Abstract
Rationale
Various dimensions of impulsivity have been linked to substance abuse and dependence, both as consequences of, and as predisposing factors to addiction. With respect to the latter, they may be quantitative indicators of liability for substance use disorders (SUD) and aid in determining underlying genetic influences. We have previously determined that inhibitory control over impulsive responding, as measured by a reversal learning task, is heritable and under substantial genetic control, however their role as explaining variables for aspects of SUD have not been well explored.
Objective
The aim of this study was to test for an association between genetically-determined differences in inhibitory control and addiction-related phenotypes, such that phenotypes of poor inhibitory control would predict propensity for elevated operant drug-seeking and –taking behaviors.
Methods
Mice from BxD strains with either good reversal learning (GRL) or poor reversal learning (PRL) ability were tested for intravenous cocaine self-administration under FR1, FR2, and FR5 reinforcement schedules. Additionally, locomotor responses to experimenter-delivered cocaine were assessed.
Results
Compared to GRL strains, PRL strains acquired self-administration behavior more rapidly and administered cocaine at greater rates under all schedules of reinforcement, without any differences in discrimination index. In addition, PRL mice also exhibited increased responding during time-out periods. PRL strains also showed larger locomotor responses to 10 or 20 mg/kg injections of cocaine.
Conclusions
These studies demonstrate that heritable strain differences in inhibitory control do influence drug self-administration, thus suggest that genetically-driven impulsivity of this type may predispose susceptibility to drug abuse and addiction.
Keywords: inhibitory control, cocaine, BxD, reversal learning, impulsivity, addiction
Substance use disorders (SUD) are characterized, in part, by both impulsive and compulsive drug-seeking and -taking behaviors; these phenomena are likely linked to compromised ability to exert effortful, inhibitory control over behavior (Groman et al. 2009; Jentsch and Taylor 1999). Initially, these impairments were attributed to neuroadaptations caused by experience with the pharmacological properties of drugs of abuse (Bornovalova et al. 2005; Calu et al. 2007; Coffey et al. 2003; Ersche et al. 2008; Fillmore and Rush 2006; Heil et al. 2006; Jentsch et al. 2002; Olausson et al. 2007; Paine et al. 2003; Roesch et al. 2007; Simon et al. 2007; Stalnaker et al. 2009; Winstanley et al. 2009), but more recently impulsivity-related traits have shown to predict susceptibility to different aspects of addiction-related behaviors (Belin et al. 2008; Boulougouris et al. 2007; Crews and Boettiger 2009; Diergaarde et al. 2008; Economidou et al. 2009; Lee et al. 2007; Perry et al. 2005; Poulos et al. 1995). These findings are consistent with conceptual theories emphasizing the notion that elevated trait impulsiveness predisposes individuals to initiate drug use and to experience the positive reinforcing effects of drugs (Dalley et al. 2011; Kreek et al. 2005; Perry and Carroll 2008), as well as to transition to more compulsive or habitual forms of drug intake with prolonged experience.
This suggests that naturally-occurring variation in impulsivity and inhibitory control traits may be useful as “endophenotypes” (Bearden and Freimer 2006; Gottesman 2003) that quantitatively predict vulnerability for the development of SUD and that can be used to investigate underlying genetic determinants. Several traits reflecting different facets of impulsivity/compulsivity, such as sensation-seeking (Laviola et al. 1999; Wills et al. 1994), risk-taking (Lejuez et al. 2002; Stout et al. 2005), poor inhibitory control and cognitive dysfunction (Bechara et al. 2002; Crews and Boettiger 2009; Grant et al. 2000; Jentsch and Taylor 1999; Rogers et al. 1999; Rogers and Robbins 2001), have been shown to be involved in substance abuse and/or addiction. Indeed, drug-dependent individuals are, on average, impaired when evaluated using procedures that measure these dimensions of behavior. Of specific relevance to the current study, discrimination-reversal learning – which measures inhibitory control over behavior - is impaired in individuals with a history of polydrug abuse involving cocaine and alcohol (Filimore & Rush, 2006), while probabilistic reversal learning is severely impaired in chronic cocaine users (Ersche et al., 2008). Moreover, some of these impulsivity-related traits have been further linked to a susceptibility to SUD, and some of these traits are heritable in humans (Aron and Poldrack 2005; Brewer and Potenza 2009; Ersche et al. 2012; Ersche et al. 2010; Groman et al. 2009).
A recent study extends these observations by (1) demonstrating that inhibitory control phenotypes, measured with a 2-choice operant spatial reversal learning procedure (Izquierdo and Jentsch 2012) that assesses the ability to effortfully withhold or disengage from impulsive or compulsive responses, are heritable in mice and (2) revealing novel genomic determinants (Laughlin et al. 2011). Using 51 BxD recombinant inbred (RI) strains derived from an intercross of the C57Bl6 and DBA strains (Peirce et al. 2004), we found that approximately 1/3 of all variance in reversal learning abilities are attribu5table to genetic factors and – of that genetic variance – approximately 1/3 was explained by a major effect quantitative trait locus on chromosome 10 (Laughlin et al. 2011). In addition to aiding in the discovery of novel genetic variants, inbred mouse panels like the BxD resource provide an opportunity for mapping genetic correlations between traits; specifically, any two phenotypes that share a common genetic basis should exhibit a pattern of correlated expression across strains that make up the panel. As such, BxD mice offer the opportunity to map the genetic relationship between heritable variation in inhibitory control and drug self-administration, explicitly testing the hypothesis described above.
In line with the notion that impairments in inhibitory control are important variables explaining susceptibility for aspects of substance abuse and dependence, the goal of the present study was to generate definitive data indicating that genetically-influenced phenotypic differences in inhibitory control predict phenotypes related to drug reinforcement. We used an extreme group approach, in which four BxD mouse strains - two exhibiting poor reversal learning/inhibitory control (BxD42 and BxD68) and two exhibiting good reversal learning/inhibitory control (BxD31 and BxD38) (Laughlin et al. 2011) – were assessed for cocaine self-administration and responding in extinction. We hypothesized that phenotypes related to poor inhibitory control would predict propensity for elevated operant drug-seeking and –taking behaviors. This study is a first step required to support the feasibility and rationale of subsequent genome-scale efforts to identify potentially similar or different genetic influences on impulsivity and drug reinforcement.
