Abstract
Rationale
Problematic drug use is associated with difficulty in exerting self-control over behaviors, and this difficulty may be a consequence of atypical morphometric characteristics that are exhibited by drug-experienced individuals. The extent to which these structural abnormalities result from drug use or reflect neurobiological-risk factors that predate drug use, however, is unknown.
Objectives
To determine how methamphetamine affects corticostriatal structure and how drug-induced changes relate to alterations in inhibitory control.
Methods
Structural magnetic resonance images and positron emission tomography (PET) scans, assessing dopamine D2-like receptor and transporter availability, were acquired in monkeys trained to acquire, retain and reverse three-choice visual discrimination problems before and after exposure to an escalating dose regimen of methamphetamine (or saline, as a control). Voxel-based morphometry was used to compare changes in corticostriatal gray matter between methamphetamine and saline exposed monkeys. The change in gray matter before and after the dosing regimen was compared to the change in the behavioral performance and in dopaminergic markers measured with PET.
Results
Methamphetamine exposure, compared to saline, increased gray matter within the right putamen. These changes were positively correlated with changes in performance of methamphetamine-exposed monkeys in the reversal phase, and were negatively correlated with alterations in D2-like receptor and DAT availability.
Conclusions
The results provide the first evidence that exposure to a methamphetamine dosing regimen that resembles human use alters the structural integrity of the striatum and that gray-matter abnormalities detected in human methamphetamine users are due, at least in part, to the pharmacological effects of drug experience.
Keywords: gray matter, striatum, prefrontal cortex, inhibitory control, methamphetamine, addiction, dopamine
INTRODUCTION
Substance use disorders are associated with cognitive abnormalities that are thought to reflect, in part, neurobiological dysfunction caused by long-term exposure to drugs of abuse. This view is supported by observations that exposure of animals to drugs can produce cognitive and neural alterations that resemble those that are exhibited by substance-dependent humans (Jentsch et al., 2002; Nader et al., 2006; Porter et al., 2011; Groman et al., 2012; Porter et al., 2012). The coupling of neural changes following drug exposure with alterations in cognition (Groman et al., 2012) underscores the functional impact of such changes on behavior.
Of the drug-induced effects on cognition, impairment in the ability to exert inhibitory control over behavior is the topic of much research (Izquierdo and Jentsch, 2012). Difficulty with stopping or withholding behaviors has been proposed to be a central feature of substance dependence (Jentsch and Taylor, 1999), and emerging evidence indicates that the relationship between inhibitory control and substance dependence is bi-directional. Specifically, pre-existing differences in inhibitory control predict future drug-taking behaviors (Dalley et al., 2007; Diergaarde et al., 2008; Perry et al., 2008; Anker et al., 2009), and chronic exposure to drugs of abuse can cause inhibitory control impairments to emerge (Jentsch et al., 2002; Schoenbaum et al., 2004; Schoenbaum and Setlow, 2005), indicating that inhibitory control deficits are both a cause and consequence of substance dependence (Groman and Jentsch, 2013). Further, variability in the degree of inhibitory-control impairments exhibited by substance-dependent individuals has been reported to predict measures of sobriety (Aharonovich et al., 2006; Turner et al., 2009), suggesting that improving inhibitory control, through behavioral or pharmacological mechanisms, may serve as effective intervention and treatments for substance dependence (Groman and Jentsch, 2011). One type of inhibitory control involves the ability to modify behavior adaptively when contingencies change, and lesions to the prefrontal cortex impair this ability in humans and animals (Dias et al., 1996; Fellows and Farah, 2003; Rygula et al., 2010), suggesting that the prefrontal cortex is critical for the maintenance of flexible, goal-directed behaviors (Clarke et al., 2004). Indeed, individual differences in gray-matter volume within the prefrontal cortex are related to performance on tasks of inhibitory control in healthy humans and monkeys (Haldane et al., 2008; Tabibnia et al., 2011; Sridharan et al., 2012). The dorsal striatum also plays a critical role in inhibitory control processes (Bellebaum et al., 2008; Castane et al., 2010; Clarke et al., 2011), suggesting that inhibitory control relies on an integrated network of nuclei within the corticostriatal system.
Inhibitory control deficits exhibited by stimulant-dependent individuals may reflect dysfunction in corticostriatal circuitry (Baicy and London, 2007). Consistent with this hypothesis, on average, samples of methamphetamine-dependent research participants have smaller gray-matter volumes in the prefrontal cortex (Thompson et al., 2004; Morales et al., 2012) and larger gray-matter volumes in the striatum (Chang et al., 2005; Jernigan et al., 2005), relative to methamphetamine-naive controls. While recent evidence suggests that some differences in gray-matter volume between stimulant abusers and healthy individuals are also detected in unaffected siblings of stimulant abusers (Ersche et al., 2012), no studies to date have examined whether exposure to methamphetamine causally alters gray matter in corticostriatal circuitry and whether these changes have a functional impact on measures of inhibitory control.
In order to address these questions, the current study was performed using voxel-based morphometry to measure gray matter structure in monkeys trained to acquire, retain and reverse visual discrimination problems before and after a 31-day escalating dose-regimen of methamphetamine (or saline) administration. Based on the available evidence, we hypothesized that exposure to methamphetamine would decrease gray matter within the prefrontal cortex, but increase gray matter within the striatum. Further, we expected that the changes in gray matter would be correlated with changes in reversal learning performance and dopaminergic markers in the same animals reported previously (Groman et al., 2012).
METHODS
Subjects
Fourteen adult, male vervet monkeys (Chlorocebus aethiops sabaeus from the UCLA Vervet Research Colony), between the ages of 5 to 9 years of age, were included in the current study. The monkeys were housed in a climate-controlled vivarium, where they had unlimited access to water and received twice-daily portions of standard monkey chow in amounts that exceeded their nutritional needs (Teklad, Harlan Laboratories). Monkeys received half of their daily portion of chow after behavioral testing (~1100 hr) and the other half in the afternoon (~1500 hr). The total amount of chow was never reduced during the experiment to increase motivation for task performance. All monkeys were maintained in accordance with the Guide for the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council, Department of Health, Education, and Welfare Publication No. (NIH) 85–23, revised 1996. The research protocols were approved by the UCLA Chancellor's Animal Research Committee.
