Abstract
Lead (Pb), arsenic (As) and manganese (Mn) are neurotoxic elements that often occur in mixtures for which practically no information is available on biomarkers (BMs) for the evaluation of exposure/effects. Exposures to these metals may increase delta-aminolevulinic acid (delta-ALA), which in itself may potentiate neurotoxicity. The objective of this study was to investigate the utility of urinary delta-ALA (delta-ALA-U) levels as BM of exposure and/or neurotoxic effects induced by this mixture. Five groups of Wistar rats were treated for 8 days with Pb (5 mg/kg), As (60 mg/L), Mn (10 mg/kg), the 3-metal mixture (same doses of the single metals), and control group. Motor activity was evaluated and 24-h urine collected before and after the treatment. 24-hours (h) after the last dose, the rats were sacrificed and the brains removed for analyses. Delta-ALA and metal levels were determined in brain and urine. Co-treated rats showed a significant (p<0.05) correlation between increased Pb, As, Mn and delta-ALA levels in the brain and decreased motor activity. Delta-ALA-U concentrations were higher in the mixture-treated group than the sum of the delta-ALA-U levels in each single-treated groups and discriminated (p<0.05) between the mixture and untreated rats. Moreover, delta-ALA-U was correlated (p<0.05) with brain delta-ALA levels. These results establish that treatments with this metal mixture exacerbate behavioral dysfunction, increasing most prominently brain Pb levels. This study is the first to establish that delta-ALA-U levels represent a sensitive BM of exposure/neurotoxic effect to this metal mixture.
Keywords: metal mixtures, lead, arsenic and manganese, neurotoxicity, biomarkers, delta-aminolevulinic acid
1. Introduction
In today's industrialized world the sources of exposure to metals in occupational settings, polluted water, foodstuffs and in the environment, are ubiquitous (Ferrer, 2003). Human exposure to metals is a problem of global magnitude (Toscano and Guilarte, 2005). Lead (Pb), arsenic (As) and manganese (Mn) are among metals/metalloids that represent major public health concerns (ATSDR 2007a, b and c). In fact, Pb remains the main environmental heavy metal pollutant (Charlet et al., 2012; Mameli et al., 2001) with estimates that combustion of leaded gasoline accounts for up to 90% of atmospheric Pb deposition. In addition, metal smelting activities contribute to Pb accumulation both in the atmosphere and soil (ATSDR, 2007c). Occupational exposures to Pb are common in the manufacturing of batteries, sheet lead and ceramic industries (Patrick, 2006). Pb poisoning from water supplies (Jemiola-Rzeminska, 2007) contaminated by mining and industrial waste (computer chips, wood preservatives and agrochemicals), as well as occupational settings (Rodríguez et al., 2003) is common. Increased As levels in the environment are mainly attributable to industrial products and wastes, agricultural pesticides and mine drainage. Occupational exposures to As also occur in industrial settings, particularly in nonferrous smelting, electronics, wood preservatives, glass manufacturing and application of arsenical pesticides (Kakkar and Jeffery, 2005). While an essential metal, excessive exposures to Mn may result from food and drinking water, as well as in clinical and occupational settings (Batterman et al., 2011; Bowler et al., 2006). The use of methylcyclopentadienyl manganese tricarbonyl (MMT), a substitute for leaded gasoline, the agricultural runoff with agrochemicals and activities, such as steeling and mining have been associated with increased Mn exposure (Martinez-Finley et al., 2012).
Given their co-existence in soil and atmosphere, exposure to Pb, As or Mn does not occur in isolation (Kordas et al., 2010). Indeed, in the real world, exposures to complex mixtures are the rule, rather than exception (Scherer, 2005). While the health sequalae of individual metal exposures may be known, there is a dearth of information on health outcomes subsequent to metal mixture exposures (Mowat and Bundy, 2002). Therefore, studying single metal exposures may fail to adequately predict health risks (Kordas et al., 2010).
Over the last several decades, the incidence of neurological diseases has increased (WHO, 2006). Given their environmental persistence and bioaccumulation (Mameli et al., 2001) and their propensity to accumulate in the brain (ATSDR, 2007a, b and c), metals have been implicated in the etiology of several neurodegenerative diseases (Weiss, 2010). Pb, As and Mn are established neurotoxic metals that readily cross the blood-brain barrier (BBB) (Balbuena and Erich, 2011; Martinez-Finley et al., 2012; Yokel, 2009). Chronic exposure to As or Pb induce peripheral motor nerve dysfunction (Blom et al., 1985; Mameli et al., 2001) with a broad range of behavioral disturbances (Gurer and Ercal, 2000; Halatek et al., 2009). Mn poisoning results in an irreversible condition known as “manganism,” a neurodegenerative disorder that resembles Parkinson disease in both symptomatology and the underlying cellular mechanisms (Ellingsen et al., 2008; Martinez-Finley et al., 2012).
Neurological disorders induced by chronic metal exposure can be progressive and manifest clinically decades after the initial exposure (Gil and Pla, 2001). The onset of neurotoxic effects is largely subtle, insidiously manifested and unidentifiable as a clearly defined disease (Shy, 1993). Accordingly, biomarkers (BMs), as observable endpoints in a continuum of events from exposure to disease, have become increasingly important for the detection and diagnosis of early poisoning (Kakkar and Jaffery, 2005; Scherer, 2005). Studies on the interactions between metals, using BMs have been recognized as necessary and timely (Wang and Fowler, 2008). Nevertheless, despite of the existence of several BMs for Pb, As or Mn induced toxicity (Cowan et al., 2009; Heitland and Köster, 2003; Higashikawa, 2000), practically no information exists on the effects of co-exposure to these three metals and potential BMs of toxicity. Useful BMs should reflect not only the exposure, but the onset of biochemical/physiological changes in cells and tissues (Scherer, 2005). With respect to metals, its detection in biological samples is the most common exposure BM (Phoon, 1998), with urinary analysis (a non-invasive procedure) representing the preferred media (Miller et al., 2004). Several attempts have been made to use urinary Pb as a surrogate of blood Pb levels, the traditional and preferred BM for the assessment of Pb exposure (Fukui et al., 1999; Patrick, 2006), but the approach is of limited value since it is not applicable on an individual basis (Fukui et al., 1999). Urinary As measurements are considered a reliable exposure BM, because urinary elimination is the major route for excretion of this metalloid (De Vizcaya-Ruiza et al., 2009). Urinary Mn levels are also of limited value, since they indicate an average level of exposure on a group basis, but are difficult to extrapolate on an individual basis (Batterman et al., 2011). As for BMs of effect, a shared target for Pb, As and Mn toxicity is heme biosynthesis (Bhadauria and Flora, 2004; Maines, 1980; Patrick, 2006). Delta-ALA is the first precursor in heme synthesis (Ennis et al., 2003), formed from glycine and succinyl CoA, in a reaction catalyzed by deltaaminolevulinic acid synthetase (ALAS). Subsequently, delta-aminolaevulinic acid dehydratase (ALAD) catalyzes the condensation of two molecules of delta- ALA to form porphobilinogen, another heme precursor (Makino et al., 2000). Suboptimal condensation of two delta-ALA molecules leads to decreased heme formation, in turn, stimulating ALAS via a negative feedback loop, resulting in increased delta-ALA levels both in circulating blood and urine (Gurer and Ercal, 2000). The best-known hematological effect of Pb is the interference with ALAD (ATSDR, 2007c; Patrick, 2006). ALAD is also a highly sensitive BM of As (Bhadauria and Flora, 2004). Little is known about the potential of Mn to interfere with heme biosynthesis; however Mn inhibits ALAS in both liver and brain (Maines, 1980). Increased plasma delta-ALA concentrations correlate with Pb-induced neurological disturbances (Gurer and Ercal, 2000; Olympio et al., 2009). ALA exerts a number of effects, including the inhibition of Na+, K+ -ATPase and adenylate cyclase activities, free radical formation followed by oxidative damage and alterations on gamma-aminobutyric acid (GABA) and glutamate uptake and release (Demasi et al., 1996; Emanuelli, 2003; Juknat et al., 1995).