Methods and Materials
Animals
Adult mice from four BxD RI strains were born on site from breeding pairs acquired from The Jackson Laboratory (Bar Harbor ME). The BXD recombinant inbred mouse panel is a collection of inbred mouse strains derived from an intercross of C57BL/6 (maternal) and DBA/2 (paternal) strains. Briefly, the founder strains were mated to produce an isogenic F1 generation. Subsequent sibling matings for 20+ generations were undertaken, resulting in recombination events leading to distinct homozygous strains, each of which carries a unique mosaic of founder (B vs. D) alleles. The resulting strains (~80) are maintained by inbreeding and are commercially available, representing a powerful genetic reference population, meaning that results from their study can be compared across researchers and time (Peirce et al. 2004).
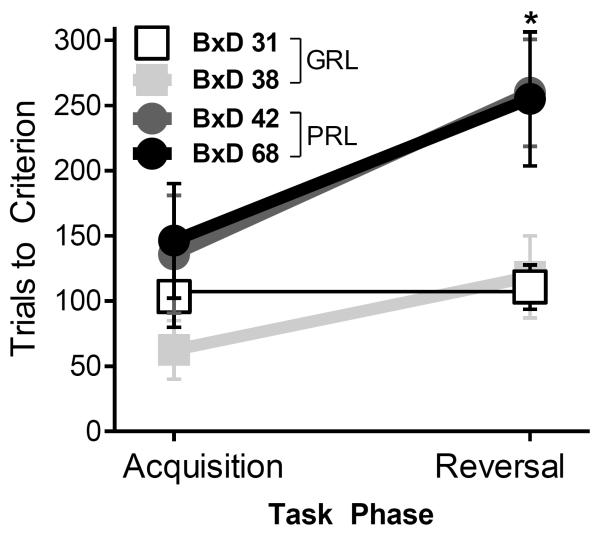
Four out of the 51 previously described strains were selected based on reversal learning task performance (Laughlin et al. 2011). Selection criteria consisted of comparable performance in the acquisition phase of the reversal learning task as well as opposite performance levels in the reversal phase, under an extreme groups approach: BxD31 and BxD38 exhibited good reversal ability (low impulsivity, good inhibitory control), while BxD42 and BxD68 exhibited poor reversal learning (high impulsivity, poor inhibitory control) (Figure 1). A total of 51 mice from these strains were included in the self-administration experiments; an additional 49 experimentally naïve mice were used to measure the psychomotor stimulant effects of cocaine.
Fig.1. Reversal Learning Performance.
Comparison of the number of trials required to reach performance criteria during acquisition and reversal task phases between GRL (BxD 31, white squares; BxD 38, light gray squares) and PRL strains (BxD 42, dark grey circles; BxD 68, black circles). Data are presented as mean ± SEM (n= 2–3 per group). * = p<0.05, difference between pooled PRL and GRL strains
All mice were weaned at 21 days of age and were group-housed by sex and strain in standard cages under a 14/10 light/dark schedule with food and water available ad libitum. Subjects ranged from 60–120 days of age and 20–30g in weight at onset of experimentation. Animal care and experimental procedures were approved by the Chancellor's Animal Research Committee at the University of California, Los Angeles and were consistent with the Public Health Service's Guide for the Care and Use of Laboratory Animals (NRC 2011).
Drugs
Cocaine base (National Institute on Drug Abuse; Rockville MD) was dissolved in a minimum quantity of concentrated hydrochloric acid before being brought to volume in 0.9% sterile saline; the pH was then adjusted to ~6.5 using 0.1M – 1M sodium hydroxide.
Reversal Learning Taske
Reversal learning was measured using a two-step spatial discrimination task, as described previously (Laughlin et al. 2011). Briefly, mice were trained and tested daily in operant conditioning chambers fitted with five horizontal nose-poke apertures and a photocell-equipped food-delivery magazine. Subjects were trained to nose-poke the central aperture to initiate trials, which illuminated the flanking apertures. Responses at one of these two apertures was reinforced with delivery of a food pellet (correct response), while responses to the other resulted in a 5-s time-out (incorrect response). Initial training ended for an individual mouse when it reached performance criteria (16 out of 20 consecutive correctly completed trials). Once criteria were reached, the reinforcement contingencies were switched in reversal sessions that commenced on the following day.
Self-Administration Experiments
Mice were handled for five days and then implanted with chronic indwelling jugular catheters under aseptic surgical technique, as described below. Subjects (BxD31=10 males; BxD38=14 males, 7 females; BxD42=14 males; BxD68=4 males, 2 females) were then tested for cocaine self-administration. Mice were not food restricted, baited or drug-primed at any point during the experiment.
Surgery
Subjects were anesthetized under an oxygen/ isoflourane (1.5–2.0%) ventilation system and supported on a circulating-water heating pad. A catheter (Camcaths, Ely, Cambridgeshire UK) was inserted through a ventral supra-jugular incision, placed 1.2cm dorsal to the heart and anchored with suture knots (SOFSILK, 4–0) above and below a silicon bead; it was tunneled subcutaneously over the shoulder and externalized through a midscapular dorsal incision. Incisions were sutured and treated with triple antibiotic ointment. Mice recovered for six days before starting self-administration.
Catheter maintenance and patency
A 20μL solution containing the antibiotic ticarcillin clavulanate (Glaxo Smith Klein, Research Triangle Park, NC, USA) (100mg/1.5mL) dissolved in heparinized (30 units/mL) 0.9% saline was flushed through the catheter immediately after implantation, daily throughout recovery, and before and after each self-administration session. Catheter patency was evaluated with a 20μL bolus infusion of 1% propofol (10mg/mL) before starting self-administration and between each experimental phase. If prominent sedation was not apparent within 3s of infusion, the mouse was removed from the study and from subsequent analyses.
Apparatus
Operant conditioning chambers enclosed in sound-attenuating cabinets were controlled by a computer running Med-PC (Med Associates; St. Albans VT); chambers were fitted with two levers that were respectively assigned as active and inactive in a counterbalanced fashion. A single-channel fluid swivel connected a pump-driven syringe (infusion speed: 10μl/s) to the indwelling catheter.
Cocaine reinforcement
Daily self-administration sessions lasted 2h and were conducted 7 days a week. Completion of the response schedule on the active lever triggered a 20μL infusion containing a unit dose of 0.5mg/kg cocaine and initiated flashing of the house light for 20s. During the 20s post-infusion period, active responses were recorded but had no programmed consequence. Responses on the inactive lever were recorded but had no programmed consequence. A maximum of 65 cocaine infusions could be obtained in each session.
