Abstract
Although aberrant DNA methylation patterning is a hallmark of cancer, the relevance of targeting DNA methyltransferases (DNMT) remains unclear for most tumors. In diffuse large B-cell lymphoma (DLBCL) we observed that chemo-resistance is associated with aberrant DNA methylation programming. Prolonged exposure to low-dose DNMT inhibitors (DNMTIs) reprogrammed chemo-resistant cells to become doxorubicin sensitive without major toxicity in vivo. Nine genes were recurrently hypermethylated in chemo-resistant DLBCL. Of these, SMAD1 was a critical contributor, and reactivation was required for chemosensitization. A phase I clinical study was performed evaluating azacitidine priming followed by standard chemoimmunotherapy in high-risk newly diagnosed DLBCL patients. The combination was well tolerated and yielded a high rate of complete remission. Pre and post azacitidine treatment biopsies confirmed SMAD1 demethylation and chemosensitization, delineating a personalized strategy for the clinical use of DNMTIs.
Keywords: Non-Hodgkin lymphoma, epigenetic, DNA methylation, DNMT inhibitor, chemoresistance
Introduction
DNA methylation patterning contains epigenetic information that encodes the transcriptional programming and phenotype of normal and malignant cells(1). Aberrant DNA hypermethylation of tumor suppressor genes can result in their inappropriate transcriptional silencing and thus contribute to loss of checkpoints and other functions in cancer. For example, CpG methylation suppresses the promoter and reduces the expression of the tumor suppressor gene CDKN2A in non-Hodgkin lymphomas (NHL)(2), an event associated with more aggressive variants of the disease(3). Inactivation of tumor suppressor pathways is an important contributor to resistance to chemotherapy in cancer(4-6), in part because the activity of most chemotherapy agents depends to a great extent on the same pro-apoptotic and pro-differentiation pathways that are disabled during carcinogenesis. Inactivation of these pathways by mutations or hypermethylation can therefore affect drug sensitivity(4, 7). Gene specific and genomic alterations in DNA methylation have been described in the various subtypes of NHL(8-14). Moreover, integrated DNA methylation and gene expression profiling identified specific methylation signatures in the activated B cell (ABC) and germinal center B cell (GCB) subtypes of Diffuse Large B Cell Lymphomas (DLBCL), suggesting that these are epigenetically distinct entities(12).
CpG dinucleotides are methylated by DNA methyltransferases (DNMT)1, DNMT3A and DNMT3B. DNMT1 is predominantly involved in maintaining, whereas DNMT3A and DNMT3B primarily mediate de novo cytosine methylation. Inhibition of DNMT activity can reverse DNA methylation and gene silencing and therefore restore expression of important gene pathways(1). 5-aza-2′-deoxycytidine and azacitidine are pyrimidine nucleoside analogues of cytosine that incorporate into DNA and irreversibly inactivate DNMT by forming a covalent bond between the 5-azacytosine ring and the enzyme(15). As a consequence, DNMTs become unable to efficiently introduce methyl groups in newly synthesized DNA strands resulting in the gradual depletion of 5-methyl-cytosines from the genome as cells divide. These studies raise the possibility that DNMTIs might be useful in tumors with active DNA replication. In this regard, tumors with high proliferative ratios like DLBCL(16) might be susceptible to these agents.
DLBCL patients treated with current standard therapy, generally consisting of rituximab administered with cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP), obtain complete response rates of approximately 75% with long-term disease free survival of approximately 60%(17). The International Prognostic Index (IPI) defines risk groups based on clinical factors at presentation, including age, stage, performance status, multiple extranodal sites, and LDH (lactate dehydrogensase) level(18). Patients with multiple risk factors have a significantly poorer outcome than average. In a minority of patients whose lymphoma recurs after initial therapy, second line therapy followed by high dose chemotherapy and autologous stem cell transplant provides a second chance for cure. However, many patients will not respond to aggressive second line treatments due to refractory disease(17). In addition, a significant number of patients may have difficulty tolerating intensive second-line therapy due to age and/or comorbidities. Despite the improvements in overall survival of patients with DLBCL with the routine addition of rituximab therapy, approximately one-third of patients have disease that is either refractory or relapses after initial therapy. The fact that the majority of these patients will die within two years of diagnosis underlines the need for new therapeutic approaches in order to improve long-term outcomes.
Taking together i) the occurrence of aberrant DNA methylation patterning in DLBCL, ii) the possibility that aberrant DNA methylation might contribute to the lymphoma phenotype and repress genes that play a role in chemo-responsiveness, and iii) the high proliferative rate of DLBCL cells, which could facilitate the mechanism of action of DNMTIs; we hypothesized that DNMTIs will be therapeutically active in this disease and most importantly will mediate re-expression of genes that induce chemosensitization. In this current study we define the responsiveness of DLBCL cells to DNMTIs, demonstrate that these drugs can indeed enhance the response to chemotherapy, and identify a molecular pathway silenced through aberrant DNA methylation that contributes to this effect in both cell lines and primary human specimens. Furthermore, we demonstrate that combination treatment with the DNMTI azacitidine and standard chemoimmunotherapy is feasible, and that DNMTI therapy results in restoration of this silenced pathway and sensitization of lymphoma to chemotherapy in patients.
Results
Decitabine induces demethylation and growth suppression in a sub-set of DLBCL cells
As a single agent in humans, DAC (5-aza-2′-deoxycytidine, decitabine) is administered at a dosage up to 30 mg/m2/day that is equivalent to ~1 μM plasma peak concentration, enough to demethylate cells in susceptible tumors(15). To characterize the responsiveness of a genetically diverse set of DLBCL cells to DNMTi, a panel of 30 DLBCL cell lines was exposed to increasing concentrations of DAC. The concentration of DAC that inhibited cell line growth by 50% (GI50) within 48 hours exceeded 2 μM in all the cell lines (Fig. 1A). There were, however, eight cell lines in which the GI25 was lower than 1 μM, ranging from 100 nM in SU-DHL5 and OCI-Ly7 to 830 nM in OCI-LY19 (Fig. 1A and Table S1), we therefore consider these cell lines as in vitro sensitive to DAC. In order to determine whether differential sensitivity correlated with intracellular drug concentration, six cell lines (OCI-Ly7, DoHH2, SU-DHL6, OCI-Ly1, SU-DHL4 and OCI-Ly3) representing the spectrum of DAC sensitivity were examined for uptake and cellular retention of H3-DAC. We found no association between intracellular H3-DAC concentration at 24 h or 48 h with their GI25 for DAC (Fig. 1B). The expression of membrane transporters and nucleoside metabolic enzymes that have been suggested to influence sensitivity of other tumor types(15) were also not associated with the response to DAC (Table S1), suggesting that the difference in sensitivity may be biological in nature.
Figure 1. Anti-lymphoma activity of DAC.
A: Scatter plot of the GI50 and GI25 values of the panel of 30 DLBCL cell lines treated for 48 hours with DAC. The bottom line represents the dose that causes significant DNA damage and the upper line represents the maximum human serum concentration of DAC. B: H3-DAC uptake at 24 h and 48 h in a panel of DLBCL with dissimilar DAC GI25 values (shown between parentheses, in μM). Cells were culture with 200 nM of H3-DAC for the indicated time. Experiment represents triplicates with SEM. C: Percentage of cells positive for phospho-H2AX in OCI-L7 and SU-DHL-5 treated for 48 h with DAC 100, 200 and 500 nM. D: Effect of DAC 100 nM 48 h on 5′-methylcytosine content (in %) as determined by HPLC-MS in OCI-Ly7 cells. E: Caspases 7 and 3 activity (RLU) determined in OCI-Ly7 cells exposed to DAC 100 nM for 24h or vehicle. F: Tumor growth curve of OCI-Ly7 xenografts in SCID mice treated with vehicle (n = 7, water) or DAC 15 mg/m2 per day for 5 days (n = 5). P-value at day 10 (T test). G: Immunohistochemistry for phospho-H2AX in the lymphoma tissues of the mice from F and doxorubicin treated mice for comparison. Upper panels represent 40X microphotographs (bar = 400 μm) and lower panels represent 100X microphotographs (bar = 100 μm). The columns represent the quantification of 5 slides per mouse segregated by treatment arm and compared to vehicle-treated mice as controls.
To determine whether doses of DAC required to induce a biological response in DLBCLs might be linked to DNA damaging effects of the DNMTIs, we exposed the more sensitive DLBCL cell lines to their respective GI25 and checked for induction of H2A.X phosphorylation by flow cytometry. While DAC 100 nM, the GI25 for SU-DHL-5 and OCI-Ly7 cells, induced minimal DNA damage (~5 % of cells) (Fig. 1C), DAC 200 nM and 500 nM, the GI25 for other cell lines, caused marked DNA damage in SU-DHL-5, OCI-Ly7 (Fig. 1C), DoHH2 and SU-DHL-7 (not shown). In OCI-Ly7 cells DAC 100 nM 48 h was enough to induce biological changes since the global 5-methyl cytosine content decreased from 15% to 10.2% (Fig 1D), with a concomitant increase in the activity of caspases 7 and 3 (Fig 1E).
These data indicate that low doses of DAC can induce DNA demethylation and cell death with minimum DNA damage in susceptible DLBCL cells. We next tested the effect of DAC in vivo in SCID mice bearing human lymphoma OCI-Ly7 xenografts. Cohorts of mice were treated with vehicle (distilled water and PBS, n = 7) or DAC 15 mg/m2 (n = 5) administered five consecutive days starting on day one. Treatment was initiated when tumors reached 75 to 100 mm3. Mice were followed until untreated tumors reached 1000 mm3. This dose of DAC significantly suppressed the growth of lymphoma xenografts (p < 0.001, T-test at day 9) (Fig. 1F), causing less than 4% DNA damage (measured by phospho-H2AX immunohistochemistry) (Fig. 1G). In contrast, the administration of doxorubicin 0.6 mg/kg caused H2AX phosphorylation in about 20% of the cells (Fig. 1G). Notably, the effect of DAC was delayed until after the five-day treatment ended and peaked at days 9-12 (Fig. 1F), suggestive of an epigenetic reprogramming effect due to progressive loss of DNA methylation in DNMTi sensitive DLBCL cells similar to that observed in vitro and in patients with myeloid malignancies(19-22). Taken together these data suggest that, although demethylating doses of DAC can induce cell death in a minority of DLBCL cell lines, a bigger proportion of them are sub-lethally affected.
