Abstract
Objective
The current study explored whether immersive virtual reality continues to reduce pain (via distraction) during more than one wound care session per patient. Patients: Thirty six patients aged 8 to 57 years (mean age of 27.7 years), with an average of 8.4% total body surface area burned (range .25 to 25.5 TBSA) received bandage changes, and wound cleaning.
Methods
Each patient received one baseline wound cleaning/debridement session with no-VR (control condition) followed by one or more (up to seven) subsequent wound care sessions during VR. After each wound care session (one session per day), worst pain intensity was measured using a Visual Analogue Thermometer (VAT), the dependent variable. Using a within subjects design, worst pain intensity VAT during wound care with no-VR (baseline, Day 0) was compared to pain during wound care while using immersive virtual reality (up to seven days of wound care during VR).
Results
Compared to pain during no-VR Baseline (Day 0), pain ratings during wound debridement were statistically lower when patients were in virtual reality on Days 1, 2 and 3, and although not significant beyond day 3, the pattern of results from Days 4, 5, and 6 are consistent with the notion that VR continues to reduce pain when used repeatedly.
Conclusions
Results from the present study suggest that VR continues to be effective when used for three (or possibly more) treatments during severe burn wound debridement.
Keywords: virtual reality, burn, wound care
INTRODUCTION
One of the greatest challenges to burn care is managing the pain associated with ongoing procedures. The pain experienced by severe burn patients during medical procedures varies widely from day to day, but is often severe in intensity1–3. Health care providers are faced with the challenge of helping severe burn patients cope with painful and distressing daily wound care and physical therapy/occupational therapy procedures1 and patients’ pain ratings during the acute phase range from mild to excruciating2. The control of such pain is complicated because, as patients are repeatedly subjected to painful medical procedures, patients understandably link the pain to anxiety1, 4. Pain during hospitalization is a strong predictor of psychological adjustment at 1-month to 2-year follow-up5. Hence pain management after burn injuries is an important part of a comprehensive treatment program6 that involves multiple modalities, including long and short-acting opiates, anxiolytics, and non-pharmacological techniques7.
Opioid analgesics remain the primary treatment for acute burn pain, but pain medications have a number of negative side effects that limit use. Heavy reliance on pain medications can increase hospital stay, and pharmacologic interventions often do not eliminate all burn pain1. Because of the repetitive, often daily nature of painful burn wound debridement/cleaning, there is also a high risk for developing a physiologic tolerance to (and possible dependence on) opioid analgesics8. Both the economic and psychological costs of using only pharmacologic analgesics for burn pain management contribute to the motivation to identify adjunctive non-pharmacologic analgesic treatments that can improve burn related pain management and reduce opioid analgesic drug use8.
Psychologically based adjuncts to pain medications are particularly attractive because they seldom have side effects. Examples of such psychological interventions include information, cognitive-behavioral interventions, distraction, and hypnosis9. In general, such approaches seem helpful in making burn pain more manageable, and can be used in addition to conventional pharmacologic analgesics. However, the pain associated with treating severe burn injuries is often so profound that, while such approaches are helpful, conventional adjunctive non-pharmacologic pain treatments currently still fall short of having any major effect on this problem.
Immersive virtual reality (VR) offers a psychologically based approach to pain that appears to have unusually powerful and promising analgesic effects. VR diverts attention away from the pain during acute procedures by immersing patients in a computer generated environment10. Typically, patients use a head mounted three dimensional visual screen to interact with the computer environment in order to draw attention away from their pain. A number of studies have demonstrated that computer generated immersive virtual reality environments are effective for reducing not only laboratory induced pain11–17, but preliminary studies suggest VR can also help reduce procedural pain from non-burn etiologies (dental pain18, 19; urological endoscopies20; cerebral palsy21; discomfort during treatment for cancer22–24; and pruritus/itching25). Most of the clinical research with VR has studied burn pain, and a number of clinical series, as well as controlled trials exploring VR distraction during medical procedures of severe burn patients have been reported, generally with good results26–35. However, these studies have tended to involve small subject samples and often focus on the pain from joint range-of-motion physical/occupational therapy exercises, pain that can be significant but is not the worst that comes with burn care. Further, the results of these studies are very often limited to one medical procedure on only one day26, 36, 37. To date, there are little data on the impact of VR on pain during multiple sessions of wound debridement and/or cleaning. One pilot study reported encouraging results from seven burn patients who received VR during wound cleaning38. If VR only works the first time it is applied to patients during severe burn wound cleaning session, its clinical value will be limited in actual care.
