Abstract
Background
Alcohol use is a well-documented risk factor for the emergence of chronic smoking behavior. Very little is known, however, about the mediating pathways through which alcohol and/or alcohol-related problems influence future smoking.
Methods
Data were drawn from the longitudinal Social and Emotional Contexts of Adolescent Smoking Patterns Study. Adolescents who had smoked under 100 cigarettes in their lifetime (n = 898; experimenters) and adolescents who had smoked over 100 cigarettes, but fewer than 5 cigarettes per day (n = 152: current smokers) were examined separately (grouping variable). Path analysis was performed to investigate the association between alcohol related problems at baseline (primary predictor) and smoking regularity at the 48 month follow-up (primary outcome), both directly and through mediating variables of smoking quantity and frequency, and nicotine dependence (averaged across these measures at 6, 15-, and 24- month assessment waves).
Results
Among experimenters, after controlling for smoking and alcohol use, the association between alcohol-related problems at baseline and smoking frequency 48 months later was fully mediated by nicotine dependence symptoms. Among current smokers, only past smoking behavior was associated with 48-month smoking frequency.
Conclusions
Alcohol-related problems are a risk factor for future smoking among novice adolescent smokers above and beyond drinking or smoking per se. By signaling sensitivity to nicotine dependence symptoms, alcohol related problems represent an easily measureable risk factor that can be used to identify and intervene with adolescents before more chronic smoking behaviors emerge.
Keywords: Alcohol Problems, Smoking, Nicotine Dependence
Introduction
The link between drinking and smoking is well established with the proposed mechanism for the association commonly described as a reciprocal influence based on the shared properties of both substances (Lajtha and Sershen, 2010; Leeman et al., 2008; Little, 2000; Littleton and Little, 2002). Third variables such as genetic factors and/or shared susceptibility through personality traits or psychological symptoms may also contribute to the association between smoking and drinking (Little, 2000).
In a study examining young adults drawn from the National Epidemiologic Study of Alcohol and Related Conditions (NESARC), cross-sectional analyses revealed that a diagnosis of alcohol dependence was associated with substantially elevated risk for nicotine dependence, even among individuals smoking fewer than 5 cigarettes per day (Dierker and Donny, 2008). More recently, cross-sectional analyses using pooled data from 5 annual National Surveys of Drug Use and Health Data (NSDUH) including novice young adult smokers showed that both alcohol abuse and dependence were associated with nicotine dependence symptoms over and above an individual’s level of smoking or drinking (Dierker, Rose, Donny and Tiffany, 2010). These studies point to the possibility that emerging symptoms associated with alcohol use rather than alcohol use per se may increase risk of chronic smoking by signaling greater liability to symptoms of nicotine dependence at very low levels of smoking exposure.
Epidemiologic research further shows that about half of under-age Americans have used alcohol with those who begin drinking in adolescence being at risk for developing alcohol dependence. According to the National Survey of Alcohol and Related conditions, among adults who began drinking before age 14, nearly half had become alcoholic dependent by the age of 21. In contrast, only 9% of people who began drinking after the age of 21 developed alcohol dependence (Lopez-Quinter et al., 2011). Problems related to the heavy use and abuse of alcohol during late adolescence has also been shown to predict later alcohol dependence diagnoses in young adulthood (Wells, Horwood and Fergusson, 2006). The temporal nature of the relationship between alcohol use and/or problems and nicotine dependence, to our knowledge, has never been investigated.
Using longitudinal data, the present study examined adolescents who had smoked under 100 cigarettes in their lifetime and adolescents who had smoked over 100 cigarettes, but fewer than 5 cigarettes per day. Specifically, we sought to investigate (1) whether alcohol problems are associated with early-emerging nicotine dependence symptoms (i.e. symptoms experienced at low levels of smoking), (2) whether alcohol problems directly or indirectly predict smoking frequency 48 months later (above and beyond measures of alcohol and tobacco use), and (3) which of several possible mediating pathways, including smoking quantity and frequency, early emerging nicotine dependence symptoms and early signs of alcohol problems, might explain these associations. We have previously demonstrated that once adolescent smokers pass the 100 cigarette milestone, it is the quantity and frequency of their smoking behavior that best predicts the establishment of chronic smoking behavior. For those having smoked less than 100 cigarettes, propensity for the development of chronic smoking is best predicted from the experience of nicotine dependence symptoms (Dierker and Mermelstein, 2010).
