Abstract
Interleukin-10 (IL-10) is an important immunomodulatory cytokine that plays an obligate role in regulating inflammatory responses. Here we demonstrated the role of IL-10 in regulating crypts length and breadth as well as maintaining the survival of epithelial cells using rhesus colon explant cultures. Anti-IL-10 antibody treatment of colon explant cultures induced increased production of inflammatory cytokines/molecules like IFNγ, TNFα, CD107a and perforin as well as increased epithelial cell apoptosis compared to media controls tested. Our results suggest that IL-10 plays a crucial role in maintaining mucosal homeostasis by regulating mucosal IFNγ and TNFα cytokine production.
Keywords: Apoptosis, Colon, Cytokine, Epithelial Cell, IL-10, Rhesus Macaque
1. Introduction
Intestinal epithelial cells (ECs) play an important role in the immune system, both as a barrier and as a first-line pathogen recognition system [1]. Increased permeability due to compromised barrier function could be relevant to mucosal transmission of HIV and generalized HIV-induced immune-cell activation and disease progression. We recently have shown increased intestinal EC apoptosis in both acute and chronically SIV-infected rhesus macaques (RMs) [2]. Interleukin-10 (IL-10) was first described as an inhibitory factor for the production of Th1 cytokines [3]. IL-10 signaling is mediated by the interaction of IL-10 and IL-10 receptor (IL-10R consists of IL-10R1, IL-10R2), resulting in tyrosine phosphorylation of JAK1 and Tyk2 that finally activates latent transcription factors like stat1, stat3 or stat5, which are essential for regulating IL-10 anti-inflammatory activities [4]. In vivo and in vitro studies with recombinant IL-10 (rIL-10) protein and neutralizing IL-10 monoclonal antibodies (MAbs) have shown pleiotropic activity of IL-10 on T-cells, natural killer cells, B-cells, activated macrophages/monocytes, mast cells, dendritic cells, and keratinocytes [4, 5]. IL-10 also has inhibitory functions on several costimulatory molecules and cytokine synthesis, nitric oxide production, and MHC class I and II expression [6]. Studies with IL-10 deficient mice have shown that resident enteric bacteria are necessary for the development of spontaneous colitis and activation of immune system [7]. Similarly, studies with Th1-mediated colitis in SCID mice have provided evidence that IL-10 plays an essential role in the function of regulatory T-cells that control intestinal inflammatory responses [8]. A recent report also has shown increased IFNγ gene expression along with increased EC apoptosis in anti-IL-10 antibody treated colon explant cultures collected from patients with colon carcinoma [9]. Several studies demonstrate that IL-10 associated immune defects contribute to intestinal inflammation in inflammatory bowel disease including Crohn’s disease and Ulcerative Colitis where inflammatory T-cell responses were detected against harmless bacterial antigens [10, 11]. Despite all these studies, the detailed role of IL-10 in regulating intestinal homeostasis of normal healthy RMs is poorly documented, where the RM model is well recognized for understanding HIV/SIV pathogenesis, drug development and vaccine design.
In this study, we have examined the role of IL-10 in regulating intestinal ECs survivability by colon explant cultures using either anti-IL-10 MAbs or rIL-10 proteins. We have quantified mucosal cytokine(s) and degranulation molecule producing cells and correlated with the total apoptotic ECs. We present evidence that mucosal IL-10 plays an important role in maintaining intestinal mucosal integrity by regulating the expression of IFNγ and TNFα cytokines in intestinal lamina propria (LP).
2. Materials and Methods
2.1. Animals and ethical statement
Eight healthy, uninfected, normal male and female Indian RMs (Macaca mulatta) between 4.6-7.5 years of age and negative for HIV-2, SIV, type D retrovirus and STLV-1 infection were used for this study. Animals were housed at the Tulane National Primate Research Center (TNPRC) and under the full care of TNPRC veterinarians in accordance with the standards incorporated in the Guide to the Care and Use of Laboratory Animals. All animal procedures were performed only with sedated animals with the approval of the Tulane Institutional Animal Care and Use Committee. Colon specimens were collected during the time of their necropsy.