The purpose of the current study was to replicate the few studies that have reported VR during burn wound care with a larger sample size. We also wished to report on the repeated use of VR analgesia with a subset of the sample. The current study addresses this issue of whether VR continues to reduce pain when the same patient receives VR during two or more burn wound debridements. Using a within-subjects design, we compared pain during a no-distraction baseline condition (conventional treatment) to pain during one (n = 36), two (n = 30), three (n = 17) and up to seven separate VR wound care sessions per patient.
METHODS AND MATERIALS
Participants were treated with VR therapy during the first 14 days of admission, and studied using a baseline-post treatment comparison. Immersive VR was limited to the period before initial surgery. Normal analgesic regimen was continued; opioid equivalent oral doses were calculated. Data collection was quantitative using self-report rating scales.
Study population
Participants were thirty-six consecutive patients who ranged in age from 8 to 57 years old (mean age = 27.7 years old, SD = 15.2) who were hospitalized in a major regional burn unit in the Netherlands from 1-01-2005 until 1-07-2008 and met the following inclusion-criteria: ability to communicate meaningfully, Dutch speaking and reading/writing ability, and an expected hospital stay in the burn unit for at least 4 days. Patients with pre-morbid psychiatric illness, physical impairments that preclude VR therapy (e.g., facial burns), a history of seizure disorders, or the need for intensive care were excluded from this study.
During the study period, 239 burn survivors who ranged in age from 8 to 57 years were admitted to the burn unit for 4 days or longer. Patients were included in the study only if they were anticipated to have a minimum of two days of burn wound care. A total of 203 patients were excluded from the study for the following reasons: 45.3% had a physical impairment like facial-burns or other technical exclusion criteria (such as wound care limited to showers; VR systems cannot get wet); 18.7% of the patients suffered from acute or chronic psychiatric symptoms at the time of admission to the hospital (information based on intake-reports); 14.8% needed intensive care; 8.9% had poor Dutch proficiency. Eighteen persons (8.9%) refused to participate in the study; five patients (2.4%) were consented but did not complete at least one VR session or baseline pain score was not registered, and two patients (1%) were excluded because of relevant comorbidity; one patient had a history of seizure disorder and one patient was blind. Persons who were excluded from the study were on average 13.2 years older (p <.01). There were no statistically significant differences with regards to gender, total burned surface area, percentage full thickness skin loss, hospital stay or number of surgical procedures.
Measures
Visual Analogue Thermometer (VAT)
The VAT is a burn specific pain rating device developed by Choiniere and colleagues41 with scientifically recognized psychometric qualities42. The Visual Analogue Thermometer (VAT) consists of a 10-cm tall visual “thermometer” representing a continuum with the ends marked “no pain” and “unbearable pain”. Subjects were asked to mark the thermometer at a point corresponding to the worst pain intensity they experienced during wound care, and distance was evaluated to the nearest mm. The VAT is based on the measurement properties of the visual analogue scale, often used as a reliable measurement tool of pain and personal well-being43–45. In the present study, worst pain VAT score during wound debridement was measured immediately after the wound dressing change procedure.
Procedure
After informed consent of the patient and, when the patient was younger than 18 years, also the informed consent of the parent(s), the patient could participate in the study. All subjects received some type of pharmacologic analgesics including opioid analgesics using the normal burn unit regimen. To determine the presence of adverse effects due to the VR, after each trial the subjects were asked whether or not they felt any nausea during the VR administration, and responses were recorded for analysis. Pre-treatment nausea was not assessed; only nausea that was perceived by the patient to be a side effect of VR treatment.
Using a within subjects design, worst pain intensity VAT during wound care with no-VR (baseline, Day 0) was compared to pain during wound care while using immersive virtual reality (up to seven days of wound care during VR). Other data relevant to predicting patients’ response to treatment were collected (e.g., personality traits); however, these data will be reported in a subsequent study.
