Abstract
It is unclear why successful quitting at time of breast cancer diagnosis should remove risk from a significant lifetime of smoking. Studies concluding this may be biased by how smoking is measured in many epidemiological cohorts. In the late 1990s, a randomized trial of diet and breast cancer outcomes enrolled early-stage female breast cancer survivors diagnosed within the previous 4 years. Smoking history and key covariate measures were available at study entry for 2953 participants. Participants were followed for an average of 7.3 years (96% response rate). There were 10.1% deaths (83% from breast cancer). At enrollment, 55.2% were never smokers, 41.2% former smokers, and 4.6% current smokers. Using current smoking status in a Cox regression, there was no increased risk for former smokers for either all-cause mortality (HR=1.11; 95% CI=0.87, 1.41; p-value = 0.42) or breast cancer mortality. However, when we categorized on extensive lifetime exposure, former smokers with 20+ pack-years of smoking (25.8%) had a significantly higher risk of both all-cause (HR=1.77; 95% CI =1.17, 2.48; p-value = 0.0007) and breast cancer specific mortality (HR=1.62; 95% CI =1.11, 2.37; p-value = 0.01). Lifetime smoking exposure, not current status should be used to assess mortality risk among former smokers.
Keywords: Breast cancer, smoking status, pack-years, mortality
Introduction
Smoking is associated with higher risk of mortality in breast cancer patients, with the increased risk reportedly limited to women who were still smoking at the time of assessment (i.e. current smokers) and not in former smokers1–9,10, suggesting that the exposure risk from smoking may be resolved by quitting. However, many of these epidemiological studies simply measured smoking status of participants without consideration of lifetime exposure. However, the health hazards from smoking accumulate with exposure.11 Additionally, there has been a major decline in daily smoking consumption within the United States.12 Should a substantial proportion of self-reported former smokers have limited experience with smoking, this might dilute the observed mortality risk for all former smokers and could explain the lack of association reported by former smokers. This report investigates the relationship between lifetime smoking history and mortality in a large cohort of early-stage breast cancer survivors to investigate whether quitting is sufficient to remove the risk from a significant lifetime history of smoking.
Material & Methods
Analyses were conducted in the Women’s Healthy Eating and Living (WHEL) study cohort of early-stage breast cancer survivors diagnosed 1991–2000 and randomly assigned to either a plant-based ‘high-vegetables-fruit-fiber and low-fat’ diet or a ‘5-a-day’ comparison group. Eligibility criteria, data collection, outcomes assessment, and trial results have been published.13 In short, participants were eligible if they had Stage I (≥1cm), II, or IIIA breast cancer diagnosed within the previous 4 years, had no evidence of cancer recurrence, had completed primary therapy, were aged 18–70 years at diagnosis, and did not have life-threatening co-morbidities. A total of 2953 (95.6%) of WHEL study participants had all the data needed and were included in this analysis. The Human Subjects Committee of the University of California, San Diego, and all participating institutions approved the study procedures.
Smoking assessment
At baseline, participants completed a brief smoking history questionnaire, which included multiple choice responses regarding age of smoking initiation and cessation, duration of smoking, and the number of cigarettes/day. We classified a lifetime history of <100 cigarettes as never smoking. Former smokers reported having quit at this baseline survey. All ever smokers reported their intensity of smoking (cigarettes/day) and the number of years they smoked regularly. Pack-years exposure was determined by multiplying duration of smoking by intensity.
Covariates assessment
Standardized questionnaires also ascertained demographic, other lifestyle characteristics, (e.g. alcohol, menopausal status) and quality of life (SF-36). Height (m) and weight (kg) were measured at enrollment clinic visit and body mass index (BMI) was calculated (kg/m2). Information on tumor characteristics, treatment received, and age at diagnosis was abstracted from medical records. Variables assessed in the analysis included: diagnosis age, obesity (BMI: <30 vs. ≥30), ethnicity (non-Hispanic white, African-American, Hispanic, Asian-American, and other), education (college graduate: yes, no), employment and marital status (yes, no), post-menopausal status (yes, no), alcohol intake (none, 1–19, and ≥20 g alcohol/day), time between diagnosis and study entry, physical and mental health summary score, radiotherapy (yes, no), chemotherapy (yes, no), Tamoxifen use (yes, no), cancer stage (I, II, and IIIA), cancer grade (differentiation: well, moderate, and poor), and estrogen receptor status (positive, negative).
