Abstract
Background and Rationale
Results from many clinical studies suggest that drug relapse and craving are often provoked by acute exposure to the self-administered drug or related drugs, drug-associated cues or contexts, or certain stressors. During the last two decades, this clinical scenario has been studied in laboratory animals by using the reinstatement model. In this model, reinstatement of drug seeking by drug priming, drug cues or contexts, or certain stressors is assessed following drug self-administration training and subsequent extinction of the drug-reinforced responding.
Objective
In this review, we first summarize recent (2009-present) neurobiological findings from studies using the reinstatement model. We then discuss emerging research topics, including the impact of interfering with putative reconsolidation processes on cue- and context-induced reinstatement of drug seeking, and similarities and differences in mechanisms of reinstatement across drug classes. We conclude by discussing results from recent human studies that were inspired by results from rat studies using the reinstatement model.
Conclusions
Main conclusions from the studies reviewed highlight: (1) the ventral subiculum and lateral hypothalamus as emerging brain areas important for reinstatement of drug seeking, (2) the existence of differences in brain mechanisms controlling reinstatement of drug seeking across drug classes, (3) the utility of the reinstatement model for assessing the effect of reconsolidation-related manipulations on cue-induced drug seeking, and (4) the encouraging pharmacological concordance between results from rat studies using the reinstatement model and human laboratory studies on cue- and stress-induced drug craving.
Keywords: context, craving, cue, extinction, drug priming, drug self-administration, reconsolidation, reinstatement, relapse, review, stress
Introduction
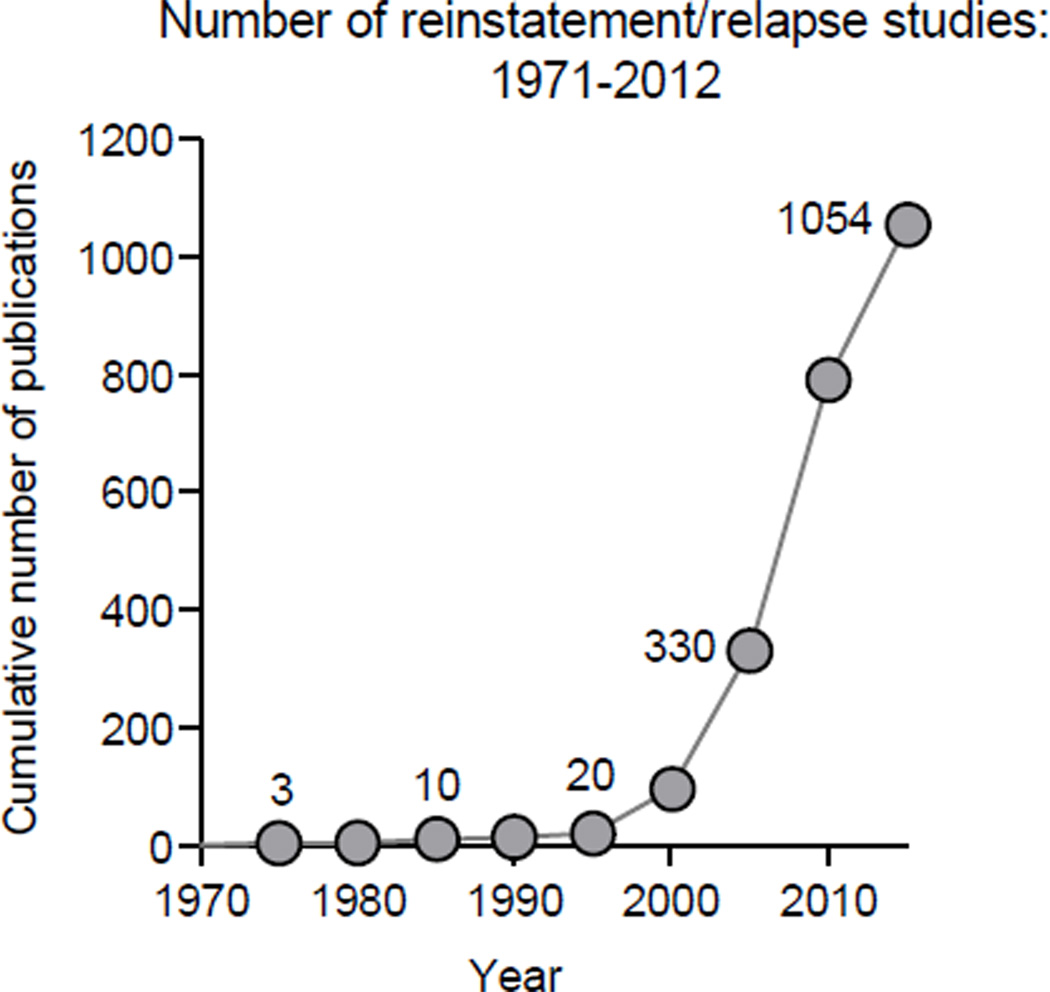
A main problem in the treatment of drug addiction is relapse to drug use after periods of abstinence (Hunt et al. 1971; O'Brien 2005). In drug addicts, drug relapse and craving during abstinence are often triggered by acute reexposure to the self-administered drug (de Wit 1996), drug-associated cues and contexts (O'Brien et al. 1992), or certain stressors (Sinha et al. 2011). This clinical scenario has been studied over the last two decades by using a reinstatement model (Shaham et al. 2003) in laboratory animals tested for reinstatement of drug seeking induced by drug priming (de Wit and Stewart 1981), discrete cues (Meil and See 1996), discriminative cues (Weiss et al. 2000), contextual cues (Crombag and Shaham 2002), or exposure to certain stressors (Shaham and Stewart 1995) (see Table 1 for glossary of terms). This phenomenological similarity or face validity, as well as the reliability of the reinstatement model in detecting reinstatement induced by these stimuli, has led to a dramatic increase in the use of this model in the addiction field (Fig. 1).
Table 1.
Glossary of terms
| Context-induced reinstatement: laboratory animals are first trained to self-administer a drug in an environment (termed context A) associated with a specific set of “background” stimuli (e.g., operant conditioning chamber fan, time of day, visual cues, tactile cues). Lever pressing is then extinguished in a different environment (termed context B) with a different set of “background” stimuli. During reinstatement testing under extinction conditions, exposure to context A previously paired with the drug reinstates lever responding responding. The procedure is based on a “renewal” procedure that has been used to assess the role of contexts in resumption of conditioned responses to aversive and appetitive cues after extinction (Bouton and Swartzentruber 1991). |
| Discrete cues-induced reinstatement: laboratory animals are first trained to self-administer a drug; each drug delivery is temporally paired with a discrete cue (e.g., tone, light). Lever pressing is then extinguished in the absence of the drug and the cue. During reinstatement testing, exposure to the discrete cue, which is earned contingently during testing, reinstates lever responding. |
| Discriminative cues-induced reinstatement: laboratory animals are trained to self-administer a drug in the presence of distinct discriminative stimuli (e.g., visual cues, olfactory cues); one set of stimuli signals drug availability (S+) and the other signals unavailability (S-). Lever pressing is then extinguished in the absence of the discriminative stimuli and the drug. During the reinstatement test, re-exposure to the S+, but not S-, reinstates operant conditioned responding. |
| Drug-priming-induced reinstatement: laboratory animals are first trained to self-administer a drug; typically each drug delivery is paired with a discrete cue. Lever pressing is then extinguished in the presence of the discrete cue. During reinstatement testing under extinction conditions (typically in the presence of the discrete cue), pre-session non-contingent priming injections of the previously self-administered drug or related drugs reinstate lever responding. |
| Reinstatement model: An animal model of drug relapse in which laboratory animals are tested for reinstatement of drug seeking induced by drug-priming, discrete cues, discriminative cues, contextual cues, or certain stressors, following drug self-administration training (typically lever-press or nose-poke for drug infusions) and subsequent extinction of the drug-reinforced responding. Less common procedural variations of the reinstatement model are operant-conditioning-based runway (Ettenberg et al. 1996) and Pavlovian-conditioning-based conditioned place preference procedures (Mueller and Stewart 2000). |
| Stress-induced reinstatement: laboratory animals are first trained to self-administer a drug; typically each drug delivery is temporally paired with a discrete cue. Lever pressing is then extinguished in the presence of the discrete cue. During reinstatement testing under extinction conditions (in the presence of the discrete cue), pre-session exposure to certain stressors (typically intermittent footshock or yohimibine injection) reinstates lever responding. |
Figure 1. Cumulative number of reinstatement/relapse studies from 1971 to 2012 (source: PubMed).
Also included in the figure are a much smaller number of studies on incubation of drug craving (Pickens et al. 2011), a phenomenon originally demonstrated in studies using the reinstatement model (Grimm et al. 2001; Neisewander et al. 2000), and studies on reinstatement of palatable food seeking that are based on the adaptation of the drug reinstatement model for the study of relapse to food seeking during dieting (Calu et al. 2013; Nair et al. 2009).
Face validity alone is not a sufficient condition for a valid animal model (Geyer and Markou 1995; Sarter and Bruno 2002). Importantly, evidence is emerging supporting the predictive validity of the reinstatement model for the treatment of relapse to heroin, alcohol, and nicotine: naltrexone, acamprosate, buprenorphine, methadone, or varenicline, which decrease drug relapse in humans, also decrease drug-priming or cue-induced reinstatement of drug seeking in rats (Bachteler et al. 2005; Le et al. 1999; Leri et al. 2004; Liu and Weiss 2002; O'Connor et al. 2010; Sorge et al. 2005); but see Liu et al. (2008) for a negative finding with bupropion, a medication for nicotine addiction. However, the predictive validity of the reinstatement model for relapse to cocaine and methamphetamine has not been established, primarily because effective medications for psychostimulant addiction and relapse are yet to be identified 1.
In this review, we first summarize recent neurobiological findings from preclinical studies using the reinstatement model. Due to space limitations, we discuss only selected mechanistic studies on brain sites and circuits that mediate the different types of reinstatement as assessed in the operant conditioning version of the reinstatement model (Shaham et al. 2003; Shalev et al. 2002). We then discuss the impact of interfering with putative reconsolidation processes on cue- and context-induced reinstatement of drug seeking, summarize similarities and differences in mechanisms of reinstatement across drug classes, and conclude by discussing recent translational human studies based on results from the reinstatement model.
2. Recent neurobiological findings
Below we discuss recent neurobiological findings from studies using the reinstatement model. Table 2 provides a summary of the main findings since 2009. Figure 2 (end of Section 2) provides a more general historical summary of brain areas involve in relapse to drug seeking, as assessed in studies using the reinstatement model since 1984 (Stewart 1984).
Table 2.
Summary of main neurobiological findings from reinstatement studies conducted from 2009 to 20112
| Cocaine | Heroin | Alcohol | Meth amphetamine |
Nicotine | |
|---|---|---|---|---|---|
|
Drug priming |
mGluR2/3, mGluR5, mGluR7, GluA2 in accumbens core |
D1 dopamine receptor in dorsal mPFC |
Dorsal mPFC and accumbens core neuronal activity |
Granular insular cortex activity |
|
| CREB, Adenosine A(2A) and mu opioid receptors in accumbens |
NR2B NMDA receptors in accumbens core |
||||
| mGluR7 in ventral pallidum | |||||
| Glutamatergic and cholinergic projections from PPTg/LDTg to VTA |
|||||
| Dorsolateral striatum neuronal activity |
|||||
|
Discrete cues |
BNST neuronal activity | D1 dopamine receptor in dorsal mPFC |
Dorsal and ventral mPFC neuronal activity |
Granular insular cortex activity |
|
| mGluR2/3, mGluR5 in accumbens core; mGluR5 in basolateral amygdala |
Accumbens core neuronal activity |
||||
| mTOR signaling in accumbens core |
|||||
| Hypocretin 1 and glutamate receptors in VTA |
|||||
| 5-HT2A, 5-HT2C receptors in ventral mPFC |
|||||
| Acute cue-induced synaptic strength and dendritic spine changes in accumbens core |
|||||
|
Discrim- inative cues |
Hypocretin 1 receptors in VTA |
NPS in lateral hypothalamus |
|||
| NPS in lateral hypothalamus and perifornical area |
|||||
| Context | Glutamate receptors in accumbens core and shell |
Ventral mPFC neuronal activity | mu opioid receptors in basolateral amygdala |
||
| mGluR1 in dorsal hippocampus |
Projections from ventral mPFC to accumbens shell |
D1 dopamine receptors and neuronal activity in accumbens core and shell |
|||
| Ventral hippocampus activity | Ventral subiculum neuronal activity |
PVT and LH | |||
| Reciprocal projections from lateral OFC to basolateral amygdala |
Projections from PVT to accumbens shell and accumbens shell to LH |
||||
| Projection from dorsal hippocampus to VTA via caudal lateral septum |
|||||
| Stress | VTA CRF and CRF1 receptors (footshock) |
Glutamate receptors in VTA (footshock) |
CRF receptors in median raphe and glucocorticoid receptors in central amygdala (yohimbine) |
Alpha-2 adreno- ceptors in central amygdala (footshock) |
|
| VTA kappa opioid receptors (cold swim stress) |
D1 dopamine receptor in accumbens shell, dorsal mPFC, and basolateral amygdala (1 d food deprivation) |
||||
| BNST neuronal activity (yohimbine) |
|||||
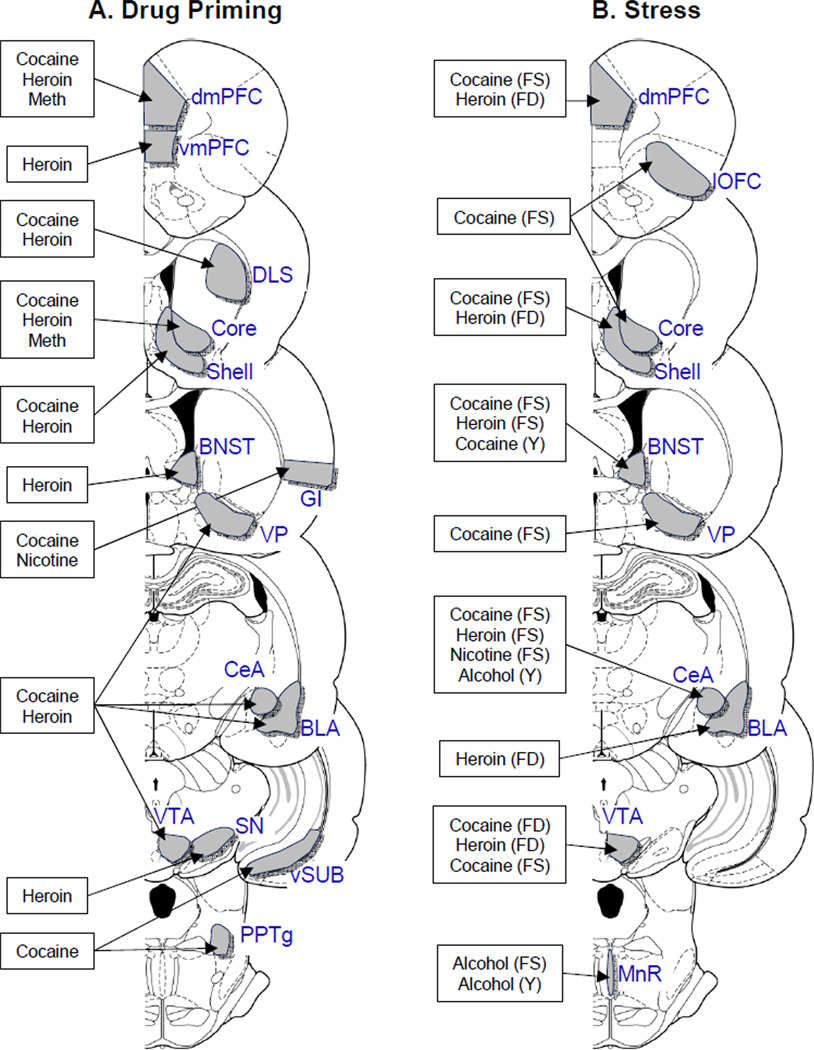
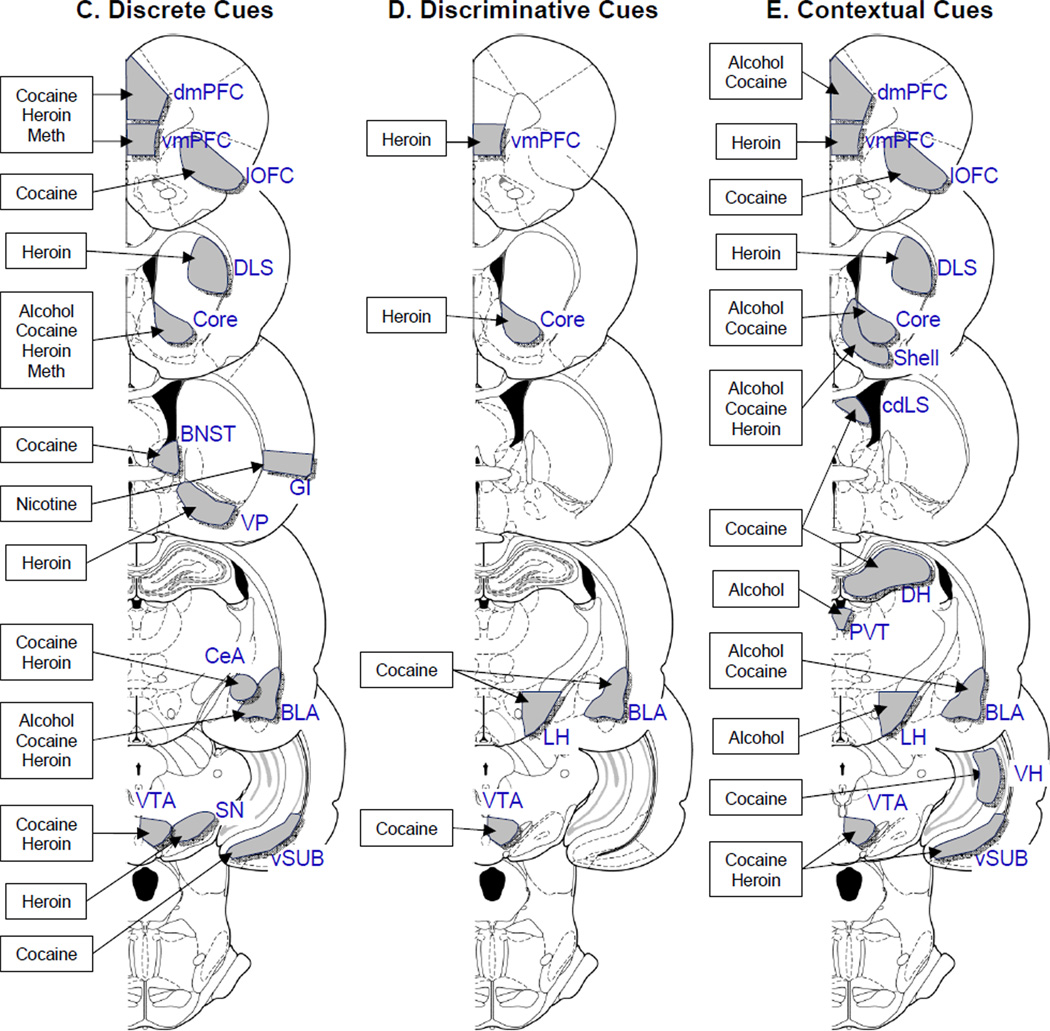
Figure 2. Brain areas involved in reinstatement of drug seeking.