All mice first trained for acquisition of cocaine self-administration under a fixed ratio 1 (FR1) schedule of reinforcement. For those that acquired and showed stable behavior, responding was evaluated under FR2 and then FR5 schedules in order to establish whether responding would increase as a function of schedule requirements – a sign that the animals were engaged in motivated drug-seeking behavior. Consistent with previous cocaine self-administration studies in mice, criteria for stable acquisition and cocaine-maintained responding (Soria et al. 2008; Thomsen and Caine 2007) under FR1 and FR2 schedules were 1) 20% or less variation in number of infusions earned and greater than 70% discrimination index (active responses divided by total responses) across 3 consecutive days, 2) 10 infusions minimum earned per session, and 3) 5 and 4 days minimum per schedule, respectively. FR5 tests were administered for 5 days (with no completion criteria). Because we did not pre-train the animals using a food reinforcer and did not, consequently, food restrict them, we expected a smaller proportion of animals to acquire self-administration. Subjects with patent catheters were considered to have failed to acquire self-administration if they earned no infusions over 7 consecutive days or if they earned fewer than 10 infusions per session over 3 consecutive days (Griffin et al. 2007; Rocha et al. 1998; Thomsen and Caine 2011; Thomsen et al. 2009).
Locomotor Activity Experiment
A separate cohort of adult female mice (BxD31=8, BxD38=22, BxD42=11, BxD68=8, all adult females) were assessed for their locomotor responses to non-contingent cocaine administration. Activity chambers consisted of large acrylic cages placed within an infrared grid (Columbus Instruments, Columbus OH); clean cages were used for each session and each subject. Mice were first habituated to the experimental setup for 30min across three consecutive days. Subsequently, subjects were tested in 1h sessions after an intra-peritoneal (IP) injection of cocaine (1mL/100g body weight) at four different doses (0, 5, 10, and 20mg/kg) using a repeated measures, cyclic Latin square design, with 2–3 days between tests. The number of photobeam breaks was automatically recorded, and activity was measured as the average activity counts, excluding the first 15min to allow for drug onset.
Data analyses
The dependent variables for self-administration data included the total number of days to reach criteria, the number of infusions earned per session, number of post-infusion responses (active responses during time-out period) and the discrimination index. These data were analyzed using a two-way repeated measures analysis of variance (ANOVA) with training day as the within-subject factor, strain (BxD31, BxD38, BxD42, BxD68) as the between-subjects factor, and sex as the covariate factor (when appropriate). The dependent variable for the locomotor activity tests was the total number of beam breaks; these data were analyzed using a two-way repeated measures ANOVA with cocaine dose (0, 5, 10, 20 mg/kg) as the within-subject factor and strain (BxD31, BxD38, BxD42, BxD68) as the between-subject factor. Upon significant overall interactions, post hoc analyses for comparisons of the number of days to reach criteria were carried out with unpaired two-tailed t tests, while a priori hypotheses were tested with unpaired one-tailed t tests. Tukey's HSD tests were used for post hoc analyses of all other self-administration measures. To identify differences in the number of mice from each strain that failed to acquire self-administration, categorical data (acquisition vs. non-acquisition) were analyzed using a Fisher's exact test. A significance level of p<0.05 was used for all statistical analyses.
Results
Reversal Learning and Cocaine SA
Reversal Learning
The BxD strains selected for the present study were chosen based on performance in a reversal learning task (Laughlin et al. 2011), in which the primary outcome measure was the number of trials required to reach performance criteria during an initial learning stage and after reversal of the reinforcement contingencies, a test of inhibitory response control (Izquierdo and Jentsch 2012). Reversal learning performance data for the selected strains were taken from the original dataset obtained in the previous experiment and re-analyzed. Consistent with the results of these 51 previously analyzed strains, there were no differences in performance during the acquisition phase between the four selected strains in the present study [F (1,3) =7.53, P=0.11] (BxD31=104±24, BxD38=63±22, BxD42=136±45, BxD68=146±44 trials to criterion); however, during reversal conditions, strain differences were apparent [F (1,3) =981, P<0.01] (Figure 1). Out of the 51 strains surveyed in our earlier study, BxD31 and BxD38 exhibited performance that placed them in the top 20% of all strains (111±17 and 119±32 trials to criterion, respectively) and are referred to, here, as “good reversal learning” (GRL) strains, while BxD42 and BxD68 exhibited reversal learning performance in the lowest 5% of the sample (260±41 and 255±51 trials to criterion, respectively) and are referred to, here, as “poor reversal learning” (PRL) strains.
Self-Administration Experiments
Approximately 75% of subjects that began self-administration testing completed all schedules of reinforcement. Subjects were removed from the experiment due to loss of catheter patency, deterioration of health or failed acquisition of self-administration (FR1 schedule). The number of mice that failed to acquire self-administration was not different between strains [p=0.82, V= 0.13] (#mice failed/#mice trained per strain: BxD31=2/12, BxD38=8/29, BxD42=3/17, BxD68=1/7).
FR1 Schedule - Acquisition
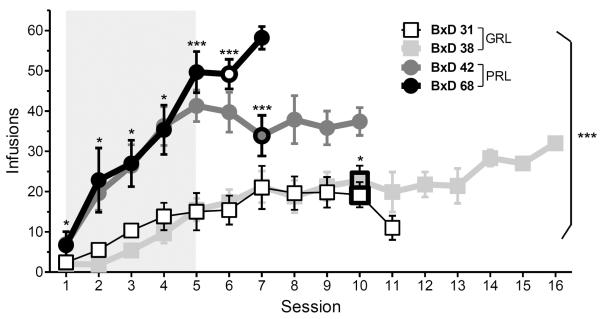
The average number of sessions required to reach criteria for stable self-administration was different between the strains [F (3, 47) =8.48, P<0.001] (Figure 2, bold shape outline). PRL strains acquired faster than GRL strains, requiring fewer sessions to reach criterion (all pair-wise comparisons: p<0.001). Because different amounts of training were required, all subsequent analyses included the data for the first five days (the minimum amount of training in all mice), as well as each mouse's last FR1 session. The average number of infusions earned per session was different between the strains, indicated by a significant strain x session interaction [F (15, 230) =4.74, p<0.0001], and a main effect of strain [F (3, 46) =23.5, p<0.0001], revealing an overall greater rate of self-administration in PRL strains compared to GRL strains (Figure 2). BxD42 mice administered a greater number of infusions on all sessions compared to GRL mice. Similarly, on all sessions except the first one, BxD68 mice earned more infusions than GRL mice. The GRL strains did not differ from one another, but mice from BxD68 differed from strain BxD42 by the end of FR1 sessions [Tukey HSD, p<0.05], suggesting that there are gradations of affectedness in the phenotypes, even within these extreme groups. Active lever responses during the 20s post-infusion timeout were also different between the strains [F (3, 34) =9.44, p<0.001] (Table 1); this effect was present in all except the first session. The strain x session interaction was not significant. Specifically, PRL strains exhibited more post-infusion responses than GRL strains (all pair-wise comparisons: p<0.05). Furthermore, while there was an expected overall gradual increase in the discrimination index (active responses divided by total responses) across acquisition in all strains, indicated by a significant effect of session [F (5,170) =8.76, p<0.01], there was no session x strain interaction, nor any main effect of strain (Table 2).
