Abstract
Scaffolds for tissue repair must provide structural and biochemical cues to initiate the complex cascade of events that lead to proper tissue formation. Incorporating genes into these scaffolds is an attractive alternative to protein delivery since gene delivery can be tunable to any DNA sequence and genes utilize the cells’ machinery to continuously produce therapeutic proteins, leading to longer lasting transgene expression and activation of autocrine and paracrine signaling that are not activated with bulk protein delivery. In this review, we discuss the importance of scaffold design and the impact of its design parameters (e.g. material, architecture, vector incorporation, biochemical cue presentation) on transgene expression and tissue repair.
Introduction
In the design of scaffolds for tissue repair, biochemical, biophysical, and cell-cell signals must be intricately orchestrated to guide the formation of healthy tissue at sites of injury or disease. Ideally, the manner in which these signals are incorporated allows for necessary changes during tissue growth. For example, the biochemical signals that contribute to the start of morphogenesis (tissue growth) are very often detrimental if they are present at the final stages of growth which, in many cases, cause pathological conditions. Thus, the biochemical signals (e.g. peptides, proteins, small molecules) must be introduced such that their activity can be regulated. Proteins are the most common bioactive signal introduced into scaffolds for tissue repair. Although delivery mechanisms have been designed to control release rates of one or multiple proteins, protein stability and cost are still major limitations. For example, the biological half-life of platelet-derived growth factor (PDGF), basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) are less than 2 [1], 3 [2], and 30 minutes [3], respectively, when injected intravenously. Thus, to achieve therapeutic success, proteins often require large doses and multiple injections [4–6]. Gene delivery has been used as an alternative to protein and protein fragment delivery [7], and it holds the advantage that a universal delivery strategy can be designed for any DNA sequence. A universal delivery strategy is not possible for growth factor delivery since the tertiary and quaternary structures are different for each protein and immobilization or other processing conditions affect each protein differently. Furthermore, the secretion of a protein by a transfected cell may be present for a longer duration. This increased residence time eliminates the need for repeated injections [8] and stimulates autocrine and paracrine signaling in tissue formation, which cannot be induced by delivery of the protein to the bulk media [9]. One major limitation of gene delivery is that the cargo is not immediately available as a bioactive signal, whereas proteins can begin their biochemical activation of targeted cells and commence tissue repair immediately after implantation. To this end, successful gene delivery and transfection depends on a series of critical steps, which take several hours to days to commence in vivo, with transgene expression peaking in the order of days after injection for naked plasmid, minicircles [10,11] and polyplexes [12]. Figure 1 details the steps that must occur for gene transfer to take place from the point of view of scaffolds for tissue repair. Figure 2 summarizes the major design characteristics for matrix based gene delivery for tissue repair. In this review, we explore the use of genes as bioactive signals to guide tissue repair from the point of view of scaffold design.
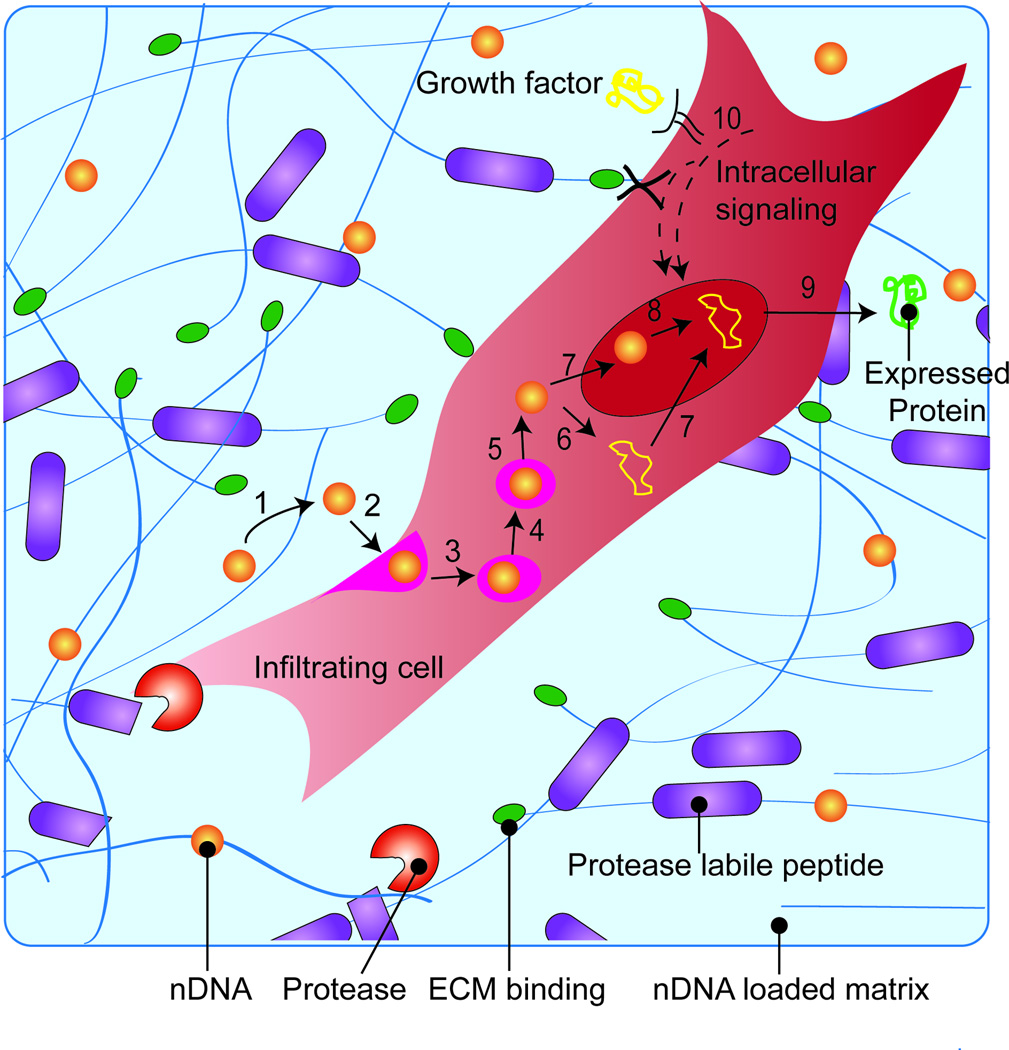
Figure 1.