Several methodologies are used to select the most adequate BMs of exposure and/or effect in determining health risk in exposed populations. Correlation analysis is a common method, where it is determined whether a significant correlation exists between the levels of a biological parameter or endpoint(s) of disease and exposure to specific chemical(s). These parameter(s) may then be used as BMs of exposure and/or effect (Casarett & Doull's, 2007). An accurate BM must have the ability to adequately identify or differentiate one condition (or outcome) from another. In fact, the growing need for rigorous evaluation of new BMs is leading to the recognition that the use of appropriate statistical techniques is essential for the accurate evaluation of their clinical relevance. The diagnostic accuracy of a BM is most commonly measured by the calculation of its sensitivity and specificity. Sensitivity is the proportion of patients who are correctly categorized as exposed or having disease, among those who truly are exposed or have the disease. Similarly, specificity is the proportion of patients who are correctly categorized as non-exposed or not having the disease among all patients who truly don't have the exposure or disease (Soreide, 2008). In this context, receiver-operating characteristic (ROC) curve analysis is emerging as a useful tool to accurately assess BMs (Cai and Pepe, 2002; Doecke et al., 2012; Shin et al., 2009; Soreide, 2008, Wu et al., 2010). ROC has been previously applied in the assessment of BMs of exposure to Pb (Sakai, 2000).
Given that Pb, As and Mn interfere with delta-ALA metabolism and that an association exists between brain delta-ALA accumulation and neurotoxicity, the present study was designed to investigate if delta-ALA-U levels represent an adequate BM of exposure and/or neurotoxic effect in rats co-treated with Pb, As and Mn. The manifestation of shared neurotoxic endpoints by Pb, As and Mn led to the hypothesis that treatments with a mixture of these metals will result in interactive toxic responses. Specifically, we tested in an in vivo rat model, whether co-exposure to these 3 metals was interactive (additive and/or synergistic), and whether correlations could be established between several BMs and behavioral outcomes.
2. Experimental Procedure
2.1. Chemicals
Chemicals were obtained from the following sources: Pb, As and Mn standards for Graphite Furnace Atomic Absorption Spectrometry (GFAAS) from Fluka, di-sodium hydrogen phosphate p.a. (Na2HPO4; ≥ 99%), hydrogen peroxide 30% (H2O2), magnesium matrix modifier for GFAAS (Mg(NO3)2 · 6H2O), nitric acid 65% suprapure (HNO3), potassium dihydrogen phosphate (KH2PO4; 99,5%), delta-aminolevulinic acid standard, sodium acetate (CH3COONa), and p-dimethylaminobenzaldehyde (C9H11NO) from Merck; acetic acid from Panreac; hydrohloric acid for ultratrace analysis (HCl); lead acetate trihydrate puriss. p.a. (C4H6O4Pb · 3H2O), manganese chloride tetrahydrate (MnCl2 · 4H2O; 99.99%) and sodium (meta) arsenite purum p.a. (AsO2Na; ≥ 99%) from Sigma.
2.2. In vivo assay
A sub-acute assay was performed in male Wistar rats (weighting 165 –206 g) from Charles River Laboratories®, Barcelona. All experiments were performed in accordance with the guiding principles of the European Community Council Directive (89/609/EEC) for the care and use of laboratory animals. The rats were housed in controlled temperature and humidity in a 12-h light/dark cycle. Their general condition was assessed on a daily basis. All animals had free access to water and food.
After a 15-day acclimation period, the animals were randomly assigned to 5 groups (6 rats/ group). Treatments were carried out according to the experimental design shown in Table 1. The administrated doses were chosen based on previous reports, showing behavioral alterations in rodents (Reddy, et al., 2003; Rodríguez et al., 2010; Vezér et al., 2005; Marreilha dos Santos et al., 2011). Behavior assays and collection of 24-h urine in metabolic cages were performed prior (pre-dosing -PD) and at the end of the experimental period. After centrifugation (2,500 rpm, 15 min) the urine samples were stored at −80°C. Prior to sacrifice (24 h after the last dose), the rats were anesthetized with pentobarbital (20 mg/kg, ip) and the brains immediately dissected out and stored at −80°C.
Table 1.
Experimental design
| Groups | Treatment Solutiona | Administration |
|---|---|---|
| Control | Sterile saline | 8 doses |
| Pb | 5 mg C4H6O4Pb/Kg b.w. | 8 doses |
| As | 60 mg AsO2Na/L | 8 days, ad libitum |
| Mn | 10 mg MnCl2/Kg b.w. | 8 doses |
| Pb + As + Mn | 5 mg C4H6O4Pb/Kg b.w. +60 mg AsO2Na/L + 10 mg MnCl2/Kg b.w. | 8 doses/days |
All treatment solutions were prepared fresh daily; Pb and Mn were administered by intraperitoneal (ip) injection and As via the drinking water.
2.3. Behavioral assays
Motor activity was assessed in an open-field apparatus (Marreilha dos Santos et al., 2012). The tests were carried out in a white box with a surrounding 30-cm-high opaque wall, the floor measuring 60 cm × 90 cm and divided into 6 equal squares by black lines. Immediately after placement in the center of the open-field, the rats’ movements were scored for five minutes. Two behavioral parameters were determined: the number of squares crossed with all paws (ambulations) and the number of times that both forelegs were raised from the floor (rearings). The rats were tested individually and after each session, the open field was thoroughly cleaned with humid cleaning tissue.
2.4. Determination of Pb, As and Mn in urine and brain
Sample preparation - 2.5 ml of each urine sample was digested with 1.25 ml of an acid mixture of 1:1 (v/v) HNO3: HCl at 100 °C for 30 min, and 80mg of each brain sample was dried for 5 h at 90°C prior to digestion with 1.5 ml of HNO3 at 100°C for 6 h, in a dry heater. The digested solutions were transferred to 25 ml volumetric flasks, the volumes were completed with deionized water and kept at 4 °C until analysis.
Graphite furnace atomic absorption spectrometry (GFAAS) - Brain and urinary Pb, As and Mn concentrations were determined by GFAAS with a PerkinElmer AAnalyst™ 700 equipped with an HGA Graphite Furnace, a programmable sample dispenser (Auto Sampler , AS 800) and WinLab 32 for AA software. Daily calibration curves for each element were obtained with standard solutions of Pb, As and Mn. Mg(NO3)2 was used as chemical modifier and added to blanks, standards and samples in equal volumes. The determined detection limits (LDs) were 1.3 ugPb/L, 7.4 ug As/L and 1.7 ug Mn/L.
All the glassware and sample cups for GFAAS were decontaminated from vestigial metals for at least 24 h in a 15% HNO3 (v/v) solution and rinsed twice with deionized water.
2.5. Determination of delta-ALA in urine and brain
One ml of supernatant (2500 rpm, 10 min) from each sample (urine or brain homogenates) was added to 1 ml of acetate buffer (pH 4.6) and 0.2 ml of acetoacetate. The samples were mixed (5 s) and incubated (100°C, 10 min) followed by the addition of 3 ml of ethyl acetate, agitation (15 s) and centrifugation (2000 rpm, 3min). A colorimetric reaction was started with Ehrlich reagent and the obtained organic phase. Delta-ALA concentrations were determined at 553 nm with an Hitachi spectrophotometer. Calibration curves were generated daily with a delta-ALA standard (Tomokuni and Ogata, 1972). The LD was 0.43 mg delta-ALA/L.
All the results were expressed as mg of Pb, As, Mn or delta-ALA per g of urinary creatinine or per g of brain protein. Urinary levels of creatinine were determined by a colorimetric method with a Randox (CR510) commercial kit, and brain protein contents according to the method described by Bradford (1976).
2.6. Statistical analysis
Data are expressed as mean ± SD. Statistical analysis was performed using SPSS 16.0 statistical package for Windows (SPSS, Inc., Chicago, IL, USA). After verification of data adequacy for parametric methods through normal distribution with Kolmogorov-Smirnov test and homogeneity of variance with Levene's test, non-parametric analysis was deemed the optimal statistical approach. All parameters were compared by Mann-Whitney tests, correlation analysis performed by the determination of Spearman's rho coefficients and areas under ROC curves were also estimated. The ROC curve is the plot of sensitivity (on the vertical axis) and 1-specificity (on the horizontal axis) for all possible thresholds of a BM in the study data set (Akobeng et al., 2007; Shin et al., 2009). Whereas a BM’ s area value close to 1 indicates an excellent BM, a curve that lies close to the diagonal (Area = 0.5) has no information content and therefore no diagnostic utility. More than one ROC curve can be present in the same plot and thus the absolute areas under each curve were compared to determine which BM has the better diagnostic performance (Warnock and Peck, 2010).
The significance of the results was considered when p values were less than 0.05.