Methods
Participants
As described elsewhere (Dierker and Mermelstein, 2010), the Social and Emotional Contexts of Adolescent Smoking Patterns (SECASPS) follows a cohort recruited from 16 Chicago-area high schools. All 9th- and 10th-grade students in these schools completed a brief screening survey of smoking behavior (N = 12,970). The sampling scheme followed an accelerated developmental design in which there was planned oversampling of adolescents in the “experimental” stage of smoking. This design had the advantage of maximizing the ability to study movement up or down from experimentation without requiring a prohibitively large sample size. The initial cohort was selected to fit the following “stages” of use: 1) never smokers; 2) former smokers (smoked in the last 12 months, but not in the last 90 days and smoked < 100 cigarettes/lifetime); 3) current experimenters (smoked in the past 90 days, < 100 cigarettes/lifetime); and 4) regular smokers (smoked in the past 30 days and smoked > 100 cigarettes/lifetime). These initial subgroups were meant to help focus recruitment efforts to achieve a cohort that was largely comprised of adolescents who had ever smoked. The definitions for each subgroup along the continuum of adolescent smoking experience were set based on both common definitions in the research literature as well as distribution of smoking experience among adolescents in the targeted 9th and 10th grades.”
Of the 3,654 students invited, 1,344 agreed to participate (37%). Of these, 1,263 (94%) completed the baseline assessment. Follow-up assessment was conducted at 6-, 15-, 24-, 33- and 48-months with 95% (n= 1,199) of the sample participating in 2 or more surveys and 86% (n=1,092) participating in the 48-month follow-up. The present analyses were based on those participants who at baseline reported smoking in the past 30 days, both experimenters (<100 cigarettes/lifetime, N=898) and current smokers (>100 cigarettes/lifetime, N=152). The mean age of this sample at baseline was 15.6 years (s.d. = 0.6). Approximately half (58%) of this sample were female and the majority (71%) were Caucasian.
Measures
Alcohol-Related Problem Scale (ARPS)
The ARPS scale (Chassin, Rogosch, and Barrera, 1991; Colder and Chassin, 1999) consisted of dichotomous endorsement of 6 items in the past year as a result of drinking alcohol. Items included 1) had a problem with, or complaints from, your family and friends; 2) been in trouble at school or work (for example, missing school or losing a job); 3) been in trouble with the police; 4) had an accident or injury; 5) awakened the morning after and found you couldn’t remember things that had happened the night before; and 6) ended up drinking more than you had expected to when you began. The ARPS was assessed at baseline, 6-, 15-, and 24-month assessment waves. Intermediate ARPS scores were calculated by averaging the scores from the 6, 15-, and 24- month assessment waves.
Alcohol frequency
Adolescents were asked at the baseline assessment wave how many times in the past 3 months they had drunk alcohol (1: zero times; 2: once/month or less; 3: more than once/month but less than once/week; 4: once/week or more but less than daily; 5: every day).
Age of Alcohol Initiation
Adolescents reported their age in years at the time of their first drink.
Nicotine Dependence Syndrome Scale (NDSS)
Nicotine dependence symptoms were measured at each assessment with a shortened version of Nicotine Dependence Syndrome Scale NDSS; (Shiffman, Waters, & Hickcox, 2004), modified for use with adolescents (Clark, 2005; Sledjeski et al., 2007; Sterling et al., 2009). Items were answered on a four-point Likert-type scale, ranging from 0 (not at all true) to 3 (very true). A total score was obtained by averaging responses to all items. An intermediate measure of nicotine dependence was obtained by averaging participants’ responses from the 6, 15, and 24-month assessment waves.
Smoking frequency and quantity
Smoking was measured with two items at baseline. Participants were asked how many days they smoked cigarettes in the past 30 days (smoking frequency) and how many cigarettes they smoked in the past 7 days (smoking quantity). Smoking frequency was assessed categorically: 0, 1, 2–3, 4–5, 6–7, 8–10, 11–20, 21–29, and 30 days smoked, and was used in all analyses as a numeric variable using the midpoint of each category. Smoking quantity was assessed numerically. Intermediate measures of smoking frequency and quantity were obtained by averaging participants’ responses from the 6, 15, and 24 months assessment waves. Smoking frequency at the 48 months follow-up was examined as the primary outcome (see Figure 1).
Figure 1. Mediating pathways of ARPS at baseline on smoking frequency at 48 months among experimenters.