Schematic overview of protein expression. For gene delivery, nDNA (1) is released from the scaffold through either hydrolysis or cellular migration (2) and internalized into the endosome (3). The endosome matures changing its oxidative and acidity resulting in endosomal escape of nDNA (4–5). nDNA can enter the nucleus (7) to be unpacked (8) or be de-coupled in the cytosol (6) for nuclear entry (7), where transcription and translation occurs (9) for protein expression. Growth factors or other bioactive signals can be used to induce intracellular signaling pathways that prime cells for transfection (10).
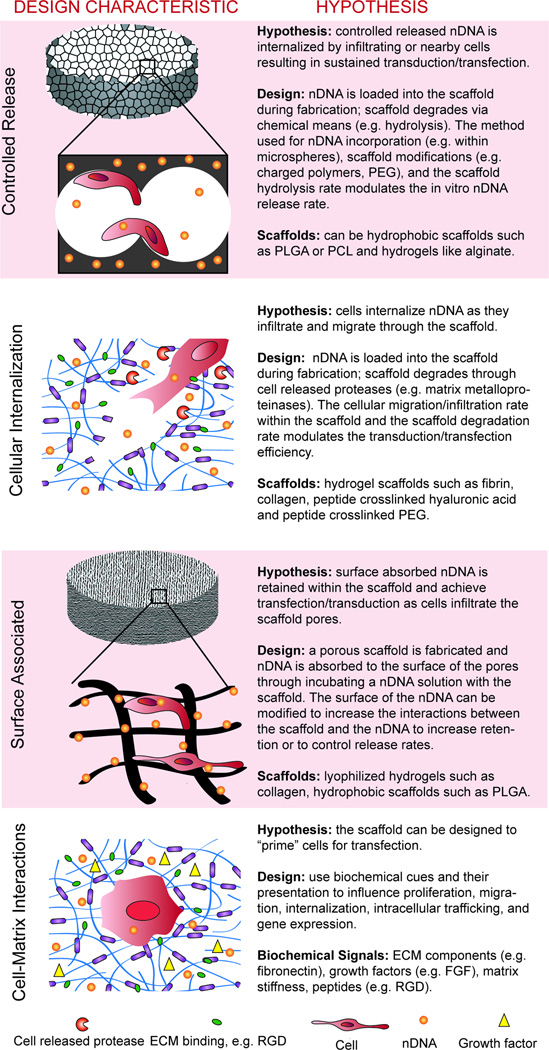
Figure 2.
The design of scaffolds for tissue repair that use genes as a bioactive signal goes beyond incorporating the nDNA into the scaffold. See text for corresponding references.
Vector Design
The two main types of vectors used for gene transfer in the context of tissue repair are plasmid DNA or modified viruses. The major design characteristics for vector design are the attenuation of the immune response, the promoters used to drive expression (Figure 3A), and the therapeutic protein expressed. Table 1 details these major design characteristics for vector design and points the reader to further reading on the subject.
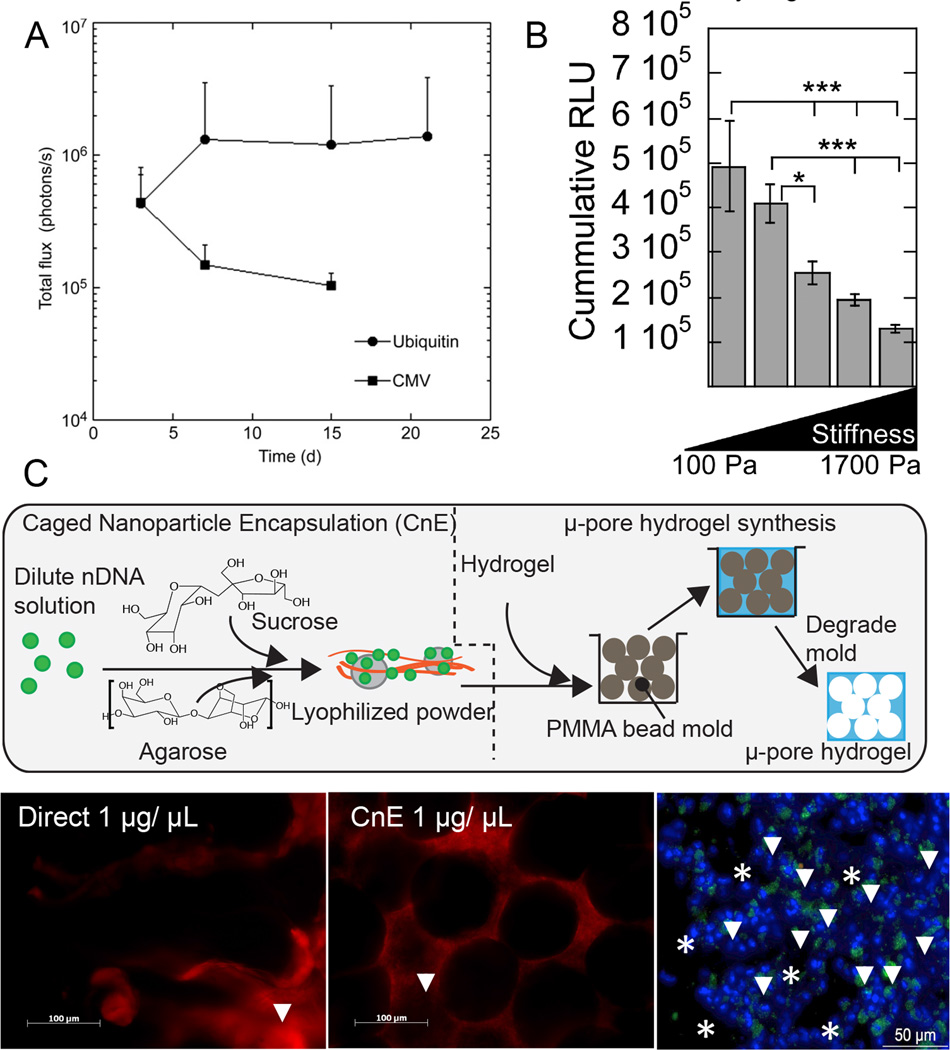
Figure 3.