Fig.2. Acquisition of Cocaine Self-administration.
Comparison of cocaine infusions (0.5mg/kg/20μL inf) earned under an FR1 schedule of reinforcement between GRL and PRL strains. Open symbols indicate the average session in which acquisition criteria was reached. Grey shaded area indicates data used for analysis. Data are presented as mean ± SEM (n= 10 BxD31, 21 BxD38, 14 BxD42, 6 BxD68). Asterisks indicate significant differences between pooled PRL and GRL strains in all pairwise comparisons: * = at least p<0.05, ***= p<0.001
Table 1.
Discrimination Index.
| FR1 REINFORCEMENT SCHEDULE | ||||||||||||
| SESSION | 1 | 2 | 3 | 4 | 5 | LAST | ||||||
| BxD 31 | 52.9 | 13.6 | 69.5 | 9.9 | 79.4 | 7.3 | 80.4 | 7.3 | 81.4 | 6.1 | 74.4 | 3.4 |
| BxD 38 | 65.8 | 7.9 | 71.1 | 8.4 | 72.3 | 6.4 | 77.0 | 5.0 | 81.0 | 5.1 | 88.2 | 2.6 |
| BxD 42 | 53.8 | 6.8 | 65.8 | 5.4 | 79.2 | 4.6 | 76.1 | 4.4 | 83.4 | 3.2 | 89.9 | 1.9 |
| BxD 68 | 66.2 | 10.9 | 79.7 | 5.4 | 82.9 | 7.2 | 86.9 | 2.4 | 89.7 | 7.0 | 87.6 | 7.2 |
| FR2 REINFORCEMENT SCHEDULE | ||||||||||||
| SESSION | 1 | 2 | 3 | 4 | ||||||||
| BxD 31 | 88.9 | 3.5 | 87.9 | 3.4 | 88.0 | 2.7 | 90.0 | 3.7 | ||||
| BxD 38 | 89.5 | 1.0 | 93.4 | 1.6 | 93.3 | 1.6 | 93.1 | 1.7 | ||||
| BxD 42 | 88.8 | 1.9 | 88.2 | 2.7 | 88.3 | 2.1 | 89.9 | 2.1 | ||||
| BxD 68 | 92.7 | 2.0 | 90.1 | 2.3 | 89.6 | 2.7 | 89.3 | 4.0 | ||||
| FR5 REINFORCEMENT SCHEDULE | ||||||||||||
| SESSION | 1 | 2 | 3 | 4 | 5 | |||||||
| BxD 31 | 87.5 | 4.2 | 86.0 | 4.0 | 86.2 | 2.8 | 90.9 | 2.0 | 89.7 | 1.0 | ||
| BxD 38 | 92.8 | 1.5 | 94.5 | 0.9 | 93.5 | 1.2 | 95.6 | 0.8 | 95.8 | 1.1 | ||
| BxD 42 | 86.2 | 3.5 | 85.6 | 4.3 | 87.7 | 3.1 | 90.2 | 1.8 | 90.4 | 1.9 | ||
| BxD 68 | 94.7 | 0.8 | 95.2 | 1.0 | 93.2 | 2.2 | 95.6 | 1.5 | 96.8 | 1.3 | ||
Averages (bold) ± SEM (italics) are expressed as the percentage of active responses (calculated as active over total responses) during FR1, FR2, and FR5 reinforcement schedules between GRL (BxD 31, BxD38) and PRL (BxD 42, BxD 68) strains.
Asterisks indicate significant differences between pooled PRL and GRL strains in all pairwise comparisons.
Table 2.
Post-infusion Responses.
| FR1 REINFORCEMENT SCHEDULE | ||||||||||||
| SESSION | 1 | 2 | 3 | 4 | 5 | LAST | ||||||
| BxD 31 | 2.0 | 0.6 | 1.5 | 0.6 | 1.7 | 0.9 | 3.3 | 1.1 | 5.4 | 2.2 | 7.4 | 2.0 |
| BxD 38 | 0.4 | 0.2 | 0.8 | 0.4 | 1.8 | 0.6 | 5.0 | 1.3 | 5.3 | 1.3 | 9.65 | 1.6 |
| BxD 42 | 1.8 | 0.5 | 9.7 | 2.6 | 11.9 | 2.5 | 14.1 | 2.4 | 14.0 | 2.8 | 14.1 | 1.8 |
| BxD 68 | 2.5 | 1.3 | 12.0 | 2.5 | 14.7 | 2.3 | 15.2 | 3.8 | 16.5 | 2.1 | 18.2 | 1.7 |
| FR2 REINFORCEMENT SCHEDULE | ||||||||||||
| SESSION | 1 | 2 | 3 | 4 | ||||||||
| BxD 31 | 8.3 | 1.8 | 7.0 | 1.4 | 7.3 | 1.6 | 6.0 | 1.5 | ||||
| BxD 38 | 8.5 | 1.3 | 7.8 | 1.3 | 7.8 | 1.1 | 7.7 | 1.1 | ||||
| BxD 42 | 7.7 | 1.0 | 9.6 | 2.0 | 9.6 | 1.8 | 10.6 | 2.2 | ||||
| BxD 68 | 8.8 | 1.6 | 10.7 | 0.9 | 11.2 | 0.9 | 12.3 | 1.5 | ||||
| FR5 REINFORCEMENT SCHEDULE | ||||||||||||
| SESSION | 1 | 2 | 3 | 4 | 5 | |||||||
| BxD 31 | 87.5 | 4.2 | 86.0 | 4.0 | 86.2 | 2.8 | 90.9 | 2.0 | 89.7 | 1.0 | ||
| BxD 38 | 92.8 | 1.5 | 94.5 | 0.9 | 93.5 | 1.2 | 95.6 | 0.8 | 95.8 | 1.1 | ||
| BxD 42 | 86.2 | 3.5 | 85.6 | 4.3 | 87.7 | 3.1 | 90.2 | 1.8 | 90.4 | 1.9 | ||
| BxD 68 | 94.7 | 0.8 | 95.2 | 1.0 | 93.2 | 2.2 | 95.6 | 1.5 | 96.8 | 1.3 | ||
Averages (bold) ± SEM (italics) are expressed as the number of active lever responses during the 20s post-infusion time-out period under FR1, FR2, and FR5 reinforcement schedules between GRL (BxD 31, BxD38) and PRL (BxD 42, BxD 68) strains.
