Abstract
BACKGROUND
Approximately 18% of pregnant women continue to smoke tobacco cigarettes throughout pregnancy. Offspring exposed to tobacco smoke in utero exhibit a higher incidence of drug use in later stages of development relative to non-exposed children. Animal models indicate that prenatal nicotine (PN) exposure alone alters the development of the mesocorticolimbic dopamine (DA) system, which, in part, organizes motivated behavior and reward. The orexin/hypocretin neuropeptide system, which originates in the lateral hypothalamus (LH), projects to key areas of the mesocorticolimbic DA pathway. Previous research suggests that orexin exerts a major influence on motivation and reward.
METHODS
The present experiments determined if intravenous (IV) PN exposure alters (1) the expression of orexin neurons and melanin-concentrating hormone (MCH; positive control) in the LH; and (2) orexin projections from the LH onto DA neurons in the ventral tegmental area (VTA). Dams were injected with IV nicotine (0.05 mg/kg/injection) or saline 3×/day during gestational days 8–21. Tissues from adult male offspring (~130 days) were examined using immunohistochemistry.
RESULTS
Relative to controls, offspring of IV PN exposure showed (1) increased numbers of orexin neurons in the LH, and no changes in the expression of MCH; and (2) increased orexin appositions on DA cells in the VTA.
CONCLUSION
The findings indicate that the influence of PN exposure is enduring, and suggests that the PN-induced modification of orexin expression on mesolimbic circuitry may contribute to the reported changes in motivated behaviors related to food and drug reward observed in offspring prenatally exposed to nicotine.
Keywords: prenatal nicotine, intravenous, orexin, lateral hypothalamus, ventral tegmental area, immunohistochemistry, rats
1. INTRODUCTION
Between 2002 and 2011, approximately 18% of women in the US reported smoking during pregnancy (~17.6 % in 2010–2011; SAMHSA, 2012). In utero tobacco smoke exposure decreases fetal growth and causes developmental abnormalities including low birth weight, decreased lung growth and pulmonary function, and increased incidence of sudden infant death syndrome (Castles et al., 1999; Cornelius and Day, 2009; DiFranza et al., 2004; Fleming and Blair, 2007). Neurocognitive deficits, such as language acquisition delays, auditory processing deficits, and attention deficit hyperactivity disorder are reported following prenatal exposure to maternal smoking (Button et al., 2007; DiFranza et al., 2004; Fergusson et al., 1998; Linnet et al., 2003). Prenatal tobacco smoke exposure also increases the likelihood of drug use during adolescence and adulthood (Buka et al., 2003; Kandel et al., 1994; Weissman et al., 1999). These findings suggest that maternal smoking has enduring effects on the neurodevelopment and motivated behavior of offspring (Buka et al., 2003; Cornelius and Day, 2009; Kandel et al., 1994).
Nicotine is the key compound in tobacco that maintains cigarette smoking behavior (Benowitz et al., 2009; Corrigall and Coen, 1989). Various animal models are therefore used to assess the effects of prenatal nicotine (PN) on brain development (Dwyer et al., 2009). In these models, nicotine alone is administered continuously by osmotic minipump, (Dwyer et al., 2008; Slotkin et al., 1987a, 1987b) orally through the animal's drinking water (Pauly et al., 2004; Schneider et al., 2010; Zhu et al., 1996, 2012), or intravenously (Lacy et al., 2012; LeSage et al., 2006). Research investigating PN exposure demonstrates that PN acts as a teratogen: exposure to continuous PN reduces brain cell number (Slotkin et al., 1987a, 1987b), and alters cell replication, cell survival, and synaptogenesis in utero, relative to control animals (Navarro et al., 1989; Slikker et al., 2005; Slotkin, 2004).
A major focus of research investigating the effects of PN exposure is on the development of neural systems that organize motivated behavior (Dwyer et al., 2009). One hypothesis being tested is that PN alone alters the development of the mesocorticolimbic dopamine (DA) system, which is primarily composed of the ventral tegmental area (VTA), the nucleus accumbens (NAcc), and the prefrontal cortex (Edwards and Koob, 2010; Everitt et al., 2008; Kalivas, 2009; Robinson and Berridge, 2003; Wise and Bozarth, 1987), and that these modifications mediate the changes in motivated behavior observed in human offspring of maternal tobacco smoking, e.g., increasing the vulnerability to drug dependence (Kandel et al., 1994; Weissman et al., 1999). PN exposure produced alterations in DA neurons in fetal and preweanling rats (Navarro et al., 1988; Ribary and Lichtensteiger, 1989) and resulted in decreased striatal DA concentrations and D2 receptors in weanlings (Richardson and Tizabi, 1994). Adolescent offspring, exposed to PN, exhibited increased c-fos expression in the infralimbic cortex and NAcc core (Park et al., 2006) and decreased nicotine-evoked DA release in the NAcc shell (Kane et al., 2004). PN altered MAPK and PI3K signaling pathways (Wei et al., 2011) and produced increased mRNA expression and protein levels of brain-derived neurotrophic factor throughout the mesocorticolimbic DA system (Harrod et al., 2011; Wei et al., 2011), relative to saline treated controls. Together these results demonstrate that PN alters the development of the motivational system and suggests that nicotine exposure via maternal smoking directly contributes to the increased drug abuse liability observed in human offspring of maternal smoke exposure (Buka et al., 2003; Cornelius and Day, 2009; Kandel et al., 1994; Weissman et al., 1999).
Other neurophysiological systems modulate the activity of the mesocorticolimbic DA system and therefore influence motivated behavior. One example is the orexin/hypocretin system (Aston-Jones et al., 2009, 2010; Cason et al., 2010; Espana et al., 2011; Kenny, 2011), which originates in the lateral hypothalamus and projects to the VTA, NAcc, and PFC (Alberto et al., 2006; Fadel and Deutch, 2002; Mondal et al., 1999; Sakurai et al., 1998), as well as numerous other structures throughout the brain (Nambu et al., 1999; Peyron et al., 1998). Orexin neurons release the neuropeptides orexin A (OxA) which binds both the orexin-1 (Ox1R) and orexin-2 (Ox2R) receptors, and orexin B (OxB) which is selective for Ox2R (Sakurai et al., 1998). Orexin neurons exhibit reciprocal connections with DA neurons within the VTA (Alberto et al., 2006; Bubser et al., 2005) and these neuropeptides modulate DA release to the PFC (Vittoz and Berridge, 2006; Vittoz et al., 2008). Orexin knockout mice and rats treated with an Ox1R antagonist show altered basal DA signaling and diminished DA responses to cocaine (Espana et al., 2010). VTA DA signaling is also critical for drug-dependent behavioral sensitization and synaptic plasticity (Bonci and Borgland, 2009; Borgland et al., 2006). Cocaine seeking (Smith et al., 2009, 2010; Zhou et al., 2008), morphine place preference (Sharf et al., 2010a) and chronic alcohol intake (Lawrence et al., 2006; Stettner et al., 2011; Voorhees and Cunningham, 2011) are associated with increased indices of orexin signaling in the LH.