Novel approaches to investigate the effect of design parameters on transgene expression. Different plasmid promoters (UbC vs. CMV) showed to have an effect on in vivo transgene expression (A), while stiffness had an inverse correlation with in vitro transgene expression in hyaluronic acid hydrogels (B). To decrease nDNA aggregation at higher nDNA concentrations, a caged nanoparticle encapsulation (CnE) technique was developed and applied to porous hydrogels for in vivo transfection (arrows show transfected cells, C).
Table 1.
Vector and carrier design characteristics.
| Consideration | Type | Details | References |
|---|---|---|---|
| Immune Response | CpG Motifs |
|
[51–53] |
| Minicircle DNA |
|
[10,54,55] | |
| Viral Capsid |
|
[56] | |
|
[41] | ||
| Promoters | Cytomegalovirus (CMV) |
|
[57] |
| Ubiquitin C (UbC) |
|
[19] | |
| Tissue-Specific |
|
[58] | |
| Bioactive Signal | Growth Factors |
|
[59] |
| Transcription Factors |
|
[33] | |
| siRNA |
|
[60] | |
| Carriers | Polyplex |
|
[30,60,61] |
| Lipoplex |
|
[14,61] | |
| Viral |
|
[15,62] | |
| Inorganic Particles |
|
[21,63] | |
Delivery of the Vector
Although naked DNA has shown success in the delivery of genes in vivo for tissue repair, the field has moved towards the use of packaged DNA (nDNA), either in synthetic particles or viruses. This review does not intend to focus on delivery vector design, however, the most commonly used delivery vectors used in the context of tissue repair are mentioned in Table 1. The reader is referred to the following recent review articles that focus on this topic [13–15].
Design of the Matrix
Although the primary focus to enhance transgene expression in vivo has been the design of the delivery vector, the matrix itself can provide alternative approaches to enhance transfection efficiency as well as promote tissue formation. Gene transfer from a matrix offers a three-dimensional distribution of complexes for more controlled, localized transfection as compared to a bolus delivery that may result in an unfavorable systemic delivery or unintended delivery to neighboring organs and tissues. In addition, delivery from a matrix can maintain the level of the vector over time, providing repeated opportunities for transfection/transduction and extending transgene expression as compared to bolus delivery. Incorporation of polyplexes into hydrogels scaffolds have shown sustained expression compared to soluble polyplexes (35 days compared to 7 days) [12]. Below, we review how the matrix has been designed to modulate transgene expression and guide tissue formation (Figure 2).
Controlled Release of nDNA
Controlled release strategies are often described as an important design parameter for matrix mediated gene transfer, with the belief that sustained release of the transfection vector achieves prolonged transgene expression over burst-released vectors. The hypothesis is that maintaining the level of the vector in the local microenvironment constant (since vector is continuously released) provides repeated opportunities for transfection/transduction resulting in sustained transgene expression. Prolonged transgene expression of a single protein is desired in situation where the tissue takes time to mature. For example, the sustained release of VEGF is necessary to promote the formation of mature vasculature [16]. To achieve controlled release, the nucleic acid is encapsulated within the scaffold during scaffold fabrication, and the release rate is controlled through modulating the degradation rate of the scaffold. In this approach, typically the scaffold degradation rate is not dependent on cellular action but rather it is chemically mediated through processes such as hydrolysis. Additionally, the scaffolds may be highly porous to allow for cellular infiltration within the scaffold such that as the DNA is released, it can reach the infiltrating cells. Poly(lactide-co-glycolide) (PLGA) scaffolds were some of the first to be used for matrix mediated gene delivery [17] and can be designed to release plasmid DNA in hours, weeks or months in vitro and have resulted in sustained transgene expression for up to 105 days [18]. Although controlled release is often cited as a desired quality to ensure long lasting expression, recent data suggest that release rate in vitro does not lead to corresponding differences of transgene expression in vivo. The Shea lab designed PLGA scaffolds with vastly different DNA release rates in vitro and showed they all achieved the same level and duration of transgene expression in vivo [19]. This suggests that in vitro release kinetics for nDNA do not correlate well with in vivo release or that sustained release of DNA is not the reason sustained transgene expression is observed. Hydrogels have also been designed to achieve controlled release. Oxidized alginate hydrogels loaded with DNA/PEI nDNA were shown to achieve sustained release in vitro and achieve enhanced revascularization in vivo [20].
