Abstract
BACKGROUND
The recent recognition that isoforms of the cellular NADPH-dependent oxidases, collectively known as the NOX protein family, participate in a wide range of physiologic and pathophysiologic processes in both the animal and plant kingdoms has stimulated interest in the identification, localization, and quantitation of their products in biological settings. Although several tools for reassuring oxidants released extracellularly are available, the specificity and selectivity of the methods for reliable analysis of intracellular oxidants have not matched the enthusiasm for studying NOX proteins.
SCOPE OF REVIEW
Focusing exclusively on superoxide anion and hydrogen peroxide produced by NOX proteins, this review describes the ideal probe for analysis of O2· and H2O2 generated extracellularly and intracellularly by NOX proteins. An overview of the components, organization, and topology of NOX proteins provides a rationale for applying specific probes for use and a context in which to interpret results and thereby construct plausible models linking NOX-derived oxidants to biological responses. The merits and shortcomings of methods currently in use to assess NOX activity are highlighted, and those assays that provide quantitation of superoxide or H2O2 are contrasted with those intended to examine spatial and temporal aspects of NOX activity.
MAJOR CONCLUSIONS
Although interest in measuring the extracellular and intracellular products of the NOX protein family is great, robust analytical probes are limited.
Several reliable methods for measurement of extracellular O2· and H2O2 by NOX proteins are available.
Chemiluminescent probes for both extracellular and intracellular O2· and H2O2 detection have shortcomings that limit their use
Options for quantitation of intracellular O2· and H2O2 are very limited
However, non-redox sensitive probes and genetically encoded reporters promise to provide spatial and temporal detection of O2· and H2O2
GENERAL SIGNIFICANCE
The widespread involvement of NOX proteins in many biological processes requires rigorous approaches to the detection, localization, and quantitation of the oxidants produced.
Keywords: NADPH oxidase, NOX protein family, superoxide anion, hydrogen peroxide
1. Introduction
Oxidants generated by cellular NADPH oxidases (NOX proteins) participate in many biological processes, serving both as critical elements of signaling pathways as well as important effector molecules [1–6]. The recognition that human neutrophils utilize oxidants to kill ingested microbes and to promote biochemical events in sterile inflammation inspired decades of work to elucidate the components, organization, regulation, and much of the biochemistry of the phagocyte NADPH oxidase [reviewed in [3, 7]]. Insights from that body of work have provided the context for subsequent work on non-phagocyte oxidases, although important differences in tissue distribution and activity of the NOX isoforms make extrapolation from the phagocyte system sometimes challenging. Appreciation of the wide distribution of NOX isoforms throughout biology has stimulated great interest in oxidants and inspired studies in a variety of scientific disciplines. On the down side, however, enthusiasm for studying the physiology and pathophysiology of NADPH oxidases has in many cases fostered dependence on assays that are not specific, selective or quantitative have spawned proposed mechanisms that are frequently fanciful and occasionally implausible. Recognition of the challenges to measuring products of the NOX proteins in biological settings and the limitations of available reporter systems [8] may help avoid the pitfalls of magical thinking.
2. The ideal probe
Probes used to measure products of NOX proteins and include both those subject to redox modification (i.e. change fluorescence or chemiluminescence when oxidized) as well as those without a redox-based mechanism for reporting. For comprehensive discussion of individual probes, the reader is referred to any of the many excellent recent reviews of the most frequently used probes that highlight the chemistry underlying their ability to detect oxidants, their shortcomings, and their applications to the measurement of reactive oxygen and reactive nitrogen species generated in biological systems [9–15]. In addition, Winterbourn provides elsewhere in this issue an updated review of the challenges of measuring O2· and H2O2. Although the cellular NADPH oxidases initiate production of a variety of oxidants, the comments that follow focus on only approaches for the detection and quantitation of superoxide anion and hydrogen peroxide generated by NOX proteins. Detection of hypochlorous acid, a major product of the phagocyte NADPH oxidase, will be reviewed in detail by Kettle elsewhere in this issue. The methods discussed in this review will be limited to those that require relatively routine analytical equipment. For that reason, methods to measure directly electron transfer such as patch clamping [16] or oxidant production using electron spin resonance [17] are not included.
To link specific products of NADPH oxidase activity with posttranslational modifications in downstream targets and specific physiologic or pathophysiologic pathways, it is essential to identify precisely the oxidant generated, which in this discussion is limited to superoxide anion and hydrogen peroxide. Ideal probes to target O2· and H2O2 should exhibit several features that are desirable irrespective of the site of oxidant production. However, reporters for intracellular oxidants require additional, specialized attributes (Table). The optimal probe should respond to low concentrations of superoxide anion or H2O2 and be sensitive, in that it is responsive over several orders of magnitude of O2· or H2O2 concentrations that span physiologic and pathophysiologic levels. Reactions between probe and target should be specific for the oxidant of interest and insensitive to pH, other reactive oxygen or nitrogen species, oxidized glutathione, or antioxidant agents. Probes should be cell-permeable, nontoxic to cells, and operate reliably at concentrations low enough to leave the cellular redox balance unaltered. With the chemistry for its reaction with the oxidant of interest defined, a probe should provide precise quantitation with very low background signal. The product of oxidant and probe should be non-reactive, thereby avoiding spurious signals from secondary downstream reactions. For optimal application, use of the probe would require neither specialized equipment nor expertise, and its output would be simple to quantify accurately.
TABLE.
Attributes of an ideal probe for O2· and H2O2
|
Some properties (e.g. 6 and 7) would be relevant only when monitoring O2· and H2O2 in intracellular compartments. The relative importance of specific attributes depends on the goal of the study, whether it is to quantitate precisely the products of NADPH oxidase activity, to identify cellular compartments in which oxidants are generated, or to monitor kinetics of oxidant production in the context of signaling cascades.
No single probe has all of these attributes, but many important goals in the study of NADPH oxidase biology can be achieved with the currently available analytical tools. require reporters that need to possess only some of these properties. For example, dissecting signaling properties of specific NOX proteins may rest on detection of the spatial and temporal aspects of specific oxidant production, with quantitation a much less important parameter. Regardless of the particular experimental setting, however, it is prudent to employ two or more assays, each relying on a different biochemical principle, to detect or measure O2· and H2O2 and to complement measurements with other experimental approaches, such as pharmacologic inhibitors, inhibitory RNA technology, or cells genetically deficient in the specific NOX isoforms or components.
3. Anatomy of a NOX protein
Authors frequently ascribe the detection of increased cytoplasmic probe activity (“ROS production”) to the presence of products of plasma or endosomal membrane NADPH oxidase activity. Correct interpretation of experimental findings and construction of plausible models for underlying mechanisms for NOX proteins requires an appreciation of the topology of electron transfer by NADPH oxidases.. NOX proteins transport electrons from NADPH on the cytoplasmic face of plasma, endosomal, or phagosomal membranes to O2 at the extracellular space or in the lumen of the endosome or phagosome, respectively [Figure 1]. Consequently, for O2· or H2O2 produced by NOX proteins to engage detectors present in the cytoplasm, the oxidant must move from its site of origin, across a membrane, and into the cytoplasm. As a charged species, O2· would require passage through an anion channel to reach the cytoplasm [18, 19]. Uncharged but nonpolar, H2O2 diffuses across membranes in mammalian cells to a very limited extent [20] but could readily enter cytoplasm through isoforms of aquaporin [21]. As discussed elsewhere [[22] and Winterbourn elsewhere in this issue] O2· and H2O2 differ in reactivity. However, the rapidity of the cellular responses attributed to NADPH oxidase-derived oxidants, often in milliseconds, and the presence of antioxidant species in cytoplasm that can readily consume H2O2 require: (1) signaling targets to be close to the oxidant source, or (2) the oxidants to initiate a cascade of posttranslational modifications that both relay signals intracellularly and prompt downstream intracellular oxidant production. Schematics that overlook these intermediate steps and depict oxidants generated by the plasma membrane-associated NADPH oxidase directly driving intracellular signaling represent implausible mechanisms.
Figure 1.
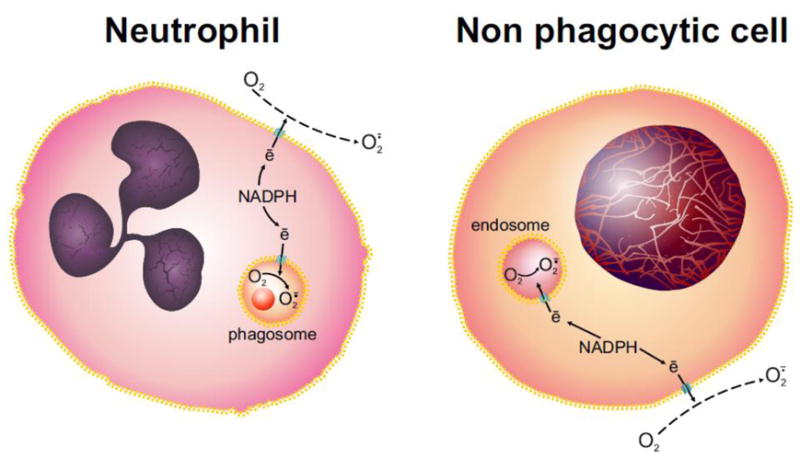
Topology of NOX proteins in phagocytes and nonphagocytes.
Operating as a electron transferases, all NOX protein family members transport electrons from NADPH, generated by the hexose monophosphate shunt, across the plasma or phagosomal membranes in neutrophils and other phagocytes and across the plasma membrane or endosomal membranes in non-phagocytic cells. In neither case do NOX proteins generate O2· directly into the cytoplasm.
3.1. The human neutrophil paradigm
Historically, the patriarch of the NOX protein family, the phagocyte NADPH oxidase, illustrates many structural and functional features that are shared by other NOX proteins. However, important differences exist in composition, subcellular distribution, and activity of non-phagocyte oxidases that likely reflect their cell- or tissue specific functions. Essentially all the O2 consumed and oxidants generated by stimulated neutrophils reflects the activity of the NADPH oxidase, with negligible contributions from mitochondria. Unassembled in resting phagocytes, the enzymatically active multicomponent phagocyte NADPH oxidase includes proteins that reside in plasma or granule membranes as well as those recruited from cytoplasm to dock with membrane components after stimulation [Figure 2] [reviewed in [3]]. The membrane component is flavocytochrome b558, a heterodimeric membrane protein composed of gp91phox and p22phox, and serves as an electron transferase with O2 as the electron acceptor. Structural stability, heme acquisition, and transport from the endoplasmic reticulum to target membranes require heterodimer formation [23–26] Gp91phox (aka NOX2), containing one molecule of flavin adenine dinucleotide (FAD) [27] and two molecules of heme, serves as the catalytic center of the phagocyte oxidase. Two electrons from NADPH are transferred to FAD, followed by two sequential single-electron reductions of two inequivalent heme groups to O2 to form two molecules of O2· [28]]. Electron transfer by the phagocyte oxidase is robust, with more than 1010 electrons translocated within 5 minutes in response to formylated peptides [29] and this magnitude of electron redistribution would depolarize the plasma or phagosomal membrane > 200 mV within milliseconds and thereby terminate oxidase activity if uncompensated. However, the action of a voltage-gated proton channel, Hvn1, compensates for >95% of the negative charge created by the translocation of electrons [29, 30].
Figure 2.