It is unlikely that PN-induced changes within the mesocorticolimbic DA system alone account for the modifications in the motivated behavior of rodents described above, given that PN exposure has been shown to modify receptor systems and signaling pathways in multiple brain regions of offspring (Harrod et al., 2011; Lawrence et al., 2006; Navarro et al., 1989; Park et al., 2006; Richardson and Tizabi, 1994; Slotkin et al., 1987a, 1987b; Stettner et al., 2011; Voorhees and Cunningham, 2011; Wei et al., 2011). The present set of experiments focused on the effects of PN on the orexin/hypocretin system because it is documented to play an important role in motivation and reward (Aston-Jones et al., 2009, 2010; Harris et al., 2005; Sharf et al., 2010b; Smith et al., 2009), and abundant evidence from adult animals suggests that the orexin system may mediate certain effects of acute or chronic nicotine administration. For example, nicotine alters orexin expression and neuronal activation (Corrigall, 2009; Kane et al., 2001, 2000; Pasumarthi and Fadel, 2008; Pasumarthi et al., 2006). Also, nicotine self-administration increases orexin receptor mRNA in the arcuate nucleus (LeSage et al., 2010), and administering nicotine directly into the LH area containing orexin neurons stimulates local glutamate and acetylcholine efflux (Pasumarthi and Fadel, 2010). In adult animals, chronic exposure to nicotine via daily IP injection (2–4 mg/kg for 14 days), resulted in increased expression of mRNA for prepro-orexin, OxA and OxB, and both orexin receptors, OX1R and OX2R, in the hypothalamus (Kane et al., 2000). Orexin signaling is also required for the maintenance of nicotine self-administration in adult rats (Hollander et al., 2008; Kenny, 2011). Moreover Boychuk and Heyward, (2011) reported that continuous PN, delivered via osmotic minipump, resulted in decreased expression of prepro-orexin mRNA in the LH of adolescent rats.
The present experiments utilized a low-dose IV PN exposure method that has been shown to alter the expression of brain-derived neurotrophic factor in offspring (Harrod et al., 2011), and to produce alterations in various behavioral assays including prepulse inhibition of the acoustic startle response (Lacy et al., 2011), sucrose-maintained responding (Lacy et al., 2012), and methamphetamine self-administration (Harrod et al., 2012). We determined if PN exposure altered the expression of orexin in the LH and in its projections to the VTA using immunohistochemical techniques in tissue from adult offspring (~130 days) that were prenatally exposed to nicotine or saline. This is the first experiment to investigate the effects of PN exposure on orexin expression in the VTA. Based on previous findings, it was hypothesized that IV PN exposure would result in altered expression of orexin in the LH and in the VTA.
2. METHODS
2.1 Animals
A total of 45 female and 15 male experimentally naïve Sprague-Dawley rats were acquired from Harlan Industries, Inc. (Indianapolis, IN). Animals were transported to the University of South Carolina, and were allowed to acclimate to a colony room located in the Department of Psychology for seven days prior to breeding. Rodent food (ProLab Rat/Mouse/Hamster Chow 3000) and water were provided ad libitum throughout the course of the experiments. All animal cages were provided with Nylabones (Nylabone, Inc.; long lasting durable chew-original; Neptune, NJ) and Nestlets (Nestlets; Ancare, Bellmore, NY), for purposes of environmental enrichment throughout the duration of the study. A Nylabone was replaced if it was thoroughly chewed, and one Nestlet nesting product was placed in the animals' cage when the cage was changed, which occurred 2×/week. The animal colony room was maintained at 21±2 °C, 50±10% relative humidity and the photoperiod was defined by a 12L:12D cycle (i.e., lights on at 0700h). The protocol for this research was approved by the Institutional Animal Care and Use Committee (IACUC) at the University of South Carolina.
2.2 Surgery: Indwelling, interiorized jugular catheters
Nulliparous females were implanted with interiorized jugular catheters as previously described (Mactutus et al., 1994). The catheters were implanted at Harlan Laboratories Inc. (Indianapolis, IN) prior to the rats' arrival at the animal care facilities at the University of South Carolina. Briefly, animals were anesthetized with a mixture of xylazine (3.3 mg/kg/ml) and ketamine hydrochloride (100 mg/kg/ml). Following anesthesia, a sterile Intracath IV catheter (Becton, Dickinson and Co., Franklin Lakes, NJ), equipped with a Luer-Lok injection cap (Medex, Inc., Carlsbad, CA) was implanted dorsally into a subcutaneous pouch. The distal end of the catheter was inserted into the left jugular vein, advanced toward the heart, and the catheter was secured with sterile suture. Animals were kept under periodic post-operative observation and returned to the colony upon recovery. On the day following surgery, catheters were flushed with 0.2 ml of heparinized saline to maintain patency.
2.3 Breeding
Following acclimation to the colony room at the University of South Carolina, females were group housed three per cage, and one male rat was introduced per cage overnight, from 1700h to 0900h. The results of the breeding from the previous night were assessed daily at 0900h. Female rats were vaginally lavaged and samples were examined under a microscope (10×). The presence of sperm and the cycle of estrous were used to determine pregnancy. If sperm was identified the animal was single-caged and this day represented gestational day (GD) 0. If no sperm was present in the lavage specimen, the cycle of estrous was recorded and the animal remained group-housed and bred until determined pregnant. The weights of the pregnant dams were recorded daily during pregnancy.
2.4 Intravenous PN treatment
Pregnant dams were assigned to either PN (0.05 mg/kg/injection), or prenatal saline (PS) groups. From gestational day (GD) 8–21, dams were administered saline (vehicle) or nicotine 3×/day, via the internalized jugular catheter. An organism's susceptibility to a teratogen can vary depending on the stage of development (Vorhees, 1986). Fertilized egg implantation occurs on approximately GD 6, and teratogens generally have an all-or-none effect on the organism during the preimplantation phase of development, which is considered to be approximately GD 1–7. GD 8–21 was chosen as the period of IV nicotine administration because this time includes the initial stages of neurogenesis and migration, synaptogenesis, gliogenesis, and myelination. Thus, this period represents a window of prenatal susceptibility for nicotine's teratogenic effects on neural development (Dwyer et al., 2008). Injections were delivered through the Luer-Lok injection cap of the subcutaneously implanted injection port. Following the first and second injections catheters were “post-flushed” with 0.2 ml of 0.9% physiological saline because 0.2 ml represents the approximate volume of the catheter. Post flush of 0.2 ml of heparinized saline post-flush was used to flush the catheter and to maintain catheter patency after the third, daily nicotine injection. All IV nicotine, saline, and post-flush injections were 20 seconds in duration. All injections were performed during the light portion of the photoperiod, and injections were administered daily at approximately 1000, 1300, and 1600 hours.
2.5 Litter composition, surrogate fostering, and postnatal testing
The day of birth was considered postnatal day (PND) 0. On PND 1, litters were culled to 10, with 5 males and 5 females whenever possible. All pups were surrogate-fostered to timed-pregnant, drug naïve dams, to prevent poor maternal care (Vorhees, 1986). To assess developmental milestones, the righting reflex, negative geotaxis, and eye opening were assessed on PNDs 3–5, 8–10, and 13–17, respectively. The righting reflex was assessed by placing pups onto their backs, and recording the time from being released to rolling over onto their stomach. The maximum latency for each animal to right itself was 25s per trial, with 3 consecutive trials, on PNDs 3–5. Negative geotaxis was assessed by placing animals at a downward 25-degree angle on a wire mesh grid. Pups were placed with their heads facing downward toward the slope of the grid, with a maximum latency of 30s for the pup to turn 180-degrees and face up the slope. Animals were tested for three consecutive trials on PNDs 8–10. Eye opening was assessed by checking each eye for degree of openness. Each animal's eyes (left and right) were checked for degree of openness across 5 consecutive days. The degree of openness was rated on a scale of 0–3: 0 = completely closed; 1 = any opening exposing the cornea, regardless of how small; 2 = cornea and pupil are exposed but eye lids are not fully open; 3 = fully open. All animals' weights were recorded on PND 1, 7, 14, and 21. Rats were weaned and pair housed, same sex, on PND 21.