Controlling Cellular Infiltration into nDNA loaded scaffolds
An alternative approach to controlled release is to design scaffolds that allow cell mediated degradation and cellular infiltration within the bulk of the scaffold. These approaches involve the use of hydrogels either naturally crosslinked (e.g. collagen [21], fibrin [22,23], gelatin [24]) or synthetically crosslinked with protease degradable peptides (e.g. PEG [25], hyaluronic acid [26,27]). The hypothesis in this case is that cells uptake the DNA as they infiltrate the scaffold and thus the transgene expression can be sustained or increased with time. This hypothesis has been proven to be true in vitro with cells embedded in DNA loaded MMP-degradable PEG [28,29] or hyaluronic acid [30] hydrogels, showing sustained transgene expression when the hydrogels were designed to enhance the cellular migration rate. The incorporation of nDNA into protease degradable scaffolds can result in aggregation either due to the interaction of nDNA with the gel precursor solutions such as in the case with fibrin or hyaluronic acid [26], or the interaction of nDNA particles with themselves as in the case for high nDNA concentrations [28]. To prevent such aggregation, a caged nanoparticle encapsulation (CnE) approach has been designed, where the nDNA are generated under dilute conditions and lyophilized in the presence of sucrose and agarose (Figure 3C). The sucrose is used as a cryo-protectant while the agarose functions as an inert polymer that prevents nDNA from interacting with the gel precursor solution and itself. This approach has been shown to result in active and non-aggregated polyplexes [26,31], resulting in transgene expression in vivo in a subcutaneous model (Figure 3C). Since synthetically crosslinked hydrogels have been shown to result in poor cellular infiltration in vivo in areas of low protease expression [27], micron sized pores have been introduced into PEG [32] and HA [27] hydrogels to enhance cellular infiltration and angiogenesis. Lentiviral vectors encoding for VEGF encapsulated in porous PEG hydrogels demonstrated blood vessel formation and lectin-positive cells at 2 and 4 weeks, while significant collagen deposition was observed by 4 weeks when compared to encapsulated lentivirus encoding for luciferase [32].
To achieve further control over transgene expression of encapsulated nDNA and prevent premature release of the nDNA, nDNA has been covalently immobilized to the scaffold backbone. In this case, the release rate and transfection efficiency are either related to the degradation rate of the scaffold (to allow nDNA release and internalization by infiltrating cells surrounding the implant [33]) or the degradation rate of the tether between the nDNA and the scaffold (which can control release rate or target a particular cell population) [34,35].
Surface Associated nDNA
A complementary or stand-alone approach to control both release and cellular infiltration involves associating the DNA to the scaffold surface through nonspecific adsorption. The hypothesis in this case is that the loosely associated DNA can achieve sufficient nDNA retention to avoid premature release, allowing transgene expression to promote tissue repair soon after implantation and the embedded DNA (if present) can prolong this expression or express a different gene. Moreover, since nDNA are adsorbed following scaffold formation, it avoids the harsh processing conditions that that may occur during scaffold synthesis and can avoid polyplex aggregation [36]. In vitro, surface associated DNA polyplexes result in enhanced transgene expression compared to embedded polyplexes [37]. Surface associated DNA was the most widely used approach to deliver DNA in vivo from scaffolds this past year. Effective gene transfer and tissue formation was demonstrated with collagen/gelatin meshes or sponges [38–40], silk fibroin scaffolds [41], PLGA multichannel bridges [42], electrospun fibers [43] and collagen/chitosan scaffolds [44] [45].These studies achieved regeneration of critical size defects in animal models and yielded similar results compared to the delivery of recombinant protein.
Biochemical Cues
Since gene transfer efficiency is correlated with cellular process such as proliferation rate, cellular infiltration rate into the scaffold, and actin/microtubule polymerization or de-polymerization, the scaffold itself can be engineered to enhance transgene expression. Integrin cell adhesion to the scaffold can be engineered to achieve enhanced cell migration and proliferation. Alginate hydrogels conjugated with various RGD densities for siRNA-mediated knockdown of eGFP demonstrated that increasing RGD density resulted in significantly higher knockdown of the targeted protein [46]. Moreover, RGD gradients and presentation (homogeneous vs. clustered) in different scaffolds have been used to influence transfection [30,47]. Hydrogel stiffness can also be used to modulate migration and gene delivery rates; stiffer gels result in slower release rates of encapsulated polyplexes and decreased cell populations, spreading, and transfection [30] (Figure 3B). ECM proteins have also shown to have a significant impact on gene transfer with different ECM molecules enhancing or inhibiting gene transfer in vitro [48,49]. Although the mechanism of the ECM mediated enhancement is not completely understood, RhoGTPases have been shown to play a significant role [48]. The co-delivery of proteins from the scaffold can be used to modulate the proliferative state of the infiltrating cells. Delivery of plasmid encoding for BMP-2 with along with recombinant bFGF encapsulated in PLG microspheres in vivo demonstrated significantly enhanced gene expression and increased blood vessel density compared to pDNA alone [50].
Future directions and Conclusion
Current tissue engineering approaches to help guide wound healing and tissue repair primarily focus on developing scaffolds to deliver bioactive signals to aid these events. In this report, we aimed to elucidate the complexity of designing gene-loaded scaffolds for tissue engineering. Although this review focused primarily on gene delivery, it is important to note that a successful scaffold may not necessarily be successful based solely on the delivery of proteins or genes, but rather a combination of both. A dual delivery hydrogel system of proteins and genes can utilize the transient expression of protein delivery, and achieve sustained expression through encapsulated or immobilized plasmids. Moreover, studies on gene incorporation, scaffold material, architecture, and presentation of biochemical cues highlight the importance of how cells experience the local microenvironment and their effect on gene transfer. It is paramount to also investigate strategies to prime cells for transfection which may include providing proliferative cues, ECM components, integrins, and better mimicking the heterogeneity of the cellular microenvironment by incorporating growth factors or plasmids in a gradient or spatially-patterned scaffold. Modulating the scaffold composition in layers may allow future investigations on delivering multiple proteins, genes, or a combination with more control over design parameters (e.g. number of polymeric layers, amount of nucleic acid deposition). As a result, careful consideration of these parameters must be taken to create a successful gene loaded scaffold for regenerative medicine and tissue repair.