3. Results
3.1. Behavioral assays
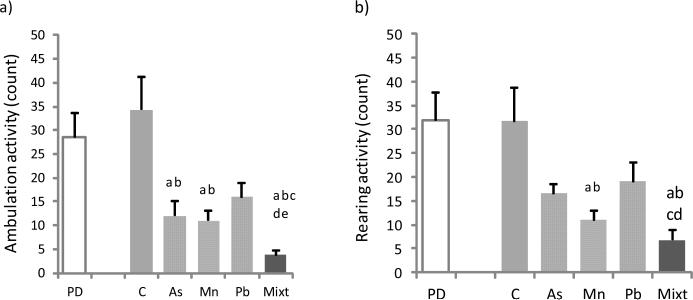
Two parameters of motor activity were determined, ambulation and rearing, prior to treatments and 24 h after the last dose. In the Pb treated group a trend towards decreased motor activity was observed when compared with the control group and with the PD group (Figs.1 a and b). The As treated group revealed similar trends to those found in the Pb treated group, but with statistical significance for ambulation (p < 0.05) (Fig.1 c). The Mn treated rats exhibited a significant decrease in ambulation and rearing compared with the two control groups (p < 0.05) (Figs.1 a and b). Rats treated with the metal mixture showed a decrease in both motor parameters that were significantly different from all the other groups (p < 0.05) (Figs.1 and b), except for rearing activity when compared with the Pb treated group (p < 0.05) (Fig.1 b).
Figs. 1 (a) and (b).
Motor activity, ambulation (a) and rearing (b) counts in groups of Wistar rats treated with As, Mn, Pb and a mixture of these metals (Mixt). Two control groups were used: prior to the administration (PD) and a sterile saline solution treated group (C). Data represent the mean ± SD (n=6). All groups were compared by Mann-Whitney U tests: a, b, c, d and e are p < 0.05 versus PD, C, As, Mn and Pb.
3.2. Brain Pb, As, Mn and delta-ALA levels
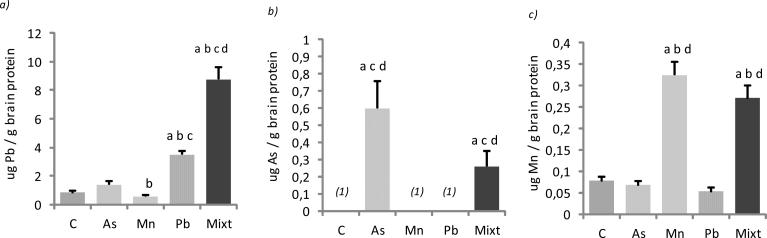
Brain Pb levels had a similar basal value in the control, As and Mn treated groups, while Pb treated rats had significantly higher brain Pb levels compared to these groups (p < 0.05) (Fig.2 a). The metal mixture group had significantly increased Pb brain levels compared to the other 4 groups (p < 0.05) (Fig.2 a). Brain As levels in the control, Pb and Mn treated groups were under the LD, and the As and metal mixture treated groups had As levels significantly higher than the other groups (p < 0.05) (Fig.2 b). Brain Mn concentrations in the control, As and Pb groups had similar values, and the Mn and metal mixture treated groups had significantly increased Mn levels compared with the other 3 groups (p < 0.05) (Fig.2 c).
Figs. 2 (a), (b) and (c).
Brain concentrations of Pb (a), As (b) and Mn (c) in groups of Wistar rats treated with As, Mn, Pb and a mixture of these metals (Mixt). A control group (C) was also used. Data represent the mean ± SD (n=6). (1) Means lower than the detection limit. All groups were compared by Mann-Whitney U tests: a, b, c, and d are p < 0.05 versus C, As, Mn and Pb.
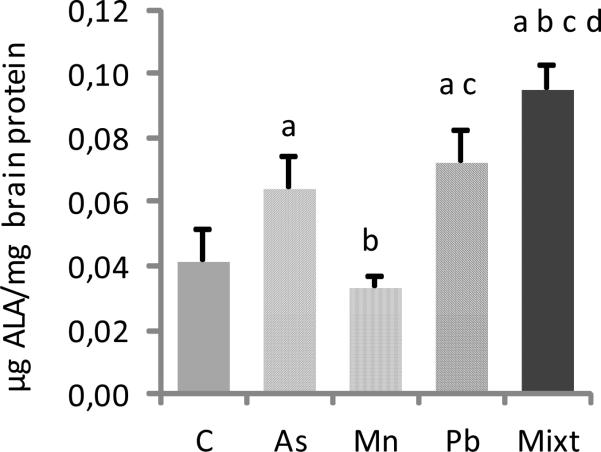
Brain delta-ALA concentrations were significantly increased in the Pb and As treated groups compared with the control (p< 0.05), whereas in the Mn treated group, delta- ALA brain levels were similar to the control (p > 0.05) (Fig.3). The highest concentrations of brain delta-ALA were observed in the metal mixture treated group, with levels significantly increased compared to all the other groups (p< 0.05) (Fig.3).
Fig.3.
Brain concentrations of delta-ALA in groups of Wistar rats treated with As, Mn, Pb and a mixture of these metals (Mixt). A control group (C) was also used. Data represent the mean ± SD (n=6). All groups were compared by Mann-Whitney U tests: a, b, c, and d are p < 0.05 versus C, As, Mn and Pb.
3.3. Urinary Pb, As, Mn and delta-ALA levels
Urinary levels of Pb, As and Mn are shown in Fig.4, expressed as mg/g of urinary creatinine. Pb levels in the Pb treated group were significantly higher compared to the control and Mn groups. The metal mixture treated rats had significantly higher levels of urinary Pb than all the other groups (p < 0.05) (Fig.4 a). A significant increase in urinary As levels was noted both in the As and metal mixture groups compared with the control, Pb and Mn treated groups (p < 0.05) (Fig.4 b). Mn urinary levels in the control, As, Pb and Mn treated groups had similar values. The metal mixture treated group had a significantly higher level of urinary Mn compared with the control, As and Mn treated groups (p < 0.05) (Fig.4 c).
Fig. 4 (a), (b) and (c).
Urinary concentrations of Pb (a), As (b) and Mn (c) in groups of Wistar rats treated with As, Mn, Pb and a mixture of these metals (Mixt). A control group (C) was also used. Data represent the mean ± SD. (1) Means lower than the detection limit. All groups were compared by Mann Whitney tests: a, b, c, and d are p < 0.05 versus C, As, Mn and Pb.
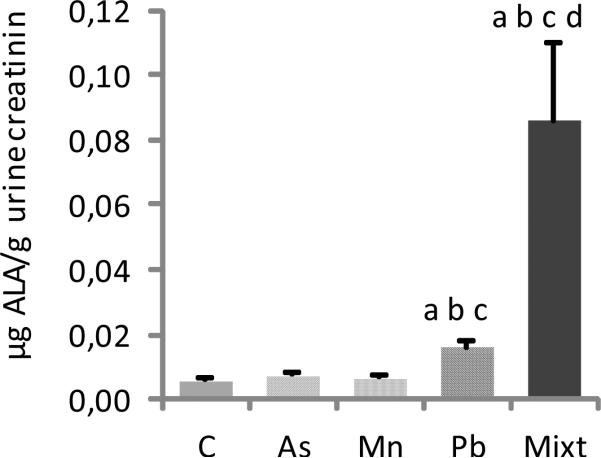
Delta-ALA-U levels were analyzed and corrected for creatinine content. The Pb and metal mixture treated rats showed a significant increase in delta-ALA levels compared with all the other groups (p < 0.05) (Fig.5). Delta-ALA-U levels in the mixture treated group were also significantly increased compared to the Pb treated group (p < 0.05) (Fig.5).
Fig.5.
Urinary concentrations of delta-ALA in groups of Wistar rats treated with As, Mn, Pb and a mixture of these metals (Mixt). A control group (C) was also used. Data represent the mean ± SD (n=6). All groups were compared by Mann-Whitney U tests: a, b, c, and d are p < 0.05 versus C, As, Mn and Pb.
3.4. ROC curves for urinary biomarkers
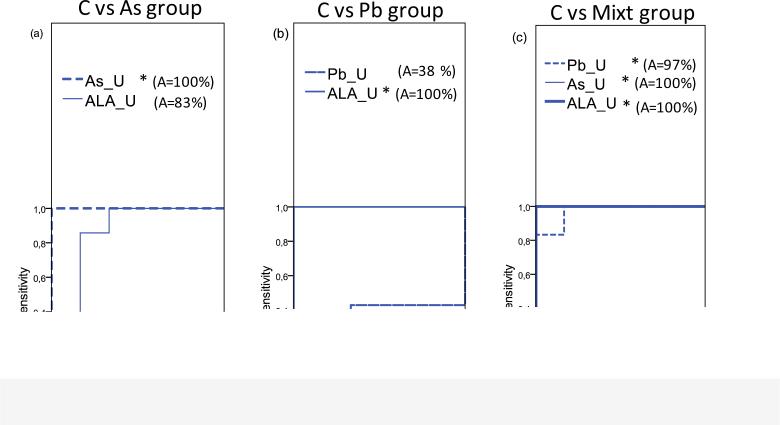
Areas under the ROC curves were determined for all urine BMs to evaluate their ability to discriminate between treatments, and specifically to differentiate controls from Pb, As, Mn or Pb/As/Mn mixture treated rats. Significant area values (p<0.05) (100%) were noted for delta-ALA-U levels, showing the ability of this BM to discriminate controls from Pb treated rats, and for urinary As levels to discriminate the controls from As treated rats (Figs.6 a and b). None of the BM was found to discriminate between Mn treated rats and controls (p>0.05; data not shown). Urinary As, Pb and delta-ALA discriminated between non-treated and mixture treated rats (p<0.05) with Pb urine levels exhibiting an area (p<0.05) of 97%, and urinary As and delta-ALA an area (p<0.05) of 100% (Fig.6 c).