Outcomes assessment
Staff queried study outcomes (i.e. breast cancer events and/or death) by telephone semi-annually. Any report of a breast cancer event or death triggered a confirmation interview (including family member) and an oncologist review of the medical record and/or death certificates. In addition, both the Social Security and the National Death Index were searched using Social Security number, name, and date of birth. At study completion, vital status was available on 96% of enrollees. Survival was the time from study enrollment (1995–2000) to death from any cause. Follow-up time was censored at the earlier of (a) time of the last documented staff contact date or (b) study completion (June 2006) for participants without an event (median follow-up time was 7.3 years, range was 0.01 – 10.8 years). For breast cancer-free survival, participants who died of causes other than breast cancer were censored at that time.
Statistical analysis
Smoking status and smoking history variables met the proportional hazard assumptions (the lines in the log minus log survival curves did not converge) and we plotted Kaplan-Meier curves of cumulative survival by categories of pack-years. Cox proportional hazard models were used to determine the mortality risk (cancer specific and all-cause) by smoking status (for current and former smokers, reference= never smokers); hazard ratios (HR) and 95% confidence intervals (95% CI) were reported.14 Covariates were assessed with a backward selection procedure and the most parsimonious models were selected when exclusion of variables did not affect the β-coefficient of the smoking status variable by more than 10%. We tested the association between smoking intensity (cigarettes/day) and duration (years of regular smoking) and noted significant co-linearity (Spearman correlation=0.95, p<0.0001). Thus, it was not appropriate to treat them as independent variables in the same model as suggested by Peto 15. Accordingly, we conducted separate analyses for each variable as well as for the combined pack-years variable. The multivariate models with pack-years data were repeated without current smokers, to test whether the hazard ratios changed significantly. Data analyses were conducted in SAS version 9.2 (Cary, NC) and all tests used were two-sided.
Results
As previously reported,10 WHEL participants were mostly non-Hispanic white (85.3%), post-menopausal (79.4%), married (70.1%), and employed (72.2%). Approximately 26% were obese, 34% were ≥55 years old, and 54% were college graduates. Most had estrogen receptor positive tumors (74.3%); the majority had stage II or IIIA tumors (62.4%). The majority received adjuvant therapy (61.5%, 69.8%, and 66.3% had radiotherapy, chemotherapy, or Tamoxifen respectively). Over a median of 7.3 years of follow-up, 297 (10.1%) participants died, of whom 249 (82.5%) died from breast cancer. The majority (55.2%) were never smokers, with 41.2% former smokers, and the remaining 4.6% current smokers.
In the current smoking status adjusted analysis, compared to never smokers, current smokers had a 59% higher risk of all-cause mortality, but not a significantly increased risk of breast cancer mortality. No increased risk was observed for former smokers (Table 1). There appeared to be a threshold of 15 cigarettes per day under which there was no increased adjusted risk. However, for both 15–24 and 25+ cigarettes per day, there was a significant increased risk of all-cause mortality with the highest risk seen with the highest intensity. While the 15–24 cigarettes per day category was associated with breast cancer deaths, such an association was not seen at the higher intensity of smoking.
Table 1.