Asterisks indicate significant differences between pooled PRL and GRL strains in all pairwise comparisons. :
at least p<0.05,
at least p<0.01,
p<0.001
FR2 Schedule
Upon change to the FR2 schedule, all strains experienced an initial decrease in infusions earned, with an increase across subsequent sessions. There was a significant main effect of strain [F (3, 36) =32.76, p<0.001], without a significant strain x session interaction (Figure 3A). This effect was driven by the relatively low number of infusions in one of the GRL strains, BxD31, as compared to PRL strains; the other GRL strain, BxD38, showed an intermediate phenotype. Nevertheless, PRL strains still maintained a larger number of infusions compared to GRL strains across all sessions (all pair-wise comparisons: p<0.05). In contrast to the acquisition phase, there were no differences between the strains in the number of active post-infusion responses made nor in discrimination index (Table 1, 2); all strains responded on the active lever approximately 90% of the time. The strain x session interactions were not significant for either measure.
Fig.3. FR2 and FR5 Cocaine Self-administration.
Comparison of cocaine infusions (0.5mg/kg/20μL inf) earned under (A) FR2 and (B) FR5 reinforcement schedules between GRL and PRL strains. Data are presented as mean ± SEM (FR2: n= 7 BxD31, 17 BxD38, 11 BxD42, 6 BxD68; FR5: n= 7 BxD31, 17 BxD38, 11 BxD42, 6 BxD68). Asterisks indicate significant differences between pooled PRL and GRL strains in all pairwise comparisons: * = at least p<0.05, **= at least p<0.01, ***= p<0.001
FR5 Schedule
A similar pattern of effects was maintained under the FR5 reinforcement schedule, with an initial decrease in response to the schedule change and a differential number of infusions between the strains; ANOVA revealed a significant strain x session interaction [F (12,132) =5.05, p<0.01] and a main effect of strain [F (3, 33) =29.7, p<0.001] (Figure 3B). Compared to GRL strains, PRL strains earned a larger number of infusions, increasingly so as the sessions progressed (all pair-wise comparisons: p<0.05). Amongst the GRL strains, BxD38 mice again earned a larger number of infusions than BxD31 mice (all pair-wise comparisons: p<0.05). Additionally, active responses during timeouts were different between the strains, indicated by a significant strain x session interaction [F (12,132) =61.1, p<0.05] and a main effect of strain [F (3, 33) =12.9, p<0.001] (Table 1). PRL mice made more post-infusion responses than GRL mice in sessions 3, 4, and 5 (all pair-wise comparisons: p<0.05). These effects were without any strain differences in discrimination index (Table 2). The strain x session interaction was also not significant.
Locomotor Activity Experiment
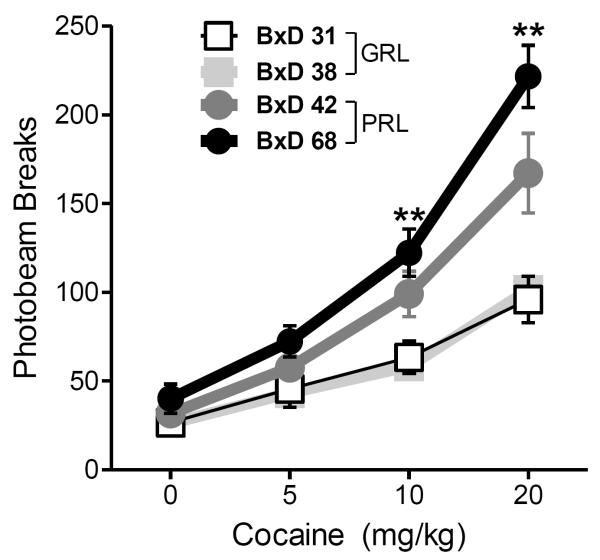
An ANOVA considering total locomotor activity, with strain and cocaine dose as independent variables and beam breaks as the dependent measure, revealed a significant strain x dose interaction [F (6,90) = 5.75, p<0.001] and a main effect of strain [F (3,45) =25.3, p<0.001] (Figure 5). Compared to GRL strains, PRL strains exhibited a larger number of beam breaks after administration of either of the two higher doses of cocaine (10, 20 mg/kg) (all pair-wise comparisons: p<0.01).
Discussion
The goal of this study was to prospectively examine the relationship between genetic differences in inhibitory response control abilities and drug reinforcement, in order to test the hypothesis that heritable variation in a cognitive process linked to impulsivity could predict the propensity to engage in addiction-related behaviors. Past studies in rats have substantiated the idea that dimensions of impulsivity predict self-administration behaviors (Boulougouris et al. 2007; Diergaarde et al. 2008; Perry et al. 2005). The present study extends this work by using select mouse strains from a genetic reference population (BxD panel) to determine the genetic correlation between these phenotypes, evidence required to evaluate the notion that inhibitory control is an endophenotype for drug reinforcement. The results demonstrate that heritable strain differences in inhibitory control do influence drug reinforcement, with strains that exhibit an impulsive/compulsive pattern of responding in a reversal learning test also demonstrating differential sensitivity to the reinforcing and psychomotor stimulant effects of cocaine. Compared to GRL strains, PRL strains self-administered cocaine at elevated rates across all schedules of reinforcement, though their ratio of active to inactive responses was not different. Additionally, PRL mice exhibited increased locomotor activity in response to higher doses of experimenter-administered cocaine, as compared to GRL strains. Together, our findings suggest that genetically-influenced deficits in a dimension of impulsivity – namely, inhibitory response control – can predispose mice to elevated rates of drug consumption.
Strain Differences in Operant Conditioning
Poor reversal ability was associated with an elevated propensity to self-administer more cocaine across increasing schedules of reinforcement. Though reversal learning procedures involve many methodological differences in task details, with different aspects of behavior measured depending upon the specific implementations (Izquierdo and Jentsch 2012), the task used in the present study assesses instrumental reward related reversal learning, in particular, and emphasizes updating of behavior in response to changes in reinforcement contingencies. Furthermore, while these types of reversal learning tasks can be comparable to tests of instrumental extinction in that both measure functions of response inhibition, they have different motivational contexts, with reversal learning involving selective suppression of one response while actively engaging in another, vs. a general inhibition of a single conditional response.