Figs.6 (a), (b) and (c).
The ability of the urinary BMs, As, Pb and delta-ALA, to discriminate between control rats (C) and the As treated group (a), C and the Pb treated group (b) and C and the mixture treated group (Mixt) (c). Any significant BMs areas were found for the Mn exposed group (data not shown). BMs'curves area are plotted by ROC (Receiver Operating Characteristics) analysis. Areas are directly proportional to the BMs discriminant capabilities; * represent significant areas (p<0.05).
3.5. Biomarkers’ correlations
Correlations of BMs were calculated by Spearman's rho coefficients for: (i) brain delta-ALA and brain metals (Pb, As, Mn), (ii) urine and brain metal levels, and (iii) urine and brain delta-ALA concentrations (Table 2). These correlations were calculated for the As, Mn, Pb and As/Mn/Pb mixture treated groups. For the single metal treatment groups, significant correlations were noted only between urinary and brain As levels in the As treated group (ρ=0.828; p<0.05) (Table 2). In contrast, in the As/Mn/Pb mixture group, significant correlations were noted between all the BMs, except for the Mn levels.
Table 2.
Correlations between brain (Br) and urinary (U) Pb, As and ALA levels in groups of Wistar rats treated with As, Mn, Pb and a mixture of these metals (Mixt).
| Spearman's rho | Group | Group | |||
|---|---|---|---|---|---|
| As | Mixt | Pb | Mixt | ||
| As-U vs As-Br | 0.828 | 0.839 | Pb-U vs Pb-Br | NS | 0.678 |
| As-U vs ALA-Br | NS | 0.62 | Pb-U vs ALA-Br | NS | 0.846 |
| As-Br vs ALA-Br | NS | 0.627 | Pb-Br vs ALA-Br | NS | 0.783 |
| ALA-Br vs ALA-U | NS | 0.788 | ALA-Br vs ALA-U | NS | 0.788 |
Data represent significant correlations expressed by Spearman's rho values (p<0.05) and “NS” represents not significant correlations.
In fact, in the metal mixture group, Pb and As urine levels were correlated with Pb and As brain levels, respectively (ρ=0.839 and ρ=0.678; p< 0.05) (Table 2), as well as with delta-ALA brain concentrations (ρ=0.620 and ρ=0.846, respectively; p< 0.05)(Table 2). Correlations between brain BMs were also noted, specifically between Pb and As levels and delta-ALA levels (ρ=0.627, ρ=0.811 and ρ=0.783, respectively; p< 0.05) (Table 2). In the metal mixture group urinary delta-ALA was correlated with brain delta-ALA (ρ=0.788; p< 0.05) (Table 2). No significant correlations were noted between brain and/or urinary Mn levels and the other BMs (data not shown).
Correlations between brain and urine BMs (Pb, As, Mn and delta-ALA) and motor activity parameters (ambulation and rearing) were also determined. In the Pb treated group, brain Pb concentrations were correlated with both motor activity parameters (ρ=−0.601 and ρ=−0.664 for ambulation and rearing counts; p<0.05) (Table 3), while delta-ALA-U concentrations were correlated with the ambulation counts (ρ=−0.626; p<0.05) (Table 3). Negative significant Spearman's coefficients (p<0.05) were noted in the As treated group between brain and urine As levels and motor activity (ρ=−0.737 and ρ=−0.816 for ambulation counts, and ρ=−0.614 and ρ=−0.698 for rearing counts, respectively) (Table 3), as well as between brain delta-ALA levels and ambulation and rearing (ρ=−0.728 and ρ=−0.713, respectively). In Mn treated rats, only brain Mn levels were correlated with the motor activity (ρ=−0.555 and ρ=−0.623 for ambulation and rearing respectively) (Table 3). In the metal mixture treated group all urine and brain BMs were correlated with motor activity, with the exception of the urinary Mn levels. As, Mn and Pb brain levels were correlated with ambulation counts (ρ=−0.816, ρ=−0.811 and ρ=−0.797, respectively) and with rearing counts (ρ=−0.829, ρ=−0.847 and ρ=−0.868; p<0.05, respectively) (Table 3). In addition, in the mixture treated group, urinary As and Pb concentrations were correlated with the ambulation counts (ρ=−0.893 and ρ=−0.923; p< 0.05), with rearing counts (ρ=−0.823 and ρ=−0.879; p<0.05) (Table 3) and with brain and urinary delta-ALA levels (ρ=−0.827 and ρ=−0.880; p<0.05) (Table 3).
Table 3.
Correlations between brain (Br) and urinary (U) As, Mn, Pb and ALA levels and motor activity, ambulation and rearing counts, in groups of Wistar rats treated with As, Mn, Pb and a mixture of these metals (Mixt).
| Spearman's rho | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Motor activity | As-Br | Mn-Br | Pb-Br | ALA-Br | As-U | Mn-U | Pb-U | ALA-U |
| As | Ambulation counts | −0.737 | - | - | −0.728 | −0.816 | - | ||
| Mn | - | −0.555 | - | - | - | ||||
| Pb | - | - | −0.601 | - | −0.626 | ||||
| Mixt | −0.816 | −0.811 | −0.797 | −0.783 | −0.893 | −0.923 | −0.827 | ||
| As | Rearing counts | −0.614 | - | - | −0.713 | −0.698 | - | ||
| Mn | - | −0.623 | - | - | - | ||||
| Pb | - | - | −0.664 | - | |||||
| Mixt | −0.829 | −0.847 | −0.868 | −0.791 | −0.833 | −0.879 | −0.780 | ||
Data represent significant correlations expressed by Spearman's rho values (p<0.05).
4. Discussion
Excessive exposure to several metals can lead to brain dysfunction (Kordas et al., 2010), yet the combined effects of metal mixtures are largely unknown (Tiffany-Castiglioni et al., 2006; Wang and Fowler, 2008). Herein, we evaluated in vivo changes in delta-ALA levels in the urine as a potential BM of exposure and/or neurotoxic effect in response to treatment with a mixture of Pb, As and Mn, which is known to coexist in the environment in particular in several occupational settings. Behavioral assays were performed in Wistar rats, and brain and urinary levels of Pb, As, Mn and delta-ALA in single metal and metal mixture treated rats were determined.
The general status of the animals was evaluated by determining their weight during the acclimatization period and after the administration of the 4th and 8th dose. All animals had a progressive weight gain during the acclimatization period that continued during the treatments, but only in the control group. The Pb and As alone treated groups body-weight stabilized, while a decrease in weight-gain was observed in Mn and the mixture exposed groups (data not shown).
Open-field spontaneous motor activity assessment is a sensitive and reliable behavioral test; it has been previously used to assess Pb, As or Mn-induced neurotoxicity (Marreilha dos Santos, 2011; Moreira et al., 2001; Sansar et al., 2011). In this study, all single metal treated rats showed decreased motor activity compared with the controls, although not attaining statistical significance for either of the motor parameters (rearing and ambulation) in the Pb treated group, and for rearing in the As treated group (Figs.1 a and b). Conflicting results on ambulation and rearing activities in Pb-exposed rats have been previously noted (Chandra et al., 1981; Reddy et al., 2003; Rocha et al., 2001; Rodrigues et al., 1996), consistent with our inability to ascertain a clear and significant effect on these behaviors in the Pb treated group. With respect to As and Mn, our results corroborate previous reports, consistent with decreased motor activities (Rodríguez et al., 2010; Vezér et al., 2005). Our data also show that treatment with the 3-metal mixture decreases ambulation and rearing activity compared with all the other groups (p<0.05) (Figs.1 a and b). This is in agreement with earlier reports, where rats simultaneously co-treated with Mn and Pb showed a significant decrease in motor activity compared with single metal treated animals (Chandra et al., 1981). Motor deficits due to As exposure have also been described (Mejía et al., 1997). Combined, these data establishes that Pb, As and Mn mixture treatment exacerbates motor dysfunction in the rat.