Drugs and Dosing Regimen
Methamphetamine hydrochloride was purchased from Sigma-Aldrich (St Louis MO). Doses of methamphetamine were prepared fresh daily in 0.9% saline and were sterile-filtered prior to administration. Injections were administered intramuscularly at a volume of 0.1 ml/kg. The dosing regimen was designed to model the escalation in both frequency of intake and cumulative daily dose reported by human users of methamphetamine (Han et al., 2011). Methamphetamine was initially administered once per day at 0.1 mg/kg, but the dose and frequency escalated across the 31 d period, with the final dose of 1.0 mg/kg being administered four times per day. Details of the dosing regimen are provided in Table 1. The initial daily dose was administered at 0830 h. During week two, a second daily dose was administered at 1630 h. During weeks three through five, the second daily dose was administered at 1330 h, and a third daily dose given at 1630 h. For the last two weeks of treatment, the second daily dose was administered at 1100 h, the third at 1330 h, and a fourth at 1600 h.
Table 1.
The dose of methamphetamine (mg/kg) given per day and at each injection during the 31 d dosing regimen. Table was originally published in Groman et al., 2012.
| Week 1 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | |
|---|---|---|---|---|---|---|---|---|
| Injection 1 | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 |
| Week 2 | Day 8 | Day 9 | Day 10 | Day 11 | Day 12 | Day 13 | Day 14 | |
|---|---|---|---|---|---|---|---|---|
| Injection 1 | 0.25 | 0.35 | 0.45 | 0.55 | 0.65 | |||
| Injection 2 | 0.25 | 0.35 | 0.45 | 0.55 | 0.65 |
| Week 3 | Day 15 | Day 16 | Day 17 | Day 18 | Day 19 | Day 20 | Day 21 | |
|---|---|---|---|---|---|---|---|---|
| Injection 1 | 0.35 | 0.45 | 0.55 | 0.65 | ||||
| Injection 2 | 0.45 | 0.55 | 0.65 | 0.75 | ||||
| Injection 3 | 0.5 | 0.6 | 0.7 | 0.8 |
| Week 4 | Day 22 | Day 23 | Day 24 | Day 25 | Day 26 | Day 27 | Day 28 | |
|---|---|---|---|---|---|---|---|---|
| Injection 1 | 0.6 | 0.7 | 0.8 | 0.9 | 1.0 | |||
| Injection 2 | 0.6 | 0.7 | 0.8 | 0.9 | 1.0 | |||
| Injection 3 | 0.6 | 0.7 | 0.8 | 0.9 | 1.0 | |||
| Injection 4 | 0.6 | 0.7 | 0.8 | 0.9 | 1.0 |
| Week 5 | Day 29 | Day 30 | Day 31 | |||||
|---|---|---|---|---|---|---|---|---|
| Injection 1 | 1.0 | 1.0 | 1.0 | |||||
| Injection 2 | 1.0 | 1.0 | 1.0 | |||||
| Injection 3 | 1.0 | 1.0 | 1.0 | |||||
| Injection 4 | 1.0 | 1.0 | 1.0 |
Discrimination Acquisition, Retention and Reversal Learning
As previously described in detail (Groman and Jentsch, 2011; Groman et al., 2012), subjects were trained to acquire, retain and reverse visual discrimination problems in a modified Wisconsin General Testing Apparatus. On each trial, monkeys were allowed to open a single box. A trial ended when the subject made a correct response to rewarded stimulus (rewarded with a small piece of fruit), an incorrect response, or an omission occurred (no response for 2 min), and a 20-sec inter-trial interval followed. The spatial position of the different visual stimuli was varied pseudorandomly across trials.
Each discrimination problem consisted of three phases: acquisition, retention and reversal. In the acquisition phase, subjects were required to learn which one of the three visual stimuli was associated with reward solely through feedback provided by the task. Once the performance criterion was met (seven correct choices within 10 consecutive trials), the session was terminated and the monkey returned to the home cage. If the monkey did not reach the performance criterion within 80 trials, the session ended and the same discrimination problem presented the following day(s) until the performance criterion was met. One day after reaching criterion, subjects were assessed in the retention phase, where the stimulus-reward contingencies remained unchanged. Immediately after reaching the performance criterion (four correct responses within five consecutive trials), unsignaled to the monkey, the reversal phase began. In the reversal phase, the stimulus-reward contingencies changed such that the previously rewarded stimulus was no longer rewarded and one of the two previously non-rewarded stimuli was rewarded. The reversal phase continued until the performance criterion was met (seven correct choices within 10 consecutive trials) or until 80 trials had been completed, whichever occurred first. The number of trials required to reach criterion in the reversal phase was the primary dependent measure.
Subjects were trained on several novel discrimination problems before and after the 31-day dosing regimen, each using novel stimuli. Drug effects on behavioral performance were determined by comparing data at three time points: (1) behavioral performance of subjects in the discrimination problem completed immediately prior to beginning the dosing regimen referred to as the “baseline assessment”; (2) performance during 3 weeks within the course of the treatment regimen (referred to as the “3 week assessment”; to limit the anorectic effects of methamphetamine, this test occurred after at least 36 h had elapsed since the last methamphetamine or saline administration); and (3) performance at 5 d after the last drug administration (referred to as the “5 d post-exposure assessment”). The baseline data for 12 of the 14 monkeys used in the current study have been reported (Groman et al., 2011), as were the behavioral data before and after the dosing regimen (Groman et al., 2012).
After completion of the dose regimen, two additional behavioral assessments were conducted, but the performance criterion was increased (nine correct choices in 10 completed trials for the acquisition, retention, and reversal phases) to augment the cognitive demands of the task. Specifically, the additional acquisition and retention training was expected to increase the likelihood of perseverative responding; these two “high-difficulty” sessions were conducted at 8 d and 2 weeks after cessation of drug administration (referred to as the “8 d post-exposure assessment” and the “2 week post-exposure assessment,” respectively).
For the purposes of the current study, changes in gray matter were compared to the change in reversal learning performance between the baseline assessment and the 5 d post-exposure assessment, as these assessments used the same performance criterion. The number of trials required to reach criterion in reversal phase of the baseline assessment was subtracted from the number of trials required to reach criterion in the reversal phase of the 5 d post-exposure assessment to calculate a behavioral difference score.