2.6 Immunohistochemistry
At PND 130, eight PN animals and six PS male rats were euthanized, and the brains were harvested. OxA and melanin-concentrating hormone (MCH) cell bodies in the lateral hypothalamus/perifornical area (LH/PFA), and orexinergic projections to the VTA and tyrosine hydroxylase (TH) expression in the VTA were measured via immunohistochemistry. Following rapid decapitation, brains were fixed overnight in 4% paraformaldehyde (pH 7.4), and transferred to a 30% sucrose/0.1M phosphate buffer solution for cryoprotection. Coronal sections through the rostro-caudal extent of the hypothalamus and VTA were serially cut (40μm) on a microtome (separated in 1:6 serial ratio; sections were 240 μm apart). Single label (orexin or MCH) and double-label (orexin and TH) immunohistochemistry procedures were performed on representative sets of tissue from the LH/PFA and VTA, respectively.
For experiment one, sections were incubated with a rabbit anti-OxA antibody (1:2000; 48 h at 4 °C; Calbiochem; La Jolla, CA, USA) followed by a biotinylated donkey anti-rabbit IgG secondary antibody (1:1000; 1.5 h at room temperature; Jackson, Inc.; West Grove, PA, USA) and horseradish peroxidase-conjugated streptavidin (1:1600; 1 h; Jackson, Inc.). In the second experiment, sections were incubated in chicken anti-MCH (1:4000; 48 h at 4 °C; BMA; Augst, Switzerland) followed by a biotinylated donkey anti-chicken IgG secondary antibody (1:1000; 1.5 h at room temperature), and horseradish peroxidase-conjugated streptavidin (1:1600; 1 h; Jackson, Inc.). For both orexin and MCH single-labeling, immunoreactivity was visualized by using a nickel-cobalt intensified diaminobenzidine solution with 0.3% hydrogen peroxide, which caused blue-black cytoplasmic labeling of orexin or MCH-positive cells.
For double-labeling, coronal sections of the VTA were first processed for OxA fiber labeling as described above and then incubated with mouse anti-TH antibody (1:5000; 48 h at 4 °C; ImmunoStar; Hudson, WI, USA) followed by unlabeled donkey anti-mouse IgG secondary antibody (1:200; 2.0 h at room temperature; Jackson, Inc.; West Grove, PA, USA) and mouse peroxidase anti-peroxidase (PAP) (1:500); 1.5 h; Jackson, Inc.). Development of these sections in plain diaminobenzidine solution and 3% hydrogen peroxide resulted in light brown cytoplasmic labeling in TH-positive VTA neurons.
2.7 Cell Counts
Single-labeled OxA and MCH counts were performed on representative sections from two rostrocaudal levels of the LH/PFA, corresponding to approximately 3.3 and 3.6 mm caudal to bregma (Paxino and Watson, 1998). Because prior work has suggested functional heterogeneity within the orexin system, with more medially-located orexin neurons implicated in arousal and stress responses and laterally-located cells more involved in reward and feeding (e.g. Harris et al., 2005; Fadel et al., 2002; Estabrooke et al., 2001), we performed counts in both the medial and lateral sectors using the fornix as the bisecting marker as previously described (Pasumarthi et al., 2006). Cells were counted at 10× magnification.
Putative orexin appositions on representative VTA DA (i.e., TH-positive) neurons were performed at 40× magnification as previously described (Pasumarthi and Fadel, 2010), with an “apposition” defined as an OxA-immunoreactive varicosity in the same focal plane as a TH-positive soma or proximal dendrite. For each animal, 20 TH-positive cells (10 each from two different levels of the VTA corresponding to approximately 5.3 and 6.3 mm caudal to bregma) were randomly selected at low (2×) magnification, where appositions were not visible. The total number of appositions on the 20 TH-positive neurons was summed for each animal and group means were calculated.
2.8 Drugs
Nicotine dihydrogen ditartrate salt (Sigma, St. Louis, MO) was weighed daily as base and dissolved in physiological saline (0.9%, Hospira, Lake Forest, IL), with an injection volume of 1ml/kg. After being dissolved in saline, the pH level of the nicotine solution was adjusted to 7.0. Heparin (APP Pharmaceuticals, Schaumburg, IL; 1000 U) was added to saline, and a 2.5% heparinized saline solution was used to flush the IV catheters once a day.
2.9 Data analysis
Analysis of variance (ANOVA) techniques were used to analyze the litter parameter and immunohistochemistry data (SPSS Inc, version 19; 2010).
2.9.1 Litter Parameters
The between-subjects factor was Prenatal Treatment (PN or PS). The within-subjects factors were GD and PND. A Prenatal Treatment (2) × GD (4) mixed-factorial ANOVA determined if there were differences between PS and PN dams on the measure of maternal weight gain. A univariate ANOVA was conducted for the total number of pups born to PN and PS dams. A Prenatal Treatment (2) × PND (3, 4, or 5) mixed- factorial ANOVA was conducted for the pup weight gain, righting reflex, negative geotaxis and eye opening data.
2.9.2 Experiment 1: Orexin and MCH cell counts in the LH/PFA
The between-subjects factor was Prenatal treatment (PN or PS). The within-subjects factor was Region (Medial and Lateral Hypothalamus). A Prenatal Treatment (2) × Region (2) mixed-factorial ANOVA was used to analyze the expression of LH/PFA orexin-positive cells of PN and PS offspring in the lateral and medial portions of the LH. The same analysis was used to analyze the expression of MCH in the LH of tissue from PN and PS animals.
2.9.3 Experiment 2: Orexin / TH appositions in the VTA
The mean number of appositions in the VTA of PN and PS rats was analyzed with a univariate ANOVA.
3. RESULTS
3.1 Litter Parameters
There were no differences between the PN and PS groups for the measures of maternal or pup weight gain. The weight gain data for the saline and nicotine dams throughout gestation and for the PN and PS pups across PND 1–21 are shown in Table 1. There were also no significant effects between PN and PS rats with regard to the number of pups born or litter composition. Additionally, there were no significant effects of PN treatment on the developmental milestones of righting reflex, negative geotaxis, or eye opening (data not shown).
Table 1.
Mean (±SEM) maternal and pup weight gain (g) for dams and offspring, respectively. The dams were injected with IV nicotine or saline, 3X/day on GD 8–21, and were weighed on GD 0, 7, 14, and 21, and pups were weighed on PND 1, 7, 14, and 21. There were no significant differences for maternal or pup weight gain.
| Maternal Weight Gain: | |||||
| GD | |||||
| 0 | 7 | 14 | 21 | ||
|
|
|||||
| Prenatal Treatment | n | ||||
| Nicotine | 9 | 0 | 22.11 (±1.90) | 51.43 (±1.81) | 140.50 (±5.23) |
| Saline | 8 | 0 | 21.27 (±2.02) | 52.93 (±1.92) | 148.62 (±5.55) |
| Pup Weight Gain: | |||||
| PND | |||||
| 1 | 7 | 14 | 21 | ||
|
|
|||||
| Prenatal Treatment | n | ||||
| PN | 76 | 0 | 8.76 (±0.17) | 22.76 (±0.44) | 40.92 (±0.67) |
| PS | 69 | 0 | 9.20 (±0.17) | 22.25 (±0.43) | 40.88 (±0.69) |
3.2 Experiment 1: Effects of IV PN on orexin expression in the LH/PFA
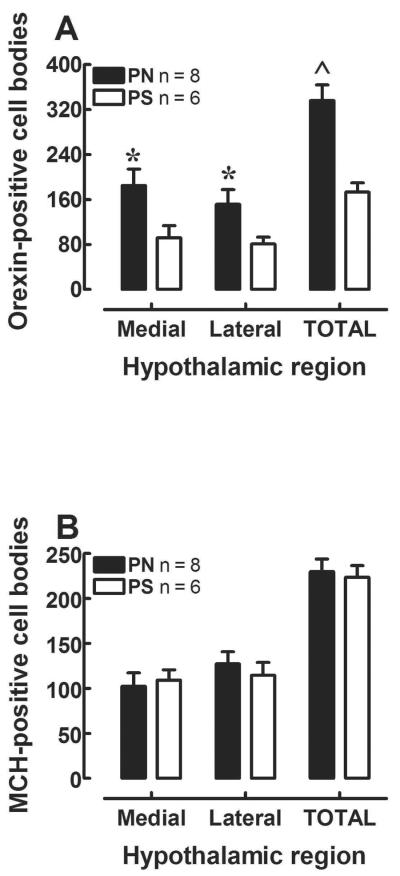
Figure 1A shows orexin-positive cell counts measured in the LH/PFA of PN and PS offspring. The ANOVA revealed that rats treated with IV PN had a greater number of orexin cells in the LH, relative to PS animals [Prenatal Treatment: F(1, 12) = 7.4, p < 0.05], and this factor did not interact with a specific region of the LH [Prenatal Treatment × Region: F(1, 12) = 1.82, p > 0.05]. The analysis also showed that there were a greater number of orexin-positive cells in the medial area relative to the lateral portion of the LH [Region: F(1, 12) = 5.5, p < 0.05], regardless of prenatal treatment. Orexin neurons were blue-black in appearance and extended from the lateral hypothalamus proper, over the fornix (which demarcated lateral and medial sectors), and into the dorsomedial hypothalamus (Figure 3).