DAC potentiates the anti-lymphoma effect of doxorubicin in chemo-sensitive cells
To explore the possibility that DAC 100 nM would make sub-lethally affected lymphoma cells more susceptible to chemotherapy, we first exposed the same 30 cell lines to a panel of drugs with known activity in DLBCL, including doxorubicin, dexamethasone, etoposide, mechlorethamine (representing alkylating agents for in vitro use) and methotrexate. Most cell lines exhibited distinct patterns of responsiveness to DNMTIs and chemotherapy drugs (Fig. 2A). This lack of cross-resistance supports the notion of combining agents to achieve more potent anti-lymphoma activity. Doxorubicin containing regimens such as RCHOP are clinically active and routinely used for the treatment of DLBCL(23-26) and we therefore focused on pharmacodynamics of combination therapy of DNMTis with doxorubicin. Individual sensitivity to each drug is a prerequisite to define synergy(27). Sensitivity to doxorubicin was established based on clinically achievable concentrations (~200 nM) of the dose administered in the CHOP regimen (50 mg/m2)(28). Four DAC sensitive cell lines (OCI-Ly7, DoHH2, SU-DHL8 and WSU-NHL) were also sensitive to doxorubicin (Fig. 2B). Exposing these cells to DAC 100 nM and doxorubicin 200 nM resulted in synergistic cell killing in all four cell lines (Fig. 2C). Synergy was also observed for the other classes of drugs included in the CHOP regimen (i.e. vincristine, dexamethasone and mechlorethamine) (Fig. S1). Moreover, the addition of DAC to the four CHOP chemotherapy drugs together allowed for up to 5.6-fold reduction in the CHOP dose as determined by the dose-reduction index(27) (Fig. 2D). The effect of DAC and doxorubicin was independent of whether the drugs were administered sequentially or concurrently (Fig. S2A,B). Synergistic killing was at least partially due to induction of apoptosis since co-administration of the two drugs induced greater caspase -7/-3 activity (Fig. 2E) and morphological changes consistent with apoptosis (not shown). Cell cycle arrest increased with the combination only in WSU-NHL cells (Fig. S2C) and plasmacytic differentiation markers increased in all the cell lines with the combination (Fig. S2D). The efficacy of DAC and doxorubicin in combination was next tested in vivo in SCID mice bearing human lymphoma OCI-Ly7 xenografts. Cohorts of mice were treated with vehicle (distilled water and PBS, n = 7), doxorubicin 0.6 mg/kg (n = 5), DAC 15 mg/m2 (n = 5) or their combination (n = 5). Treatment was initiated when tumors reached 75 to 100 mm3. Doxorubicin was administered twice a week for two weeks (4 doses in total) and DAC was administered five consecutive days starting on day one. Mice were followed for 30 days or until tumors reached 1000 mm3, and the area under the curve for tumor growth was calculated. At day 10, while doxorubicin suppressed the growth of lymphoma xenografts (p = 0.002, T-test), DAC and the combination were significantly more effective than doxorubicin (p < 0.001, T-test) (Fig. 2F). After 20 days of follow-up it was evident that mice treated with the combination showed slower progression of tumor growth compare to DAC alone (p = 0.025, T-test) (Fig. 2F).
Figure 2. DAC synergizes with doxorubicin in sensitive cells.
A: normalized heat map representation of the GI50 or GI25 values (for DAC) of the panel of 30 DLBCL cell lines treated for 48 hours by five chemotherapy drugs with effect in DLBCL. Cells are ranked (form lowest to highest, from top to bottom) based on GI25 values for DAC. Scale is normalized for each drug. B: plot of doxorubicin log GI50 values (Y-axis) vs. DAC log GI25 values (X-axis) in a panel of 12 cell lines. The dotted green line represents 250 nM as the cutoff to define sensitivity (lower portion) vs. resistance (upper portion) to doxorubicin in cell lines. The degree of sensitivity to DAC is represented by grey scale as in A. C: isobolograms (GI90) for four DAC and doxorubicin sensitive DLBCL cell lines (SU-DHL8, DoHH2, OCI-Ly7 and WSU-NHL) tested for the combination of these drugs. Squares represent each cell line with their relative position to the additive line indicating the result from the combination. Cells below the line show synergistic combination. D: dose reduction plot for CHOP GI90(fold DAC to vehicle, x-axis) in the panel of four chemosensitive cell lines after exposure to DAC for 48 h compared to their respective vehicle treated cells. Change in GI90 is represented by fold to vehicle. The red arrow indicates the favorable dose reduction zone. Data represent mean of triplicates experiments. E: activity of caspase-7/-3 in DAC and doxorubicin sensitive DLBCL cells exposed to vehicle, doxorubicin 100 nM, DAC 100 nM or the concomitant combination of drugs for 24 h. Data is presented relative to vehicle and normalized to the number of cells. F: AUC of tumor growth curves in OCI-Ly7 xenografted mice treated with vehicle (PBS, n = 7), doxorubicin 0.6 mg/kg twice a week (n = 5), DAC 15 mg/m2 daily (n = 5) or their combination (n = 5). Treatment was initiated when tumors reached ~100 mm3, drugs were administered for 10 days and mice were further followed without treatment until the end of the experiment at day 20. The left panel represents the AUC from day 1 till day 10 with all the mice alive. P values represent the comparison of tumor AUC volumes to vehicle. The right panel represents the AUC of surviving mice from day 10 to day 20. P values represent the comparison of tumor AUC volumes. Data is presented as mean with 95% CI. G: Graphical representation of the combinatorial effect of DAC and doxorubicin in five primary DLBCL cases. CD19+ DLBCL cells were exposed to DAC, doxorubicin and the combination of drugs in two dose levels. The combinatorial effect was calculated using the fractional product method and represented in the y-axis as mean of 4 replicates with SEM.
To determine whether our results obtained in DLBCL cell lines could be extended to primary human DLBCL cells we obtained single cell suspensions from the biopsies of five confirmed unselected DLBCL patients. CD19 positive DLBCL cells were isolated and co-cultured with a feeder layer of HK dendritic cells in a dual chamber. Each primary DLBCL specimen was exposed to DAC 100 or 300 nM, doxorubicin 600 or 1200 nM, and the combination of the drugs in duplicates (along with four replicates for control and vehicle-treated cells). After 48 hours exposure, cell proliferation was determined by a metabolic assay. Consistent with the low proliferation rate (5 to 20%) and therefore low drug incorporation, there was little response of these specimens to DAC alone (Fig. S3). However doxorubicin yielded between 10 and 76%, and 7 and 96% loss of viability after 600 and 1200 nM concentrations respectively (Fig. S3). In order to calculate synergy, we used the fractional product method(29). This method is appropriate to evaluate combination of drugs with dissimilar mechanism of action (such as epigenetic and cytotoxic agents), administered in combination at pre-defined doses(29). We found that combination treatment was synergistic in 4/5 cases at 600 nM and 5/5 cases at 1200 nM of doxorubicin (Fig. 2G). Therefore, for chemo-sensitive DLBCL cells combination therapy with DAC and doxorubicin resulted in enhanced therapeutic efficacy in vitro and in vivo.
Prolonged DAC administration induces reprogramming of refractory DLBCL cells
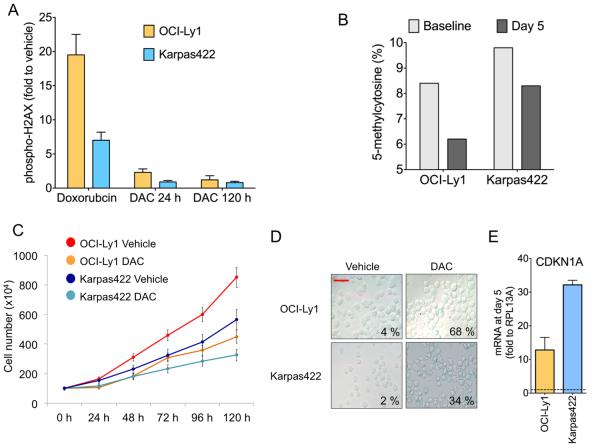
We next examined whether DNMTI could restore responsiveness to doxorubicin in chemotherapy resistant DLBCL cells. The OCI-Ly1 and Karpas422 cell lines were obtained from DLBCL patients that failed to respond to several chemotherapy regimens(30, 31), and maintained their resistant phenotype when propagated in vitro (Fig. 2B), therefore representing refractory DLBCLs. The GI50 of DAC for these chemo-resistant DLBCL cells was in the low micromolar range (Fig. 1A) known to be associated with DNA-damaging (off-target) effects(32). Accordingly, exposure of OCI-Ly1 and Karpas422 refractory DLBCL cell lines to GI50 range DAC caused evident H2AX phosphorylation similar to that induced by doxorubicin (Fig. S4A). To ascertain whether DAC induces chemo-sensitization independent of DNA damage in refractory DLBCL cell lines, we administered DNMTi at a sub-GI50 dose (i.e. 100 nM) known to cause minimal DNA damage (Fig. 1C) for five days, since epigenetic reprogramming induced by DNMTI may take a longer time to manifest(22). This exposure had little impact on H2AX phosphorylation (Fig. 3A), but did induce hypomethylation and decreased the growth rate in both cell lines (Fig. 3B and 3C), increasing doubling time from 39 h to 61 h and from 50.6 h to 67 h in OCI-Ly1 and Karpas422, respectively. These changes were accompanied by induction of senescence-associated morphological changes and β-galactosidase activity in OCI-Ly1 from 4% at baseline to 68% at day 5, and from 2% to 34% at the same time point in Karpas422 (Fig. 3D), with similar increases observed by immunoblotting (Fig. S4B). In addition, there was an increase in PARP cleavage, suggesting induction of apoptosis as well (Fig. S4B). On the other hand, there was no evidence of differentiation (measured by CD38+ and CD138+ flow cytometry) (Fig. S4C) nor cell cycle arrest (Fig. S4D). These features are consistent with the recently characterized senescence-like phenotype termed senescence with incomplete growth arrest (SWING)(33) in which cells proliferate a slower rate. Another component of the SWING phenotype is the up-regulation of CDKN1A(33), which we also found to be induced at the mRNA (Fig. 3E) and protein (Fig. S4E) levels in these cell lines treated with 100 nM of DAC for 5 days. Development of this particular phenotype is further supported by an increase in p16 (CDKN2A) (Fig. S4E). SWING is associated with increased sensitivity to doxorubicin-induced genotoxic stress(33), a feature that could be capitalized on for chemosensitization.
Figure 3. DAC induces DNA demethylation and senescence-like phenotype in chemoresistant cells.