MRI scanning procedures
Each subject underwent structural magnetic resonance (MR) imaging twice: 2–3 weeks prior to initiating the dosing regimen (referred to as the `Baseline' scan) and 3½ weeks after completion of the dosing regimen (referred to as the `Post-Dosing Regimen' scan). On scan days, monkeys received an intramuscular injection of ketamine hydrochloride (10 mg/kg) and atropine sulfate (0.01 mg/kg). Once the monkey was sedated, an endotracheal tube was placed to provide inhalation of 2–3% isoflurane gas (in 100% O2) for the duration of the scan (approximately 1 hr). Nine T1-weighted volumes with three-dimensional, magnetization-prepared, rapid-acquisition, gradient-echo (MPRAGE) images were acquired (TR=1900 ms TE=4.38 ms, FOV=96 mm, flip angle 15 degrees, voxel size 0.5 mm, 248 slices, slice thickness 0.5 mm) using a 1.5-T Siemens Sonata scanner and an 8-channel, high-resolution, knee-array coil (Invivo Corporation).
PET scanning procedures
Three PET scans were collected, as previously described (Groman et al., 2012): (1) ~2 weeks prior to initiating the dosing regimen (referred to as the “baseline scan”), (2) ~2 weeks after the last drug administration to examine the immediate effects of methamphetamine (referred to as “2-week post exposure scan”) and (3) ~7 weeks after the last drug administration to examine the stability of neural changes (referred to as the “7-week post exposure scan”). PET scanning was completed using [11C]WIN-35428 and [18F]fallypride as radioligands for assessing DAT and D2-like receptor availability, respectively. For the current study, D2-like and DAT availability measurements collected at the 2-week post-exposure scan were used, as these measurements were closest in time to when the second MR image was collected. Difference scores were calculated by subtracting the availability measurements obtained at the 2-week post-exposure scan from the baseline scan.
Data processing
MR image pre-processing
The nine MPRAGE sequences were motion-corrected using Statistical Parametric Mapping 5 (SPM; Institute of Neurology, University College London, London, England) and averaged. The images were aligned such that the anterior and posterior commissures were in the same plane, field-bias corrected and manually skull-stripped.
Developing tissue class priors for MR image segmentation in vervet monkey
Tissue class priors, which indicate the likelihood of finding a given tissue class at a given location, were created by manually defining and smoothing (3 mm FWHM Gaussian kernel) gray matter, white matter, and cerebrospinal fluid on a vervet monkey template. Ten vervet monkey scans, collected for a different study (Fears et al., 2009), were segmented using SPM5, with the following changes to the default parameters: human tissue classification priors were replaced with the maps of tissue classification created from the vervet template, no affine regularization was conducted, and the sampling distance was changed from 3 mm to 1 mm. The resulting segmentations were averaged to create probability maps of gray matter, white matter and CSF.
Processing of MR images
Baseline and post-dosing regimen MR images for each subject were moved into a position located halfway between the two images, using Advanced Normalization Tools (Avants et al., 2011), and the baseline and post-dosing scans were averaged to create a mean image. The mean, baseline, and post-dosing regimen MR images were then segmented into gray matter, white matter and CSF, using SPM5. The following segmentation parameters were changed from the default settings: human priors were replaced with the vervet monkey priors, no affine regularization was conducted, and the sampling distance was changed from 3 mm to 1 mm. Diffeomorphic Anatomical Registration using Exponential Lie Algebra (DARTEL) was then used to register each subject's mean gray-matter image to template space. Baseline and post-dosing regimen gray-matter images were moved to template space using the nonlinear deformation parameters generated by the registration of each subject's mean gray-matter image to template space (summarized in Figure 1). In the resulting gray-matter images, intensity at each voxel represents the proportion of gray matter with a region.
Fig. 1.
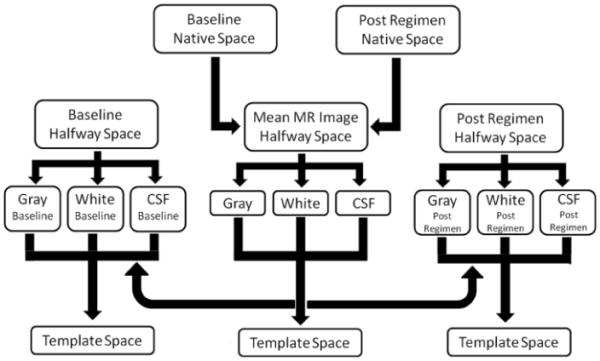
A diagram of the processing stream used in the current study for the structural MR images.
Reconstruction and processing of PET images
PET images were reconstructed as previously described (Groman et al., 2012). Activity was extracted from each subjects' PET image using previously defined regions of interest and imported into the PMOD kinetic analysis program (PMOD v3.15). Time-activity curves were fit using the Multilinear Reference Tissue Model (Ichise et al., 2003), using the activity from the cerebellum as the reference. K2′, the rate constant of tracer transfer from the reference region (cerebellum) to plasma, was extracted from the high-activity areas of the caudate nucleus and putamen and averaged together. The time-activity curves were then refit with MRTM2 using the average fixed k2′ value applied to all brain regions.
Statistical analysis
In order to determine whether exposure to methamphetamine had a different effect on gray matter than saline treatment, difference images were created by subtracting the intensity of gray-matter images obtained from the post-dosing regimen scan from that of the baseline scan. These difference images were then smoothed using a 5 mm FWHM Gaussian function (McLaren et al., 2010). A general linear model (GLM) was created to statistically test the following: 1) whether the difference in gray matter within saline-exposed monkeys was greater than that in methamphetamine-exposed monkeys, 2) whether the difference in gray matter within methamphetamine-exposed monkeys was greater than that in saline-exposed monkeys 3) whether the difference in gray matter between the baseline and post-dosing regimen was statistically significant in saline-exposed monkeys and 4) whether the difference in gray matter between baseline and post-dosing regimen was statistically significant in methamphetamine-exposed monkeys. The GLM was then implemented in FSL RANDOMISE v2.1 tool (Permutation-based nonparametric inference, Oxford University, Oxford UK) with a variance smoothing of 5 mm (FWHM Gaussian). Threshold-free cluster enhancement (TFCE) (Smith and Nichols, 2009) was used to detect significant clusters of change; this method provides the ability to perform cluster-based inference without the need to specify an arbitrary cluster-forming threshold, as is necessary when using Gaussian random field theory. For each analysis, 10,000 randomization runs were performed. Statistical maps were thresholded at p < 0.05 and corrected for the search volume contained in the following regions of interest, selected based on evidence that gray-matter volume or density in methamphetamine-dependent individuals is altered in these regions (Thompson et al., 2004; Chang et al., 2005; Jernigan et al., 2005; Morales et al., 2012): orbitofrontal cortex, ventral medial prefrontal cortex, cingulate cortex, insula, caudate, putamen and ventral striatum.