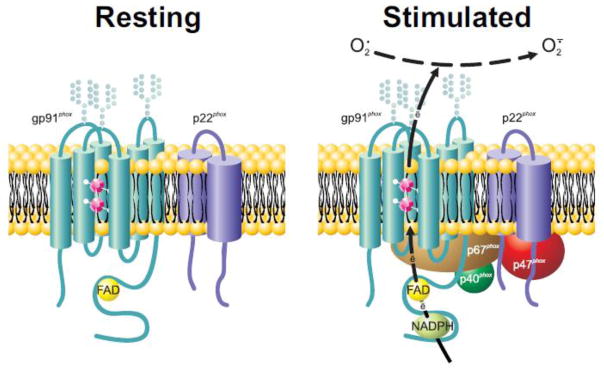
Anatomy of the phagocyte NADPH oxidase.
The membrane component of the phagocyte NADPH oxidase, flavocytochrome b558, is a heterodimeric heme-containing flavoprotein comprised of NOX2 (aka gp91phox) and p22phox located in unstimulated neutrophils in the plasma membrane and membranes of secretory vesicles and specific granulesIn stimulated neutrophils, cytoplasmic components are recruited to the membrane (see text), and NADPH binds to NOX2 and shuttles electrons to FAD and across the membrane via the two inequivalent hemes (magenta dodecahedrons) linked between transmembrane helices in NOX2. Consequently, oxygen undergoes single electron reduction to form O2·.
Transformation of the unassembled and inactive state to an active enzyme assembled on phagosomal or plasma membranes reflects agonist-dependent conformational changes in the cytoplasmic components that result in their translocation. Too extensive to review here, elegant studies have elucidated many features of phagocyte oxidase assembly, including conformational changes in flavocytochrome b558 and surrounding membrane, phosphorylation of multiple sites on p47phox, phosphorylation of p67phox and p22phox, and contributions of the PX domains of p47phox and p40phox [31–36]. Whereas sustained activity requires a source of cytoplasmic NADPH [37], the mechanisms underlying termination of the phagocyte oxidase are not defined [38].
The initial product of the phagocyte oxidase is O2·, which dismutates spontaneously or via reactions involving myeloperoxidase [39], to yield H2O2. As much as 40% of the O2 consumed by human neutrophils can be recovered as HOCl, the product of myeloperoxidase and H2O2 in the presence of chloride anion [reviewed in [40]]. Methods for quantitation of HOCl and its derivatives are presented by Kettle elsewhere in this issue.
3.2 Beyond phagocytes, the NOX protein family
Identification of mox1 (now known as NOX1) as a homologue of human gp91phox by the Lambeth lab [41] heralded the recognition that rather than being a property unique to phagocytic immune cells, the phagocyte NADPH oxidase is a member of a family of NADPH-dependent oxidases, the NOX protein family. NOX protein family members are conserved throughout both plant and animal kingdoms, with many cells possessing more than one isoform [5, 6, 42, 43]. Likewise impressive is the remarkably broad range of biological processes served by NOX proteins, including not only host defense, as best exemplified by NOX2, but also thyroid hormone synthesis, respiratory and gastrointestinal mucosal host defense, regulation of gene expression and cell growth, cell death, angiogenesis, cardiomyocyte differentiation, regulation of blood pressure, maintenance of normal pancreatic beta cell function, and maturation of sperm [reviewed in [6]].
Like the phagocyte oxidase, all NOX proteins are flavoproteins that operate as electron transferases, transporting electrons derived from cytoplasmic NADPH across a membrane to the electron acceptor oxygen [Figure 3]. However, with regard to both structural composition and organization, dependence on cofactors, and function, the non-phagocyte NOX proteins exhibit notable exceptions to the human neutrophil paradigm. The structural organization of NOX proteins has been reviewed in detail recently [2, 3], although a brief overview of some key points relevant to their oxidant production merit mention in the context of this review.
Figure 3.
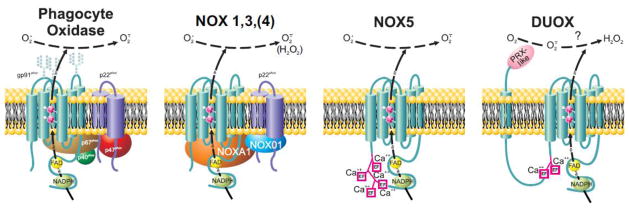
Composition of activated NOX protein family members in membranes.
Individual NOX proteins vary in their requirements for associated p22phox, cytosolic cofactors, and calcium-binding EF hands. In addition, DUOX1 and 2 have an extracellular peroxidase-like (PRX-like) domain and associated transmembrane helix not present in other NOX protein family members. Whereas the phagocyte oxidase (NOX2) and NOX1, 3, and 5 produce O2· extracellularly, only H2O2 is recovered extracellularly from NOX4, DUOX1 or DUOX [see text for details].
With regard to the structure of the NOX protein itself, NOX5, DUOX1, and DUOX2 differ from other family members in possessing EF hands that bind calcium and regulate activation [44–47]. Duox has as an additional unique structural feature an additional transmembrane loop and extended extracellular domain at its amino terminus. The >540 amino acid extracellular sequence shares 19–20% identity at the amino acid level with myeloperoxidase, prompting its frequently being called the “peroxidase-like domain”. Although the C. elegans DUOX protein supports peroxidase-mediated chemistry [48], the human DUOX proteins lack biochemical evidence for peroxidase activity, either as holoprotein or isolated extracellular domains [49, 50]. Pertinent to the discussion regarding the oxidants released extracellularly by DUOX, the extracellular domain of DUOX lacks superoxide-dismutase activity [49].
Only NOX1, NOX3, and NOX4 share with NOX2 a required association with p22phox, which contributes to proper trafficking to the target membrane and provide a potential docking site for cytoplasmic oxidase components [51–54]. NOX proteins differ as well with respect to essential cytoplasmic components. Like NOX2, NOX1 and NOX3 require recruitment of cytosolic proteins that organize the multicomponent oxidase at the plasma membrane and that are part of the catalytic center of the assembled enzyme. NOX organizing protein 1 (NOXO1) and NOX activating protein 1 (NOXA1) are functional and structural homologues of p47phox and p67phox, respectively [55, 56]. NOX4 maybe be negatively regulated by NOXA1 [57], and no cytoplasmic elements are required for the activity of NOX5 and DUOX. Whereas DUOXes do not require cytoplasmic proteins for activity per se, they do depend on maturation factors DUOXA1 and DUOXA2 for proper targeting to membranes during biosynthesis of DUOX1 and DUOX2, respectively [58].
In contrast to the phagocyte oxidase, NOX4, NOX5, and DUOX are constitutively active. With respect to the oxidants generated by NOX proteins, nonphagocyte NOX proteins NOX1, NOX3 and NOX5 produce O2· [reviewed in [6]. However, oxidant production by NOX4 and DUOX present incompletely understood behavior. In the case of NOX4, transfected 293 HEK cells, COS-7 cells, or renal tubular cells produce H2O2 extracellularly [59–63] and no detectable extracellular O2·, except from mesangial cells [64, 65]. Mutations in the first intracellular loop (B loop) eliminate activity, whereas those in the terminal extracellular loop (E loop) reduces H2O2 and increases O2· detected extracellularly [61]. Data from studies of intracellular oxidant production by NOX4 have been interpreted as demonstrating O2· production [61, 63–70], but the assays used lack specificity for measurement of O2· (see later). However, Serrander et al. reported that intracellular reduction of nitroblue tetrazolium (NBT) in 293 HEK cells expressing an inducible NOX4 construct without any evidence for O2· detected in the cytoplasm [62]. Similarly, NOX4-dependent NBT reduction has been detected in airway smooth muscle cells exposed to TGF-β [71]. It is plausible that O2· production in those transfectants occurs in a membrane-bound cytoplasmic compartment that contains NBT but is inaccessible to the other probes used. Subcellular localization of many of the nonphagocyte NOX proteins has been challenging because of the paucity of reliable and validated antibodies, a shortcoming that adds to the ambiguity and uncertainty engendered by limitations of probes to measure intracellular oxidants.
As noted for NOX4, H2O2 is the oxidant detected extracellularly by cells expressing transfected DUOX or by cells expressing endogenous DUOX (e.g. thyroid, airway or colonic epithelium, lung cancer cell lines) [50, 57, 72–75]. Heterodimer formation, proper targeting, and extracellular H2O2 production depend on the integrity of the associated DUOX (i.e. DUOXA1 with DUOX1 and DUOXA2 with DUOX2) [50, 74, 76]. When DUOX is trapped intracellularly, oxidant production is disrupted, with studies suggesting that O2· rather than H2O2 is produced and leaked extracellularly [50]. A comprehensive study employing chimeric constructs of DUOXA1 and DUOXA2 expressed in COS-7 cells to identify structural determinants of oxidant production in the DUOX system [76] suggest that the second intracellular loop and cytoplasmic terminus of DUOXA1 are responsible for H2O2 production by DUOX1, whereas the amino terminus of DUOXA2 dictates oxidant production by DUOX2; wild-type DUOXA2 supports H2O2 production and amino terminal mutants result in O2· by DUOX2. As with the analysis of intracellular O2· by NOX4, the shortcomings inherent in the probes used to detect O2· undermine confidence that structure-function relationships between NOX4 and DUOX and their products are fully understood.
4. Monitoring activities of cellular NADPH oxidases
Both the specific activity of the phagocyte NADPH oxidase and the ease with which large numbers of normal neutrophils can be obtained make detailed analysis and quantitative examination of the phagocyte NADPH oxidase possible. One can quantitate activity of the phagocyte NADPH oxidase by measuring hexose monophosphate shunt activity (source of NADPH and electrons driving the oxidase), consumption of the electron recipient O2, or products released into the extracellular space. However, quantitation of products generated within phagosomes or intracellularly by nonphagocyte NOX proteins is challenging. The merits and shortcomings of currently available probes have been comprehensively presented recently in several excellent reviews [see [9–15]].
4.1 Oxygen consumption
Measuring oxygen consumption is free of competing reactions or confounding influences caused by intermediate reporter substrates or their associated activity. In addition, O2 consumption reflects oxidase activity whether oxidants are generated intra- or extracellularly. Cells suspended in buffer are placed in the sample chamber (37°C) of a Clark electrode, and the decrease in current is monitored continuously after addition of buffer or agonist. Current flow is directly proportional to the concentration of dissolved oxygen equilibrating across the electrode membrane. Since the system is calibrated with each run, the concentration of dissolved oxygen can be determined by the current at any given time, thereby providing a continuous measurement of oxygen consumption.
Measurement of oxygen consumption with a traditional Clark electrode is not without its shortcomings. Inherently, the response of the Clark electrode is sluggish, as the oxygen concentration in the cell suspension needs to equilibrate across the probe membrane with the electrolyte solution, the compartment in which the oxygen content is directly measured. Vigorous mixing of the cell suspension promotes rapid equilibration and optimal measurements,, and the standard Clark electrode requires relatively large numbers of cells in suspension and a robust cellular response, as achieved by neutrophils but perhaps not when cells expressing isoforms of the nonphagocyte oxidase are studied. In principle, NOX protein activity in non-phagocytes should be amenable to study by quantitating oxygen consumption by stimulated cells. However, the level of NADPH oxidase activity in many cells of interest is lower than that in neutrophils, and mitochondria contribute to the overall cellular oxygen consumption to a much greater extent in non-phagocytes than they do in neutrophils. However, microplate-based respirometry can be performed with adherent cells, providing measurements that mirror those obtained with a Clark electrode [77, 78]. Although not yet applied specifically to the study of NOX proteins, this technology has been used to measure mitochondrial oxygen consumption by stimulated murine embryonal fibroblasts [79] and murine macrophages [80], and should be easily adapted to the study of NADPH oxidase activity in adherent cells, both phagocytic and non-phagocytic.
