Abstract
Objective
Obesity, insulin resistance and diabetes disproportionately affect African American (AA) women. Abnormal adipose tissue free fatty acid (FFA) release is associated with these conditions. Resting energy expenditure (REE) and sex predict FFA release in Caucasians, but whether this is true in AA is unknown. We sought to understand the sex-specific relationships between FFA release, REE and race.
Design and Methods
100 adults (47% AA, 50% male, age 32±8y (mean ± SD)) from 3 different centers underwent duplicate measures of FFA release ([U-13C] palmitate) and REE (indirect calorimetry). Body composition was determined by DXA and abdominal imaging.
Results
AA participants had lower REE, but similar FFA concentrations and flux compared with Caucasian participants. The significant predictors of palmitate release were REE, sex, and race. REE and FFA flux were correlated in both sexes and both races. In a multiple-linear regression analysis with palmitate flux as the dependent variable and REE, sex, race, total fat mass, fat free mass, and insulin as independent variables, REE was the only independent predictor of FFA release in men. Both REE and race predicted palmitate flux in women.
Conclusions
FFA flux is related to REE, but the relationship differs in AA and Caucasian women.
Keywords: lipolysis, race, energy expenditure, obesity
Introduction
African Americans (AA) have a greater incidence and prevalence (1,2) of obesity and obesity related morbidity than Caucasians (3). Some studies indicate AA women are more insulin resistant than Caucasian women (3,4). An upper-body fat distribution, specifically with increased visceral fat, predicts a greater risk of metabolic and cardiovascular complications. However, the risk for diabetes and cardiovascular disease in AA women with upper-body obesity (UBO) is not necessarily greater than similarly obese Caucasian women (3,5). In fact, a risk factor profile without major metabolic abnormalities and that is quite similar to that of lower-body obese (LBO) AA women is reported (6,7), although this may be confounded by the lower fasting plasma triglyceride concentrations in AA women independent of insulin resistance (8,9). Whether this is related to differences in adipose tissue metabolism is not clear. Circulating free fatty acids (FFA), originating from adipose tissue lipolysis, mediate at least some of the metabolic risk related to UBO; many of the metabolic abnormalities associated with high risk obesity can be reproduced by experimentally raising plasma FFA (10,11). Moreover, the possibility that abnormal adipose tissue metabolism contributes to the racial health disparities due to obesity in AA women is supported by in vitro (12,13) and in situ (microdialysis) studies that find racial differences with respect to basal and insulin suppressed lipolysis (14,15). On the other hand, there appears to be no clear racial differences in the ability of insulin to suppress systemic lipolysis in women in vivo (8); we found no studies testing for differences between AA women and men (8,16). Hence, despite more prevalent and high risk obesity in AA women, it remains to be determined whether the characteristics of FFA metabolism are similar in AA and Caucasian men and women (17,18).
Although there are numerous approaches for analyzing FFA kinetic data (per kg body weight, per kg fat free mass, per kg fat mass), we found that postabsorptive FFA flux in Caucasian adults is most closely related to resting energy expenditure (REE). In addition, we reported that women have significantly greater FFA release relative to REE than men (19). These findings suggest that postabsorptive FFA release is responsive to lean tissue oxidative needs and that Caucasian women have greater non-oxidative FFA disposal than Caucasian men. Given that AA adults have lower REE than Caucasian adults (20,21), in these studies we examined whether the relationship between FFA release and REE is different in AA and Caucasian women and men. To address this question we measured palmitate flux and REE in AA and Caucasian women and men in duplicate after 2 weeks of the same weight maintenance diet. The primary aim was to compare by race the sex-specific relationship between palmitate flux and REE.
Methods
One hundred non-diabetic adults (47% AA, 50% male) were recruited to participate at Clinical Research Centers (CRC) at the Mayo Clinic, Rochester, Minnesota, National Institutes of Health (NIH), Bethesda, Maryland and Washington University at St. Louis, Missouri (Mayo 62%, NIH 25%, and Washington University at St. Louis 13%). The study protocol was approved by Institutional Review Boards at each institution. All volunteers gave informed consent. Participants were nonsmokers and were not taking any medication known to affect either FFA or glucose metabolism. All participants were weight stable for the previous 3 months and had a normal oral glucose tolerance test prior to entering the study. The women were premenopausal. Volunteers were recruited such that approximately one-half of the men and women were lean and one-half were obese and that a wide range of body fat distribution was included within the obese group. All volunteers had normal hematologic indices, liver and renal function tests. Results for the Caucasian participants from Mayo Clinic were previously published (19).
