Abstract
Objective
The signaling protein CD40 and its ligand, CD40L, are thought to contribute to atherosclerotic plaque formation and rupture. We sought to determine their utility as markers of cerebral atherosclerosis and neurological dysfunction.
Methods
We recruited 82 patients with acute cerebral infarction (ACI) and classified each as having large-artery atherosclerosis (LAA, 30), small-artery occlusion (36), or cardioaortic embolism (16). We also recruited 17 patients who had carotid artery stenosis (CAS) without stroke and 20 healthy individuals as controls. CD40L expression on peripheral blood monocytes (PBMCs) was detected by direct immunofluorescence with flow cytometry, and serum soluble CD40L (sCD40L) was measured by ELISA.
Results
CD40L expression on PBMCs was highest in the LAA group, followed by that in the CAS group (both P<0.01 versus control). It was also higher in patients with atherosclerotic infarction than in those without atherosclerosis (P<0.05). PBMC CD40L expression was sensitive and specific for detecting atherosclerosis and LAA cerebral infarction and was superior to serum C-reactive protein for predicting cerebral atherosclerosis (P<0.01). Serum sCD40L was higher in patients with ACI than in healthy controls (P<0.01) or patients with CAS (P<0.01) and correlated with increased disability on three scales (all P<0.01).
Conclusions
We conclude that patients with ACI have an upregulated CD40–CD40L system, which could be used as a clinical biomarker for assessing atherosclerotic instability and severity of neurological dysfunction.
Keywords: Atherosclerosis, Biomarker, CD40 ligand, C-reactive protein, Stroke
Introduction
Atherosclerosis is a complex chronic inflammatory disorder associated with the concomitant actions of lipids and immune-regulated inflammatory pathways.1 The signaling protein CD40 and its ligand, CD40L (recently renamed CD154), could play an important role in atherosclerotic plaque formation and rupture.2 Indeed, data implicate this CD40–CD40L system in the pathogenesis of inflammation and thrombosis.3 CD40 and CD40L are expressed primarily in human atherosclerotic lesions but not in uninvolved arteries or veins.4 Binding of CD40L to CD40 induces human vascular endothelial cells to express adhesion molecules such as selectin-E, vascular cell adhesion molecule, and intercellular adhesion molecule-1 and to release chemotactic factors such as interleukin-8, RANTES (regulated upon activation, normal T-cell expressed and secreted), and macrophage inflammatory protein.5 Blockade of CD40–CD40L interactions with antibodies in ApoE−/− mice caused the plaques to undergo compositional changes: T cells, macrophages, and lipids decreased; collagenous fibers increased; and the plaques did not progress significantly.6 In addition, blocking CD40–CD40L interactions in mice limited atheroma development and altered features of atherosclerotic plaques associated with stability in humans.7 These results indicate that CD40 and CD40L are critical to the development, progression, and complications of atherosclerosis and might participate in instability and rupture of atherosclerotic lesions.
CD40L is present in two forms, cell membrane-bound CD40L (mCD40L) and soluble CD40L (sCD40L). sCD40L is produced when mCD40L is cleaved by a proteolytic enzyme. CD40L is expressed mainly on CD4+ T cells, platelets, monocytes, macrophages, B cells, and natural killer cells.8 When it binds to CD40 on vascular cells such as endothelial cells, it causes the expression of adhesion molecules and the release of inflammatory cytokines (e.g. interleukin-6) and procoagulant tissue factor.3 Platelet-associated CD40L presents at the cell surface only after platelet activation. sCD40L is then released into the blood from activated platelets.9 Studies have shown elevated levels of sCD40L in patients with unstable angina10 and coronary and cerebral vascular disease.11
C-reactive protein (CRP) is an inflammatory biomarker that independently predicts future vascular events.12 Although high-sensitivity (hs)-CRP is reported to be the best marker for predicting cardiovascular events among the inflammatory biomarkers, including serum amyloid A, interleukin-6, and soluble intercellular adhesion molecule type-1 in healthy postmenopausal women,13 clinical evidence is insufficient to justify the measurement of CRP in the routine evaluation of cerebrovascular disease risk in primary prevention.14
Ischemic stroke has many etiologies, and accurate classification of the cause is critical to determining outcome, recurrence rate, and strategies for secondary stroke prevention. It has been reported that CD40L expression in platelets and serum sCD40L levels are increased in patients with acute cerebral infarction (ACI).15 Our previous study showed that CD40 and CD40L were expressed in carotid atherosclerotic plaques in the shoulder areas, especially in patients with stroke.16 Here, we used an evidence-based classification algorithm known as SSS-TOAST (the Stop Stroke Study Trial of Org 10172 in Acute Stroke Treatment)17 to determine whether levels of CD40L correlate with stroke subtypes and severity of neurological dysfunction. Our research focuses on the expression of CD40L on peripheral blood monocytes (PBMCs) and serum levels of sCD40L and hs-CRP.