It is well established that As, Mn and Pb perturb brain function (ATSDR, 2007a and c; Marreilha dos Santos et al., 2011). In the present study, brain Pb, As and Mn levels were significantly higher in each of the single treated groups compared with the controls (Figs.2 a, b and c) and correlated with the decrease in motor activity (Table 3). Corroborating earlier reports (Donoghue, 2004; Landrigan et al., 2007; Ramteke et al., 2009; Reckziegel et al., 2011; Rodríguez et al., 2003 and 2010), increased brain Pb, As or Mn levels were also associated with neurobehavioral deficits. Significant correlations were noted between Pb, As and Mn brain levels and motor activity in the metal mixture group (Table 3), consistent with studies reporting decreased motor activity in rats co-exposed to Pb/Mn or Pb/As (Chandra et al., 1981; Mejía et al., 1997). These results suggest an association between brain co-accumulation of these three metals and the observed behavioral changes.
Brain As and Mn concentrations in rats treated with the metal mixture were higher than in controls, but nearly similar to those found in the single metal treated groups (Figs.2 b and c). Notably, brain Pb levels in the metal mixture treated group were significantly higher than in any of the other groups (Fig.2 a). Consistent with our results, higher brain Pb levels were previously reported in rats exposed to the binary mixtures of Pb/Mn and Pb/As compared to rats only exposed to Pb (Chandra et al. 1981; Mejía et al., 1997). Combined, these observations suggest that co-exposure to Pb and other metals increase brain Pb accumulation. It has yet to be determined whether this effect is associated with upregulation of Pb-specific transporters, increased leakiness of the neurovasculature and/or reduced clearance of brain Pb. Treatment with the metal mixture resulted in the highest brain Pb accumulation. Although Mn and Pb share the same mechanism of transport as Fe, namely the DMT-1 transporter (Aschner, 2006; Zheng et al, 2012) and since there was an increase of Mn and Pb brain levels, it was possible that a decrease in Fe brain concentrations might have occurred. The relationship between exposure to the metal mixture and alterations in levels of other metals in the brain, such as Fe, has yet to be determined.
Pb interferes with heme synthesis, promoting ALAS and inhibiting ALAD activity, thus increasing blood delta-ALA levels (Ennis et al., 2003) and As decreases blood ALAD activity as well (Bhadauria and Flora, 2004). In the present study, Pb and As treated rats showed increased brain delta-ALA levels compared with the control (Fig.3); however, no correlations between brain delta-ALA and the levels of each of these metals in response to single metal treatment were noted (Table 2). It is likely that in Pb treated rats the administered doses were insufficiently high to reach the requisite brain levels that trigger significant delta-ALA accumulation. Consistent with this observation, the treatment failed to cause a significant behavioral dysfunction (Table 2 and Figs.1 a and b). Brain delta-ALA concentrations in the As treated group were correlated with decreased motor activity, suggesting that the behavioral changes were induced by the As itself (Fig. 2b and Table 3) and/or by delta-ALA (Fig. 3). Practically no information exists on the effects of Mn on brain delta-ALA levels. Our results showed that Mn treated rats and controls have similar brain delta-ALA levels (Fig.3).
Thus, we posit that the observed decrease in motor activity in Mn treated animals is secondary to the accumulation of Mn in the brain (Fig. 2c and Table 3). In the metal mixture treated group brain delta-ALA levels, were the highest and significantly different from all the other treatment groups (Fig.3). Moreover, brain delta-ALA concentrations were correlated with increased brain Pb and As levels (Table 2). It is recognized that the bioavailability of Pb in tissues may be affected by the presence of high affinity Pb-binding proteins (Rocha et al., 1995). A higher retention of Pb in the brain along with the presence of As, may possibly explain why brain delta-ALA concentrations increased in this group. In addition, delta-ALA-U levels were correlated with brain delta-ALA levels (Table 2). In vitro experiments also established that brain delta-ALA is slowly metabolized once incorporated into neurons (Juknat et al., 1995). Therefore, we posit that blood-borne delta-ALA may enter in the brain, leading to its accumulation in the co-treated group.
Delta-ALA's deleterious effects in the brain have been broadly described (Ennis et al., 2003). Common neurotoxic mechanisms are shared by Pb, As and Mn; these include interference with GABAergic systems and the induction of oxidative stress, conditions known to interfere with motor behavior (Adonaylo and Oteiza, 1999; Anderson et al., 2008; Demasi et al., 1996; Erikson et al., 2004; Flora et al., 2005; Jomova and Valko, 2011; Lelli et al., 2005; Milatovic et al., 2009; Reckziegel et al., 2011; Rodriguez et al., 2003; Rodríguez et al., 2010; Struzynska and Sulkowski, 2004; Wong et al 1991). In fact, ALA competes for the binding of GABA to GABA receptors (Muller, 2004) in several brain regions. In addition, the observed increase of delta-ALA in brain may not only decrease dopamine uptake (Kovalev et al., 1988) but also, owing to its pro-oxidant properties (Demasi et al., 1996), oxidize dopamine per se. Dopamine receptors play a major role in modulating the control of motor function in rats (Nagai, et al., 2012; Wong et al 1991). The consequences of the increase of delta-ALA, the decrease of dopamine and GABA in brain cells may also explain the observed decrease in motor activity.
Indeed, correlations were found between motor activity and brain delta-ALA, Pb, As and Mn levels (Table 2), suggesting that these four parameters interfered with behavior in the metal mixture treated group (Figs.1 a and b).
The use of urine samples for the determination of BMs in humans is a simple and non-invasive method; thus, urinary delta-ALA, Pb, As and Mn levels were determined to assess their utility as peripheral BMs of exposure and/or neurotoxic effects. Upon single metal exposure, urinary As in the As treated group was higher than in controls (Fig.4 b). In addition, this BM was the only one that showed a significant area in the ROC curve analysis (Fig.6 b) and was correlated with the motor activity (Table 3). Urine represents the major route of As excretion (ATSDR, 2007a); thus, these results confirm its reliability in differentiating As treated from non-treated rats, and suggests its utility as a BM of As-induced neurotoxicity. Even though urinary Pb levels in the Pb treated group trended higher than in the controls, no statistically significant differences were noted (Fig.4 a). Pb is excreted in both urine and feces, but urinary Pb is considered a poor BM of exposure (ATSDR, 2007c), consistent with our results. In the mixture treated group, urinary Pb and Mn levels were the highest in comparison to all the other groups (Fig.4), establishing that the metal mixture increases the excretion rates of Pb and Mn. Our results suggest that treatment with the mixture Pb/As/Mn can alter the toxicokinetic process of each of the metals and consequently the toxicity of the mixture. A significant ROC curve area was obtained for urinary Pb in the mixture treated group (Fig.6 a and b), where the urinary Pb area was higher than in the group exposed to Pb alone, possibly due to increased Pb excretion in the co-treated animals. In contrast, As urine concentrations in the single treated as well as in the mixture treated groups was analogous. In agreement, the areas of the ROC curve in these groups were also analogous. Concerning the urinary Mn levels, ROC analysis confirmed the “weakness” of this BM (ASTDR, 2007c) to correctly differentiate non-treated from co-treated rats (data not shown). As far as single metal treatments, delta-ALA-U changes were observed only in the Pb treated group and accordingly a significant (p<0.05) (Fig. 6) area under the ROC curve was obtained for this BM. Delta-ALA-U levels are established BMs of Pb exposure, representing a preferred BM over urinary or blood Pb levels (ATSDR, 2007c), thus supporting our results. However, further analyses at various doses are needed in order to clarify the relationship between Pb-induced neurotoxic effects and delta-ALA-U levels (Figs. 1 and 5).
Metal mixture treatment led to increased delta-ALA-U levels, which were higher than the sum of the levels for each of the three single metals treated groups.
In fact, along with the known effects of Pb on the accumulation of delta-ALA, increased urinary excretion of total porphyrins is described in As exposed subjects (Marchiset-Ferley et al., 2012). We believe that in the co-exposed group of rats, a decrease in heme levels may induce delta-ALA accumulation through negative feedback control. These events might not be enough to significantly modify delta-ALA urinary levels of single exposed As rats, but may explain the results observed for the mixture exposed group. Concerning Mn, further studies will be needed to clarify the relation between the exposure to this metal and the levels of urinary delta-ALA (Maines, 1980)(Fig. 5).
