Abstract
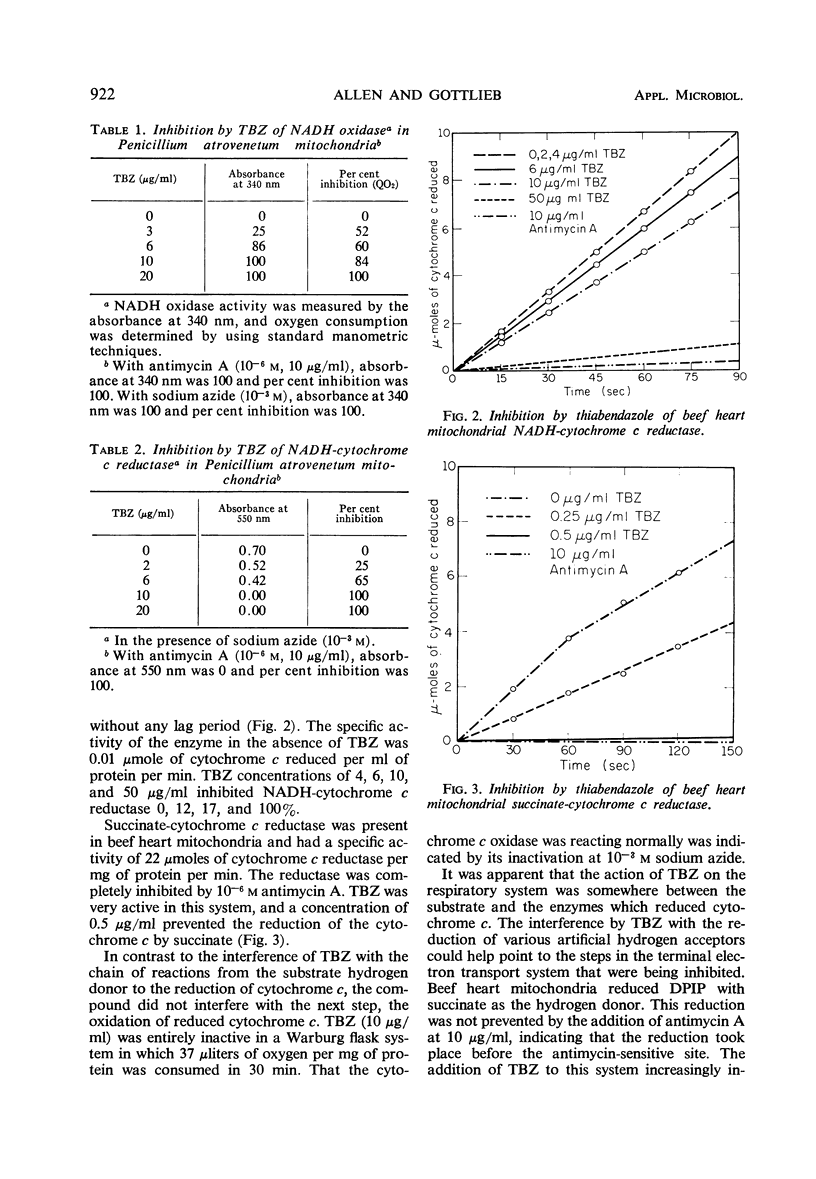
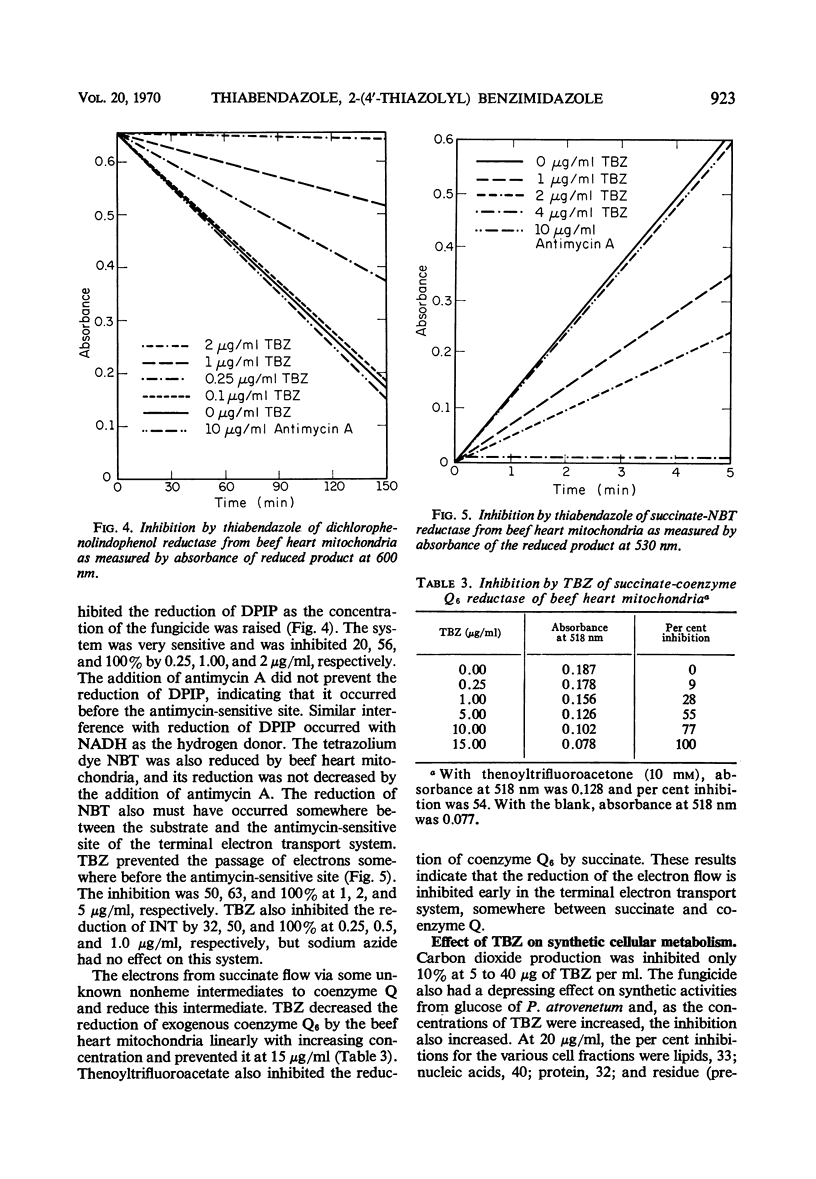
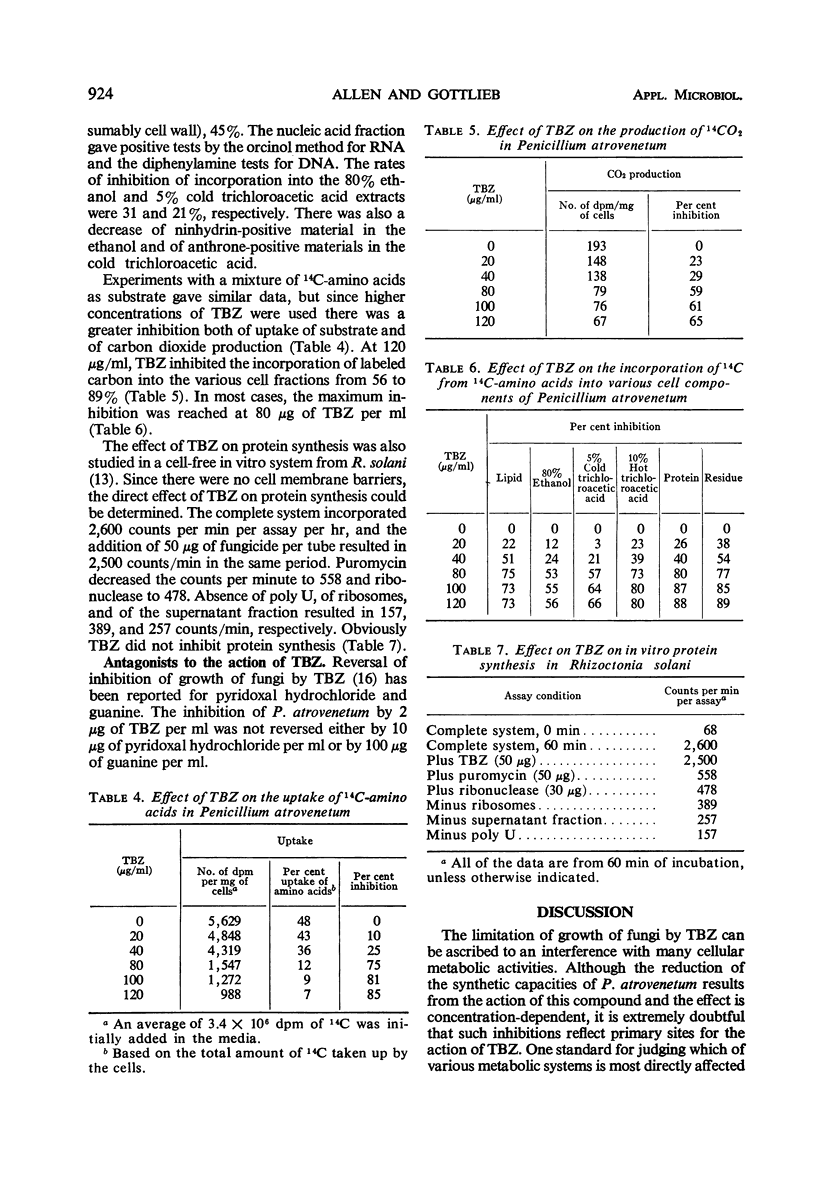
Thiabendazole, 2-(4′-thiazolyl) benzimidazole (TBZ) inhibited the growth of Penicillium atrovenetum at 8 to 10 μg/ml. Oxygen consumption with exogenous glucose was inhibited at 20 μg/ml, but endogenous respiration required more than 100 μg/ml. TBZ inhibited completely the following systems of isolated heart or fungus mitochondria: reduced nicotinamide adenine dinucleotide oxidase, succinic oxidase, reduced nicotinamide adenine dinucleotide-cytochrome c reductase, and succinic-cytochrome c reductase at concentrations of 10, 167, 10, and 0.5 μg/ml, respectively. Cytochrome c oxidase was not inhibited. Antimycin A and sodium azide caused the