Figure 1.
(A) Mean (±SEM) orexin-positive cell counts for the PS and PN groups are presented for the Medial and Lateral divisions of the orexin cell population. PN animals showed significantly more orexin-positive cell counts in the medial and lateral hypothalamic regions; * indicates p<0.05 vs. PS group within the same hypothalamic sector. The total orexin-positive cell counts for the PS and PN groups are shown on the right side of the graph; ^ indicates the significant main effect of prenatal treatment. (B) Mean (±SEM) Melanin-concentrating hormone-positive cell counts for the PS and PN groups are presented for Medial and Lateral divisions of the MCH cell population.
Figure 3.
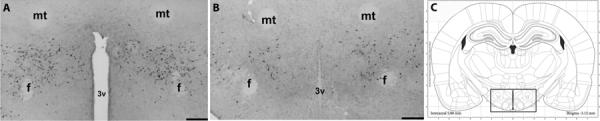
Orexin expression in hypothalamus. (A) Low-power hemispheric view (left) showing orexin immunoreactive cells (black) around the fornix, median forbrain bundle, and third ventricle in an LH/PFA coronal section from a PN animal. (B) LH/PFA from control animal (center). (C) Coronal hemisection schematic of cell count area (modified from Paxinos and Watson, 1998) in LH/PFA (right). Box indicates general area where counts were made. Scale bars = 1000 μm (A&B).
The ANOVA conducted on the MCH data revealed no differences in expression of the MCH-positive neurons between the PN and PS groups [Prenatal Treatment: F(1, 12) = 0.04, p = 0.846; region: F(1, 12) = 1.5, p = 0.240; Prenatal Treatment × Region: F<1.0, p > 0.05]. Figure 1B shows MCH cells measured in the LH/PFA of PN and PS offspring. As with orexin labeling, nickel/cobalt-enhanced diaminobenzidine immunostaining resulted in blue-black MCH-positive cell bodies which were found in a medial-lateral continuum in the posterior hypothalamus, but also extended more dorsally toward the subincertal nucleus and zona incerta (Figure 4).
Figure 4.
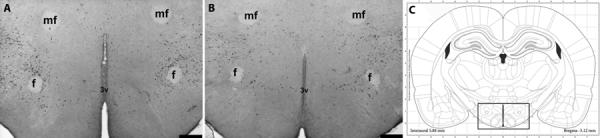
MCH expression in hypothalamus. (A) Low-power hemispheric view (left) showing MCH immunoreactive cells (black) around the fornix, median forbrain bundle, and third ventricle in an LH/PFA coronal section from a PN animal. Pictomicrographs representing MCH-immunoreactive cells in the rostrocaudal extent of the LH/PFA. (B) LH/PFA from control animal (center). (C) Coronal hemisection schematic of cell count area (modified from Paxinos and Watson, 1998) in LH/PFA (right). Box indicates general area where counts were made. Scale bars = 1000 μm (A&B).
3.3 Experiment 2: Effects of IV PN on orexin / TH appositions in the VTA
Virtually all TH-positive (i.e., dopaminergic) neurons in the VTA received multiple apparent appositional contacts from orexin fibers. These appositions had the appearance of punctate black varicosities arising from orexin-positive fibers juxtaposed against the cell bodies and proximal dendrites of light brown, TH-immunoreactive profiles (Figure 5). Quantitatively, the PN group exhibited an increased number of orexin-positive appositions per VTA DA neuron relative to the PS group, and this difference was statistically significant [F(1,12) = 6.5, p < 0.05]. These data are shown in Figure 2.
Figure 5.
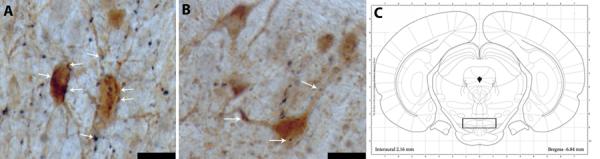
Orexin immunoreactive fibers in the VTA. (A) High magnification view showing a dense immune-reactive fiber plexus (nickel-cobalt enhanced black fibers) enmeshing a cluster of TH-positive neurons (lightly stained cell bodies in the VTA of a PN animal (left). Orexin fibers in the VTA contain numerous varicosities and frequent appositions with TH-immunoreactive cells (thick arrows) and dendrites (thin arrows). (B) VTA from control PS rat. (center). (C) Coronal hemisection schematic of cell count area (modified from Paxinos and Watson, 1998) in VTA (right). Scale bars = 50 μm (A&B). Box indicates general area where counts were made. In all cases, images were imported into Adobe Photoshop (v 5.5), where minor revisions to contrast and brightness were made.
Figure 2.
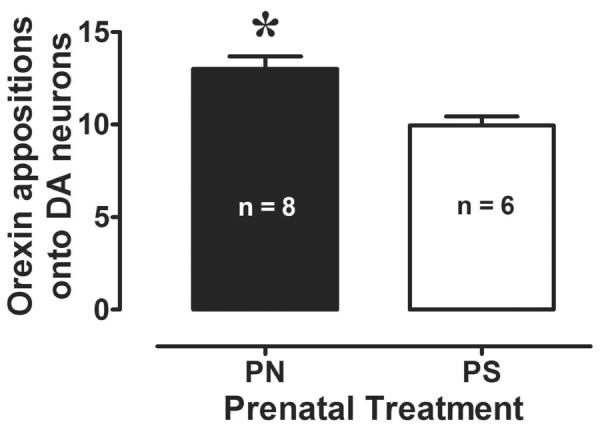
Mean (±SEM) total orexin-positive appositions on VTA DA neurons for the PS and PN groups. Numbers represent the average summed total (appositions/neuron) for each animal based on total appositions counted on twenty randomly-selected TH-positive neurons. The PN group exhibited an increased number of orexin and TH appositions in the VTA relative to the PS group. * indicates a significant difference between PS and PN animals, p<0.05.
4. DISCUSSION
The present experiments investigated if IV PN exposure resulted in modification of orexin/hypocretin expression in the LH and the VTA of adult, male offspring. Our results indicate that the low-dose IV PN exposure resulted in increased orexin expression, compared to saline controls. PN-exposed rats had significantly more orexin-positive cells in the LH, as well as more orexin appositions onto DA neurons in the VTA, compared to PS animals. PN exposure did not increase expression of MCH-positive cells in the LH, indicating that the effect of PN is not ubiquitous among cell populations of the LH.