Methods
Patients
We recruited 82 patients with ACI who were admitted to our department between December 2006 and December 2007. Patients were included if their first ACI event had occurred within 1 week, as confirmed by cranial CT or MRI. Patients were excluded from the study if they had inflammatory or infectious diseases, malignant tumors, or autoimmune diseases.
All patients were evaluated by clinical symptoms, physical examination, cranial CT or MRI, and cerebral magnetic resonance angiography or cerebral angiography. Based on the SSS-TOAST criteria,14 we classified patients with ACI as having large-artery atherosclerosis (LAA), small-artery occlusion (SAO), or cardioaortic embolism (CAE). We also recruited age- and sex-matched patients who had carotid artery stenosis (CAS) but no history of ischemic stroke event, as confirmed by cerebral MRI or cerebral angiography, during the previous 3 months. These subjects were selected from patients referred to the outpatient clinic or the Department of Neurology. The control group consisted of 20 age- and sex-matched healthy subjects who had had no clinical signs of peripheral, coronary, or cerebral vascular disease within the 6 months preceding study entrance. The study was approved by the local ethics committee. All subjects signed the informed consent.
Neurological function of patients was assessed with the National Institutes of Health Stroke Scale (NIHSS), activities of daily living were assessed with the Barthel Index, and disability was assessed with the modified Rankin scale.
PBMC isolation and culture
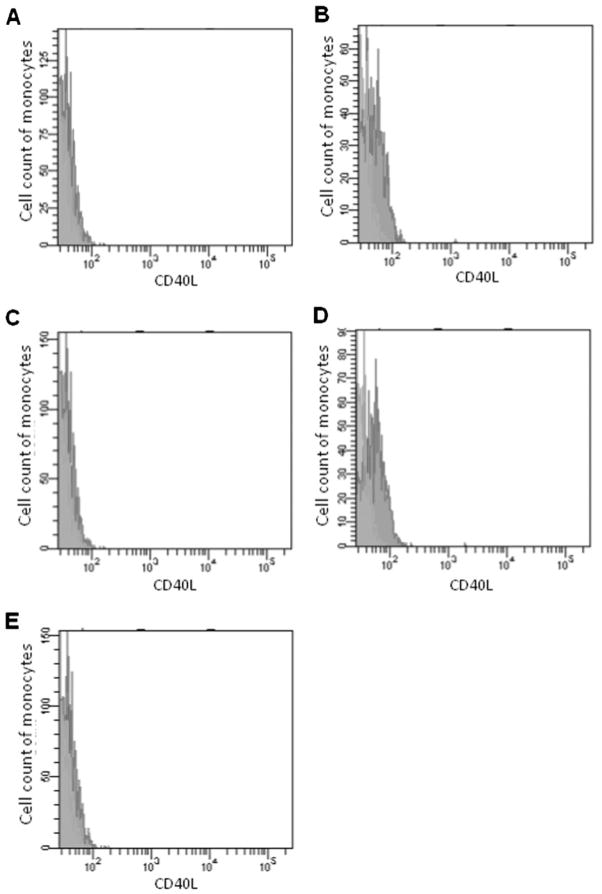
Peripheral blood was drawn from the patients 1–7 days after symptom onset. For each enrolled subject, PBMCs were isolated from 2 ml of blood by density gradient centrifugation. PBMCs were adjusted to a concentration of 1×106 cells/ml with cold phosphate-buffered saline (PBS). One hundred-microliter aliquots of PBMCs were blocked with mouse serum for 10 minutes and then incubated with 10 μl of CD154-FITC fluorescent antibody at 4°C for 40 minutes in the dark. The cells were washed twice in PBS and then resuspended with 300 μl of PBS. Flow cytometry was used to count out 5000 cells (Cell Quest 3.0 software; Fig. 1A). CD40L expression was assessed by mean fluorescence intensity (Fig. 1). Each sample was tested with another fluorescent antibody labeled with isotype IgG as a negative control. All of the sample tests were finished within 6 hours.