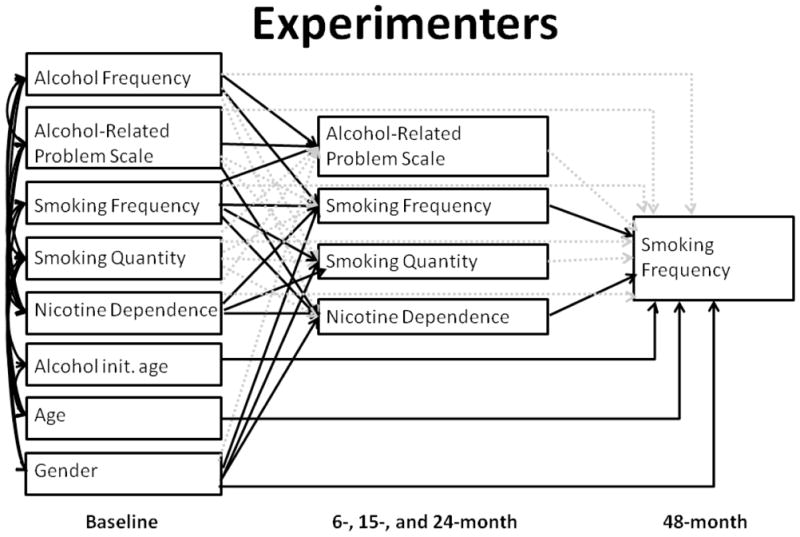
Baseline variables are shown in the left column, intermediate variables (from 6, 15, and 24-month assessment waves) are shown in the middle column, and the outcome (smoking frequency) is on the right. Solid lines indicate significant (p<.05) paths, and dotted lines are not significant.
Analyses
Path analysis was performed to investigate both direct effects and mediating pathways of alcohol problems, smoking quantity, smoking frequency, and nicotine dependence symptoms, controlling for direct effects of baseline alcohol frequency, age, age of alcohol initiation, and gender. Mediating pathways were modeled simultaneously and included intermediate (averaged across 6, 15, and 24 month) alcohol problems, smoking frequency, smoking quantity, and nicotine dependence symptoms. A multiple-group model was run with both smoking groups (experimenters and current smokers). Finally, gender differences in path coefficients were examined by comparing the model fit of the constrained model (which constrains all path coefficients to be equal for males and females) with the fit of the unconstrained model (which allows path coefficients to differ across gender).
Results
Though the χ2 of the multi-group path model was high (χ2=116.423, degrees of freedom (df)=44, p<.001), several other fit indices demonstrated a good fit. (χ2/df=2.646; Comparative Fit Index (CFI) =.986; Tucker-Lewis Index (TLI) =.942; root mean square error of approximation (RMSEA)=.040). The fit index was evaluated based on the following guidelines: χ2/d.f.≤5 (Marsh and Hocevar, 1985; Wheaton, Muthen, Alwin and Summers, 1977), CFI≥0.95 (Hu and Bentler, 1998), TLI≥0.9 (Bentler and Bonett, 1980), and RMSEA≤.05 (M. W. Browne & Cudeck, 1992). Among experimenters, the association between alcohol-related problems at baseline and smoking frequency 48 months later was mediated through intermediate nicotine dependence symptoms (Figure 1; Table 1). This indirect effect through nicotine dependence accounted for a 39.4% change in the total effect of alcohol-related problems. Though baseline alcohol related problems were associated with alcohol related problems averaged across the 6, 15 and 24 month assessments (B=.422, p<.001), neither was directly associated with smoking frequency at 48 months.
Table 1. Standardized path coefficients from the path analysis examining direct and mediated effects of ARPS.
Top: direct effects from baseline values to 48-month smoking frequency (outcome). Middle: Mediating pathways from baseline values to intermediate values. Bottom: Mediating pathways from intermediate values to 48-month smoking frequency. Standardized path coefficients and their p-values are shown for experimenters (left column; corresponding to Figure 1) and current smokers (right column; corresponding to Figure 2). Bold: p<.05.