Coronal sections of the rat brain (Paxinos and Watson 2005) depicting brain areas implicated in reinstatement of drug seeking induced by drug-priming (A), certain stressors (B), and discrete (C), discriminative (D), and contextual cues (E). Data only include studies using the operant self-administration variation of the reinstatement model in which brain lesions, reversible inactivation, and intracranial drug injection methods were used. The drug label indicates the self-administered drug (A, alcohol; C, cocaine; H, heroin; M, methamphetamine. The citation list is limited to original empirical findings within a given drug class and reinstating stimulus.
Abbreviations of brain areas: BLA, Basolateral nucleus of the Amygdala; BNST; bed nucleus of the stria terminalis; CeA, Central nucleus of the Amygdala; cd-LS, Lateral Septum, caudodorsal Lateral Septum; DH, dorsal hippocampus; DLS, dorsolateral striatum; dmPFC and vmPFC, dorsal and ventral medial prefrontal cortex; GI, Granular Insular Cortex; LH, Lateral Hypothalamus; lOFC, lateral Orbitofrontal Cortex; MnR, Median Raphe; PPTg, Pedunculo-pontine Tegmental nucleus; PVT, Paraventricular Thalamus; SN, substantia nigra; VH, ventral hippocampus; vSUB, ventral subiculum; VP, ventral pallidum; VTA, ventral tegmental area.
Other abbreviations: A, alcohol; C, cocaine; H, heroin; M, methamphetamine; N, nicotine; FD, food deprivation; FS, footshock; CS, cold swim; Y, yohimbine.
(A) Drug priming: Dorsal mPFC: cocaine (McFarland and Kalivas 2001), heroin (Rogers et al. 2008), methamphetamine (Rocha and Kalivas 2010). Ventral mPFC: cocaine (Pentkowski et al. 2010), heroin (Rogers et al. 2008). Dorsolateral striatum: cocaine (Gabriele and See 2011), heroin (Rogers et al. 2008). Accumbens: cocaine (Self et al. 1998), heroin (Stewart and Vezina 1988). Accumbens core: cocaine (Cornish et al. 1999), heroin (Rogers et al. 2008), methamphetamine (Rocha and Kalivas 2010). Accumbens shell: cocaine (Anderson et al. 2003), heroin (Rogers et al. 2008). BNST: heroin (Rogers et al. 2008). Granular insular cortex: cocaine (Alleweireldt et al. 2006), nicotine (Forget et al. 2010). Ventral pallidum: cocaine (McFarland and Kalivas 2001), heroin (Rogers et al. 2008). Central amygdala: cocaine (Alleweireldt et al. 2006), heroin (Rogers et al. 2008). Basolateral amygdala: cocaine (Alleweireldt et al. 2006), heroin (Rogers et al. 2008). Substantia nigra: heroin (Rogers et al. 2008). VTA: cocaine (McFarland and Kalivas 2001; Stewart 1984), heroin (Stewart 1984). Ventral subiculum: cocaine (Sun and Rebec 2003; Vorel et al. 2001). PPTg/LDT: cocaine (Schmidt et al. 2009).
(B) Stress: Dorsal mPFC: Footshock: cocaine (Capriles et al. 2003; McFarland et al. 2004); Food deprivation: heroin (Tobin et al. 2013). Lateral OFC: Footshock: cocaine (Capriles et al. 2003). Accumbens: Footshock: cocaine (Xi et al. 2004). Accumbens core: Footshock: cocaine (McFarland et al. 2004). Accumbens shell: Footshock: cocaine (McFarland et al. 2004). Food deprivation: heroin (Tobin et al. 2013). BNST: Footshock: cocaine (Erb and Stewart 1999), heroin (Shaham et al. 2000b); Yohimbine: cocaine (Buffalari and See 2011). Ventral pallidum: Footshock: cocaine (McFarland et al. 2004). Central amygdala: Footshock: cocaine (Erb and Stewart 1999), heroin (Shaham et al. 2000a), nicotine (Yamada and Bruijnzeel 2011); Yohimbine: Alcohol (Simms et al. 2012). Basolateral amygdala: Food deprivation: heroin (Tobin et al. 2013). VTA: Footshock: cocaine (McFarland et al. 2004; Wang et al. 2005), heroin (Wang et al. 2012); Swim stress: (Graziane et al. 2013). Median Raphe: Footshock: alcohol (Le et al. 2002); yohimbine: alcohol (Le et al. 2012).
(C) Discrete cues: Dorsal mPFC: cocaine: (McLaughlin and See 2003), heroin (Rogers et al. 2008), methamphetamine (Rocha and Kalivas 2010). Ventral mPFC: cocaine: (Pentkowski et al. 2010), heroin (Rogers et al. 2008), methamphetamine (Rocha and Kalivas 2010). Lateral OFC: cocaine: (Fuchs et al. 2004b). Dorsolateral striatum: heroin (Rogers et al. 2008). Accumbens core: alcohol (Sinclair et al. 2012), cocaine (Fuchs et al. 2004a), heroin (Bossert et al. 2007), methamphetamine (Rocha and Kalivas 2010). BNST: cocaine (Buffalari and See 2011). Granular insular cortex: nicotine (Forget et al. 2010). Ventral pallidum: heroin (Rogers et al. 2008). Central amygdala: cocaine (Kruzich and See 2001), heroin (Rogers et al. 2008). Basolateral amygdala: alcohol (Sinclair et al. 2012), cocaine (Meil and See 1996), heroin (Rogers et al. 2008). Substantia nigra: heroin (Rogers et al. 2008). VTA: cocaine (Mahler et al. 2012b), heroin (Rogers et al. 2008). Ventral subiculum: cocaine (Sun and Rebec 2003).
(D) Discriminative cues: Ventral mPFC: heroin (Alvarez-Jaimes et al. 2008). Accumbens core: heroin (Alvarez-Jaimes et al. 2008). Basolateral amygdala: cocaine (Yun and Fields 2003). Lateral Hypothalamus: cocaine (Kallupi et al. 2012). VTA: cocaine (James et al. 2011).
(E) Contextual cues: Dorsal mPFC: alcohol (Willcocks and McNally 2013), cocaine (Fuchs et al. 2005). Ventral mPFC: heroin (Bossert et al. 2011). Lateral OFC: cocaine (Lasseter et al. 2011). Dorsolateral striatum: heroin (Bossert et al. 2009). Accumbens: cocaine (Fuchs et al. 2008). Accumbens core: alcohol (Chaudhri et al. 2009). Accumbens shell: alcohol (Chaudhri et al. 2009), heroin (Bossert et al. 2007). Caudo-dorsal lateral septum: cocaine (Luo et al. 2011). Dorsal Hippocampus: cocaine (Luo et al. 2011). Paraventricular thalamus: alcohol (Hamlin et al. 2009). Basolateral amygdala: alcohol (Marinelli et al. 2010), cocaine (Fuchs et al. 2005). Lateral Hypothalamus: alcohol (Marchant et al. 2009). VTA: cocaine (Luo et al. 2011), heroin (Bossert et al. 2004). Ventral hippocampus: cocaine (Lasseter et al. 2010). Ventral subiculum: heroin (Bossert and Stern 2013).
Drug priming
Summary of main findings prior to 2009
Early pioneering studies of Stewart and colleagues (Stewart 1984; Stewart and Vezina 1988) and subsequent studies (Anderson et al. 2003; Bachtell et al. 2005) indicate that dopamine neurons in ventral tegmental area (VTA) that project to nucleus accumbens shell play a critical role in reinstatement of drug seeking induced by cocaine priming and possibly heroin priming as well (Schmidt et al. 2005; Shalev et al. 2002). Over the last 12 years, Kalivas and colleagues have identified a critical role of glutamate projections from dorsal (prelimbic, anterior cingulate) medial prefrontal cortex (mPFC) to accumbens core in reinstatement induced by both cocaine priming (Cornish and Kalivas 2000; Kalivas and McFarland 2003) and heroin priming (LaLumiere and Kalivas 2008). Previous studies using reversible inactivation methods and local injections of dopamine receptor antagonists have also identified a role of other brain areas in cocaine-priming-induced reinstatement, including ventral subiculum (Sun and Rebec 2003), ventral pallidum (McFarland and Kalivas 2001), and central and basolateral amygdala (Alleweireldt et al. 2006). A previous study by Rogers et al. (2008) in which the authors used a muscimol-baclofen reversible inactivation procedure (McFarland and Kalivas 2001), indicate a role of many brain areas in heroin-priming-induced reinstatement, including all of the above mentioned areas, as well as substantia nigra, bed nucleus of stria terimalis (BNST), and mediodorsal thalamus (MDT). More recent studies (since 2009) have expanded on the above findings.
Cocaine priming
Glutamate-related mechanisms in nucleus accumbens and ventral pallidum
Kalivas and colleagues further explored glutamatergic mechanisms in accumbens core that mediate cocaine-priming-induced reinstatement. In previous studies, they reported that cocaine self-administration decreases basal glutamate levels in accumbens and that reversing this effect by systemic injections of N-acetylcysteine (NAC), a cystine prodrug that increases extracellular glutamate by stimulating the glial cystine/glutamate exchanger, decreases cocaine-priming-induced synaptic glutamate release in accumbens and reinstatement of drug seeking (Baker et al. 2003). Kupchik et al. (2012) replicated this effect and also reported that accumbens injections of NAC mimic the systemic effect of the drug on basal glutamate levels and cocaine-priming-induced reinstatement. These authors performed additional in vivo and in vitro mechanistic studies, which point towards a role for both mGluR2/3 and mGluR5 in the effect of NAC on accumbens glutamate transmssion and reinstatement.
Mahler et al. (2012a) provided additional support for a role of accumbens glutamate in cocaine-priming-induced reinstatement. They reported that systemic injections of modafinil, a cognitive enhancer that increases extracellular dopamine (Madras et al. 2006), decreases cocaine-priming-induced reinstatement. This is an unexpected finding because systemic injections of dopamine reuptake blockers typically reinstate cocaine seeking (De Vries et al. 1998; Schmidt et al. 2005; Spealman et al. 1999). However, Mahler et al. (2012a) also reported that, like NAC, modafinil prevents the reduction in basal levels of glutamate in accumbens after withdrawal from cocaine, which, as discussed above, is the mechanism underlying NAC’s effect on cocaine-priming-induced reinstatement. The authors suggested that the similar effect of NAC and modafinil on cocaine-priming-induced reinstatement is due to the fact that the both drugs normalize tonic extrasynaptic glutamate transmission, controlled by the glial glutamate-cystine exchanger, leading to stimulation of mGluR2 located on presynaptic neurons, a cellular event that prevents the acute effect of cocaine priming on synaptic glutamate release, which mediates cocaine-priming-induced reinstatement (Kupchik et al. 2012; Mahler et al. 2012a).
There is also evidence for a role of accumbens core mGluR5 and mGluR7, and ventral pallidum mGluR7 in cocaine-priming-induced reinstatement. Injections of mGluR5 antagonists into accumbens core (Wang et al. 2013) or shell (Kumaresan et al. 2009) decrease cocaine-priming-induced reinstatement. In a comprehensive study, Li et al. (2010) found that injections of an mGluR7 allosteric agonist into accumbens core or ventral pallidum decrease cocaine-priming-induced reinstatement. The mGluR7 agonist also caused a slow-onset long-lasting increase in extracellular glutamate in accumbens and blocked both cocaine-priming-induced increases in accumbens glutamate release and cocaine-induced reinstatement; these effects were reversed by an mGluR7 antagonist or an mGluR2/3 antagonist. Thus, it appears that the effect of the mGluR7 allosteric agonist on cocaine-priming-induced reinstatement is mediated by a mechanism similar to the one mediating the effect of NAC on this reinstatement.
Two other molecular mechanisms associated with glutamate receptor signaling and trafficking have been implicated in cocaine-priming-induced reinstatement. Wang et al. (2013) reported that accumbens core injections of a compound that block Homer binding with mGluR5 receptors, which normally promote signaling at this receptor, decreases this reinstatement. Wiggins et al. (2011) reported that activation of accumbens core integrins signaling, which is associated with GluA2-containing AMPA receptor trafficking, decreases cocaine-priming-induced reinstatement. A question for future research is whether the two findings are mechanistically connected to each other or to the glutamatergic mechanism normalized by NAC that mediates cocaine-priming-induced reinstatement (Baker et al. 2003).
Other mechanisms in nucleus accumbens
Mu opioid receptors (MOR)
Simmons and Self (2009) provided evidence implicating accumbens MORs in cocaine-priming-induced reinstatement. They reported that local injections of the selective MOR agonist DAMGO or beta-endorphin (which preferentially binds to MOR; (Akil et al. 1984)) reinstated cocaine seeking while local injections of the selective MOR antagonist CTAP decreased cocaine-priming-induced reinstatement.
Adenosine A2A receptors
O'Neill et al. (2012) provided evidence implicating accumbens adenosine A2A receptors, which co-localize with D2-family dopamine receptors in the striatum (Ferre et al. 1997), in cocaine-priming-induced reinstatement. They reported that local injections of the A2A receptor antagonist MSX-3 reinstated cocaine seeking while the A2A receptor agonist CGS 21680 decreased cocaine-priming-induced reinstatement. These data extend earlier results of Bachtell et al. (2009) on the inhibitory effect of systemic injections of CGS 21680 on this reinstatement.
CREB (cAMP response element binding protein)
There is evidence that CREB activity in accumbens shell negatively modulates cocaine reward, as assessed in the CPP procedure (Carlezon et al. 2005). Recently, Larson et al. (2011) assessed the effect of viral-mediated CREB overexpression or down-regulation in accumbens on cocaine self-administration and reinstatement induced by drug priming, discrete cues, and intermittent footshock. They found that CREB overexpression increases the rewarding effects of cocaine (indicated by a shift to the left in the dose-response curve and increased progressive ratio responding) while CREB down-regulation has the opposite effect. However, while CREB overexpression potentiated cocaine-priming-induced reinstatement at a higher (10 mg/kg) but not a lower (5 mg/kg) dose, overexpression had no effect on discrete-cue- or footshock-induced reinstatement. Additionally, down-regulation of accumbens CREB, which decreases endogenous CREB activity, had no effect on reinstatement induced by cocaine priming, discrete cues, or footshock. Taken together, the results of this comprehensive study established a critical role of accumbens CREB activity in ongoing cocaine self-administration but not cocaine-induced reinstatement. This conclusion is in agreement with results from previous studies demonstrating dissociable mechanisms of cocaine self-administration versus reinstatement (Kalivas and McFarland 2003; Shalev et al. 2002). The Larson et al. (2011) study is also of general interest to the addiction field, because to our knowledge, the study provides the first demonstration of opposite modulation of drug reward in the same brain area by an intracellular protein in the two most widely used behavioral procedures to assess drug reward: CPP and drug self-administration.
Glutamate transmission in VTA and PPTg/LDTg
Schmidt et al. (2009) reported that injections of the AMPA/kainate receptor antagonist CNQX into pedunculopontine tegmental nucleus (PPTg) and laterodorsal tegmental nucleus (LDTg) decrease cocaine-priming-induced reinstatement; PPTg and LDTg nuclei send glutamatergic and cholinergic projections to VTA (Geisler et al. 2007). These authors also found that VTA injections of CNQX, as well as scopolamine and mecamylamine, muscarinic and nicotinic acetylcholine receptor antagonists, respectively, decrease cocaine-priming-induced reinstatement (Schmidt et al. 2009). These findings suggest that the PPTg/LDTg projection to VTA plays a role in cocaine-priming-induced reinstatement.
5-HT transmission in mPFC
Pentkowski et al. (2010) reported that injections of the 5-HT2C receptor agonist MK212 into prelimbic or infralimbic mPFC decreased cocaine-priming-induced reinstatement, an effect reversed by local injections of the 5-HT2C receptor antagonist SB242084. In contrast, MK212 injections into the anterior cingulate area (part of the dorsal mPFC) were ineffective. To our knowledge these data are the first to implicate ventral mPFC areas (infralimbic and ventral part of prelimbic) in cocaine-priming-induced reinstatement.