Figure 1.
Detection of CD40L-expressing peripheral blood monocytes by flow cytometry. Monocytes gated by flow cytometry and expression of CD40L were assessed by the mean fluorescence intensity. Green area represents isotype control and red area represents CD40L detection. The x-axis represents CD40L fluorescence intensity and the y-axis represents the number of blood monocytes. (A) Normal control. (B) Carotid artery stenosis only. (C) Small-artery occlusion infarct. (D) Large-artery atherosclerotic infarct. (E) Cardioaortic cerebral embolism infarct.
Detection of serum sCD40L and hs-CRP levels
We analyzed serum sCD40L by ELISA using commercial kits and reagents. All assays had intra-assay and inter-assay coefficients of variation <5% and <10%, respectively. The hs-CRP level of each group was detected by an ultrasensitive nephelometric method with automatic biochemistry.
Statistical analysis
The data were analyzed by SPSS 10.0 statistical software and are presented as mean±standard deviation. The NIHSS, Barthel Index, and modified Rankin scale scores are expressed as medians with interquartile ranges (25th and 75th percentiles). One-way comparisons were made with one-factor analysis of variance, two-way comparisons of homogeneity of variance with least significant difference, and multiple comparison of heterogeneity of variance with Dunnett’s C test. Non-parametric data were analyzed with the Wilcoxon rank-sum test. The Spearman correlation was used to determine the strength of association between the variables. Comparisons of sensitivity and specificity were made with a Chi-square test. Statistical significance was set at a value of P<0.05.
Results
Study population demographics
The baseline characteristics of the subjects are summarized in Table 1. Patients with ACI included 46 men and 36 women with a mean age of 62.1±12.3 years (range: 34–91 years). The LAA group comprised 30 patients (19 male, 11 female) with a mean age of 62.7±12.3 years (range: 45–81 years); the SAO group comprised 36 patients (20 male, 16 female) with a mean age of 61.9±11.8 years (range: 46–91 years); and the CAE group comprised 16 patients (7 male, 9 female) with a mean age of 63.0±14.0 years (range: 34–76 years). The CAS group included 17 patients (10 male, 7 female) with a mean age of 61.8±10.1 years (range: 44–88 years), and the control group included 20 patients (13 male, 7 female) with a mean age of 60.9±11.8 years (range: 42–82 years). There were no significant differences in sex or age between the groups (all P>0.05).
Table 1.
Characteristics of patients and controls
| Characteristic | Control (n=20) | CAS (n=17) | LAA (n=30) | SAO (n=36) | CAE (n=16) |
|---|---|---|---|---|---|
| Age in years (SD) | 60.9 (11.8) | 61.8 (10.1) | 62.7 (12.3) | 61.9 (11.8) | 63.0 (14.0) |
| Sex, male (%) | 13 (65.00) | 10 (58.82) | 19 (63.33) | 20 (55.56) | 7 (43.75) |
| Hypertension (%) | 11 (55.00) | 11 (64.71) | 31 (81.58) | 15 (60.00)* | 5 (62.5) |
| Cardiac disease (%) | 3 (15.00) | 3 (17.65) | 7 (18.92)** | 4 (16.00)** | 8 (100) |
| Diabetes mellitus (%) | 4 (20.00) | 4 (23.53) | 10 (27.03) | 6 (24.00) | 2 (25.00) |
| Dyslipidemia (%) | 7 (35.00) | 8 (47.06) | 19 (51.35) | 11 (44.00) | 2 (25.00) |
| Smoking (%) | 6 (30.00) | 6 (35.29) | 20 (54.05) | 8 (32.00) | 2 (25.00) |
| Alcohol drinking (%) | 4 (20.00) | 4 (23.53) | 9 (24.32) | 7 (28.00) | 1 (12.50) |
Note: CAS, carotid artery stenosis only; LAA, large-artery atherosclerosis; SAO, small-artery occlusion; CAE, cardioaortic embolism.