Protocol
For two weeks prior to the study days and for the two days of the study, participants were given weight maintenance diets (40% carbohydrate, 40% fat, 20% protein) prepared in metabolic kitchens. On day 14, the volunteers were admitted to a CRC for a three day-two night stay. The evening meal was provided at 1800 h on the day of admission and a snack, providing ~ 10% of daily energy needs, was given at 2100 h. A catheter was placed in an antecubital vein and kept patent with a 0.9% saline infusion.
On each of the two study days [U-13C]palmitate was administered in the antecubital vein as a continuous intravenous infusion from 0630h to 0730h as described previously (22). Resting energy expenditure and respiratory exchange ratio (RER) were measured each morning from 0700h to 0730h before the participants arose from bed. The metabolic carts were calibrated before the measurements. The indirect calorimeters used were: DeltaTrac Metabolic Cart: Sensormedics, Inc, Yorba Linda, CA (Mayo); TrueOne 2400, ParvoMedics, Sandy, Utah (NIH and Washington University). Blood samples were obtained from an arterialized hand vein prior to the start of the [U-13C]palmitate infusion and at 30, 40, 50 and 60 min afterwards. By collecting samples prior to the start of the tracer infusion each day we could account for any residual tracer in the plasma FFA pool (none was found). Samples were transferred to chilled tubes, placed on ice, centrifuged within 10 min, and stored at −70 °C. All samples were analyzed in the Mayo Clinic Metabolomics Core Laboratory. After the study was completed each day the volunteer was given a protocol breakfast and allowed to resume usual activities. Participants returned to the CRC at 1700h, consumed the evening meal and snack as described above and repeated the study the next morning. Because the studies at NIH and Washington University included two, sequential study days rather than the four sequential days for the Mayo studies (19), we used only the first two days of the data from the Mayo studies to assure comparable data quality from each center. In order to balance participant burden with the goal of achieving a good estimate of average palmitate flux we used our existing data from the group of volunteer who underwent 4 studies (19) to determine the added variability we would incur from performing fewer studies. We found that performing only one study would result in an average absolute difference from the mean of four studies of 19% (which was deemed unacceptable), whereas the average absolute differences for 2 and 3 studies were 9 and 4%, respectively. We therefore decided to ask the additional volunteers at NIH and Washington University to undergo only 2 studies. Sixty-two participants (12 AA) were studied at Mayo, 25 (22 AA) were studied at NIH and 13 AA were studied at Washington University.
Body composition
Total body fat and fat free mass (FFM) was measured by dual-energy x-ray absorptiometry (DXA) scanning at Mayo (Lunar Radiation Corp, Madison, WI) (23), NIH and Washington University (Hologic QDR 4500, Hologic, Inc., Bedford, MA). Intra-abdominal and subcutaneous abdominal fat masses were assessed by abdominal scans; at Mayo and NIH by a single-slice computed tomography (CT) scan of the abdomen at the L2–3 level (24), at Washington University by MRI at the L4–L5 interspace.
Analysis of samples
Insulin concentrations were measured using chemiluminescent sandwich assays (Sanofi Diagnostics Pasteur Inc., Chaska, Minnesota, USA), and catecholamines were measured using HPLC with electrochemical detection. A test of identical samples revealed that norepinephrine concentrations measured at NIH were slightly greater than at Mayo but correlated well between the two sites. Therefore, plasma norepinephrine concentrations were excluded from the multiple regression analysis. Plasma triglyceride concentrations were measured using an automated analyzer and standard enzymatic technique. All analyses of 13C enrichment of plasma palmitate and palmitate concentration were performed by LC/MS (25).
Calculations
Palmitate turnover rates were calculated from the mean enrichment values and tracer infusion rates (19,22) using steady-state formulas. The average of each individual’s flux from both study days was used for statistical analysis.