4.2 Extracellular O2· and H2O2
Extracellular O2· and H2O2 can be quantified precisely in several ways. For measurement of extracellular O2·, we favor using superoxide dismutase-inhibitable reduction of ferricytochrome C, as initially applied to human neutrophils by the Babior laboratory [81] and used as well to quantitate extracellular superoxide production by nonphagocyte NOX proteins (e.g. [50, 53, 59–62, 64, 65, 82]). The O2· released extracellularly reduces ferricytochrome C to ferrocytochrome C with 1:1 stoichiometry, the amount of reduced ferricytochrome C can be measured spectrophotometrically at 550 nm. For every experimental condition, an identical sample that also contains superoxide dismutase (SOD) must be assayed in parallel. Using the millimolar extinction coefficient at 550 nm for the difference between reduced and oxidized ferricytochrome C [21.1 mM−1 cm−1 [83]], nmoles of O2· produced can be calculated from the difference in absorbances in the absence and presence of SOD. The very sharp peak of reduced ferricytochrome C at 550 nm requires special attention. Unless using a spectrophotometer with a monochromator that is precisely tuned to the correct wavelength or an instrument with a very narrow bandpass filter will underestimate the amount of O2· detected. It is essential to perform the SOD-containing samples in parallel for all conditions, as other biological substances may reduce ferricytochrome C, including ascorbate, glutathione, and several reductases, a confounding issue particularly problematic with broken cell systems. Although reoxidation of ferrocytochrome C back to ferricytochrome C by H2O2 is possible anddoes not occur to a significant extent in most settings, the addition of catalase will eliminate the activity if problematic. The SOD-inhibitable reduction of ferricytochrome C can be used in either continuous [84] or discontinuous [81] assays, and can be adapted to a microtiter plate format [85]. Use of the latter requires adjustment for the light path in the calculation of the amount of O2·, in accordance with Beer’s law; whereas the light path is 1 cm in the standard spectrophotometric cuvette format, it is 0.6 cm for the microtiter plate assay. Others probes can be used to detect O2· using the operational definition of SOD-inhibitable response. Chemiluminsence-based probes are discussed later, but extracellular O2· production can be measured spectrophotometrically as SOD-inhibitable reduction of WST-1, a cell impermeable sulfonated tetrazolium salt [86]. Its greater reactivity in comparison with ferricytochrome C may reflect signal amplification secondary to the contribution of intermediate radicals rather than greater sensitivity to O2· Hydropropidine, a membrane impermeant fluorogenic probe derived from propidium iodide, has recently been employed to detect extracellular O2· by stimulated murine macrophages [87]. Use of SOD-inhibitable fluorescence of hydropropidine compares favorably with O2· detection using ferricytochrome C and merits further study.
Extracellular H2O2 can be quantitated in two general ways. Using a hydrogen peroxide electrode, H2O2 in solution can be measured polarigraphically, using the same principles as were discussed earlier with regard to measuring oxygen consumption. The sensitivity of the electrode allows precise and rapid measurement of extracellular H2O2 with very little consumption of the H2O2 and without the use of targets to react with or capture the H2O2 [88]. Cells are suspended in a chamber maintained at 37°C with continuous stirring and the change in current in solution, reflecting changes in H2O2 concentration, are monitored continuously. Alternatively, one can exploit the ability of H2O2 to oxidize susceptible probes, including scopoletin [89], homovanillic acid (HVA) [90], phenol red [91, 92] or Amplex red (N-acetyl-3,7-dihydroxyphenoxazine) [93], in the presence of horseradish peroxidase (HRP). Whereas the fluorescence of scopoletin decreases after oxidation, oxidized products of HVA, phenol red, and Amplex all increase, thereby providing a more sensitive assay. Developed initially to measure NOX2 activity, all four probes have been used to measure extracellular H2O2 production by NOX4 [59, 60, 62] and DUOX [50, 57, 72–74, 76]. Note that accurate detection of H2O2 by these assays requires addition of SOD, because O2· reacts with the probe, resulting in underestimation of H2O2 produced [94].
The oxidation of Amplex red is irreversible, has a stoichiometry of 1:1 [93], and is sensitive to low levels of H2O2, with a 5 pmol lower limit of detection and 30 pmol lower limit of quantitation [13, 95]. In a 100 μl reaction volume in a microtiter plate format, 5 pmols of H2O2 from 2 × 103 phorbol myristate acetate-stimulated neutrophils can be reliably quantitated [93]. Using absorbance readings from experimental samples and a standard curve of defined concentrations of H2O2 in the presence of Amplex red and HRP, the amount of H2O2 generated extracellularly can be calculated.
In many ways, H2O2 detection using HRP and Amplex red mirrors O2· measurements using SOD-inhibitable reduction of ferricytochrome C as a method to quantitate extracellular products of the NADPH oxidase. However, one important difference in use of the two assays needs to be kept in mind if using a broken cell system to study any of the NOX family members. The broken cell system is an experimental approach that combines enriched, purified, or recombinant subcellular oxidase components in the presence of NADPH and an amphiphile to recapitulate the activity of the NADPH oxidase of phagocytes [[96–100] and reviewed [101]]. Its development and application to the study of phagocytes made possible the identification of cytosolic oxidase components [102–105] and the dissection of many other features of the phagocyte NADPH oxidase. Whereas the ferricytochrome C assay can be used to measure O2· production by the broken cell system, NADPH and other reduced pyridine nucleotides can directly oxidize Amplex Red in an HRP-dependent fashion [106]. Although the reaction of NADPH and Amplex Red is slow and limited [95], caution should be exercised when interpreting data from studies utilizing this probe in broken cell NADPH oxidase systems. Use of lower concentrations of NADP+ and inclusion of an NADPH-generating system can minimize this problem (Reviewer, personal communication).
4.3 Intracellular O2· and H2O2
Intracellular oxidants produced by NOX proteins have been associated with a wide range of biological process, both in phagocytes and nonphagocytic cells, although the specific compartments in which oxidant generation occurs differs in these two cell types. Nearly all the oxidant generation is within phagosomes when neutrophils ingest opsonized particles at low particle-to-neutrophil ratios (1:1 to 5:1) in the absence of agents that poison the cytoskeleton (e.g. cytochalasins). Commercially available redox-sensitive probes that are coupled to carrier substrates are frequently used to monitor intraphagosomal oxidant production following phagocytosis. For example, Oxyburst Green (Molecular Probes) represents a complex of dihydrofluorescein (DCFH2), bovine serum albumin (BSA), and rabbit antibody to BSA that engages the phagocyte Fc receptor, thereby accessing the phagosome when particles are ingested. Although such oxidation-sensitive conjugates can detect events in neutrophil phagosomes that are dependent on the NADPH oxidase and myeloperoxidase (MPO), the biochemistry in the phagosomes of human neutrophils is remarkably complex and the identity of the reactive species generated therein incomplete [reviewed [40, 107, 108]. Furthermore, there are significant shortcomings to using DCFH2 to detect products of NADPH oxidases (see below). No probes that are currently available allow precise or specific quantitation of O2· or H2O2 generated within phagosomes.
Oxidants generated by nonphagocyte oxidases in endosomes have been implicated in a variety of signaling cascades. Consequently, there is a need to access intracellular compartments in order to quantitate oxidants generated therein. Substrates used to detect oxidants within intracellular compartments include both oxidant-sensitive and non-redox probes.
4.3.1 Oxidant-sensitive probes
4.3.1.1 Chemiluminescence
Stimulated neutrophils emit light in a fashion that requires an intact NADPH oxidase and enzymatically active MPO for maximum chemiluminescence [109–111]. Many different luminescent substrates have been used to amplify the light emitted from stimulated neutrophils, including lucigenin (bis-N-methyl acridinium nitrate), luminol (5-amino-2,3-dihydro-1,4-phthalazinedione), and isoluminol (6-amino-2,3-dihydro-1,4-phthalazinedione) in order to improve detection. For example, use of luminol allows detection of responses from 2 × 104 stimulated neutrophils [112], thus providing a valuable tool in situations when neutrophils are scarce, as when studying cells from children or patients with neutropenia. The cell-permeable agents luminol and lucigenin are commonly used to detect oxidant production both intracellularly and extracellularly, whereas isoluminol, which does not penetrate into cells, can serve to probe the extracellular space [reviewed in [113]]. The addition of HRP makes isoluminol-enhanced chemiluminescence by stimulated neutrophils solely dependent on the activity of the NADPH oxidase and able to detect responses of as few as 250 cells [114]. Because neutrophils require a functional NADPH oxidase and active MPO to emit optimal luminol-enhanced chemiluminescence [111, 115], abnormalities in either the oxidase (e.g. chronic granulomatous disease) or MPO (e.g. MPO deficiency) can result in abnormal neutrophil responses when luminol is used. Lucigenin-enhanced chemiluminescence is not dependent on MPO [110]. Lucigenin, luminol, and related or enhanced substrates, including Diogenes [National Diagnostics} and L-012 [116] have been employed in a variety of settings to measure O2· derived from non-phagocyte NOX proteins, both extracellularly as SOD-inhibitable responses and intracellularly [50, 59, 63, 69, 70, 76]. Despite their capacity to detect and amplify signals associated with activation of the NADPH oxidase, the use of chemiluminescent probes to monitor or measure O2· or H2O2 has serious limitations that undermine use as analytical tools. The chemistry underlying the emission of luminescence in the presence of oxidants is complicated and incompletely unraveled. Because of uncertainties about the identify of reactive species detected and concerns about the inherent behavior of substrates used to amplify the chemiluminescence (see Winterbourn elsewhere in this issue), many with expertise in free radical biology are skeptical about the use of these probes [reviewed in [10, 11, 14, 15, 110, 117–119]]. None of these probes is specific, selective, or quantifiable and all have significant shortcomings. For example, lucigenin interacts with a variety of nucleophiles and reducing agents that can generate the single electron product of lucigenin reduction (LC·+), which in turn generates O2· [10, 117, 118] and produces luminescence. After detailed examination of its chemistry [review [120]], luminol fares no better than lucigenin as a probe for O2· and hydrogen peroxide production by NADPH oxidases. In biological systems, the multiplicity of interfering substrates in intracellular compartments is too great to allow luminol to serve as a specific probe for O2· or H2O2 production by stimulated neutrophils or in broken cell superoxide generating systems derived from neutrophils [121, 122]. However, luminol-enhanced chemiluminescence may yield useful information. Detection of products of the NADPH oxidase released extracellularly can be monitored as isoluminol-enhanced chemiluminescence, which requires O2· production [114]. However, neither the specific reactive species responsible nor the stoichiometry of the reactions with isoluminol is known, leaving the assay of limited utility. Luciferin (coelenterazine) derivatives, including the methoxy derivative MCLA, show promise as a substrate for O2· detection [123, 124]. In contrast to lucigenin, MCLA does not undergo redox cycling [125]. However, it is cell impermeable, thus limiting application of SOD-inhibitable MCLA chemiluminescence to the detection of extracellular O2·. 3.1.2 Redox responsive fluorescent probes
In addition to the luminescent probes, substrates that become fluorescent when oxidized, just as discussed earlier with using Amplex red, are widely used to monitor oxidant production [reviewed [9–12, 14, 15, 126–130]]. Most commonly used among these redox-sensitive fluorescent probes are dihydrofluorescein (DCFH2), dihydrorhodamine (DH2R), and hydroethidine (HE). Because cells are loaded with a cell-permeable form of the probe, the efficiency of uptake and the rate of leakage of the reporter become additional variables to consider when using these probes to compare cellular responses under different experimental conditions.