To determine the dependency between changes in gray matter and changes in the number of trials required to reach criterion in the reversal phase in the methamphetamine-exposed monkeys, the difference in the number of trials required to reach criterion in the reversal phase before and after the dosing regimen was regressed against the difference in gray matter using RANDOMISE with variance smoothing of 5 mm (FWHM) and, based on the results of the analysis described above, confined to the search volume of the bilateral putamen. Ten thousand randomization runs were performed, and TFCE was used to detected significant correlation clusters. Statistical maps were thresholded at p<0.05 and corrected for the entire search volume of the putamen.
The relationship between changes in gray matter and changes in D2-like receptor and DAT availability were examined by regressing the difference in the availability of these dopaminergic markers within the right putamen (2-week post-exposure scan - baseline) against the difference in gray matter using RANDOMISE with variance-smoothing of 5 mm (FWHM) and, based on the results of analysis described above, confined to the search volume of the bilateral putamen. Ten thousand randomization runs were performed, and TFCE used to detected significant correlation clusters. Statistical maps were thresholded at p<0.05 and corrected for the entire search volume of the putamen. Effect size maps (Cohen's d) were created by taking the square root of the mean difference in intensity of the gray-matter images in methamphetamine-exposed monkeys to that of saline-exposed monkeys divided by the pooled standard deviation in the difference values.
Correlation coefficients, obtained from Pearson's product-moment correlations, were compared between data from methamphetamine- and saline-exposed monkeys using Fisher's r-to-z transform.
RESULTS
Brain morphometry
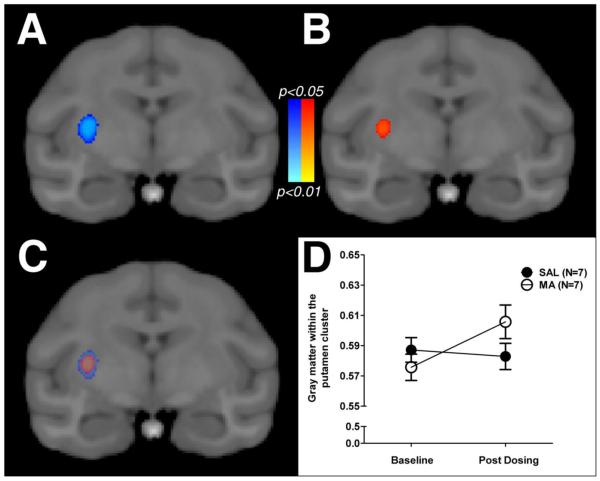
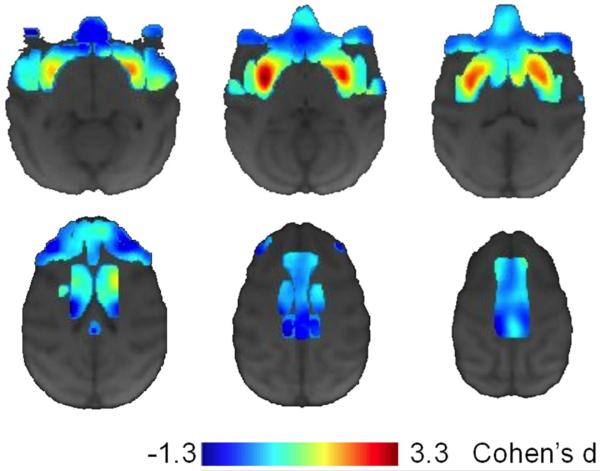
Voxel-wise analysis examining whether methamphetamine produced changes in gray matter that differed from changes in the control condition detected a significant cluster, located within the caudal portion of the right putamen (Figure 2A). A similar cluster was observed in the left putamen; however, it did not survive corrections for multiple comparisons. The simple-effects contrasts examining the difference in gray matter in saline or methamphetamine monkeys indicated that the interaction was due to a significant change in gray matter within the right putamen of methamphetamine-exposed monkeys (Figure 2B), as no significant clusters were detected in the saline-exposed monkeys. Values extracted from the significant interaction cluster revealed that, on average, gray matter increased in methamphetamine-exposed monkeys by 5.2% (+/−1.2% SEM), while gray matter decreased in saline-exposed monkeys by 0.8% (+/− 1.5%; SEM). The overlap between the significant interaction cluster and simple-effect cluster are plotted in Figure 2C, and the raw gray-matter intensity values are plotted in Figure 2D. Effect size (Cohen's d) maps, comparing changes in gray matter between methamphetamine- and saline-exposed monkeys within the regions of interest (Fig. 3), allow for qualitative comparison of differences between groups that did not reach the prescribed statistical threshold.
Fig. 2.
Statistical maps (p values) for the voxel-wise analysis of changes in gray matter with exposure to an escalating dose regimen of saline (SAL; N=7) or methamphetamine (MA; N=7).The significant time-by-drug exposure interaction detected in the right putamen is presented in panel A and the simple effects contrast presented in panel B. The overlap between the interaction cluster and the simple effect cluster is presented in panel C. For illustrative purposes, the intensity of the gray-matter images at the baseline and post-dosing scan in monkeys exposed to saline (closed circles) or methamphetamine (open circles) are presented in panel D.
Fig. 3.
Effect size maps (Cohen's d) comparing the change in gray matter between methamphetamine- and saline-exposed monkeys. Cooler values are indicative of gray matter loss (darker to lighter blue represents lower to greater negative effects) in the methamphetamine-exposed monkeys compared to the saline-exposed monkeys, while hotter values are indicative of increases in gray matter (darker to lighter red represents lower to greater positive effects) in methamphetamine-exposed monkeys compared to saline-exposed monkeys.
Acquisition, retention and reversal performance
As previously described (Groman, Lee et al., 2012), analysis of behavioral performance of monkeys before, during and after the dosing regimen revealed no significant differences between the groups for the number of trials required to reach criterion in the acquisition phase or retention phase. However, the number of trials required to reach criterion in the reversal phase significantly diverge between the groups across the behavioral assessments (group by discrimination session: p<0.001). Post-hoc analyses confirmed that this was due to an significant increase in the number of trials required to reach criterion in the methamphetamine-exposed monkeys between baseline and the three-week assessment (p<0.001); however, no other pairwise comparisons were significant (all p's>0.81).