| Path | Experimenters | Current Smokers | ||
|---|---|---|---|---|
| Standardized path coefficient | p | Standardized path coefficient | p | |
| Baseline to outcome | ||||
| ARPS → 48-month smoking freq. | −.043 | .240 | .008 | .932 |
| Alcohol freq. → 48-month smoking freq. | −.051 | .140 | −.033 | .696 |
| Smoking freq. → 48-month smoking freq. | −.061 | .225 | .419 | .003 |
| Smoking quant. → 48-month smoking freq. | .089 | .051 | −.426 | <.001 |
| NDSS → 48-month smoking freq. | −.005 | .911 | −.124 | .337 |
| Age of alc. init. → 48-month smoking freq. | −.075 | .035 | −.040 | .624 |
| Age at baseline → 48-month smoking freq. | .067 | .013 | −.088 | .276 |
| Gender → 48-month smoking freq. | .118 | <.001 | .090 | .246 |
| Baseline to intermediate | ||||
| ARPS → int. ARPS | .422 | <.001 | .528 | <.001 |
| ARPS → int. smoking freq. | .028 | .401 | −.136 | .062 |
| ARPS → int. smoking quant. | .025 | .468 | −.019 | .803 |
| ARPS → int. NDSS | .063 | .046 | .011 | .877 |
| Alcohol freq. → int. ARPS | .227 | <.001 | .114 | .121 |
| Alcohol freq. → int. smoking freq. | .084 | .010 | .120 | .097 |
| Alcohol freq. → int. smoking quant. | .054 | .118 | .047 | .545 |
| Alcohol freq. → int. NDSS | .007 | .824 | .054 | .458 |
| Smoking freq. → int. ARPS | .117 | .014 | .009 | .935 |
| Smoking freq. → int. smoking freq. | .363 | <.001 | .671 | <.001 |
| Smoking freq. → int. smoking quant. | .287 | <.001 | .191 | .087 |
| Smoking freq. → int. NDSS | .220 | <.001 | .303 | .004 |
| Smoking quant. → int. ARPS | −.063 | .157 | −.058 | .497 |
| Smoking quant. → int. smoking freq. | .043 | .335 | −.049 | .561 |
| Smoking quant. → int. smoking quant. | .067 | .153 | .343 | <.001 |
| Smoking quant. → int. NDSS | −.009 | .834 | −.118 | .162 |
| NDSS → int. ARPS | −.002 | .949 | .165 | .096 |
| NDSS → int. smoking freq. | .157 | <.001 | −.040 | .683 |
| NDSS → int. smoking quant. | .131 | <.001 | .092 | .384 |
| NDSS → int. NDSS | .414 | <.001 | .440 | <.001 |
| Gender → int. ARPS | .026 | .370 | .180 | .007 |
| Gender → int. smoking freq. | .090 | .002 | .004 | .947 |
| Gender→ int. smoking quant. | .131 | <.001 | .009 | .901 |
| Gender→ int. NDSS | .115 | <.001 | .004 | .948 |
| Intermediate to outcome | ||||
| Int. ARPS → 48-month smoking freq. | .048 | .189 | −.184 | .090 |
| Int. smoking freq. → 48-month smoking freq. | .367 | <.001 | .183 | .208 |
| Int. smoking quant. → 48-month smoking freq. | .013 | .785 | .240 | .038 |
| Int. NDSS → 48-month smoking freq. | .193 | <.001 | .093 | .515 |
Age of alc. init: Age in years of first drink.
Alcohol freq: Drinking frequency in past 90 days.
ARPS: Score on Alcohol-Related Problem Scale.
Gender: 1=female, 2=male
NDSS: average of 10 items in past 30 days.
Smoking freq: # days smoked/past 30 days.
Smoking quant: # cigarettes smoked/past 7 days.
Among current smokers, alcohol-related problems were not associated with future smoking behavior (Figure 2; Table 1). Instead, only past smoking behavior was associated with 48-month smoking frequency: that is, baseline smoking frequency was directly associated with the outcome (B=.419, p=.003), and the effect of baseline smoking quantity was partially mediated by intermediate smoking quantity (direct: B=−.426, p<.001; baseline to intermediate smoking quantity: B=.343, p<.001; intermediate smoking quantity to 48-month smoking frequency: B=.240, p=.038).
Figure 2. Mediating pathways of ARPS at baseline on smoking frequency at 48 months among current smokers.