A: quantification of H2AX phosphorylation (represented as fold compared to vehicle) for OCI-Ly1 and Karpas422 cells treated with doxorubicin 600 nM for 24 h and DAC 100 nM for 24 h and 120 h. B: global 5-methylcytosine content in OCI-Ly1 and Karpas422 cells baseline and treated with DAC 100 nM for 5 days. The y-axis represent the 5-methylcytosine content in percentage. C: cell growth plot for OCI-Ly1 and Karpas422 cells treated with vehicle (water) or DAC 100 nM for up to 120 h. The y-axis represents the number of cells (×104) and the x-axis hours of treatment. D: microphotographs of OCI-Ly1 and Karpas422 cells treated with vehicle (water) or DAC 100 nM for 5 days stained for β-galactosidase activity (bar = 25 μm). The number indicates the mean of positive (senescent) cells over total cells in five fields. E: transcript abundance (fold to vehicle, y axis) of CDKN1A in Karpas422 and OCI-Ly1 cells treated with 100 nM of DAC for 5 days. Data represent the mean of experimental triplicates with 95% CI.
DAC enables refractory DLBCL cells to regain chemosensitivity
OCI-Ly1 and Karpas422 cells tolerate doxorubicin induced DNA damage (H2AX phosphorylation) (Fig. 3A) with relatively little cell death (18% and 26% respectively). Accordingly, induction of caspase-7 and -3 activity was only observed at doxorubicin doses ≥ 600 nM in OCI-Ly1 cells and ≥ 300 nM in Karpas422 cells (Fig. 4A), which exceed the serum concentration commonly achieved in the CHOP regimen (i.e. ~200 nM)(28). In contrast, pre-treatment of these cells with the five day 100 nM dosing schedule of DAC induced caspase-7 and -3 cleavage even with a concentration as low as 37.5 nM of doxorubicin (Fig. 4A) and decreased the doxorubicin GI50 by 5.3 and 15-fold in OCI-Ly1 and Karpas422 respectively (Fig. 4B). Chemo-sensitization was likewise observed with azacitidine (not shown). A similar effect was observed for the two other doxorubicin resistant cell lines SC1 and RL (Fig. 4B). In order to determine whether DNMTi enhance the effect of doxorubicin in a resistant DLBCL cell line in vivo, we treated OCI-Ly1 cell xenografted mice (n = 20) with the same schedule and dose employed for the more sensitive OCI-Ly7 tumors (as in Fig 2F). In this case, concurrent DAC and doxorubicin failed to achieve significant effect compared to each drug alone (Fig. S5A), suggesting that epigenetic reprogramming drugs may also require time to sensitize tumors to DNA damaging agents in vivo. A second cohort of OCI-Ly1 xenografted SCID mice (n = 31) was therefore treated with a sequential schedule. Once tumors reached 75 to 100 mm3, mice were randomized to 3 groups and treated with vehicle (n = 8, PBS), doxorubicin 0.6 mg/kg (n = 8) and DAC 15 mg/m2 (n = 15). After 10 days, the 15 DAC-treated mice were again randomized to vehicle (n = 8, PBS) or doxorubicin (n = 7). During the 20 day period, animals in the doxorubicin only arm were administered the drug twice a week for a total of 5 doses, DAC alone was administered daily for 10 days, and the combination consisted in 10 consecutives days of DAC alone followed by 2 doses of doxorubicin. In this case, tumor growth was indeed more potently suppressed by the DAC-doxo combination compared to each drug alone (p = 0.008 for DAC and p < 0.001 for doxorubicin) (Fig. 4C). This regimen caused no increased in DNA damage (measured by phosphorylation of H2AX) in tumors treated with DAC as compared to vehicle alone (Fig. 4D) but increased the DNA damage caused by doxorubicin alone (Fig. 4D). There was no evidence of toxicity to normal tissues based on histological examination (not shown) suggesting that the combination can be safely administered. To further explore toxicity, we administered the same regimen to immunocompetent C57BL/6 mice (n = 5 combination and 5 vehicle control). We again could not detect any evidence of organ damage by tissue macroscopic examination and microscopic analysis including bone marrow, liver and heart (Fig. S5B). No animals died. One mouse had a decrease in total white blood cells counts without evidence of bone marrow damage, and all mice presented elevation of total bilirubin levels without evidence of liver toxicity by microscopic examination (Fig. S5B,C). We next obtained single cell suspensions from the biopsies of four clinically documented relapsed and chemo-resistant DLBCL cases. CD19 isolated cells were sequentially exposed to DAC 100 nM or vehicle for 48 h, followed by either doxorubicin 600 nM, doxorubicin 1200 nM or vehicle for an additional 48 h. Notably, all cases were resistant to doxorubicin 600 nM and two cases were also resistant to doxorubicin 1200 nM (Fig. 4E). However, after DAC 100 nM all the samples were more sensitive to doxorubicin 600 nM and three samples were more sensitive to doxorubicin 1200 nM (Fig. 4E). Therefore, DNMTi can safely overcome chemotherapy resistance in vitro and in vivo, and in cell lines and primary DLBCLs and may represent a potential therapeutic strategy to improve outcome for patients with high-risk disease.
Figure 4. Five day exposure to DAC sensitizes cells to doxorubicin.
A: activity of caspase-7/3 (y axis, fold to control) in OCI-Ly1 and Karpas422 cells pre-exposed to vehicle or DAC 100 nM for 5 days and treated with water (UT) or five concentrations of doxorubicin. B: dose reduction plot for doxorubicin GI50 (fold DAC to vehicle, x-axis) in the panel of eight doxorubicin resistant cell lines after exposure to DAC for 5 days compared to their respective vehicle treated cells. Change in GI50 is represented by fold to vehicle. The red arrow indicates the favorable dose reduction zone. Data represent mean of triplicates experiments. C: AUC of tumor growth curves in OCI-Ly1 xenografted mice treated with vehicle (PBS, n = 8), doxorubicin 0.6 mg/kg twice a week (n = 8), DAC 15 mg/m2 daily (n = 8) or their sequential combination (n = 7). Treatment was initiated when tumors reached ~100 mm3, drugs were administered sequentially during the first 10 days and mice were further followed without treatment until the end of the experiment at day 20. P values represent the comparison of tumor AUC volumes to vehicle and between DAC and the combination. D: Immunohistochemistry for phospho-H2AX in the lymphoma tissues of the mice from C. Left panels represent 40× photographs (bar = 400 μm) and right panels represent 100× photographs (bar = 100 μm). Quantification of positive cells (in %) is shown as inserts. E: CD19+ single-cell suspensions from lymph node biopsies of four refractory DLBCL specimens were exposed to DAC 100 nM for 48 h followed by either doxorubicin 0.6 μM, doxorubicin 1.2 μM or vehicle for additional 48 h. Cell viability at 96 h (normalized to 48 h of vehicle or DAC-treated cells) is shown on the y-axis. The experiment was carried out in duplicates.
SMAD1 methylation associates with chemoresistant phenotypes in DLBCL
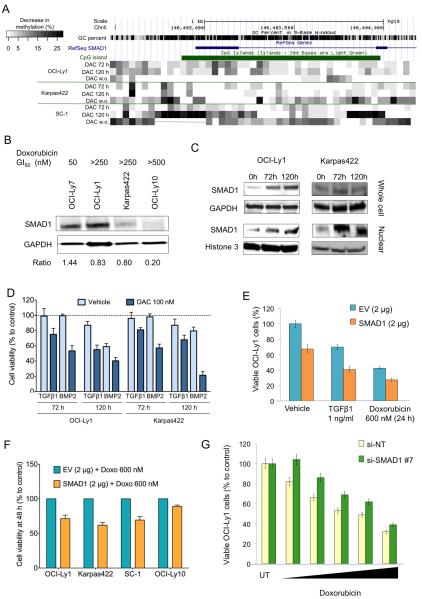
In order to explore the nature of the epigenetically encoded chemoresistance in DLBCL cells we first measured intracellular accumulation of anthracycline in our eight doxorubicin resistant cell lines. All the chemoresistant cell lines accumulated intracellular anthracycline to levels similar to doxorubicin-sensitive cells (Fig. S6) which was approximately one order of magnitude higher than cells engineered to express a MDR phenotype (Fig. S6). We hence postulated that chemo-resistance might be epigenetically mediated. To identify candidate epigenetically silenced genes that might contribute to doxorubicin resistance, we performed gene expression and DNA methylation profiling (using the HELP assay) of a panel of eight doxorubicin-resistant vs. six doxorubicin-sensitive cells (from Fig. 2A and Table S1). Nine unique genes were found to be both reproducibly and specifically hypermethylated and repressed in resistant cell lines (fold difference >1.5, representing ~20% difference in methylation(12)) (Fig 5A and Table S2). To determine whether these genes were in fact silenced by DNA methylation, the chemoresistant cell lines OCI-Ly1 and Karpas422 were treated with DAC 100 nM for 5 days and transcript abundance measured by QPCR. GAPDH and HPRT were used as controls (non differentially methylated or expressed). Among the nine genes, we found that VAV3, ETV6 and SMAD1 where the most upregulated, with >5 fold induction (Fig. 5B). SMAD family proteins mediate the actions of TGFB in suppressing lymphocyte proliferation and inducing differentiation and apoptosis(34). SMAD1/5/8 also mediates chemotherapy-induced senescence in cancer cells(35, 36) a phenotype that we observed in OCI-Ly1 and Karpas422 cells treated with low-dose of DAC (Fig. 3D and S4). Quantitative DNA methylation analysis (Sequenom Epityping) of the SMAD1 locus confirmed the increased CpG methylation status of this locus in doxorubicin-resistant cell lines OCI-Ly1, Karpas422 and OCI-Ly10 vs. chemo-sensitive OCI-Ly7 cells (Fig. 5C). To determine whether this association was also present in patients, we analyzed SMAD1 methylation status in 22 primary DLBCL cases, of which 11 were refractory and 11 sensitive to R-CHOP. We found an increase in CpG methylation of SMAD1 in the refractory vs. sensitive cases (Fig. 5D, p = 0.016, Mann-Whitney test). To determine the prevalence of SMAD1 methylation in DLBCL patients, we performed HELP assays in a cohort of 231 unselected cases. We found that 75% of DLBCL patients show 5% or less SMAD1 methylation (Fig. 5E). Reciprocally, 70% of the cases from a cohort of 248 DLBCL patients, showed phospho-SMAD1 expression in 20% or more cells by immunohistochemistry using tissue microarrays (Fig. S7). These proportions are overall consistent with the frequency of patients who initially respond to chemotherapy(17). To further determine the association of SMAD1 with chemoresistance phenotype, we analyzed SMAD1 methylation and expression according to DLBCL cell of origin status (i.e. GCB vs. ABC type, the latter of which are more chemotherapy resistant). The databases included (i) a publicly available DNA methylation profiling dataset consisting of 57 newly diagnosed DLBCLs (40 GCB and 17 ABC) treated with R-CHOP(13); and (ii) two publicly available DLBCL gene expression microarray datasets including 201 DLBCLs (108 GCB and 93 ABC)(37) and 119 DLBCL (85 GCB and 34 ABC)(38), respectively. We observed that SMAD1 was more frequently highly methylated in ABC vs. GCB DLBCLs (p = 0.018, t-test) (Fig. 5F) and was also expressed at lower levels in ABC vs. GCB cases (7.3 ± 0.8 vs. 8.4 ± 1 respectively, p = 2.24 × 10−5 and 5.1 ± 0.14 vs. 5.8 ± 0.14, respectively, p = 0.001, t-tests) (Fig. 5G). These data suggest that in DLBCL patients, SMAD1 hypermethylation and lower expression are associated with a more chemoresistant phenotype.