Statistical Analyses
Unless stated otherwise, data is presented as mean ± SD. Students t-tests and Pearson correlations were performed. Multiple regression analyses, both non-stepped and stepwise forward, were performed using log transformed palmitate flux as the dependent variable and race, REE, fat free mass, fat mass and insulin as independent variables. Parameter estimates were back-transformed after statistical calculations, while 95% Confidence Intervals (CI) were calculated from the log transformed standard error before back-transformation. Thus, parameter estimates and CI’s represent relative changes (ratio change) per unit absolute change in the independent parameters. Only pre-specified independent variables were entered into the analyses; a P < 0.05 was required to keep the independent variable in the mode. Because epinephrine values were available for only 77% of subjects separate regression analyses were performed for this subset.
Results
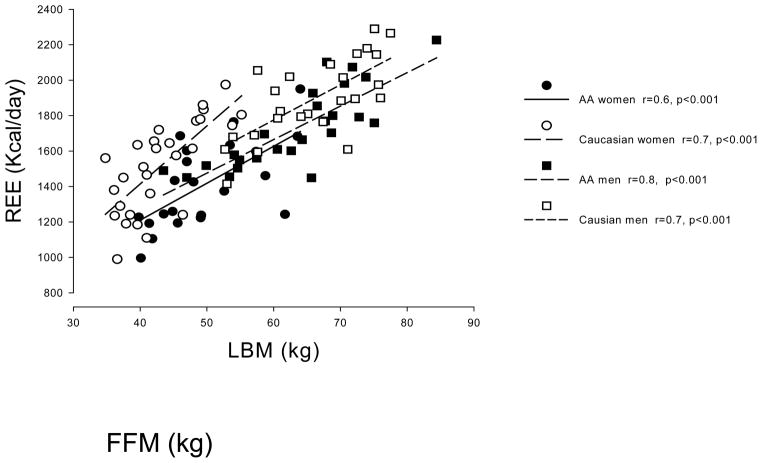
Demographic and metabolic characteristics are presented in Table 1. The expected sex differences in body composition were observed. In addition, AA women and men had lower REE and plasma triglycerides than Caucasian women and men, respectively. BMI was similar in AA and Caucasian men but greater in AA women than in Caucasian women. P values for comparisons between men and women are not provided in Table 1. For both AA and Caucasians, men had more FFM, higher REE’s, lesser percent body fat (all P < 0.001) and higher triglycerides (P < 0.05). Caucasian men had a larger visceral fat area than Caucasian women (P < 0.01), whereas AA women had greater abdominal subcutaneous fat area than AA men (P<0.05). To understand whether there were significant site differences in the indirect calorimetry and body composition measurements, we examined the correlations between REE and FFM for AA and Caucasian men and women (Figure 1). Correlations were highly significant (p<0.001) in all groups. A non-stepped multiple regression analysis with REE as the dependent variable and FFM, race, site, and sex as independent variables showed that FFM and race were significant predictors of REE, whereas site and sex were not (data not shown).
Table 1.
Demographic and Metabolic Characteristics by Race and Sex
| Variable | Caucasian Men | AA Men | P value | Caucasian Women | AA Women | P value |
|---|---|---|---|---|---|---|
| n=25 | n=25 | n=28 | n=22 | |||
| Age (years) | 30±7 | 35±6 | <0.01 | 30±8 | 34±7 | 0.08 |
| BMI (kg/m2) | 26.9±4.3 | 26.8±5.3 | 0.94 | 26.1±5.6 | 30.5±6.6 | 0.01 |
| Percent fat (%) | 22.5±7.4 | 20.2±7.2 | 0.29 | 37.1±8.8 | 37.2±9.4 | <0.001 |
| Fat mass (kg) | 20.2±9.2 | 19.3±10.1 | 0.74 | 27.5±12.6 | 32.8±13.9 | 0.002 |
| FFM (kg) | 66.1±8.0 | 63.2±9.7 | 0.27 | 43.1±5.8 | 49.7±7.5 | 0.027 |
| Visceral fat area (cm2) | 75 (27–274) | 83 (5–240) | 0.34 | 41 (21–174) | 46 (7–203) | 0.91 |
| Abdominal subcutaneous fat area (cm2) | 110 (40–404) | 88 (22–422) | 0.24 | 143 (52–527) | 223 (19–671) | 0.25 |
| RER | 0.83±0.04 | 0.83±0.04 | 0.80 | 0.82±0.04 | 0.84±0.04 | 0.047 |
| REE (kcal/day) | 1895±226 | 1726±226 | 0.01 | 1515±259 | 1413±244 | <0.001 |
| Palmitate (μmol/L) | 89±24 | 92±27 | 0.67 | 89±24 | 104±28 | 0.16 |
| Palmitate flux (μmol/min) | 89±23 | 93±30 | 0.82 | 90±28 | 83±30 | 0.21 |
| Triglycerides (mg/dL)* | 191+96 | 84+31 | <0.01 | 126+66 | 55+23 | <0.001 |
RER – respiratory exchange ratio; REE – resting energy expenditure;
Results from Mayo and NIH. The values are raw, unadjusted data. BMI was greater in AA women than all other groups, therefore, rather than performing a non-paired T tests for comparison with Caucasian women, comparisons for women were performed using multiple regression analysis with BMI and race as independent factors (BMI as continuous variable; race as categorical variable). Thus, the P values for race comparisons for women are adjusted for BMI.