These results suggest that the mixture treatment leads to delta-ALA-U changes that exceed the additive effects of the individual metals. The observed increased ALA excretion in urine may also be supported by the fact that ALA reabsorption is a saturable process and takes place only under low ALA concentrations (O'Flaherty et al., 1981). In this case, in the mixture group, and in accordance with our results, we believe that the reabsorption is minimal and has no effect on the metal-induced effects on ALA urinary levels, as the secretion pathway is dominating.
Moreover, ROC curve analysis revealed that delta-ALA-U could reliably identify co- exposed rats (Fig.6 c). In addition, in this group, delta-ALA-U was correlated with Pb, As and Mn brain levels, as well as with motor activity changes (Tables 3). Accordingly, delta-ALA-U concentration may serve as a reliable BM of both exposure and neurotoxic effect induced by the mixture of Pb, As and Mn.
5. Conclusions
We investigated the utility of delta-ALA-U as exposure and/or neurotoxicity BM in response to Pb, As or Mn treatments, as well as to a mixture of these 3 metals. Brain Pb and delta-ALA levels were higher in rats treated with a mixture of these metals than after single metal exposure, suggesting that Pb plays a key role in the excessive accumulation of brain delta-ALA. In addition, the entrance of blood-borne delta-ALA into the brain may also contribute to its accumulation. Treatment with the metal mixture led to a marked decrease in motor activity. Given the existence of shared mechanisms for delta-ALA, Pb, As and Mn induced neurotoxicity, the behavioral changes are likely multifactorial and stem from (1) the increase in brain Pb levels and its deposition along with As and Mn, and (2) the accumulation of neurotoxic delta-ALA. Finally, in the co-treated group, delta-ALA-U levels were higher than the sum of delta-ALA levels in the single treatment groups, establishing that the exposure to the mixture induced a synergistic effect on this urinary BM. Further studies are planned to evaluate if higher doses of this mixture induce additive or synergistic behavioral changes. In summary, the results presented herein establish that delta-ALA-U can identify co-exposed rats and correlates with their behavioral changes. These observations establish that delta-ALA-U levels can serve as a sensitive peripheral BM of exposure and neurotoxicity upon exposure to a mixture of Pb, As and Mn. Further translation of these observations to human populations, namely to occupational cohorts, will establish the suitability of these parameters as BMs of exposure and toxicity in response to single or metal mixture exposures.
Highlights.
Co-treatment with Pb, As and Mn led to behavioral toxicity greater than upon each single metal treatment
Total delta-ALA urinary levels in metal co-treated rats is greater than the additive effect
Urinary delta-ALA in the metal co-treated group correlates with brain delta-ALA and behavioral toxicity
Urinary delta-ALA levels represent a sensitive biomarker of exposure/neurotoxic effect to this metal mixture.
Acknowledgments
The authors wish to thank the financial support by FCT strategic project Pest-OE/SAU/UI4013/2011, for the I-Med.UL, Faculty of Pharmacy, University of Lisbon, and from the National Institute of Health (NIH ES R01 10563, P30 ES 000267). The authors also wish to thank Doctor Maria Eduarda Mendes for her advises with the atomic absorption spectrometry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None of the authors has any financial interest or conflict of interest related to the manuscript.
References
- Adonaylo VN, Oteiza PI. Pb2 promotes lipid oxidation and alterations in membrane physical properties. Toxicology. 1999;132:19–32. doi: 10.1016/s0300-483x(98)00134-6. [DOI] [PubMed] [Google Scholar]
- Akobeng AK. Understanding diagnostic tests 3: Receiver operating characteristic curves. Acta Paediatr. 2007;96(5):644–647. doi: 10.1111/j.1651-2227.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- Anderson JG, Fordahl SC, Cooney PT, Weaver TL, Colyer CL, Erikson KM. Manganese exposure alters extracellular GABA, GABA receptor and transporter protein and mRNA levels in the developing rat brain. Neurotoxicology. 2008;29:1044–1053. doi: 10.1016/j.neuro.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR a. U.S. Department of Health and Human Services Public Health Service Agency for Toxic Substances and Disease Registry. Toxicological Profile for Arsenic. 2007 CAS#: 7440-38-2. http://www.atsdr.cdc.gov/toxprofiles, 25 Feb 2013. [PubMed]
- ATSDR b. U.S. Department of Health and Human Services Public Health Service Agency for Toxic Substances and Disease Registry. Toxicological Profile For Lead. 2007 CAS# 7439-92-1. http://www.atsdr.cdc.gov/toxprofiles, 25 Feb 2013.
- Aschner M. The transport of manganese across the blood–brain barrier. NeuroToxicology. 2006;27:311–314. doi: 10.1016/j.neuro.2005.09.002. [DOI] [PubMed] [Google Scholar]
- ATSDR c. U.S. Department of Health and Human Services Public Health Service Agency for Toxic Substances and Disease Registry. Toxicological Profile For Manganese. 2007 CAS# 7439-96-5. http://www.atsdr.cdc.gov/toxprofiles, 25 Feb 2013.
- Balbuena P, Li W, Ehrich M. Assessments of tight junction proteins occludin, claudin 5 and scaffold proteins ZO1 and ZO2 in endothelial cells of the rat blood–brain barrier: Cellular responses to neurotoxicants malathion and lead acetate. Neurotoxicology. 2011;32:58–67. doi: 10.1016/j.neuro.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Batterman S, Su FC, Jia C, Naidoo RN, Robins T, Naik I. Manganese and lead in children's blood and airborne particulate matter in Durban, South Africa. Sci Total Environ. 2011;409:1058–1068. doi: 10.1016/j.scitotenv.2010.12.017. [DOI] [PubMed] [Google Scholar]
- Bhadauria S, Flora SJ. Arsenic induced inhibition of delta-aminolevulinate dehydratase activity in rat blood and its response to meso 2,3-dimercaptosuccinic acid and monoisoamyl DMSA. Biomed Environ Sci. 2004;17(1):101–108. [PubMed] [Google Scholar]
- Blom S, Lagerkvist B, Linderholm H. Arsenic exposure to smelter workers. Clinical and neurophysiological studies. Scand J Work Environ Health. 1985;11:265–269. doi: 10.5271/sjweh.2227. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1986;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bowler RM, Gysens S, Diamond E, Nakagawa S, Drezgic M, Roels HA. Manganese exposure: Neuropsychological and neurological symptoms and effects in welders. Neurotoxicology. 2006;27:315–326. doi: 10.1016/j.neuro.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Cai T, Pepe MS. Semiparametric Receiver Operating Characteristic Analysis to Evaluate Biomarkers for Disease. J Amer Statist Assoc. 2002;97(460):1–10. [Google Scholar]
- Casarett & Doull's . Toxicology: The Basic Science of Poisons. seventh Ed. McGraw-Hill; 2007. [Google Scholar]
- Chandra SV, Mohd AM, Saxena DK, Murthy RC. Behavioral and neurochemical changes in rats simultaneously exposed to manganese and lead. Arch Toxicol. 1981;49:49–56. doi: 10.1007/BF00352071. [DOI] [PubMed] [Google Scholar]
- Charlet L, Chapron Y, Faller P, Kirsch R, Stone AT, Baveye PC. Neurodegenerative diseases and exposure to the environmental metals Mn, Pb, and Hg . Coord Chem Rev. 2012;256:2147–2163. [Google Scholar]
- Cowan DM, Zheng W, Zou Y, Shi X, Chen J, Rosenthal FS, Fan Q. Manganese exposure among smelting workers: Relationship between blood manganese–iron ratio and early onset neurobehavioral alterations. Neurotoxicology. 2009;30(6):1214–1222. doi: 10.1016/j.neuro.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vizcaya-Ruiza A, Barbiera O, Ruiz-Ramos R, Cebrian ME. Biomarkers of oxidative stress and damage in human populations exposed to arsenic. Mutat Res. 2009;674:85–92. doi: 10.1016/j.mrgentox.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Demasi M, Penatti CAA, Delucia R, Bechara EJH. The prooxidant effect of 5-aminolevulinic acid in the brain tissue of rats: implications in neuropsychiatric manifestations in porphyrias. Free Radic Biol Med. 1996;20(3):291–299. doi: 10.1016/0891-5849(95)02035-7. [DOI] [PubMed] [Google Scholar]