VR Technology
In this study the Cybermind Hi-Res900ST™ -3D Head Mounted Display with a field of view of (Low-tech) 31.2° diagonal degree was used with an integrated audio system and controlled by a FasTrak® control-box: the Polhemus FasTrak 6 DOF motion tracking system and StarTech 4 port VGA/Video splitter, connected with a Dell® 650 Precision Workstation with Pentium IV processor, 2.392 Ghz (2 processors: HyperThreading Technology); Physical Memory: 2047 MB, Free memory: 1829 MB; Operating System: Windows 2000, SP 3 + Virtools® Web Player Network Configuration; Display Mode: 1024 × 768 (32 bit); NVIDIA Quadro FX 1000 (126/128 MB) graphic card; IVR-software, SnowWorld version 2.1 (2003) was used (www.vrpain.com).
Pain medications
Patients were medicated for pain with paracetamol during hospital stay from admission to discharge and dosing was determined by age. The mean dose recorded in the patient sample was 32.8 mg/kg per 24 hours (min-max: 16.7–62.0; SD 10.7; Median 31.5). For background, breakthrough and procedural pain, patients also used opioid analgesics including enteral morphine, transdermal fentanyl, intramuscular piritramide, and/or oral tramadol. To calculate total opioid analgesic usage, doses were standardized by calculating the oral morphine equivalent dose (OME) dose per 24 hours. The following formula was used: morphine suppositorium mg/24 hours multiplied by 1.0, transdermal fentanyl mcg/hour multiplied by 3.6, intramuscalar piritramide mg/24 hours multiplied by 0.8, and oral tramadol mg/24 hour divided by 3.0. In addition to the several opioid and non-opioid analgesic medications administered regularly for background pain, enteral morphine and piritramide were administered on a PRN (as needed) basis to address procedural pain. The mean opioid analgesic usage for background pain in our sample per kilogram body weight per 24 hours was 1.60 mg (min-max: 1.05 – 2.57; SD 0.5; Median 1.42). The mean procedural opioid analgesic usage in our sample per kilogram body weight was 0.16 mg (min-max: 0.11 – 0.42; SD 0.1; Median 0.13).
Statistical analyses
Differences between pain intensity during no-VR (baseline) and pain intensity during VR was tested using within-subjects paired t-tests. All patients were compared to a baseline of wound care with medication only and no VR. However, this baseline often did not occur on the first day of hospitalization, and patients often had several wound care sessions before their baseline day (i.e., before the first study day). The baseline wound care occurred within the first two weeks of hospitalization and was based on factors such as when patients were out of intensive care and stable and alert enough to participate in the study. All effects are reported as significant at p < .05. All analyses were executed using SPSS Statistics version 20 (release 20.0.1).
RESULTS
Thirty six patients participated in the study (i.e., completed at least one “no VR” baseline and one VR treatment). These patients were predominantly male (83.8%) and were, on average, 27.9 years old (SD = 14.8). Patients had an average length of hospital stay of 23.2 days (5–63; SD = 11.8), had undergone, between admission and discharge, but after the VR study period, on average 0.78 (0–2; SD = 0.75) surgical procedures, and their TBSA ranged from 0.25 to 25.5% (M = 8.4, SD = 6.6). The percentage full-thickness skin loss ranged from 0–16% (M = 2.8; SD = 4.1). Participants used VR on at least one and up to seven consecutive days (M = 2.8; SD =1.4) during wound dressing procedures. Thirty-six patients (100%) completed at least one wound care session in VR, 30 patients (83%) used VR during two or more wound care sessions and 17 patients (47%) used VR for three or more VR wound care sessions (see Table 1). None of the patients reported side effects of VR intervention (e.g., nausea). Although not formally assessed, some patients reported mild pre-treatment nausea that was thought to be associated with opioid analgesic use. In no cases did patients report a worsening of nausea or attribute it to VR use. No correlations were found between the reduction in pain ratings and demographic variables such as age, sex, duration of hospital stay, or percentage of (deep) burns.
Table 1.