Figure 1.
The relationship between fat free mass (FFM) and resting energy expenditure (REE) for African-American (AA) and Caucasian men and women are depicted. The relationships were different by race (P < 0.05), but not by sex or site.
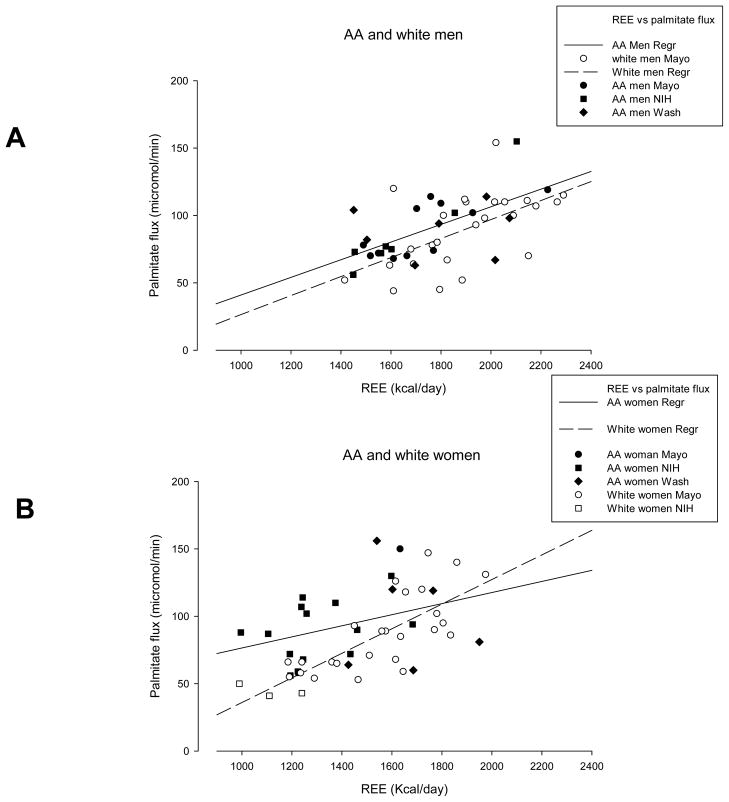
Compared with Caucasian men, AA men had similar palmitate concentrations and flux (Table 1). Similar findings were observed in Caucasian women compared with AA women. Relationships between palmitate flux and REE were significant in all groups as depicted in Figure 2. Of note, RER was similar across the range of REE in each group. We tested whether site was a factor influencing the relationship between palmitate flux and REE by performing a multiple regression analysis with palmitate flux as dependent variable and REE, site, race, and sex as independent variables. The analysis revealed that REE (p<0.001), race (p=0.002), and sex (p<0.001), but not site (p=0.17), significantly predicted palmitate flux.
Figure 2.
Panel A depicts the relationships between resting energy expenditure (REE) and palmitate flux for African-American (AA) and Caucasian men. There was a significant, positive correlation between REE and palmitate flux in both groups and the relationship was not significantly different between AA and Caucasian men. Panel B depicts the relationships between resting energy expenditure (REE) and palmitate flux for African-American (AA) and Caucasian women. There was a significant, positive correlation between REE and palmitate flux in both AA and Caucasian women and the relationship was significantly different between AA and Caucasian women.