Adjusted associations of smoking with mortality (all-cause and breast cancer specific) in a cohort of US breast cancer survivors (n = 2953) enrolled in the Women’s Healthy Eating and Living (WHEL) Study.
| Variable | N | Death due to all-causes (events = 297) | Death due to breast cancer (events = 245 ) | ||||
|---|---|---|---|---|---|---|---|
| Event | HR (95%CI) | P-value | Event | HR (95%CI) | P-value | ||
| Smoking status | |||||||
| Never smokers | 1602 | 151 | Reference | 130 | Reference | ||
| Current smokers | 135 | 19 | 1.59 (0.98, 2.59) | 0.06 | 12 | 1.23 (0.67, 2.24) | 0.51 |
| Former smokers | 1216 | 127 | 1.11 (0.87, 1.41) | 0.42 | 103 | 1.08 (0.82, 1.40) | 0.60 |
|
| |||||||
| No. of cigarettes/day | |||||||
| Never smokers | 1602 | 151 | Reference | 130 | Reference | ||
| < 5 | 365 | 28 | 0.82 (0.55, 1.24) | 0.36 | 25 | 0.88 (0.57, 1.36) | 0.57 |
| 5–14 | 425 | 38 | 0.95 (0.60, 1.36) | 0.79 | 30 | 0.90 (0.60, 1.34) | 0.60 |
| 15–24 | 364 | 48 | 1.44 (1.03, 2.01) | 0.03 | 40 | 1.44 (1.00, 2.07) | 0.05 |
| 25 + | 197 | 32 | 1.67 (1.13, 2.47) | 0.01 | 20 | 1.28 (0.79, 2.06) | 0.31 |
| Years of smoking | |||||||
| Never smokers | 1602 | 151 | Reference | 130 | Reference | ||
| < 10 | 516 | 37 | 0.79 (0.54, 1.14) | 0.20 | 32 | 0.86 (0.54, 1.18) | 0.17 |
| 10 – 19 | 361 | 42 | 1.22 (0.86, 1.72) | 0.26 | 39 | 1.34 (0.93, 1.93) | 0.12 |
| 20 – 29 | 288 | 26 | 0.98 (0.63, 1.48) | 0.91 | 20 | 0.90 (0.52, 1.34) | 0.47 |
| 30 + | 186 | 41 | 2.42 (1.67, 3.50) | <0.0001 | 24 | 1.77 (1.11, 2.81) | 0.02 |
|
| |||||||
| Pack-years | |||||||
| 0 | 1602 | 151 | Reference | 130 | Reference | ||
| 0.1 – 10.0 | 747 | 63 | 0.92 (0.68, 1.23) | 0.57 | 55 | 0.95 (0.68, 1.30) | 0.73 |
| 10.1 – 20.0 | 238 | 21 | 0.93 (0.58, 1.47) | 0.76 | 17 | 0.90 (0.54. 1.51) | 0.73 |
| 20+ | 366 | 62 | 1.81 (1.32, 2.47) | 0.0002 | 43 | 1.54 (1.07, 2.32) | 0.02 |
Covariates in the models: age, cancer stage, tumor grade, estrogen receptor status at diagnosis; chemotherapy and Tamoxifen use post-diagnosis; race, obesity, education, menopausal status, alcohol consumption, physical and mental health at trial entry; time between diagnosis and study entry
HR = hazard ratio; CI = confidence interval
Duration of smoking was also investigated and a significant risk for both breast cancer and all-cause mortality was observed for those with 30+ years of smoking. The combined pack- years variable was tested and a threshold of 20+ pack-years was associated with both a 54% increase in breast cancer mortality (HR=1.54; 95% CI=1.07, 2.32) and an 81% increase in all-cause mortality (HR=1.81; 95% CI= 1.32–2.47). For former smokers over this threshold (30%) the observed significant increases were similar to the full model (58% and 71% respectively, data not shown).
Kaplan-Meier curves for survival (Figure 1A) and breast cancer free survival (Figure 1B) indicate that poorer outcomes were observed for smokers with a 20+ pack-years history early on during the follow-up period and increased over time.
Figure 1.