P=0.040 for LAA versus SAO.
P<0.001 for CAE versus LAA and for CAE versus SAO.
Table 2 shows the relative levels of neurological disability in patients with ACI as assessed by the NIHSS, Barthel Index, and modified Rankin scale. Both the NIHSS score and the Barthel Index were significantly higher in the CAE group than in the LAA group and significantly higher in the LAA group than in the SAO group, indicating that disability was the greatest in the CAE group. The modified Rankin scale score was significantly higher in the CAE group than in the LAA and SAO groups (both P<0.05), but the difference between the LAA and SAO groups was not significant (P=0.052).
Table 2.
Neurological function and disability in patients with acute cerebral infarction
| Functionality test | SAO (n=36) | LAA (n=30) | CAE (n=16) |
|---|---|---|---|
| NIHSS | 3.5 (2, 6) | 9 (7, 13)** | 12.5 (6, 28.75)**,†† |
| Barthel Index | 90 (55, 100) | 30 (20, 61.25)* | 22.5 (0, 50)**,†† |
| Modified Rankin scale | 2 (1, 4) | 4 (3.75, 4) | 4 (4, 5)*† |
Note: Data are presented as medians, with 25th and 75th percentiles given in parentheses.
SAO, small-artery occlusion; LAA, large-artery atherosclerosis; CAE, cardioaortic embolism; NIHSS, National Institutes of Health Stroke Scale.
P<0.05 versus SAO.
P<0.01 versus SAO.
P<0.05 versus LAA.
P<0.01 versus LAA.
Measurement of CD40L expression on PBMCs by indirect immunofluorescence flow cytometry
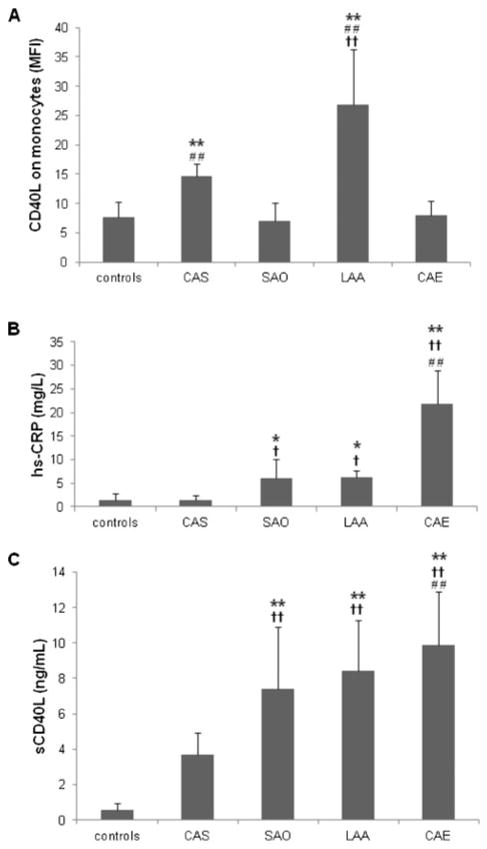
The mean fluorescence intensity of CD40L expression was 7.65±2.64 in normal controls, 14.59±2.18 in the CAS group, 7.08±3.09 in the SAO group, 26.87±9.43 in the LAA group, and 7.94±2.46 in the CAE group. CD40L expression on PBMCs was significantly higher in the LAA group than in the other ACI groups (SAO and CAE), CAS group, or normal controls (all P<0.01). It also was significantly higher in the CAS group than in the control, SAO, or CAE groups (all P<0.01; Fig. 2A). There was no significant difference in CD40L expression on PBMCs between the normal controls and SAO and CAE groups (all P>0.05).
Figure 2.