PRL mice exhibited increased rates of drug-reinforced responding, even in response to escalation of the response requirement. The observed strain differences are not likely due to traits such as general learning ability or performance during extinction conditions. All strains were able to discriminate the active vs. inactive levers equally well and learned the initial operant response in the reversal learning test with equal efficiencies (Laughlin et al. 2011). In addition, mice from one of the GRL strains (BxD38) and both PRL strains demonstrate comparable response accuracy and variability in a 5-choice serial reaction time task (Malkki et al. 2010). Moreover, compared to other BxD strains, mice from BxD31 (GRL) exhibit greater rates of initial magazine checking in a food-reinforced operant conditioning task, which could represent enhanced learning in this strain (Loos et al. 2012). That said, if learning ability influenced self-administration rates, GRL strains, not PRL strains, would acquire faster; in fact, we found the opposite: PRL strains acquired cocaine self-administration more readily and made more responses overall.
Conditioned reinforcement may also be an influence on increased response rates, as drug-associated stimuli can exert differential control of drug-related behavior. Indeed, another dimension of behavior linked with both impulse control and incentive motivational processes is the propensity of individuals to behave as “sign-trackers” (Saunders and Robinson 2010). Aside from having a propensity to attribute incentive salience to reward-related cues, “sign-trackers”' have shown to exhibit several traits and behaviors that may contribute to addiction vulnerability, including but not limited to: increased impulsivity/behavioral disinhibition as measured by a 2-choice serial reaction time task and a differential reinforcement of low rates of responding (DRL) task, increased susceptibility to cue-induced reinstatement and increased resistance to extinction (when a drug-related cue is presented) (Lovic et al. 2011). As discussed above, enhanced behavioral control by the drug-related cue could explain some aspects of behavior in PRL mice, thereforethe relationship between reversal learning phenotypes and sign-tracking propensity deserves additional study.
Pharmacokinetic vs. Pharmacodynamic Differences?
Theoretically, greater intake and stimulant effects of cocaine in PRL strains could be due to be differential pharmacokinetics or pharmacodynamics. In this case, pharmacokinetic differences are less likely, as previous studies of the two parental strains (C57BL/6J, DBA/2J) have revealed no differences in brain cocaine concentrations after either intravenous self-administration or intra-peritoneal experimenter-administered cocaine (Rocha et al. 1998; van der Veen et al. 2007). Similarly, reports comparing these two strains have also found higher maximal locomotor stimulant effects of cocaine in DBA/2J mice, without any significant differences in the ED50 (Rocha et al. 1998; Tolliver et al. 1994). Collectively, these data do not provide strong support for pharmacokinetic differences in the parental strains used to create the BxD panel that could explain the differences in cocaine responses reported here.
Alternatively, there is reason to expect that pharmacodynamic differences explain the effects observed here. In our earlier study, we reported that reversal learning abilities varied negatively with brain dopamine D2-like receptor number, an effect similar to that reported in non-human primates (Groman et al. 2012). BxD strains exhibiting poor reversal learning had relatively lower Bmax estimates for D2 receptors in multiple brain regions. Dopamine D2-like receptor availability is also lower in drug-dependent persons (Lee et al. 2009; Volkow et al. 2001) and in humans and animals with impulsivity-related traits that predispose for addictions (Buckholtz et al. 2010; Dalley et al. 2007). One simple hypothesis is that the decrease in D2 receptor number and function leads directly to augmented responding for cocaine reinforcement; this hypothesis is well supported by pharmacological and mutant mouse studies (Caine et al. 2002). At present, it is not clear whether the strain differences in cocaine reinforcement reported here represent strain differences in dose-response curves. Only more systematic dose-response studies will clarify this issue.
Relevance to Other Forms of Impulsive/Compulsive Behavior
In previous studies, other measures of impulsive behavior –namely, premature or anticipatory responding in a 5-choice serial reaction time task and the tendency to discount delayed rewards– have shown to predict alcohol intake or intravenous drug self-administration (Boulougouris et al. 2007; Diergaarde et al. 2008; Mitchell et al. 2006; Perry et al. 2005). With respect to past studies in rats, the data are unable to define whether the relationship is mediated by genetic correlation or by non-genetic factors. Studies in inbred mouse strains that segregate differences in impulsivity-related traits have sometimes found hypothesized relationships to drug and alcohol consumption (Gubner et al. 2010; Moschak et al. 2012). Indeed, when compared with GRL strains, PRL mice made more responses during the post-infusion time-out periods. This is reminiscent of the persistent drug-seeking in rats that occurs during periods of known drug unavailability, which has been reported to be predictive of compulsive drug seeking (Deroche-Gamonet et al. 2004).
Our studies provide unequivocal data for the hypothesis that genetically-influenced differences in inhibitory response control predict levels of drug intake, meaning that the genetic influences on this dimension of impulsivity and on drug-directed behaviors are at least partially shared. In our laboratory, on-going genome wide association studies using the hybrid mouse diversity panel (Ghazalpour et al. 2012) seek to identify the specific genomic loci that mediate this relationship.
Summary
In summary, strains with a phenotype of poor inhibitory control over impulsive responses, measured in a reversal learning test, exhibit increased rates of consumption and sensitivity to the psychomotor effects of cocaine. These effects are not easily explained by differences in general operant conditioning rates, by increased motor activity or by pharmacokinetic mechanisms. Further experiments are needed to more fully explore the relationship between inhibitory control and behavior reflective of different aspects of drug abuse and addiction, as well as to develop more accurate mechanistic accounts of our findings. Nevertheless, these findings provide the first direct evidence in support of the hypothesis that genetically-influenced deficits in this type of inhibitory control may be predisposing factors for susceptibility to substance abuse and dependence.
Fig.4. Differential effects of non-contingent cocaine administration on locomotor activity between BxD strains.
Values represent number of horizontal beam breaks as a function of dose. Data are presented as mean ± SEM (n= 10 BxD31, 24 BxD38, 13 BxD42, 8 BxD68). Asterisks indicate significant differences between pooled PRL and GRL strains in all pairwise comparisons: **= at least p<0.01
Acknowledgements
These studies were supported by several components of the UCLA Consortium for Neuropsychiatric Phenomics (UL1-DE019580, RL1-MH083270, PL1-NS062410) and PHS grant R01-DA031852 awarded to JDJ. MCC was supported by the UCLA Training Program in Translational Neuroscience of Drug Abuse (T32-DA024635). In addition, we are grateful to Yao-Ying Ma for surgical technical support. All experiments comply with the current laws of the United States of America.
Footnotes
Conflict of Interest Disclosure All authors report no potential conflicts of interest.