Figure 5. SMAD1 is methylated in DLBCLs with chemoresistant phenotype.
A: Intersection plot showing nine common hypermethylated (HELP > 1.5) and low abundance (GE < 1.5) genes from a panel of doxorubicin resistant DLBCL cell lines. B: transcript abundance (fold to vehicle, y axis) of nine hypermethylated and low abundant genes in Karpas422 and OCI-Ly1 cells treated with 100 nM of DAC for 72 h and 120 h. HPRT and GAPDH are negative controls. C: representation of SMAD1 gene methylation in DLBCL cell lines with distinct sensitivity to doxorubicin (more sensitive on top, more resistant on bottom). Data is presented as percent of methylation (0 to 100%) of a gene region accordingly to the RefSeq representation. D: representation of SMAD1 gene methylation in DLBCL patient specimens with distinct sensitivity to RCHOP (11 responsives vs. 11 refractories). Data is presented as percent of methylation (median with IQR) for each group. E: SMAD1 methylation determined by HELP assay in 231 DLBCL patients. Bars represent the frequency of patients per SMAD1 methylation category. Number of patients per category is shown on top. F: graphical representation of SMAD1 methylation (HpaII/MspI ratio, y axis) of 17 ABC DLBCL cases vs. 40 GCB DLBCL cases. The red line represents the mean. P values for the comparison between ABC and GCB cases are shown on top. G: gene expression probeset intensity for SMAD1 between 93 ABC DLBCL cases and 108 GCB DLBCL cases (left) and between 34 ABC DLBCL cases and 85 GCB DLBCL cases (right). The red line represents the mean. P values for the comparison between ABC and GCB cases are shown on top.
Reactivation of SMAD1 contributes to chemosensitization of DLBCL cells
We next wished to determine whether SMAD1 reactivation contributes to chemosenstization induced by DAC. Exposure of OCI-Ly1 and Karpas422 cells to DAC 100 nM for up to 120 h induced 5% to 18% reduction of SMAD1 methylation (Fig. 6A) for most CpGs, concordant with the increase in SMAD1 transcript abundance. A similar SMAD1 demethylation effect was detected in SC-1 (Fig. 6A). The area more susceptible to demethylation included an upstream CpG shore(39, 40) of 500 bp and a CpG island compassing from SMAD1 promoter region to exon 2 (Fig. 6A). To determine the stability of the effect of DAC on SMAD1 demethylation, DLBCL cells were treated as before (DAC 100 nM for 5 days) followed by a DAC washout period of 5 days and analyzed for SMAD1 methylation. During the washout period, cells continued to proliferate at much lower rate than untreated cells, increasing doubling time from 39 h (untreated) to 58 h and from 50.6 h (untreated) to 77 h in OCI-Ly1 and Karpas422, respectively. After drug washout, SMAD1 demethylation was sustained or even increased in Karpas422 and SC-1, but not in OCI-Ly1 cells (Fig. 6A). These results suggest that DNMTI effects can be sustained even beyond the period of exposure to these drugs. We next analyzed SMAD1 protein levels in response to DNMTi. We observed an association between SMAD1 protein abundance and doxorubicin sensitivity (Fig. 6B) and a concurrent increase in nuclear SMAD1 protein abundance in Karpas422 and OCI-Ly1 cells exposed to 100 nM DAC (Fig. 6C). In DLBCL and FL cells, SMAD1, which is normally associated with BMP signaling, is aberrantly activated following TGFβ stimulation(41). Loss of TGFβ antiproliferative response, normally occurring in T and B lymphocytes(42, 43), has been reported in NHLs, specifically in T cell lymphomas(44, 45) and Small Lymphocytic Lymphoma/Chronic Lymphocytic Leukemias(42). OCI-Ly1 and Karpas422 grown in serum-depleted medium showed a modest response to the inhibitory effect of BMP2 and TGFβ1. However, exposure to 100 nM of DAC for up to 5 days sensitized these cells, in a time-dependent manner, to the inhibitory effect of these ligands by ~20 to 35% in Karpas422 and ~20 to 60% in OCI-Ly1 cells compared to vehicle (Fig. 6D and Fig. S8A). In addition, OCI-Ly1 cells engineered to overexpress SMAD1 to levels similar to those induced by DNMTI, displayed reduced proliferation in a dose-dependent manner (Fig S8B). After four days of transfection with SMAD1, OCI-Ly1 cells decreased their proliferation rate by 2.6 times (Fig. S8C). Moreover, overexpression of exogenous SMAD1 in these refractory cells (OCI-Ly1) rendered them more susceptible to the inhibitory effects of TGFβ1 and doxorubicin (Fig. 6E). A similar effect was found in Karpas422 and SC-1 cells and with lower magnitude in OCI-Ly10 cells (Fig. 6F). Conversely, SMAD1 knockdown further increased the resistance to doxorubicin in OCI-Ly1 cells (Fig. 6G and S9A) and, to lesser extend, in Karpas422 (Fig. S9B). Taken together, these results suggest that epigenetic silencing of SMAD1 confers a growth advantage and contributes to chemoresistance of DLBCL cells.
Figure 6. Reactivation of epigenetically silenced SMAD1 contributes to chemosensitization.
A: representation of the change in the methylation (in %) of SMAD1 in chemoresistant OCI-Ly1, Karpas422 and SC-1 cells after treatment with DAC for 72 h, 120 h and 120 h followed by a washout period of 120 h (w.o.) compared to baseline methylation levels. B: immunoblot for SMAD1 (and GAPDH as control) for cells with dissimilar GI50 for doxorubicin (shown on top). The ratio for the density of SMAD1 over GAPDH is shown at the bottom. C: immunoblot for SMAD1 in OCI-Ly1 and Karpas422 treated with vehicle (0 h) and DAC for 72 h and 120 h. Whole cell lysate and nuclear fraction are shown on top and bottom, respectively. GAPDH for whole cell lysates and histone 3 for nuclear extracts were used as controls. D: Cell viability of OCI-Ly1 and Karpas422 cells treated with vehicle or DAC 100 nM for 72 and 120 h and treated with TFGB1 and BMP2 for additional 24 h. Data represents experimental triplicates. E: Differential viability of OCI-Ly1 cells transfected with SMAD1 or empty-vector (EV) plasmids and treated with water (vehicle), TGFB1 or doxorubicin 600 nM for 24 h. Experiment represent the mean of triplicates with SEM. F: Cell viability determined in OCI-Ly1, Karpas422, SC-1 and OCI-Ly10 cells transfected with SMAD1 2 μg or empty vector 2 μg (EV) for 24 h and subsequently treated with doxorubicin 600 nM or vehicle for additional 24 h. Results are relative to EV + doxorubicin 600 nM set as 100% viability. G: Viability of OCI-Ly1 after 48 h of transfection with si-NT or si-SMAD1 and treated with vehicle (UT) or several concentrations doxorubicin for 24 h (10, 30, 125, 250 and 500 nM)
Combination of DNMTI with R-CHOP is feasible in newly diagnosed high-risk DLBCL patients
Based on our pre-clinical results, we initiated a phase I clinical trial in patients with newly diagnosed DLBCL in order to test the feasibility, efficacy, and biological effects of 5 day exposure to DNMTI (in this case azacitidine) administered prior to R-CHOP. Azacitidine was administered subcutaneously at escalating doses (25 mg/m2, 50 mg/m2, or 75 mg/m2 daily for 5 days), followed by standard R-CHOP chemotherapy administered on day 8, with cycles repeated every 21 days for 6 cycles. We used a modified Continuous Reassessment Model to determine dose escalation. Enrollment has been completed, with twelve patients treated (Fig. 7A). Eleven of twelve patients were of high intermediate or high risk by the IPI, and one patient of low intermediate risk. Median age was 65 years (range 43-83 years). All but one patient was over age 60. The combination therapy was well tolerated. Two of 10 patients treated at the highest dose level experienced dose limiting toxicity (DLT): one developed reactivation of hepatitis C, and the other experienced prolonged neutropenia, which subsequently resolved. All patients experienced transient grade 3 or 4 neutropenia, and four patients developed grade 3 neutropenic fever. Six patients underwent modification of planned therapy. In addition to the two patients developing DLT, who discontinued azacitidine at the time of these events, one patient refused the last cycle of R-CHOP, another refused the last cycle of azacitidine, one patient discontinued azacitidine (but completed planned R-CHOP) after experiencing a gastrointestinal hemorrhage related to lymphoma response in a large gastric mass, and one patient had a 3-day dose delay due to grade 2 thrombocytopenia on the first day of cycle 3 (Fig. 7A). Eleven of 12 patients have achieved a complete response (Fig. 7A,B), and 10 remain in remission at median follow up of 13 months (range 5 to 28 months). One of the patients who relapsed had discontinued both azacitidine and rituximab after 2 treatment cycles due to hepatitis C reactivation.
Figure 7. DLBCL patients treated with azacitidine show SMAD1 demethylation and chemosensitization.