Although the neurophysiological basis of the increase in orexin neurons cannot be determined from the present data, one possibility is that PN exposure interferes with the neuronal pruning process during postnatal development, leaving more orexin neurons in the LH. Indeed, the number of orexin neurons may reach a maximal level before 10 weeks of age, and then decrease significantly by 20 weeks (Sawai et al., 2010), the approximate age of our animals. A qualitatively similar phenomenon has been described for orexin axons and terminals in the locus coeruleus (Steininger et al., 2004). The IV nicotine procedure used in the present experiment altered the expression of brain-derived neurotrophic factor (BDNF) in the mesocorticolimbic system of adolescent offspring (Harrod et al., 2011). BDNF is important for cell survival (Barde, 1989; Davies et al., 1986a, b), and high levels of BDNF are found in the hypothalamus (Katoh-Semba et al., 1997; Kawamoto et al., 1996). Given that PN exposure alters BDNF protein levels (Harrod et al., 2011) and BDNF mRNA (Wei et al., 2011), it may enhance survival of hypothalamic orexin neurons via a BDNF-dependent mechanism. The increased number of immunoreactive cell bodies in the LH may also be a function of the detectability of OxA using immunohistochemical methods. It is possible that PN exposure produced elevated transcription of the neuropeptide as has been described for chronic nicotine treatment in adult animals (Kane et al., 2000), increasing the immunohistochemical detectability of low orexin-expressing neurons without an actual increase in neuronal number. Thus, in general, there may have been orexin containing neurons that were sub-threshold for detection, and the influence of PN on these particular neurons was to increase the amount of neuropeptide, which may result in increased neurons in the LH and projections to the VTA. It is important to note that none of the offspring in the present experiment were exposed to nicotine or any other drug following prenatal exposure. This indicates that the increased orexin expression observed during adulthood is an enduring result of nicotine's effect on neurodevelopment that occurs during GD 8–21.
Orexin plays a role in motivation and reward (Alberto et al., 2006; Aston-Jones et al., 2009; Choi et al., 2010; Espana et al., 2010; Lawrence et al., 2006). In adult rats, orexin transmission is required for the maintenance of nicotine self-administration (Hollander et al., 2008). Orexin input to the VTA, in particular, plays a crucial role in psychostimulant-induced behavioral sensitization and synaptic plasticity (Borgland et al., 2006). Given the role of the orexin system in addiction-related processes (Mahler et al., 2012), our findings of increased orexin neurons and input to VTA DA neurons as a function of PN exposure may have profound implications for addictive and impulsive behaviors. The increased number of orexin appositions onto the VTA observed in PN animals suggests a greater orexin-mediated influence on motivation of offspring exposed to PN. A novel hypothesis from the present experiment is that the PN-induced increase in orexin expression in the VTA contributes, in part, to changes in motivated behavior reported in offspring prenatally exposed to IV nicotine.
Indeed, IV PN exposure alters motivated behavior in adult and adolescent rodent offspring. For example, behavioral studies show that IV PN-exposed offspring exhibit alterations in the motivation to respond for sucrose and drug reinforcement. Adult rats exposed to IV PN exhibited similar inverted U-shaped response curves when tested on various concentrations of sucrose in adulthood (i.e., 0, 3, 10, 30, and 56%; FR-3). The same rats showed higher break points when the same sucrose concentrations were tested with PR schedules of reinforcement, indicating that the rats were more motivated for sucrose than controls (Lacy et al., 2012). In another study, IV PN- and PS-exposed adult rats were trained to self-administer methamphetamine on an FR-3. After demonstrating stable responding, animals were tested with novel doses of the reinforcer on the same schedule of reinforcement. The PN and PS groups showed inverted U-shaped dose response curves that are standard for psychostimulant drugs (Yokel, 1987); however, the PN rats' dose response curve was shifted to the left, indicating that PN rats were more sensitive to the reinforcing effects IV methamphetamine compared to PS controls (Harrod et al., 2012).
Previous experiments report that PN induced changes in motivated behavior, as well. In these studies, PN was delivered continuously, via osmotic mini-pump, and adolescent offspring were the subjects of the behavioral experiments. Levin et al. (2006) show that female rats exposed to continuous PN or PS acquired nicotine self-administration on a FR1 schedule during adolescence, and there were no differences between PN and PS rats on this measure; however, PN rats self-administered more nicotine than PS controls under conditions of reinstatement. Franke et al. (2008) report that adolescent male offspring exposed to continuous PN responded less for sucrose on fixed-ratio schedules of reinforcement, and there were no differences between PN and PS animals when tested on PR. Moreover, in a separate experiment, PN rats required a higher concentration of IV cocaine than PS animals to acquire stable self-administration when continuous reinforcement was available. The latter findings differ from those of the Lacy et al. (2011) and Harrod et al. (2012) studies because they suggest that adolescent PN-exposed offspring are less sensitive to the stimulus properties of sucrose and cocaine than PS rats. These seemingly contradictory findings may be due to differences in the age at testing. Adolescence and adulthood represent different stages of neural development (Andersen et al., 2000; Chambers et al., 2003; Spear, 2000), as the adolescent brain experiences substantial changes in dopamine and dopamine receptor expression within motivation and reward circuitry. Other differences include the route of PN administration, and the amount of nicotine delivered. Despite these procedural and methodological differences, it remains clear that PN exposure induces motivational changes for abused drugs in offspring of various ages.
Regarding the orexin system, the extent to which PN-induced changes in hypothalamic and ventral tegmental orexin affect the motivation for sucrose or drug reward are not known. Interestingly, one previous study showed that PN exposure decreased prepro-orexin expression in the LH (Boychuk and Hayward, 2011). While this study indicates that PN decreases expression of prepro-orexin mRNA within the hypothalamus, the authors also report no differences in expression between groups in the dorsomedial hypothalamus. The methods of the present study differ from the Boychuk and Hayward (2011) experiment in several ways. The Boychuk and Hayward (2011) study investigated adolescent offspring. Additionally, continuous nicotine was administered, and the exposure period extended into postnatal development. In the current study pups were surrogate fostered to timed-pregnant, drug naïve dams to prevent postpartum exposure to nicotine, because postnatal and adolescent nicotine exposure alone has been shown to alter various neurochemical and behavioral outcomes (Bracken et al., 2011; Counotte et al., 2012; Rinker et al., 2011; Weaver et al., 2012). Our findings indicate that the PN-induced increase in orexin expression was initiated in the prenatal period, which was GD 8–21. Nonetheless, these experiments provide converging evidence that two different routes of PN exposure altered orexin expression in the LH. Further studies are needed to elucidate the functional and behavioral consequences of increased orexin levels in the LH and VTA of nicotine exposed animals.
There are advantages and disadvantages of using various models of PN administration. Regarding the IV method, the advantages are that IV injection of nicotine mimics the pharmacokinetics of tobacco smoke inhalation and allows for 100% bioavailability of nicotine (Benowitz et al., 2009); less daily exposure of nicotine is needed relative to other exposure models, and three daily IV injections, administered 3 hours apart, allows for accumulation of nicotine levels throughout the day (the half-life for IV nicotine, 0.05 mg/kg/injection, is ~49 minutes; Booze et al., 1999). A disadvantage of this model is that only one of the 4000 constituents of tobacco smoke is administered to the dam. Also, the IV method does not mimic the pulmonary uptake and delayed release of nicotine exhibited in tobacco smokers (Rose et al., 1999).
Data from the current study indicate that low-dose, IV PN exposure increases orexin production in the LH and in the axon terminals that project to the VTA. The enduring nature of the IV PN-induced effects is profound given that the increased levels of the neuropeptide orexin were detected in animals that developed well into adulthood. This suggests that increased orexin input to the VTA is a possible mechanism by which PN alters the development and function of the mesocorticolimbic dopamine pathway, increasing the vulnerability to drug dependence if recreational drug use is initiated.