Paired t-test results of Baseline VAT pain scores/Opioid-usage (OME/kg/day) compared with VR VAT pain score/Procedural Opioid-usage of the 1st-7th day VR
| VAT pain scores | Procedural Opioid usage | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| On | df (n− 1 ) | t | p | SD diff. | t | p | SD diff. |
| Day 1 | 35 | 2.939 | .006 | 2.23 | 0.793 | .433 | 0.05 |
| Day 2 | 29 | 2.145 | .040 | 2.17 | 0.792 | .435 | 0.05 |
| Day 3 | 16 | 2.262 | .038 | 2.22 | −1.444 | .168 | 0.05 |
| Day 4 | 6 | −0.034 | .974 | 2.22 | −1.508 | .175 | 0.07 |
| Day 5 | 2 | 4.214 | .052 | 0.81 | * | 1.000 | 0.00 |
| Day 6 | 2 | 4.105 | .055 | 1.21 | * | 1.000 | 0.00 |
| Day 7 | 0 | * | * | * | * | * | * |
Scores are equal or not analyzable (n=1)
VR and burn Pain
Procedural VAT pain scores
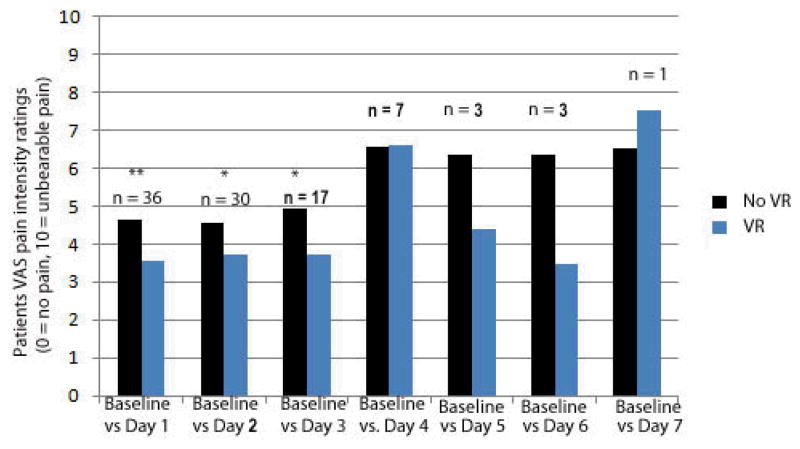
Using within-subjects paired t-tests, each patient’s pain during wound care with no VR (baseline, day zero) was compared to the same patient’s pain ratings during wound care during VR on up to seven treatment days (see Figure 1).
Figure 1.
Virtual Reality distraction remained effective over multiple treatments.
DISCUSSION
Results of the current study show that VR reduced the amount of pain reported on more than one dressing change/wound debridement session per patient. To our knowledge, this is the first large multi-patient study to test whether VR analgesia remains effective for reducing pain during burn wound care, when VR is used more than once per burn patient.
Although the current study provides encouraging preliminary evidence, additional research is needed. The within-subjects design used in the present study generally has more statistical power than a between-groups design. However, future studies using a between groups experimental design are still needed. The declines reported in the present study could be potentially partially due to historical factors (e.g., due to natural healing) and might lead to overestimation of the amount of VR analgesia. A between-groups design could isolate the influences of VR more carefully. Although we were able to report that pain ratings went down with repeated use of VR, only 83% received VR for two or more treatments, and only 47% of the sample received VR three or more sessions. However, this is the largest sample VR wound care study to date.
The software used in this study (SnowWorld 2003) appears to be powerful in its ability to capture attention and is particularly appropriate for patients with burn injuries (given the illusion of coolness that is encouraged); however, this is one of the early worlds that has been created for medical and pain applications in general, (a more recent version of this software is now available; www.vrpain.com). The VR helmet used in the present study is an earlier “low tech” model, and has a relatively narrow field of view (31 degrees diagonal). This limits the amount of peripheral vision stimulated, and patients can see the hospital room out of their peripheral vision. Future studies using a higher quality (e.g., wider field of view) VR helmet now available (e.g., the MX90 with 90 degrees diagonal field of view, from www.nvisinc.com) will likely show even stronger analgesic effects14. As hardware and software technology improves, the ability to reduce pain using immersive virtual reality is likely to increase in the future (see review46).
To date, the current study is the largest sample of hospitalized burn patients to receive VR distraction while undergoing bandage changes/wound debridement. The present findings are encouraging regarding the potential for developing VR into a powerful analgesic technique that does not have pharmacological side effects. Research to date suggests VR has few if any side effects (there is the potential of motion sickness which has thus far been avoided46).
The size of the analgesic effects in the current study and the finding that VR continued to reduce pain when used repeatedly (i.e., over multiple debridement sessions) represent an encouraging advance in the field. Future studies further exploring how effectively VR reduces pain when used for multiple debridement sessions are justified and needed. For example, a patient with a large burn may require 30 or more wound care sessions lasting 30 minutes or more per day and in addition, may require 30–60 or more physical/occupational therapy range-of-motion sessions. Whether VR continues to be effective under those conditions is an important topic for future research.