Correlation of changes in inhibitory control with changes in gray matter morphometry
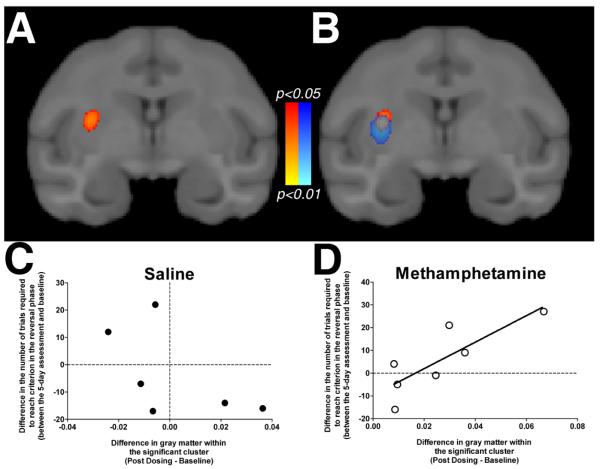
To determine whether the change in putamen gray matter detected in the methamphetamine-exposed monkeys was behaviorally relevant, we tested for a correlation between the change in the number of trials required to reach criterion in the reversal phase after methamphetamine exposure and the difference in gray matter within the putamen. The voxel-wise analysis of this regression revealed a significant cluster located within the caudal portion of the right putamen (Figure 4A), overlapping with the time-by-dosing regimen cluster detected above (Figure 4B). The change in gray matter was positively correlated with the change in reversal-learning performance, such that the increased gray matter was associated with a greater number of trials needed to reach criterion in the reversal phase in methamphetamine-exposed monkeys (Figure 4D). The relationship was not statistically significant in saline-exposed monkeys (Figure 4C).
Fig. 4.
Statistical maps (p values) for the voxel-wise regression of changes in gray matter within the putamen on change in reversal learning performance. The change in the number of trials required to reach criterion in the reversal phase was positively related to the change in gray matter, which is presented in panel A. This cluster overlapped with the time-by-dosing regimen interaction on gray matter as presented in panel B. The relationship was only detected in the methamphetamine-exposed monkeys (panel D), as the change in gray matter did not significantly correlate with the change in reversal learning performance within the saline-exposed monkeys (panel 4C). Scatter plots are for illustrative purposes only.
Covariation of dopaminergic markers with gray-matter morphometry
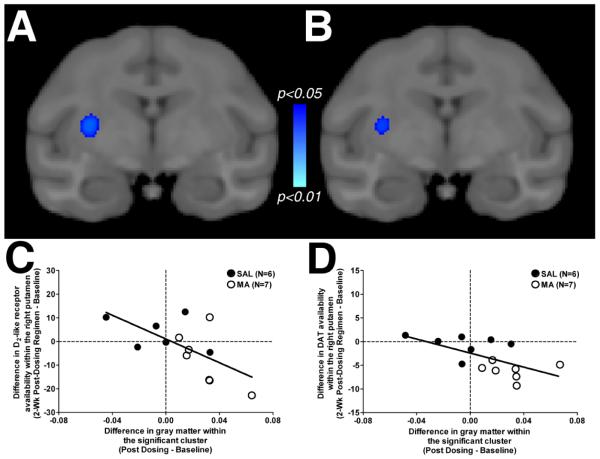
Previously, we demonstrated that exposure to an escalating dose of methamphetamine significantly reduced D2-like receptor and DAT within the striatum of the animals tested here (Groman et al., 2012). To determine whether changes in markers of the dopamine system were correlated with changes in gray matter, the change in these dopaminergic markers within the right putamen before and after the dosing regimen was regressed against changes in gray matter within the putamen. The voxel-wise analysis revealed a significant cluster located within the caudal portion of the right putamen for the change in D2-like receptor availability (Figure 5A) as well as the change in DAT availability (Figure 5B). The correlation coefficients did not differ between saline- or methamphetamine-exposed monkeys for D2-like receptor availability (Figure 5C) or for DAT availability (Figure 5D).
Fig. 5.
Statistical maps (p values) from the voxel-wise regression of changes in gray matter on changes in D2-like receptor availability (panel A) and changes in DAT availability (panel B) within the putamen. Scatter plots demonstrating the relationship between the difference in gray matter (X axis) and the difference in D2-like receptor availability (Y axis; panel C) or difference in DAT availability (Y axis; panel D) are presented in both saline-exposed monkeys (closed circles) and methamphetamine-exposed monkeys (open circles).
DISCUSSION
These data provide the first evidence that exposure to a prolonged, escalating dosing regimen of methamphetamine designed to mimic aspects of human use patterns produces long-lasting alterations in the structural integrity of the striatum that can be measured in vivo with sMRI, and suggest that the structural abnormalities previously detected in methamphetamine-dependent humans are, at least in part, a consequence of drug use. The degree of change in gray matter within the right putamen correlated with change in reversal-learning performance, providing evidence that the structural alterations have a functional impact on inhibitory control, a process that may be central to addiction. Finally, changes in dopaminergic markers within the putamen were correlated with the change in gray matter, suggesting that the methamphetamine-induced biochemical alterations may be related to the structural change.
Escalation of methamphetamine administration results in increased gray matter in right putamen
Previous studies have indicated that gray-matter volume within the putamen is greater in methamphetamine-dependent humans compared to control subjects (Chang et al., 2005; Jernigan et al., 2005; Churchwell et al., 2012). Although these morphometric differences have long been presumed to be an effect of chronic drug use (Chang et al., 2007), recent evidence has suggested that greater putamen gray-matter volume may be present prior to drug use (Ersche et al., 2012), with these structural differences possibly influencing the development of substance abuse and/or dependence. The results of the current study support the former hypothesis, demonstrating that gray matter in the putamen increased following exposure to methamphetamine in a manner similar to the differences detected in human-methamphetamine users. This is not to say that gray-matter volume differences in the putamen are not both a cause and consequence of stimulant dependence.