DCFH2 reacts very slowly with O2· and H2O2 [10] and inefficiently with HOCl [131] but can be oxidized by H2O2 in the presence of catalysts, including metals (e.g. iron and copper), peroxidases, and species with peroxidase-like activity (e.g. catalase, hemoglobin, myoglobin, and superoxide dismutase) [10]. Thus, the susceptibility of DCFH2 to oxidation by constituents in cytoplasm can obfuscate interpretation of data from intracellular measurements. For example, DCF fluorescence during neural cell death was initially interpreted as evidence linking reactive oxidant production with apoptosis [132]. However, cytochrome c released from mitochondria during apoptosis [133, 134] can directly oxidize DCFH2 [135], providing a mechanism for increasing DCF fluorescence independent of oxidant production. In similar ways, competing reactions between DCFH2 and cytoplasmic contents have proved troublesome when using this probe to monitor oxidant production in cardiovascular tissue [reviewed [13, 14].
DHR shares many of the properties of DCFH2, most notably the lack of reactivity with O2· and H2O2 in the absence of a catalyst [127, 131]. Both HOCl and chloramines can oxidize DHR, and the latter reaction is particularly efficient in the presence of iodide [136]. Consequently, DHR has a special application in studies of neutrophil function. As neutrophils from patients with CGD fail to promote DHR oxidation [137], flow cytometry of DHR oxidation by stimulated human neutrophils can be used to screen subjects for the presence of CGD [138]. The assay can be performed on as little as 400 μl of anticoagulated whole blood, making it very useful for evaluating the small volumes of blood often available from very young children. The assay can identify both affected patients and carriers of X-linked disease, as the latter have two populations of circulating cells, normal and defective. It is critical to recognize that DHR oxidation in this assay depends on the activity of endogenous MPO [139]. Consequently, neutrophils from patients with complete MPO deficiency will have abnormal DHR oxidation that resembles that of patients with CGD [140]. Neutrophils from subjects with partial MPO deficiency have sufficient peroxidase activity to support oxidation of DHR and appear as normal neutrophils in flow cytometry.
Interest in delineating the contribution of NOX proteins to signaling provides a strong incentive for identifying and quantitating intracellular superoxide and H2O2 in nonphagocytic cells. The redox probe hydroethidine (HE) has several properties distinct from those of DCFH2 and DHR that make it suitable for use as a quantitative analytical tool. In contrast to lucigenin, luminol, and DCH2F, there are no known reactions between the product of HE and O2· with oxygen, thereby eliminating a mechanism for spontaneous O2· generation [94]. O2· reacts more readily with HE than with DCFH2 and DHR, with rate of ~ 2 × 106 M−1s−1 [129], and generates a specific product, 2-hydroxythidium (2-OH-E+), that can be quantitated [128, 141, 142]. Like other radical probes, HE can produce background fluorescence secondary to amine oxidation by air and light. Deuteration at the α-amine carbon-hydrogen bond appears to alleviate this problem [143], although the modified radical oxidant probes are not currently commercially available.
HE can also react with H2O2 in the presence of peroxidases [144] or nonenzymatically with non-peroxidase heme proteins [e.g. cytochromes, hemoglobin, myoglobin [145]], thereby competing for O2· and complicating quantitation of O2· by HE fluorescence alone. Although such reactions undermine the application of confocal fluorescence microscopy to monitor specifically O2· product as changes in HE fluorescence [130, 146], methods have been developed to recover and quantitate 2-OH-E+ in lysates or stimulated cells. Because ethidium (E+), the two-electron oxidation product of HE unrelated to O2· generation [141], and 2-OH-E+ have overlapping spectra, HPLC analysis and quantitation are requisites for the selective determination of O2·-generated products [128, 147]. It is important to recognize that HE will be competing with intracellular proteins, such as SOD, that can interact readily with O2·, likely resulting in 2-OH-E+ quantitation underestimating the amount of O2· produced in situ [147]. For this and other theoretical concerns [147], it is best to consider 2-OH-E+ measurement as a semiquantitative determination of intracellular O2· production. However, results using this assay parallel those obtained when O2· is detected as SOD-inhibitable reduction of ferricytochrome C, as clearly demonstrated in studies of endothelial cells in intact vascular tissue [142]. Furthermore, 2-OH-E+ is stable, allowing samples to be stored frozen and analyzed at a later time [13]. Thus, the detection and quantitation of 2-OH-E+ by HPLC provide a specific and selective means to compare relative O2· levels in cells under varied experimental conditions.
4.3.2 Non-redox probes
With the exception of electrodes to monitor oxygen consumption and hydrogen peroxide production, the probes discussed thus far depend on their redox properties for use as detectors of O2· and H2O2. Whereas modification of redox-sensitive probes by oxidants depends on catalysts such as HRP, generation of radicals as intermediates, and oxidation of the reporter molecule, non-redox probes exploit very different properties.
4.3.2.1 Boronate-based probes
In addition to these redox-sensitive probes, small molecules containing fluorophores and a boronate ester have been used to detect H2O2 in biological systems, exploiting the reactivity of H2O2 with arylboronates to generate phenols [148]. The strategy underlying the design of these probes rests on positioning the boronic ester coupled to a fluorophores in such a conformation that the structure is a colorless, non-fluorescent lactone until it reacts with H2O2, which transforms the arylboronate to a phenol and generating a fluorescent product [149]. Modifications in the specific fluorescent probes included in the boronate structure provide a variety of reporting molecules from which to choose for specific applications [150]. A variety of boronate-based, water-soluble fluorochromes have been generated and applied to detect H2O2 at micromolar concentrations [151] as well as NO and H2S [152], both in standard experimental settings as well as high throughput assays [reviewed in [150, 151, 153–155]. However, some of the probes react slowly with H2O2 [k ~ 1 to 2 M−1s−1 [156] compared to their reactivity with ONOO− or HOCl, oxidant species relevant in the context of biological systems with NADPH oxidases [120, 151, 153, 157]. For example, reactivity of some boronates with HOCl is 1000-fold faster than that with H2O2, with both oxidants producing the same fluorescent product [122]. Furthermore, the probes do not provide quantitative data, although new approaches measuring spectral ratios by mass spectrometry are promising, as recently applied to measuring H2O2 from mitochondria in living Drosophila [120].
The Chang lab has extended use of the boronate derivatives to include caged luciferin, Peroxy Caged Luciferin-1 (PCL-1) to detect and image H2O2 in the context of acute inflammation in response to endotoxin challenge [158]. As presented earlier, H2O2 oxidizes an aryl boronic acid to phenol, but the result with PCL-1 is the release of part of luciferin. D-cysteine, the other half of luciferin, is release in response to a caspase 8 activity probe, thereby providing a sensitive method to monitor endotoxin-elicited oxidant production Applications to study cell ex vivo as well as intact animals hold special promise for such tools.
4.3.2.2 Genetically based reporters
Challenges facing the utility of the boronate-based probes, namely selectivity in distinguishing between reactive oxygen and reactive nitrogen species, and reversibility are addressed in part by genetically encoded probes [review [15, 126]]. Probes such as roGFP use site-directed mutants of GFP that undergo a conformational change when oxidants promote disulfide bond formation in the chromophore [159]. However, roGFP is not selective for H2O2 and, like many of these probes, more accurately reflects the redox state of the cellular compartment in which it resides than it does the presence or amount of a specific oxidant. A variation on fluorescent redox-sensitive probes such as roGFP is HyPer, a fluorescent probe that is sensitive, reversible, and selective for H2O2 [160]. HyPer exploits the properties of oxyR, a peroxide sensor that responds to local changes in H2O2 concentration to initiate transcription of antioxidant genes in Escherichia coli [161]. A circularly permutated form of the fluorescent protein YFP (cpYFP) was introduced into the regulatory domain of oxyR, such that oxidation of critical cysteines in cpYFP results in conformational changes that alter its two excitation maxima in opposing directions; the excitation at 420 nm decreases while that at 500 nm increases, thus enabling HyPer to function as a ratiometric probe [160]. Although sensitive to pH, HyPer oxidation-dependent changes in fluorescence are reversible and are selective for H2O2, as the probe is unaffected by O2·, NO, ONOO, or oxidized glutathione [160]. As an example of an application of this methodology to the study the generation of intracellular oxidants by non-phagocyte NOX proteins, investigators used HyPer-ER, a modified reporter that targets endoplasmic reticulum (ER), to examine H2O2 production mediated by NOX4 in the ER of human umbilical vein endothelial cells [162].
Concerned that the sensitivity of HyPer to pH limits reliable detection of oxidants in intracellular compartments in which pH would change during cell activation, the Geiszt group developed two novel, genetically-encoded probes that utilize fluorescence resonance energy transfer (FRET) to report the presence and changes in intracellular H2O2 [163]. The sensor utilizes two redox-sensitive proteins from Sacchromyces cervesiae, oxidant receptor 1 (Orp1) and yeast activator protein 1 (Yap1), that together constitute a two-component H2O2-sensing system; the peroxidase Orp1 catalyzes oxidation of the transcription factor Yap1 in the presence of H2O2, thereby providing the yeast with the capacity to respond transcriptionally to oxidant stress [164]. The Geiszt lab engineered two fusion proteins, using variants of Cerulean and Venus fluorescent proteins and domains of Orp1 and Yap1, to create constructs that exhibit inverse FRET when oxidized. Concurrent determination of ratiometric FRET measurements and use of confocal fluorescence microscopy permit both qualitative detection of H2O2 production as well as identification of the cellular compartment from which it originates. The authors applied the method to detect H2O2 intracellularly after NOX2 and DUOX1 activation as well as H2O2 that diffused into cells from an extracellular source, as would be relevant to paracrine signaling by oxidants [165]. This approach shows promise for spatial and temporal localization of H2O2 within cells, although it is not a quantitative assay.
4.3.2.3 Other probes
Probes based on deprotection of fluorescein derivatives have been developed to measure O2· [166]. The bis(2,4-dinitrobenzenesulfonyl) fluoresceins increase fluorescence in the presence of O2· generated by xanthine-xanthine oxidase or by stimulated neutrophils. However, unstimulated neutrophils as well as glutathione alone reduce the probe, both undesirable features. Additional modification of this family of protected fluorescein derivatives will be needed before they can be applied to study NOX protein activity.
Nanotechnology has been applied to measuring intracellular reactive oxygen species in biological systems, using fluorescein conjugates immobilized on gold nanoprobes. For example, hyaluronic acid that has been modified to resist reduction by glutathione can be immobilized to gold nanoprobes and introduced into cells to monitor intracellular production of reactive oxygen species [167, 168], exploiting oxidant-dependent degradation of hyaluronic acid [169]. As sensitive as DCF, the gold nanoprobes were used to measure intracellular oxidant generation by macrophages challenged with nanogram amounts of endotoxin [167]. However, details of the specific oxidants detected (O2·, OH, H2O2, ONOO−, or −OCl) in biological settings await further definition.
5. Summary
Cellular NADPH oxidases participate in a wide range of important biological processes. Furthermore, they are distributed in nearly all cell types and tissues, and many cells express more than one NOX protein isoform. Together, their wide distribution and scientific importance have stimulated interest in the detection and quantitation of products of stimulated NOX proteins in biological systems. However, the quality of the available analytical tools to measure NOX protein products does not match the enthusiasm for their study. Approaches to quantitate oxygen consumption, extracellular release of O2· (using SOD-inhibitable reduction of ferricytochrome C) or H2O2 (using HRP plus Amplex Red), and intracellular O2· production (using HPLC to quantitate 2-OH-E+) provide reliable assessment of NADPH oxidase activity in a given population of cells. Spatial and temporal localization in individual cells or tissue depends on probes that often lack specificity, selectivity, or the potential for precise quantitation. However, their use in conjunction with other probes and their interpretation with the recognition of their shortcomings can provide valuable qualitative insights.