References
- Aron AR, Poldrack RA. The cognitive neuroscience of response inhibition: relevance for genetic research in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1285–92. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Freimer NB. Endophenotypes for psychiatric disorders: ready for primetime? Trends Genet. 2006;22:306–13. doi: 10.1016/j.tig.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Hindes A. Decision-making and addiction (part II): myopia for the future or hypersensitivity to reward? Neuropsychologia. 2002;40:1690–705. doi: 10.1016/s0028-3932(02)00016-7. [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–5. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornovalova MA, Daughters SB, Hernandez GD, Richards JB, Lejuez CW. Differences in impulsivity and risk-taking propensity between primary users of crack cocaine and primary users of heroin in a residential substance-use program. Exp Clin Psychopharmacol. 2005;13:311–8. doi: 10.1037/1064-1297.13.4.311. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Dalley JW, Robbins TW. Effects of orbitofrontal, infralimbic and prelimbic cortical lesions on serial spatial reversal learning in the rat. Behav Brain Res. 2007;179:219–28. doi: 10.1016/j.bbr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Brewer J, Potenza MN. The neurobiology and genetics of impulse control disorders: relationships to drug addictions. Biochem Pharmacol. 2009;75:63–75. doi: 10.1016/j.bcp.2007.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Shelby ES, Smith CE, Kessler RM, Zald DH. Dopaminergic network differences in human impulsivity. Science. 2010;329:532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mello NK, Patel S, Bristow L, Kulagowski J, Vallone D, Saiardi A, Borrelli E. Role of dopamine D2-like receptors in cocaine self-administration: studies with D2 receptor mutant mice and novel D2 receptor antagonists. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:2977–88. doi: 10.1523/JNEUROSCI.22-07-02977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calu DJ, Stalnaker TA, Franz TM, Singh T, Shaham Y, Schoenbaum G. Withdrawal from cocaine self-administration produces long-lasting deficits in orbitofrontal-dependent reversal learning in rats. Learn Mem. 2007;14:325–8. doi: 10.1101/lm.534807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp Clin Psychopharmacol. 2003;11:18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacology, biochemistry, and behavior. 2009;93:237–47. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–94. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–70. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science (New York, NY) 2004;305:1014–7. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer AN, De Vries TJ. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry. 2008;63:301–8. doi: 10.1016/j.biopsych.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Economidou D, Pelloux Y, Robbins TW, Dalley JW, Everitt BJ. High impulsivity predicts relapse to cocaine-seeking after punishment-induced abstinence. Biol Psychiatry. 2009;65:851–6. doi: 10.1016/j.biopsych.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. Abnormal brain structure implicated in stimulant drug addiction. Science. 2012;335:601–4. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Roiser JP, Robbins TW, Sahakian BJ. Chronic cocaine but not chronic amphetamine use is associated with perseverative responding in humans. Psychopharmacology. 2008;197:421–31. doi: 10.1007/s00213-007-1051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Turton AJ, Pradhan S, Bullmore ET, Robbins TW. Drug addiction endophenotypes: impulsive versus sensation-seeking personality traits. Biol Psychiatry. 2010;68:770–3. doi: 10.1016/j.biopsych.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR. Polydrug abusers display impaired discrimination-reversal learning in a model of behavioural control. J Psychopharmacol. 2006;20:24–32. doi: 10.1177/0269881105057000. [DOI] [PubMed] [Google Scholar]
- Ghazalpour A, Rau CD, Farber CR, Bennett BJ, Orozco LD, van Nas A, Pan C, Allayee H, Beaven SW, Civelek M, Davis RC, Drake TA, Friedman RA, Furlotte N, Hui ST, Jentsch JD, Kostem E, Kang HM, Kang EY, Joo JW, Korshunov VA, Laughlin RE, Martin LJ, Ohmen JD, Parks BW, Pellegrini M, Reue K, Smith DJ, Tetradis S, Wang J, Wang Y, Weiss JN, Kirchgessner T, Gargalovic PS, Eskin E, Lusis AJ, Leboeuf RC. Hybrid mouse diversity panel: a panel of inbred mouse strains suitable for analysis of complex genetic traits. Mamm Genome. 2012 doi: 10.1007/s00335-012-9411-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II. The Endophenotype Concept in Psychiatry: Etymology and Strategic Intentions. American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38:1180–7. doi: 10.1016/s0028-3932(99)00158-x. [DOI] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Randall PK, Middaugh LD. Intravenous cocaine self-administration: individual differences in male and female C57BL/6J mice. Pharmacology, biochemistry, and behavior. 2007;87:267–79. doi: 10.1016/j.pbb.2007.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, James AS, Jentsch JD. Poor response inhibition: at the nexus between substance abuse and attention deficit/hyperactivity disorder. Neuroscience and biobehavioral reviews. 2009;33:690–8. doi: 10.1016/j.neubiorev.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, Lee B, Seu E, James AS, Feiler K, Mandelkern MA, London ED, Jentsch JD. Dysregulation of D(2)-mediated dopamine transmission in monkeys after chronic escalating methamphetamine exposure. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:5843–52. doi: 10.1523/JNEUROSCI.0029-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubner NR, Wilhelm CJ, Phillips TJ, Mitchell SH. Strain differences in behavioral inhibition in a Go/No-go task demonstrated using 15 inbred mouse strains. Alcohol Clin Exp Res. 2010;34:1353–62. doi: 10.1111/j.1530-0277.2010.01219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil SH, Johnson MW, Higgins ST, Bickel WK. Delay discounting in currently using and currently abstinent cocaine-dependent outpatients and non-drug-using matched controls. Addict Behav. 2006;31:1290–4. doi: 10.1016/j.addbeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Jentsch JD. Reversal learning as a measure of impulsive and compulsive behavior in addictions. Psychopharmacology. 2012;219:607–20. doi: 10.1007/s00213-011-2579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Olausson P, De La Garza R, 2nd, Taylor JR. Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2002;26:183–90. doi: 10.1016/S0893-133X(01)00355-4. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–90. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–7. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Laughlin RE, Grant TL, Williams RW, Jentsch JD. Genetic dissection of behavioral flexibility: reversal learning in mice. Biological psychiatry. 2011;69:1109–16. doi: 10.1016/j.biopsych.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviola G, Adriani W, Terranova ML, Gerra G. Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animal models. Neuroscience & Biobehavioral Reviews. 1999;23:993–1010. doi: 10.1016/s0149-7634(99)00032-9. [DOI] [PubMed] [Google Scholar]