Because we found evidence of racial differences in the relationship between REE and FFA flux, further analyses were performed. A non-stepped multiple linear regression analysis showed that REE was a significant independent predictor of palmitate flux in men, whereas race, fat free mass, fat mass (or visceral mass), and insulin concentrations did not enter into the final model (Table 2). A stepwise, forward multiple regression analysis provided results similar to those observed with the non-stepped analysis (Table 3) and a similar analysis using data from the subset of participants (n=32) in whom plasma epinephrine concentrations were available did not detect an effect of epinephrine.
Table 2.
Multiple linear regression analysis by sex and race: Dependent variable: Palmitate Flux
| Caucasian and AA Men | |||
|---|---|---|---|
| R2=0.37, adjR2=0.32 | |||
| Variable | Parameter estimate | 95% CI | P-Value |
| Race | 0.88 | 0.76–1,01 | 0.08 |
| REE | 1.0007 | 1.0002–1.0012 | 0.005 |
| Fat Free Mass | 1.00 | 0.99–1.01 | 0.94 |
| Fat Mass | 1.00 | 1.00–1.01 | 0.33 |
| Intercept | 26 | 15–45 | <0.001 |
|
| |||
| Caucasian and AA Women | |||
| R2=0.42, adjR2=0.37 | |||
| Variable | Parameter estimate | 95% CI | P-Value |
| Race | 0.85 | 0.69–1,05 | 0.13 |
| REE | 1.0007 | 1.0002–1.0011 | 0.009 |
| Fat Free Mass | 1.00 | 0,98–1.02 | 0.79 |
| Fat Mass | 1.00 | 0.99–1.01 | 0.44 |
| Intercept | 32 | 14–72 | <0,01 |
Results of a non-stepped, multiple linear regression analysis using REE, race, fat free mass, fat mass (or visceral mass), and insulin concentrations as potential independent predictors of palmitate flux; only significant predictors are show.
Table 3.
Forward stepwise multiple regression analysis by sex and race: Dependent variable: Palmitate Flux
| Caucasian and AA Men | |||
|---|---|---|---|
| R2=0.31, adjR2=0.29 | |||
| Variable | Parameter estimate | 95% CI | P-Value |
| REE | 1.0007 | 1.0004–1.0010 | <0.001 |
| Intercept | 25 | 15–43 | <0.001 |
|
| |||
| Caucasian and AA Women | |||
| R2=0.41, adjR2=0.39 | |||
| Variable | Parameter estimate | 95% CI | P-Value |
| Race | 0.80 | 0.69–0.94 | 0.008 |
| REE | 1.0008 | 1.0005–1.0011 | <0.001 |
| Intercept | 33 | 21–53 | <0.001 |
Results of a stepwise, forward multiple linear regression analysis using REE, race, fat free mass, fat mass (or visceral mass), and insulin concentrations as potential independent predictors of palmitate flux; only significant predictors are show.
Multiple-linear regression analyses similar to those used for the data from men revealed that palmitate flux in women was significantly and independently predicted by REE in the non-stepped model (Table 2). The stepwise forward regression analysis gave similar results, but revealed that race was also a significant predictor of palmitate flux (Table 3). The stepwise forward multiple regression analysis of data from the participants (n=35) in whom plasma epinephrine concentrations were available did not indicate that epinephrine was a significant predictor of palmitate flux in women.
Discussion
These studies were undertaken to determine whether the relationship between adipose tissue lipolysis and REE differs between AA and Caucasian women and men. To accomplish this we performed duplicate measurements of postabsorptive palmitate flux and REE in combination with measurements of body composition, plasma insulin and catecholamine concentrations. The novel findings from this study are: 1) FFA flux is related to REE in both AA and Caucasian men and women; 2) the relationship between FFA flux and REE is significantly different in AA and Caucasian women. Within the ranges of BMI we studied, body fat (total and visceral), FFM and fasting insulin concentrations did not relate to FFA flux when REE was taken into account. These results should help to improve the understanding of differences in metabolic risk of obese AA women compared with Caucasian women.