A: Demographics, toxicity and response of patients enrolled in the Phase I trial of azacitidine priming followed by R-CHOP. IPI: International Prognostic Index, DLT: dose limiting toxicity, CR: complete response, PR: partial response, PD: progressive disease, PFS: progression-free survival (in months). B: Pre and post cycle 5 or 6 treatment PET-CT scan images from two patients that achieved complete response. In the upper panel the patient had a large hypermetabolic lymphoma mass affecting the right lung associated with malignant pleural effusion. In the lower panel the patient had a large hypermetabolic lymphoma mass in the mediastinal region. C: global 5-methylcytosine content (in percentage, y axis) of CD19+ cells in a patient (Pt. 1) treated with azacitidine for five days compared to the pre-treatment CD19+ cells. Pt. 1 is the same patient in all the panels. D: representation of the change in the methylation (in %) of SMAD1 (as determined by MassArray) in six patients treated as in B. E: SMAD1 transcript abundance in cells from Pt. 1 and Pt. 2 before and after 5 days of treatment with azacitidine (biopsy taken at day 7). Data is normalized to RPL13A and represented as fold to pre-treatment. F: phospho-SMAD1 expression in lymphoma tissues from Pt. 3 and Pt. 4 before and after 5 days of treatment with azacitidine (bar = 100 μm). G: the CD19+ cells obtained pre and post-azacitidine from the Pt. 1 were exposed ex vivo to doxorubicin 100 nM, rituximab 80 μg/mL and CHOP (combination of mechlorethamine, doxorubicin, dexamethasone and vincristine) for 48 h and analyzed for viability. Cell viability (represented as percentage of vehicle-treated cells) is shown on the y-axis. The experiment was carried out in triplicates.
We obtained CD19 purified DLBCL cells from the serial biopsy specimens of patients who consented to this procedure immediately before the first treatment and after the fifth day of treatment with azacitidine. Patient 1 yielded sufficient cells to perform global cytosine methylation analysis by LC-MS-MS at both time points and exhibited decrease in global cytosine methylation of a magnitude comparable to that shown for cell lines treated with DAC in vitro (Fig. 7C, compare to Fig. 1D). In samples from six patients, we were able to measure SMAD1 methylation by Sequenom Epityping. Compared to the respective pre-treatment time-points, we observed a decrease in SMAD1 methylation after treatment with azacitidine in all patients (Fig. 7D). For some patients, enough material was available to determine SMAD1 expression either by QPCR or immunohistochemistry. These studies yielded 15-fold and 1.5-fold increase in SMAD1 mRNA abundance (Fig. 7E) and increase in phospho-SMAD1 protein expression (Fig. 7F) after azacitidine respectively. Moreover, the greater yield of cells obtained from patient 1 allowed us to determine whether the administration of azacitidine rendered the tumor more sensitive to chemotherapy. Purified DLBCL tumor cells obtained from the patient pre- and post-azacitidine treatment were exposed ex vivo to doxorubicin, rituximab and CHOP-like combination chemotherapy and cell viability was determined after 48 h of exposure. We observed a large increase in the fraction of dead cells in the post-azacitidine sample compared to the pre-azacitidine sample (Fig. 7G). The chemosensitization effect was particularly evident for doxorubicin, where the excess of killing in the post-treatment sample was 55% for 100 nM of doxorubicin (p<0.01, paired T-test) (Fig. 7G). Taken together, our results show that sequential DNMTI followed by R-CHOP can be safely administered to newly diagnosed older patients with high-risk disease, resulting in high rates of complete clinical response and confirm the chemosensitization effect identified in pre-clinical studies associated with reactivation of SMAD1.
Discussion
One major barrier to the eradication of DLBCL with anthracycline-based chemotherapy is the ability of certain lymphomas to exhibit resistance to these drugs. Anthracycline resistance may be intrinsic or acquired and may occur through multiple mechanisms. These mechanisms may be classified in two major groups, (i) decreased drug accumulation and/or increased drug inactivation, for example via upregulation of the MDR/TAP (multidrug resistance/antigen peptide transporter) genes; this mechanism has been characterized in cell and animal models but its relevance to clinical resistance remains unclear in most tumor types(46-49); and (ii) biological mechanisms that prevent cell death from occurring after anthracycline-induced damage. Accumulating evidence indicates that the capacity of most cancers to resist the cytotoxic effects of chemotherapy is more closely connected to genetic and epigenetic abnormalities that affect critical cell cycle checkpoint and cell death pathways than to specific mechanisms of resistance unique to each agent(50, 51). Restoring non-functional pathways in certain tumor cells may increase their susceptibility to cancer chemotherapy more efficiently than improving drug-target interactions. For example, abrogation of the function of the tumor suppressor TP53 may compromise the capacity of lymphoma cells to induce cell cycle arrest and/or to trigger apoptosis in response to DNA damaging agents(6, 52, 53). Along these lines, herein we show that the TGFβ pathway transducer SMAD1 is silenced through aberrant DNA methylation, and that this silencing contributes to chemotherapy resistance in DLBCL.
TGFβ belongs to a superfamily of polypeptide growth factors that control the growth, proliferation, differentiation and apoptosis of normal and malignant cells of multiple lineages(34). Normal and neoplastic B cells have receptors for TGFβ and can synthesize and secrete several of these ligands(34, 54). The cellular actions of the various TGFβ receptors are mediated through SMAD proteins, among which SMAD1 and SMAD5 are preferentially involved in the BMP-dependent pathways, while SMAD2 and SMAD3 are involved in the TGFB pathway(54). However, non-canonical activation can lead to the formation of mixed complexes(34, 54, 55). With the exception of certain EBV-positive B cell lymphomas, the loss of the TGFB (and BMP) antiproliferative response is a hallmark in NHLs(41, 54). Although genetic mutations in SMADs are rare in hematological malignancies, a recent publication suggested that SMAD5 could be silenced through miR-155 in DLBCLs(41). Our results indicate that in DLBCL, the other member of the complex, SMAD1, could be epigenetically silenced through aberrant DNA methylation. BMP6, another component of the TGFβ SMAD1/5/8 pathway, was also found to be hypermethylated in DLBCL patients(8). Other transcriptional mechanisms may impair TGFβ signaling in DLBCL; for example, the transcriptional repressor BCL6 was found to disrupt the SMAD4/p300 interaction and repress SMAD4 in Burkitt Lymphoma(56). DNA methylation-mediated repression of SMAD1 is functionally relevant to chemoresistance, since treatment of DLBCL cells with low-dose long-term DAC de-repressed SMAD1 and restored responsiveness to the growth inhibitory effect of TGFB signaling and chemotherapy agents. Moreover, a similar effect was induced by ectopically expressing SMAD1 in these cells. Conversely, downregulation of SMAD1 was sufficient to partially rescue DLBCL cells from DAC-induced anti-lymphoma effects.
We noted that low dose DAC induced a senescence-like phenotype in DLBCL cells. Stress-induced and replicative senescence constitute strong antiproliferative responses to protect normal cells from oncogenic events(57). Senescent cells are physiologically characterized by terminal growth arrest and exhibit particular morphological and biochemical features(57). Some of these features, such as expression of SA-β-gal, are also induced upon chemotherapy treatment of cancer cells. The role of senescence in the response to chemotherapy treatment is controversial, since these cells are terminally arrested (not proliferating) making them apparently less susceptible to the effect of classical chemotherapy drugs that target proliferating cells(57). However, several pre-clinical studies showed that tumor cells with a senescence phenotype are more susceptible to genotoxic agents(57-60). This may be due to changes in death threshold associated with defective DNA damage response and possibly other effects in senescent cells(57). However, it is also possible that cancer cells activate a different senescence-like program than normal cells. Along these lines, recent studies suggest that activation of oncogenes in tumor cells, which like DLBCL cells express endogenous telomerase activity(61) and commonly lack critical regulators of cell cycle progression, may be sufficient to trigger morphological and biochemical manifestations of senescence but cannot induce full growth arrest(33). When this senescence-like program is activated, cells have impaired DNA damage response making them more susceptible to genotoxic stresses induced by doxorubicin and other compounds(33). We find that this program, named senescence with incomplete growth arrest (SWING)(33), is induced in DLBCL cells by low-doses of DAC, and that these cells exhibited more pronounced DNA damage and cell death when subsequently exposed to chemotherapy treatments. The suppression of the DNA damage response in the SWING phenotype is critically dependent on the CDNK1A pathway(33). In this regard, it is possible that up-regulation of CDNK1A upon induction of the SWING phenotype in DLBCL cells depends on the function of SMAD1, since CDKN1A is a target gene of the BMP/SMAD pathway(62).
Despite their efficacy in myelodysplasia(63), the need for optimal dose and schedule as well as pharmacodynamic markers has been a major challenge for clinical translation of DNMTIs for DLBCL patients. Moreover, a phase I trial of DNMTIs for lymphoma patients following a classical approach for anticancer agents, i.e. the use of maximal tolerated dose, showed a low therapeutic index for these drugs(64). We pursued an alternative approach in our clinical trial: that of using DNMTI therapy to “prime” DLBCL prior to administration of chemotherapy. We hypothesized that pretreatment with the DNMTI would result in demethylation and re-expression of genes that play a role in modulating chemotherapy sensitivity in the lymphoma cells. We found that the combination was tolerable, even in older patients, with manageable toxicity. Furthermore, preliminary efficacy results were promising in this high-risk population.
Our results suggest a more rational way to incorporate these drugs for clinical use based on their pharmacodynamics in DLBCL cells. Specifically we note that (i) in agreement with a recent publication(22), doses of DNMTIs that induce DNA demethylation are about ten orders of magnitude lower than those causing significant DNA damage, (ii) gene demethylation followed by phenotypic changes (re-programming) are critical to chemosensitization, and (iii) marked ex vivo chemosensitization is accompanied by demethylation and expression of specific genes but modest global hypomethylation, suggesting that reactivation of specific pathways (i.e. TGFB) may be more relevant for the final outcome.
DNA replication and proliferation can also influence the final effect of DNMTIs. The high proliferative rate of DLBCLs offers the opportunity for DNMTis to readily incorporate into the DNA of these tumor cells and block DNMT activity. Most DLBCL cell lines have doubling times of between 18 and 36 hours, and hence DNMTIs are able to induce chemo-sensitization in vitro and in vivo. DLBCLs in humans are also highly proliferative and we accordingly observed a similar chemo-sensitization effect when the drugs were administered to patients. Ex vivo, primary human DLBCLs proliferate much less, and so the effect of DAC was less pronounced. As clinical trials of low dose DNMTIs proceed, it will be important for correlative studies to carefully examine the association between tumor proliferative rates with the level of chemo-sensitization induced by these drugs. Equally important to the rational deployment of DNMTIs to the clinic is the identification of predictive markers that can guide the selection of these drugs for patients most likely to respond. In this regard, we found SMAD1 hypermethylation in lymphoma patients to be associated with more chemoresistant subtypes of DLBCLs (ABC-DLBCLs) and poorer overall survival. Whether SMAD1 methylation status will help to predict response to DNMTIs, R-CHOP or other treatments will require adequately powered prospective clinical trials. The research presented herein provides a mechanism-based rationale for the study of DNMTI in DLBCLs, a biological explanation (SMAD1 hypermethylation and silencing) for chemotherapy resistance and poor outcome in DLBCL, and early-phase clinical therapeutic findings that will guide the design of larger scale clinical trials exploring this strategy to overcome chemotherapy resistance and potentially improve outcomes for patients with this disease.