- Lee B, Groman S, London ED, Jentsch JD. Dopamine D2/D3 receptors play a specific role in the reversal of a learned visual discrimination in monkeys. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2007;32:2125–34. doi: 10.1038/sj.npp.1301337. [DOI] [PubMed] [Google Scholar]
- Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Mumford JA, Bokarius AV, Dahlbom M, Mukherjee J, Bilder RM, Brody AL, Mandelkern MA. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:14734–40. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, Strong DR, Brown RA. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART) J Exp Psychol Appl. 2002;8:75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Loos M, Staal J, Pattij T, Neuro BMPC, Smit AB, Spijker S. Independent genetic loci for sensorimotor gating and attentional performance in BXD recombinant inbred strains. Genes Brain Behav. 2012;11:147–56. doi: 10.1111/j.1601-183X.2011.00754.x. [DOI] [PubMed] [Google Scholar]
- Lovic V, Saunders BT, Yager LM, Robinson TE. Rats prone to attribute incentive salience to reward cues are also prone to impulsive action. Behav Brain Res. 2011;223:255–61. doi: 10.1016/j.bbr.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkki HA, Donga LA, de Groot SE, Battaglia FP, Neuro BMPC, Pennartz CM. Appetitive operant conditioning in mice: heritability and dissociability of training stages. Front Behav Neurosci. 2010;4:171. doi: 10.3389/fnbeh.2010.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SH, Reeves JM, Li N, Phillips TJ. Delay discounting predicts behavioral sensitization to ethanol in outbred WSC mice. Alcohol Clin Exp Res. 2006;30:429–37. doi: 10.1111/j.1530-0277.2006.00047.x. [DOI] [PubMed] [Google Scholar]
- Moschak TM, Stang KA, Phillips TJ, Mitchell SH. Behavioral inhibition in mice bred for high vs. low levels of methamphetamine consumption or sensitization. Psychopharmacology. 2012;222:353–65. doi: 10.1007/s00213-012-2650-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC . Guide for the Care and Use of Laboratory Animals. The National Academic Press; Washington, D.C.: 2011. [Google Scholar]
- Olausson P, Jentsch JD, Krueger DD, Tronson NC, Nairn AC, Taylor JR. Orbitofrontal cortex and cognitive-motivational impairments in psychostimulant addiction: evidence from experiments in the non-human primate. Annals of the New York Academy of Sciences. 2007;1121:610–38. doi: 10.1196/annals.1401.016. [DOI] [PubMed] [Google Scholar]
- Paine TA, Dringenberg HC, Olmstead MC. Effects of chronic cocaine on impulsivity: relation to cortical serotonin mechanisms. Behav Brain Res. 2003;147:135–47. doi: 10.1016/s0166-4328(03)00156-6. [DOI] [PubMed] [Google Scholar]
- Peirce JL, Lu L, Gu J, Silver LM, Williams RW. A new set of BXD recombinant inbred lines from advanced intercross populations in mice. BMC Genet. 2004;5:7. doi: 10.1186/1471-2156-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology (Berl) 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology. 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Poulos CX, Le AD, Parker JL. Impulsivity predicts individual susceptibility to high levels of alcohol self-administration. Behav Pharmacol. 1995;6:810–814. [PubMed] [Google Scholar]
- Rocha BA, Odom LA, Barron BA, Ator R, Wild SA, Forster MJ. Differential responsiveness to cocaine in C57BL/6J and DBA/2J mice. Psychopharmacology. 1998;138:82–88. doi: 10.1007/s002130050648. [DOI] [PubMed] [Google Scholar]
- Roesch MR, Takahashi Y, Gugsa N, Bissonette GB, Schoenbaum G. Previous cocaine exposure makes rats hypersensitive to both delay and reward magnitude. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:245–50. doi: 10.1523/JNEUROSCI.4080-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, Baker NB, Hunter J, Carthy T, Booker E, London M, Deakin JF, Sahakian BJ, Robbins TW. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 1999;20:322–39. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Robbins TW. Investigating the neurocognitive deficits associated with chronic drug misuse. Current opinion in neurobiology. 2001;11:250–7. doi: 10.1016/s0959-4388(00)00204-x. [DOI] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. A cocaine cue acts as an incentive stimulus in some but not others: implications for addiction. Biol Psychiatry. 2010;67:730–6. doi: 10.1016/j.biopsych.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Mendez IA, Setlow B. Cocaine exposure causes long-term increases in impulsive choice. Behavioral neuroscience. 2007;121:543–9. doi: 10.1037/0735-7044.121.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria G, Barbano MF, Maldonado R, Valverde O. A reliable method to study cue-, priming-, and stress-induced reinstatement of cocaine self-administration in mice. Psychopharmacology. 2008;199:593–603. doi: 10.1007/s00213-008-1184-x. [DOI] [PubMed] [Google Scholar]
- Stalnaker TA, Takahashi Y, Roesch MR, Schoenbaum G. Neural substrates of cognitive inflexibility after chronic cocaine exposure. Neuropharmacology 56 Suppl. 2009;1:63–72. doi: 10.1016/j.neuropharm.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout JC, Rock SL, Campbell MC, Busemeyer JR, Finn PR. Psychological processes underlying risky decisions in drug abusers. Psychol Addict Behav. 2005;19:148–57. doi: 10.1037/0893-164X.19.2.148. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. Intravenous drug self-administration in mice: practical considerations. Behavior genetics. 2007;37:101–18. doi: 10.1007/s10519-006-9097-0. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. False positive in the intravenous drug self-administration test in C57BL/6J mice. Behav Pharmacol. 2011;22:239–47. doi: 10.1097/FBP.0b013e328345f8f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Hall FS, Uhl GR, Caine SB. Dramatically decreased cocaine self-administration in dopamine but not serotonin transporter knock-out mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:1087–92. doi: 10.1523/JNEUROSCI.4037-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolliver BK, Belknap JK, Woods WE, Carney JM. Genetic analysis of sensitization and tolerance to cocaine. J Pharmacol Exp Ther. 1994;270:1230–8. [PubMed] [Google Scholar]
- van der Veen R, Piazza PV, Deroche-Gamonet V. Gene-environment interactions in vulnerability to cocaine intravenous self-administration: a brief social experience affects intake in DBA/2J but not in C57BL/6J mice. Psychopharmacology (Berl) 2007;193:179–86. doi: 10.1007/s00213-007-0777-0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–21. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Wills TA, Vaccaro D, McNamara G. Novelty seeking, risk taking, and related constructs as predictors of adolescent substance use: an application of Cloninger's theory. J Subst Abuse. 1994;6:1–20. doi: 10.1016/s0899-3289(94)90039-6. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Bachtell RK, Theobald DE, Laali S, Green TA, Kumar A, Chakravarty S, Self DW, Nestler EJ. Increased impulsivity during withdrawal from cocaine self-administration: role for DeltaFosB in the orbitofrontal cortex. Cerebral cortex. 2009;19:435–44. doi: 10.1093/cercor/bhn094. [DOI] [PMC free article] [PubMed] [Google Scholar]