Our results are consistent with previous reports showing significantly lower REE (20,21) and plasma triglycerides (3,9,26) in AA women and men compared with their Caucasian counterparts. By expanding the measurements to include measures of FFA flux we were able to test one of the major functions of adipocytes – the export of lipid fuel via lipolysis. Very few studies have examined differences in systemic lipolysis between AA and Caucasians. Albu et al. (8) found similar basal and insulin suppressed FFA turnover in viscerally obese AA and Caucasian women, whereas Racette et al. (16) found lower basal and epinephrine stimulated systemic lipolysis in abdominally obese AA women compared with abdominally obese Caucasian women. Neither of these results seems to explain why we found AA women differed from Caucasian women with respect to the REE and FFA flux relationship.
African American women suffer disproportionately from obesity-associated conditions such as type 2 diabetes mellitus/insulin resistance, cardiovascular disease, and hypertension (3,7), but at a higher BMI level than Caucasian women (6). The AA women participating in this study had significantly lower REE relative to FFM, and those with the lowest REE’s had somewhat greater palmitate flux relative to REE than Caucasian women (Figure 2, panel B). To the extent that greater fasting FFA availability may contribute to metabolic dysfunction, our data seem to indicate AA women with the lowest REE’s are at higher risk. It is worth noting that despite greater palmitate flux relative to REE in some AA women, their plasma triglycerides were significantly lower than Caucasian women. We found no trend for AA women with lower REE’s to have higher plasma triglyceride concentrations (data not shown), suggesting that the statistically significant, but modest differences in FFA flux between AA and Caucasian women is not driving hepatic triglycerides synthesis and secretion in the former group.
One explanation for the differences in palmitate flux in the AA women could be that these participants were slightly underfed during the 2 week feeding period prior to the study (27). However, we find this unlikely because RER was similar over the entire range of REE and the RER was not reduced compared with that of Caucasian women. Moreover, there was no weight loss in the AA women during the 2 weeks preceding the study where meals were provided for the participants. Therefore, factors other than REE may be increasing lipolysis slightly in the AA women. Importantly, our results were not affected by omitting the subgroup of AA women with the greatest BMI from the analysis. We did not find that plasma catecholamine concentrations explained the different relationship between REE and palmitate flux in the AA and Caucasian women, but assay differences between NIH and Mayo may have made it more difficult to detect such a relationship.
The study has some limitations. First, the experiments were conducted at 3 different sites and between-site differences in some of the experimental approaches or on site measures might be confounding. We find this unlikely because participants of different sex and race - irrespective of site - fell well within the overall groups in the various regression analyses performed. In addition, the expected relationships between FFM and REE were found and site did not predict the relationships we examined in a multivariate regression analysis. Second, the study does not allow one to derive a cause-effect mechanism between REE and FFA flux. We have previously speculated on how and why REE may be so well linked to FFA flux (19), and noted that neither increasing (11) or decreasing (28) circulating FFA experimentally has effects on REE. Thus, although in AA men and Caucasian women and men REE is the factor that best relates to overnight postabsorptive FFA release from adipose tissue, we do not have additional insights or data that might explain this relationship beyond the possibility that greater REE (FFA consumption) might lower ambient FFA concentrations, calling into play the effects of FFA concentrations on lipolytic and anti-lipolytic hormones in a counter-regulatory manner.
In conclusion, our results imply significant differences in the relationship between energy needs and mobilization of FFA between Caucasian and AA women, but not men. The previously described significance of REE as an important determinant of lipolysis was confirmed and extended to include AA men and women. These findings are of importance to our understanding of abnormalities of lipid metabolism among sexes and races, especially when AA women are included.
Table 4.
Forward stepwise multiple regression analysis by sex and race: Dependent variable: Palmitate Flux (N=32 men/35 women)
| Caucasian and AA Men | |||
|---|---|---|---|
| R2=0.38, adjR2=0.36 | |||
| Variable | Parameter estimate | 95% CI | P-Value |
| REE | 1.0009 | 1.0005–1.0013 | <0.001 |
| Intercept | 16 | 8–35 | <0.001 |
|
| |||
| Caucasian and AA Women | |||
| R2=0.36, adjR2=0.34 | |||
| Variable | Parameter estimate | 95% CI | P-Value |
| REE | 1.0005 | 1.0001–1-0008 | 0.012 |
| Fat Free Mass | 1.0193 | 1.0066–1.0320 | 0.005 |
| Intercept | 17 | 10–31 | <0.001 |
Results of a stepwise, forward multiple linear regression analysis using REE, race, fat free mass, fat mass (or visceral mass), epinephrine and insulin concentrations as potential independent predictors of palmitate flux; only significant predictors are show.