2.2. Colon explant experiments
Colon specimens approximately 8 cm in length were collected in ice-cold HBSS and were processed as described previously [12]. In brief, tissues were rinsed immediately and cut into approximately 2-4 mm2 fragments and placed in ice-cold RPMI-1640. Finally explants were cultured for 6h in 2ml RPMI-1640 containing BSA (0.01%), fungizone (1%), HEPES (25mM), and antibiotics (200μg/ml streptomycin and 200U/ml penicillin) in the presence of 5% CO2 at 37°C. Mucosal explants were treated with either anti-IL-10 MAbs (5μg/ml, BioLegend), rIL-10 protein (50ng/ml, BioLegend) or isotype control (5μg/ml, BioLegend). Explants without any treatment or isotype controls were treated as media-only (negative) controls. Initially we cultured colon explant tissues collected from sacrificed normal Indian RMs for 0h, 6h, 12h, and 24h without any antibody/protein treatment and there were no significant changes in cell death or morphology between 6h cultures compared to 0h cultures. However, increased death and changes in morphology were evident in colon explants kept beyond 6h (data not shown). Brefeldin A (Sigma) was added 1h after treatment for in situ detection of cytokines and degranulation molecules. After incubation, explant cultures were either cryopreserved in OCT or embedded in paraffin after proper fixation as previously described [2]. Tissue sections of 5 μm thick were processed from paraffin blocks and stained with Hematoxylin and Eosin (H&E).
2.3. Immunofluorescence and immunoperoxidase staining
Tissue sections were processed for immunofluorescent staining with one or a combination of primary antibodies (Supplementary Table 1) as described earlier [2]. In brief, tissue sections were stained sequentially for 2-3 colors by incubating first with the primary antibody for 1h, washed and stained further with Alexa Flour 488-conjugated secondary antibodies (1:1000 dilution, Invitrogen) for 30 min. Similarly, the slides were further stained with another primary antibody followed by Alexa Fluor 568-conjugated secondary antibodies (1:1000 dilution, Invitrogen). Nuclear staining was performed with anti-nuclear ToPro-3 antibodies (1μM, Invitrogen). Stained tissue sections were mounted using Prolong® Gold antifade medium (Invitrogen) and scanned for imaging using a TCS SP2 confocal laser scanning microscope (Leica, Germany) equipped with three lasers. Negative control slides were incorporated in each experiment either by omitting the primary antibody or using isotype IgG1 and IgG (H+L) controls [2] (Supplementary Figure 1). ImageJ (version 1.46, NIH, USA) and Adobe Photoshop CS5 Extended (USA) were used to assign colors to the channels collected. For quantification of intestinal apoptotic ECs, minimum 10 fields were imaged using Nuance FX multispectral imaging system at 500-720nm spectral range and assigned color using Nuance Version 2.10 software (CRi, USA). Active caspase-3+ (AC3+, marker for apoptotic cells) enterocytes were expressed in percentages of the total enterocytes (ToPro-3+Cytokeratin+).
An average of five fields (400X magnification) were manually counted in each stained mucosal explant tissue for quantifying cytokines and degranulation molecules. The sites for all immunohistochemistry evaluations were selected randomly from each tissue and counted by two different individuals to avoid bias.
2.4. Morphometric analysis
Paraffin embedded colon explant tissues were used for morphometric analysis. Slides were stained for H&E and measured for crypt length and breadth using Image-Pro Plus, v4.5 software as outlined previously [13].
2.5. Statistics
Graphical presentation and statistical analysis of the data were performed using GraphPad Prism (Version 5.0f, GraphPad software, CA). Results between experimental groups were compared using nonparametric Kruskal-Wallis test. Dunn’s multiple comparison test was used for post hoc analysis. The correlation between the frequency of cytokine expressing cells and percentages of apoptotic enterocytes from all treatments was calculated using nonparametric Spearman’s rank correlation. Differences were considered statistically significant when the P value was <0.05.