Highlights.
We present a detailed description of gene loaded scaffold design for tissue repair.
Infiltrating cells are transfected through released DNA or matrix residing DNA.
Surface associated of DNA via nonspecific adsorption is a popular current technique.
Biochemical cues may be used to “prime” cells for transgene expression.
Acknowledgements
We would like to thank Talar Tokatlian for helpful discussions and our funding from the National Institutes of Heath NHLBI (R01 HL110592) and the National Science Foundation CBET division (0747539 CAREER). We apologize to all the scientists that we were unable to cite due to space limitations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Papers of particular interest, published within the period of review have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Bowen-Pope DF, Malpass TW, Foster DM, Ross R. Platelet-derived growth factor in vivo: levels, activity, and rate of clearance. Blood. 1984;64(2):458–469. [PubMed] [Google Scholar]
- 2.Edelman ER, Nugent MA, Karnovsky MJ. Perivascular and intravenous administration of basic fibroblast growth factor: vascular and solid organ deposition. Proc Natl Acad Sci U S A. 1993;90(4):1513–1517. doi: 10.1073/pnas.90.4.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eppler SM, Combs DL, Henry TD, Lopez JJ, Ellis SG, Yi JH, Annex BH, McCluskey ER, Zioncheck TF. A target-mediated model to describe the pharmacokinetics and hemodynamic effects of recombinant human vascular endothelial growth factor in humans. Clin Pharmacol Ther. 2002;72(1):20–32. doi: 10.1067/mcp.2002.126179. [DOI] [PubMed] [Google Scholar]
- 4.Simons M, Annex BH, Laham RJ, Kleiman N, Henry T, Dauerman H, Udelson JE, Gervino EV, Pike M, Whitehouse MJ, Moon T, et al. Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2: double-blind, randomized, controlled clinical trial. Circulation. 2002;105(7):788–793. doi: 10.1161/hc0802.104407. [DOI] [PubMed] [Google Scholar]
- 5.Simons M, Ware JA. Therapeutic angiogenesis in cardiovascular disease. Nat Rev Drug Discov. 2003;2(11):863–871. doi: 10.1038/nrd1226. [DOI] [PubMed] [Google Scholar]
- 6.Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, Shah PK, Willerson JT, Benza RL, Berman DS, Gibson CM, et al. The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation. 2003;107(10):1359–1365. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- 7.Gauglitz GG, Jeschke MG. Combined gene and stem cell therapy for cutaneous wound healing. Mol Pharm. 2011;8(5):1471–1479. doi: 10.1021/mp2001457. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal A, Mallapragada SK. Synthetic sustained gene delivery systems. Curr Top Med Chem. 2008;8(4):311–310. [PubMed] [Google Scholar]
- 9.Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML. Autocrine VEGF Signaling Is Required for Vascular Homeostasis. Cell. 2007;130(4):691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang M, Chen Z, Hu S, Jia F, Li Z, Hoyt G, Robbins RC, Kay MA, Wu JC. Novel minicircle vector for gene therapy in murine myocardial infarction. Circulation. 2009;120(11) Suppl:S230–S237. doi: 10.1161/CIRCULATIONAHA.108.841155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilber A, Frandsen JL, Wangensteen KJ, Ekker SC, Wang X, McIvor RS. Dynamic gene expression after systemic delivery of plasmid DNA as determined by in vivo bioluminescence imaging. Hum Gene Ther. 2005;16(11):1325–1332. doi: 10.1089/hum.2005.16.1325. [DOI] [PubMed] [Google Scholar]
- 12.Meilander-Lin NJ, Cheung PJ, Wilson DL, Bellamkonda RV. Sustained in vivo gene delivery from agarose hydrogel prolongs nonviral gene expression in skin. Tissue Eng. 2005;11(3–4):546–555. doi: 10.1089/ten.2005.11.546. [DOI] [PubMed] [Google Scholar]
- 13.Jafari M, Soltani M, Naahidi SN, Karunaratne D, Chen P. Nonviral Approach for Targeted Nucleic Acid Delivery. Current Medicinal Chemistry. 2012;19(2):197–208. doi: 10.2174/092986712803414141. [DOI] [PubMed] [Google Scholar]
- 14.Tros de Ilarduya C, Sun Y, Düzgüneş N. Gene delivery by lipoplexes and polyplexes. European Journal of Pharmaceutical Sciences. 2010;40(3):159–170. doi: 10.1016/j.ejps.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Giacca M, Zacchigna S. Virus-mediated gene delivery for human gene therapy. Journal of Controlled Release. 2012;161(2):377–388. doi: 10.1016/j.jconrel.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Bramfeld H, Sabra G, Centis V, Vermette P. Scaffold Vascularization: A Challenge for Three-Dimensional Tissue Engineering. Current Medicinal Chemistry. 2010;17(33):3944–3967. doi: 10.2174/092986710793205327. [DOI] [PubMed] [Google Scholar]
- 17.Shea LD, Smiley E, Bonadio J, Mooney DJ. DNA delivery from polymer matrices for tissue engineering. Nat Biotechnol. 1999;17(6):551–554. doi: 10.1038/9853. [DOI] [PubMed] [Google Scholar]
- 18.Jang JH, Rives CB, Shea LD. Plasmid delivery in vivo from porous tissue-engineering scaffolds: transgene expression and cellular transfection. Mol Ther. 2005;12(3):475–483. doi: 10.1016/j.ymthe.2005.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Avilés MO, Lin C-H, Zelivyanskaya M, Graham JG, Boehler RM, Messersmith PB, Shea LD. The contribution of plasmid design and release to in vivo gene expression following delivery from cationic polymer modified scaffolds. Biomaterials. 2010;31(6):1140–1147. doi: 10.1016/j.biomaterials.2009.10.035. •• In this report, a layer of PLG microspheres with cationic polymers were coated with PD in a PLGA matrix. In vitro studies showed that the PD coating yielded a smaller initial burst release. In vivo transgene expression showed no statistical difference with unmodified matrices. CMV promoter yields a burst in initial transgene expression, while the UbC promoter achieves a comparable, sustained transgene expression in vivo.