It is certain that probes better able to provide quantitation as well as localization of intracellular O2· and H2O2 will be available in the future. Until that time, if limited to the use of fluorescent or luminescent reporters, it is best to employ more than one method to assess NOX protein activity, where the underlying principles of the assay differ and to complement measurements with the use of well-characterized pharmacologic inhibitors or genetic approaches that target the specific NOX protein of interest. Until we have probes that precisely meet our needs, we must rely on sound understanding of the principles and topology of NOX proteins, rigorous experimental design, and careful interpretation of results to unravel the contributions of cellular NADPH oxidase to biology.
Highlights.
NADPH oxidases participate in diverse biological processes throughout nature
Available methods to detect superoxide anion and H2O2 have shortcomings
Attention to the topology of NADPH oxidases is essential to unravel their function
Acknowledgments
Work in the Nauseef laboratory is supported by funding from National Institute of Health grants AI70958 and AI044642, and a Merit Review grant from the Veterans Administration.
Abbreviations used
- CGD
chronic granulomatous disease
- cpYFP
circularly permutated form of the yellow fluorescent protein
- DCFH2
dihydrofluorescein
- DH2R
dihydrorhodamine
- DUOX
dual oxidase (1 or 2)
- DUOXA
dual oxidase maturation factor (1 or 2)
- fMLF
formyl methionylleucylphenylalanine
- FRET
fluorescence resonance energy transfer
- GFP
green fluorescent protein
- HE
hydroethidine
- HOCl
hypochlorous acid
- HRP
horseradish peroxidase
- HVA
homovanillic acid
- MPO
myeloperoxidase
- NBT
nitroblue tetrazolium
- NOX
NADPH oxidase protein
- 2-OH-E+
2-hydroxythidium
- PMA
phorbol myristate acetate
- SOD
superoxide dismutase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Takac I, Schröder K, Brandes RP. The NOX family of NADPH oxidases: friend or foe of the vascular system? Curr Hypertens Rep. 2011 doi: 10.1007/s11906-011-0238-3. ahead of print. [DOI] [PubMed] [Google Scholar]
- 2.Sumimoto H. Structure, regulation, and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS Journal. 2008;275:3249–3277. doi: 10.1111/j.1742-4658.2008.06488.x. [DOI] [PubMed] [Google Scholar]
- 3.Nauseef WM. Biological roles for the NOX family NADPH oxidases. J Biol Chem. 2008;283:16961–16965. doi: 10.1074/jbc.R700045200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nauseef WM. Nox enzymes in immune cells. Semin Immunopathol. 2008;30:195–208. doi: 10.1007/s00281-008-0117-4. [DOI] [PubMed] [Google Scholar]
- 5.Bedard K, Lardy B, Krause KH. NOX family NADPH oxidases: not just in mammals. Biochemie. 2007;89:1107–1112. doi: 10.1016/j.biochi.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 7.Holland SM. Chronic granulomatous disease. Hematol Oncol Clin North Am. 2013;27:89–99. doi: 10.1016/j.hoc.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy MP, Holmgren A, Larsson NG, Halliwell B, Chang CJ, Kalyanaraman B, Rhee SG, Thornalley PJ, Partridge L, Gerns D, Nyström T, Belousov V, Schumaker PT, Winterbourn CC. Unraveling the biological roles of reactive oxygen species. Cell Metabolism. 2011;13:361–366. doi: 10.1016/j.cmet.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalyanaraman B, Darley-Usmar V, Davies KJ, Dennery PA, Forman HJ, Grisham MB, Mann GE, Moore K, Roberts LJ, 2nd, Ischiropoulos H. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic Biol Med. 2012;52:1–6. doi: 10.1016/j.freeradbiomed.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wardman P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: progress, pitfalls, and prospects. Free Radic Biol Med. 2007;43:995–1022. doi: 10.1016/j.freeradbiomed.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 11.Maghzal GJ, Krause KH, Stocker R, Jaquet V. Detection of reactive oxygen species derived from the family of NOX NADPH oxidases. Free Radic Biol Med. 2012;53:1903–1918. doi: 10.1016/j.freeradbiomed.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Dikalov SI, Harrison DG. Methods for Detection of Mitochondrial and Cellular Reactive Oxygen Species. Antioxid Redox Signal. 2012 doi: 10.1089/ars.2012.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dikalov S, Griendling KK, Harrison DG. Measurement of reactive oxygen species in cardiovascular studies. Hypertension. 2007;49:717–727. doi: 10.1161/01.HYP.0000258594.87211.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarpey MM, Fridovich I. Methods of detection of vascular reactive species: nitric oxide, superoxide, hydrogen peroxide, and peroxynitrite. Circ Res. 2001;89:224–236. doi: 10.1161/hh1501.094365. [DOI] [PubMed] [Google Scholar]
- 15.Rhee SG, Chang TS, Jeong W, Kang D. Methods for detection and measurement of hydrogen peroxide inside and outside of cells. Mol Cells. 2010;29:539–549. doi: 10.1007/s10059-010-0082-3. [DOI] [PubMed] [Google Scholar]
- 16.Morgan D, DeCoursey TE. Analysis of electrophysiological properties and responses of neutrophils. Methods Mol Biol. 2007;412:139–175. doi: 10.1007/978-1-59745-467-4_11. [DOI] [PubMed] [Google Scholar]
- 17.Kohno M. Applications of electron spin resonance spectrometry for reactive oxygen species and reactive nitrogen species research. J Clin Biochem Nutr. 2010;47:1–11. doi: 10.3164/jcbn.10-13R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lynch RE, Fridovich I. Permeation of the erythrocyte stroma by superoxide radical. J Biol Chem. 1978;253:4697–4699. [PubMed] [Google Scholar]
- 19.Roos D, Eckmann CM, Yazdanbakhsh M, Hamers MN, de Boer M. Excretion of superoxide by phagocytes measured with cytochrome c entrapped in resealed erythrocyte ghosts. J Biol Chem. 1984;259:1770–1775. [PubMed] [Google Scholar]
- 20.Bienert GP, Schjoerring JK, Jahn TP. Membrane transport of hydrogen peroxide. Biochem Biophys Acta. 2006;1758:994–1003. doi: 10.1016/j.bbamem.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 21.Miller EW, Dickinson BC, Chang CJ. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc Natl Acad Sci USA. 2010;107:15681–15686. doi: 10.1073/pnas.1005776107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winterbourn CC. Reconciling the chemistry and biology of reactive oxygen species. Nature Chem Biology. 2008;4:278–286. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- 23.Biberstine-Kinkade KJ, DeLeo FR, Epstein RI, LeRoy BA, Nauseef WM, Dinauer MC. Heme-ligating histidines in flavocytochrome b 558. J Biol Chem. 2001;276:31105–31112. doi: 10.1074/jbc.M103327200. [DOI] [PubMed] [Google Scholar]
- 24.DeLeo FR, Burritt JB, Yu L, Jesaitis AJ, Dinauer MC, Nauseef WM. Processing and maturation of flavocytochrome b558 includes incorporation of heme as a prerequisite for heterodimer assembly. J Biol Chem. 2000;275:13986–13993. doi: 10.1074/jbc.275.18.13986. [DOI] [PubMed] [Google Scholar]
- 25.Yu L, DeLeo FR, Biberstine-Kinkade KJ, Renee J, Nauseef WM, Dinauer MC. Biosynthesis of flavocytochrome b558. J Biol Chem. 1999;274:4364–4369. doi: 10.1074/jbc.274.7.4364. [DOI] [PubMed] [Google Scholar]
- 26.Yu L, Quinn MT, Cross AR, Dinauer MC. Gp91 phox is the heme binding subunit of the superoxide-generating NADPH oxidase. Proc Natl Acd Sci USA. 1998;95:7993–7998. doi: 10.1073/pnas.95.14.7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cross AR, Jones OTG, Garcia R, Segal AW. The association of FAD with the cytochrome b-245 of human neutrophils. Biochem J. 1982;208:759–763. doi: 10.1042/bj2080759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cross AR, Segal AW. The NADPH oxidase of professional phagocytes-prototype of the NOX electron transport chain systems. Biochim Biophys Acta. 2004;1657:1–22. doi: 10.1016/j.bbabio.2004.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeCoursey TE. Voltage-gated proton channels find their dream job managing the respiratory burst in phagocytes. Physiology. 2010;25:27–40. doi: 10.1152/physiol.00039.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demaurex N, El Chemaly A. Physiological roles of voltage-gated proton channels in leukocytes. J Physiol. 2010;588:4659–4665. doi: 10.1113/jphysiol.2010.194225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor RM, Riesselman MH, Lord CI, Gripentrog JM, Jesaitis AJ. Anionic lipid-induced conformational changes in human phagocyte flavocytochrome b precede assembly and activation of the NADPH oxidase complex. Arch Biochem Biophys. 2012;52:24–31. doi: 10.1016/j.abb.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 32.Minakami R, Maehara Y, Kamakura S, Kumano O, Miyano K, Sumimoto H. Membrane phospholipid metabolism during phagocytosis in human neutrophils. Genes Cells. 2010;15:409–424. doi: 10.1111/j.1365-2443.2010.01393.x. [DOI] [PubMed] [Google Scholar]
- 33.Ellson CD, Davidson K, Ferguson GJ, O’Connor R, Stephens LR, Hawkins PT. Neutrophils from p40 phox-1- mice exhibit severe defects in NADPH oxidase regulation and oxidant-dependent bacterial killing. J Exp Med. 2006;203:1927–1937. doi: 10.1084/jem.20052069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ellson C, Davidson K, Anderson K, Stephens LR, Hawkins PT. Ptdlns3P binding to the PX domain of p40 phox is a physiological signal in NADPH oxidase activation. The EMBO Journal. 2006;25:4468–4478. doi: 10.1038/sj.emboj.7601346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanai F, Liu H, Field S, Akbary H, Matsuo T, Brown G, Cantley L, Yaffe M. The PX domains of p47phox and p40phox bind to lipid products of PI(3)K. Nat Cell Biol. 2001;3:675–678. doi: 10.1038/35083070. [DOI] [PubMed] [Google Scholar]
- 36.El Benna J, Dang PM, Gougerot-Pocidalo MA, Marie JC, Braut-Boucher F. p47phox, the phagocyte NADPH oxidase/NOX2 organizer: structure, phosphorylation and implication in diseases. Exp Mol Med. 2009;41:217–225. doi: 10.3858/emm.2009.41.4.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen HJ, Chovaniec ME. Superoxide production by digitonin-stimulated guinea pig granulocytes. J Clin Invest. 1978;61:1088–1096. doi: 10.1172/JCI109008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeCoursey TE, Ligeti E. Regulation and termination of NADPH oxidase activity. Cell Mol Life Sci. 2005;62:2173–2193. doi: 10.1007/s00018-005-5177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winterbourn CC, Kettle AJ. Redox reactions and microbial killing in the neutrophil phagosome. Antioxid Redox Signal. 2013;18:642–660. doi: 10.1089/ars.2012.4827. [DOI] [PubMed] [Google Scholar]