Acknowledgments
This study was financially supported primarily by the Dutch Burns Foundation grant number 05.112. Secondary support was from the Scan Design Foundation by Inger and Jens Bruun, and the following NIH grants: 1R01AR054115-01A1, R01GM042725-17A1. Thanks to Hunter Hoffman PhD and Sam Sharar MD for their valuable comments and Alison Schultz for help preparing the manuscript. We thank all the participants and the nurses of the Burn Centre of the Martini Hospital Groningen for their collaboration.
References
- 1.Wiechman Askay S, Patterson DR, Sharar SR, et al. Pain management in patients with burn injuries. International Review of Psychiatry. 2009 Dec;21(6):522–530. doi: 10.3109/09540260903343844. [DOI] [PubMed] [Google Scholar]
- 2.Summer GJ, Puntillo KA, Miaskowski C, et al. Burn injury pain: the continuing challenge. Journal of Pain. 2007 Jul;8(7):533–548. doi: 10.1016/j.jpain.2007.02.426. [DOI] [PubMed] [Google Scholar]
- 3.Choiniere M, Melzack R, Rondeau J, et al. The pain of burns: Characteristics and correlates. Journal of Trauma. 1989 Nov;29(11):1531–1539. doi: 10.1097/00005373-198911000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Byers JF, Bridges S, Kijek J, LaBorde P. Burn patients’ pain and anxiety experiences. Journal of Burn Care and Rehabilitation. 2001 Mar-Apr;22(2):144–149. doi: 10.1097/00004630-200103000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Patterson DR, Tininenko JR, Ptacek JT. Pain during burn hospitalization predicts long-term outcome. Journal of Burn Care and Research. 2006 Sep-Oct;27(5):719–726. doi: 10.1097/01.BCR.0000238080.77388.FE. [DOI] [PubMed] [Google Scholar]
- 6.Esselman PC. Burn rehabilitation: An overview. Archives of Physical Medicine and Rehabilitation. 2007 Dec;88(12 Suppl 2):S3–6. doi: 10.1016/j.apmr.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 7.Sharar SR, Patterson DR. Burn Pain. In: Fishman SM, Ballantyne JC, Rathmell JP, editors. Bonica’s Management of Pain. 4. Philadelphia, PA: Lippincott, Williams, & Wilkins; 2009. [Google Scholar]
- 8.Sharar SR, Miller W, Teeley A, et al. Applications of virtual reality for pain management in burn-injured patients. Expert Review of Neurotherapeutics. 2008 Nov;8(11):1667–1674. doi: 10.1586/14737175.8.11.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patterson DR. Non-opioid-based approaches to burn pain. Journal of Burn Care and Rehabilitation. 1995 May-Jun;16(3 Pt 2):372–376. doi: 10.1097/00004630-199505001-00007. [DOI] [PubMed] [Google Scholar]
- 10.Patterson DR, Tininenko JR, Schmidt AE, et al. Virtual reality hypnosis: A case report. International Journal of Clinical and Experimental Hypnosis. 2004 Jan;52(1):27–38. doi: 10.1076/iceh.52.1.27.23925. [DOI] [PubMed] [Google Scholar]
- 11.Patterson DR, Hoffman HG, Garcia Palacios A, et al. Analgesic effects of posthypnotic suggestions and virtual reality distraction on thermal pain. Journal of Abnormal Psychology. 2006 Nov;115(4):834–841. doi: 10.1037/0021-843X.115.4.834. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman HG, Richards TL, Coda B, et al. Modulation of thermal pain-related brain activity with virtual reality: Evidence from fMRI. Neuroreport. 2004 Jun 7;15(8):1245–1248. doi: 10.1097/01.wnr.0000127826.73576.91. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman HG, Richards TL, Van Oostrom T, et al. The analgesic effects of opioids and immersive virtual reality distraction: Evidence from subjective and functional brain imaging assessments. Anesthesia and Analgesia. 2007 Dec;105(6):1776–1783. doi: 10.1213/01.ane.0000270205.45146.db. table of contents. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman HG, Seibel EJ, Richards TL, et al. Virtual reality helmet display quality influences the magnitude of virtual reality analgesia. Journal of Pain. 2006 Nov;7(11):843–850. doi: 10.1016/j.jpain.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman HG, Sharar SR, Coda B, et al. Manipulating presence influences the magnitude of virtual reality analgesia. Pain. 2004 Sep;111(1–2):162–168. doi: 10.1016/j.pain.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Wender R, Hoffman HG, Hunner HH, et al. Interactivity influences the magnitude of virtual reality analgesia. Journal of Cybertherapy and Rehabilitation. 2009 Spring;2(1):27–33. [PMC free article] [PubMed] [Google Scholar]