Heroin, methamphetamine, and nicotine priming
Heroin priming
During the last three years, two studies have assessed brain mechanisms of heroin-priming-induced reinstatement. See (2009) reported that blockade of dopamine D1 receptors in dorsal mPFC decreases this reinstatement, providing additional support for the role of this brain area in heroin-priming-induced reinstatement (Rogers et al. 2008). Shen et al. (2011) found that heroin priming causes LTP-like changes in field excitatory postsynaptic potentials in accumbens core neurons after mPFC stimulation, and reverses the decrease in accumbens core spine density after extinction training (prior to reinstatement). The authors then provided a functional significance for these results by reporting that either blockade of accumbens core NR2B-containing NMDA receptors or reduction in accumbens NR2B gene expression decreases heroin-priming-induced reinstatement. The results of this elegant study extend earlier findings of LaLumiere et al. (2008) on the role of glutamate projections from dorsal mPFC to accumbens core in heroin-priming-induced reinstatement.
Methamphetamine priming
Rocha and Kalivas (2010) used a muscimol-baclofen reversible inactivation procedure to study the role of mPFC and accumbens sub-regions in methamphetamine-priming-induced reinstatement. They reported that, like cocaine priming (McFarland and Kalivas 2001) this reinstatement is decreased by inactivation of either dorsal mPFC or accumbens core but not ventral mPFC or accumbens shell.
Nicotine priming
Le Foll and colleagues reported that pharmacological (muscimol+baclofen) or electrical inactivation of the granular insula decreases nicotine-priming-induced reinstatement (Forget et al. 2010; Pushparaj et al. 2013). These data, and similar results described below on the effect of these manipulations on discrete-cue-induced reinstatement of nicotine seeking, indicate an important role of the granular insula cortex in reinstatement of nicotine seeking.
Conclusions
The results from the studies reviewed above provide additional mechanistic insight on the role of glutamate-related mechanisms in accumbens core in drug-priming-induced reinstatement of cocaine and heroin seeking. The results from these studies also demonstrate a role of glutamate transmission in ventral pallidum, VTA, and PPTg/LDTg. There is also evidence for a role of accumbens MOR and adenosine A2A receptors, as well as 5-HT transmission in both ventral and dorsal mPFC regions in cocaine-priming-induced reinstatement. Recent evidence also implicates dorsal mPFC and accumbens core in methamphetamine-priming induced reinstatement, dorsal mPFC dopamine in heroin-priming-induced reinstatement, and granular insula cortex in nicotine priming-induced reinstatement.
Discrete cues
Summary of main findings prior to 2009
Previous studies reported that reversible inactivation (muscimol+baclofen or tetrodotoxin) of dorsal (but not ventral) mPFC, accumbens core (but not shell), BLA and CeA, or dorsolateral striatum decreases discrete cue-induced reinstatement of cocaine seeking (Feltenstein and See 2008; See 2005). Previous studies also reported that reversible inactivation of these brain areas, as well as dorsal (but not ventral) BNST, ventral mPFC, ventral pallidum, or substantia nigra decrease discrete-cue-induced reinstatement of heroin seeking (Feltenstein and See 2008; Rogers et al. 2008; See 2005). Below, we discuss recent findings on mechanisms of discrete-cue-induced reinstatement of drug seeking.
Role of glutamate in accumbens, VTA, and mPFC in discrete-cue-induced reinstatement of cocaine seeking
Kalivas and colleagues reported that accumbens core injections of NAC or a Homer-mGluR5 binding antagonist (that inhibits glutamate transmission) decrease discrete-cue-induced reinstatement of cocaine seeking (Kupchik et al. 2012; Wang et al. 2013). This group also demonstrated that the inhibitory effect of systemic NAC injections on cue-induced cocaine seeking is reversed by accumbens core injections of an mGluR2/3 antagonist, indicating a role of these receptors (presumably mGluR2 located on presynaptic glutamatergic neurons) in the inhibitory effect of NAC (see discussion in the drug priming section) on cue-induced cocaine seeking (Moussawi et al. 2011). Furthermore, using optogenetic procedures, they also reported that inhibition of glutamatergic projections from dorsal mPFC to accumbens core decreases this reinstatement (Stefanik et al. 2013). Finally, Sinclair et al. (2012) reported that blockade of mGluR5 in accumbens core (or basolateral amygdala) decreases discrete-cue-induced reinstatement of cocaine seeking.
Mahler et al. (2012b) reported that blockade of VTA AMPA receptors decreases discrete-cue-induced reinstatement. A question that arises from these results is which glutamatergic projection/s to VTA (Geisler et al. 2007) are activated during the reinstatement test. Mahler and Aston-Jones (2012) addressed this question using retrograde tracer injections into VTA (cholera toxin B, CTb) in combination with detection of Fos-activated neurons after the discrete-cue-induced reinstatement test. Double-labeling (co-expression) of CTb with Fos in VTA projection areas was assessed, and the double-labeling data suggest that projections (presumably glutamatergic) from lateral hypothalamus and BNST, but not mPFC, are activated during the reinstatement test. The functional role of these projections in discrete-cue-induced reinstatement is a subject for future research.
Role of mPFC serotonin in discrete-cue-induced reinstatement of cocaine seeking
Neisewander and colleagues have demonstrated an unexpected role of ventral but not dorsal mPFC serotonin in discrete-cue-induced reinstatement of cocaine seeking (Pentkowski et al. 2010; Pockros et al. 2011). They found that injections of a 5-HT2C agonist into ventral, but not dorsal, mPFC or a 5-HT2A antagonist into ventral mPFC decrease this reinstatement. They proposed that the net effect of their pharmacological manipulations is inhibition of glutamatergic projection neurons, because 5-HT2C receptors are located primarily on GABA interneurons (Liu et al. 2007) whereas 5-HT2A receptors are primarily located on glutamatergic projection neurons (Willins et al. 1997). However, in the absence of follow-up studies on the projection areas involved in the inhibitory effect of the serotonergic drugs in ventral mPFC, the findings should be interpreted with caution, especially in reference to the glutamatergic projection from this brain area to accumbens shell (Sesack et al. 1989). This is because, as mentioned above, reversible inactivation of accumbens core but not shell decreases discrete-cue-induced reinstatement of cocaine seeking (See 2005). The data of Neisewander and colleagues are also surprising because of the previous finding that reversible inactivation of dorsal but not ventral mPFC decreases this reinstatement (See 2005). However, dissociable neuroanatomical effects on reinstatement by reversible inactivation procedures versus selective pharmacological drugs (dopamine receptor antagonists) have also been demonstrated for accumbens core versus shell (Anderson et al. 2003; McFarland and Kalivas 2001). Thus, conclusions from circuitry-based studies that rely only on reversible inactivation procedures should be made with caution.
Other mechanisms of discrete-cue-induced reinstatement of drug seeking
Cocaine
Buffalari and See (2011) reported that reversible inactivation of dorsal BNST also decreases discrete-cue-induced reinstatement of cocaine seeking. Mahler et al. (2012b) reported that blockade of VTA hypocretin 1 receptor decreases discrete-cue-induced reinstatement of cocaine seeking. Wang et al. (2010) reported a role of accumbens core mammalian target of rapamycin signaling pathway (mTOR) in discrete-cue-induced reinstatement of cocaine seeking. Finally, in a recent study, Gipson et al. (2013) reported that discrete-cue-induced cocaine seeking rapidly (within 15 min) enlarges spine head diameter in accumbens core and increases synaptic strength, as assessed by measuring AMPA/NMDA current ratio in whole-cell patch recordings in accumbens slices. These authors also demonstrated that muscimol-baclofen inactivation of dorsal mPFC reversed both discrete-cue-induced reinstatement and acute cue-induced changes in dendritic spines and AMPA/NMDA current ratio. These data indicate that activation of dorsal mPFC-to-accumbens core glutamatergic projection mediates the acute cue-induced morphological changes and synaptic strength in accumbens core.
Heroin, methamphetamine, and nicotine
See (2009) reported that blockade of D1-family in receptors in dorsal mPFC decreases discrete-cue-induced reinstatement of heroin seeking. Rocha (2010) reported that reversible inactivation of accumbens core (but not shell), or dorsal or ventral mPFC, decreases discrete-cue-induced reinstatement of methamphetamine seeking. Forget et al. (2010) and Pushparaj et al. (2013) reported that reversible inactivation of granular insular cortex decreases discrete-cue-induced reinstatement of nicotine seeking.
Summary
As with drug priming-induced reinstatement, glutamate transmission in accumbens core plays an important role in discrete cue-induced-reinstatement of cocaine seeking, and a recent study demonstrates that the glutamatergic projection from mPFC to accumbens core is critical for this reinstatement. Recent data also indicate that glutamate projections from mPFC to accumbens mediate discrete-cue-induced rapid changes in dendritic spines and synaptic strength (increased AMPA/NMDA current ratio) in accumbens core that potentially mediate cocaine seeking. Within the ventral mPFC, neuronal activity and serotonin receptors are involved in discrete cue-induced reinstatement of methamphetamine and cocaine seeking, respectively. In the dorsal mPFC, D1 receptors are critical for discrete-cue-induced reinstatement of heroin seeking. Outside of the PFC to accumbens pathway, glutamate transmission in VTA plays a role in discrete cue-induced-reinstatement of cocaine seeking, and neuronal activity in the BNST and granular insula cortex contributes to such reinstatement of cocaine and nicotine seeking, respectively.
Discriminative cues
Summary of main findings prior to 2009
Previous studies in which investigators used Fos expression, systemic injections of dopamine receptor antagonists, and dopamine microdialysis suggest a role of accumbens core, mPFC, and basolateral amygdala in discriminative-cue-induced reinstatement of cocaine seeking (Weiss 2005); additionally, basolateral amygdala lesions decrease this reinstatement (Yun and Fields 2003). Discriminative-cue-induced reinstatement of heroin seeking is decreased by blockade of CB1 receptors in ventral mPFC or accumbens core, but not basolateral amygdala (Alvarez-Jaimes et al. 2008).
Role of hypocretin and neuropeptide S (NPS)
During the last three years, evidence has emerged for a role of hypocretin (de Lecea et al. 1998) and NPS (Xu et al. 2004) in discriminative-cue-induced reinstatement of drug seeking. James et al. (2011) reported that blockade of hypocretin 1 receptors in VTA but not paraventricular thalamus (PVT) decreases discriminative-cue-induced reinstatement of cocaine seeking. Additional evidence for a role of hypocretin in this reinstatement was demonstrated in the studies of Ciccocioppo and colleagues on the role of NPS in discriminative cue-induced reinstatement of drug seeking (Cannella et al. 2009; Kallupi et al. 2010; Kallupi et al. 2012).
Kallupi et al. (2010) reported that ventricular injections of NPS induce Fos in hypocertin 1 neurons in lateral hypothalamus, perifornical area, and dorsomedial hypothalamus. NPS injections into these areas potentiated discriminative-cue-induced reinstatement of cocaine seeking, with the most potent effect occurring with lateral hypothalamus injections (Kallupi et al. 2010). NPS injections into the lateral hypothalamus also potentiated discriminative-cue-induced reinstatement of alcohol seeking; this effect was reversed by systemic injections of hypocretin 1 receptor antagonist (Cannella et al. 2009; Kallupi et al. 2010). Subsequently, these authors reported that injections of an NPS receptor antagonist into lateral hypothalamus and perfornical area decrease discriminative-cue-induced reinstatement of cocaine seeking, demonstrating a role of endogenous NPS receptors in this reinstatement (Kallupi et al. 2012).
Context
Summary of main findings prior to 2009
Previous studies indicate a role of several brain sites in context-induced reinstatement of drug seeking (Crombag et al. 2008). We have identified a role of glutamate transmission in VTA as well as dopamine and glutamate transmission in accumbens shell in context-induced reinstatement of heroin seeking (Bossert et al. 2006; Bossert et al. 2004; Bossert et al. 2007). Fuchs and colleagues have identified a role of basolateral, dorsal mPFC, dorsal hippocampus, and accumbens core and shell in context-induced reinstatement of cocaine seeking (Fuchs et al. 2007; Fuchs et al. 2005; Fuchs et al. 2008). Studies of McNally and colleagues have suggested a role of the lateral hypothalamus in alcoholic beer and cocaine seeking (Hamlin et al. 2008; Hamlin et al. 2007). During the last three years, investigators have extended these findings. We discuss the new findings separately for heroin, cocaine, and alcohol.
Heroin
Ventral mPFC to accumbens shell projections
We studied the role of the glutamatergic projection from ventral mPFC to accumbens shell in context-induced reinstatement of heroin seeking (Bossert et al. 2011; Bossert et al. 2012). We first found that reversible inactivation of ventral, but not dorsal, mPFC decreases context-induced reinstatement of heroin seeking (Bossert et al. 2011), an effect mimicked by selectively inactivating ventral mPFC Fos-activated neurons using the novel Daun02 inactivation method (Koya et al. 2009). Subsequently, we found that context-induced reinstatement was associated with increased Fos expression in ventral mPFC neurons, including those projecting to accumbens shell (as assessed by double-labeling of Fos with Fluorogold, a retrograde tracer). We confirmed the functional role of this projection in context-induced reinstatement by demonstrating that reversible inactivation of ventral mPFC in one hemisphere combined with dopamine D1 receptor blockade into contralateral or ipsilateral accumbens shell decreases this reinstatement (Bossert et al. 2012).
Dorsolateral striatum and ventral subiculum
We found that blockade of D1-family receptors in dorsolateral (but not dorsomedial) striatum, and reversible inactivation of ventral subiculum, but not posterior CA1 area, decreases this reinstatement (Bossert and Stern 2013; Bossert et al. 2009). The former finding implicates that dopaminergic projection from the substantia nigra to dorsal striatum (Ungerstedt 1971) in context-induced reinstatement. Based on our previous findings above on the critical role of accumbens shell in context-induced reinstatement, the latter finding potentially implicates the glutamatergic projection from ventral subiculum to accumbens shell (Groenewegen et al. 1987) in this reinstatement.
Cocaine
Accumbens, orbitofrontal cortex (OFC), and basolateral amygdala
Fuchs and colleagues continued their studies examining brain mechanisms of context-induced reinstatement of cocaine seeking. Xie et al. (2012) reported that blockade of AMPA/kainite receptors in accumbens core or shell (medial or lateral sub-regions) decreases context-induced reinstatement, extending earlier findings (Fuchs et al. 2008). Lasseter et al. (2009) reported that inactivation of lateral, but not medial, orbitofrontal cortex (OFC) decreases context-induced reinstatement. Lasseter et al. (2011) also used an anatomical disconnection procedure to demonstrate a role of reciprocal projections (both ipsilateral and contralateral) between OFC and basolateral amygdala in this reinstatement.
Dorsal and ventral hippocampus
Lasseter et al. (2010) also reported that inactivation of ventral hippocampus, but not posterior dorsal hippocampus or dentate gyrus, decreases context-induced reinstatement. Xie et al. (2010) reported that blockade of mGlu1 receptors in dorsal hippocampus decreases context-induced reinstatement, extending a previous finding (Fuchs et al. 2005). Additional evidence for a critical role of dorsal hippocampus in context-induced reinstatement is obtained by Luo et al. (2011). They used electrophysiology, anatomical tracing, reversible inactivation, and ‘disconnection’ procedures to demonstrate a role of projections from CA3 dorsal hippocampus area to VTA, via the lateral septum, in context-induced reinstatement of cocaine seeking. Thus, both dorsal and ventral regions of the hippocampus appear to be critical for context-induced reinstatement of cocaine seeking.
Alcohol
McNally and colleagues continued their investigation of the role of sub-regions of hypothalamus and thalamus and their projections in context-induced reinstatement of alcoholic beer seeking in the drug (A) context, as well as extinction of alcohol seeking in the non-drug (B) context (Marchant et al. 2012; Millan et al. 2011).
Role of PVT and its projections to accumbens shell in reinstatement and extinction
Hamlin et al. (2009) reported that PVT lesions decrease context-induced reinstatement; additionally, this reinstatement was associated with activation of PVT projections to accumbens shell (as assessed by double-labeling of Fos-CTb in PVT after CTb injections into accumbens shell). Marchant et al. (2010) used Fos-CTb double-labeling and reported data suggesting a role of projections from medial dorsal hypothalamus (MDH, perifornical and dorsomedial nuclei) prodynorphin/hypocretin neurons to PVT in controlling extinction responding in context B. They also reported that injections of a kappa opioid receptor agonist into PVT blocks reinstatement in context A. Since PVT is critical for context-induced reinstatement (Hamlin et al. 2009), the authors proposed that kappa opioid receptor-mediated inhibition of PVT is important for inhibiting alcohol seeking in the non-drug extinction context.
Role of accumbens shell projections to lateral hypothalamus (LH) in reinstatement and extinction
Marchant et al. (2009) reported that reversible inactivation of LH decreases context-induced reinstatement. Activation of afferents to LH during context-induced reinstatement was assessed using retrograde tracer injections (CTb) into LH in combination with detection of Fos activated neurons. They reported two anatomically and functionally distinct projections: ventral accumbens shell--lateral hypothalamus projections that were activated during context-induced reinstatement testing (in the drug (A) context), and dorsomedial accumbens shell--lateral hypothalamus projections that were activated in the non-drug, extinction (B) context. In a single context design (i.e., rats are trained, undergo extinction, and tested in the same context), Millan et al. (2010) demonstrated a functional role of ventral accumbens shell in extinction-induced inhibition of alcohol seeking. They reported that reversible inactivation of ventral accumbens shell reinstates extinguished alcohol seeking. Accumbens shell inactivation induced Fos in both hypocretin and CART neurons in lateral hypothalamus, and concurrent inactivation of lateral hypothalamus inhibited reinstatement induced by accumbens shell inactivation. The authors speculated that accumbens shell projections to lateral hypothalamus control behavioral inhibition following extinction training by inhibiting lateral hypothalamic neuropeptide expressing neurons, whose activation promotes alcohol seeking.
