not reliably distinguish conditions present on admission from those that developed during hospitalization. Finally, while volume is a surrogate for quality, it is not itself a direct measure of quality. Indeed, while hospital and surgical volumes were associated with certain clinical outcomes, only a portion of the racial variation seen in these outcomes could be attributed to hospital/surgeon volume.
Despite these limitations, this all-payer dataset, including all RPs from non-Federal hospitals in three diverse States, over a 12-year period provided sufficient detail to identify racial differences in important quality indicators. This study identifies Black men as a population at risk for receipt of lower-quality care, providing a potential target for interventions aimed at improving the quality of surgical care, including increasing access to high-volume providers.
Conclusions
We demonstrated that, compared to Whites, Blacks had substantially lower utilization of HVHs and HVSs for RP, and less optimal inpatient clinical outcomes. This suggests that there are gaps between Blacks and Whites in the quality of care for PCa, which may contribute to observed racial variation in PCa outcomes. Efforts to improve quality of care for RP should include increasing access to high-quality PCa treatment centers for all patients.
Supplementary Material
Acknowledgments
Sources of Financial Support: National Institute of Environmental Health Sciences K12 ES15855, the National Center for Research Resources/ National Institutes of Health via the Vanderbilt CTSA grant UL1 RR024975
Footnotes
Relevant Financial Disclosures: None
Note: the opinions expressed in this paper are those of the authors and do not necessarily reflect the position of the US Agency for Healthcare Research and Quality or of the US Department of Health and Human Services.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Ries LAG, Melbert D, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975-2005, National Cancer Institute. Bethesda, MD: 2008. http://seer.cancer.gov/csr/1975_2005/ Accessed August 5, 2011. [Google Scholar]
- 3.Cohen JH, Schoenbach VJ, Kaufman JS, et al. Racial differences in clinical progression among Medicare recipients after treatment for localized prostate cancer (United States) Cancer Causes Control. 2006;17:803. doi: 10.1007/s10552-006-0017-7. [DOI] [PubMed] [Google Scholar]
- 4.Albain KS, Unger JM, Crowley JJ, et al. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst. 2009;101:984. doi: 10.1093/jnci/djp175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tehranifar P, Neugut AI, Phelan JC, et al. Medical advances and racial/ethnic disparities in cancer survival. Cancer Epidemiol Biomarkers Prev. 2009;18:2701. doi: 10.1158/1055-9965.EPI-09-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donabedian A. The quality of care. How can it be assessed? JAMA. 1988;260:1743. doi: 10.1001/jama.260.12.1743. [DOI] [PubMed] [Google Scholar]
- 7.Spencer BA, Steinberg M, Malin J, et al. Quality-of-care indicators for early-stage prostate cancer. J Clin Oncol. 2003;21:1928. doi: 10.1200/JCO.2003.05.157. [DOI] [PubMed] [Google Scholar]
- 8.Barocas DA, Mitchell R, Chang SS, et al. Impact of surgeon and hospital volume on outcomes of radical prostatectomy. Urol Oncol. 2010;28:243. doi: 10.1016/j.urolonc.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Healthcare Cost and Utilization Project (HCUP) Agency for Healthcare Research and Quality; Rockville, MD: Jun, 2011. HCUP Databases. www.hcup-us.ahrq.gov/sidoverview.jsp. Accessed August 5, 2011. [PubMed] [Google Scholar]
- 10.Coffey R, Barrett M, Houchens R, et al. Methods Applying AHRQ Quality Indicators to Healthcare Cost and Utilization Project (HCUP) Data for the Eighth (2010) National Healthcare Quality Report (NHQR) and National Healthcare Disparities Report (NHDR) HCUP Methods Series Report # 2010-06. 2010 http://www.hcup-us.ahrq.gov/reports/methods.jsp. Accessed September 1, 2011.