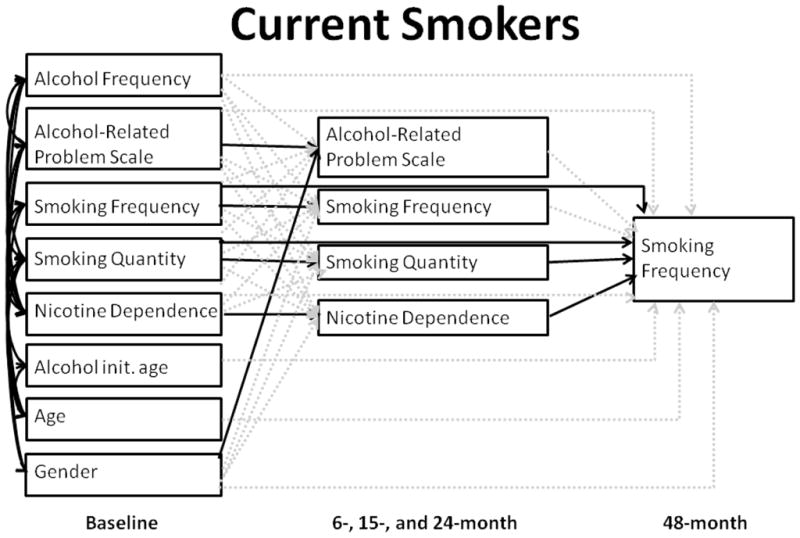
Baseline variables are shown in the left column, intermediate variables (from 6, 15, and 24-month assessment waves) are shown in the middle column, and the outcome (smoking frequency) is on the right. Solid lines indicate significant (p<.05) paths, and dotted lines are not significant.
When nested models were run to evaluate the effect of removing the mediating pathway of alcohol related problems, model fit was found to marginally decrease (χ2=3.554, df=1, p=.059), which may suggest that this mediating pathway is potentially an important component of the path model. In testing for gender differences, the constrained model (in which male and female path coefficients were set to be equal) did not have a significantly worse fit than the unconstrained model (in which path coefficients were allowed to vary between genders). Thus, there is no significant evidence for gender differences in path coefficients within each smoking group.
Discussion
After statistical control for both smoking and drinking behavior, among experimenters, it was alcohol-related problems, rather than alcohol use, that was found to be associated with smoking frequency 48 months later, and this relationship was fully mediated through nicotine dependence symptoms. Among current smokers, only past smoking behavior was associated with smoking frequency 48-months later.
Among experimenters, the presence of alcohol related problems may serve as a signal for enhanced liability to nicotine dependence symptoms above and beyond both how much an adolescent has smoked or drank. The link between alcohol use and smoking has most often been discussed in terms of the role of each behavior in increasing risk of exposure to the other substance or as general markers of risk taking (Jessor and Jessor, 1977). In contrast, the present findings suggest that it is alcohol-related problems, rather than alcohol use per se, that forecasts the development of more regular smoking behaviors, and that this association can be partially explained by the emergence of nicotine dependence symptoms that are independent of smoking quantity and frequency.
Neurobiological changes associated with the emergence of alcohol-related problems may increase the propensity for similar changes related to nicotine dependence. For example, preclinical data suggest animals that develop tolerance to the effects of one substance may also become tolerant to the effects of the other as demonstrated with behavioral data (Funk, Marinelli, and Lee, 2006). Alternately, a third variable may be related to risk for both alcohol problems and nicotine dependence (Li, Volkow, Baler, and Egli, 2007). For example, selectively breeding mice for sensitivity to one substance results in animals with heightened sensitivity to the other substance, suggesting that shared genetic risk factors (Bien and Burge, 1990; Collins, 1990; Littleton and Little, 2002; Prendergast, Rogers, Barron, Bardo and Littleton, 2002). Notably, we did not see evidence that smoking behavior and tobacco dependence forecast intermediate alcohol problems among current smokers, an observation seemingly at odds with a simple, symmetrical third variable account. Overall, the present findings confirm that alcohol-related problems likely play a role in the experience of nicotine dependence symptoms either as a cause and/or signal of sensitivity to nicotine dependence symptoms, over and above exposure to either substance.
We have previously shown that among recent experimenters at baseline, mother’s ever-smoking is partially mediated through intermediate nicotine dependence and smoking frequency Selya, Dierker, Rose, Hedeker and Mermelstein, 2012). That is, mother’s ever-smoking was both directly associated with 48-month smoking frequency among adolescents, and was also indirectly associated with this outcome through early-emerging nicotine dependence and smoking frequency shortly following initiation. This may indicate that sensitivity to nicotine is at least partially heritable. Additionally, the current findings suggest that this possible genetic susceptibility to dependence may extend across different substances.