Role of amygdala projections to accumbens shell in extinction
Millan et al. (2011) also reported that while blockade of AMPA receptors in accumbens shell has no effect on context-induced reinstatement (i.e., responding in context A), this blockade reinstates alcohol seeking in the non-drug extinction (B) context. They then demonstrated that the latter effect can be mimicked by disconnection of glutamatergic projections from basolateral amygdala to accumbens shell. This finding suggests an unexpected role of this projection in inhibition of alcohol seeking, since this projection has previously been implicated in promoting reward seeking (Everitt and Wolf 2002; Kelley and Berridge 2002).
Role of basolateral amygdala and accumbens core and shell in reinstatement
Marinelli et al. (2010) reported that basolateral amygdala, but not dorsal hippocampus, blockade of mu opioid receptors decreases context-induced reinstatement of alcohol seeking. Chaudhri et al. (2009) reported that this reinstatement is decreased by blockade of dopamine D1-family receptors in either accumbens core or shell. Using a Pavlovian approach variation of the ABA renewal procedure (Chaudhri et al. 2008), they also demonstrated that reversible inactivation of both accumbens sub-regions decreases context-induced reinstatement (Chaudhri et al. 2010). The data from these studies lead to different conclusions on the role of accumbens shell and basolateral amygdala in promoting versus inhibiting alcohol seeking than the findings of McNally and colleagues. The reasons for the different results (and conclusions) in these studies are unknown but may be related to the use of different pharmacological agents or drug injections at different rostral-caudal accumbens and basolateral amygdala subregions. It is also possible that McNally and colleagues identify different neurobiological substrates, because their rats are water-restricted in the home-cage and lever press for low alcohol concentrations dissolved in palatable non-alcoholic beer. In contrast, Marinelli et al. (2010) and Chaudhri et al. (2009; 2010) use non-deprived rats that lever press for higher concentrations of alcohol dissolved in water.
Conclusions
The data reviewed indicate that accumbens core and shell glutamate and dopamine receptors play an important role in context-induced reinstatement of cocaine, alcohol, and heroin (shell only) seeking. Other overlapping brain areas include basolateral amygdala (cocaine and alcohol) and ventral subiculum/hippocampus (cocaine and heroin context). The data reviewed also indicate that (1) projections from ventral mPFC to accumbens shell are critical for context-induced reinstatement of heroin seeking, (2) projections from PVT to accumbens shell and accumbens shell to LH play are critical for context-induced reinstatement of alcohol seeking, and (3) reciprocal projections from OFC to basolateral amygdala are critical for context-induced reinstatement of cocaine seeking.
Stress
Summary of main findings prior to 2009
Since the mid-1990s, we and others have used the reinstatement model to study mechanisms of stress-induced reinstatement of drug seeking (Shaham et al. 2000a; Shalev et al. 2010). In the operant conditioning version of the reinstatement model, investigators have used three main stressors: intermittent footshock (Shaham and Stewart 1995), acute one-day food deprivation (Shalev et al. 2000), and yohimbine (Lee et al. 2004; Shepard et al. 2004), an alpha-2 adrenoceptor antagonist that induces stress- and anxiety-like responses in humans and laboratory animals (Bremner et al. 1996a; b). Yohimbine also induces heroin and alcohol craving in drug addicts (Stine et al. 2002; Umhau et al. 2011), and was also recently shown to increase opiate intake in humans in the laboratory (Greenwald et al. 2013). The brain sites and circuits of food-deprivation and yohimbine-induced reinstatement were not explored before 2009. For intermittent footshock-induced reinstatement, results from early studies indicate a role of corticotropin-releasing factor (CRF) and glutamate in VTA (Wang et al. 2005), and a role of CRF projections from central amygdala to BNST, as well as noradrenaline, in these brain areas (Erb et al. 2001; Shaham et al. 2003). Results from studies using reversible inactivation, dopamine receptor antagonists, and microdialysis also indicate a role for multiple brain sites in intermittent-footshock-induced reinstatement of cocaine seeking, including dorsal (but not ventral) mPFC, OFC, accumbens core and shell, and ventral pallidum, as well as the glutamatergic projections from dorsal mPFC to accumbens core (Capriles et al. 2003; McFarland et al. 2004; Xi et al. 2004). Below, we discuss recent studies on brain sites and neurotransmitters involved in intermittent-footshock-, yohimbine-, and food-deprivation-induced reinstatement of drug seeking. We also discuss results from a very recent study (Graziane et al. 2013) in which investigators used a cold swim stressor that was given 24 h before the reinstatement test (Conrad et al. 2010).
Intermittent footshock
Role of CRF and glutamate transmission in VTA
Recent studies further characterized VTA mechanisms of footshock-induced reinstatement. Wang et al. (2012) reported that footshock-induced reinstatement of heroin seeking is associated with increased glutamate (and dopamine) release in VTA, and that local blockade of ionotropic glutamate receptors prevents this reinstatement. These data extend previous results with cocaine-trained rats (Wang et al. 2005). These authors (Wang et al. 2009) also reported that while VTA perfusion of hypocretin 1 reinstates cocaine seeking, this effect is not blocked by CRF receptor antagonists; conversely, blockade of hypocretin-1 receptors does not block CRF-dependent footshock-induced reinstatement. These results rule out a role of VTA hypocretin 1 receptor in this reinstatement.
Blacktop et al. (2011) reported that VTA injections of CRF reinstate cocaine seeking in rats with a history of extended (6 h/d) but not limited access (2 h/d) to self-administered cocaine; this effect was decreased by VTA injections of selective CRF1 but not CRF2 receptor antagonists. Additionally, these authors reported that footshock-induced reinstatement is decreased by blockade of CRF1 but not CRF2 receptors. These findings are in agreement with previous results on the effect of systemic or ventricular CRF1 but not CRF2 receptor antagonists on footshock-induced reinstatement of drug seeking (Shalev et al. 2010). Blacktop et al. (2011) results are also in agreement with the expression of CRF1 (moderate) and CRF2 (very low/not detected) receptors in VTA, and the selective affinity of CRF to CRF1 versus CRF2 receptors (Bale and Vale 2004; Van Pett et al. 2000).
However, these results are opposite to those of Wang et al. (2007) who reported that blockade of CRF2 but not CRF1 receptors in VTA decreases footshock-induced reinstatement of cocaine seeking. The reasons for these discrepant results are unknown. Blacktop et al. (2011) suggested that duration of drug access (4 h/d versus 6 h/d) might play a role. However, it is difficult to envision that this parametric difference will cause a switch from CRF2- to CRF1-dependent mechanisms.
Role of noradrenaline in central amygdala
Yamada et al. (2011) reported that central amygdala injections of alpha-2 adrenoceptor agonists (clonidine or dexmedetomidine) but not beta- or alpha-1 adrenoceptor antagonists (propranolol or prazosin, respectively) decrease footshock-induced reinstatement of nicotine seeking. These data suggest that the central amygdala is a brain site sensitive to the potent effect of alpha-2 adrenoceptor agonists on footshock-induced reinstatement of drug seeking (Erb et al. 2000; Shaham et al. 2000a). However, clonidine is a highly lipophilic drug; thus, in the absence of anatomical control injections, the role of adjacent brain sites cannot be ruled out. Additionally, the negative data with propranolol is surprising based on the previous results that blockade of central amygdala beta-adrenoceptors decreases footshock-induced reinstatement of cocaine seeking (Leri et al. 2002).
Yohimbine
Recent studies have begun to assess brain sites of yohimbine-induced reinstatement.
Role of CRF in median raphe
Le et al. (2012) reported that median raphe injections of the non-selective CRF receptor antagonist d-Phe-CRF decrease yohimbine-induced reinstatement of alcohol seeking, a finding extending a previous result for footshock-induced reinstatement (Le et al. 2002).
Role of BNST
Buffalari et al. (2011) reported that reversible inactivation of the BNST decreases yohimbine-induced reinstatement of cocaine seeking. Simms et al. (2012) reported that central amygdala injections of the glucocorticoid receptor antagonist mifepristone decrease yohimbine-induced reinstatement of alcohol seeking. This effect, which likely involves rapid non-genomic effects of this antagonist, is somewhat surprising because, at least for intermittent footshock, previous studies have shown that the effect of this stressor on reinstatement is independent of corticosterone secretion (Erb et al. 1998; Shaham et al. 1997). Simms et al. (2012) data, however, suggest that mechanisms of yohimbine- and footshock-induced reinstatement are at least partially dissociable. Support for this notion comes from the finding that ventral noradrenergic bundle lesions, previously shown to decrease footshock-induced reinstatement (Shaham et al. 2000b), have no effect on yohimbine-induced reinstatement (Le et al. 2009).
Food deprivation
Recent studies have begun to assess the brain mechanisms of food-deprivation-stress-induced reinstatement of heroin seeking (Shalev et al. 2000). Tobin et al. (2013) recently performed a comprehensive study assessing the role of dopamine D1 receptors in different brain regions in acute 1-day food-deprivation-induced reinstatement of heroin seeking. They found that injections of the D1-family receptor antagonist SCH 23390 into accumbens shell, dorsal mPFC, and basolateral amygdala, but not accumbens core or ventral mPFC, decrease this reinstatement (Tobin et al. 2013). An interpretation issue in this study is that SCH 23390 is also a 5-HT2C (formerly 5-HT1C) receptor agonist (Millan et al. 2001). Thus, the role of this receptor in the effects of SCH 23390 cannot be ruled out, especially in light of the finding that that systemic injections of the 5-HT2C receptor agonist Ro60-0175 decrease yohimbine-induced reinstatement of cocaine seeking (Fletcher et al. 2008).
Cold swim stress: role of kappa opioid receptors in VTA
Results from previous studies demonstrate that kappa opioid receptor agonists reinstate cocaine seeking in monkeys (Valdez et al. 2007) and cocaine conditioned place preference (CPP) in mice (Redila and Chavkin 2008). Additionally, systemic injections of kappa opioid receptor antagonists decrease reinstatement of cocaine CPP induced by acute pre-session swim stress or social defeat stress (Bruchas et al. 2010; Carey et al. 2007; Redila and Chavkin 2008). Using the operant conditioning version of the reinstatement model in rats, Conrad et al. (2010) reported that acute exposure to 4 to 4.5 min cold (4–5°C) swim stress caused modest but persistent rei nstatement of cocaine seeking in test sessions performed 1, 2, and 3 days after stress exposure.
In a recent study, Graziane (2013) have used the same swim stress to investigate the role of kappa-opioid-receptor-related mechanisms in VTA in stress-induced reinstatement of cocaine seeking. In slice electrophysiology experiments, the authors found that antagonism of kappa opioid receptors reverses cold-swim-stress-induced blockade of LTP at GABAergic synapses on dopamine neurons in VTA, a cellular process that normally inhibits VTA dopamine neurons. In contrast, antagonism of kappa opioid receptors had no effect on swim-stress-induced potentiation of excitatory synapses on dopamine neurons. They also reported that blockade of kappa opioid receptors in VTA by nor-BNI decreases swim-stress-induced reinstatement of cocaine seeking. These data suggest that stress-induced blockade of LTP at GABAergic synapses in VTA plays a role in stress-induced reinstatement of cocaine seeking. A question for future research is whether this mechanism also plays a role in reinstatement of cocaine seeking induced by acute pre-session exposure to stressors like intermittent footshock or yohimbine.
Summary
Glutamate and CRF transmission in VTA (heroin and cocaine) and alpha-2 adrenoceptors in central amygdala (nicotine) play a role in intermittent footshock-induced reinstatement. CRF receptors in median raphe (alcohol), glucocorticoid receptors in central amygdala (alcohol), and BNST neuronal activity (cocaine) play a role in yohimbine-induced reinstatement of drug seeking. Dopamine D1 receptors in accumbens shell, dorsal mPFC, and basolateral amygdala play a role in acute (1 day) food deprivation-induced reinstatement of heroin seeking. Finally, kappa opioid receptors in VTA play a role in the delayed effect (1–3 days) of cold-swim-stress on reinstatement of cocaine seeking.
3. Emerging research topics and translational research
Reconsolidation and cue-induced reinstatement of drug seeking
Background and summary of main findings prior to 2009
Drug use and relapse involves learned associations between drug-associated discrete and contextual cues and drug taking, as well as the drug’s pharmacological effects (O'Brien et al. 1992; Stewart et al. 1984). Two main mechanisms for the establishment and persistence of memories for these cues are consolidation, a time-dependent process that leads to permanent storage of newly acquired memory (McGaugh 1966; 2000), and reconsolidation, a time-dependent process in which consolidated memory objects are rendered transiently malleable shortly after their reactivation (Dudai 2006; Misanin et al. 1968; Nader et al. 2000). Since the publication of two excellent papers on the effect of molecular and pharmacological manipulations that interfere with reconsolidation of cue memories on Pavlovian and instrumental responses to cocaine cues (Lee et al. 2005; Miller and Marshall 2005a), many studies have assessed the role of reconsolidation processes in responding to drug cues (Diergaarde et al. 2008; Milton and Everitt 2012; Sorg 2012; Torregrossa and Taylor 2012). Main findings prior to 2009 are that activation of NMDA receptors and the immediate early gene zif268 in basolateral amygdala and extracellular signal-regulated kinase (ERK) in accumbens core play critical roles in reconsolidation of memories for cocaine cues (Lee et al. 2005; Lee et al. 2006; Miller and Marshall 2005a; Milton et al. 2008).
Below we discuss selected recent mechanistic studies on brain sites and circuits involved in reconsolidation of memories for drug cues in which the operant conditioning self-administration variation of the reinstatement model was used. In these studies, rats first undergo drug self-administration. Next, they undergo extinction of lever responding in the absence of the discrete cues or context (extinction in a novel context). Rats are then exposed to the discrete cues or context in a short extinction session (a memory reactivation/retrieval manipulation) and immediately after that they receive the experimental manipulation designed to interfere with reconsolidation of cue or context memory. Subsequently, rats are tested for discrete-cue- or context-induced reinstatement of drug seeking. Control conditions in these studies include either exposure to the experimental manipulation without discrete cue or context exposure, or delayed exposure to the manipulation (several hours) after the short memory reactivation/retrieval session.
Recent findings
Sanchez et al. (2010) reported that post-retrieval session basolateral amygdala injections of an inhibitor of protein kinase A (PKA) decrease subsequent discrete-cue-induced but not drug-priming-induced reinstatement of cocaine seeking. These data provide additional evidence for a role of basolateral amygdala in reconsolidation of cocaine cue memories. Sanchez et al. (2010) data also suggest that memories for cocaine cues and cocaine’s interoceptive effects are dissociable. However, this conclusion should be made with caution, because only a single cocaine priming dose was used.
Additional potential evidence for a role of basolateral amygdala in reconsolidation of memories for discrete cues associated with cocaine self-administration derives from a study of Xue et al. (2012). These authors have used a memory retrieval-extinction procedure, previously shown to impair reconsolidation of memories for fear cues (Monfils et al. 2009), and demonstrated that this procedure decreases spontaneous recovery (the resumption of the extinguished conditioned response that occurs after time has passed following the conclusion of extinction), drug priming-, and context-induced reinstatement of heroin and cocaine seeking. For cocaine, the retrieval-extinction manipulation was associated with decreases in protein kinase M zeta, a molecule that plays a role in maintenance of long-term memory (Sacktor 2011), in basolateral amygdala. However, as discussed by Xue et al. (2012), based on the available data, it is unclear whether the inhibitory effect of the retrieval-extinction manipulation on reinstatement of drug seeking is due to interference with reconsolidation, potentiation of consolidation of extinction memories, or both processes.
In this regard, results from a recent report of Millan et al. (2013) suggest that, at least for context-induced reinstatement, interference with reconsolidation is not the critical learning mechanism for the inhibitory effect of the retrieval-extinction manipulation on reinstatement. These authors first confirmed the findings of Xue et al. (2012) by demonstrating that context-induced reinstatement of alcohol seeking is attenuated by exposing rats to a short retrieval session (10 min cue exposure under extinction conditions) that was followed 70 min later by a longer 50-min extinction session. However, Millan et al. (2013) also demonstrated that reversing the order of the retrieval and extinction sessions decreases subsequent context-induced reinstatement. The results from the reversed condition are incompatible with a reconsolidation account of the retrieval-extinction manipulation (Monfils et al. 2009). One possibility is that the retrieval-extinction manipulation enhances consolidation of extinction memories, resulting in ‘deepened’ extinction. However, Millan et al. (2013) also showed that the retrieval-extinction manipulation paradoxically increased re-acquisition of alcohol self-administration. This result suggests that rather than interfering with reconsolidation of context memories or potentiating consolidation of extinction learning, the retrieval-extinction procedure (and the reversed condition) leads to inhibition of reinstatement by facilitating discrimination between reinforced and non-reinforced test sessions (Hutton-Bedbrook and McNally 2013). While the specific mechanism is still unknown, the results of Millan et al. (2013) question the potential clinical utility of the retrieval-extinction procedure for relapse prevention.
In a series of elegant studies, Fuchs and colleagues modified the context-induced reinstatement procedure to evaluate the neuroanatomical basis of cocaine context memory reconsolidation. Fuchs et al. (2009) first reported that injections of the protein synthesis inhibitor anisomycin into basolateral amygdala after short re-exposure to the cocaine-paired context (context A, a reactivation/retrieval manipulation) decrease subsequent context-induced reinstatement of cocaine seeking. Subsequently, Wells et al. (2012) reported that injections of the ERK inhibitor U0126 into basolateral amygdala after cocaine-context memory reactivation decrease subsequent context-induced reinstatement. In contrast, post-context reactivation injections of U0126 into accumbens core were ineffective. Accumbens core and local ERK activity in this area are involved in cocaine-context memory reconsolidation, as assessed in the CPP procedure (Miller and Marshall 2005b; Theberge et al. 2010). Thus, the data of Wells et al. (2012) suggest that mechanisms of reconsolidation of memories of contexts associated with cocaine self-administration are dissociable from those associated with non-contingent cocaine exposure.