- 40.Klebanoff SJ, Kettle AJ, Rosen H, Winterbourn CC, Nauseef WM. Myeloperoxidase: a front-line defender against phagocytosed microorganisms. J Leuk Biol. 2013;93:185–198. doi: 10.1189/jlb.0712349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK, Lambeth JD. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 42.Aguirre J, Lambeth JD. Nox enzymes from fungus to fly to fish and what they tell us about nox function in mammals. Free Rad Biol& Med. 2010;49:1342–1353. doi: 10.1016/j.freeradbiomed.2010.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leto TL, Morand S, Hurt D, Ueyama T. Targeting and regulation of reactive oxygen species generation by NOX family NADPH oxidases. Antioxidants & Redox Signaling. 2009;11:2607–2619. doi: 10.1089/ars.2009.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ameziane-El-Hassani R, Morand S, Boucher JL, Frapart YM, Apostolou D, Agnandji D, Gnidehou S, Ohayon R, Noël-Hudson MS, Francon J, Lalaoui K, Virion A, Dupuy C. Dual oxidase-2 has an intrinsic Ca 2+-dependent H 2 0 2 -generating activity. J Biol Chem. 2005;280:30046–30054. doi: 10.1074/jbc.M500516200. [DOI] [PubMed] [Google Scholar]
- 45.Donko A, Peterfi Z, Sum A, Leto T, Geiszt M. Dual oxidases. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2005;360:2301–2308. doi: 10.1098/rstb.2005.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bedard K, Jaquet V, Krause KH. NOX5: from basic biology to signaling and disease. Free Rad Biol& Med. 2012;52:725–734. doi: 10.1016/j.freeradbiomed.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 47.Bánfi B, Tirone F, Durussel I, Knisz J, Moskwa P, Molnar GZ, Krause KH, Cox JA. Mechanism of Ca 2+ activation of the NADPH oxidase 5 (NOX5) J Biol Chem. 2004;279:18583–18591. doi: 10.1074/jbc.M310268200. [DOI] [PubMed] [Google Scholar]
- 48.Edens WA, Sharling L, Cheng G, Shapira R, Kinkade JM, Lee T, Edens HA, Tang X, Sullards C, Flaherty DB, Benian GM, Lambeth JD. Tyrosine cross-linking of extracellular matrix is catalyzed by Duox, a multidomain oxidase/peroxidase with homology to the phagocyte oxidase subunit gp91 phox. J Cell Biol. 2001;154:879–891. doi: 10.1083/jcb.200103132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meitzler JL, Ortiz de Montellano PR. Caenorhabditis elegans and human dual oxidase 1 (DUOX1) “peroxidase” domains. Insights into heme binding and catalytic activity. J Biol Chem. 2009;284:18634–18643. doi: 10.1074/jbc.M109.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morand S, Ueyama T, Tsujibe S, Saito N, Korzeniowska A, Leto TL. Duox maturation factors form cell surface complexes with Duox affecting the specificity of reactive oxygen species generation. FASEB J. 2009;23:1205–1218. doi: 10.1096/fj.08-120006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ambasta RK, Kumar P, Griendling KK, Schmidt HHHW, Busse R, Brandes RP. Direct interaction of the novel nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem. 2004;279:45935–45941. doi: 10.1074/jbc.M406486200. [DOI] [PubMed] [Google Scholar]
- 52.Ueno N, Takeya R, Miyano K, Kikuchi H, Sumimoto H. The NADPH oxidase NOX3 constitutively produces superoxide in a p22 phox -dependent manner: its regulation by oxidase organizers and activators. J Biol Chem. 2005;280:23328–23339. doi: 10.1074/jbc.M414548200. [DOI] [PubMed] [Google Scholar]
- 53.Nakano Y, Banfi B, Jesaitis AJ, Dinauer MC, Allen LA, Nauseef WM. Critical roles for p22 phox in the structural maturation and subcellular targeting of Nox3. Biochem J. 2007;403:97–108. doi: 10.1042/BJ20060819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.von Löhneysen K, Noack D, Jesaitis AJ, Dinauer MC, Knaus UG. Mutational analysis reveals distinct features of the Nox4-p22 phox complex. J Biol Chem. 2008;283:35273–35282. doi: 10.1074/jbc.M804200200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Geiszt M, Lekstrom K, Witta J, Leto TL. Proteins homologous to p47 phox and p67 phox support superoxide production by NAD(P)H oxidase 1 in colon epithelial cells. J Biol Chem. 2003;278:20006–20012. doi: 10.1074/jbc.M301289200. [DOI] [PubMed] [Google Scholar]
- 56.Takeya R, Ueno N, Kami K, Taura M, Kohjima M, Izaki T, Nunoi H, Sumimoto H. Novel human homologues of p47 phox and p67 phox participate in activation of superoxide-production NADPH oxidases. J Biol Chem. 2003;278:25234–25246. doi: 10.1074/jbc.M212856200. [DOI] [PubMed] [Google Scholar]
- 57.Pacquelet S, Lehmann M, Luxen S, Regazzoni K, Frausto M, Noack D, Knaus UG. Inhibitory action of NoxA1 on dual oxidase activity in airway cells. J Biol Chem. 2008;283:24649–24658. doi: 10.1074/jbc.M709108200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grasberger H, Refetoff S. Identification of the maturation factor for dual oxidase. J Biol Chem. 2006;281:18269–18272. doi: 10.1074/jbc.C600095200. [DOI] [PubMed] [Google Scholar]
- 59.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 60.von Löhneysen K, Noack D, Hayes P, Friedman JS, Knaus UG. Constitutive NADPH oxidase 4 activity resides in the composition of the B-loop and the penultimate C terminus. J Biol Chem. 2012;287:8737–8745. doi: 10.1074/jbc.M111.332494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takac I, Schröder K, Zhang L, Lardy B, Anilkumar N, Lambeth JD, Shah AM, Morel F, Brandes RP. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J Biol Chem. 2011;286:13304–13313. doi: 10.1074/jbc.M110.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Fórró L, Schlegel W, Krause KH. Nox4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J. 2007;406:105–114. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.New DD, Block K, Bhandhari B, Gorin Y, Abboud HE. IGF-I increases the expression of fibronectin by Nox4-dependent Akt phosphorylation in renal tubular epithelial cells. Am J Physiol Cell Physiol. 2012;302:C122–130. doi: 10.1152/ajpcell.00141.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gorin Y, block K, Hernandez J, Bhandari B, Wagner B, Barnes JL, Abboud HE. Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem. 2005;280:39616–39626. doi: 10.1074/jbc.M502412200. [DOI] [PubMed] [Google Scholar]
- 65.Gorin Y, Ricono JM, Kim NH, Bhandari B, Choudhury GG, Abboud HE. Nox4 mediates angiotensin II-induced activation of Akt/protein kinase B in mesangial cells. Am J Physiol Renal Physiol. 2003;285:F219–F229. doi: 10.1152/ajprenal.00414.2002. [DOI] [PubMed] [Google Scholar]
- 66.Spencer NY, Yan Z, Boudreau RL, Zhang Y, Luo M, Li Q, tian X, Shah AM, Davisson RL, Davidson B, Banfi B, Engelhardt JF. Control of hepatic nuclear superoxide production by glucose 6-phosphate dehydrogenase and NADPH oxidase-4. J Biol Chem. 2011;286:8977–8987. doi: 10.1074/jbc.M110.193821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Galijasevic S, Maitra D, Lu T, Sliskovic I, Abdulhamid I, Abu-Soud HM. Myeloperoxidase interaction with peroxynitrite: chloride deficiency and heme depletion. Free Rad Biol& Med. 2009;47:431–439. doi: 10.1016/j.freeradbiomed.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maalouf RM, Eid AA, Gorin YC, block K, Escobar GP, Bailey S, Abboud HE. Nox4-derived reactive oxygen species mediate cardiomyocyte injury in early type 1 diabetes. Am J Physiol Cell Physiol. 2012;302:C597–C604. doi: 10.1152/ajpcell.00331.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boudreau HE, Casterline BW, Rada B, Korzeniowska A, Leto TL. Nox4 involvement in TGF-beta and SMAD3-driven induction of the epithelial-to-mesenchymal transition and migration of breast epithelial cells. Free Radic Biol Med. 2012;53:1489–1499. doi: 10.1016/j.freeradbiomed.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mandal CC, Ganapathy S, Gorin Y, Mahadev K, Block K, Abboud HE, Harris SE, Ghosh-Choudhury G, Ghosh-Choudhury N. Reactive oxygen species derived from Nox4 mediate BMP2 gene transcription and osteoblast differentiation. Biochem J. 2011;433:393–402. doi: 10.1042/BJ20100357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sturrock A, Huecksteadt TP, Norman K, Sanders K, Murphy TM, Chitano P, Wilson K, Hoidal JR, Kennedy TP. Nox4 mediates TGF-β1-induced retinoblastoma protein phosphorylation, proliferation, and hypertrophy in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1543–L1555. doi: 10.1152/ajplung.00430.2006. [DOI] [PubMed] [Google Scholar]
- 72.Raad H, Eskalli Z, Corvilain B, Miot F, De Deken X. Thyroid hydrogen peroxide production is enhanced by the Th2 cytokines, IL-4 and IL-13, through increased expression of the dual oxidase 2 and its maturation factor DUOXA2. Free Radic Biol Med. 2013;56:216–225. doi: 10.1016/j.freeradbiomed.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 73.Fink K, Martin L, Mukawera E, Chartier S, De Deken X, Brochiero E, Miot F, Grandvaux N. IFNb/TNFa synergism induces a non-canonical STAT2/IRF9-dependent pathway triggering a novel DUOX2 NADPH Oxidase-mediated airway antiviral response. Cell Res. 2013 doi: 10.1038/cr.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Luxen S, Noack D, Frausto M, Davantuere S, Torbett BE, Knaus UG. Heterodimerization controls localization of duox-duoxA NADPH oxidases in airway cells. J Cell Science. 2009;122:1238–1247. doi: 10.1242/jcs.044123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moskwa P, Lorentzen D, Excoffon KJ, Zabner J, McCray PB, Jr, Nauseef WM, Dubuy C, Banfi B. A novel host defense system of airways is defective in cystic fibrosis. Am J Respir Crit Care Med. 2007;175:174–183. doi: 10.1164/rccm.200607-1029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hoste C, Dumont JE, Miot F, de Deken X. The type of DUOX-dependent ROS production is dictated by defined sequences in DUOXA. Exp Cell Res. 2012;318:2353–2364. doi: 10.1016/j.yexcr.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 77.Ferrick DA, Neilson A, Beeson C. Advances in measuring cellular bioenergetics using extracellular flux. Drug discovery today. 2008;13:268–274. doi: 10.1016/j.drudis.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 78.Gerencser AA, Neilson A, Choi SW, Edman U, Yadava N, Oh RJ, Ferrick DA, Nicholls DG, Brand MD. Quantitative microplate-based respirometry with correction for oxygen diffusion. Anal Chem. 2009;81:6868–6878. doi: 10.1021/ac900881z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shimada K, Crother TR, Karlin J, Chen S, Chiba N, Ramanujan VK, Vergnes L, Ojcius DM, Arditi M. Caspase-1 dependent IL-1beta secretion is critical for host defense in a mouse model of Chlamydia pneumoniae lung infection. PLoS One. 2011;6:e21477. doi: 10.1371/journal.pone.0021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bulua AC, Simon A, Maddipati R, Pelletier M, Park H, Kim KY, Sack MN, Kastner DL, Siegel RM. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS) J Exp Med. 2011;208:519–533. doi: 10.1084/jem.20102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Babior BM, Kipnes RS, Curnutte JT. Biological defence mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973;52:741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Teshima S, Rokutan K, Nikawa T, Kishi K. Guinea pig gastric mucosal cells produce abundant superoxide anion through an NADPH oxidase-like system. Gastroenterology. 1998;115:1186–1196. doi: 10.1016/s0016-5085(98)70090-3. [DOI] [PubMed] [Google Scholar]