3. Results and Discussion
3.1. Intramucosal IL-10 blocking induces crypt morphological changes and apoptosis in colon explants
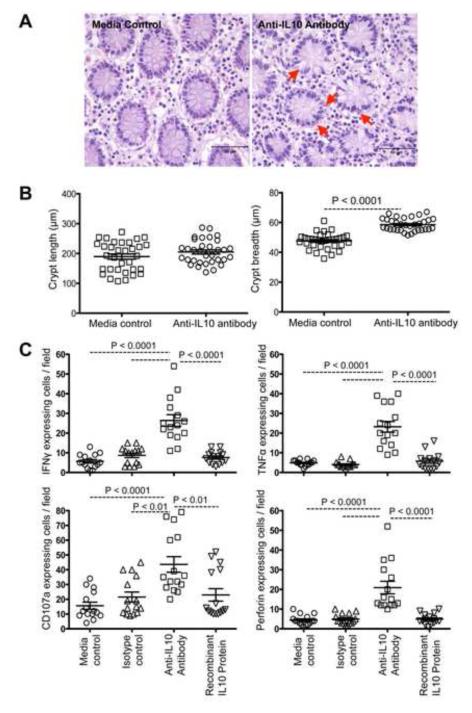
The lamina propria was mildly expanded with moderately increased lymphocytes and plasma cells in the anti-IL-10 MAb treated explants compared to controls (Figure 1A). Neither isotype MAb or rIL-10 protein treated colonic explants showed any pathological changes. However, numbers of cells in apoptosis were increased both in LP and crypts, characterized by pyknotic nuclei and eosinophilic cytoplasm. Many goblet cells in crypts had moderate to severe cytoplasmic vacuolar degeneration. We also observed a significant dilatation of crypt breadth in anti-IL-10 MAbs treated explants (P<0.0001) but no changes in crypt length (P=0.253) compared to media controls (Figure 1B) in three independent experiments. There were no morphological changes in explants treated with rIL-10 protein. These in vitro studies demonstrated that endogenous IL-10 in mucosal tissues is important in maintaining normal epithelial morphology.
Figure 1.
Treatment with anti-IL-10 monoclonal antibodies (MAbs) induces damage to colonic crypts and increased inflammatory cytokine production. (A) Destruction of colonic epithelial cells was observed in explant cultures in the presence of anti-IL-10 MAbs compared to media alone explants in H&E stained sections. Red arrows indicate disruption of the epithelial cell layer of the crypts. (B) Significant differences in colonic crypt breadth were observed between anti-IL-10 antibodies treated explants and controls compared to colonic crypt length in treatment groups (n=3; 11 crypts were measured for length and breadth for each sample). (C) Treatment with anti-IL-10 MAbs induces increased IFNγ, TNFα, CD107a and perforin production. The horizontal lines denote the mean frequencies (± standard errors) of each treatment group. Statistically significant differences between each treatment group are shown.
3.2. Anti-IL-10 antibody treatment leads to increased expression of proinflammatory, Th1 cytokines and degranulation molecules in lamina propria lymphocytes and increased apoptosis of enterocytes
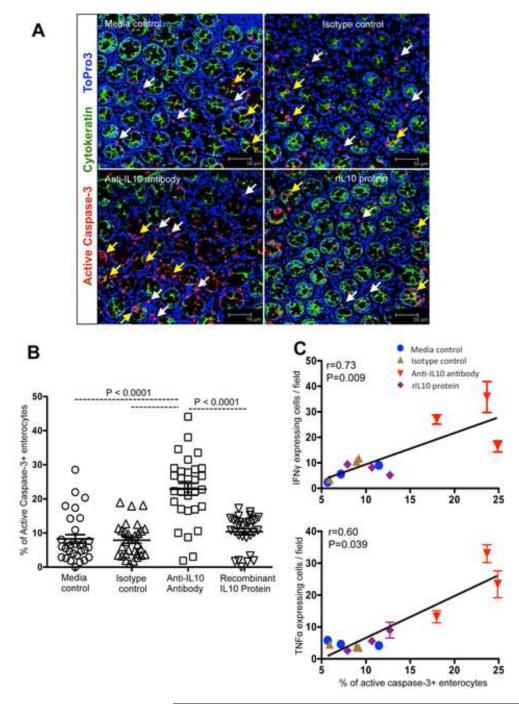
IL-10 is an important immunoregulatory cytokine that plays a key role in the control of inflammation [6, 14]. Earlier studies in mice have shown that anti-mouse IL-10 MAbs neutralized IL-10 in vivo and decreased IL-10 levels in serum nearly 75% [15-17]. A recent report with colon explant collected from patients undergoing surgery for colon carcinoma had also shown that IL-10 depletion can be achieved by administering neutralizing anti-IL-10 MAbs in culture conditions [9]. We quantified in situ proinflammatory (TNFα), Th1 (IFNγ) cytokines, and degranulation molecule (CD107a and Perforin) expression in mucosal explant tissues treated with either rIL-10 protein or anti-IL-10 MAbs in comparison with media and isotype controls. Endogenous IL-10 blocking with anti-IL-10 