In another study, Ramirez (2009) provided evidence suggesting that dorsal hippocampus, but not dorsal mPFC or dorsolateral caudate putamen, is involved in reconsolidation of cocaine context memories. However, interpretation of data from this study is complicated by the fact that post-context reactivation injections of the sodium channel blocker tetrodotoxin, but not the protein synthesis inhibitor anisomycin (the classical manipulation in consolidation/reconsolidation studies), into dorsal hippocampus decreased subsequent context-induced reinstatement. Finally, Wells (2011) combined their context reactivation procedure with an anatomical disconnection procedure (Everitt et al. 1991; Gaffan et al. 1993) to provide data suggesting that a brain circuit that includes the basolateral amygdala and dorsal hippocampus is necessary for reconsolidation of cocaine context memories. They reported that context-induced reinstatement of cocaine seeking is decreased in rats previously injected unilaterally with anisomycin into basolateral amygdala combined with unilateral injection of muscimol+baclofen into dorsal hippocampus of the contralateral side immediately after the context reactivation session. This inhibitory effect on context-induced reinstatement was not observed after ipsilateral injections of anisomycin (basolateral amygdala) and muscimol+baclofen (dorsal hippocampus) (Wells et al. 2011).
Conclusions
Results from recent studies confirm a role of basolateral amygdala in reconsolidation of memories for discrete cocaine cues, and further demonstrate a role of this brain area in reconsolidation of memories for contextual cocaine cues. Local basolateral amygdala molecular mechanisms involved in memory reconsolidation include PKA for discrete cues and ERK for contextual cues. Recent evidence also suggests a potential role of dorsal hippocampus in reconsolidation of memories for cocaine-associated contexts. A question for future research is whether these reconsolidation-related mechanisms generalize to other drugs of abuse.
Similarities and differences in neuronal mechanisms of reinstatement across drug classes
Background and summary of main findings prior to 2009
As mentioned above, early studies of Stewart and colleagues and subsequent studies suggest that the mesolimbic dopamine system plays a critical role in drug-priming-induced reinstatement of heroin and cocaine seeking (Schmidt et al. 2009; Self 2004; Self and Nestler 1998; Stewart et al. 1984; Stewart and Vezina 1988). These findings, and a series of influential theoretical reviews in the mid-1980s and early 1990s, which emphasized similarities in addiction mechanisms across drug classes (Robinson and Berridge 1993; Stewart et al. 1984; Wise and Bozarth 1987), have led to the widely accepted notion of commonalities in mechanisms of reinstatement of drug seeking across drug classes (Kalivas et al. 2009; Kalivas and McFarland 2003; Shaham et al. 2003). Indeed, a large body of data supports this notion.
Evidence for similarities
At the behavioral level, exposure to drug priming, different types of cues (discrete, discriminative, or contextual), or certain stressors (intermittent footshock or yohimbine) reliably reinstate heroin, cocaine, alcohol, methamphetamine, or nicotine seeking (Bossert et al. 2005; Bruijnzeel 2012; Feltenstein and See 2008; Le and Shaham 2002; Weiss 2005). There is also substantial evidence for neuropharmacological similarities in mechanisms of reinstatement of drug seeking across drug classes.
For example, the glutamatergic projection from dorsal mPFC to accumbens is critical for drug-priming-induced reinstatement of cocaine and heroin seeking (LaLumiere and Kalivas 2008; McFarland and Kalivas 2001), and possibly methamphetamine seeking as well (Rocha and Kalivas 2010). Extrahypothalamic CRF transmission, neuroadrenaline transmission, or dorsal mPFC activity is critical for intermittent-footshock-induced reinstatement of cocaine and heroin seeking (Kalivas and McFarland 2003; Shaham et al. 2003). Basolateral amygdala or accumbens core activity is critical for discrete cue-induced reinstatement of cocaine, heroin, and alcohol seeking (Feltenstein and See 2008; Marinelli et al. 2010). Dorsal mPFC activity is critical for discrete-cue-induced reinstatement of heroin, cocaine, methamphetamine, and MDMA seeking (Ball and Slane 2012; Feltenstein and See 2008; Rocha and Kalivas 2010). Ventral subiculum or dorsolateral striatum activity is critical for context-induced reinstatement of heroin and cocaine seeking (Bossert and Stern 2013; Bossert et al. 2009; Fuchs et al. 2006; Lasseter et al. 2010).
Evidence for differences
Evidence is emerging in recent years for some dissociation between the brain sites and projections controlling reinstatement of drug seeking across drug classes (Badiani et al. 2011). For example, reversible inactivation of VTA, dorsal mPFC, accumbens core, or ventral pallidum, but not ventral mPFC, accumbens shell, substantia nigra, central and basolateral amygdala, or mediodorsal thalamus decreases cocaine-priming-induced reinstatement (McFarland and Kalivas 2001). On the other hand, reversible inactivation of any of the above brain areas, as well as the BNST, decrease heroin-priming-induced reinstatement (Rogers et al. 2008).
Another example is that context-induced reinstatement of cocaine or alcohol seeking is mediated by mPFC and accumbens sub-regions that are functionally dissociable from those involved in context-induced reinstatement of heroin seeking. Thus, context-induced reinstatement of cocaine or alcohol seeking is decreased by reversible inactivation of dorsal but not ventral mPFC (Fuchs et al. 2005; Willcocks and McNally 2013) while reversible inactivation of ventral but not dorsal mPFC decreases context-induced reinstatement of heroin seeking (Bossert et al. 2011). Furthermore, context-induced reinstatement of cocaine or alcohol seeking is decreased by reversible inactivation (for cocaine) or dopamine receptor blockade (for alcohol) of either accumbens core or shell (Fuchs et al. 2008), while context-induced reinstatement of heroin seeking is decreased by inhibiting glutamatergic transmission or dopamine receptor blockage in accumbens shell but not core (Bossert et al. 2006; Bossert et al. 2007).
Additionally, reinstatement of cocaine or alcohol seeking is triggered by reversible inactivation of ventral mPFC or accumbens shell (Fuchs et al. 2008; Millan et al. 2010; Peters et al. 2008) and attenuated (cocaine) by ventral mPFC AMPA receptor activation (Peters et al. 2008). Functionally disconnecting these two brain regions by unilateral inhibition of ventral mPFC and simultaneous contralateral inactivation of accumbens shell mimics reinstatement of cocaine seeking induced by bilateral inactivation of either brain area (Peters et al. 2008). These findings suggest that projections from ventral mPFC to accumbens shell inhibit cocaine seeking after extinction (Peters et al. 2009). In contrast, we have found that reversible inactivation of ventral mPFC (muscimol+baclofen injections) combined with D1-family receptor blockade in accumbens shell of either contralateral or ipsilateral hemisphere has no effect on reinstatement of heroin seeking after extinction (Bossert et al. 2012). Furthermore, the same disconnection manipulation decreased context-induced reinstatement of heroin seeking. Taken together, our data and those of Peters et al. (2009) suggest that activation of ventral mPFC—accumbens shell projection has opposite effects on heroin seeking versus cocaine seeking. Table 3 provides a summary of results for the effect of different manipulations of ventral and dorsal mPFC on reinstatement across drug classes.
Table 3.
Effect of inhibition of ventral or dorsal mPFC on extinction and reinstatement across drug classes.
| Extinction | Discrete cue reinstatement |
Discriminative cue reinstatement |
Context reinstatement |
Drug priming reinstatement |
Stress reinstatement |
|
|---|---|---|---|---|---|---|
|
Dorsal mPFC Cocaine |
− | ↓ | ↓ | ↓ | ↓ | |
| Heroin | − | ↓ | ↓ | |||
| Meth- amphetamine |
− | ↓ | ↓ | |||
| Alcohol | − | ↓ | ||||
|
Ventral mPFC Cocaine |
↑ | ↓ | − | − | − | |
| Heroin | − | ↓ | ↓ | ↓ | ↓ | |
| Meth- amphetamine |
− | ↓ | − | |||
| Alcohol | − | − |
Each symbol represents the behavioral effect of a manipulation designed to decrease mPFC activity or function. ↑, increased drug seeking; ↓, decreased drug seeking; −, no effect on drug seeking. The data are based on the following references: Cocaine: (Capriles et al. 2003; Fuchs et al. 2005; McFarland et al. 2004; McFarland and Kalivas 2001; McLaughlin and See 2003; Pentkowski et al. 2010; Peters et al. 2008; Stefanik et al. 2013); Heroin: (Alvarez-Jaimes et al. 2008; Bossert et al. 2011; LaLumiere and Kalivas 2008; Rogers et al. 2008; See 2009); Methamphetamine: (Rocha and Kalivas 2010); Alcohol: (Willcocks and McNally 2013).
Finally, additional support for the notion that relapse to opiate and psychostimulant seeking can involve dissociable mechanism is derived from studies on incubation of drug craving (time-dependent increases in cue-induced drug seeking after withdrawal). This phenomenon was originally discovered in studies on cue-induced reinstatement of cocaine seeking (Grimm et al. 2001; Neisewander et al. 2000) and intermittent footshock-stress-induced reinstatement of heroin seeking (Shalev et al. 2001). We do not provide in this review an update on mechanisms of incubation of drug craving, because we covered this topic in two recent reviews (Marchant et al. 2013; Pickens et al. 2011). Additionally, in recent mechanistic studies we and others primarily assessed cue-induced drug seeking in a single extinction session at different withdrawal days, not cue-induced reinstatement after extinction. Regarding similarities and differences, however, three pieces of data suggest dissociable mechanisms of incubation of opiate versus psychostimulant craving. GDNF (glial cell line-derived neurotrophic factor) activity in VTA is critical for incubation of cocaine but not heroin craving (Airavaara et al. 2011; Lu et al. 2009). Additionally, BDNF (brain-derived neurotrophic factor) in VTA and accumbens has been implicated in incubation of cocaine craving (Grimm et al. 2003; Li et al. 2013; Lu et al. 2004) but not heroin craving (Theberge et al. 2012). Finally, we recently found that chronic delivery of the selective toll-like receptor 4 (TLR4) receptor antagonist (+)-naltrexone during the withdrawal phase decreases the development of incubation of heroin but not methamphetamine craving (Theberge et al. 2013).
Conclusions
Taken together, while a large body of evidence supports the accepted dogma of similarities in mechanisms of reinstatement of drug seeking across drug classes, emerging recent evidence suggest the existence of some mechanistic differences, particularly in reference to context-induced reinstatement of drug seeking and incubation of drug craving. As discussed elsewhere (Badiani et al. 2011), we proposed that different psychological states induced by cocaine versus heroin, and by extension, the psychological states induced by cues and contexts associated with the two drugs might account for the mechanistic differences discussed above. Specifically, studies using the runway model by Ettenberg and colleagues suggest that heroin induces classical approach behavior similar to that seen with food rewards, while cocaine induces approach-avoidance behavior (Ettenberg 2009; Ettenberg and Geist 1993). We speculate that the partial dissociation in brain circuits controlling cocaine versus heroin seeking may mirror this difference in motivational states (Badiani et al. 2011).
Translational human studies based on results from the reinstatement model
As mentioned in the Introduction, several medications that have been used to treat opiate, nicotine, and alcohol addiction decrease drug-priming and cue-induced reinstatement of drug seeking. These findings support the predictive validity of the reinstatement model (Epstein et al. 2006), but they fall short of demonstrating the translational value of the model. This is because the medications were retrospectively assessed in the reinstatement model after they have demonstrated efficacy in human drug addicts. In other words, the medications demonstrated ‘postdictive’ validity, a situation in which effective medications in humans were subsequently found to also be effective in the animal model (Goldstein and Simpson 2002). To date, there are no instances of ‘prospective’ predictive validity with the model; that is, medications/drugs found effective in the reinstatement model that were subsequently found to prevent drug relapse in humans. However, there are several examples of pharmacological similarities between results from rat studies using the reinstatement model and follow-up human laboratory studies on stress-, cue-, and drug-induced craving and other self-report measures, which are to some degree predictive of subsequent drug relapse (Back et al. 2010; Sinha et al. 2006; Sinha et al. 2011). We discuss below these examples.
N-acetylcysteine (NAC)
As mentioned above, NAC injections decrease cocaine-priming- and discrete-cue-induced reinstatement of cocaine seeking in rats (Baker et al. 2003; Kupchik et al. 2012; Reichel et al. 2011). In an initial human study based on the rat studies, LaRowe et al. (2007) reported that NAC modestly decreases the subjective desire to use cocaine and interest in cocaine; however, NAC had no effect on cue-induced cocaine craving. In a second study, Amen et al. (2011) reported that 4-day treatment with NAC decreases craving induced by non-contingent priming cocaine injections. These data, however, should be interpreted with great caution because of the very small number of subjects (n=4 per condition) that participated in this preliminary study. Results from other preliminary studies with low number of subjects suggest that NAC either has weak effect or no effect on measures of drug taking and drug craving in nicotine (Knackstedt et al. 2009) or methamphetamine (Grant et al. 2010) addicts (for a review see Berk et al. (2013). Together, whether or not NAC reliably decreases drug-priming- or cue-induced cocaine craving or other measures of drug craving and relapse is an empirical question that should be determined in future studies. Such studies are of particular importance in light of the recent negative findings for NAC’s effect on cocaine-priming-induced reinstatement in primates (Bauzo et al. 2012).
Alpha-2 adrenoceptor agonists
In several studies, investigators have assessed the effect of alpha-2 adrenoceptor agonists (clonidine, lofexidine, guanbenz) that inhibit central noradrenaline cell firing and release (Abercrombie et al. 1988; Aghajanian and VanderMaelen 1982) on stress-induced reinstatement. These drugs potently inhibit intermittent footshock-induced reinstatement of heroin, cocaine, speedball (a heroin-cocaine mixture), alcohol, and nicotine seeking in rats (Erb et al. 2000; Highfield et al. 2001; Le et al. 2005; Shaham et al. 2000b; Zislis et al. 2007). Based on these rat studies, Sinha and colleagues, and Preston, Epstein and colleagues assessed the effect of alpha-2 adrenoceptor agonists on stress-induced drug craving in human addicts in the laboratory. The human studies have also assessed the effect of these drugs on cue-induced drug craving.
The first human study on the effect of an alpha-2-adrenergic agonist (lofexidine) on stress- and cue-induced craving was conducted in opiate-dependent individuals in naltrexone treatment (Sinha et al. 2007); naltrexone is an opiate receptor antagonist that is approved in the treatment of opioid addiction but it is not used widely because of poor compliance (Julius 1976). In this small laboratory and clinical outcomes study, Sinha et al. (2007) found that 4 weeks of daily 2.4 mg lofexidine decreases stress-induced opiate craving, anger ratings, and basal heart rates, as well as improves opiate relapse outcomes; they also found that lofexidine decreases cue-induced opiate craving.
In a follow-up study, Fox et al., (2012b) assessed whether chronic 4 week administration of the alpha-2 adrenoceptor agonist guanfacine (up to 3 mg/day) would decrease guided imagery-stress−, cue- and stress+cue-induced drug craving, as well as anxiety in cocaine-dependent individuals who also use alcohol and nicotine. They found that guanfacine decreases stress- and cue-induced nicotine craving. Guanfacine also modestly decreased cue-induced cocaine craving, anxiety and arousal, but not stress-induced craving. The latter data, however, are difficult to interpret because the stress manipulation caused a weak craving response in the placebo group; thus, the negative finding may reflect a floor effect.
In a recent study with a larger sample, Jobes et al. (2011) assessed the effect of clonidine on stress- and cue-induced craving in cocaine users that were randomly assigned to three groups receiving placebo or clonidine (0.1 or 0.2 mg, orally) under double-blind conditions. The stress and cue manipulations were standard auditory-imagery scripts of stress-related and drug-cue related situations. These authors reported that both clonidine doses strongly decrease stress-induced cocaine craving, while only the high clonidine dose decreases cue-induced craving.
Finally, it should be noted that in the initial rat studies, the alpha-2 adrenoceptor agonists selectively decreased footshock-stress-induced reinstatement but not cue- or drug-priming-induced reinstatement (Erb et al. 2000; Highfield et al. 2001), but see Smith and Aston-Jones (2011) for recent positive results for cue-induced reinstatement of cocaine seeking. In contrast, in the human studies the alpha-2 adrenoceptor agonists consistently decreased cue-induced drug craving (Fox et al. 2012b; Jobes et al. 2011; Sinha et al. 2007). A possible reason for these different findings is that in the rat, cue exposure likely primarily causes an appetitive motivational state with little or no stress component (Feltenstein and See 2008; See 2005). In contrast, in the human, laboratory cue exposure also induces an alpha-2 adrenoceptor agonist sensitive stress-like physiological (e.g., increased cortisol and heart rate) and psychological (e.g., increased subjective ratings of anxiety, anger, and irritability) states that are similar to those induced by exposure to stressors (Sinha et al. 1999; Sinha et al. 2000).
Prazosin
In a recent study, Le et al. (2011) reported that the alpha-1 adrenoceptor antagonist prazosin blocks both intermittent footshock- and yohimbine-induced reinstatement of alcohol seeking. In an independent parallel human study, Fox et al. (2012a) assessed the effect of placebo or prazosin (16 mg/day for 4 weeks) on stress- and cue-induced alcohol craving, anxiety, and negative emotions. They reported that prazosin decreases stress-induced craving and negative emotions. However, while prazosin decreased cue-induced negative emotion self-reports, it had no effect on cue-induced craving. The latter negative data might be due to a floor effect, because of the weak cue-induced craving in the placebo group.