Comparisons of CD40L and high-sensitivity C-reactive protein (hs-CRP) between healthy controls and patients with carotid artery stenosis (CAS), small-artery occlusion (SAO), large-artery atherosclerosis (LAA), and cardioaortic embolism (CAE). (A) Mean fluorescence intensity (MFI) of CD40L on peripheral blood monocytes as assessed by flow cytometry. **P<0.01 versus control; ††P<0.01 versus CAS; ##P<0.01 versus SAO or CAE. (B) Serum hs-CRP levels. *P<0.05, **P<0.01 versus control; †P<0.05, ††P<0.01 versus CAS; ##P<0.01 versus SAO or LAA. (C) Serum sCD40L levels. **P<0.01 versus control; †† P<0.01 versus CAS; ##P<0.01 versus SAO.
Serum hs-CRP level
Serum hs-CRP levels were 1.44±1.18 mg/l in normal controls, 1.42±1.01 mg/l in the CAS group, 5.99± 4.07 mg/l in the SAO group, 6.18±1.42 mg/l in the LAA group, and 21.98±7.04 mg/l in the CAE group. Among subtypes of ACI, serum hs-CRP was significantly higher in the CAE group than in any other group (all P<0.01), and significantly higher in the SAO and LAA groups than in the normal control and CAS groups (all P<0.05; Fig. 2B). There was no significant difference in serum hs-CRP level between the SAO and LAA groups (P>0.05) or between the CAS group and normal controls (P>0.05).
CD40L on PBMCs and serum hs-CRP in CAS and ACI
Of the 99 patients, 47 had cerebral atherosclerosis (CAS and LAA). When the upper bounds of 95% confidence intervals of 8.89 and 1.99 for normal controls were used as cutoff points for CD40L and hs-CRP, respectively, the sensitivity of CD40L for cerebral atherosclerosis was 100% and the specificity was 72.31%; the sensitivity of hs-CRP was 51.06% and the specificity was 40.68%. Hence, CD40L had greater sensitivity and specificity for predicting cerebral atherosclerosis than did hs-CRP (P<0.01).
Serum sCD40L levels detected by ELISA
Serum sCD40L was 0.56±0.40 ng/ml in normal controls, 3.71±1.19 ng/ml in the CAS group, 7.41±3.49 ng/ml in the SAO group, 8.41±2.89 ng/ ml in the LAA group, and 9.99±3.01 ng/ml in the CAE group. The serum sCD40L level was significantly higher in the CAS, SAO, LAA, and CAE groups than in the normal controls (all P<0.01; Fig. 2C). It was also higher in all groups with ACI (SAO, LAA, and CAE) than in the CAS-only group (all P<0.01), and it was higher in the CAE group than in the SAO group (P<0.01). There was no significant difference in serum sCD40L between the CAE group and the LAA group (P=0.066) or between the LAA group and the SAO group (P=0.118).
Correlations between serum hp-CRP, CD40L, sCD40L, and neurological dysfunction
CD40L expression on PBMCs had a positive linear correlation with NIHSS score (Spearman rho=0.282, P<0.05; Fig. 3A) and a negative linear correlation with Barthel Index score (Spearman rho=−0.253, P<0.05; Fig. 3B). Similarly, serum hs-CRP level had a positive linear correlation with NIHSS score (Spearman rho=0.433, P<0.01; Fig. 3C) and a negative linear correlation with Barthel Index score (Spearman rho=0.569, P<0.01; Fig. 3D). There was a positive linear correlation between serum sCD40L and NIHSS score (Spearman rho=0.237, P<0.05; Fig. 3E), but no correlation between serum sCD40L and Barthel Index in ACI patients (Spearman rho= −0.178, P>0.05).
Figure 3.
Correlations between CD40L expression on PBMCs, serum hp-CRP, serum sCD40L, and neurological dysfunction. CD40L expression on PBMCs was positively correlated with NIHSS score (Spearman rho=0.282, P<0.05; A) and negatively correlated with Barthel Index score (Spearman rho=−0.253, P<0.05; B). Serum hs-CRP level was positively correlated with NIHSS score (Spearman rho=0.433, P<0.01; C) and negatively correlated with Barthel Index score (Spearman rho=0.569, P<0.01; D). There was a positive linear correlation between serum sCD40L and NIHSS score (Spearman rho=0.237, P<0.05, E).