Methods
Cell lines
DLBCL cell lines OCI-Ly1, OCI-Ly4, OCI-Ly10 and OCI-Ly7 were grown in medium containing 90% Iscove’s and 10% FCS (20% FCS for OCI-Ly10) and supplemented with penicillin G and streptomycin. DLBCL cell lines DB, DoHH2, Farage, HT, Karpas422, Karpas231, NU-DUL-1, OCI-Ly3, OCI-Ly8, OCI-Ly18, OCI-Ly19, OZ, RL, RC-K8, RI-1, SU-DHL4, SU-DHL5, SU-DHL6, SU-DHL7, SU-DHL8, SU-DHL10, SC1, Toledo, VAL1, WSU-NHL, and WSU-DLBCL-2 were grown in medium containing 90% RPMI and 10% FCS supplemented with penicillin G and streptomycin, L-glutamine and HEPES. Cell lines were obtained from the ATCC, DMSZ or the Ontario Cancer Institute. We perform monthly testing for mycoplasm sp. and other contaminants and quarterly cell identification by SNP.
Single locus DNA methylation assays
Total genomic DNA was extracted from 4 × 106 DLBCL cells and CD19+ patient samples using the PureLink mini kit (Invitrogen) and eluted in RNAse-free water. EpiTYPER assays (Sequenom, CA) were performed on bisulfite-converted DNA. Bisulfite conversion was performed using EZ DNA Methylation kit from Zymo Research (Irvine, CA). EpiTYPER primers were designed to cover CpG islands associated with the respective HpaII amplifiable fragments. All primers were designed using Sequenom EpiDesigner beta software (http://www.epidesigner.com/). Primer sequences are shown in Table S3.
Cellular uptake and retention of H3-DAC
Cell lines OCI-Ly3, OCI-Ly1, DoHH2, SUDHL4 and SUDHL6 were incubated with H3-decitabine 200 nM in complete medium for 24 and 48 h when cellular radioactivity was determined in counts per minute. Data is presented normalized to OCI-Ly1 with SEM.
Cellular uptake and retention of daunorubicin
Cellular uptake of doxorubicin (hydroxy-daunorubicin) was determined by uptake of daunorubicin as previously published(65). Briefly, cell lines OCI-Ly1, OCI-Ly10, OCI-Ly18, Karpas231, Karpas422, SC1, RL, VAL1, CCRF-CEM/VBL100 (MDR+)(66) and CCRF-CEM (MDR−) were incubated with vehicle, daunorubicin 500 nM and 2.5 μM in complete medium at 37 C for 1 h and washed 3 times with PBS. Fluorescence (PE-A channel) was determined with a flow cytometer (LSAR II BD Biosciences).
Growth inhibition determination
DLBCL cell lines were grown at concentrations sufficient to keep untreated cells in exponential growth over the complete drug exposure time. Cell viability was determined using a fluorometric resazurin reduction method (CellTiter-Blue, Promega) and trypan blue automatic method (TC10, BioRad). Fluorescence (Ex560nm/Em590nm) was determined with the Synergy4 microplate reader (BioTek). The number of viable cells was calculated by using the linear least-squares regression of the standard curve. The fluorescence was determined for three replicates per treatment condition and normalized to their respective controls (vehicle treated cells). To plot dose-effect curves CompuSyn software (Biosoft) was used, and drug concentrations that inhibits the growth of the cell lines by 25% and 50% compared to control (GI25 and GI50, respectively) were determined. Data were presented as the mean GI50 or GI25 with a 95% confidence interval for duplicate experiments. Data is normalized to cellular doubling time (Table S1). To determine synergism in primary cells we used the Webb’s fractional product method(29). This method is based on the equation Z = X + Y (1-X), were Z is the expected effect of the combination, and X and Y represent the effect of each drug alone. If Z is equal to the actual effect of the combination, then the relation is additive; if Z is higher then is less than additive and if Z is lower then is more than additive (synergistic). Data is presented as mean of 4 replicates with SEM.
Array-based methylation analysis
The HELP assay was performed as previously published(12). Briefly, one microgram of high-molecular-weight DNA was digested overnight with isoschizomer enzymes HpaII and MspI, respectively (New England Biolabs). DNA fragments were purified with phenol/chloroform, resuspended in 10 mM Tris-HCl pH 8.0, and used immediately to set up the ligation reaction with MspI/HpaII-compatible adapters and T4 DNA ligase. Ligation-mediated PCR was performed with enrichment for the 200 to 2000 base pair products and was submitted for hybridization (Roche). We used the HG 17 human promoter custom array covering 25626 HpaII amplifiable fragments within the promoters of the genes. Data quality control and analysis were performed as described previously. Probe sets with intensity less than 2.5 mean absolute deviation of the random probe sets on the array were marked as missing values. After quality control processing, a median normalization was performed on each array by subtracting the median log-ratio (HpaII/MspI) of that array (resulting in median log-ratio of 0 for each array). The HELP array probesets for SMAD1 are MSPI0406S00245618 and MSPI0406S00245619. Correlation of HELP data with percent of methylation was done as published(12).
DNA damage analysis
Cells: DNA damage (double-strand breaks) was determined by quantification of phosphorylation of H2A.X (Ser139) using a fluorescent-based assay (Millipore) following the manufacturer instructions with modification for suspension cells. The nuclear dye (Hoechst 33342) was used to determine cell number for normalization. Data is presented as fold H2A.X phosphorylation in treated cells vs. vehicle (water) at the indicated exposure times. Tissues: phosphorylation of H2A.X (Ser139) (Millipore, clone EP854Y) was determined by immunohistochemistry as previously described(67) with modifications. Briefly, antigen retrieval was performed in EDTA buffer pH: 9 at 95 °C (in an steamer) for 15 minutes. Cases were blinded scored by two researchers.
Caspase-7 and -3 activity
The activity of caspase-7 and -3 was determined using the Apo-ONE caspase 3/7 assay (Promega) following the manufacturer instructions. DLBCL cells were treated in triplicates with vehicle, TGFβ1, BMP2, doxorubicin, decitabine or their combinations at indicated concentrations and time points. The presence of caspase-3 and -7 activity allows the R110 group to become intensely fluorescent (Exitation499nm/Emission521nm) that is measured using the Synergy4 microplate reader (BioTek). Caspase-3 and -7 activity was related to the cell number determined by CellTiter-Blue (Promega) in a multiplex assay.
Cellular senescence
Cellular features of senescence were determined in DLBCL cells after 72 and 120 h of administration of decitabine100 nM (once per day) or water using the senescence β-galactosidase kit (Cell Signaling) following the manufacturer instructions with modifications for suspension cells. Cells were stained at 37 C for at least 6 h. Pictures were taken using an Olympus DP72 camera in a Zeiss Axioscope microscope. Experiments were carried out in triplicates.
Immunoblotting
Lysates from DLBCL cells were prepared using 50 mM Tris pH 7.4, 150 mM NaCl and 1% NP-40 lysis buffer. Lysates for nuclear and cytoplasmatic fractions were obtained using a fractionation kit (Biovision) following the manufacturer instructions. Protein concentrations were determined using the BCA kit (Pierce). Twenty five to 50 micrograms of protein lysates were loaded in a SDS-PAGE gels, transferred to PVDF membranes, and probed with primary antibodies: mouse monoclonal to SMAD1 (Santa-Cruz), mouse rabbit polyclonal to GAPDH (Santa-Cruz), mouse monoclonal to PARP-1 (F-2) (Santa-Cruz), mouse monoclonal to p21 (DCS-60) (Santa Cruz) and mouse monoclonal to p16 (F-12) (Santa Cruz). Densitometry values were obtained by using the ImageJ 1.40g software (NIH).
SMAD1 small-interfering RNA
Predesigned siRNA targeting SMAD1 (Invitrogen catalog s8394, s8395 and s8396) and control (a sequence with no significant similarity to any vertebrate gene, Invitrogen 12935-110 Lo GC Duplex 2) were obtained from Invitrogen. Cells were transfected with 2 μM of siRNA by electroporation (Amaxa electroporator) using the transfection buffer recommended by the manufacturer (Amaxa) or subjected to the procedure without siRNA (mock control).
Transient transfection of SMAD1 into DLBCL cells
eGFP (backbone control) or eGFP-SMAD1 plasmids were transfected at several concentrations into DLBCL cell lines by electroporation (Amaxa nucleofector, Amaxa). Cells were left to recover for 12 h, resuspended in complete growth medium and treated with drugs for 24 h.
Supplementary Material
Statement of Significance.
The problem of chemoresistance DLBCL remains the most urgent challenge in the clinical management of patients with this disease. We describe a mechanism-based approach towards the rational translation of DNA methyltransferase inhibitors for the treatment of high-risk DLBCL.
Acknowledgments
We thank J. Masague (Memorial Sloan Kettering Cancer Center, New York, NY) for providing SMAD1 plasmid vector. We thank O. Ammerpohl and R. Siebert for their help in analyzing methylation patterns in patients, and Martin Krzywinski for help in data visualization. We thank Celgene for providing the study drug azacitidine.
Financial support:
This work was funded in part by grants LLS SCOR 7017-9 (to A.M.), K08 CA127353 (to R.S.), LLS 6304-11 (to R.S.) NIH CA129831 and CA129831-03S1 (both to L.A.G.). S. B is supported in part by funds from the Clinical Translational Science Center (CTSC), National Center for Advancing Translational Sciences (NCATS) grant #UL1-RR024996. A.M. is supported by the Burroughs-Wellcome Foundation and the Chemotherapy Foundation. A.M. and J.P.L. are supported by the V Foundation. R.L.E. is supported by the Charles, Lillian and Betty Neuwirth Clinical Scholar Award. L.C. is a Raymond and Beverly Sackler Scholar and Scholar of the American Society of Hematology. L.C. is supported by the Irma T. Hirschl Trust and the Doris Duke Charitable Foundation.