MAbs significantly increased IFNγ, TNFα, CD107a and perforin expression in LP cells compared to controls (P<0.01 to P<0.0001; Figure 1C). Representative immunofluorescent images also indicated up-regulation of IFNγ, TNFα, perforin and CD107a cytokines in mucosal sites (Supplementary Figures 2 and 3). There were no significant differences in cytokine expression in explants when treated with rIL-10 protein compared to controls (P>0.05; Figure 1C). A significantly increased percentage of apoptotic enterocytes (AC3+Cytokeratin+Topro-3+) were observed in anti-IL-10 MAb treated explant cultures compared to others (P<0.0001; Figures 2A-B). Percentages of apoptotic enterocytes correlated with cytokine expressing cells/field for both treated and untreated explant tissues. Nonparametric Spearman’s rank correlation coefficient analysis of these independent measures showed a highly significant positive correlation of IFNγ and TNFα molecules and EC apoptosis (Figure 2C). Despite an increase in perforin and CD107a degranulation molecules expression in anti-IL10 MAb-treated explants compared to other groups, we were unable to detect a significant correlation between the percentages of perforin positive cells and EC undergoing apoptosis (r=0.49, p=0.104). There was also no correlation between CD107a positive cells and percentages of apoptotic ECs (r=0.37, p=0.228). Cytokines, like IFNγ, TNFα, Perforin and CD107a, based on their target cells, can result in either antiviral activity or damage to mucosal surfaces. Therefore, ex vivo studies with colon explants showed that IL-10 plays a crucial role in maintaining mucosal integrity and homeostasis, while blocking of endogenous mucosal IL-10 may lead to increases in proinflammatory, Th1 cytokines, and degranulation molecules resulting in both intestinal LP cell and EC apoptosis, which may potentially compromise intestinal epithelial barrier and integrity. Loss of ECs and increased inflammation in inflammatory bowel disease is also mediated by increased effector T-cell responses like IFNγ and TNFα [18]. Th1 cells producing increased IFNγ were thought to be responsible for early enteropathy in an experimental colitis model, where disease progression is also mediated by intact mucosal IL-17 producing cells [19]. Evidence from in vitro studies with murine small and large intestine has shown that IL-10 binds to IL-10 receptors and directly regulates ECs viability, anti-inflammatory activity in the epithelium, and blocks IFNγ-induced MHC class II upregulation [20]. The present data show that mucosal IL-10 also plays a role in maintaining intestinal homeostasis through its anti-inflammatory activities. We also show the pathogenic effects of anti-IL-10 MAbs treatment on ECs was accompanied by production of several other cytokines including IFNγ, TNFα, perforin and CD107a, but we cannot exclude the role of other cytokine(s) that might be playing an important role in regulating EC apoptosis and maintaining intestinal homeostasis.
Figure 2.