Conclusions
Studies using the reinstatement model in rats have led to human laboratory studies on the effect of several drugs (approved for human use for other indications) on stress-, cue-, and drug-priming-induced craving in human addicts. The available data with NAC are not sufficient to conclude that the drug is effective against cue- or drug-priming-induced craving. Similarly, the results with prazosin, which decreased stress- but not cue-induced alcohol craving in a relatively small sample, require an independent replication. On the other hand, a more consistent pattern of results has emerged with alpha-2 adrenoceptor agonists that decrease both stress- and cue-induced craving to opiates, cocaine, and nicotine. A question for future research is whether chronic treatment with alpha-2 adrenoceptor agonists would also prevent stress-induced relapse in the addict’s environment. Ongoing studies at both Yale and NIDA intramural research programs will answer this question in the future.
Acknowledgments
Research was supported by the National Institute on Drug Abuse, Intramural Research Program. The authors declare that they do not have any conflicts of interest (financial or otherwise) related to the data presented in this manuscript. We thank Robyn St. Laurent for editorial corrections. The review is dedicated to the Waletzky family.
Footnotes
We do not provide a lengthy discussion on the validity of the reinstatement model, a topic which we and others have discussed in detail in previous reviews (Epstein D, Preston K, Stewart J, Shaham Y (2006) Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology 189: 1–16Epstein DH, Preston KL (2003) The reinstatement model and relapse prevention: a clinical perspective. Ibid.168: 31–41Katz J, Higgins S ibid.The validity of the reinstatement model of craving and relapse to drug use. 21–30Shaham Y, Shalev U, Lu L, de Wit H, Stewart J ibid.The reinstatement model of drug relapse: history, methodology and major findings. 3–20.
References
- Abercrombie ED, Keller RW, Jr., Zigmond MJ. Characterization of hippocampal norepinephrine release as measured by microdialysis perfusion: pharmacological and behavioral studies. Neuroscience. 1988;27:897–904. doi: 10.1016/0306-4522(88)90192-3. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, VanderMaelen CP. alpha 2-adrenoceptor-mediated hyperpolarization of locus coeruleus neurons: intracellular studies in vivo. Science. 1982;215:1394–1396. doi: 10.1126/science.6278591. [DOI] [PubMed] [Google Scholar]
- Airavaara M, Pickens CL, Stern AL, Wihbey KA, Harvey BK, Bossert JM, Liu QR, Hoffer BJ, Shaham Y. Endogenous GDNF in ventral tegmental area and nucleus accumbens does not play a role in the incubation of heroin craving. Addict Biol. 2011;16:261–272. doi: 10.1111/j.1369-1600.2010.00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker JM. Endogenous opioids: biology and function. Annu Rev Neurosci. 1984;7:223–255. doi: 10.1146/annurev.ne.07.030184.001255. [DOI] [PubMed] [Google Scholar]
- Alleweireldt AT, Hobbs RJ, Taylor AR, Neisewander JL. Effects of SCH-23390 infused into the amygdala or adjacent cortex and basal ganglia on cocaine seeking and self-administration in rats. Neuropsychopharmacology. 2006;31:363–374. doi: 10.1038/sj.npp.1300794. [DOI] [PubMed] [Google Scholar]
- Alvarez-Jaimes L, Polis I, Parsons LH. Attenuation of cue-induced heroin-seeking behavior by cannabinoid CB1 antagonist infusions into the nucleus accumbens core and prefrontal cortex, but not basolateral amygdala. Neuropsychopharmacology. 2008;33:2483–2493. doi: 10.1038/sj.npp.1301630. [DOI] [PubMed] [Google Scholar]
- Amen SL, Piacentine LB, Ahmad ME, Li SJ, Mantsch JR, Risinger RC, Baker DA. Repeated N-acetyl cysteine reduces cocaine seeking in rodents and craving in cocaine-dependent humans. Neuropsychopharmacology. 2011;36:871–878. doi: 10.1038/npp.2010.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SM, Bari AA, Pierce RC. Administration of the D1-like dopamine receptor antagonist SCH-23390 into the medial nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug-seeking behavior in rats. Psychopharmacology. 2003;168:132–138. doi: 10.1007/s00213-002-1298-5. [DOI] [PubMed] [Google Scholar]
- Bachteler D, Economidou D, Danysz W, Ciccocioppo R, Spanagel R. The effects of acamprosate and neramexane on cue-induced reinstatement of ethanol-seeking behavior in rat. Neuropsychopharmacology. 2005;30:1104–1110. doi: 10.1038/sj.npp.1300657. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Self DW. Effects of adenosine A2A receptor stimulation on cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2009;206:469–478. doi: 10.1007/s00213-009-1624-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtell RK, Whisler K, Karanian D, Self DW. Effects of intranucleus accumbens shell administration of dopamine agonists and antagonists on cocaine-taking and cocaine-seeking behaviors in the rat. Psychopharmacology. 2005;183:41–53. doi: 10.1007/s00213-005-0133-1. [DOI] [PubMed] [Google Scholar]
- Back SE, Hartwell K, DeSantis SM, Saladin M, McRae-Clark AL, Price KL, Moran-Santa Maria MM, Baker NL, Spratt E, Kreek MJ, Brady KT. Reactivity to laboratory stress provocation predicts relapse to cocaine. Drug Alcohol Depend. 2010;106:21–27. doi: 10.1016/j.drugalcdep.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiani A, Belin D, Epstein D, Calu D, Shaham Y. Opiate versus psychostimulant addiction: the differences do matter. Nat Rev Neurosci. 2011;12:685–700. doi: 10.1038/nrn3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Ball KT, Slane M. Differential involvement of prelimbic and infralimbic medial prefrontal cortex in discrete cue-induced reinstatement of 3,4-methylenedioxymethamphetamine (MDMA; ecstasy) seeking in rats. Psychopharmacology (Berl) 2012;224:377–385. doi: 10.1007/s00213-012-2762-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauzo RM, Kimmel HL, Howell LL. The cystine-glutamate transporter enhancer N-acetyl-L-cysteine attenuates cocaine-induced changes in striatal dopamine but not self-administration in squirrel monkeys. Pharmacol Biochem Behav. 2012;101:288–296. doi: 10.1016/j.pbb.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk M, Malhi GS, Gray LJ, Dean OM. The promise of N-acetylcysteine in neuropsychiatry. Trends Pharmacol Sci. 2013 doi: 10.1016/j.tips.2013.01.001. (in press) [DOI] [PubMed] [Google Scholar]
- Blacktop JM, Seubert C, Baker DA, Ferda N, Lee G, Graf EN, Mantsch JR. Augmented cocaine seeking in response to stress or CRF delivered into the ventral tegmental area following long-access self-administration is mediated by CRF receptor type 1 but not CRF receptor type 2. J Neurosci. 2011;31:11396–11403. doi: 10.1523/JNEUROSCI.1393-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: An update and clinical implications. Eur J Pharmacol. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Gray SM, Lu L, Shaham Y. Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology. 2006;31:2197–2209. doi: 10.1038/sj.npp.1300977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Liu SY, Lu L, Shaham Y. A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J Neurosci. 2004;24:10726–10730. doi: 10.1523/JNEUROSCI.3207-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci. 2007;27:12655–12663. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Stern AL. Role of ventral subiculum in context-induced reinstatement of heroin seeking in rats. Addict Biol. 2013 doi: 10.1111/adb.12015. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Stern AL, Theberge FR, Cifani C, Koya E, Hope BT, Shaham Y. Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. Nat Neurosci. 2011;14:420–422. doi: 10.1038/nn.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Stern AL, Theberge FR, Marchant NJ, Wang HL, Morales M, Shaham Y. Role of projections from ventral medial prefrontal cortex to nucleus accumbens shell in context-induced reinstatement of heroin seeking. J Neurosci. 2012;32:4982–4891. doi: 10.1523/JNEUROSCI.0005-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Wihbey KA, Pickens CL, Nair SG, Shaham Y. Role of dopamine D(1)-family receptors in dorsolateral striatum in context-induced reinstatement of heroin seeking in rats. Psychopharmacology. 2009;206:51–60. doi: 10.1007/s00213-009-1580-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Swartzentruber D. Sources of relapse after extinction in Pavlovian and instrumental learning. Clin Psychol Rev. 1991;11:123–140. [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: I. preclinical studies. Synapse. 1996a;23:28–38. doi: 10.1002/(SICI)1098-2396(199605)23:1<28::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: II.clinical studies. Synapse. 1996b;23:39–51. doi: 10.1002/(SICI)1098-2396(199605)23:1<39::AID-SYN5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW. Tobacco addiction and the dysregulation of brain stress systems. Neurosci Biobehav Rev. 2012;36:1418–1441. doi: 10.1016/j.neubiorev.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari DM, See RE. Inactivation of the bed nucleus of the stria terminalis in an animal model of relapse: effects on conditioned cue-induced reinstatement and its enhancement by yohimbine. Psychopharmacology. 2011;213:19–27. doi: 10.1007/s00213-010-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calu DJ, Chen YW, Kawa AB, Nair SG, Shaham Y. The use of the reinstatement model to study relapse to palatable food seeking during dieting. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.04.030. (accepted pending minor revisions) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannella N, Economidou D, Kallupi M, Stopponi S, Heilig M, Massi M, Ciccocioppo R. Persistent increase of alcohol-seeking evoked by neuropeptide S: an effect mediated by the hypothalamic hypocretin system. Neuropsychopharmacology. 2009;34:2125–2134. doi: 10.1038/npp.2009.37. [DOI] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2003;168:66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- Carey AN, Borozny K, Aldrich JV, McLaughlin JP. Reinstatement of cocaine place-conditioning prevented by the peptide kappa-opioid receptor antagonist arodyn. Eur J Pharmacol. 2007;569:84–89. doi: 10.1016/j.ejphar.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr., Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Janak PH. Context-induced relapse of conditioned behavioral responding to ethanol cues in rats. Biol Psychiatry. 2008;64:203–210. doi: 10.1016/j.biopsych.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Janak PH. Ethanol seeking triggered by environmental context is attenuated by blocking dopamine D1 receptors in the nucleus accumbens core and shell in rats. Psychopharmacology. 2009;207:303–314. doi: 10.1007/s00213-009-1657-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Schairer WW, Janak PH. Separable roles of the nucleus accumbens core and shell in context- and cue-induced alcohol-seeking. Neuropsychopharmacology. 2010;35:783–791. doi: 10.1038/npp.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, McCutcheon JE, Cotterly LM, Ford KA, Beales M, Marinelli M. Persistent increases in cocaine-seeking behavior after acute exposure to cold swim stress. Biol Psychiatry. 2010;68:303–305. doi: 10.1016/j.biopsych.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Duffy P, Kalivas PW. A role for nucleus accumbens glutamate transmission in the relapse to cocaine-seeking behavior. Neuroscience. 1999;93:1359–1367. doi: 10.1016/s0306-4522(99)00214-6. [DOI] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20 doi: 10.1523/JNEUROSCI.20-15-j0006.2000. RC89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag H, Bossert JM, Koya E, Shaham Y. Context-induced relapse to drug seeking: a review. Trans R Soc Lond B: Biol Sci. 2008;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Shaham Y. Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav Neurosci. 2002;116:169–173. doi: 10.1037//0735-7044.116.1.169. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer AN, Binnekade R, Mulder AH, Vanderschuren LJ. Drug-induced reinstatement of heroin- and cocaine-seeking behaviour following long-term extinction is associated with expression of behavioural sensitization. Eur J Neurosci. 1998;10:3565–3571. doi: 10.1046/j.1460-9568.1998.00368.x. [DOI] [PubMed] [Google Scholar]
- de Wit H. Priming effects with drugs and other reinforcers. Exp Clin Psychopharmacol. 1996;4:5–10. [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology. 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Diergaarde L, Schoffelmeer AN, De Vries TJ. Pharmacological manipulation of memory reconsolidation: towards a novel treatment of pathogenic memories. Eur J Pharmacol. 2008;585:453–457. doi: 10.1016/j.ejphar.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Dudai Y. Reconsolidation: the advantage of being refocused. Curr Opin Neurobiol. 2006;16:174–178. doi: 10.1016/j.conb.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Epstein D, Preston K, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology. 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Preston KL. The reinstatement model and relapse prevention: a clinical perspective. Psychopharmacology. 2003;168:31–41. doi: 10.1007/s00213-003-1470-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J. Alpha-2 adrenergic agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology. 2000;23:138–150. doi: 10.1016/S0893-133X(99)00158-X. [DOI] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J Neurosci. 1998;18:5529–5536. doi: 10.1523/JNEUROSCI.18-14-05529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. Stress-induced relapse to drug seeking in the rat: Role of the bed nucleus of the stria terminalis and amygdala. Stress. 2001;4:289–303. doi: 10.3109/10253890109014753. [DOI] [PubMed] [Google Scholar]
- Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci. 1999;19 doi: 10.1523/JNEUROSCI.19-20-j0006.1999. RC35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A. The runway model of drug self-administration. Pharmacol Biochem Behav. 2009;91:271–277. doi: 10.1016/j.pbb.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A, Geist TD. Qualitative and quantitative differences in the operant runway behavior of rats working for cocaine and heroin reinforcement. Pharmacol Biochem Behav. 1993;44:191–198. doi: 10.1016/0091-3057(93)90298-8. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, MacConell LA, Geist TD. Effects of haloperidol in a response-reinstatement model of heroin relapse. Psychopharmacology. 1996;124:205–210. doi: 10.1007/BF02246658. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Morris KA, O'Brien A, Robbins TW. The basolateral amygdala-ventral striatal system and conditioned place preference: further evidence of limbic-striatal interactions underlying reward-related processes. Neuroscience. 1991;42:1–18. doi: 10.1016/0306-4522(91)90145-e. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Wolf ME. Psychomotor stimulant addiction: a neural systems perspective. J Neurosci. 2002;22:3312–3320. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. The neurocircuitry of addiction: an overview. Br J Pharmacol. 2008;154:261–274. doi: 10.1038/bjp.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre S, Fredholm BB, Morelli M, Popoli P, Fuxe K. Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci. 1997;20:482–487. doi: 10.1016/s0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Rizos Z, Sinyard J, Tampakeras M, Higgins GA. The 5-HT(2C) receptor agonist Ro60-0175 reduces cocaine self-administration and reinstatement induced by the stressor yohimbine, and contextual cues. Neuropsychopharmacology. 2008;33:1402–1412. doi: 10.1038/sj.npp.1301509. [DOI] [PubMed] [Google Scholar]
- Forget B, Pushparaj A, Le Foll B. Granular insular cortex inactivation as a novel therapeutic strategy for nicotine addiction. Biol Psychiatry. 2010;68:265–271. doi: 10.1016/j.biopsych.2010.01.029. [DOI] [PubMed] [Google Scholar]
- Fox HC, Anderson GM, Tuit K, Hansen J, Kimmerling A, Siedlarz KM, Morgan PT, Sinha R. Prazosin effects on stress- and cue-induced craving and stress response in alcohol-dependent individuals: preliminary findings. Alcohol Clin Exp Res. 2012a;36:351–360. doi: 10.1111/j.1530-0277.2011.01628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Seo D, Tuit K, Hansen J, Kimmerling A, Morgan PT, Sinha R. Guanfacine effects on stress, drug craving and prefrontal activation in cocaine dependent individuals: preliminary findings. J Psychopharmacol. 2012b;26:958–972. doi: 10.1177/0269881111430746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Bell GH, Ramirez DR, Eaddy JL, Su ZI. Basolateral amygdala involvement in memory reconsolidation processes that facilitate drug context-induced cocaine seeking. Eur J Neurosci. 2009;30:889–900. doi: 10.1111/j.1460-9568.2009.06888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci. 2006;26:3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, Bell GH. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci. 2007;26:487–498. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, See RE. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2004a doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MP, See RE. Differential Involvement of Orbitofrontal Cortex Subregions in Conditioned Cue-Induced and Cocaine-Primed Reinstatement of Cocaine Seeking in Rats. The Journal of Neuroscience. 2004b;24:6600–6610. doi: 10.1523/JNEUROSCI.1924-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Ramirez DR, Bell GH. Nucleus accumbens shell and core involvement in drug context-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2008;200:545–556. doi: 10.1007/s00213-008-1234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriele A, See RE. Lesions and reversible inactivation of the dorsolateral caudate-putamen impair cocaine-primed reinstatement to cocaine-seeking in rats. Brain Res. 2011;1417:27–35. doi: 10.1016/j.brainres.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffan D, Murray EA, Fabre-Thorpe M. Interaction of the amygdala with the frontal lobe in reward memory. Eur J Neurosci. 1993;5:968–975. doi: 10.1111/j.1460-9568.1993.tb00948.x. [DOI] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Markou A. Animal models of psychiatric disorders. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology. New York: Raven Press; 1995. pp. 787–798. [Google Scholar]