- 17.Dahlquist LM, Weiss KE, Law EF, et al. Effects of videogame distraction and a virtual reality type head-mounted display helmet on cold pressor pain in young elementary school-aged children. Journal of Pediatric Psychology. 2010 Jul;35(6):617–625. doi: 10.1093/jpepsy/jsp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furman E, Jasinevicius TR, Bissada NF, et al. Virtual reality distraction for pain control during periodontal scaling and root planing procedures. Journal of the American Dental Association. 2009 Dec;140(12):1508–1516. doi: 10.14219/jada.archive.2009.0102. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman HG, Garcia-Palacios A, Patterson DR, et al. The effectiveness of virtual reality for dental pain control: a case study. CyberPsychology and Behavior. 2001 Aug;4(4):527–535. doi: 10.1089/109493101750527088. [DOI] [PubMed] [Google Scholar]
- 20.Wright JL, Hoffman HG, Sweet RM. Virtual reality as an adjunctive pain control during transurethral microwave thermotherapy. Urology. 2005 Dec;66(6):1320. doi: 10.1016/j.urology.2005.06.123. [DOI] [PubMed] [Google Scholar]
- 21.Steele E, Grimmer K, Thomas B, et al. Virtual reality as a pediatric pain modulation technique: A case study. CyberPsychology and behavior. 2003 Dec;6(6):633–638. doi: 10.1089/109493103322725405. [DOI] [PubMed] [Google Scholar]
- 22.Schneider SM, Prince-Paul M, Allen MJ, et al. Virtual reality as a distraction intervention for women receiving chemotherapy. Oncology Nursing Forum. 2004 Jan-Feb;31(1):81–88. doi: 10.1188/04.ONF.81-88. [DOI] [PubMed] [Google Scholar]
- 23.Gershon J, Zimand E, Pickering M, et al. A pilot and feasibility study of virtual reality as a distraction for children with cancer. Journal of the American Academy of Child and Adolescent Psychiatry. 2004 Oct;43(10):1243–1249. doi: 10.1097/01.chi.0000135621.23145.05. [DOI] [PubMed] [Google Scholar]
- 24.Wolitzky K, Fivush R, Zimand E, et al. Effectiveness of virtual reality distraction during a painful medical procedure in pediatric oncology patients. Psychology and Health. 2005;20(6):817–824. [Google Scholar]
- 25.Leibovici V, Magora F, Cohen S, et al. Effects of virtual reality immersion and audiovisual distraction techniques for patients with pruritus. Pain Research and Management. 2009 Jul-Aug;14(4):283–286. doi: 10.1155/2009/178751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan EA, Chung JW, Wong TK, et al. Application of a virtual reality prototype for pain relief of pediatric burn in Taiwan. Journal of Clinical Nursing. 2007 Apr;16(4):786–793. doi: 10.1111/j.1365-2702.2006.01719.x. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman HG, Doctor JN, Patterson DR, et al. Use of virtual reality as an adjunctive treatment of adolescent burn pain during wound care: A case report. Pain. 2000;85(1–2):305–309. doi: 10.1016/s0304-3959(99)00275-4. [DOI] [PubMed] [Google Scholar]
- 28.Hoffman HG, Patterson DR, Carrougher GJ. Use of virtual reality for adjunctive treatment of adult burn pain during physical therapy: A controlled study. Clinical Journal of Pain. 2000;16(3):244–250. doi: 10.1097/00002508-200009000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Hoffman HG, Patterson DR, Carrougher GJ, et al. Effectiveness of virtual reality-based pain control with multiple treatments. Clinical Journal of Pain. 2001;17(3):229–235. doi: 10.1097/00002508-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Hoffman HG, Patterson DR, Magula J, et al. Water-friendly virtual reality pain control during wound care. Journal of Clinical Psychology. 2004 Feb;60(2):189–195. doi: 10.1002/jclp.10244. [DOI] [PubMed] [Google Scholar]