Acknowledgments
Role of funding source
Funding for this study was provided by the National Institute on Drug Abuse, DA021287 (SBH), National Institute of Health, 5 T32GM091740, National Institute on Aging AG030646 (JRF) and the University of South Carolina Research Productivity Scholar grant, KA-21 (SBH). None of these funding sources had a further role in the preparation of the experimental procedures; writing, or the submission of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Steven Harrod and Jim Fadel designed the study. Ryan Lacy and Steven Harrod conducted the prenatal treatments and Amanda Morgan and Ryan Lacy managed the surrogate fostering procedures and care of litters and the developing offspring. Jim Fadel and Emily Stanley wrote the protocol for tissue preparation and the immunohistochemistical methods. Amanda Morgan conducted the statistical analyses and wrote the first version of the manuscript; and all authors edited subsequent versions. The final version was approved by all authors. The authors would like to thank Bonnie Barte, Davis Weinberg, and Shelly Maxwell for their technical assistance with the experiment.
Conflict of interest statement
All authors declare they have no conflict of interest.
REFERENCES
- Alberto CO, Trask RB, Quinlan ME, Hirasawa M. Bidirectional dopaminergic modulation of excitatory synaptic transmission in orexin neurons. J. Neurosci. 2006;26:10043–10050. doi: 10.1523/JNEUROSCI.1819-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37:167–169. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Moorman DE, Richardson KA. Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharmacology. 2009;56(Suppl. 1):112–121. doi: 10.1016/j.neuropharm.2008.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, Richardson KA. Lateral hypothalamic orexin/hypocretin neurons: a role in reward-seeking and addiction. Brain Res. 2010;1314:74–90. doi: 10.1016/j.brainres.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barde YA. Trophic factors and neuronal survival. Neuron. 1989;2:1525–1534. doi: 10.1016/0896-6273(89)90040-8. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Hukkanen J, Jacob P., 3rd Nicotine chemistry, metabolism, kinetics and biomarkers. Handb. Exp. Pharmacol. 2009:29–60. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonci A, Borgland S. Role of orexin/hypocretin and CRF in the formation of drug-dependent synaptic plasticity in the mesolimbic system. Neuropharmacology. 2009;56(Suppl. 1):107–111. doi: 10.1016/j.neuropharm.2008.07.024. [DOI] [PubMed] [Google Scholar]
- Booze RM, Welch MA, Wood ML, Billings KA, Apple SR, Mactutus CF. Behavioral sensitization following repeated intravenous nicotine administration: gender differences and gonadal hormones. Pharmacol. Biochem. Behav. 1999;64:827–839. doi: 10.1016/s0091-3057(99)00169-0. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Boychuk CR, Hayward LF. Prenatal nicotine exposure alters postnatal cardiorespiratory integration in young male but not female rats. Exp. Neurol. 2011;232:212–221. doi: 10.1016/j.expneurol.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracken AL, Chambers RA, Berg SA, Rodd ZA, McBride WJ. Nicotine exposure during adolescence enhances behavioral sensitivity to nicotine during adulthood in Wistar rats. Pharmacol. Biochem. Behav. 2011;99:87–93. doi: 10.1016/j.pbb.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubser M, Fadel JR, Jackson LL, Meador-Woodruff JH, Jing D, Deutch AY. Dopaminergic regulation of orexin neurons. Eur. J. Neurosci. 2005;21:2993–3001. doi: 10.1111/j.1460-9568.2005.04121.x. [DOI] [PubMed] [Google Scholar]
- Buka SL, Shenassa ED, Niaura R. Elevated risk of tobacco dependence among offspring of mothers who smoked during pregnancy: a 30-year prospective study. Am. J. Psychiatry. 2003;160:1978–1984. doi: 10.1176/appi.ajp.160.11.1978. [DOI] [PubMed] [Google Scholar]
- Button TM, Maughan B, McGuffin P. The relationship of maternal smoking to psychological problems in the offspring. Early Hum. Dev. 2007;83:727–732. doi: 10.1016/j.earlhumdev.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason AM, Smith RJ, Tahsili-Fahadan P, Moorman DE, Sartor GC, Aston-Jones G. Role of orexin/hypocretin in reward-seeking and addiction: implications for obesity. Physiol. Behav. 2010;100:419–428. doi: 10.1016/j.physbeh.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castles A, Adams EK, Melvin CL, Kelsch C, Boulton ML. Effects of smoking during pregnancy. Five meta-analyses. Am. J. Prev. Med. 1999;16:208–215. doi: 10.1016/s0749-3797(98)00089-0. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am. J. Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DL, Davis JF, Fitzgerald ME, Benoit SC. The role of orexin-A in food motivation, reward-based feeding behavior and food-induced neuronal activation in rats. Neuroscience. 2010;167:11–20. doi: 10.1016/j.neuroscience.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Cornelius MD, Day NL. Developmental consequences of prenatal tobacco exposure. Curr. Opin. Neurol. 2009;22:121–125. doi: 10.1097/WCO.0b013e328326f6dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA. Hypocretin mechanisms in nicotine addiction: evidence and speculation. Psychopharmacol. (Berl.) 2009;206:14. doi: 10.1007/s00213-009-1588-2. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacol. (Berl.) 1989;99:473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Counotte DS, Goriounova NA, Moretti M, Smoluch MT, Irth H, Clementi F, Schoffelmeer AN, Mansvelder HD, Smit AB, Gotti C, Spijker S. Adolescent nicotine exposure transiently increases high-affinity nicotinic receptors and modulates inhibitory synaptic transmission in rat medial prefrontal cortex. FASEB J. 2012;26:1810–1820. doi: 10.1096/fj.11-198994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies AM, Thoenen H, Barde YA. Different factors from the central nervous system and periphery regulate the survival of sensory neurones. Nature. 1986a;319:497–499. doi: 10.1038/319497a0. [DOI] [PubMed] [Google Scholar]
- Davies AM, Thoenen H, Barde YA. The response of chick sensory neurons to brain-derived neurotrophic factor. J. Neurosci. 1986b;6:1897–1904. doi: 10.1523/JNEUROSCI.06-07-01897.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children's health. Pediatrics. 2004;113:1007–1015. [PubMed] [Google Scholar]
- Dwyer JB, Broide RS, Leslie FM. Nicotine and brain development. Birth Defects Res. C. Embryo Today. 2008;84:30–44. doi: 10.1002/bdrc.20118. [DOI] [PubMed] [Google Scholar]
- Dwyer JB, McQuown SC, Leslie FM. The dynamic effects of nicotine on the developing brain. Pharmacol. Ther. 2009;122:125–139. doi: 10.1016/j.pharmthera.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. J. Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Koob GF. Neurobiology of dysregulated motivational systems in drug addiction. Future Neurol. 2010;5:393–401. doi: 10.2217/fnl.10.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana RA, Melchior JR, Roberts DC, Jones SR. Hypocretin 1/orexin A in the ventral tegmental area enhances dopamine responses to cocaine and promotes cocaine self-administration. Psychopharmacol. (Berl.) 2011;214:415–426. doi: 10.1007/s00213-010-2048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana RA, Oleson EB, Locke JL, Brookshire BR, Roberts DC, Jones SR. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur. J. Neurosci. 2010;31:336–348. doi: 10.1111/j.1460-9568.2009.07065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111:379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Woodward LJ, Horwood LJ. Maternal smoking during pregnancy and psychiatric adjustment in late adolescence. Arch. Gen. Psychiatry. 1998;55:721–727. doi: 10.1001/archpsyc.55.8.721. [DOI] [PubMed] [Google Scholar]