- Gipson CD, Kupchik YM, Shen H, Reissner KJ, Thomas CA, Kalivas PW. Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron. 2013;77:867–872. doi: 10.1016/j.neuron.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Simpson JC. Validity: Definition and Applications to Psychiatric Research. In: Tsuang MT, Tohen M, editors. Textbook in Psychiatric Epidemiology. 2nd ed. New York: Wiley-Liss; 2002. pp. 149–163. [Google Scholar]
- Grant JE, Odlaug BL, Kim SW. A double-blind, placebo-controlled study of N-acetyl cysteine plus naltrexone for methamphetamine dependence. Eur Neuropsychopharmacol. 2010;20:823–828. doi: 10.1016/j.euroneuro.2010.06.018. [DOI] [PubMed] [Google Scholar]
- Graziane NM, Polter AM, Briand LA, Pierce RC, Kauer JA. Kappa opioid receptors regulate stress-induced cocaine seeking and synaptic plasticity. Neuron. 2013;77:942–954. doi: 10.1016/j.neuron.2012.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald MK, Lundahl LH, Steinmiller CL. Yohimbine increases opioid-seeking behavior in heroin-dependent, buprenorphine-maintained individuals. Psychopharmacology (Berl) 2013;225:811–824. doi: 10.1007/s00213-012-2868-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Vermeulen-Van der Zee E, te Kortschot A, Witter MP. Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience. 1987;23:103–120. doi: 10.1016/0306-4522(87)90275-2. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, Choi EA, McNally GP. Paraventricular thalamus mediates context-induced reinstatement (renewal) of extinguished reward seeking. Eur J Neurosci. 2009;29:802–812. doi: 10.1111/j.1460-9568.2009.06623.x. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, McNally GP. Renewal of extinguished cocaine-seeking. Neuroscience. 2008;151:659–670. doi: 10.1016/j.neuroscience.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Newby J, McNally GP. The neural correlates and role of D1 dopamine receptors in renewal of extinguished alcohol-seeking. Neuroscience. 2007;146:525–536. doi: 10.1016/j.neuroscience.2007.01.063. [DOI] [PubMed] [Google Scholar]
- Highfield D, Yap J, Grimm J, Shalev U, Shaham Y. Repeated lofexidine treatment attenuates stress-induced, but not drug cues-induced reinstatement of a heroin-cocaine mixture (speedball) seeking in rats. Neuropsychopharmacology. 2001;25:320–331. doi: 10.1016/S0893-133X(01)00227-5. [DOI] [PubMed] [Google Scholar]
- Hunt WA, Barnett LW, Branch LG. Relapse rates in addiction programs. J Clin Psychol. 1971;27:455–456. doi: 10.1002/1097-4679(197110)27:4<455::aid-jclp2270270412>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Hutton-Bedbrook K, McNally GP. The promises and pitfalls of retrieval-extinction procedures in preventing relapse to drug seeking. Front Psychiatry. 2013;4:14. doi: 10.3389/fpsyt.2013.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Charnley JL, Levi EM, Jones E, Yeoh JW, Smith DW, Dayas CV. Orexin-1 receptor signalling within the ventral tegmental area, but not the paraventricular thalamus, is critical to regulating cue-induced reinstatement of cocaine-seeking. Int J Neuropsychopharmacol. 2011;14:684–690. doi: 10.1017/S1461145711000423. [DOI] [PubMed] [Google Scholar]
- Jobes ML, Ghitza UE, Epstein DH, Phillips KA, Heishman SJ, Preston KL. Clonidine blocks stress-induced craving in cocaine users. Psychopharmacology (Berl) 2011;218:83–88. doi: 10.1007/s00213-011-2230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D. NIDA’s naltrexone research program. NIDA Res Monogr. 1976;9:5–11. doi: 10.1037/e497452006-004. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Lalumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56(Suppl 1):169–173. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology. 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kallupi M, Cannella N, Economidou D, Ubaldi M, Ruggeri B, Weiss F, Massi M, Marugan J, Heilig M, Bonnavion P, de Lecea L, Ciccocioppo R. Neuropeptide S facilitates cue-induced relapse to cocaine seeking through activation of the hypothalamic hypocretin system. Proc Natl Acad Sci USA. 2010;107:19567–19572. doi: 10.1073/pnas.1004100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallupi M, de Guglielmo G, Cannella N, Li HW, Calo G, Guerrini R, Ubaldi M, Renger JJ, Uebele VN, Ciccocioppo R. Hypothalamic Neuropeptide S receptor blockade decreases discriminative cue-induced reinstatement of cocaine seeking in the rat. Psychopharmacology. 2012 doi: 10.1007/s00213-012-2910-y. (in press) [DOI] [PubMed] [Google Scholar]
- Katz J, Higgins S. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology. 2003;168:21–30. doi: 10.1007/s00213-003-1441-y. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, LaRowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, Markou A, Kalivas PW. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry. 2009;65:841–845. doi: 10.1016/j.biopsych.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya E, Golden SA, Harvey BK, Guez-Barber DH, Berkow A, Simmons DE, Bossert JM, Nair SG, Uejima JL, Marin MT, Mitchell TB, Farquhar D, Ghosh SC, Mattson BJ, Hope BT. Targeted disruption of cocaine-activated nucleus accumbens neurons prevents context-specific sensitization. Nat Neurosci. 2009;12:1069–1073. doi: 10.1038/nn.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruzich PJ, See RE. Differential Contributions of the Basolateral and Central Amygdala in the Acquisition and Expression of Conditioned Relapse to Cocaine-Seeking Behavior. The Journal of Neuroscience. 2001;21 doi: 10.1523/JNEUROSCI.21-14-j0002.2001. RC155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaresan V, Yuan M, Yee J, Famous KR, Anderson SM, Schmidt HD, Pierce RC. Metabotropic glutamate receptor 5 (mGluR5) antagonists attenuate cocaine priming- and cue-induced reinstatement of cocaine seeking. Behav Brain Res. 2009;202:238–244. doi: 10.1016/j.bbr.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupchik YM, Moussawi K, Tang XC, Wang X, Kalivas BC, Kolokithas R, Ogburn KB, Kalivas PW. The effect of N-acetylcysteine in the nucleus accumbens on neurotransmission and relapse to cocaine. Biol Psychiatry. 2012;71:978–986. doi: 10.1016/j.biopsych.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28:3170–3177. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRowe SD, Myrick H, Hedden S, Mardikian P, Saladin M, McRae A, Brady K, Kalivas PW, Malcolm R. Is cocaine desire reduced by N-acetylcysteine? Am J Psychiatry. 2007;164:1115–1117. doi: 10.1176/ajp.2007.164.7.1115. [DOI] [PubMed] [Google Scholar]
- Larson EB, Graham DL, Arzaga RR, Buzin N, Webb J, Green TA, Bass CE, Neve RL, Terwilliger EF, Nestler EJ, Self DW. Overexpression of CREB in the nucleus accumbens shell increases cocaine reinforcement in self-administering rats. J Neurosci. 2011;31:16447–16457. doi: 10.1523/JNEUROSCI.3070-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Ramirez DR, Xie X, Fuchs RA. Involvement of the lateral orbitofrontal cortex in drug context-induced reinstatement of cocaine-seeking behavior in rats. Eur J Neurosci. 2009;30:1370–1381. doi: 10.1111/j.1460-9568.2009.06906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Wells AM, Xie X, Fuchs RA. Interaction of the basolateral amygdala and orbitofrontal cortex is critical for drug context-induced reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacology. 2011;36:711–720. doi: 10.1038/npp.2010.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Xie X, Ramirez DR, Fuchs RA. Sub-region specific contribution of the ventral hippocampus to drug context-induced reinstatement of cocaine-seeking behavior in rats. Neuroscience. 2010;171:830–839. doi: 10.1016/j.neuroscience.2010.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le A, Shaham Y. Neurobiology of relapse to alcohol in rats. Pharmacol Ther. 2002;94:137–156. doi: 10.1016/s0163-7258(02)00200-0. [DOI] [PubMed] [Google Scholar]
- Le AD, Funk D, Coen K, Li Z, Shaham Y. Role of corticotropin-releasing factor in the median raphe nucleus in yohimbine-induced reinstatement of alcohol seeking in rats. Addict Biol. 2012 doi: 10.1111/j.1369-1600.2011.00374.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Funk D, Harding S, Juzytsch W, Fletcher PJ. The role of noradrenaline and 5-hydroxytryptamine in yohimbine-induced increases in alcohol-seeking in rats. Psychopharmacology. 2009;204:477–488. doi: 10.1007/s00213-009-1481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Funk D, Juzytsch W, Coen K, Navarre BM, Cifani C, Shaham Y. Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology. 2011;218:89–99. doi: 10.1007/s00213-011-2178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Fletcher PJ, Shaham Y. The role of corticotropin-releasing factor in the median raphe nucleus in relapse to alcohol. J Neurosci. 2002;22:7844–7849. doi: 10.1523/JNEUROSCI.22-18-07844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology. 2005;179:366–373. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- Le AD, Poulos CX, Harding S, Watchus W, Juzytsch W, Shaham Y. Effects of naltrexone and fluoxetine on alcohol self-administration and reinstatement of alcohol seeking induced by priming injections of alcohol and exposure to stress in rats. Neuropsychopharmacology. 1999;21:435–444. doi: 10.1016/S0893-133X(99)00024-X. [DOI] [PubMed] [Google Scholar]
- Lee B, Tiefenbacher S, Platt DM, Spealman RD. Pharmacological blockade of alpha(2)-arenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacology. 2004;29:686–693. doi: 10.1038/sj.npp.1300391. [DOI] [PubMed] [Google Scholar]
- Lee JL, Di Ciano P, Thomas KL, Everitt BJ. Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron. 2005;47:795–801. doi: 10.1016/j.neuron.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ. Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci. 2006;26:5881–5887. doi: 10.1523/JNEUROSCI.0323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced, but not cocaine-induced reinstatement, by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leri F, Tremblay A, Sorge RE, Stewart J. Methadone maintenance reduces heroin- and cocaine-induced relapse without affecting stress-induced relapse in a rodent model of poly-drug use. Neuropsychopharmacology. 2004;29:1312–1320. doi: 10.1038/sj.npp.1300435. [DOI] [PubMed] [Google Scholar]
- Li X, DeJoseph MR, Urban JH, Bahi A, Dreyer JLGEM, Ford KA, Ferrario CR, Loweth JA, Wolf ME. Different roles of BDNF in nucleus accumbens core versus shell during the incubation of cue-induced cocaine craving and its long-term maintenance. J Neurosci. 2013;33:1130–1142. doi: 10.1523/JNEUROSCI.3082-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Li J, Gardner EL, Xi ZX. Activation of mGluR7s inhibits cocaine-induced reinstatement of drug-seeking behavior by a nucleus accumbens glutamate-mGluR2/3 mechanism in rats. J Neurochem. 2010;114:1368–1380. doi: 10.1111/j.1471-4159.2010.06851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Bubar MJ, Lanfranco MF, Hillman GR, Cunningham KA. Serotonin2C receptor localization in GABA neurons of the rat medial prefrontal cortex: implications for understanding the neurobiology of addiction. Neuroscience. 2007;146:1677–1688. doi: 10.1016/j.neuroscience.2007.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Caggiula AR, Palmatier MI, Donny EC, Sved AF. Cue-induced reinstatement of nicotine-seeking behavior in rats: effect of bupropion, persistence over repeated tests, and its dependence on training dose. Psychopharmacology (Berl) 2008;196:365–375. doi: 10.1007/s00213-007-0967-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Dempsey J, Liu SY, Bossert JM, Shaham Y. A single infusion of brain-derived neurotrophic factor into the ventral tegmental area induces long-lasting potentiation of cocaine seeking after withdrawal. J Neurosci. 2004;24:1604–1611. doi: 10.1523/JNEUROSCI.5124-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Wang X, Wu P, Xu C, Zhao M, Morales M, Harvey BK, Hoffer BJ, Shaham Y. Role of ventral tegmental area glial cell line-derived neurotrophic factor in incubation of cocaine craving. Biol Psychiatry. 2009;66:137–145. doi: 10.1016/j.biopsych.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G. Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science. 2011;333:353–357. doi: 10.1126/science.1204622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madras BK, Xie Z, Lin Z, Jassen A, Panas H, Lynch L, Johnson R, Livni E, Spencer TJ, Bonab AA, Miller GM, Fischman AJ. Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. J Pharmacol Exp Ther. 2006;319:561–569. doi: 10.1124/jpet.106.106583. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Aston-Jones GS. Fos activation of selective afferents to ventral tegmental area during cue-induced reinstatement of cocaine seeking in rats. J Neurosci. 2012;32:13309–13326. doi: 10.1523/JNEUROSCI.2277-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Hensley-Simon M, Tahsili-Fahadan P, Lalumiere RT, Thomas C, Fallon RV, Kalivas PW, Aston-Jones G. Modafinil attenuates reinstatement of cocaine seeking: role for cystine-glutamate exchange and metabotropic glutamate receptors. Addict Biol. 2012a doi: 10.1111/j.1369-1600.2012.00506.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Aston-Jones G. Interactions between VTA orexin and glutamate in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2012b doi: 10.1007/s00213-012-2681-5. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Furlong TM, McNally GP. Medial dorsal hypothalamus mediates the inhibition of reward seeking after extinction. J Neurosci. 2010;30:14102–14115. doi: 10.1523/JNEUROSCI.4079-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Hamlin AS, McNally GP. Lateral hypothalamus is required for context-induced reinstatement of extinguished reward seeking. J Neurosci. 2009;29:1331–1342. doi: 10.1523/JNEUROSCI.5194-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Li X, Shaham Y. Recent developments in animal models of drug relapse. Curr Opin Neurobiol. 2013 doi: 10.1016/j.conb.2013.01.003. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Millan EZ, McNally GP. The hypothalamus and the neurobiology of drug seeking. Cell Mol Life Sci. 2012;69:581–597. doi: 10.1007/s00018-011-0817-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Le AD. Opioid receptors in the basolateral amygdala but not dorsal hippocampus mediate context-induced alcohol seeking. Behav Brain Res. 2010;211:58–63. doi: 10.1016/j.bbr.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. Time-dependent processes in memory storage. Science. 1966;153:1351–1358. doi: 10.1126/science.153.3742.1351. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory--a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology. 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- Meil WM, See RE. Conditioned cued recovery of responding following prolonged withdrawal from self-administered cocaine in rats: an animal model of relapse. Behav Pharmacol. 1996;7:754–763. [PubMed] [Google Scholar]
- Millan EZ, Furlong TM, McNally GP. Accumbens shell-hypothalamus interactions mediate extinction of alcohol seeking. J Neurosci. 2010;30:4626–4635. doi: 10.1523/JNEUROSCI.4933-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan EZ, Marchant NJ, McNally GP. Extinction of drug seeking. Behav Brain Res. 2011;217:454–462. doi: 10.1016/j.bbr.2010.10.037. [DOI] [PubMed] [Google Scholar]
- Millan EZ, McNally GP. Accumbens shell AMPA receptors mediate expression of extinguished reward seeking through interactions with basolateral amygdala. Learn Mem. 2011;18:414–421. doi: 10.1101/lm.2144411. [DOI] [PubMed] [Google Scholar]
- Millan EZ, Milligan-Saville J, McNally GP. Memory retrieval, extinction, and reinstatement of alcohol seeking. Neurobiol Learn Mem. 2013;101:26–32. doi: 10.1016/j.nlm.2012.12.010. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Newman-Tancredi A, Quentric Y, Cussac D. The “selective” dopamine D1 receptor antagonist, SCH23390, is a potent and high efficacy agonist at cloned human serotonin2C receptors. Psychopharmacology (Berl) 2001;156:58–62. doi: 10.1007/s002130100742. [DOI] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005a;47:873–884. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005b;47:873–884. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Milton AL, Everitt BJ. The persistence of maladaptive memory: addiction, drug memories and anti-relapse treatments. Neurosci Biobehav Rev. 2012;36:1119–1139. doi: 10.1016/j.neubiorev.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Milton AL, Lee JL, Butler VJ, Gardner R, Everitt BJ. Intra-amygdala and systemic antagonism of NMDA receptors prevents the reconsolidation of drug-associated memory and impairs subsequently both novel and previously acquired drug-seeking behaviors. J Neurosci. 2008;28:8230–8237. doi: 10.1523/JNEUROSCI.1723-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misanin JR, Miller RR, Lewis DJ. Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science. 1968;160:554–555. doi: 10.1126/science.160.3827.554. [DOI] [PubMed] [Google Scholar]
- Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science. 2009;324:951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Zhou W, Shen H, Reichel CM, See RE, Carr DB, Kalivas PW. Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse. Proc Natl Acad Sci U S A. 2011;108:385–390. doi: 10.1073/pnas.1011265108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D, Stewart J. Cocaine-induced conditioned place preference: reinstatement by priming injections of cocaine after extinction. Behav Brain Res. 2000;115:39–47. doi: 10.1016/s0166-4328(00)00239-4. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, LeDoux JE. The labile nature of consolidation theory. Nat Rev Neurosci. 2000;1:216–219. doi: 10.1038/35044580. [DOI] [PubMed] [Google Scholar]
- Nair SG, Adams-Deutsch T, Epstein DH, Shaham Y. The neuropharmacology of relapse to food seeking: methodology, main findings, and comparison with relapse to drug seeking. Prog Neurobiol. 2009;89:18–45. doi: 10.1016/j.pneurobio.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien CP. Anticraving medications for relapse prevention: a possible new class of psychoactive medications. Am J Psychiatry. 2005;162:1423–1431. doi: 10.1176/appi.ajp.162.8.1423. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, McLellan AT, Ehrman R. Classical conditioning in drug-dependent humans. Ann N Y Acad Sci. 1992;654:400–415. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- O'Connor EC, Parker D, Rollema H, Mead AN. The alpha4beta2 nicotinic acetylcholine-receptor partial agonist varenicline inhibits both nicotine self-administration following repeated dosing and reinstatement of nicotine seeking in rats. Psychopharmacology. 2010;208:365–376. doi: 10.1007/s00213-009-1739-5. [DOI] [PubMed] [Google Scholar]