Unlike previous studies that have detected gray-matter abnormalities in the prefrontal cortex of methamphetamine dependent humans (Thompson et al., 2004; Morales et al., 2012), prefrontal gray matter was not significantly different in monkeys exposed to methamphetamine than in monkeys exposed to saline. The discrepancy between the human studies and the current study could reflect a variety of factors. One possibility is that changes in prefrontal gray matter produced by 31 days of methamphetamine exposure may be on a smaller scale than that those in putamen, requiring a larger sample size than that used here to detect the change (as evidenced by the effect size maps presented in Figure 3). Changes in prefrontal cortical structure may occur at a slower rate than those in the putamen, emerging only after extensive periods of drug use (several years). Supporting this hypothesis is evidence that prefrontal gray-matter volume/density in heroin- and cannabis-dependent individuals is negatively correlated with duration of drug use (Yuan et al., 2009; Stone et al., 2012). It is also possible that some of the abnormalities in prefrontal cortex detected in methamphetamine-dependent individuals are attributable to factors other than methamphetamine exposure. For example, a large proportion of methamphetamine-dependent individuals smoke tobacco cigarettes, and some abnormalities in prefrontal gray-matter volume observed in methamphetamine-dependent individuals may be attributable to cigarette smoking (Morales et al., 2012). Finally, given that prefrontal gray-matter volume is smaller in drug-naive individuals who are at greater than normal risk for developing alcoholism (Benegal et al., 2007), it is also possible that the differences in prefrontal gray-matter volume observed in methamphetamine abusers predate drug use may reflect and increased vulnerability to developing methamphetamine dependence.
Potential mechanisms for the methamphetamine-induced increase in putamen gray matter
Although several studies have indicated that gray matter in the striatum is altered in methamphetamine-dependent individuals (Chang et al., 2005; Jernigan et al., 2005; Churchwell et al., 2012; Ersche et al., 2012; Morales et al., 2012), the mechanism by which these alterations occur is unknown. Greater gray-matter volume/density in the putamen may be indicative of increased neuronal density (Tulloch et al., 2011), alterations in the morphology of the existing neurons (e.g., increased spine density) (Jedynak et al., 2007), or, as had been previously proposed, an inflammatory response to methamphetamine exposure (Kousik et al., 2012). In animals, administration of methamphetamine increases activation of astroglia, microglia and other markers of inflammation that have been proposed to mediate the neurotoxic effects of methamphetamine on the dopamine system (Asanuma et al., 2003), and administration of drugs that prevent microglial activation prevents the methamphetamine-induced dopamine alterations (Thomas and Kuhn, 2005). Measurements of microglial activation in methamphetamine-dependent people suggest that reactive gliosis can persist for at least two years following the initiation of abstinence from methamphetamine (Sekine et al., 2008). The changes in gray matter detected in the current study were still present 3 weeks after the final dose of methamphetamine was administered and were correlated with changes in measures of D2-like and DAT availability, suggesting that changes in putamen morphometry are long-lasting and co-occur with changes in the putamen dopamine system, possibly arising from inflammation.
The increase in gray matter observed here was greater in the right putamen, with a similar, nonsignificant trend observed in the left putamen; however, nonsignificant decreases in gray matter were observed in the caudate nucleus (see effect size map, Figure 3). An emerging literature suggest that exposure to methamphetamine has differential effects in striatal subregions. Repeated exposure to methamphetamine increases spine density on medium spiny neurons in the dorsolateral striatum, but decreases spine density in the immediately adjacent dorsomedial striatum (Jedynak et al., 2007), the rodent striatal subregions believed to be orthologous to the primate putamen and caudate, respectively. Methamphetamine exposure also results in alterations in the vasculature of the dorsal but not ventral striatum (Kousik et al., 2011), possibly producing a spatially restricted hypoxic environment and inflammatory response. Data from human studies suggest that stimulant-dependent individuals have smaller gray-matter volume in the caudate than stimulant-naive individuals (Morales et al., 2012; Moreno-Lopez et al., 2012), in contrast to findings indicating greater volume in the putamen. Taken together, this evidence suggests that methamphetamine exposure differentially alters regions within the striatum, which may differentially alter the behavioral effects of methamphetamine by simultaneously altering the neural circuits that underlie goal-directed (dorsomedial striatum) and habitual (dorsolateral striatum) behaviors. However, additional studies with larger samples are needed.
Correlation of methamphetamine-induced changes in gray matter with changes in inhibitory control
Emerging evidence indicates that the ability to exert inhibitory control over behaviors relies on several regions within the corticostriatal circuit (Castane et al., 2010). Lesions to the orbitofrontal cortex and dorsal striatum produce similar impairments in reversal-learning performance, indicating that the ability to modify behaviors adaptively, upon a change in contingencies, depends upon the coordinated activity of nuclei within the corticostriatal circuit. In the current study, the methamphetamine-induced increases in gray matter in the putamen were positively correlated with the change in reversal-learning performance, demonstrating that the morphometric changes had a detrimental impact on a behavioral process that is altered in human methamphetamine users (Ghahremani et al., 2011).
Conversely, striatal gray-matter volume in human methamphetamine-dependent subjects is negatively correlated with measures of cognitive function (Chang et al., 2005; Jan et al., 2012). The discrepancy between the current findings and this observation may reflect temporal differences in the effects of methamphetamine. For example, during the initial phases of drug use, the effect of methamphetamine on the putamen may be the greatest, producing the largest inflammatory response and alterations to the dopamine system. Consistent with this hypothesis, in adolescent methamphetamine abusers, number of lifetime doses of methamphetamine are positively correlated with gray-matter volume in the putamen (Churchwell et al., 2012). In adults who abuse methamphetamine, however, years of methamphetamine abuse are negatively correlated with gray-matter volume in the putamen (Chang et al., 2005), suggesting that as use of the drug continues, tolerance develops and compensatory mechanisms are recruited as an adaption to the persistent presence of methamphetamine, which may then impact cognitive processes in a different way than when methamphetamine use initially began.
These data highlight the neural and behavioral variability that exists between individuals in response to methamphetamine. Despite receiving identical doses and cumulative amounts of methamphetamine, the impact of methamphetamine on gray matter was minimal in some individuals (<2%) and great in others (~11%) suggesting that unidentified biological and/or genetic factors may mediate the neural response of individuals to methamphetamine. The current study found that changes in gray matter within the methamphetamine-exposed monkeys correlated with the changes in reversal learning performance, suggesting that individual differences in methamphetamine-induced gray matter changes have a functional and meaningful impact on behaviors that may contribute to the pathophysiology of methamphetamine dependence.