- 20.Kong H, Kim E, Huang Y-C, Mooney D. Design of Biodegradable Hydrogel for the Local and Sustained Delivery of Angiogenic Plasmid DNA. Pharmaceutical Research. 2008;25(5):1230–1238. doi: 10.1007/s11095-007-9526-7. [DOI] [PubMed] [Google Scholar]
- 21.Shin S, Shea LD. Lentivirus Immobilization to Nanoparticles for Enhanced and Localized Delivery From Hydrogels. Mol Ther. 2010;18(4):700–706. doi: 10.1038/mt.2009.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kidd ME, Shin S, Shea LD. Fibrin hydrogels for lentiviral gene delivery in vitro and in vivo. Journal of Controlled Release. 2012;157(1):80–85. doi: 10.1016/j.jconrel.2011.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raut SD, Lei P, Padmashali RM, Andreadis ST. Fibrin-mediated lentivirus gene transfer: Implications for lentivirus microarrays. Journal of Controlled Release. 2010;144(2):213–220. doi: 10.1016/j.jconrel.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saito T, Tabata Y. Preparation of gelatin hydrogels incorporating small interfering RNA for the controlled release. J Drug Target. 2012;20(10):864–872. doi: 10.3109/1061186X.2012.725170. [DOI] [PubMed] [Google Scholar]
- 25.Shepard JA, Wesson PJ, Wang CE, Stevans AC, Holland SJ, Shikanov A, Grzybowski BA, Shea LD. Gene therapy vectors with enhanced transfection based on hydrogels modified with affinity peptides. Biomaterials. 2011;32(22):5092–5099. doi: 10.1016/j.biomaterials.2011.03.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lei Y, Rahim M, Ng Q, Segura T. Hyaluronic acid and fibrin hydrogels with concentrated DNA/PEI polyplexes for local gene delivery. J Control Release. 2011;153(3):255–261. doi: 10.1016/j.jconrel.2011.01.028. • In this report, a CnE technique was applied to fibrin and hyaluronic acid hydrogel systems. Hydrogels were loaded with higher DNA concentrations without aggregation via CnE. DNA/PEI polyplexes loaded in HA and fibrin hydrogels achieved gene transfer in vivo.
- 27. Tokatlian T, Cam C, Siegman SN, Lei Y, Segura T. Design and characterization of microporous hyaluronic acid hydrogels for in vitro gene transfer to mMSCs. Acta Biomaterialia. 2012;8(11):3921–3931. doi: 10.1016/j.actbio.2012.07.014. • In this report, porous hyaluronic acid hydrogels were utilized to study in vitro gene transfer. CnE allowed loading of LPEI/pDNA polyplexes into porous gels without aggregation. Studies showed sustained release of polyplexes in the presence of cells. In vitro transgene expression showed an increase in GLuc expression over time. No significant difference could be observed between gels with various pore sizes.
- 28.Lei Y, Segura T. DNA delivery from matrix metalloproteinase degradable poly (ethylene glycol) hydrogels to mouse cloned mesenchymal stem cells. Biomaterials. 2009;30(2):254–265. doi: 10.1016/j.biomaterials.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shepard JA, Huang A, Shikanov A, Shea LD. Balancing cell migration with matrix degradation enhances gene delivery to cells cultured three-dimensionally within hydrogels. J Control Release. 2010;146(1):128–135. doi: 10.1016/j.jconrel.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gojgini S, Tokatlian T, Segura T. Utilizing Cell–Matrix Interactions To Modulate Gene Transfer to Stem Cells Inside Hyaluronic Acid Hydrogels. Molecular Pharmaceutics. 2011;8(5):1582–1591. doi: 10.1021/mp200171d. •• In this article, HA hydrogel parameters were varied; was studied. Increasing N/P ratio increased in vitro gene transfection but resulted in toxicity. Intermediate RGD clusters (0.4RGD/HA molecule) showed optimal transgene expression. Intermediate RGD concentrations (100uM) showed higher levels of transgene expression. Softer hydrogels resulted in enhanced gene transfer.