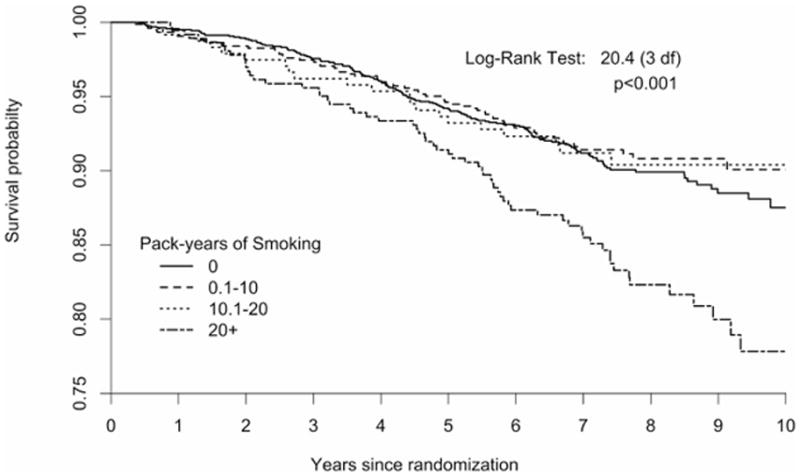
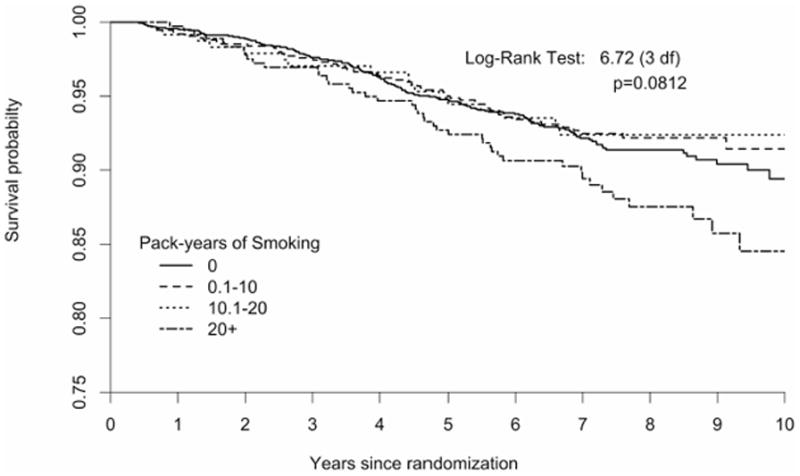
Time to death from any-cause (Figure 1A) and from breast cancer (Figure 1B) by pack-years of exposure to smoking in a cohort of US breast cancer survivors followed for a mean of 7.3 years (n =1602 never smokers; 0.1–10 pack-years=747, 10.1–20 pack-years=238, and >20 pack- years=366).
Discussion
Similar to other studies, using only current smoking status at enrollment suggested that smoking was associated with all-cause mortality, but not breast cancer mortality, and there was no increased risk among former smokers. However, a very different conclusion is reached when both intensity and duration of smoking are considered. There would appear to be no increased risk provided that the intensity of smoking was not more than 15 cigarettes per day and the duration was less than 30 years. Indeed, these two variables were highly co-linear, which may account for the anomaly in dose response with smoking intensity. Using a combined variable, the threshold under which there was no increased risk of a poorer health outcome was 20+ pack-years. Regardless of current smoking status, women with this level of experience with cigarette smoking had a 54% higher risk of dying from breast cancer during the study period. The risk of dying from any cause during the study period for this group was estimated to be 80% higher for current smokers and slightly less for former smokers (70%).
These data indicate that epidemiological and clinical studies need to collect at least a brief smoking history in their studies. That so many studies have only reported smoking status is surprising given the strong dose-response relationship that has been reported so often for mortality for many different cancers.16 Smoking seems to have become the variable that does not need to be measured in treatment trials, with a recent study noting that only 20% of clinical trials of co-operative groups even measure smoking status. 8 Yet, if a significant smoking history is associated with a 50% increase in breast cancer mortality, not including this variable in the analysis will significantly bias study findings. In this study, even though very few women were current smokers, over one third had 20+ pack-years of smoking history.