The current findings should be interpreted within the context of study limitations. First, the detection of significant results for the current smokers may have been limited by sample size (n = 152). The relatively small sample size also limited the complexity of the model, and prevented including additional risk factors for smoking, e.g. race/ethnicity. Given that individual from different ethnicities may disproportionately bear health consequences associated with tobacco use, more targeted selection in future studies will assist in understanding how early sensitivities may contribute. Additionally, these results are directly tied to the measurement of nicotine dependence according to the NDSS, and as such mainly reflect drive and tolerance dimensions. Furthermore, the current results may under-report risk associated with future smoking, in that adolescents who dropped out of the study had higher smoking rates at baseline. Finally, while we were able to examine an array of alcohol related problems, a more formal assessment of alcohol abuse and dependence symptoms was not available.
Despite these limitations, the current study has a number of strengths. First, this study is one of few longitudinal samples available to date that includes a large group of youth at the earliest stages of smoking exposure. Further, to our knowledge, this is the first study to longitudinally investigate the association between alcohol-related problems and nicotine dependence as one of several mediating pathways for future smoking behavior. As such, the present study adds to accumulating evidence showing substantial individual variability in nicotine dependence symptoms based on the presence or absence of alcohol-related problems, not accounted for by variability in substance use. If causally associated, these findings would suggest that treatment of alcohol-related problems may prevent or reduce the early emergence of nicotine dependence among novice smokers. If instead, alcohol related problems are a signal of sensitivity for nicotine dependence, best accounted for by a third variable, then adolescents with alcohol-related problems during their early exposures to smoking represents an important subgroup that may benefit from intervention that directly targets this association (Kalman, Kim, DiGirolamo, Smelson and Ziedonis, 2010).
The current findings suggest that individual differences in early-emerging nicotine dependence symptoms may drive smoking patterns at low levels of use, whereas for more experienced smokers, past smoking behavior is a better predictors of future behavior. Risk factors for chronic smoking are traditionally considered to function by increasing active exposure to cigarettes. In contrast, this study adds to a small but growing body of literature supporting an alternate type of risk factor: one which causes or signals future smoking, is present well before established smoking patterns emerge, and is unlinked to the level of smoking exposure (Dierker and Donny, 2008).
Acknowledgments
The research described was supported by Award Number P01CA098262 (Mermelstein) from the National Cancer Institute, by grants R01 DA022313-01A2, R01 DA022313-02S1 (Dierker) and R21DA024260 (Rose) from the National Institute on Drug Abuse, and by Center Grant P50 DA010075 awarded to Penn State University. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute, the National Institute on Drug Abuse or the National Institutes of Health.
Footnotes
Conflict of interest declaration: none
References
- Bentler PM, Bonett DG. Significance tests and goodness of fit in the analysis of covariance structures. Psychol Bull. 1980;88:588–606. [Google Scholar]
- Bien TH, Burge R. Smoking and Drinking - A literature review. Int J Addict. 1990;25:1429–1454. doi: 10.3109/10826089009056229. [DOI] [PubMed] [Google Scholar]
- Browne MW, Cudeck R. Alternative ways of assessing model fit. Sociol Method Res. 1992;21:230–258. [Google Scholar]
- Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing structural equation models. Newbury Park, CA: Sage; 1993. pp. 136–162. [Google Scholar]
- Chassin L, Rogosch F, Barrera M. Substance use and symptomatology among adolescent children of alcoholics. J Abnormal Psych. 1991;100:449–463. doi: 10.1037//0021-843x.100.4.449. [DOI] [PubMed] [Google Scholar]
- Colder CR, Chassin L. The psychosocial characteristics of alcohol users versus problem users: Data from a study of adolescents at risk. Dev Psychopathol. 1999;11:321–348. doi: 10.1017/s0954579499002084. [DOI] [PubMed] [Google Scholar]