- 31.Hoffman HG, Patterson DR, Seibel E, et al. Virtual reality pain control during burn wound debridement in the hydrotank. Clinical Journal of Pain. 2008 May;24(4):299–304. doi: 10.1097/AJP.0b013e318164d2cc. [DOI] [PubMed] [Google Scholar]
- 32.Hoffman HG, Patterson DR, Soltani M, et al. Virtual reality pain control during physical therapy range of motion exercises for a patient with multiple blunt force trauma injuries. CyberPsychology and Behavior. 2009 Feb;12(1):47–49. doi: 10.1089/cpb.2008.0056. [DOI] [PubMed] [Google Scholar]
- 33.Schmitt YS, Hoffman HG, Blough DK, et al. A randomized, controlled trial of immersive virtual reality analgesia, during physical therapy for pediatric burns. Burns. 2011 Feb;37(1):61–68. doi: 10.1016/j.burns.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharar SR, Carrougher GJ, Nakamura D, et al. Factors influencing the efficacy of virtual reality distraction analgesia during postburn physical therapy: preliminary results from 3 ongoing studies. Archives of Physical Medicine and Rehabilitation. 2007 Dec;88(12 Suppl 2):S43–49. doi: 10.1016/j.apmr.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Carrougher GJ, Hoffman HG, Nakamura DY, et al. The effect of virtual reality on pain and range of motion in adults with burn injuries. Journal of Burn Care and Research. 2009 Sep-Oct;30(5):785–791. doi: 10.1097/BCR.0b013e3181b485d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maani CV, Hoffman HG, Fowler M, et al. Combining ketamine and virtual reality pain control during severe burn wound care: one military and one civilian patient. Pain Medicine. 2011 Apr;12(4):673–678. doi: 10.1111/j.1526-4637.2011.01091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Twillert B, Bremer M, Faber AW. Computer-generated virtual reality to control pain and anxiety in pediatric and adult burn patients during wound dressing changes. Journal of Burn Care and Research. 2007 Sep-Oct;28(5):694–702. doi: 10.1097/BCR.0B013E318148C96F. [DOI] [PubMed] [Google Scholar]
- 38.Das DA, Grimmer KA, Sparnon AL, et al. The efficacy of playing a virtual reality game in modulating pain for children with acute burn injuries: A randomized controlled trial. BMC Pediatrics. 2005;5(1):1. doi: 10.1186/1471-2431-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flores A, Hoffman HG, Russell W, et al. Longer, multiple virtual reality pain distraction treatments of Hispanic and Caucasian children with large severe burns. CyberTherapy Conference; San Diego, CA. 2008. [Google Scholar]
- 40.Hoffman HG, Patterson DR, Carrougher GJ, et al. The effectiveness of virtual reality pain control with multiple treatments of longer durations: A case study. International Journal of Human-Computer Interaction. 2001;13(1):1–12. [Google Scholar]
- 41.Choiniere M, Auger FA, Latarjet J. Visual analogue thermometer: A valid and useful instrument for measuring pain in burned patients. Burns. 1994 Jun;20(3):229–235. doi: 10.1016/0305-4179(94)90188-0. [DOI] [PubMed] [Google Scholar]
- 42.Choiniere M, Amsel R. A visual analogue thermometer for measuring pain intensity. Journal of Pain and Symptom Management. 1996 May;11(5):299–311. doi: 10.1016/0885-3924(95)00204-9. [DOI] [PubMed] [Google Scholar]
- 43.Scott J, Huskisson EC. Graphic representation of pain. Pain. 1976 Jun;2(2):175–184. [PubMed] [Google Scholar]
- 44.Huskisson EC. Measurement of pain. Lancet. 1974 Nov 9;2(7889):1127–1131. doi: 10.1016/s0140-6736(74)90884-8. [DOI] [PubMed] [Google Scholar]
- 45.Clarke PRF, Spear FG. Reliability and sensitivity in the self-assessment of well-being. Bulletin of the British Psychological Society. 1964;17(18A):55. [Google Scholar]
- 46.Hoffman HG, Chambers GT, Meyer WJ, 3rd, et al. Virtual reality as an adjunctive non-pharmacologic analgesic for acute burn pain during medical procedures. Annals of Behavioral Medicine. 2011 Jan 25;41:183–191. doi: 10.1007/s12160-010-9248-7. [DOI] [PMC free article] [PubMed] [Google Scholar]