- Doecke JD, Laws SM, Faux NG, et al. Blood-based protein biomarkers for diagnosis of Alzheimer Disease. Arch Neurol. 2012;69(10):1318–1325. doi: 10.1001/archneurol.2012.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue AM. Occupational health hazards in mining: an overview. Occup Med. 2004;54:283–289. doi: 10.1093/occmed/kqh072. [DOI] [PubMed] [Google Scholar]
- Ellingsen DG, Konstantinov R, Bast-Pettersen R, Merkurjeva L, Chashchin M, Thomassen Y, Chashchin V. A neurobehavioral study of current and former welders exposed to manganese. Neurotoxicology. 2008;29:48–59. doi: 10.1016/j.neuro.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Emanuelli T, Pagel FW, Porciúncula LO, Souza DO. Effects of 5-aminolevulinic acid on the glutamatergic neurotransmission. Neurochem Int. 2003;42:115–121. doi: 10.1016/s0197-0186(02)00074-8. [DOI] [PubMed] [Google Scholar]
- Ennis SR, Novotny A, Xiang J, Shakui P, Masada T, Stummer W, Smith DE, Keep RF. Transport of 5-aminolevulinic acid between blood and brain. Brain Res. 2003;959:226–234. doi: 10.1016/s0006-8993(02)03749-6. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Dobson AW, Dorman DC, Aschner M. Manganese exposure and induced oxidative stress in the rat brain. Sci Total Environ. 2004:334–335. 409–416. doi: 10.1016/j.scitotenv.2004.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer A. Metal poisoning. An Sist Sanit Navar. 2003;26(1):141–153. [PubMed] [Google Scholar]
- Flora SJS, Bhadauria S, Pant SC, Dhaked RK. Arsenic induced blood and brain oxidative stress and its response to some thiol chelators in rats. Life Sci. 2005;77:2324–2337. doi: 10.1016/j.lfs.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Fukui Y, Miki M, Ukai H, Okamoto S, Takada S, Higashikawa K, Ikeda M. Urinary lead as a possible surrogate of blood lead among workers occupationally exposed to lead. Int Arch Occup Environ Health. 1999;72:516–520. doi: 10.1007/s004200050409. [DOI] [PubMed] [Google Scholar]
- Gil F, Pla A. Biomarkers as biological indicators of xenobiotic exposure. J Appl Toxicol. 2001;21:245–255. doi: 10.1002/jat.769. [DOI] [PubMed] [Google Scholar]
- Gurer H, Ercal N. Can antioxidants be beneficial in the treatment of lead poisoning? Free Radic Biol Med. 2000;29(10):927–945. doi: 10.1016/s0891-5849(00)00413-5. [DOI] [PubMed] [Google Scholar]
- Halatek T, Sinczuk-Walczak H, Rabieh S, Wasowicz W. Association between occupational exposure to arsenic and neurological, respiratory and renal effects. Toxicol Appl Pharm. 2009;239:193–199. doi: 10.1016/j.taap.2009.04.022. [DOI] [PubMed] [Google Scholar]
- Heitland P, Köster HD. Fast determination of arsenic species and total arsenic in urine by HPLC-ICP-MS: Concentration ranges for unexposed German inhabitants and clinical case studies. J Anal Toxicol. 2003;32:308–313. doi: 10.1093/jat/32.4.308. [DOI] [PubMed] [Google Scholar]
- Higashikawa K, Furuki K, Takada S, Okamoto S, Ukai H, Yuasa T, Ikeda M. Blood lead level to induce significant increase in urinary d-aminolevulinic acid level among lead-exposed workers: A statistical approach. Ind Health. 2000;38:181–188. doi: 10.2486/indhealth.38.181. [DOI] [PubMed] [Google Scholar]
- Jemiola-Rzeminska M, Rivera C, Suwalsky M, Strzalka K. Interaction of arsenic compounds with model phospholipid membranes. Thermochim Acta. 2007;458:132–137. [Google Scholar]
- Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283:65–87. doi: 10.1016/j.tox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Juknat AA, Kotler ML, Batlle AM. High delta-aminolevulinic acid uptake in rat cerebral cortex: effect on porphyrin biosynthesis. Comp Biochem Physiol. 1995;111(1):143–150. doi: 10.1016/0742-8413(94)00085-8. [DOI] [PubMed] [Google Scholar]
- Kakkar P, Jaffery FN. Biological markers for metal toxicity. Environ Toxicol Pharm. 2005;19:335–349. doi: 10.1016/j.etap.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Kordas K, Queirolo EI, Ettinger AS, Wright RO, Stoltzfus RJ. Prevalence and predictors of exposure to multiple metals in preschool children from Montevideo, Uruguay. Sci Total Environ. 2010;408:4488–4494. doi: 10.1016/j.scitotenv.2010.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalev GI, Kerecsen L, Gankina EM, Goldina OA, Eremenko AV, Kudrin VS. Nonreceptor component of aminoacidergic regulation of dopamine release in rat brain. Ann. Ist.Super. Sanità. 1988;24(3):491–494. [Google Scholar]
- Landrigan P, Nordberg M, Lucchini R, Nordberg G, Grandjean P, Iregren A, Alessio L. The Declaration of Brescia on Prevention of the Neurotoxicity of Metals. Am J Ind Med. 2006;50(10):709–711. doi: 10.1002/ajim.20404. [DOI] [PubMed] [Google Scholar]
- Lelli SM, San Martín de Viale LC, Mazzetti MB. Response of glucose metabolism enzymes in an acute porphyria model: Role of reactive oxygen species. Toxicology. 2005;216:49–58. doi: 10.1016/j.tox.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Maines MD. Regional distribution of the enzymes of haem biosynthesis and the inhibition of 5-aminolaevulinate synthase by manganese in the rat brain. Biochem J. 1980;190:315–321. doi: 10.1042/bj1900315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S, Tsruta H, Takata T. Relationship between blood lead level and urinary ALA level in workers exposed to very low levels of lead. Ind Health. 2000;38:95–98. doi: 10.2486/indhealth.38.95. [DOI] [PubMed] [Google Scholar]
- Mameli O, Caria MA, Melis F, Solinas A, Tavera C, Ibba A, Tocco M, Flore C, Randaccio FS. Neurotoxic effect of lead at low concentrations. Brain Res Bull. 2001;55(2):269–275. doi: 10.1016/s0361-9230(01)00467-1. [DOI] [PubMed] [Google Scholar]
- Marchiset-Ferlay N, Savanovitch C, Sauvant-Rochat MP. What is the best biomarker to assess arsenic exposure via drinking water? Environ Int. 2012;39(1):150–171. doi: 10.1016/j.envint.2011.07.015. [DOI] [PubMed] [Google Scholar]
- Marreilha dos Santos AP, Lopes Santos M, Batoréu MC, Aschner M. Prolactin is a peripheral marker of manganese neurotoxicity. Brain Res. 2011;1382:282–290. doi: 10.1016/j.brainres.2011.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marreilha dos Santos AP, Lucas Rui L., Andrade Vanda, Mateus M. Luísa, Milatovic Dejan, Aschner Michael, Batoreu M. Camila. Protective effects of ebselen (Ebs) and para-aminosalicylic acid (PAS) against manganese (Mn)-induced neurotoxicity. Toxicol Appl Pharmacol. 2012;258(3):394–402. doi: 10.1016/j.taap.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Martinez-Finley E J, Chakraborty S, Fretham SJB, Aschner M. Cellular transport and homeostasis of essential and nonessential metals. Metallomics. 2012;4:593–605. doi: 10.1039/c2mt00185c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejía JJ, Diáz-Barriga F, Calderón J, Ríos C, Jiménez-Capdeville ME. Effects of lead–arsenic combined exposure on central monoaminergic systems. Neurotoxicol Teratol. 1997;19(6):489–497. doi: 10.1016/s0892-0362(97)00066-4. [DOI] [PubMed] [Google Scholar]
- Milatovic D, Milatovic SZ, Gupta RC, Yu Y, Aschner M. Oxidative damage and neurodegeneration in manganese-induced neurotoxicity. Toxicol Appl Pharm. 2009;240:219–225. doi: 10.1016/j.taap.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RC, Brindle E, Holman DJ, Shofer J, Klein NA, Soules MR, O'Connor KA. Comparison of specific gravity and creatinine methods for normalizing urinary reproductive hormone concentrations. Clin Chem. 2004;50:924–932. doi: 10.1373/clinchem.2004.032292. [DOI] [PubMed] [Google Scholar]
- Moreira EG, Vassilieff I, Vassilieff VS. Developmental lead exposure: behavioral alterations in the short and long term. Neurotoxicol Teratol. 2001;23:489–495. doi: 10.1016/s0892-0362(01)00159-3. [DOI] [PubMed] [Google Scholar]