- Collins AC. Interactions of ethanol and nicotine at the receptor level. Recent developments in alcoholism: an official publication of the American Medical Society on Alcoholism, the Research Society on Alcoholism, and the National Council on Alcoholism. 1990;8:221–231. [PubMed] [Google Scholar]
- Dierker L, Donny E. The role of psychiatric disorders in the relationship between cigarette smoking and DSM-IV nicotine dependence among young adults. Nicotine Tob Res. 2008;10:439–446. doi: 10.1080/14622200801901898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierker L, Mermelstein R. Early Emerging Nicotine-Dependence Symptoms: A Signal of Propensity for Chronic Smoking Behavior in Adolescents. J Pediat. 2010;156:818–822. doi: 10.1016/j.jpeds.2009.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierker LC, Rose JS, Donny E, Tiffany S. Alcohol use as a signal for sensitivity to nicotine dependence among recent onset smokers. Addict Behav. 2011;36:421–426. doi: 10.1016/j.addbeh.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Marinelli PW, Le AD. Biological processes underlying co-use of alcohol and nicotine: Neuronal mechanisms, crosstolerance, and genetic factors. Alcohol Res Health. 2006;29:186–192. [PMC free article] [PubMed] [Google Scholar]
- Hu L-t, Bentler PM. Fit indices in covariance structure modeling: sensitivity to underparametrized model misspecification. Psychol Methods. 1998;3(4):424–453. [Google Scholar]
- Jessor R, Jessor L. Problem Behavior and Psychosocial Development - A Longitudinal Study of Youth. New York: Academic Press; 1977. [Google Scholar]
- Kalman D, Kim S, DiGirolamo G, Smelson D, Ziedonis D. Addressing tobacco use disorder in smokers in early remission from alcohol dependence: The case for integrating smoking cessation services in substance use disorder treatment programs. Clin Psychol Rev. 2010;30:12–24. doi: 10.1016/j.cpr.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajtha A, Sershen H. Nicotine: Alcohol Reward Interactions. Neurochem Res. 2010;35:1248–1258. doi: 10.1007/s11064-010-0181-8. [DOI] [PubMed] [Google Scholar]
- Leeman RF, McKee SA, Toll BA, Krishnan-Sarin S, Cooney JL, Makuch RW, O’Malley SS. Risk factors for treatment failure in smokers: Relationship to alcohol use and to lifetime history of an alcohol use disorder. Nicotine Tob Res. 2008;1012:1793–1809. doi: 10.1080/14622200802443742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TK, Volkow ND, Baler RD, Egli M. The biological bases of nicotine and alcohol co-addiction. Biol Psychiatry. 2007;61:1–3. doi: 10.1016/j.biopsych.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Little HJ. Behavioral mechanisms underlying the link between smoking and drinking. Alcohol Res Health. 2000;24:215–224. [PMC free article] [PubMed] [Google Scholar]
- Littleton J, Little H. Interactions between alcohol and nicotine dependence: A summary of potential mechanisms and implications for treatment. Alcohol Clin Exp Res. 2002;26:1922–1924. doi: 10.1097/01.ALC.0000040941.36050.A7. [DOI] [PubMed] [Google Scholar]
- Lopez-Quintero C, de los Cobos JP, Hasin DS, Okuda M, Wang S, Grant BF, Blanco C. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: Results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Drug Alcohol Depend. 2011;115:120–130. doi: 10.1016/j.drugalcdep.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh HW, Hocevar D. Application of confirmatory factor analysis to the study of self-concept: first- and higher order factor models and their invariance across groups. Psychol Bull. 1985;97(3):562–582. [Google Scholar]
- Prendergast MA, Rogers D, Barron S, Bardo MT, Littleton JM. Ethanol and nicotine: A pharmacologic balancing act? Alcohol Clin Exp Res. 2002;26:1917–1918. doi: 10.1097/01.ALC.0000040846.27378.80. [DOI] [PubMed] [Google Scholar]
- Selya AS, Dierker LC, Rose JS, Hedeker D, Mermelstein RJ. Risk factors for adolescent smoking: parental smoking and the mediating role of nicotine dependence. Drug Alcohol Depend. 2012;124(3):311–318. doi: 10.1016/j.drugalcdep.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Waters AJ, Hickcox M. The Nicotine Dependence Syndrome Scale: A multidimensional measure of nicotine dependence. Nicotine Tob Res. 2004;6:327–348. doi: 10.1080/1462220042000202481. [DOI] [PubMed] [Google Scholar]
- Wells JE, Horwood LJ, Fergusson DM. Stability and instability in alcohol diagnosis from ages 18 to 21 and ages 21 to 25 years. Drug Alcohol Depend. 2006;81:157–165. doi: 10.1016/j.drugalcdep.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Wheaton B, Muthen B, Alwin DF, Summers GF. Assessing reliability and stability in panel models. Sociol Methodol. 1977;8:84–136. [Google Scholar]