Epithelial cell (EC) apoptosis correlates with markers of cytotoxicity. (A) Lamina propria cells and enterocytes apoptosis were detected by multi-label immunofluorescent confocal microscopy, where increased apoptosis of colonic ECs was detected in anti-IL-10 antibody-treated cultures compared to other treatment groups (scale bars 50μm). Apoptotic ECs and lamina propria cells are indicated by yellow and white arrows respectively. (B) Scattered plots (with mean ± standard errors) of apoptotic ECs in colonic explant cultures with different treatments are shown (n=3; A minimum 10 fields were measured for each sample). Statistically significant differences between each group of treatment are shown. (C) A positive correlation of apoptotic ECs with mucosal markers of cytotoxicity is shown (n=3). Spearman’s rank correlation coefficient analysis of cytokine molecules/field and percentages of apoptotic enterocytes from all treatment groups of colon explant culture was performed using GraphPad Prism.
In conclusion, our results demonstrate a potential protective role of mucosal IL-10 in regulating macaque intestinal mucosal integrity and homeostasis. It is important to monitor and further explore the role of mucosal IL-10 in regulating HIV/SIV pathogenesis and HIV/SIV enteropathy, which may lead to the development of improved therapeutic strategies to prevent epithelial cell damage and systemic immune activation during acute and chronic HIV/SIV infection.
Supplementary Material
Supplementary Figure 1. Colon explants (media control) show minimal to negative false positive cells when stained with secondary antibody only and detected by multi-labeled immunofluorescent confocal microscopy (scale bars 75μm).
Supplementary Figure 2. IL-10 blocking results in increased INFγ (A) and TNFα (B) producing cells in colonic explant cultures detected by multi-labeled immunofluorescent confocal microscopy (Scale bars 50μm). Different treatment groups in colon explants are shown in the inset of each picture. Arrow indicates INFγ or TNFα positive cells in LP region of colon explants.
Supplementary Figure 3. IL-10 blocking results in increased Perforin (A) and CD107a (B) producing cells in colonic explant cultures detected by multi-labeled immunofluorescent confocal microscopy (Scale bars 50 and 20μm respectively). Different treatment groups in colon explants are shown in the inset of each picture. Arrow indicates perforin or CD107a positive cells in LP region of colon explants.
IL-10 plays an obligate role in regulating inflammatory responses.
Anti-IL-10 antibody treatment induced increased epithelial cell apoptosis of colon.
IL-10 maintains mucosal homeostasis by regulating cytokines/degranulation molecules.
ACKNOWLEDGEMENTS
We thank Maury Duplantis, Dot Kubler, Carol Coyne, Cecily Conerly Midkiff and all animal care staff of the department of veterinary medicine for their technical assistance. We also thank Dr. Rudolf Bohm for help with this study. The work was supported by NIH grants P20 GM103458-09, R21 AI080395 (BP).
Abbreviations
- AC-3
active caspase-3
- IL-10
interleukin-10
- HIV
human immunodeficiency virus
- Th1
T-helper 1
- JAK1
janus kinase 1
- Tyk2
tyrosine kinase 2
- SCID
severe combined immunodeficiency
- EC
epithelial cells
- RM
rhesus macaque
- MAb
monoclonal antibody
- rIL-10
recombinant IL-10
- SIV
simian immunodeficiency virus
Footnotes
CONFLICT OF INTEREST: The authors have declared that no competing interests exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annual review of physiology. 2009;71:241–60. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- [2].Pan D, Das A, Liu D, Veazey RS, Pahar B. Isolation and characterization of intestinal epithelial cells from normal and SIV-infected rhesus macaques. PloS one. 2012;7:e30247. doi: 10.1371/journal.pone.0030247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. The Journal of experimental medicine. 1989;170:2081–95. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mosser DM, Zhang X. Interleukin-10: new perspectives on an old cytokine. Immunological reviews. 2008;226:205–18. doi: 10.1111/j.1600-065X.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tangsinmankong N, Day NK, Good RA, Haraguchi S. Monocytes are target cells for IL-10 induction by HIV-1 Nef protein. Cytokine. 2000;12:1506–11. doi: 10.1006/cyto.2000.0741. [DOI] [PubMed] [Google Scholar]