Abbreviations list
- DAC
5-aza-2′-deoxycytidine, decitabine
- DLBCL
diffuse large B-cell lymphoma
- DLT
dose limiting toxicity
- DNMT
DNA methyltransferases
- DNMTI
DNMT inhibitors
- IPI
international prognostic index
- SWING
senescence with incomplete growth arrest
Footnotes
Potential conflict of interest:
J.P.L. has served as a consultant for Genentech and Celgene (makers of rituximab and azacitidine). R.L.E. is currently an employee of Genentech. All the other authors declare no conflict of interest.
Additional Materials and Methods are shown as supplementary material
Accession number. Expression and methylation profiling data from DLBCL cell lines have been deposited in Gene Expression Omnibus (GEO) and are accessible through accession number GSE28744.
References
- 1.Berdasco M, Esteller M. Aberrant epigenetic landscape in cancer: how cellular identity goes awry. Dev Cell. 2010;19:698–711. doi: 10.1016/j.devcel.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Martinez-Delgado B, Fernandez-Piqueras J, Garcia MJ, Arranz E, Gallego J, Rivas C, et al. Hypermethylation of a 5′ CpG island of p16 is a frequent event in non-Hodgkin’s lymphoma. Leukemia. 1997;11:425–8. doi: 10.1038/sj.leu.2400579. [DOI] [PubMed] [Google Scholar]
- 3.Pinyol M, Cobo F, Bea S, Jares P, Nayach I, Fernandez PL, et al. p16(INK4a) gene inactivation by deletions, mutations, and hypermethylation is associated with transformed and aggressive variants of non-Hodgkin’s lymphomas. Blood. 1998;91:2977–84. [PubMed] [Google Scholar]
- 4.Gordian E, Ramachandran K, Singal R. Methylation mediated silencing of TMS1 in breast cancer and its potential contribution to docetaxel cytotoxicity. Anticancer Res. 2009;29:3207–10. [PubMed] [Google Scholar]
- 5.Schmitt CA, McCurrach ME, de Stanchina E, Wallace-Brodeur RR, Lowe SW. INK4a/ARF mutations accelerate lymphomagenesis and promote chemoresistance by disabling p53. Genes Dev. 1999;13:2670–7. doi: 10.1101/gad.13.20.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cerchietti LC, Polo JM, Da Silva GF, Farinha P, Shaknovich R, Gascoyne RD, et al. Sequential transcription factor targeting for diffuse large B-cell lymphomas. Cancer research. 2008;68:3361–9. doi: 10.1158/0008-5472.CAN-07-5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lonning PE. Genes causing inherited cancer as beacons to identify the mechanisms of chemoresistance. Trends Mol Med. 2004;10:113–8. doi: 10.1016/j.molmed.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Daibata M, Nemoto Y, Bandobashi K, Kotani N, Kuroda M, Tsuchiya M, et al. Promoter hypermethylation of the bone morphogenetic protein-6 gene in malignant lymphoma. Clin Cancer Res. 2007;13:3528–35. doi: 10.1158/1078-0432.CCR-06-2766. [DOI] [PubMed] [Google Scholar]
- 9.Esteller M, Gaidano G, Goodman SN, Zagonel V, Capello D, Botto B, et al. Hypermethylation of the DNA repair gene O(6)-methylguanine DNA methyltransferase and survival of patients with diffuse large B-cell lymphoma. J Natl Cancer Inst. 2002;94:26–32. doi: 10.1093/jnci/94.1.26. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Nagai H, Ohno T, Yuge M, Hatano S, Ito E, et al. Aberrant DNA methylation of p57(KIP2) gene in the promoter region in lymphoid malignancies of B-cell phenotype. Blood. 2002;100:2572–7. doi: 10.1182/blood-2001-11-0026. [DOI] [PubMed] [Google Scholar]
- 11.Nakamichi I, Tomita Y, Zhang B, Sugiyama H, Kanakura Y, Fukuhara S, et al. Correlation between promoter hypermethylation of GSTP1 and response to chemotherapy in diffuse large B cell lymphoma. Ann Hematol. 2007;86:557–64. doi: 10.1007/s00277-007-0299-1. [DOI] [PubMed] [Google Scholar]
- 12.Shaknovich R, Geng H, Johnson NA, Tsikitas L, Cerchietti L, Greally JM, et al. DNA methylation signatures define molecular subtypes of diffuse large B-cell lymphoma. Blood. 2010;116:e81–9. doi: 10.1182/blood-2010-05-285320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaknovich R, Melnick A. Epigenetics and B-cell lymphoma. Curr Opin Hematol. 2011;18:293–9. doi: 10.1097/MOH.0b013e32834788cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiozawa E, Takimoto M, Makino R, Adachi D, Saito B, Yamochi-Onizuka T, et al. Hypermethylation of CpG islands in p16 as a prognostic factor for diffuse large B-cell lymphoma in a high-risk group. Leuk Res. 2006;30:859–67. doi: 10.1016/j.leukres.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Momparler RL. Pharmacology of 5-Aza-2′-deoxycytidine (decitabine) Seminars in hematology. 2005;42:S9–16. doi: 10.1053/j.seminhematol.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Yoon DH, Choi DR, Ahn HJ, Kim S, Lee DH, Kim SW, et al. Ki-67 expression as a prognostic factor in diffuse large B-cell lymphoma patients treated with rituximab plus CHOP. Eur J Haematol. 85:149–57. doi: 10.1111/j.1600-0609.2010.01467.x. [DOI] [PubMed] [Google Scholar]
- 17.Elstrom RL, Martin P, Ostrow K, Barrientos J, Chadburn A, Furman R, et al. Response to second-line therapy defines the potential for cure in patients with recurrent diffuse large B-cell lymphoma: implications for the development of novel therapeutic strategies. Clinical lymphoma, myeloma & leukemia. 2010;10:192–6. doi: 10.3816/CLML.2010.n.030. [DOI] [PubMed] [Google Scholar]
- 18.A predictive model for aggressive non-Hodgkin’s lymphoma The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. The New England journal of medicine. 1993;329:987–94. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 19.Figueroa ME, Skrabanek L, Li Y, Jiemjit A, Fandy TE, Paietta E, et al. MDS and secondary AML display unique patterns and abundance of aberrant DNA methylation. Blood. 2009;114:3448–58. doi: 10.1182/blood-2009-01-200519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blum W, Marcucci G. Targeting epigenetic changes in acute myeloid leukemia. Clinical advances in hematology & oncology: H&O. 2005;3:855–65. 82. [PubMed] [Google Scholar]
- 21.Klisovic RB, Stock W, Cataland S, Klisovic MI, Liu S, Blum W, et al. A phase I biological study of MG98, an oligodeoxynucleotide antisense to DNA methyltransferase 1, in patients with high-risk myelodysplasia and acute myeloid leukemia. Clin Cancer Res. 2008;14:2444–9. doi: 10.1158/1078-0432.CCR-07-1320. [DOI] [PubMed] [Google Scholar]
- 22.Tsai HC, Li H, Van Neste L, Cai Y, Robert C, Rassool FV, et al. Transient low doses of DNA-demethylating agents exert durable antitumor effects on hematological and epithelial tumor cells. Cancer cell. 2012;21:430–46. doi: 10.1016/j.ccr.2011.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gams RA, Rainey M, Dandy M, Bartolucci AA, Silberman H, Omura G. Phase III study of BCOP v CHOP in unfavorable categories of malignant lymphoma: a Southeastern Cancer Study Group trial. J Clin Oncol. 1985;3:1188–95. doi: 10.1200/JCO.1985.3.9.1188. [DOI] [PubMed] [Google Scholar]
- 24.Jones SE, Grozea PN, Metz EN, Haut A, Stephens RL, Morrison FS, et al. Superiority of adriamycin-containing combination chemotherapy in the treatment of diffuse lymphoma: a Southwest Oncology Group study. Cancer. 1979;43:417–25. doi: 10.1002/1097-0142(197902)43:2<417::aid-cncr2820430203>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 25.Bishop JF, Wiernik PH, Wesley MN, Kaplan RS, Diggs CH, Barcos MP, et al. A randomized trial of high dose cyclophosphamide, vincristine, and prednisone plus or minus doxorubicin (CVP versus CAVP) with long-term follow-up in advanced non-Hodgkin’s lymphoma. Leukemia. 1987;1:508–13. [PubMed] [Google Scholar]
- 26.Gordon LI, Harrington D, Andersen J, Colgan J, Glick J, Neiman R, et al. Comparison of a second-generation combination chemotherapeutic regimen (m-BACOD) with a standard regimen (CHOP) for advanced diffuse non-Hodgkin’s lymphoma. N Engl J Med. 1992;327:1342–9. doi: 10.1056/NEJM199211053271903. [DOI] [PubMed] [Google Scholar]
- 27.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacological reviews. 2006;58:621–81. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 28.Erttmann R, Erb N, Steinhoff A, Landbeck G. Pharmacokinetics of doxorubicin in man: dose and schedule dependence. Journal of cancer research and clinical oncology. 1988;114:509–13. doi: 10.1007/BF00391502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prichard MN, Shipman C., Jr. A three-dimensional model to analyze drug-drug interactions. Antiviral research. 1990;14:181–205. doi: 10.1016/0166-3542(90)90001-n. [DOI] [PubMed] [Google Scholar]
- 30.Chang H, Blondal JA, Benchimol S, Minden MD, Messner HA. p53 mutations, c-myc and bcl-2 rearrangements in human non-Hodgkin’s lymphoma cell lines. Leukemia & lymphoma. 1995;19:165–71. doi: 10.3109/10428199509059672. [DOI] [PubMed] [Google Scholar]
- 31.Dyer MJ, Fischer P, Nacheva E, Labastide W, Karpas A. A new human B-cell non-Hodgkin’s lymphoma cell line (Karpas 422) exhibiting both t (14;18) and t(4;11) chromosomal translocations. Blood. 1990;75:709–14. [PubMed] [Google Scholar]
- 32.Palii SS, Van Emburgh BO, Sankpal UT, Brown KD, Robertson KD. DNA methylation inhibitor 5-Aza-2′-deoxycytidine induces reversible genome-wide DNA damage that is distinctly influenced by DNA methyltransferases 1 and 3B. Molecular and cellular biology. 2008;28:752–71. doi: 10.1128/MCB.01799-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sherman MY, Meng L, Stampfer M, Gabai VL, Yaglom JA. Oncogenes induce senescence with incomplete growth arrest and suppress the DNA damage response in immortalized cells. Aging cell. 2011;10:949–61. doi: 10.1111/j.1474-9726.2011.00736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Isufi I, Seetharam M, Zhou L, Sohal D, Opalinska J, Pahanish P, et al. Transforming growth factor-beta signaling in normal and malignant hematopoiesis. J Interferon Cytokine Res. 2007;27:543–52. doi: 10.1089/jir.2007.0009. [DOI] [PubMed] [Google Scholar]