Discussion
Inflammation is an important factor in the development of atherosclerosis and plaque instability.9 The interaction between CD40 and CD40L may accelerate atherogenesis, enlarge atheromatous plaques, and promote thrombotic occlusion of the artery by inducing proinflammatory cytokines, metalloproteinases, and procoagulant tissue factor.3 Our results showed that CD40L expression in circulating monocytes was higher in patients with CAS than in normal controls. CD40L on PBMCs also was higher in patients with the LAA stroke type than in patients with any other type of ACI or pure CAS. These results are consistent with a previous report showing that patients with LAA and diabetes mellitus have higher levels of CD40L than do patients with SAO18 and suggest that circulating monocytes with high CD40L expression may be associated with cerebral atherosclerosis and plaque instability. Platelets, macrophages, T cells, endothelial cells, and smooth muscle cells in atherosclerotic lesions can express CD40L.4,8 Binding of CD40 in these cell types induces the expression of various molecules considered relevant to atherogenesis, including adhesion molecules, pro-inflammatory cytokines, and chemokines. It also induces matrix-degrading activities and likely promotes plaque activation, plaque rupture, thrombosis, and ischemia.19 Entry of monocytes with high CD40L expression into atherosclerotic plaques could cause plaque instability.
CRP has been widely accepted as a potent risk indicator that can independently predict future cardiovascular events.20 It has been reported that plasma levels of the inflammatory marker hs-CRP are associated with cervicocephalic atherosclerosis load in patients who have experienced an ischemic stroke or transient ischemic attack.21 Elevated CRP also is associated with an increased risk of ischemic stroke.22,23 However, ischemic stroke has many etiologies. Our analysis of serum hs-CRP level in subtypes of ACI showed that serum hs-CRP was higher in the CAE group than in the other ACI groups, suggesting that hs-CRP is not a reliable biomarker for cerebral atherosclerosis. Previous studies have shown that an elevated serum CRP level reflects an increased tendency for plaque rupture and a high atherosclerotic burden.24,25 However, we did not observe significant differences in the serum hs-CRP level between the CAS group and normal controls. This discrepancy further suggests that serum hs-CRP is not a reliable biomarker for cerebral atherosclerosis. Alternatively, the level of association is too low to be detected in our small sample size. In a previous report, postmortem data revealed that individuals who had died suddenly from atherothrombi or a cardiac event with stable or unstable plaque had CRP levels that were higher than those of individuals who had died of unnatural or non-cardiac causes.25 Although we found that serum hs-CRP levels correlated with the severity of neurological dysfunction, our results indicate that CD40L in circulating monocytes is more sensitive and specific than serum hs-CRP for detecting atherosclerosis. Hence, CD40L may be a better biomarker for detecting cerebral atherosclerosis and plaque instability.
Serum sCD40L, which is derived primarily from activated platelets, has biological activity.26 An elevated sCD40L level has been reported to predict future cardiovascular events in healthy women27 and recurrent events and death in patients with acute coronary syndromes.28 We have shown previously that the levels of P-selectin, tissue plasminogen activator antigen, and plasminogen activator inhibitor-1 activity are significantly higher and tissue plasminogen activator activity significantly lower in patients with LAA than in control subjects, indicating that patients with LAA have an increase in platelet activation and a decrease in fibrinolysis.29 Consistent with previous reports,10 our data showed that the amount of serum sCD40L released from activated platelets was higher in patients who had ACI than in patients who had CAS but had not experienced a stroke. Likewise, sCD40L was higher in patients who had CAS without stroke than in healthy controls. Further analyses of CD40L, sCD40L level, and neurological function of patients with ACI showed that CD40L expression in circulating monocytes and sCD40L level were correlated with neurological dysfunction and severity of ACI. These results support a link between the CD40–CD40L system and the underlying inflammatory and hypercoagulable state in patients with cerebral atherosclerosis.
Limitations of our study include the variability in length of time between symptom onset and sample collection and the small number of patients with ACI. The results need to be confirmed in a larger sample of patients. Additional study is also needed to determine whether interventions that downregulate CD40L expression on circulating monocytes or serum sCD40L level could benefit patients with cerebral atherosclerosis and acute ischemic stroke.