It has been argued that intensity and duration should not be highly co-linear in most studies and so the pack-years variable is not the best one to include in the analysis15. However, in the United States there has been a major decline in the average intensity of smoking over the past 50 years12. Importantly, the leading edge of this decline appears to have been a reduction in the prevalence of moderate to heavy smoking across successive birth cohorts. As a result, age is strongly associated with intensity. As duration of smoking is a function of age, it is to be expected that intensity and duration will be co-linear in a US population during this time period. That the WHEL study had a wide age eligibility range (18–70 years) would further ensure co-linearity.
Unfortunately, many epidemiological studies only measure current smoking status1–7, 9 and not lifetime exposure. Many of the early epidemiological studies on smoking used the pack-years of smoking measure to take into account both duration and intensity of smoking, but this requires additional survey questions. However, a design decision to limit measurement to only smoking status has important consequences for hypothesis testing in epidemiological studies. When smoking is a covariate of a hypothesized relationship, using smoking status in the model is unlikely to achieve the goal of removing confounding from smoking exposure. This inadequate adjustment will bias the hazard estimate for the exposure of interest. Should the true effect of the variable of interest be modest, then this approach could affect the significance of the test. When smoking is the exposure of interest, using smoking status will either underestimate or obscure the mortality risk, particularly for former smokers.
The strength of this analysis includes the large sample size, availability of key covariates, and a long and almost complete follow-up with a significant number of outcome events. A limitation is that this study enrolled mainly white, highly educated women who were early-stage breast cancer survivors who volunteered for a diet intervention trial and so has limited generalizability to other populations. The calculation of pack-years of smoking is limited as the intensity assessment is only focused on reported recent average smoking. Given that it has been documented that smokers have reduced their daily consumption, particularly since the rapid diffusion of smoke-free workplace policies, recent consumption will be an underestimate of true consumption.12, 17 The WHEL cohort was a young cohort with half of the population under 52 years of age, however 87% of the study population were over 40 years of age. As smoking initiation usually occurs in the teenage years18 and smoking is very addictive, there was ample opportunity for a 40 year old to accrue 20+ pack-years of smoking. As mentioned above, there was an age gradient in the pack-years variable.
In summary, the measures of intensity and duration of past smoking are co-linear and this study indicates that it is very important to use a combined exposure variable in any analysis of the effect of smoking on breast cancer mortality. When only current smoking status is used in analyses, the results suggest that those who have quit smoking are no longer at any increased risk from their smoking behavior. However, a combined exposure variable indicates that women with a history of 20+ pack-years have a 50% increased breast cancer mortality risk even after they have quit smoking. This suggests that use of a current status measure of smoking could bias conclusions of the relationship between smoking and breast cancer incidence as well.
Novelty and impact.
Many studies with breast cancer survivors only measure smoking status and not lifetime exposure (pack-years), and as a result report that former smokers do not have an elevated mortality risk. This report of breast cancer survivors has an important clinical message as it shows that former smokers with a 20+ pack-years have a significantly higher mortality risk (breast specific and all-cause) compared to both lifetime non-smokers and former smokers with < 20 pack-years.
Acknowledgments
The Women’s Healthy Eating and Living (WHEL) Study, was initiated with the support from the Walton Family Foundation and continued with funding from the National Cancer Institute (Grant CA 69375). Some of the data were collected using General Clinical Research Centers on NIH grants M01-RR00070, M01-RR00079, and M01-RR00827. For this analysis, Dr. Pierce was supported by Komen Foundation Grant No. 100988.
Footnotes
Conflict of Interests
No financial conflicts of interest were reported by the authors of this paper.