- 35.Buckley S, Shi W, Driscoll B, Ferrario A, Anderson K, Warburton D. BMP4 signaling induces senescence and modulates the oncogenic phenotype of A549 lung adenocarcinoma cells. American journal of physiology Lung cellular and molecular physiology. 2004;286:L81–6. doi: 10.1152/ajplung.00160.2003. [DOI] [PubMed] [Google Scholar]
- 36.Su D, Zhu S, Han X, Feng Y, Huang H, Ren G, et al. BMP4-Smad signaling pathway mediates adriamycin-induced premature senescence in lung cancer cells. The Journal of biological chemistry. 2009;284:12153–64. doi: 10.1074/jbc.M807930200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lenz G, Wright G, Dave SS, Xiao W, Powell J, Zhao H, et al. Stromal gene signatures in large-B-cell lymphomas. The New England journal of medicine. 2008;359:2313–23. doi: 10.1056/NEJMoa0802885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monti S, Savage KJ, Kutok JL, Feuerhake F, Kurtin P, Mihm M, et al. Molecular profiling of diffuse large B-cell lymphoma identifies robust subtypes including one characterized by host inflammatory response. Blood. 2005;105:1851–61. doi: 10.1182/blood-2004-07-2947. [DOI] [PubMed] [Google Scholar]
- 39.Doi A, Park IH, Wen B, Murakami P, Aryee MJ, Irizarry R, et al. Differential methylation of tissue- and cancer-specific CpG island shores distinguishes human induced pluripotent stem cells, embryonic stem cells and fibroblasts. Nature genetics. 2009;41:1350–3. doi: 10.1038/ng.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Irizarry RA, Ladd-Acosta C, Wen B, Wu Z, Montano C, Onyango P, et al. The human colon cancer methylome shows similar hypo- and hypermethylation at conserved tissue-specific CpG island shores. Nature genetics. 2009;41:178–86. doi: 10.1038/ng.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rai D, Kim SW, McKeller MR, Dahia PL, Aguiar RC. Targeting of SMAD5 links microRNA-155 to the TGF-beta pathway and lymphomagenesis. Proc Natl Acad Sci U S A. 107:3111–6. doi: 10.1073/pnas.0910667107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Douglas RS, Capocasale RJ, Lamb RJ, Nowell PC, Moore JS. Chronic lymphocytic leukemia B cells are resistant to the apoptotic effects of transforming growth factor-beta. Blood. 1997;89:941–7. [PubMed] [Google Scholar]
- 43.Kehrl JH, Wakefield LM, Roberts AB, Jakowlew S, Alvarez-Mon M, Derynck R, et al. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. The Journal of experimental medicine. 1986;163:1037–50. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kadin ME, Cavaille-Coll MW, Gertz R, Massague J, Cheifetz S, George D. Loss of receptors for transforming growth factor beta in human T-cell malignancies. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:6002–6. doi: 10.1073/pnas.91.13.6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schiemann WP, Pfeifer WM, Levi E, Kadin ME, Lodish HF. A deletion in the gene for transforming growth factor beta type I receptor abolishes growth regulation by transforming growth factor beta in a cutaneous T-cell lymphoma. Blood. 1999;94:2854–61. [PubMed] [Google Scholar]
- 46.Ashmore SM, Thomas DG, Darling JL. Does P-glycoprotein play a role in clinical resistance of malignant astrocytoma? Anticancer Drugs. 1999;10:861–72. doi: 10.1097/00001813-199911000-00001. [DOI] [PubMed] [Google Scholar]
- 47.Cantwell BM, Bozzino JM, Corris P, Harris AL. The multidrug resistant phenotype in clinical practice; evaluation of cross resistance to ifosfamide and mesna after VP16-213, doxorubicin and vincristine (VPAV) for small cell lung cancer. Eur J Cancer Clin Oncol. 1988;24:123–9. doi: 10.1016/0277-5379(88)90242-8. [DOI] [PubMed] [Google Scholar]
- 48.Friedenberg WR, Rue M, Blood EA, Dalton WS, Shustik C, Larson RA, et al. Phase III study of PSC-833 (valspodar) in combination with vincristine, doxorubicin, and dexamethasone (valspodar/VAD) versus VAD alone in patients with recurring or refractory multiple myeloma (E1A95): a trial of the Eastern Cooperative Oncology Group. Cancer. 2006;106:830–8. doi: 10.1002/cncr.21666. [DOI] [PubMed] [Google Scholar]
- 49.Friedenberg WR, Tallman MS, Brodsky I, Paietta E, Rowe JM, Lee SJ, et al. Modified VAD and PSC-833 in the treatment of resistant or relapsing chronic lymphocytic leukemia (E4996): a trial of the Eastern Cooperative Oncology Group. Leuk Res. 2004;28:813–9. doi: 10.1016/j.leukres.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 50.Indran IR, Tufo G, Pervaiz S, Brenner C. Recent advances in apoptosis, mitochondria and drug resistance in cancer cells. Biochim Biophys Acta. 2011;1807:735–45. doi: 10.1016/j.bbabio.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 51.Fodale V, Pierobon M, Liotta L, Petricoin E. Mechanism of cell adaptation: when and how do cancer cells develop chemoresistance? Cancer J. 2011;17:89–95. doi: 10.1097/PPO.0b013e318212dd3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Phan RT, Dalla-Favera R. The BCL6 proto-oncogene suppresses p53 expression in germinal-centre B cells. Nature. 2004;432:635–9. doi: 10.1038/nature03147. [DOI] [PubMed] [Google Scholar]
- 53.Foroutan B, Ruf AA, Costall B, Anderson D. An in vitro model to study chemoresistance in non-Hodgkin’s lymphoma patients over-expressing mutant p53. J Pharmacol Toxicol Methods. 2007;55:151–8. doi: 10.1016/j.vascn.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 54.Dong M, Blobe GC. Role of transforming growth factor-beta in hematologic malignancies. Blood. 2006;107:4589–96. doi: 10.1182/blood-2005-10-4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Munoz O, Fend F, de Beaumont R, Husson H, Astier A, Freedman AS. TGFbeta-mediated activation of Smad1 in B-cell non-Hodgkin’s lymphoma and effect on cell proliferation. Leukemia. 2004;18:2015–25. doi: 10.1038/sj.leu.2403485. [DOI] [PubMed] [Google Scholar]
- 56.Wang D, Long J, Dai F, Liang M, Feng XH, Lin X. BCL6 represses Smad signaling in transforming growth factor-beta resistance. Cancer Res. 2008;68:783–9. doi: 10.1158/0008-5472.CAN-07-0008. [DOI] [PubMed] [Google Scholar]
- 57.Singh R, George J, Shukla Y. Role of senescence and mitotic catastrophe in cancer therapy. Cell division. 2010;5:4. doi: 10.1186/1747-1028-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Dong X, Liu A, Zer C, Feng J, Zhen Z, Yang M, et al. siRNA inhibition of telomerase enhances the anti-cancer effect of doxorubicin in breast cancer cells. BMC cancer. 2009;9:133. doi: 10.1186/1471-2407-9-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seluanov A, Mittelman D, Pereira-Smith OM, Wilson JH, Gorbunova V. DNA end joining becomes less efficient and more error-prone during cellular senescence. Proc Natl Acad Sci U S A. 2004;101:7624–9. doi: 10.1073/pnas.0400726101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Santarosa M, Del Col L, Tonin E, Caragnano A, Viel A, Maestro R. Premature senescence is a major response to DNA cross-linking agents in BRCA1-defective cells: implication for tailored treatments of BRCA1 mutation carriers. Molecular cancer therapeutics. 2009;8:844–54. doi: 10.1158/1535-7163.MCT-08-0951. [DOI] [PubMed] [Google Scholar]
- 61.Chiu KC, Fine M, Ikle D, Slovak ML, Arber DA. Telomerase activity and proliferation index in aggressive mature B-cell lymphoma: comparison to germinal center phenotypic markers. Human pathology. 2003;34:1259–64. doi: 10.1016/j.humpath.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 62.Yamato K, Hashimoto S, Imamura T, Uchida H, Okahashi N, Koseki T, et al. Activation of the p21(CIP1/WAF1) promoter by bone morphogenetic protein-2 in mouse B lineage cells. Oncogene. 2001;20:4383–92. doi: 10.1038/sj.onc.1204572. [DOI] [PubMed] [Google Scholar]
- 63.Kihslinger JE, Godley LA. The use of hypomethylating agents in the treatment of hematologic malignancies. Leukemia & lymphoma. 2007;48:1676–95. doi: 10.1080/10428190701493910. [DOI] [PubMed] [Google Scholar]
- 64.Blum KA, Liu Z, Lucas DM, Chen P, Xie Z, Baiocchi R, et al. Phase I trial of low dose decitabine targeting DNA hypermethylation in patients with chronic lymphocytic leukaemia and non-Hodgkin lymphoma: dose-limiting myelosuppression without evidence of DNA hypomethylation. Br J Haematol. 2010;150:189–95. doi: 10.1111/j.1365-2141.2010.08213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tapiero H, Fourcade A, Vaigot P, Farhi JJ. Comparative uptake of adriamycin and daunorubicin in sensitive and resistant friend leukemia cells measured by flow cytometry. Cytometry. 1982;2:298–302. doi: 10.1002/cyto.990020506. [DOI] [PubMed] [Google Scholar]
- 66.Cass CE, Janowska-Wieczorek A, Lynch MA, Sheinin H, Hindenburg AA, Beck WT. Effect of duration of exposure to verapamil on vincristine activity against multidrug-resistant human leukemic cell lines. Cancer Res. 1989;49:5798–804. [PubMed] [Google Scholar]
- 67.Cerchietti LC, Lopes EC, Yang SN, Hatzi K, Bunting KL, Tsikitas LA, et al. A purine scaffold Hsp90 inhibitor destabilizes BCL-6 and has specific antitumor activity in BCL-6-dependent B cell lymphomas. Nature medicine. 2009;15:1369–76. doi: 10.1038/nm.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.