- 83.Massey V. The microestimation of succinate and the extinction coefficient of cytochrome c. Biochim Biophys Acta. 1959;34:255–256. doi: 10.1016/0006-3002(59)90259-8. [DOI] [PubMed] [Google Scholar]
- 84.Newburger PE, Chovaniec ME, Cohen HJ. Activity and activation of the granulocyte superoxide-generating system. Blood. 1980;55:85–92. [PubMed] [Google Scholar]
- 85.Pick E, Mizel D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J Immunol Meth. 1981;46:211–226. doi: 10.1016/0022-1759(81)90138-1. [DOI] [PubMed] [Google Scholar]
- 86.Tan AS, Berridge MV. Superoxide produced by activated neutrophils efficiently reduces the tetrazolium salt, WST-1 to produce a soluble formazan: a simple colorimetric assay for measuring respiratory burst activation and for screening anti-inflammatory agents. J Immunol Methods. 2000;238:59–68. doi: 10.1016/s0022-1759(00)00156-3. [DOI] [PubMed] [Google Scholar]
- 87.Michalski R, Zielonka J, Hardy M, Joseph J, Kalyanaraman B. Hydropropidine: a novel, cell-impermeant fluorogenic probe for detecting extracellular superoxide. Free Radic Biol Med. 2013;54:135–147. doi: 10.1016/j.freeradbiomed.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Test ST, Weiss SJ. Quantitative and temporal characterization of the extracellular H2O2 pool generated by human neutrophils. J Biol Chem. 1984;259:399–405. [PubMed] [Google Scholar]
- 89.Root RK, Metcalf JA. H2O2 release from human granulocytes during phagocytosis. J Clin Invest. 1977;60:1266–1279. doi: 10.1172/JCI108886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ruch W, Cooper PH, Baggiolini M. Assay of H 2 O 2 production by macrophages and neutrophils with homovanillic acid and horse-radish peroxidase. J Immunol Methods. 1983;63:347–357. doi: 10.1016/s0022-1759(83)80008-8. [DOI] [PubMed] [Google Scholar]
- 91.Pick E, Keisari Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods. 1980;38:161–170. doi: 10.1016/0022-1759(80)90340-3. [DOI] [PubMed] [Google Scholar]
- 92.Smith RJ, Bowman BJ. Generation of hydrogen peroxide by human neutrophils: effects of soluble stimuli and requirements for divalent cations. Clin Immunol Immunopathol. 1982;24:194–203. doi: 10.1016/0090-1229(82)90231-8. [DOI] [PubMed] [Google Scholar]
- 93.Zhou M, Diwu Z, Panchuk-Voloshina N, Haugland RP. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal Biochem. 1997;253:162–168. doi: 10.1006/abio.1997.2391. [DOI] [PubMed] [Google Scholar]
- 94.Kettle AJ, Carr AC, Winterbourn CC. Assays using horseradish peroxidase and phenolic substrates require superoxide dismutase for accurate determination of hydrogen peroxide production by neutrophils. Free Radic Biol Med. 1994;17:161–164. doi: 10.1016/0891-5849(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 95.Mishin V, Gray JP, Heck DE, Laskin DL, Laskin JD. Application of the Amplex red/horseradish peroxidase assay to measure hydrogen peroxide generation by recombinant microsomal enzymes. Free Radic Biol Med. 2010;48:1485–1491. doi: 10.1016/j.freeradbiomed.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bromberg Y, Pick E. Unsaturated fatty acids stimulate NADPH-dependent superoxide production by cell-free system derived from macrophages. Cell Immunol. 1984;88:213–221. doi: 10.1016/0008-8749(84)90066-2. [DOI] [PubMed] [Google Scholar]
- 97.Heynemann RA, Vercauteren RE. Activation of a NADPH-dependent oxidase from horse polymorphonuclear leukocytes in a cell-free system. J Leukoc Biol. 1984;36:751–759. doi: 10.1002/jlb.36.6.751. [DOI] [PubMed] [Google Scholar]
- 98.McPhail LC, Shirley PS, Clayton CC, Snyderman R. Activation of the respiratory burst enzyme from human neutrophils in a cell-free system: evidence for a soluble cofactor. J Clin Invest. 1985;75:1735–1739. doi: 10.1172/JCI111884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Curnutte JT. Activation of human neutrophil nicotinamide adenine dinucleotide phosphate, reduced (triphosphopyridine nucleotide, reduced) oxidase by arachidonic acid in a cell-free system. J Clin Invest. 1985;75:1740–1743. doi: 10.1172/JCI111885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Clark RA, Leidal KG, Pearson DW, Nauseef WM. NADPH oxidase of human neutrophils: Subcellular localization and characterization of an arachidonate-activatable superoxide- generating system. J Biol Chem. 1987;262:4065–4074. [PubMed] [Google Scholar]
- 101.Molshanski-Mor S, Mizrahi A, Ugolev Y, Dahan I, Berdichevsky Y, Pick E. Cell-free assays: the reductionist approach to the study of NADPH oxidase assembly, or “all you wanted to know about cell-free assays but did not dare to ask”. Methods Mol Biol. 2007;412:385–428. doi: 10.1007/978-1-59745-467-4_25. [DOI] [PubMed] [Google Scholar]
- 102.Volpp BD, Nauseef WM, Clark RA. Two cytosolic neutrophil NADPH oxidase components absent in autosomal chronic granulomatous disease. Science. 1988;242:1295–1298. doi: 10.1126/science.2848318. [DOI] [PubMed] [Google Scholar]
- 103.Nunoi H, Rotrosen D, Gallin JI, Malech HL. Two forms of autosomal chronic granulomatous disease lack distinct neutrophil cytosol factors. Science. 1988;242:1298–1301. doi: 10.1126/science.2848319. [DOI] [PubMed] [Google Scholar]
- 104.Abo A, Pick E, Hall A, Totty N, Teahan CG, Segal AW. Activation of the NADPH oxidase involves the small GTP-binding protein p21 rac1. Nature. 1991;353:668–670. doi: 10.1038/353668a0. [DOI] [PubMed] [Google Scholar]
- 105.Wientjes FB, Hsuan JJ, Totty NF, Segal AW. p40phox, a third cytosolic component of the activation complex of the NADPH oxidase to contain src homology 3 domains. Biochem J. 1993;296:557–561. doi: 10.1042/bj2960557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Votyakova TV, Reynolds IJ. Detection of hydrogen peroxide with Amplex Red: interference by NADH and reduced glutathione auto-oxidation. Arch Biochem Biophys. 2004;431:138–144. doi: 10.1016/j.abb.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 107.Hurst JK. What really happens in the neutrophil phagosome? Free Radic Biol Med. 2012;53:508–520. doi: 10.1016/j.freeradbiomed.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Winterbourn CC, Hampton MB, Livesey JH, Kettle AJ. Modeling the reactions of superoxide and myeloperoxidase in the neutrophil phagosome. J Biol Chem. 2006;281:39860–39869. doi: 10.1074/jbc.M605898200. [DOI] [PubMed] [Google Scholar]
- 109.Allen RC, Stjernholm RL, Steele RH. Evidence for the generation of an electronic excitation state(s) in human polymorphonuclear leukocytes and its participation in bactericidal activity. Biochem Biophys Res Commun. 1972;47:679–684. doi: 10.1016/0006-291x(72)90545-1. [DOI] [PubMed] [Google Scholar]
- 110.Allen RC. Phagocytic leukocyte oxygenation activities and chemiluminescence: a kinetic approach to analysis. Methods Enzymol. 1986;133:449–493. doi: 10.1016/0076-6879(86)33085-4. [DOI] [PubMed] [Google Scholar]
- 111.Rosen H, Klebanoff SJ. Chemiluminescence and superoxide production by myeloperoxidase-deficient leukocytes. J Clin Invest. 1976;58:50–60. doi: 10.1172/JCI108458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stevens P, Winston DJ, Van Dyke K. In vitro evaluation of opsonic and cellular granulocyte function by luminol-dependent chemiluminescence: utility in patients with severe neutropenia and cellular deficiency states. Infect Immun. 1978;22:41–51. doi: 10.1128/iai.22.1.41-51.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dahlgren C, Karlsson A, Bylund J. Measurement of respiratory burst products generated by professional phagocytes. Methods Mol Biol. 2007;412:349–363. doi: 10.1007/978-1-59745-467-4_23. [DOI] [PubMed] [Google Scholar]
- 114.Lundqvist H, Dahlgren C. Isoluminol-enhanced chemiluminescence: a sensitive method to study the release of superoxide anion from human neutrophils. Free Radic Biol Med. 1996;20:785–792. doi: 10.1016/0891-5849(95)02189-2. [DOI] [PubMed] [Google Scholar]
- 115.Dahlgren C, Stendahl O. Role of myeloperoxidase in luminol-dependent chemiluminescence of polymorphonuclear leukocytes. Infect Immun. 1983;39:736–741. doi: 10.1128/iai.39.2.736-741.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Daiber A, August M, Baldus S, Wendt M, Oelze M, Sydow K, Kleschyov AL, Munzel T. Measurement of NAD(P)H oxidase-derived superoxide with the luminol analogue L-012. Free Radic Biol Med. 2004;36:101–111. doi: 10.1016/j.freeradbiomed.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 117.Liochev SI, Fridovich I. Lucigenin as mediator of superoxide production: revisited. Free Radic Biol Med. 1998;25:926–928. doi: 10.1016/s0891-5849(98)00121-x. [DOI] [PubMed] [Google Scholar]
- 118.Liochev SI, Fridovich I. Lucigenin (bis-N-methylacridinium) as a mediator of superoxide anion production. Arch Biochem Biophys. 1997;337:115–120. doi: 10.1006/abbi.1997.9766. [DOI] [PubMed] [Google Scholar]
- 119.Vilim V, Wilhelm J. What do we measure by a luminol-dependent chemiluminescence of phagocytes? Free Radic Biol Med. 1989;6:623–629. doi: 10.1016/0891-5849(89)90070-1. [DOI] [PubMed] [Google Scholar]
- 120.Cocheme HM, Quin C, McQuaker SJ, Cabreiro F, Logan A, Prime TA, Abakumova I, Patel JV, Fearnley IM, James AM, Porteous CM, Smith RA, Saeed S, Carre JE, Singer M, Gems D, Hartley RC, Partridge L, Murphy MP. Measurement of H2O2 within living Drosophila during aging using a ratiometric mass spectrometry probe targeted to the mitochondrial matrix. Cell Metab. 2011;13:340–350. doi: 10.1016/j.cmet.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Faulkner K, Fridovich I. Luminol and lucigenin as detectors for O 2. Free Rad Biol& Med. 1993;15:447–451. doi: 10.1016/0891-5849(93)90044-u. [DOI] [PubMed] [Google Scholar]
- 122.Samuni A, Krishna CM, Cook J, Black CD, Russo A. On radical production by PMA-stimulated neutrophils as monitored by luminol-amplified chemiluminescence. Free Radic Biol Med. 1991;10:305–313. doi: 10.1016/0891-5849(91)90037-4. [DOI] [PubMed] [Google Scholar]
- 123.Lucas M, Solano F. Coelenterazine is a superoxide anion-sensitive chemiluminescent probe: Its usefulness in the assay of respiratory burst in neutrophils. Anal Biochem. 1992;206:273–277. doi: 10.1016/0003-2697(92)90366-f. [DOI] [PubMed] [Google Scholar]
- 124.Teranishi K. Luminescence of imidazo[1,2-a]pyrazin-3(7H)-one compounds. Bioorg Chem. 2007;35:82–111. doi: 10.1016/j.bioorg.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 125.Tarpey MM, White CR, Suarez E, Richardson G, Radi R, Freeman BA. Chemiluminescent detection of oxidants in vascular tissue. Lucigenin but not coelenterazine enhances superoxide formation. Circ Res. 1999;84:1203–1211. doi: 10.1161/01.res.84.10.1203. [DOI] [PubMed] [Google Scholar]
- 126.Meyer AJ, Dick TP. Fluorescent protein-based redox probes. Antioxid Redox Signal. 2010;13:621–650. doi: 10.1089/ars.2009.2948. [DOI] [PubMed] [Google Scholar]