Acknowledgments
We acknowledge the technical assistance of the staff of the Mayo Clinic GCRC. This work was supported by grants DK40484, DK50456 and UL1 RR024150 from the U.S. Public Health Service; by DK 37948, DK 56341, UL1 RR024992 (SK) from the U.S. Public Health Service; by grants from the Novo Nordic Foundation (to SN), and by the Mayo Foundation. Anne E. Sumner and Bernard V. Miller III were supported by the intramural program of NIDDK. Hana Turkova was supported by the intramural program of NICHD.
Footnotes
Disclosure
The authors have no conflict of interest to disclose.
Author Contributions
SN, AES, BVM3rd, SK performed studies, analyzed data, edited manuscript; MDJ designed studies, analyzed data, edited manuscript; SN performed the tedious statistical analysis; HT analyzed samples and data, edited manuscript.
References
- 1.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999–2002. Diabetes Care. 2006;29:1263–8. doi: 10.2337/dc06-0062. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 3.Dowling HJ, Pi-Sunyer FX. Race-dependent health risks of upper body obesity. Diabetes. 1993;42:537–43. [PubMed] [Google Scholar]
- 4.Chow CC, Periwal V, Csako G, et al. Higher acute insulin response to glucose may determine greater free fatty acid clearance in African-American women. J Clin Endocrinol Metab. 2011;96:2456–63. doi: 10.1210/jc.2011-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stevens J, Keil JE, Rust PF, Tyroler HA, Davis CE, Gazes PC. Body mass index and body girths as predictors of mortality in black and white women. Arch Intern Med. 1992;152:1257–62. [PubMed] [Google Scholar]
- 6.Sumner AE, Kushner H, Sherif KD, Tulenko TN, Falkner B, Marsh JB. Sex differences in African-Americans regarding sensitivity to insulin’s glucoregulatory and antilipolytic actions. Diabetes Care. 1999;22:71–7. doi: 10.2337/diacare.22.1.71. [DOI] [PubMed] [Google Scholar]
- 7.Sumner AE. The relationship of body fat to metabolic disease: influence of sex and ethnicity. Gend Med. 2008;5:361–71. doi: 10.1016/j.genm.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albu JB, Curi M, Shur M, Murphy L, Matthews DE, Pi-Sunyer FX. Systemic resistance to the antilipolytic effect of insulin in black and white women with visceral obesity. Am J Physiol. 1999;277:E551–E60. doi: 10.1152/ajpendo.1999.277.3.E551. [DOI] [PubMed] [Google Scholar]
- 9.Sumner AE, Cowie CC. Ethnic differences in the ability of triglyceride levels to identify insulin resistance. Atherosclerosis. 2008;196:696–703. doi: 10.1016/j.atherosclerosis.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 10.Ferrannini E, Barrett EJ, Bevilacqva S, DeFronzo RA. Effect of fatty acids on glucose production and utilization in man. J Clin Invest. 1983;72:1737–47. doi: 10.1172/JCI111133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boden G, Jadali F, White J, et al. Effects of fat on insulin-stimulated carbohydrate metabolism in normal men. J Clin Invest. 1991;88:960–6. doi: 10.1172/JCI115399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowling HJ, Fried SK, Pi-Sunyer FX. Insulin resistance in adipocytes of obese women: effects of body fat distribution and race. Metabolism. 1995;44:987–95. doi: 10.1016/0026-0495(95)90094-2. [DOI] [PubMed] [Google Scholar]
- 13.Johnson J, Fried SK, Pi-Sunyer FX, Albu JB. Impaired insulin action in subcutaneous adipocytes from women with visceral obesity. Am J Physiol. 2001;280:E40–E9. doi: 10.1152/ajpendo.2001.280.1.E40. [DOI] [PubMed] [Google Scholar]