- Fleming P, Blair PS. Sudden infant death syndrome and parental smoking. Early Hum. Dev. 2007;83:721–725. doi: 10.1016/j.earlhumdev.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Franke RM, Park M, Belluzzi JD, Leslie FM. Prenatal nicotine exposure changes natural and drug-induced reinforcement in adolescent male rats. Eur. J. Neurosci. 2008;27:2952–2961. doi: 10.1111/j.1460-9568.2008.06253.x. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Harrod SB, Lacy RT, Morgan AJ. Offspring of prenatal IV nicotine exposure exhibit increased sensitivity to the reinforcing effects of methamphetamine. Front. Pharmacol. 2012;3:116. doi: 10.3389/fphar.2012.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrod SB, Lacy RT, Zhu J, Hughes BA, Perna MK, Brown RW. Gestational IV nicotine produces elevated brain-derived neurotrophic factor in the mesocorticolimbic dopamine system of adolescent rat offspring. Synapse. 2011;65:1382–1392. doi: 10.1002/syn.20975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ. Insular hypocretin transmission regulates nicotine reward. Proc. Natl. Acad. Sci. U. S. A. 2008;105:19480–19485. doi: 10.1073/pnas.0808023105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. Perspective: the manifest destiny of cocaine research. Neuropsychopharmacology. 2009;34:1089–1090. doi: 10.1038/npp.2009.9. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Wu P, Davies M. Maternal smoking during pregnancy and smoking by adolescent daughters. Am. J. Public Health. 1994;84:1407–1413. doi: 10.2105/ajph.84.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane JK, Parker SL, Li MD. Hypothalamic orexin-A binding sites are downregulated by chronic nicotine treatment in the rat. Neurosci. Lett. 2001;298:1–4. doi: 10.1016/s0304-3940(00)01730-4. [DOI] [PubMed] [Google Scholar]
- Kane JK, Parker SL, Matta SG, Fu Y, Sharp BM, Li MD. Nicotine up-regulates expression of orexin and its receptors in rat brain. Endocrinology. 2000;141:3623–3629. doi: 10.1210/endo.141.10.7707. [DOI] [PubMed] [Google Scholar]
- Kane VB, Fu Y, Matta SG, Sharp BM. Gestational nicotine exposure attenuates nicotine-stimulated dopamine release in the nucleus accumbens shell of adolescent Lewis rats. J. Pharmacol. Exp. Ther. 2004;308:521–528. doi: 10.1124/jpet.103.059899. [DOI] [PubMed] [Google Scholar]
- Katoh-Semba R, Takeuchi IK, Semba R, Kato K. Distribution of brain-derived neurotrophic factor in rats and its changes with development in the brain. J. Neurochem. 1997;69:34–42. doi: 10.1046/j.1471-4159.1997.69010034.x. [DOI] [PubMed] [Google Scholar]
- Kawamoto Y, Nakamura S, Nakano S, Oka N, Akiguchi I, Kimura J. Immunohistochemical localization of brain-derived neurotrophic factor in adult rat brain. Neuroscience. 1996;74:1209–1226. doi: 10.1016/0306-4522(96)00245-x. [DOI] [PubMed] [Google Scholar]
- Kenny PJ. Tobacco dependence, the insular cortex and the hypocretin connection. Pharmacol. Biochem. Behav. 2011;97:700–707. doi: 10.1016/j.pbb.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy RT, Hord LL, Morgan AJ, Harrod SB. Intravenous gestational nicotine exposure results in increased motivation for sucrose reward in adult rat offspring. Drug Alcohol Depend. 2012;124:299–306. doi: 10.1016/j.drugalcdep.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy RT, Mactutus CF, Harrod SB. Prenatal IV nicotine exposure produces a sex difference in sensorimotor gating of the auditory startle reflex in adult rats. Int. J. Dev. Neurosci. 2011;29:153–161. doi: 10.1016/j.ijdevneu.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br. J. Pharmacol. 2006;148:752–759. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSage MG, Gustaf E, Dufek MB, Pentel PR. Effects of maternal intravenous nicotine administration on locomotor behavior in pre-weanling rats. Pharmacol. Biochem. Behav. 2006;85:575–583. doi: 10.1016/j.pbb.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSage MG, Perry JL, Kotz CM, Shelley D, Corrigall WA. Nicotine self-administration in the rat: effects of hypocretin antagonists and changes in hypocretin mRNA. Psychopharmacol. (Berl.) 2010;209:203–212. doi: 10.1007/s00213-010-1792-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Lawrence S, Petro A, Horton K, Seidler FJ, Slotkin TA. Increased nicotine self-administration following prenatal exposure in female rats. Pharmacol. Biochem. Behav. 2006;85:669–674. doi: 10.1016/j.pbb.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnet KM, Dalsgaard S, Obel C, Wisborg K, Henriksen TB, Rodriguez A, Kotimaa A, Moilanen I, Thomsen PH, Olsen J, Jarvelin MR. Maternal lifestyle factors in pregnancy risk of attention deficit hyperactivity disorder and associated behaviors: review of the current evidence. Am. J. Psychiatry. 2003;160:1028–1040. doi: 10.1176/appi.ajp.160.6.1028. [DOI] [PubMed] [Google Scholar]
- Mactutus CF, Herman AS, Booze RM. Chronic intravenous model for studies of drug (Ab)use in the pregnant and/or group-housed rat: an initial study with cocaine. Neurotoxicol. Teratol. 1994;16:183–191. doi: 10.1016/0892-0362(94)90116-3. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Moorman DE, Sartor GC, Aston-Jones G. Multiple roles for orexin/hypocretin in addiction. Prog. Brain Res. 2012;198:79–121. doi: 10.1016/B978-0-444-59489-1.00007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal MS, Nakazato M, Date Y, Murakami N, Hanada R, Sakata T, Matsukura S. Characterization of orexin-A and orexin-B in the microdissected rat brain nuclei and their contents in two obese rat models. Neurosci. Lett. 1999;273:45–48. doi: 10.1016/s0304-3940(99)00624-2. [DOI] [PubMed] [Google Scholar]
- Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain Res. 1999;827:243–260. doi: 10.1016/s0006-8993(99)01336-0. [DOI] [PubMed] [Google Scholar]
- Navarro HA, Seidler FJ, Schwartz RD, Baker FE, Dobbins SS, Slotkin TA. Prenatal exposure to nicotine impairs nervous system development at a dose which does not affect viability or growth. Brain Res. Bull. 1989;23:187–192. doi: 10.1016/0361-9230(89)90146-9. [DOI] [PubMed] [Google Scholar]
- Navarro HA, Seidler FJ, Whitmore WL, Slotkin TA. Prenatal exposure to nicotine via maternal infusions: effects on development of catecholamine systems. J. Pharmacol. Exp. Ther. 1988;244:940–944. [PubMed] [Google Scholar]
- Park MK, Loughlin SE, Leslie FM. Gestational nicotine-induced changes in adolescent neuronal activity. Brain Res. 2006;1094:119–126. doi: 10.1016/j.brainres.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Pasumarthi RK, Fadel J. Activation of orexin/hypocretin projections to basal forebrain and paraventricular thalamus by acute nicotine. Brain Res. Bull. 2008;77:367–373. doi: 10.1016/j.brainresbull.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasumarthi RK, Fadel J. Stimulation of lateral hypothalamic glutamate and acetylcholine efflux by nicotine: implications for mechanisms of nicotine-induced activation of orexin neurons. J. Neurochem. 2010;113:1023–1035. doi: 10.1111/j.1471-4159.2010.06666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasumarthi RK, Reznikov LR, Fadel J. Activation of orexin neurons by acute nicotine. Eur. J. Pharmacol. 2006;535:172–176. doi: 10.1016/j.ejphar.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Pauly JR, Sparks JA, Hauser KF, Pauly TH. In utero nicotine exposure causes persistent, gender-dependant changes in locomotor activity and sensitivity to nicotine in C57Bl/6 mice. Int. J. Dev. Neurosci. 2004;22:329–337. doi: 10.1016/j.ijdevneu.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribary U, Lichtensteiger W. Effects of acute and chronic prenatal nicotine treatment on central catecholamine systems of male and female rat fetuses and offspring. J. Pharmacol. Exp. Ther. 1989;248:786–792. [PubMed] [Google Scholar]