Future directions for understanding the pathophysiology of methamphetamine dependence
These data support the notion that greater putamen gray-matter volume in methamphetamine-dependent than in methamphetamine-naïve individuals is, in part, a consequence of drug use; however, the etiology and reversibility of these methamphetamine-induced changes remains unknown. Future studies in animals, combining sMRI assessments of brain structure with histological measurements have the potential to link gross anatomical changes to changes at the cellular level. In humans, multimodal neuroimaging, incorporating measures of microglial activation (Sekine et al., 2008) may be used to test the hypothesis that changes in gray matter reflect inflammation. Since the current study found that exposure to methamphetamine produced an changes in morphometry that functionally impacted behaviors previously shown to predict aspects of sobriety in substance-dependent individuals (Aharonovich et al., 2006; Turner et al., 2009), determining the etiology of these morphometric changes may identify novel targets for the treatment of methamphetamine dependence.
Acknowledgements
This study was supported primarily by Public Health Service grants P20-DA022539, R03-DA020598, T32-DA024635, F31-DA028812, and F31-DA033117 with additional support from the Consortium for Neuropsychiatric Phenomics at UCLA. The Consortium is funded by PHS grants UL1-DE019580 and RL1-MH083270.
References
- Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend. 2006;81:313–322. doi: 10.1016/j.drugalcdep.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Perry JL, Gliddon LA, Carroll ME. Impulsivity predicts the escalation of cocaine self-administration in rats. Pharmacol Biochem Behav. 2009;93:343–348. doi: 10.1016/j.pbb.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma M, Tsuji T, Miyazaki I, Miyoshi K, Ogawa N. Methamphetamine-induced neurotoxicity in mouse brain is attenuated by ketoprofen, a non-steroidal anti-inflammatory drug. Neurosci Lett. 2003;352:13–16. doi: 10.1016/j.neulet.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54:2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baicy K, London ED. Corticolimbic dysregulation and chronic methamphetamine abuse. Addiction. 2007;102(Suppl 1):5–15. doi: 10.1111/j.1360-0443.2006.01777.x. [DOI] [PubMed] [Google Scholar]
- Bellebaum C, Koch B, Schwarz M, Daum I. Focal basal ganglia lesions are associated with impairments in reward-based reversal learning. Brain. 2008;131:829–841. doi: 10.1093/brain/awn011. [DOI] [PubMed] [Google Scholar]
- Benegal V, Antony G, Venkatasubramanian G, Jayakumar PN. Gray matter volume abnormalities and externalizing symptoms in subjects at high risk for alcohol dependence. Addict Biol. 2007;12:122–132. doi: 10.1111/j.1369-1600.2006.00043.x. [DOI] [PubMed] [Google Scholar]
- Castane A, Theobald DE, Robbins TW. Selective lesions of the dorsomedial striatum impair serial spatial reversal learning in rats. Behav Brain Res. 2010;210:74–83. doi: 10.1016/j.bbr.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007;102(Suppl 1):16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- Chang L, Cloak C, Patterson K, Grob C, Miller EN, Ernst T. Enlarged striatum in abstinent methamphetamine abusers: a possible compensatory response. Biol Psychiatry. 2005;57:967–974. doi: 10.1016/j.biopsych.2005.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell JC, Carey PD, Ferrett HL, Stein DJ, Yurgelun-Todd DA. Abnormal striatal circuitry and intensified novelty seeking among adolescents who abuse methamphetamine and cannabis. Dev Neurosci. 2012;34:310–317. doi: 10.1159/000337724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Hill GJ, Robbins TW, Roberts AC. Dopamine, but not serotonin, regulates reversal learning in the marmoset caudate nucleus. J Neurosci. 2011;31:4290–4297. doi: 10.1523/JNEUROSCI.5066-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304:878–880. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci. 1996;110:872–886. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer AN, De Vries TJ. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry. 2008;63:301–308. doi: 10.1016/j.biopsych.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. Abnormal brain structure implicated in stimulant drug addiction. Science. 2012;335:601–604. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- Fears SC, Melega WP, Service SK, Lee C, Chen K, Tu Z, Jorgensen MJ, Fairbanks LA, Cantor RM, Freimer NB, Woods RP. Identifying heritable brain phenotypes in an extended pedigree of vervet monkeys. J Neurosci. 2009;29:2867–2875. doi: 10.1523/JNEUROSCI.5153-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain. 2003;126:1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- Ghahremani DG, Tabibnia G, Monterosso J, Hellemann G, Poldrack RA, London ED. Effect of modafinil on learning and task-related brain activity in methamphetamine-dependent and healthy individuals. Neuropsychopharmacology. 2011;36:950–959. doi: 10.1038/npp.2010.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, Jentsch JD. Cognitive control and the dopamine D(2) -like receptor: a dimensional understanding of addiction. Depress Anxiety. 2011;29:295–306. doi: 10.1002/da.20897. [DOI] [PubMed] [Google Scholar]
- Groman SM, Jentsch JD. Identifying the molecular basis of inhibitory control deficits in addictions: Neuroimaging in non-human primates. Current Opinion in Neurobiology. 2013 doi: 10.1016/j.conb.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, Lee B, Seu E, James AS, Feiler K, Mandelkern MA, London ED, Jentsch JD. Dysregulation of d2-mediated dopamine transmission in monkeys after chronic escalating methamphetamine exposure. J Neurosci. 2012;32:5843–5852. doi: 10.1523/JNEUROSCI.0029-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, Lee B, London ED, Mandelkern MA, James AS, Feiler K, Rivera R, Dahlbom M, Sossi V, Vandervoort E, Jentsch JD. Dorsal Striatal D2-Like Receptor Availability Covaries with Sensitivity to Positive Reinforcement during Discrimination Learning. J Neurosci. 2011;31:7291–7299. doi: 10.1523/JNEUROSCI.0363-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldane M, Cunningham G, Androutsos C, Frangou S. Structural brain correlates of response inhibition in Bipolar Disorder I. J Psychopharmacol. 2008;22:138–143. doi: 10.1177/0269881107082955. [DOI] [PubMed] [Google Scholar]