- Mowat FS, Bundy KJ. Experimental and mathematical/computational assessment of the acute toxicity of chemical mixtures from the Microtox assay. Adv Environ Res. 2002;6:547–558. [Google Scholar]
- Muller WE, Snyder SH. Delta-aminolevulinic acid: Influences on synaptic GABA receptor binding may explain CNS symptoms of porphyria. Annals of Neurology. 1997;2(4):340–342. doi: 10.1002/ana.410020415. [DOI] [PubMed] [Google Scholar]
- Nagai Y, Minamimoto T, Ando K, Obayashi S, Ito H, Ito N, Suhara T. Correlation between decreased motor activity and dopaminergic degeneration in the ventrolateral putamen in monkeys receiving repeated MPTP administrations: a positron emission tomography study. Neurosci Res. 2012;73(1):61–67. doi: 10.1016/j.neures.2012.02.007. [DOI] [PubMed] [Google Scholar]
- O'Flaherty EJ, Hammond PB, Taylor E. Renal reabsorption and secretion of delta-aminolevulinic acid in the rat. Fundam Appl Toxicol. 1981;1(3):278–281. doi: 10.1016/s0272-0590(81)80128-5. [DOI] [PubMed] [Google Scholar]
- Olympio KPK, Gonçalves C, Günther WM, Bechara EJH. Neurotoxicity and aggressiveness triggered by low-level lead in children: a review. Pan Am J Public Health. 2009;26(3):266–75. doi: 10.1590/s1020-49892009000900011. [DOI] [PubMed] [Google Scholar]
- Patrick L. Lead toxicity, a review of the literature. Part I: Exposure, evaluation, and treatment. Altern Med Rev. 2006;11(1):2–22. [PubMed] [Google Scholar]
- Phoon HW. Manganese expousure and biological indicators. Sing Med J. 1998;29:93–94. [PubMed] [Google Scholar]
- Ramteke SG, Meshram CM, Nanda A. Neurological complications in manganese mine workers. J Neurol Sci. 2009;285:57–154. [Google Scholar]
- Reckziegel P, Dias VT, Benvegnú D, Boufleur N, Silva Barcelos RC, Segat HJ, Pase CS, Dos Santos CM, Flores EM, Bürger ME. Locomotor damage and brain oxidative stress induced by lead exposure are attenuated by gallic acid treatment. Toxicol Lett. 2011;203(1):74–81. doi: 10.1016/j.toxlet.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Reddy GR, Basha R, Devi CB, Suresh A, Baker JL, Shafeek A, Heinz J, Chetty CS. Lead induced effects on acetylcholinesterase activity in cerebellum and hippocampus of developing rat. Int J Devl Neuroscience. 2003;21:347–352. doi: 10.1016/s0736-5748(03)00071-6. [DOI] [PubMed] [Google Scholar]
- Rocha JBT, Pereira ME, Emanuell T, Christofari RS, Souza DO. Effect of treatment with mercury chloride and lead acetate second stage of rapid postnatal brain growth on delta-aminolevulinic acid dehydratase (ALA-D) activity in brain, liver, kidney and blood of suckling rats. Toxicology. 1995;100:27–37. doi: 10.1016/0300-483x(95)03054-j. [DOI] [PubMed] [Google Scholar]
- Rocha JB, Rocha LK, Emanuelli T, Pereira ME. Effect of mercuric chloride and lead acetate treatment during the second stage of rapid post-natal brain growth on the behavioral response to chlorpromazine and on delta-ALA-D activity in weaning rats. Toxicol Lett. 2001;125(1-3):143–50. doi: 10.1016/s0378-4274(01)00435-0. [DOI] [PubMed] [Google Scholar]
- Rodrigues AL, Rocha JB, Mello CF, Souza D. Effect of perinatal lead exposure on rat behaviour in open-field and two-way avoidance tasks. Pharmacol Toxicol. 1996;79(3):150–6. doi: 10.1111/j.1600-0773.1996.tb00259.x. [DOI] [PubMed] [Google Scholar]
- Rodríguez VM, Jiménez-Capdeville ME, Giordano M. The effects of arsenic exposure on the nervous system. Toxicol Lett. 2003;145:1–18. doi: 10.1016/s0378-4274(03)00262-5. [DOI] [PubMed] [Google Scholar]
- Rodríguez VM, Limón-Pacheco JH, Carrizales L. Mendoza-Trejo MS and Giordano M. Chronic exposure to low levels of inorganic arsenic causes alterations in locomotor activity and in the expression of dopaminergic and antioxidant systems in the albino rat. Neurotoxicol Teratol. 2010;32:640–647. doi: 10.1016/j.ntt.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Sakai T. Biomarkers of lead exposure. Ind Health. 2000;38:127–142. doi: 10.2486/indhealth.38.127. [DOI] [PubMed] [Google Scholar]
- Sansar W, Ahboucha S, Gamrani H. Chronic lead intoxication affects glial and neural systems and induces hypoactivity in adult rat. Acta Histochem. 2011;113:601–607. doi: 10.1016/j.acthis.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Scherer G. Biomonitoring of inhaled complex mixtures – Ambient air, diesel exhaust and cigarette smoke. Experim Toxicol Pathol. 2005;57:75–110. doi: 10.1016/j.etp.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Shin S, Coulter B, San Diego CA. ROC analysis for the evaluation of continuous biomarkers: Existing tools and new features in SAS® 9.2. 2009 http://pharmasug.org/download/papers/SP09.pdf, 25 Feb 2013.
- Shy CM. Epidemiological studies of neurotoxic, reproductive, and carcinogenic effects of complex mixtures. Environ Health Perspect. 1993;101(4):183–188. doi: 10.1289/ehp.93101s4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Søreide K. Receiver-operating characteristic (ROC) curve analysis in diagnostic, prognostic and predictive biomarker research. J Clin Pathol. 2009;62(1):1–5. doi: 10.1136/jcp.2008.061010. [DOI] [PubMed] [Google Scholar]
- Struzynska L, Sulkowski G. Relationships between glutamine, glutamate, and GABA in nerve endings under Pb-toxicity conditions. J Inorg Biochem. 2004;98:951–958. doi: 10.1016/j.jinorgbio.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Tiffany-Castiglioni E, Hong S, Qian Y, Tang Y, Donnelly KC. In vitro models for assessing neurotoxicity of mixtures. Neurotoxicology. 2006;27:835–839. doi: 10.1016/j.neuro.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Tomokuni K, Ogata M. Simple method for determination of urinary delta-aminolevulinic acid as an index of lead exposure. Clin Chem. 1972;18(12):1534–1536. [PubMed] [Google Scholar]
- Toscano CD, Guilarte TR. Lead neurotoxicity: From exposure to molecular effects. Brain Res Rev. 2005;49:529–554. doi: 10.1016/j.brainresrev.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Vezér T, Pappa A, Hoykb Z, Vargab C, Nárayc M, Nagymajtényi L. Behavioral and neurotoxicological effects of subchronic manganese exposure in rats. Environ Toxicol Pharm. 2005;19:797–810. doi: 10.1016/j.etap.2004.12.046. [DOI] [PubMed] [Google Scholar]
- Wang G, Fowler BA. Roles of biomarkers in evaluating interactions among mixtures of lead, cadmium and arsenic. Toxicol Appl Pharm. 2008;233:92–99. doi: 10.1016/j.taap.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Warnock DG, Peck CP. A roadmap for biomarker qualification. Nat Biotechnol. 2010;28:444–445. doi: 10.1038/nbt0510-444. [DOI] [PubMed] [Google Scholar]
- Weiss B. Lead manganese and methylmercury as risk factors for neurobehavioral impairment in advanced age. Int J Alzheimers Dis. 2010;11:1–11. doi: 10.4061/2011/607543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong LS, Eshel G, Dreher J, Ong J, Jackson DM. Role of dopamine and GABA in the control of motor activity elicited from the rat nucleus accumbens. Pharmacol Biochem Behav. 1991;38(4):829–835. doi: 10.1016/0091-3057(91)90250-6. [DOI] [PubMed] [Google Scholar]
- WHO Neurological disorders public health challenges. 2006 [Google Scholar]
- Wu Y, Yang L, Su T, Wang C, Liu G, Li XM. Pathological significance of a panel of urinary biomarkers in patients with drug-induced tubulointerstitial nephritis. Clin J Am Soc Nephrol. 2010;5(11):1954–1959. doi: 10.2215/CJN.02370310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokel RA. Manganese flux across the blood-brain barrier. Neuromolecular Med. 2009;11(4):297–310. doi: 10.1007/s12017-009-8101-2. [DOI] [PubMed] [Google Scholar]
- Zheng G, Chen J, Zheng W. Relative contribution of CTR1 and DMT1 in copper transport by the blood – CSF barrier: Implication in manganese-induced neurotoxicity. Toxicol Appl Pharmacol. 2012;260:285–293. doi: 10.1016/j.taap.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]