usual inhibition patterns for both fungus and heart terminal electron transport systems. In the presence of antimycin, the fungicide inhibited completely succinate-dichloro-phenolindophenol reductase and succinate-2, 2-di-p-nitrophenyl-(3, 3-dimethoxy-4, 4-biphenylene-5, 5-diphenylditetrazolium)-reductase at 2 and 4 μg of TBZ per ml, respectively. Coenzyme Q reductase required 15 μg/ml. TBZ reduced the uptake by P. atrovenetum of glucose and amino acids and decreased the synthesis of various cell components. At 120 μg/ml, the incorporation of labeled carbon from amino acids-U-14C was decreased: lipid, 73%; nucleic acids, 80%; protein, 80%; and a residual fraction, 89%. TBZ did not inhibit peptide synthesis in a cell-free protein-synthesizing system from Rhizoctonia solani. Probably the primary site of inhibition is the terminal electron transport system and other effects are secondary.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CRANE F. L., GLENN J. L., GREEN D. E. Studies on the electron transfer system. IV. The electron transfer particle. Biochim Biophys Acta. 1956 Dec;22(3):475–487. doi: 10.1016/0006-3002(56)90058-0. [DOI] [PubMed] [Google Scholar]
- KIKUCHI G., BARRON E. S. G. Electron transport system in Fusarium lini. Arch Biochem Biophys. 1959 Sep;84:96–105. doi: 10.1016/0003-9861(59)90557-0. [DOI] [PubMed] [Google Scholar]
- RAMASARMA T., LESTER R. L. Studies on the electron transport system. XXIV. The reduction and oxidation of exogenous coenzyme Q. J Biol Chem. 1960 Nov;235:3309–3314. [PubMed] [Google Scholar]