- Han E, Paulus MP, Wittmann M, Chung H, Song JM. Hair analysis and self-report of methamphetamine use by methamphetamine dependent individuals. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:541–547. doi: 10.1016/j.jchromb.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Ichise M, Liow JS, Lu JQ, Takano A, Model K, Toyama H, Suhara T, Suzuki K, Innis RB, Carson RE. Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab. 2003;23:1096–1112. doi: 10.1097/01.WCB.0000085441.37552.CA. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Jentsch JD. Reversal learning as a measure of impulsive and compulsive behavior in addictions. Psychopharmacology (Berl) 2012;219:607–620. doi: 10.1007/s00213-011-2579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan RK, Lin JC, Miles SW, Kydd RR, Russell BR. Striatal Volume Increases in Active Methamphetamine-Dependent Individuals and Correlation with Cognitive Performance. Brain Sci. 2012;2:553–572. doi: 10.3390/brainsci2040553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedynak JP, Uslaner JM, Esteban JA, Robinson TE. Methamphetamine-induced structural plasticity in the dorsal striatum. Eur J Neurosci. 2007;25:847–853. doi: 10.1111/j.1460-9568.2007.05316.x. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Olausson P, De La Garza R, 2nd, Taylor JR. Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology. 2002;26:183–190. doi: 10.1016/S0893-133X(01)00355-4. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC, Archibald SL, Fennema-Notestine C, Mindt MR, Marcotte TD, Heaton RK, Ellis RJ, Grant I. Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am J Psychiatry. 2005;162:1461–1472. doi: 10.1176/appi.ajp.162.8.1461. [DOI] [PubMed] [Google Scholar]
- Kousik SM, Napier TC, Carvey PM. The effects of psychostimulant drugs on blood brain barrier function and neuroinflammation. Front Pharmacol. 2012;3:121. doi: 10.3389/fphar.2012.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousik SM, Graves SM, Napier TC, Zhao C, Carvey PM. Methamphetamine-induced vascular changes lead to striatal hypoxia and dopamine reduction. Neuroreport. 2011;22:923–928. doi: 10.1097/WNR.0b013e32834d0bc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren DG, Kosmatka KJ, Kastman EK, Bendlin BB, Johnson SC. Rhesus macaque brain morphometry: a methodological comparison of voxel-wise approaches. Methods. 2010;50:157–165. doi: 10.1016/j.ymeth.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales AM, Lee B, Hellemann G, O'Neill J, London ED. Gray-matter volume in methamphetamine dependence: cigarette smoking and changes with abstinence from methamphetamine. Drug Alcohol Depend. 2012;125:230–238. doi: 10.1016/j.drugalcdep.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Lopez L, Catena A, Fernandez-Serrano MJ, Delgado-Rico E, Stamatakis EA, Perez-Garcia M, Verdejo-Garcia A. Trait impulsivity and prefrontal gray matter reductions in cocaine dependent individuals. Drug Alcohol Depend. 2012;125:208–214. doi: 10.1016/j.drugalcdep.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, Ehrenkaufer R, Mach RH. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006;9:1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- Perry JL, Nelson SE, Carroll ME. Impulsive choice as a predictor of acquisition of IV cocaine self- administration and reinstatement of cocaine-seeking behavior in male and female rats. Exp Clin Psychopharmacol. 2008;16:165–177. doi: 10.1037/1064-1297.16.2.165. [DOI] [PubMed] [Google Scholar]
- Porter JN, Gurnsey K, Jedema HP, Bradberry CW. Latent vulnerability in cognitive performance following chronic cocaine self-administration in rhesus monkeys. Psychopharmacology (Berl) 2012;226:139–146. doi: 10.1007/s00213-012-2903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JN, Olsen AS, Gurnsey K, Dugan BP, Jedema HP, Bradberry CW. Chronic cocaine self-administration in rhesus monkeys: impact on associative learning, cognitive control, and working memory. J Neurosci. 2011;31:4926–4934. doi: 10.1523/JNEUROSCI.5426-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rygula R, Walker SC, Clarke HF, Robbins TW, Roberts AC. Differential contributions of the primate ventrolateral prefrontal and orbitofrontal cortex to serial reversal learning. J Neurosci. 2010;30:14552–14559. doi: 10.1523/JNEUROSCI.2631-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B. Cocaine makes actions insensitive to outcomes but not extinction: implications for altered orbitofrontal-amygdalar function. Cereb Cortex. 2005;15:1162–1169. doi: 10.1093/cercor/bhh216. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Saddoris MP, Ramus SJ, Shaham Y, Setlow B. Cocaine-experienced rats exhibit learning deficits in a task sensitive to orbitofrontal cortex lesions. Eur J Neurosci. 2004;19:1997–2002. doi: 10.1111/j.1460-9568.2004.03274.x. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Sugihara G, Takei N, Yoshikawa E, Nakamura K, Iwata Y, Tsuchiya KJ, Suda S, Suzuki K, Kawai M, Takebayashi K, Yamamoto S, Matsuzaki H, Ueki T, Mori N, Gold MS, Cadet JL. Methamphetamine causes microglial activation in the brains of human abusers. J Neurosci. 2008;28:5756–5761. doi: 10.1523/JNEUROSCI.1179-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Sridharan A, Willette AA, Bendlin BB, Alexander AL, Coe CL, Voytko ML, Colman RJ, Kemnitz JW, Weindruch RH, Johnson SC. Brain volumetric and microstructural correlates of executive and motor performance in aged rhesus monkeys. Front Aging Neurosci. 2012;4:31. doi: 10.3389/fnagi.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JM, Bhattacharyya S, Barker GJ, McGuire PK. Substance use and regional gray matter volume in individuals at high risk of psychosis. Eur Neuropsychopharmacol. 2012;22:114–122. doi: 10.1016/j.euroneuro.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Tabibnia G, Monterosso JR, Baicy K, Aron AR, Poldrack RA, Chakrapani S, Lee B, London ED. Different forms of self-control share a neurocognitive substrate. J Neurosci. 2011;31:4805–4810. doi: 10.1523/JNEUROSCI.2859-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DM, Kuhn DM. Attenuated microglial activation mediates tolerance to the neurotoxic effects of methamphetamine. J Neurochem. 2005;92:790–797. doi: 10.1111/j.1471-4159.2004.02906.x. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulloch IK, Afanador L, Zhu J, Angulo JA. Methamphetamine induces striatal cell death followed by the generation of new cells and a second round of cell death in mice. Curr Neuropharmacol. 2011;9:79–83. doi: 10.2174/157015911795017281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner TH, LaRowe S, Horner MD, Herron J, Malcolm R. Measures of cognitive functioning as predictors of treatment outcome for cocaine dependence. J Subst Abuse Treat. 2009;37:328–334. doi: 10.1016/j.jsat.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Zhu Z, Shi J, Zou Z, Yuan F, Liu Y, Lee TM, Weng X. Gray matter density negatively correlates with duration of heroin use in young lifetime heroin-dependent individuals. Brain Cogn. 2009;71:223–228. doi: 10.1016/j.bandc.2009.08.014. [DOI] [PubMed] [Google Scholar]