References
- 1.Fentiman IS, Allen DS, Hamed H. Smoking and prognosis in women with breast cancer. Int J Clin Pract. 2005;59:1051–1054. doi: 10.1111/j.1742-1241.2005.00581.x. [DOI] [PubMed] [Google Scholar]
- 2.Hellmann SS, Thygesen LC, Tolstrup JS, Gronbaek M. Modifiable risk factors and survival in women diagnosed with primary breast cancer: Results from a prospective cohort study. Eur J Cancer Prev. 2010;19:366–373. doi: 10.1097/CEJ.0b013e32833b4828. [DOI] [PubMed] [Google Scholar]
- 3.Dal Maso L, Zucchetto A, Talamini R, et al. Effect of obesity and other lifestyle factors on mortality in women with breast cancer. Int J Cancer. 2008;123:2188–2194. doi: 10.1002/ijc.23747. [DOI] [PubMed] [Google Scholar]
- 4.Holmes MD, Murin S, Chen WY, Kroenke CH, Spiegelman D, Colditz GA. Smoking and survival after breast cancer diagnosis. Int J Cancer. 2007;120:2672–2677. doi: 10.1002/ijc.22575. [DOI] [PubMed] [Google Scholar]
- 5.Sagiv SK, Gaudet MM, Eng SM, et al. Active and passive cigarette smoke and breast cancer survival. Ann Epidemiol. 2007;17:385–393. doi: 10.1016/j.annepidem.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Manjer J, Andersson I, Berglund G, et al. Survival of women with breast cancer in relation to smoking. Eur J Surg. 2000;166:852–858. doi: 10.1080/110241500447227. [DOI] [PubMed] [Google Scholar]
- 7.Calle EE, Miracle-McMahill HL, Thun MJ, Heath CW., Jr Cigarette smoking and risk of fatal breast cancer. Am J Epidemiol. 1994;139:1001–1007. doi: 10.1093/oxfordjournals.aje.a116939. [DOI] [PubMed] [Google Scholar]
- 8.Warren GW, Kasza KA, Reid ME, Cummings KM, Marshall JR. Smoking at diagnosis and survival in cancer patients. Int J Cancer. 2013;132:401–10. doi: 10.1002/ijc.27617. [DOI] [PubMed] [Google Scholar]
- 9.Barnett GC, Shah M, Redman K, Easton DF, Ponder BA, Pharoah PD. Risk factors for the incidence of breast cancer: do they affect survival from the disease? J Clin Oncol. 2008;26:3310–3316. doi: 10.1200/JCO.2006.10.3168. [DOI] [PubMed] [Google Scholar]
- 10.Braithwaite D, Izano M, Moore DH, et al. Smoking and survival after breast cancer diagnosis: a prospective observational study and systematic review. Breast Cancer Res Treat. 2012;136:521–533. doi: 10.1007/s10549-012-2276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.USDHHS. How Tobacco Smoke Causes Disease: The Biological and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Rockville, MD: 2010. [PubMed] [Google Scholar]
- 12.Pierce JP, Messer K, White MM, Cowling DW, Thomas DP. Prevalence of heavy smoking in California and the United States, 1965–2007. JAMA. 2011;305:1106–1112. doi: 10.1001/jama.2011.334. [DOI] [PubMed] [Google Scholar]
- 13.Pierce JP, Faerber S, Wright FA, et al. A randomized trial of the effect of a plant-based dietary pattern on additional breast cancer events and survival: the Women’s Healthy Eating and Living (WHEL) Study. Control Clin Trials. 2002;23:728–756. doi: 10.1016/s0197-2456(02)00241-6. [DOI] [PubMed] [Google Scholar]
- 14.Telke SE, Eberly LE. Statistical hypothesis testing: associating patient characteristics with an incident condition: Kaplan-Meier curves, hazard ratios, and cox proportional hazards regression. J Wound Ostomy Continence Nurs. 2011;38:621–626. doi: 10.1097/WON.0b013e31823428a8. [DOI] [PubMed] [Google Scholar]
- 15.Peto J. That the effects of smoking should be measured in pack-years: misconceptions 4. Br J Cancer. 2012 Jul 24;107:406–407. doi: 10.1038/bjc.2012.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.USDHHS. The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta, GA: U.S. Dept. of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004. [Google Scholar]
- 17.Fichtenberg CM, Glantz SA. Effect of smoke-free workplaces on smoking behaviour: Systematic review. BMJ. 2002;325:188. doi: 10.1136/bmj.325.7357.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.USDHHS. Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General. Rockville, MD: 2012. [PubMed] [Google Scholar]