- 127.Royall JA, Ischiropoulos H. Evaluation of 2′,7′-dichlorofluorescin and dihydrorhodamine 123 as fluorescent probes for intracellular H2O2 in cultured endothelial cells. Arch Biochem Biophys. 1993;302:348–355. doi: 10.1006/abbi.1993.1222. [DOI] [PubMed] [Google Scholar]
- 128.Zhao H, Joseph J, Fales HM, Sokoloski EA, Levine RL, Vasquez-Vivar J, Kalyanaraman B. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc Natl Acad Sci U S A. 2005;102:5727–5732. doi: 10.1073/pnas.0501719102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zielonka J, Sarna T, Roberts JE, Wishart JF, Kalyanaraman B. Pulse radiolysis and steady-state analyses of the reaction between hydroethidine and superoxide and other oxidants. Arch Biochem Biophys. 2006;456:39–47. doi: 10.1016/j.abb.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zielonka J, Kalyanaraman B. Hydroethidine- and MitoSOX-derived red fluorescence is not a reliable indicator of intracellular superoxide formation: another inconvenient truth. Free Radic Biol Med. 2010;48:983–1001. doi: 10.1016/j.freeradbiomed.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Crow JP. Dichlorodihydrofluorescein and dihydrorhodamine 123 are sensitive indicators of peroxynitrite in vitro: implications for intracellular measurement of reactive nitrogen and oxygen species. Nitric Oxide. 1997;1:145–157. doi: 10.1006/niox.1996.0113. [DOI] [PubMed] [Google Scholar]
- 132.Kane DJ, Sarafian TA, Anton R, Hahn H, Gralla EB, Valentine JS, Ord T, Bredesen DE. Bcl-2 inhibition of neural death: decreased generation of reactive oxygen species. Science. 1993;262:1274–1277. doi: 10.1126/science.8235659. [DOI] [PubMed] [Google Scholar]
- 133.Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 134.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 135.Burkitt MJ, Wardman P. Cytochrome C is a potent catalyst of dichlorofluorescin oxidation: implications for the role of reactive oxygen species in apoptosis. Biochem Biophys Res Commun. 2001;282:329–333. doi: 10.1006/bbrc.2001.4578. [DOI] [PubMed] [Google Scholar]
- 136.Dypbukt JM, Bishop C, Brooks WM, Thong B, Eriksson H, Kettle AJ. A sensitive and selective assay for chloramine production by myeloperoxidase. Free Radic Biol Med. 2005;39:1468–1477. doi: 10.1016/j.freeradbiomed.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 137.Kohl A, Roesler J, Döcke WD, Valet G, Volk HD. Cytofluorometric assessment of phagosomal oxidation and the mode of inheritance in patients suffering from chronic granulomatous disease. Agents Actions. 1991;32:134–136. doi: 10.1007/BF01983341. [DOI] [PubMed] [Google Scholar]
- 138.Vowells SJ, Sekhsaria S, Malech HL, Shalit M, Fleisher TA. Flow cytometric analysis of the granulocyte respiratory burst: a comparison study of fluorescent probes. J Immunol Methods. 1995;178:89–97. doi: 10.1016/0022-1759(94)00247-t. [DOI] [PubMed] [Google Scholar]
- 139.Henderson LM, Chappell JB. Dihydrorhodamine 123: a fluorescent probe for superoxide generation? Eur J Biochem. 1993;217:973–980. doi: 10.1111/j.1432-1033.1993.tb18328.x. [DOI] [PubMed] [Google Scholar]
- 140.Mauch L, Lun A, O’Gorman MRG, Harris JS, Schulze I, Zychlinsky A, Fuchs T, Oelschlägel U, Brenner S, Kutter D, Rösen-Wolff A, Roesler J. Chronic granulomatous disease (CGD) and complete myeloperoxidase deficiency both yield strongly reduced dihydrorhodamine 123 test signals but can be easily discerned in routine testing for CGD. Clin Chem. 2007;53:890–896. doi: 10.1373/clinchem.2006.083444. [DOI] [PubMed] [Google Scholar]
- 141.Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vasquez-Vivar J, Kalyanaraman B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med. 2003;34:1359–1368. doi: 10.1016/s0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]
- 142.Fink B, Laude K, McCann L, Doughan A, Harrison DG, Dikalov S. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am J Physiol Cell Physiol. 2004;287:C895–902. doi: 10.1152/ajpcell.00028.2004. [DOI] [PubMed] [Google Scholar]
- 143.Kundu K, Knight SF, Lee S, Taylor WR, Murthy N. A significant improvement of the efficacy of radical oxidant probes by the kinetic isotope effect. Angew Chem Int Ed Engl. 2010;49:6134–6138. doi: 10.1002/anie.201002228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Patsoukis N, Papapostolou I, Georgiou CD. Interference of non-specific peroxidases in the fluorescence detection of superoxide radical by hydroethidine oxidation: a new assay for H2O2. Anal Bioanal Chem. 2005;381:1065–1072. doi: 10.1007/s00216-004-2999-x. [DOI] [PubMed] [Google Scholar]
- 145.Papapostolou I, Patsoukis N, Georgiou CD. The fluorescence detection of superoxide radical using hydroethidine could be complicated by the presence of heme proteins. Anal Biochem. 2004;332:290–298. doi: 10.1016/j.ab.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 146.Zielonka J, Srinivasan S, Hardy M, Ouari O, Lopez M, Vasquez-Vivar J, Avadhani NG, Kalyanaraman B. Cytochrome c-mediated oxidation of hydroethidine and mito-hydroethidine in mitochondria: identification of homo- and heterodimers. Free Radic Biol Med. 2008;44:835–846. doi: 10.1016/j.freeradbiomed.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zielonka J, Vasquez-Vivar J, Kalyanaraman B. Detection of 2-hydroxyethidium in cellular systems: a unique marker product of superoxide and hydroethidine. Nat Protoc. 2008;3:8–21. doi: 10.1038/nprot.2007.473. [DOI] [PubMed] [Google Scholar]
- 148.Ainley ADCF. Studies of the boron-carbon linkage. Part I. The oxidation and nitration of phenylboric acid. J Chem Soc. 1930:2171–2181. [Google Scholar]
- 149.Chang MC, Pralle A, Isacoff EY, Chang CJ. A selective, cell-permeable optical probe for hydrogen peroxide in living cells. J Am Chem Soc. 2004;126:15392–15393. doi: 10.1021/ja0441716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dickinson BC, Huynh C, Chang CJ. A palette of fluorescent probes with varying emission colors for imaging hydrogen peroxide signaling in living cells. J Am Chem Soc. 2010;132:5906–5915. doi: 10.1021/ja1014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Miller EW, Albers AE, Pralle A, Isacoff EY, Chang CJ. Boronate-based fluorescent probes for imaging cellular hydrogen peroxide. J Am Chem Soc. 2005;127:16652–16659. doi: 10.1021/ja054474f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lin VS, Lippert AR, Chang CJ. Cell-trappable fluorescent probes for endogenous hydrogen sulfide signaling and imaging H2O2-dependent H2S production. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1302193110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Miller EW, Tylyathan O, Isacoff EY, Chang CJ. Molecular imaging of hydrogen peroxide produced for cell signaling. Nature Chem Biology. 2007;3:263–267. doi: 10.1038/nchembio871. [DOI] [PubMed] [Google Scholar]
- 154.Sikora A, Zielonka J, Lopez M, Joseph J, Kalyanaraman B. Direct oxidation of boronates by peroxynitrite: mechanism and implications in fluorescence imaging of peroxynitrite. Free Radic Biol Med. 2009;47:1401–1407. doi: 10.1016/j.freeradbiomed.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Miller EW, Chang CJ. Fluorescent probes for nitric oxide and hydrogen peroxide in cell signaling. Curr Opin Chem Biol. 2007;11:620–625. doi: 10.1016/j.cbpa.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zielonka J, Sikora A, Hardy M, Joseph J, Dranka BP, Kalyanaraman B. Boronate probes as diagnostic tools for real time monitoring of peroxynitrite and hydroperoxides. Chem Res Toxicol. 2012;25:1793–1799. doi: 10.1021/tx300164j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zielonka J, Zielonka M, Sikora A, Adamus J, Joseph J, Hardy M, Ouari O, Dranka BP, Kalyanaraman B. Global profiling of reactive oxygen and nitrogen species in biological systems: high-throughput real-time analyses. J Biol Chem. 2012;287:2984–2995. doi: 10.1074/jbc.M111.309062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Van de Bittner GC, Bertozzi CR, Chang CJ. Strategy for dual-analyte luciferin imaging: in vivo bioluminescence detection of hydrogen peroxide and caspase activity in a murine model of acute inflammation. J Am Chem Soc. 2013;135:1783–1795. doi: 10.1021/ja309078t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Dooley CT, Dore TM, Hanson GT, Jackson WC, Remington SJ, Tsien RY. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J Biol Chem. 2004;279:22284–22293. doi: 10.1074/jbc.M312847200. [DOI] [PubMed] [Google Scholar]
- 160.Belousov VV, Fradkov AF, Lukyanov KA, Staroverov DB, Shakhbazov KS, Terskikh AV, Lukyanov S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nature Methods. 2006;3:281–286. doi: 10.1038/nmeth866. [DOI] [PubMed] [Google Scholar]
- 161.Zheng M, Aslund F, Storz G. Activation of the OxyR transcription factor by reversible disulfide bond formation. Science. 1998;279:1718–1721. doi: 10.1126/science.279.5357.1718. [DOI] [PubMed] [Google Scholar]
- 162.Wu RF, Ma Z, Liu Z, Terada LS. Nox4-derived H2O2 mediates endoplasmic reticulum signaling through local Ras activation. Mol Cell Biol. 2010;30:3553–3568. doi: 10.1128/MCB.01445-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Enyedi B, Zana M, Donko A, Geiszt M. Spatial and Temporal Analysis of NADPH Oxidase-Generated Hydrogen Peroxide Signals by Novel Fluorescent Reporter Proteins. Antioxid Redox Signal. 2012 doi: 10.1089/ars.2012.4594. [DOI] [PubMed] [Google Scholar]
- 164.Mason JT, Kim SK, Knaff DB, Wood MJ. Thermodynamic basis for redox regulation of the Yap1 signal transduction pathway. Biochemistry. 2006;45:13409–13417. doi: 10.1021/bi061136y. [DOI] [PubMed] [Google Scholar]
- 165.Haurani MJ, Pagano PJ. Adventitial fibroblast reactive oxygen species as autacrine and paracrine mediators of remodeling: bellwether for vascular disease? Cardiovasc Res. 2007;75:679–689. doi: 10.1016/j.cardiores.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 166.Maeda H, Yamamoto K, Nomura Y, Kohno I, Hafsi L, Ueda N, Yoshida S, Fukuda M, Fukuyasu Y, Yamauchi Y, Itoh N. A design of fluorescent probes for superoxide based on a nonredox mechanism. J Am Chem Soc. 2005;127:68–69. doi: 10.1021/ja047018k. [DOI] [PubMed] [Google Scholar]
- 167.Lee H, Lee K, In-Kyung K, Park TG. Fluorescent gold nanoprobe sensitive to intracellular reactive oxygen species. Advanced Functional Materials. 2009;19:1884–1890. [Google Scholar]
- 168.Lee K, Lee H, Lee KW, Park TG. Optical imaging of intracellular reactive oxygen species for the assessment of the cytotoxicity of nanoparticles. Biomaterials. 2011;32:2556–2565. doi: 10.1016/j.biomaterials.2010.11.072. [DOI] [PubMed] [Google Scholar]
- 169.Soltes L, Mendichi R, Kogan G, Schiller J, Stankovska M, Arnhold J. Degradative action of reactive oxygen species on hyaluronan. Biomacromolecules. 2006;7:659–668. doi: 10.1021/bm050867v. [DOI] [PubMed] [Google Scholar]