- 31.Lei YG, Huang SX, Sharif-Kashani P, Chen Y, Kavehpour P, Segura T. Incorporation of active DNA/cationic polymer polyplexes into hydrogel scaffolds. Biomaterials. 2010;31(34):9106–9116. doi: 10.1016/j.biomaterials.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shepard JA, Virani FR, Goodman AG, Gossett TD, Shin S, Shea LD. Hydrogel macroporosity and the prolongation of transgene expression and the enhancement of angiogenesis. Biomaterials. 2012;33(30):7412–7421. doi: 10.1016/j.biomaterials.2012.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trentin D, Hall H, Wechsler S, Hubbell JA. Peptide-matrix-mediated gene transfer of an oxygen-insensitive hypoxia-inducible factor-1alpha variant for local induction of angiogenesis. Proc Natl Acad Sci U S A. 2006;103(8):2506–2511. doi: 10.1073/pnas.0505964102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim HS, Yoo HS. MMPs-responsive release of DNA from electrospun nanofibrous matrix for local gene therapy: In vitro and in vivo evaluation. Journal of Controlled Release. 2010;145(3):264–271. doi: 10.1016/j.jconrel.2010.03.006. • In this article, PCL-PEG nanofibers and LPEI/pDNA were tethered via MMP-cleavable peptide linkers. In the presence of MMP-2, DNA and LPEI were quickly released from the nanofibers. In vivo studies showed LPEI/pDNA NF achieved significantly higher gene expression.
- 35.Tokatlian T, Shrum CT, Kadoya WM, Segura T. Protease degradable tethers for controlled and cell-mediated release of nanoparticles in 2- and 3-dimensions. Biomaterials. 2010;31(31):8072–8080. doi: 10.1016/j.biomaterials.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Segura T, Chung PH, Shea LD. DNA delivery from hyaluronic acid-collagen hydrogels via a substrate-mediated approach. Biomaterials. 2005;26(13):1575–1584. doi: 10.1016/j.biomaterials.2004.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saul JM, Linnes MP, Ratner BD, Giachelli CM, Pun SH. Delivery of non-viral gene carriers from sphere-templated fibrin scaffolds for sustained transgene expression. Biomaterials. 2007;28(31):4705–4716. doi: 10.1016/j.biomaterials.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 38.Chang PC, Seol YJ, Cirelli JA, Pellegrini G, Jin Q, Franco LM, Goldstein SA, Chandler LA, Sosnowski B, Giannobile WV. PDGF-B gene therapy accelerates bone engineering and oral implant osseointegration. Gene Ther. 2009;17(1):95–104. doi: 10.1038/gt.2009.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rose LC, Kucharski C, Uludag H. Protein expression following non-viral delivery of plasmid DNA coding for basic FGF and BMP-2 in a rat ectopic model. Biomaterials. 2012;33(11):3363–3374. doi: 10.1016/j.biomaterials.2012.01.031. • In this report, gelatin or collagen sponges with polyplexes to study protein expression in vivo. PEI25 achieved GFP expression in collagen;PEI-LA had transfection only in gelatin. Plasmids with a carrier resulted in higher bFGF expression than naked plasmids.
- 40.Chang SCN, Chung H-Y, Tai C-L, Chen PKT, Lin T-M, Jeng L-B. Repair of large cranial defects by hBMP-2 expressing bone marrow stromal cells: Comparison between alginate and collagen type I systems. Journal of Biomedical Materials Research Part A. 2010;94A(2):433–441. doi: 10.1002/jbm.a.32685. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Wu C, Luo T, Li S, Cheng X, Miron RJ. Synthesis and inflammatory response of a novel silk fibroin scaffold containing BMP7 adenovirus for bone regeneration. Bone. 2012;51(4):704–713. doi: 10.1016/j.bone.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 42. Tuinstra HM, Aviles MO, Shin S, Holland SJ, Zelivyanskaya ML, Fast AG, Ko SY, Margul DJ, Bartels AK, Boehler RM, Cummings BJ, et al. Multifunctional, multichannel bridges that deliver neurotrophin encoding lentivirus for regeneration following spinal cord injury. Biomaterials. 2012;33(5):1618–1626. doi: 10.1016/j.biomaterials.2011.11.002. • In this article, PLG multichannel bridges were loaded with lentivirus-hydroxylapatite nanoparticles. In vivo implantation showed significant transgene expression within 7 days. Bridges with vectors encoding for NT3/BDNF had more axons and myelination over time.
- 43.Yang Y, Xia T, Chen F, Wei W, Liu C, He S, Li X. Electrospun Fibers with Plasmid bFGF Polyplex Loadings Promote Skin Wound Healing in Diabetic Rats. Molecular Pharmaceutics. 2011;9(1):48–58. doi: 10.1021/mp200246b. [DOI] [PubMed] [Google Scholar]
- 44.Guo R, Xu S, Ma L, Huang A, Gao C. Enhanced angiogenesis of gene-activated dermal equivalent for treatment of full thickness incisional wounds in a porcine model. Biomaterials. 2010;31(28):7308–7320. doi: 10.1016/j.biomaterials.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 45.Luo T, Zhang W, Shi B, Cheng X, Zhang Y. Enhanced bone regeneration around dental implant with bone morphogenetic protein 2 gene and vascular endothelial growth factor protein delivery. Clinical Oral Implants Research. 2012;23(4):467–473. doi: 10.1111/j.1600-0501.2011.02164.x. [DOI] [PubMed] [Google Scholar]
- 46. Khormaee S, Ali OA, Chodosh J, Mooney DJ. Optimizing siRNA efficacy through alteration in the target cell-adhesion substrate interaction. J Biomed Mater Res A. 2012;100(10):2637–2643. doi: 10.1002/jbm.a.34202. • In this report, the effects of RGD density and matrix stiffness on gene silencing were studied in vitro. Higher RGD density achieved better siRNA knockdown.
- 47. Orsi S, Guarnieri D, De Capua A, Netti PA. Gene-activated and cell-migration guiding PEG matrices based on three dimensional patterning of RGD peptides and DNA complexes. Acta Biomater. 2012;8(9):3228–3240. doi: 10.1016/j.actbio.2012.05.010. •• In this report, PEG matrices were 3D spatially patterned with RGD peptides and PEI/pDNA polyplexes. GFP+ cells were found in regions of higher RGD and DNA concentrations over time. The percentage of transfected cells depended on RGD and DNA concentration. At the same DNA concentration higher RGD concentrations had more transfected cells. Cell migration across the RGD gradient resulted in more GFP+ cells over time.