- 11.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Begg CB, Riedel ER, Bach PB, et al. Variations in morbidity after radical prostatectomy. N Engl J Med. 2002;346:1138. doi: 10.1056/NEJMsa011788. [DOI] [PubMed] [Google Scholar]
- 13.Hu JC, Nelson RA, Wilson TG, et al. Perioperative complications of laparoscopic and robotic assisted laparoscopic radical prostatectomy. J Urol. 2006;175:541. doi: 10.1016/S0022-5347(05)00156-4. [DOI] [PubMed] [Google Scholar]
- 14.Harrell FE. Regression Modeling Strategies, with Applications to Linear Models, Survival Analysis and Logistic Regression. New York: Springer; 2001. [Google Scholar]
- 15.Latini DM, Elkin EP, Cooperberg MR, et al. Differences in clinical characteristics and disease-free survival for Latino, African American, and non-Latino white men with localized prostate cancer: data from CaPSURE. Cancer. 2006;106:789. doi: 10.1002/cncr.21675. [DOI] [PubMed] [Google Scholar]
- 16.Danley KL, Richardson JL, Bernstein L, et al. Prostate cancer: trends in mortality and stage-specific incidence rates by racial/ethnic group in Los Angeles County, California (United States) Cancer Causes Control. 1995;6:492. doi: 10.1007/BF00054156. [DOI] [PubMed] [Google Scholar]
- 17.Clegg LX, Li FP, Hankey BF, et al. Cancer survival among US whites and minorities: a SEER (Surveillance, Epidemiology, and End Results) Program population-based study. Arch Intern Med. 2002;162:1985. doi: 10.1001/archinte.162.17.1985. [DOI] [PubMed] [Google Scholar]
- 18.Godley PA, Schenck AP, Amamoo MA, et al. Racial differences in mortality among Medicare recipients after treatment for localized prostate cancer. J Natl Cancer Inst. 2003;95:1702. doi: 10.1093/jnci/djg094. [DOI] [PubMed] [Google Scholar]
- 19.Krupski TL, Kwan L, Afifi AA, et al. Geographic and socioeconomic variation in the treatment of prostate cancer. J Clin Oncol. 2005;23:7881. doi: 10.1200/JCO.2005.08.755. [DOI] [PubMed] [Google Scholar]
- 20.Klabunde CN, Potosky AL, Harlan LC, et al. Trends and black/white differences in treatment for nonmetastatic prostate cancer. Medical care. 1998;36:1337. doi: 10.1097/00005650-199809000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Zeliadt SB, Potosky AL, Etzioni R, et al. Racial disparity in primary and adjuvant treatment for nonmetastatic prostate cancer: SEER-Medicare trends 1991 to 1999. Urology. 2004;64:1171. doi: 10.1016/j.urology.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 22.Harlan LC, Potosky A, Gilliland FD, et al. Factors associated with initial therapy for clinically localized prostate cancer: prostate cancer outcomes study. J Natl Cancer Inst. 2001;93:1864. doi: 10.1093/jnci/93.24.1864. [DOI] [PubMed] [Google Scholar]
- 23.Platz EA, Rimm EB, Willett WC, et al. Racial variation in prostate cancer incidence and in hormonal system markers among male health professionals. J Natl Cancer Inst. 2000;92:2009. doi: 10.1093/jnci/92.24.2009. [DOI] [PubMed] [Google Scholar]
- 24.Pettaway CA. Racial differences in the androgen/androgen receptor pathway in prostate cancer. J Natl Med Assoc. 1999;91:653. [PMC free article] [PubMed] [Google Scholar]
- 25.Freeman VL, Durazo-Arvizu R, Arozullah AM, et al. Determinants of mortality following a diagnosis of prostate cancer in Veterans Affairs and private sector health care systems. Am J Public Health. 2003;93:1706. doi: 10.2105/ajph.93.10.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onega T, Duell EJ, Shi X, et al. Race versus place of service in mortality among Medicare beneficiaries with cancer. Cancer. 2010;116:2698. doi: 10.1002/cncr.25097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du XL, Fang S, Coker AL, et al. Racial disparity and socioeconomic status in association with survival in older men with local/regional stage prostate carcinoma: findings from a large community-based cohort. Cancer. 2006;106:1276. doi: 10.1002/cncr.21732. [DOI] [PubMed] [Google Scholar]
- 28.Robbins AS, Yin D, Parikh-Patel A. Differences in prognostic factors and survival among White men and Black men with prostate cancer, California, 1995-2004. Am J Epidemiol. 2007;166:71. doi: 10.1093/aje/kwm052. [DOI] [PubMed] [Google Scholar]
- 29.Gooden KM, Howard DL, Carpenter WR, et al. The effect of hospital and surgeon volume on racial differences in recurrence-free survival after radical prostatectomy. Medical care. 2008;46:1170. doi: 10.1097/MLR.0b013e31817d696d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jayadevappa R, Chhatre S, Johnson JC, et al. Association between ethnicity and prostate cancer outcomes across hospital and surgeon volume groups. Health Policy. 2011;99:97. doi: 10.1016/j.healthpol.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.