- Richardson SA, Tizabi Y. Hyperactivity in the offspring of nicotine-treated rats: role of the mesolimbic and nigrostriatal dopaminergic pathways. Pharmacol. Biochem. Behav. 1994;47:331–337. doi: 10.1016/0091-3057(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Rinker JA, Hutchison MA, Chen SA, Thorsell A, Heilig M, Riley AL. Exposure to nicotine during periadolescence or early adulthood alters aversive and physiological effects induced by ethanol. Pharmacol. Biochem. Behav. 2011;99:7–16. doi: 10.1016/j.pbb.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu. Rev. Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Coleman RE. Arterial nicotine kinetics during cigarette smoking and intravenous nicotine administration: implications for addiction. Drug Alcohol Depend. 1999;56:99–107. doi: 10.1016/s0376-8716(99)00025-3. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Sawai N, Ueta Y, Nakazato M, Ozawa H. Developmental and aging change of orexin-A and - B immunoreactive neurons in the male rat hypothalamus. Neurosci. Lett. 2010;468:51–55. doi: 10.1016/j.neulet.2009.10.061. [DOI] [PubMed] [Google Scholar]
- Schneider T, Bizarro L, Asherson PJ, Stolerman IP. Gestational exposure to nicotine in drinking water: teratogenic effects and methodological issues. Behav. Pharmacol. 2010;21:206–216. doi: 10.1097/fbp.0b013e32833a5bb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharf R, Guarnieri DJ, Taylor JR, DiLeone RJ. Orexin mediates morphine place preference, but not morphine-induced hyperactivity or sensitization. Brain Res. 2010a;1317:24–32. doi: 10.1016/j.brainres.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharf R, Sarhan M, Dileone RJ. Role of orexin/hypocretin in dependence and addiction. Brain Res. 2010b;1314:130–138. doi: 10.1016/j.brainres.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slikker W, Jr., Xu ZA, Levin ED, Slotkin TA. Mode of action: disruption of brain cell replication, second messenger, and neurotransmitter systems during development leading to cognitive dysfunction--developmental neurotoxicity of nicotine. Crit. Rev. Toxicol. 2005;35:703–711. doi: 10.1080/10408440591007421. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol. Appl. Pharmacol. 2004;198:132–151. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Cho H, Whitmore WL. Effects of prenatal nicotine exposure on neuronal development: selective actions on central and peripheral catecholaminergic pathways. Brain Res. Bull. 1987a;18:601–611. doi: 10.1016/0361-9230(87)90130-4. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Orband-Miller L, Queen KL, Whitmore WL, Seidler FJ. Effects of prenatal nicotine exposure on biochemical development of rat brain regions: maternal drug infusions via osmotic minipumps. J. Pharmacol. Exp. Ther. 1987b;240:602–611. [PubMed] [Google Scholar]
- Smith SG, Davis WM. A method for chronic intravenous drug administration in the rat. In: Ehrenpreis S, Neidle A, editors. Methods in Narcotics Research. Marcel Dekker; New York: 1975. pp. 3–21. [Google Scholar]
- Smith RJ, See RE, Aston-Jones G. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. Eur. J. Neurosci. 2009;30:493–503. doi: 10.1111/j.1460-9568.2009.06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Tahsili-Fahadan P, Aston-Jones G. Orexin/hypocretin is necessary for context-driven cocaine-seeking. Neuropharmacology. 2010;58:179–184. doi: 10.1016/j.neuropharm.2009.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Steininger TL, Kilduff TS, Behan M, Benca RM, Landry CF. Comparison of hypocretin/orexin and melanin-concentrating hormone neurons and axonal projections in the embryonic and postnatal rat brain. J. Chem. Neuroanat. 2004;27:165–181. doi: 10.1016/j.jchemneu.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Stettner GM, Kubin L, Volgin DV. Antagonism of orexin 1 receptors eliminates motor hyperactivity and improves homing response acquisition in juvenile rats exposed to alcohol during early postnatal period. Behav. Brain Res. 2011;221:324–328. doi: 10.1016/j.bbr.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: 2012. (NSDUH Series H-44). HHS Publication No. (SMA) 12-4713. [Google Scholar]
- Vittoz NM, Berridge CW. Hypocretin/orexin selectively increases dopamine efflux within the prefrontal cortex: involvement of the ventral tegmental area. Neuropsychopharmacology. 2006;31:384–395. doi: 10.1038/sj.npp.1300807. [DOI] [PubMed] [Google Scholar]
- Vittoz NM, Schmeichel B, Berridge CW. Hypocretin /orexin preferentially activates caudomedial ventral tegmental area dopamine neurons. Eur. J. Neurosci. 2008;28:1629–1640. doi: 10.1111/j.1460-9568.2008.06453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorhees CM, Cunningham CL. Involvement of the orexin/hypocretin system in ethanol conditioned place preference. Psychopharmacol. (Berl.) 2011;214:805–818. doi: 10.1007/s00213-010-2082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV. Handbook of Behavioral Teratology. Plenum Press; New York, NY: 1986. pp. 23–48. [Google Scholar]
- Weaver MT, Geier CF, Levin ME, Caggiula AR, Sved AF, Donny EC. Adolescent exposure to nicotine results in reinforcement enhancement but does not affect adult responding in rats. Drug Alcohol Depend. 2012;125:307–312. doi: 10.1016/j.drugalcdep.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Wang J, Dwyer JB, Mangold J, Cao J, Leslie FM, Li MD. Gestational nicotine treatment modulates cell death/survival-related pathways in the brains of adolescent female rats. Int. J. Neuropsychopharmacol. 2011;14:91–106. doi: 10.1017/S1461145710000416. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Warner , Wickramaratne PJ, Kandel DB. Maternal smoking during pregnancy and psychopathology in offspring followed to adulthood. J. Am. Acad. Child Adolesc. Psychiatry. 1999;38:892–899. doi: 10.1097/00004583-199907000-00020. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol. Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Yokel RA. Intravenous self-administration: Response rates, the effects of pharmacological challenges, and drug preference. In: Bozarth MA, editor. Methods of Assessing the Reinforcing Properties of Abused Drugs. Springer-Verlag; New York: 1987. pp. 1–33. [Google Scholar]
- Zhou Y, Cui CL, Schlussman SD, Choi JC, Ho A, Han JS, Kreek MJ. Effects of cocaine place conditioning, chronic escalating-dose “binge” pattern cocaine administration and acute withdrawal on orexin/hypocretin and preprodynorphin gene expressions in lateral hypothalamus of Fischer and Sprague-Dawley rats. Neuroscience. 2008;153:1225–1234. doi: 10.1016/j.neuroscience.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Takita M, Konishi Y, Sudo M, Muramatsu I. Chronic nicotine treatment delays the developmental increase in brain muscarinic receptors in rat neonate. Brain Res. 1996;732:257–260. doi: 10.1016/0006-8993(96)00704-4. [DOI] [PubMed] [Google Scholar]
- Zhu J, Zhang X, Xu Y, Spencer TJ, Biederman J, Bhide PG. Prenatal nicotine exposure mouse model showing hyperactivity, reduced cingulate cortex volume, reduced dopamine turnover, and responsiveness to oral methylphenidate treatment. J. Neurosci. 2012;32:9410–9418. doi: 10.1523/JNEUROSCI.1041-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]