- 14.van der Merwe MT, Crowther NJ, Schlaphoff GP, et al. Lactate and glycerol release from the subcutaneous adipose tissue of obese urban women from South Africa; important metabolic implications. J Clin Endocrinol Metab. 1998;83:4084–91. doi: 10.1210/jcem.83.11.5255. [DOI] [PubMed] [Google Scholar]
- 15.van der Merwe MT, Jansson PA, Crowther NJ, et al. Lactate and glycerol release from subcutaneous adipose tissue in black and white lean men. [Erratum appears in J Clin Endocrinol Metab 2000 Apr;85(4):1670–1] J Clin Endocrinol Metab. 1999;84:2888–95. doi: 10.1210/jcem.84.8.5927. [DOI] [PubMed] [Google Scholar]
- 16.Racette SB, Horowitz JF, Mittendorfer B, Klein S. Racial differences in lipid metabolism in women with abdominal obesity. Am J Physiol Regul Integr Comp Physiol. 2000;279:R944–R50. doi: 10.1152/ajpregu.2000.279.3.R944. [DOI] [PubMed] [Google Scholar]
- 17.Guo ZK, Hensrud DD, Johnson CM, Jensen MD. Regional postprandial fatty acid metabolism in different obesity phenotypes. Diabetes. 1999;48:1586–92. doi: 10.2337/diabetes.48.8.1586. [DOI] [PubMed] [Google Scholar]
- 18.Jensen MD, Haymond MW, Rizza RA, Cryer PE, Miles JM. Influence of body fat distribution on free fatty acid metabolism in obesity. J Clin Invest. 1989;83:1168–73. doi: 10.1172/JCI113997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nielsen S, Guo Z, Albu JB, Klein S, O’Brien PC, Jensen MD. Energy expenditure, sex, and endogenous fuel availability in humans. J Clin Invest. 2003;111:981–8. doi: 10.1172/JCI16253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albu J, Shur M, Curi M, Murphy L, Heymsfield SB, Pi-Sunyer F. Resting metabolic rate in obese, premenopausal black women. Am J Clin Nutr. 1997;66:531–8. doi: 10.1093/ajcn/66.3.531. [DOI] [PubMed] [Google Scholar]
- 21.Weinsier RL, Hunter GR, Zuckerman PA, et al. Energy expenditure and free-living physical activity in black and white women: comparison before and after weight loss. Am J Clin Nutr. 2000;71:1138–46. doi: 10.1093/ajcn/71.5.1138. [DOI] [PubMed] [Google Scholar]
- 22.Guo Z, Nielsen S, Burguera B, Jensen MD. Free fatty acid turnover measured using ultralow doses of [U-13C]palmitate. J Lipid Res. 1997;38:1888–95. [PubMed] [Google Scholar]
- 23.Jensen MD, Kanaley JA, Roust LR, et al. Assessment of body composition with use of dual-energy x-ray absorptiometry: evaluation and comparison with other methods. Mayo Clin Proc. 1993;68:867–73. doi: 10.1016/s0025-6196(12)60695-8. [DOI] [PubMed] [Google Scholar]
- 24.Jensen MD, Kanaley JA, Reed JE, Sheedy PF. Measurement of abdominal and visceral fat with computed tomography and dual-energy x-ray absorptiometry. Am J Clin Nutr. 1995;61:274–8. doi: 10.1093/ajcn/61.2.274. [DOI] [PubMed] [Google Scholar]
- 25.Persson X-MT, Blachnio-Zabielska AU, Jensen MD. Rapid measurement of plasma free fatty acid concentration and isotopic enrichment using LC/MS. J Lipid Res. 2010;51:2761–5. doi: 10.1194/jlr.M008011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Danadian K, Lewy V, Janosky JJ, Arslanian S. Lipolysis in African-American children: is it a metabolic risk factor predisposing to obesity? J Clin Endocrinol Metab. 2001;86:3022–6. doi: 10.1210/jcem.86.7.7626. [DOI] [PubMed] [Google Scholar]
- 27.Jensen MD, Bajnarek J, Lee SY, Nielsen S, Koutsari C. Relationship between postabsorptive respiratory exchange ratio and plasma free fatty acid concentrations. J Lipid Res. 2009;50:1863–9. doi: 10.1194/jlr.M900021-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Segerlantz M, Bramnert M, Manhem P, Laurila E, Groo LC. Inhibition of the rise in FFA by acipimox partially prevents GH-induced insulin resistance in GH-deficient adults. J Clin Endocrinol Metab. 2001;86:5813–8. doi: 10.1210/jcem.86.12.8096. [DOI] [PubMed] [Google Scholar]