- 48. Dhaliwal A, Maldonado M, Lin C, Segura T. Cellular Cytoskeleton Dynamics Modulates Non-Viral Gene Delivery through RhoGTPases. PLoS One. 2012;7(4):e35046. doi: 10.1371/journal.pone.0035046. • In this article, the role of RhoGTPases in polyplex uptake was studied via ECM-coated surfaces in vitro. Fibronectin-coated surfaces resulted in mMSC RhoGTPase and CdC42 activation. RhoGTPase inactivation via difficile toxin B showed inhibited transgene expression. RhoGTPase activation on collagen I-coated plates enhanced trangene expression.
- 49.Dhaliwal A, Maldonado M, Han Z, Segura T. Differential uptake of DNA–poly (ethylenimine) polyplexes in cells cultured on collagen and fibronectin surfaces. Acta Biomaterialia. 2010;6(9):3436–3447. doi: 10.1016/j.actbio.2010.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Riddle KW, Kong HJ, Leach JK, Fischbach C, Cheung C, Anseth KS, Mooney DJ. Modifying the proliferative state of target cells to control DNA expression and identifying cell types transfected in vivo. Mol Ther. 2007;15(2):361–368. doi: 10.1038/sj.mt.6300017. [DOI] [PubMed] [Google Scholar]
- 51.Scheule RK. The role of CpG motifs in immunostimulation and gene therapy. Advanced Drug Delivery Reviews. 2000;44(2–3):119–134. doi: 10.1016/s0169-409x(00)00090-9. [DOI] [PubMed] [Google Scholar]
- 52.Gill DR, Pringle IA, Hyde SC. Progress and prospects: the design and production of plasmid vectors. Gene Ther. 2009;16(2):165–171. doi: 10.1038/gt.2008.183. [DOI] [PubMed] [Google Scholar]
- 53.Yew NS, Zhao H, Wu IH, Song A, Tousignant JD, Przybylska M, Cheng SH. Reduced Inflammatory Response to Plasmid DNA Vectors by Elimination and Inhibition of Immunostimulatory CpG Motifs. Mol Ther. 2000;1(3):255–262. doi: 10.1006/mthe.2000.0036. [DOI] [PubMed] [Google Scholar]
- 54.Darquet AM, Rangara R, Kreiss P, Schwartz B, Naimi S, Delaere P, Crouzet J, Scherman D. Minicircle: an improved DNA molecule for in vitro and in vivo gene transfer. Gene Ther. 1999;6(2):209–218. doi: 10.1038/sj.gt.3300816. [DOI] [PubMed] [Google Scholar]
- 55.Chen ZY, He CY, Ehrhardt A, Kay MA. Minicircle DNA vectors devoid of bacterial DNA result in persistent and high-level transgene expression in vivo. Mol Ther. 2003;8(3):495–500. doi: 10.1016/s1525-0016(03)00168-0. [DOI] [PubMed] [Google Scholar]
- 56.Asuri P, Bartel MA, Vazin T, Jang J-H, Wong TB, Schaffer DV. Directed Evolution of Adeno-associated Virus for Enhanced Gene Delivery and Gene Targeting in Human Pluripotent Stem Cells. Mol Ther. 2012;20(2):329–338. doi: 10.1038/mt.2011.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nguyen AT, Dow AC, Kupiec-Weglinski J, Busuttil RW, Lipshutz GS. Evaluation of Gene Promoters for Liver Expression by Hydrodynamic Gene Transfer. Journal of Surgical Research. 2008;148(1):60–66. doi: 10.1016/j.jss.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Papadakis ED, Nicklin SA, Baker AH, White SJ. Promoters and control elements: designing expression cassettes for gene therapy. Curr Gene Ther. 2004;4(1):89–113. doi: 10.2174/1566523044578077. [DOI] [PubMed] [Google Scholar]
- 59.Koria P. Delivery of Growth Factors for Tissue Regeneration and Wound Healing. BioDrugs. 2012;26(3):163–175. doi: 10.2165/11631850-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 60.Scholz C, Wagner E. Therapeutic plasmid DNA versus siRNA delivery: Common and different tasks for synthetic carriers. Journal of Controlled Release. 2012;161(2):554–565. doi: 10.1016/j.jconrel.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 61.Morille M, Passirani C, Vonarbourg A, Clavreul A, Benoit J-P. Progress in developing cationic vectors for non-viral systemic gene therapy against cancer. Biomaterials. 2008;29(24–25):3477–3496. doi: 10.1016/j.biomaterials.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 62.Ginsburg DS, Thyagarajan B, Phillips JE, Calos MP. Gene Delivery by Viruses. Encyclopedia of Life Sciences. 2005:1–5. [Google Scholar]
- 63. Curtin CM, Cunniffe GM, Lyons FG, Bessho K, Dickson GR, Duffy GP, O'Brien FJ. Innovative collagen nano-hydroxyapatite scaffolds offer a highly efficient non-viral gene delivery platform for stem cell-mediated bone formation. Adv Mater. 2012;24(6):749–754. doi: 10.1002/adma.201103828. •• In this report, nano-hydroxyapatite particles (<200nm) were incorporated into collagen scaffolds. nHA-luc achieved higher transfection efficiency than other CaP transfection kits. nHA-BMP2 in vitro studies showed enhanced calcium and phosphate deposition. Collagen and phosphate deposition was enhanced in coll-nHA compared to coll-alone. Collagen matrices with nHA-BMP2 enhanced transfection and osteocalcin expression.