In conclusion, our results indicate that elevated CD40L expression on PBMCs is associated with carotid atherosclerosis and plaque instability. Furthermore, CD40L was more sensitive than was hs-CRP as a predictor of cerebral atherosclerosis. Elevated serum sCD40L level had a positive correlation with the severity of cerebral infarction and an inverse correlation with patients’ ability to carry out activities of daily living. Together, these findings suggest that the CD40–CD40L system could be used as a clinical biomarker for evaluating cerebral atherosclerosis, plaque instability, and the etiology of ACI.
Acknowledgments
This work was supported by Zhejiang Provincial Natural Science Foundation of China (LY12H09008)and Technology Development Fund (03DZl9708), and NIH (K01AG031926, R01AT007317, R01NS078026). We thank Claire Levine for assistance with this manuscript.
References
- 1.Ross R. Atherosclerosis — an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Hassan GS, Merhi Y, Mourad W. CD40 ligand: a neo-inflammatory molecule in vascular diseases. Immunobiology. 2012;217:521–32. doi: 10.1016/j.imbio.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Schönbeck U, Libby P. CD40 signaling and plaque instability. Circ Res. 2001;89:1092–103. doi: 10.1161/hh2401.101272. [DOI] [PubMed] [Google Scholar]
- 4.Mach F, Schonbeck U, Sukhova GK, Bourcier T, Bonnefoy JY, Pober JS, et al. Functional CD40 ligand is expressed on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for CD40–CD40 ligand signaling in atherosclerosis. Proc Natl Acad Sci USA. 1997;94:1931–6. doi: 10.1073/pnas.94.5.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansson GK, Robertson AK, Soderberg-Naucler C. Inflammation and atherosclerosis. Annu Rev Pathol. 2006;1:297–329. doi: 10.1146/annurev.pathol.1.110304.100100. [DOI] [PubMed] [Google Scholar]
- 6.Mach F, Schonbeck U, Sukhova GK, Atkinson E, Lippy P. Reduction of atherosclerosis in mice by inhibition of CD40 signalling. Nature. 1998;394:200–3. doi: 10.1038/28204. [DOI] [PubMed] [Google Scholar]
- 7.Lutgens E, Gorelik L, Daemen MJ, de Muinck ED, Grewal IS, Koteliansky VE, et al. Requirement for CD154 in the progression of atherosclerosis. Nat Med. 1999;5:1313–6. doi: 10.1038/15271. [DOI] [PubMed] [Google Scholar]
- 8.Schönbeck U, Libby P. The CD40/CD154 receptor/ligand dyad. Cell Mol Life Sci. 2001;58:4–43. doi: 10.1007/PL00000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andre P, Nannizzi-Alaimo L, Prasad SK, Phillips DR. Platelet-derived CD40L: the switch-hitting player of cardiovascular disease. Circulation. 2002;106:896–9. doi: 10.1161/01.cir.0000028962.04520.01. [DOI] [PubMed] [Google Scholar]
- 10.Linden MD, Furman MI, Frelinger AL, III, Fox ML, Barnard MR, Li Y, et al. Indices of platelet activation and the stability of coronary artery disease. J Thromb Haemost. 2007;5:761–5. doi: 10.1111/j.1538-7836.2007.02462.x. [DOI] [PubMed] [Google Scholar]
- 11.Lobbes MB, Lutgens E, Heeneman S, Cleutjens KB, Kooi ME, van Engelshoven JM, et al. Is there more than C-reactive protein and fibrinogen? The prognostic value of soluble CD40 ligand, interleukin-6 and oxidized low-density lipoprotein with respect to coronary and cerebral vascular disease. Atherosclerosis. 2006;187:18–25. doi: 10.1016/j.atherosclerosis.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 13.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 14.Di Napoli M, Schwaninger M, Cappelli R, Ceccarelli E, Di Gianfilippo G, Donati C, et al. Evaluation of C-reactive protein measurement for assessing the risk and prognosis in ischemic stroke: a statement for health care professionals from the CRP Pooling Project members. Stroke. 2005;36:1316–29. doi: 10.1161/01.STR.0000165929.78756.ed. [DOI] [PubMed] [Google Scholar]