- [6].Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annual review of immunology. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- [7].Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infection and immunity. 1998;66:5224–31. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. The Journal of experimental medicine. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jarry A, Bossard C, Bou-Hanna C, Masson D, Espaze E, Denis MG, et al. Mucosal IL-10 and TGF-beta play crucial roles in preventing LPS-driven, IFN-gamma-mediated epithelial damage in human colon explants. The Journal of clinical investigation. 2008;118:1132–42. doi: 10.1172/JCI32140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Anderson CA, Massey DC, Barrett JC, Prescott NJ, Tremelling M, Fisher SA, et al. Investigation of Crohn’s disease risk loci in ulcerative colitis further defines their molecular relationship. Gastroenterology. 2009;136:523–9. e3. doi: 10.1053/j.gastro.2008.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Franke A, Balschun T, Karlsen TH, Sventoraityte J, Nikolaus S, Mayr G, et al. Sequence variants in IL10, ARPC2 and multiple other loci contribute to ulcerative colitis susceptibility. Nature genetics. 2008;40:1319–23. doi: 10.1038/ng.221. [DOI] [PubMed] [Google Scholar]
- [12].Dame MK, Bhagavathula N, Mankey C, DaSilva M, Paruchuri T, Aslam MN, et al. Human colon tissue in organ culture: preservation of normal and neoplastic characteristics. vitro cellular & developmental biology Animal. 2010;46:114–22. doi: 10.1007/s11626-009-9247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sehm J, Lindermayer H, Dummer C, Treutter D, Pfaffl MW. The influence of polyphenol rich apple pomace or red-wine pomace diet on the gut morphology in weaning piglets. Journal of animal physiology and animal nutrition. 2007;91:289–96. doi: 10.1111/j.1439-0396.2006.00650.x. [DOI] [PubMed] [Google Scholar]
- [14].O’Shea JJ, Murray PJ. Cytokine signaling modules in inflammatory responses. Immunity. 2008;28:477–87. doi: 10.1016/j.immuni.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wagner RD, Maroushek NM, Brown JF, Czuprynski CJ. Treatment with anti-interleukin-10 monoclonal antibody enhances early resistance to but impairs complete clearance of Listeria monocytogenes infection in mice. Infection and immunity. 1994;62:2345–53. doi: 10.1128/iai.62.6.2345-2353.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hsieh CS, Heimberger AB, Gold JS, O’Garra A, Murphy KM. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an alpha beta T-cell-receptor transgenic system. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:6065–9. doi: 10.1073/pnas.89.13.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ishida H, Hastings R, Kearney J, Howard M. Continuous anti-interleukin 10 antibody administration depletes mice of Ly-1 B cells but not conventional B cells. The Journal of experimental medicine. 1992;175:1213–20. doi: 10.1084/jem.175.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nature reviews Immunology. 2003;3:521–33. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- [19].Elson CO, Cong Y, Weaver CT, Schoeb TR, McClanahan TK, Fick RB, et al. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 2007;132:2359–70. doi: 10.1053/j.gastro.2007.03.104. [DOI] [PubMed] [Google Scholar]
- [20].Denning TL, Campbell NA, Song F, Garofalo RP, Klimpel GR, Reyes VE, et al. Expression of IL-10 receptors on epithelial cells from the murine small and large intestine. International immunology. 2000;12:133–9. doi: 10.1093/intimm/12.2.133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Colon explants (media control) show minimal to negative false positive cells when stained with secondary antibody only and detected by multi-labeled immunofluorescent confocal microscopy (scale bars 75μm).
Supplementary Figure 2. IL-10 blocking results in increased INFγ (A) and TNFα (B) producing cells in colonic explant cultures detected by multi-labeled immunofluorescent confocal microscopy (Scale bars 50μm). Different treatment groups in colon explants are shown in the inset of each picture. Arrow indicates INFγ or TNFα positive cells in LP region of colon explants.
Supplementary Figure 3. IL-10 blocking results in increased Perforin (A) and CD107a (B) producing cells in colonic explant cultures detected by multi-labeled immunofluorescent confocal microscopy (Scale bars 50 and 20μm respectively). Different treatment groups in colon explants are shown in the inset of each picture. Arrow indicates perforin or CD107a positive cells in LP region of colon explants.