- O'Neill CE, LeTendre ML, Bachtell RK. Adenosine A2A receptors in the nucleus accumbens bi-directionally alter cocaine seeking in rats. Neuropsychopharmacology. 2012;37:1245–1256. doi: 10.1038/npp.2011.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5 edn. Amsterdam: Elsevier Academic Press; 2005. [Google Scholar]
- Pentkowski NS, Duke FD, Weber SM, Pockros LA, Teer AP, Hamilton EC, Thiel KJ, Neisewander JL. Stimulation of medial prefrontal cortex serotonin 2C (5-HT(2C)) receptors attenuates cocaine-seeking behavior. Neuropsychopharmacology. 2010;35:2037–2048. doi: 10.1038/npp.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008;28:6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of incubation of drug craving. Trends Neurosci. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockros LA, Pentkowski NS, Swinford SE, Neisewander JL. Blockade of 5-HT2A receptors in the medial prefrontal cortex attenuates reinstatement of cue-elicited cocaine-seeking behavior in rats. Psychopharmacology. 2011;213:307–320. doi: 10.1007/s00213-010-2071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushparaj A, Hamani C, Yu W, Shin DS, Kang B, Nobrega JN, Le Foll B. Electrical stimulation of the insular region attenuates nicotine-taking and nicotine-seeking behaviors. Neuropsychopharmacology. 2013;38:690–698. doi: 10.1038/npp.2012.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez DR, Bell GH, Lasseter HC, Xie X, Traina SA, Fuchs RA. Dorsal hippocampal regulation of memory reconsolidation processes that facilitate drug context-induced cocaine-seeking behavior in rats. Eur J Neurosci. 2009;30:901–912. doi: 10.1111/j.1460-9568.2009.06889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redila VA, Chavkin C. Stress-induced reinstatement of cocaine seeking is mediated by the kappa opioid system. Psychopharmacology. 2008;200:59–70. doi: 10.1007/s00213-008-1122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Moussawi K, Do PH, Kalivas PW, See RE. Chronic N-acetylcysteine during abstinence or extinction after cocaine self-administration produces enduring reductions in drug seeking. J Pharmacol Exp Ther. 2011;337:487–493. doi: 10.1124/jpet.111.179317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rocha A, Kalivas PW. Role of the prefrontal cortex and nucleus accumbens in reinstating methamphetamine seeking. Eur J Neurosci. 2010;31:903–909. doi: 10.1111/j.1460-9568.2010.07134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JL, Ghee S, See RE. The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience. 2008;151:579–588. doi: 10.1016/j.neuroscience.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor TC. How does PKMzeta maintain long-term memory? Nat Rev Neurosci. 2011;12:9–15. doi: 10.1038/nrn2949. [DOI] [PubMed] [Google Scholar]
- Sanchez H, Quinn JJ, Torregrossa MM, Taylor JR. Reconsolidation of a cocaine-associated stimulus requires amygdalar protein kinase A. J Neurosci. 2010;30:4401–4407. doi: 10.1523/JNEUROSCI.3149-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Bruno JP. Animal models in biological psychiatry. In: D'haenen H, den Boer JA, Willner P, editors. Biological psychiatry. Hoboken, NJ: Johns Willey & Sons Ltd; 2002. pp. 1–8. [Google Scholar]
- Schmidt HD, Anderson SM, Famous KR, Kumaresan V, Pierce RC. Anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. Eur J Pharmacol. 2005;526:65–76. doi: 10.1016/j.ejphar.2005.09.068. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Famous KR, Pierce RC. The limbic circuitry underlying cocaine seeking encompasses the PPTg/LDT. Eur J Neurosci. 2009;30:1358–1369. doi: 10.1111/j.1460-9568.2009.06904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE. Neural substrates of cocaine-cue associations that trigger relapse. Eur J Pharmacol. 2005;526:140–146. doi: 10.1016/j.ejphar.2005.09.034. [DOI] [PubMed] [Google Scholar]
- See RE. Dopamine D1 receptor antagonism in the prelimbic cortex blocks the reinstatement of heroin-seeking in an animal model of relapse. Int J Neuropsychopharmacol. 2009;12:431–436. doi: 10.1017/S1461145709000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self DW. Regulation of drug-taking and -seeking behaviors by neuroadaptations in the mesolimbic dopamine system. Neuropharmacology. 2004;47(Suppl 1):242–255. doi: 10.1016/j.neuropharm.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Self DW, Genova LM, Hope BT, Barnhart WJ, Spencer JJ, Nestler EJ. Involvement of cAMP-dependent protein kinase in the nucleus accumbens in cocaine self administration and relapse of cocaine-seeking behavior. JNeurosci. 1998;18:1848–1859. doi: 10.1523/JNEUROSCI.18-05-01848.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self DW, Nestler EJ. Relapse to drug-seeking: neural and molecular mechanisms. Drug Alcohol Depend. 1998;51:49–69. doi: 10.1016/s0376-8716(98)00065-9. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Rev. 2000a;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, Stewart J. Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J Neurosci. 1997;17:2605–2614. doi: 10.1523/JNEUROSCI.17-07-02605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Highfield D, Delfs JM, Leung S, Stewart J. Clonidine blocks stress-induced reinstatement of heroin seeking in rats: an effect independent of the locus coeruleus noradrenergic neurons. Eur J Neurosci. 2000b;12:292–302. doi: 10.1046/j.1460-9568.2000.00899.x. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Stress reinstates heroin-seeking in drug-free animals: An effect mimicking heroin, not withdrawal. Psychopharmacology. 1995;119:334–341. doi: 10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- Shalev U, Erb S, Shaham Y. Role of CRF and other neuropeptides in stress-induced reinstatement of drug seeking. Brain Res. 2010;1314:15–28. doi: 10.1016/j.brainres.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Shalev U, Highfield D, Yap J, Shaham Y. Stress and relapse to drug seeking in rats: studies on the generality of the effect. Psychopharmacology. 2000;150:337–346. doi: 10.1007/s002130000441. [DOI] [PubMed] [Google Scholar]
- Shalev U, Morales M, Hope B, Yap J, Shaham Y. Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology. 2001;156:98–107. doi: 10.1007/s002130100748. [DOI] [PubMed] [Google Scholar]
- Shen H, Moussawi K, Zhou W, Toda S, Kalivas PW. Heroin relapse requires long-term potentiation-like plasticity mediated by NMDA2b-containing receptors. Proc Natl Acad Sci USA. 2011;108:19407–19412. doi: 10.1073/pnas.1112052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Simmons D, Self DW. Role of mu- and delta-opioid receptors in the nucleus accumbens in cocaine-seeking behavior. Neuropsychopharmacology. 2009;34:1946–1957. doi: 10.1038/npp.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Haass-Koffler CL, Bito-Onon J, Li R, Bartlett SE. Mifepristone in the central nucleus of the amygdala reduces yohimbine stress-induced reinstatement of ethanol-seeking. Neuropsychopharmacology. 2012;37:906–918. doi: 10.1038/npp.2011.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair CM, Cleva RM, Hood LE, Olive MF, Gass JT. mGluR5 receptors in the basolateral amygdala and nucleus accumbens regulate cue-induced reinstatement of ethanol-seeking behavior. Pharmacol Biochem Behav. 2012;101:329–335. doi: 10.1016/j.pbb.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Catapano D, O'Mally S. Stress-induced craving and stress responses in cocaine dependent individuals. Psychopharmacology. 1999;142:343–351. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O'Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology. 2000;152:140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63:324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Sinha R, Kimmerling A, Doebrick C, Kosten TR. Effects of lofexidine on stress-induced and cue-induced opioid craving and opioid abstinence rates: preliminary findings. Psychopharmacology. 2007;190:569–574. doi: 10.1007/s00213-006-0640-8. [DOI] [PubMed] [Google Scholar]
- Sinha R, Shaham Y, Heilig M. Translational and reverse translational research on the role of stress in drug craving and relapse. Psychopharmacology. 2011;218:69–82. doi: 10.1007/s00213-011-2263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. alpha(2) Adrenergic and imidazoline receptor agonists prevent cue-induced cocaine seeking. Biol Psychiatry. 2011;70:712–719. doi: 10.1016/j.biopsych.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg BA. Reconsolidation of drug memories. Neurosci Biobehav Rev. 2012;36:1400–1417. doi: 10.1016/j.neubiorev.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge RE, Rajabi H, Stewart J. Rats maintained chronically on buprenorphine show reduced heroin and cocaine seeking in tests of extinction and drug-induced reinstatement. Neuropsychopharmacology. 2005;30:1681–1692. doi: 10.1038/sj.npp.1300712. [DOI] [PubMed] [Google Scholar]
- Spealman RD, Barrett-Larimore RL, Rowlett JK, Platt DM, Khroyan TV. Pharmacological and environmental determinants of relapse to cocaine-seeking behavior. Pharmacol Biochem Behav. 1999;64:327–336. doi: 10.1016/s0091-3057(99)00049-0. [DOI] [PubMed] [Google Scholar]
- Stefanik MT, Moussawi K, Kupchik YM, Smith KC, Miller RL, Huff ML, Deisseroth K, Kalivas PW, Lalumiere RT. Optogenetic inhibition of cocaine seeking in rats. Addict Biol. 2013;18:50–53. doi: 10.1111/j.1369-1600.2012.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J. Reinstatement of heroin and cocaine self-administration behavior in the rat by intracerebral application of morphine in the ventral tegmental area. Pharmacol Biochem Behav. 1984;20:917–923. doi: 10.1016/0091-3057(84)90017-0. [DOI] [PubMed] [Google Scholar]
- Stewart J, de Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev. 1984;91:251–268. [PubMed] [Google Scholar]
- Stewart J, Vezina P. A comparison of the effects of intra-accumbens injections of amphetamine and morphine on reinstatement of heroin intravenous self-administration behavior. Brain Res. 1988;457:287–294. doi: 10.1016/0006-8993(88)90698-1. [DOI] [PubMed] [Google Scholar]
- Stine SM, Southwick SM, Petrakis IL, Kosten TR, Charney DS, Krystal JH. Yohimbine-induced withdrawal and anxiety symptoms in opioid-dependent patients. Biol Psychiatry. 2002;51:642–651. doi: 10.1016/s0006-3223(01)01292-6. [DOI] [PubMed] [Google Scholar]
- Sun W, Rebec GV. Lidocaine inactivation of ventral subiculum attenuates cocaine-seeking behavior in rats. J Neurosci. 2003;23:10258–10264. doi: 10.1523/JNEUROSCI.23-32-10258.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theberge FR, Li X, Kambhampati S, Pickens CL, Bossert JM, Baumann MH, Hutchinson MR, Rice RC, Watkins LR, Shaham Y. Effect of chronic delivery of the Toll-like receptor 4 antagonist (+)-Naltrexone on incubation of heroin craving. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2012.12.019. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theberge FR, Milton AL, Belin D, Lee JL, Everitt BJ. The basolateral amygdala and nucleus accumbens core mediate dissociable aspects of drug memory reconsolidation. Learn Mem. 2010;17:444–453. doi: 10.1101/lm.1757410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theberge FR, Pickens CL, Goldart E, Fanous S, Hope BT, Liu QR, Shaham Y. Association of time-dependent changes in mu opioid receptor mRNA, but not BDNF, TrkB, or MeCP2 mRNA and protein expression in the rat nucleus accumbens with incubation of heroin craving. Psychopharmacology. 2012;224:559–571. doi: 10.1007/s00213-012-2784-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin S, Sedki F, Abbas Z, Shalev U. Antagonism of the dopamine D1-like receptor in mesocorticolimbic nuclei attenuates acute food deprivation-induced reinstatement of heroin seeking in rats. Eur J Neurosci. 2013;37:972–981. doi: 10.1111/ejn.12112. [DOI] [PubMed] [Google Scholar]
- Torregrossa MM, Taylor JR. Learning to forget: manipulating extinction and reconsolidation processes to treat addiction. Psychopharmacology. 2012 doi: 10.1007/s00213-012-2750-9. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umhau JC, Schwandt ML, Usala J, Geyer C, Singley E, George DT, Heilig M. Pharmacologically induced alcohol craving in treatment seeking alcoholics correlates with alcoholism severity, but is insensitive to acamprosate. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2010.253. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerstedt U. Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiol Scand. 1971;(Suppl 367):1–48. doi: 10.1111/j.1365-201x.1971.tb10998.x. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Platt DM, Rowlett JK, Ruedi-Bettschen D, Spealman RD. Kappa agonist-induced reinstatement of cocaine seeking in squirrel monkeys: a role for opioid and stress-related mechanisms. J Pharmacol Exp Ther. 2007;323:525–533. doi: 10.1124/jpet.107.125484. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL. Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science. 2001;292:1175–1178. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, You ZB, Rice KC, Wise RA. Stress-induced relapse to cocaine seeking: roles for the CRF(2) receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology. 2007;193:283–294. doi: 10.1007/s00213-007-0782-3. [DOI] [PubMed] [Google Scholar]
- Wang B, You ZB, Wise RA. Reinstatement of cocaine seeking by hypocretin (orexin) in the ventral tegmental area: independence from the local corticotropin-releasing factor network. Biol Psychiatry. 2009;65:857–862. doi: 10.1016/j.biopsych.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, You ZB, Wise RA. Heroin self-administration experience establishes control of ventral tegmental glutamate release by stress and environmental Stimuli. Neuropsychopharmacology. 2012 doi: 10.1038/npp.2012.167. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Luo YX, He YY, Li FQ, Shi HS, Xue LF, Xue YX, Lu L. Nucleus accumbens core mammalian target of rapamycin signaling pathway is critical for cue-induced reinstatement of cocaine seeking in rats. J Neurosci. 2010;30:12632–12641. doi: 10.1523/JNEUROSCI.1264-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Moussawi K, Knackstedt L, Shen H, Kalivas PW. Role of mGluR5 neurotransmission in reinstated cocaine-seeking. Addict Biol. 2013;18:40–49. doi: 10.1111/j.1369-1600.2011.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F. Neurobiology of craving, conditioned reward and relapse. Curr Opin Pharmacol. 2005;5:9–19. doi: 10.1016/j.coph.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smit DL, Ben-Shahar O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci USA. 2000;97:4321–4326. doi: 10.1073/pnas.97.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells AM, Arguello AA, Xie X, Blanton MA, Lasseter HC, Reittinger AM, Fuchs RA. Extracellular Signal-Regulated Kinase in the Basolateral Amygdala, but not the Nucleus Accumbens Core, is Critical for Context-Response-Cocaine Memory Reconsolidation in Rats. Neuropsychopharmacology. 2012 doi: 10.1038/npp.2012.238. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells AM, Lasseter HC, Xie X, Cowhey KE, Reittinger AM, Fuchs RA. Interaction between the basolateral amygdala and dorsal hippocampus is critical for cocaine memory reconsolidation and subsequent drug context-induced cocaine-seeking behavior in rats. Learn Mem. 2011;18:693–702. doi: 10.1101/lm.2273111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins A, Smith RJ, Shen HW, Kalivas PW. Integrins modulate relapse to cocaine-seeking. J Neurosci. 2011;31:16177–16184. doi: 10.1523/JNEUROSCI.3816-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcocks AL, McNally GP. The role of medial prefrontal cortex in extinction and reinstatement of alcohol-seeking in rats. Eur J Neurosci. 2013;37:259–268. doi: 10.1111/ejn.12031. [DOI] [PubMed] [Google Scholar]
- Willins DL, Deutch AY, Roth BL. Serotonin 5-HT2A receptors are expressed on pyramidal cells and interneurons in the rat cortex. Synapse. 1997;27:79–82. doi: 10.1002/(SICI)1098-2396(199709)27:1<79::AID-SYN8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Xi ZX, Gilbert J, Campos AC, Kline N, Ashby CR, Jr., Hagan JJ, Heidbreder CA, Gardner EL. Blockade of mesolimbic dopamine D3 receptors inhibits stress-induced reinstatement of cocaine-seeking in rats. Psychopharmacology. 2004;176:57–65. doi: 10.1007/s00213-004-1858-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Lasseter HC, Ramirez DR, Ponds KL, Wells AM, Fuchs RA. Subregion-specific role of glutamate receptors in the nucleus accumbens on drug context-induced reinstatement of cocaine-seeking behavior in rats. Addict Biol. 2012;17:287–299. doi: 10.1111/j.1369-1600.2011.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Ramirez DR, Lasseter HC, Fuchs RA. Effects of mGluR1 antagonism in the dorsal hippocampus on drug context-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology. 2010;208:1–11. doi: 10.1007/s00213-009-1700-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YL, Reinscheid RK, Huitron-Resendiz S, Clark SD, Wang Z, Lin SH, Brucher FA, Zeng J, Ly NK, Henriksen SJ, de Lecea L, Civelli O. Neuropeptide S: a neuropeptide promoting arousal and anxiolytic-like effects. Neuron. 2004;43:487–497. doi: 10.1016/j.neuron.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Xue YX, Luo YX, Wu P, Shi HS, Xue LF, Chen C, Zhu WL, Ding ZB, Bao YP, Shi J, Epstein DH, Shaham Y, Lu L. A memory retrieval-extinction procedure to prevent drug craving and relapse. Science. 2012;336:241–245. doi: 10.1126/science.1215070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H, Bruijnzeel AW. Stimulation of alpha2-adrenergic receptors in the central nucleus of the amygdala attenuates stress-induced reinstatement of nicotine seeking in rats. Neuropharmacology. 2011;60:303–311. doi: 10.1016/j.neuropharm.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun IA, Fields HL. Basolateral amygdala lesions impair both cue- and cocaine-induced reinstatement in animals trained on a discriminative stimulus task. Neuroscience. 2003;121:747–757. doi: 10.1016/s0306-4522(03)00531-1. [DOI] [PubMed] [Google Scholar]
- Zislis G, Desai TV, Prado M, Shah HP, Bruijnzeel AW. Effects of the CRF receptor antagonist D-Phe CRF(12–41) and the alpha2-adrenergic receptor agonist clonidine on stress-induced reinstatement of nicotine-seeking behavior in rats. Neuropharmacology. 2007;53:958–966. doi: 10.1016/j.neuropharm.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]