- 15.Garlichs CD, Kozina S, Fateh-Moghadam S, Handschu R, Tomandl B, Stumpf C, et al. Upregulation of CD40–CD40 ligand (CD154) in patients with acute cerebral ischemia. Stroke. 2003;34:1412–8. doi: 10.1161/01.STR.0000074032.64049.47. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Wang Z, Wu T, Deng B, Ding S, Chen H, et al. The roles of CD40–CD40L in carotid atherosclerotic plaque stability and their correlation with matrix metalloproteinase 9. Chin J Neurol. 2009;42:390–5. [Google Scholar]
- 17.Ay H, Furie KL, Singhal A, Smith WS, Sorensen AG, Koroshetz WJ, et al. An evidence-based causative classification system for acute ischemic stroke. Ann Neurol. 2005;58:688–97. doi: 10.1002/ana.20617. [DOI] [PubMed] [Google Scholar]
- 18.Tsai NW, Chang WN, Shaw CF, Jan CR, Chang HW, Huang CR, et al. Levels and value of platelet activation markers in different subtypes of acute non-cardio-embolic ischemic stroke. Thromb Res. 2009;124:213–8. doi: 10.1016/j.thromres.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 19.Antoniades C, Bakogiannis C, Tousoulis D, Antonopoulos AS, Stefanadis C. The CD40/CD40 ligand system: linking inflammation with atherothrombosis. J Am Coll Cardiol. 2009;54:669–77. doi: 10.1016/j.jacc.2009.03.076. [DOI] [PubMed] [Google Scholar]
- 20.Bisoendial RJ, Boekholdt SM, Vergeer M, Stroes ES, Kastelein JJ. C-reactive protein is a mediator of cardiovascular disease. Eur Heart J. 2010;31:2087–91. doi: 10.1093/eurheartj/ehq238. [DOI] [PubMed] [Google Scholar]
- 21.Kim SJ, Choe YH, Bang OY. Are stroke biomarkers seeing brain vessels in patients with ischemic stroke?: a C-reactive protein and homocysteine study. Stroke. 2011;42:1464–8. doi: 10.1161/STROKEAHA.110.607432. [DOI] [PubMed] [Google Scholar]
- 22.Woodward M, Lowe GD, Campbell DJ, Colman S, Rumley A, Chalmers J, et al. Associations of inflammatory and hemostatic variables with the risk of recurrent stroke. Stroke. 2005;36(10):2143–7. doi: 10.1161/01.STR.0000181754.38408.4c. [DOI] [PubMed] [Google Scholar]
- 23.Mario DN, Francesca P, Vittorio B. C-reactive protein in ischemic stroke: an independent prognostic factor. Stroke. 2001;32:917–24. doi: 10.1161/01.str.32.4.917. [DOI] [PubMed] [Google Scholar]
- 24.Burke AP, Tracy RP, Kolodgie F, Malcom GT, Zieske A, Kutys R, et al. Elevated C-reactive protein values and atherosclerosis in sudden coronary death: association with different pathologies. Circulation. 2002;105:2019–23. doi: 10.1161/01.cir.0000015507.29953.38. [DOI] [PubMed] [Google Scholar]
- 25.Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med. 1997;336:1276–82. doi: 10.1056/NEJM199705013361802. [DOI] [PubMed] [Google Scholar]
- 26.Andre P, Prasad KS, Denis CV, He M, Papalia JM, Hynes RO, et al. CD40L stabilizes arterial thrombi by a beta3 integrin —dependent mechanism. Nat Med. 2002;8:247–52. doi: 10.1038/nm0302-247. [DOI] [PubMed] [Google Scholar]
- 27.Schönbeck U, Varo N, Libby P, Buring J, Ridker PM. Soluble CD40L and cardiovascular risk in women. Circulation. 2001;104:2266–8. doi: 10.1161/hc4401.099447. [DOI] [PubMed] [Google Scholar]
- 28.Heeschen C, Dimmeler S, Hamm CW, van den Brand MJ, Boersma E, Zeiher AM, et al. Soluble CD40 ligand in acute coronary syndromes. N Engl J Med. 2003;348:1104–11. doi: 10.1056/NEJMoa022600. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Li J, Liu Q. Association between platelet activation and fibrinolysis in acute stroke patients. Neurosci Lett. 2005;384:305–9. doi: 10.1016/j.neulet.2005.04.090. [DOI] [PubMed] [Google Scholar]