Abstract
Introduction
Camphorquinone (CQ) is a photoinitiator that triggers polymerization of light-curing materials such as dental adhesives and composites. CQ does not become a part of the polymer network, suggesting that CQ can be leached out into surrounding environment including dental pulp and exert adversary effects on tissues. In order to understand the mechanisms of CQ-induced side effects, we investigated the effect of CQ on cell viability, cytokine secretion, and odontogenic differentiation of dental pulp stem cells in vitro.
Methods
Cell viability was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay after CQ exposure. Western blotting was performed for p16INK4A, p21WAF1, and p53. Secretory cytokines were evaluated using the membrane–enzyme-linked immunosorbent assay as well as conventional and quantitative reverse-transcription polymerase chain reaction. The effects of CQ on odontogenic differentiation were evaluated using alkaline phosphatase and alizarin red S staining methods.
Results
CQ treatment suppressed the proliferation of DPSCs and induced the expression of p16INK4A, p21WAF1, and p53. Levels of proinflammatory cytokines (eg, interleukin 6, interleukin 8, and matrix metalloproteinase-3 [MMP3]) were increased by CQ treatment. CQ also inhibited odontogenic differentiation and mineralization capacities of DPSC and MC3T3-E1 cells.
Conclusions
Our study showed that CQ may trigger pulpal inflammation by inducing proinflammatory cytokine production from the pulpal cells and may impair odontogenic differentiation of dental pulp cells, resulting in pulpal irritation and inflammation.
Keywords: Camphorquinone, dental pulp stem cells, inflammation, odontogenic differentiation, pulpal wound healing
Camphorquinone (CQ) is a photoinitiator commonly incorporated in the vast majority of modern dental composite materials including composites and dental adhesives (DAs) (1). CQ uses the visible light-curing systems to initiate the polymerization process (2). When irradiated with visible light in the range of 460–480 nm, CQ generates free radicals, one of the major forms of reactive oxygen species (ROS), in the presence of coinitiators such as tertiary aromatic amines. This free radical initiates the polymerization of monomers such as 2-hydroxyl-ethyl-methacrylate (HEGMA), triethyleneglycol dimethyacrylate, or 2,2-bis(4-[2-hydroxy-3-methacryloxypropoxy] phenyl)propane (3).
Although CQ is one of the important components in dental composite materials, CQ does not become incorporated into the resin polymers, suggesting that there is a likelihood of CQ being leached out into the local environment. CQ is one of the leachable substances in dental materials (4, 5), and it was suggested that the maximum concentration of 14 mmol/L CQ potentially could be leached out into the oral cavity after polymerization (6).
Substances leached out from dental materials were shown to be cytotoxic to cells (7), and several studies have been conducted to examine the cytotoxic effects of CQ on cells in vitro. Atsumi et al (8, 9) found that irradiated CQ induced ROS production and inhibited the proliferation of cells. Irradiated CQ also caused DNA damage because of ROS generation as determined using a cell-free system (10). The cytotoxic activity of CQ was shown to be correlated with ROS production (11). Recent studies showed that nonirradiated CQ also has cytotoxic effects, presumably because of its activation in cells by intracellular mechanisms (6). Indeed, nonirradiated CQ was found to generate ROS, leading to DNA alteration in cells (9–13). Collectively, these studies suggest that both irradiated and nonirradiated CQ exert cytotoxic effects.
The cytotoxic effects of CQ on cell viability have been well documented; however, the molecular events associated with the inhibition of cell proliferation or its effects on the differentiation and mineralization potential of dental pulp cells are unknown. In this study, we test the hypothesis that the inhibition of pulp cell proliferation by CQ is linked with an increased expression of cell cycle regulatory genes and inflammatory cytokines. Consequently, exposure to CQ impairs the odontogenic differentiation capacity of dental pulp cells.
Materials and Methods
Reagents, Cells, and Cell Culture
Primary dental pulp stem cells (DPSCs) were kindly provided by Dr Songtao Shi (Herman Ostrow School of Dentistry, University of Southern California, Los Angeles, CA). MC3T3-E1 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA). DPSCs and MC3T3-E1 cells were cultured in the basal medium containing 10% fetal bovine serum (FBS) in α-Minimum Essential Medium (MEM) (Invitrogen, Grand Island, NY). To induce these cells to undergo differentiation and mineralization, cells were cultured in the induction medium (IM) that contains 10% FBS, 100 µmol/L ascorbic acid 2-phosphate, 10 mmol/L β-glycerophosphosphate, and 1.8 mmol/L KH2PO4 (Sigma-Aldrich Inc, St Louis, MO) in α-MEM (14). CQ and HEMA were purchased from Sigma-Aldrich and prepared in ethanol.
MTT Assay
Cell viability in response to CQ treatment was accessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) Cell Proliferation Assay (ATCC) according to the manufacturer’s protocol. DPSCs were treated with CQ or HEMA for 3 days in the range of 12,500–50 µmol/L. The plates were read at 570 nm using the ELx800 Absorbance Microplate Reader (BioTek, Winooski, VT).
Western Blotting
Cells were lysed and subjected to Western blotting as described previously (14). The following antibodies were used: p53 (DO-1), p21 (C-19), p16 (C-20), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (0411) from Santa Cruz Biotechnology (Santa Cruz, CA).
Conventional and Real-time Quantitative Reverse-transcription Polymerase Chain Reaction
The total RNA was isolated, and complementary DNA was made as described previously (14). Quantitative reverse-transcription polymerase chain reaction (qRT-PCR) was performed in triplicates for each sample with LC480 SYBR Green I master (Roche, Indianapolis, IN) using universal cycling conditions on LightCycler 480 (Roche). A total of 45 cycles were executed, and the second derivative Cq value determination method was used to compare fold differences. For the conventional RT-PCR, a total of 35 cycles were executed. The primer sequences are available upon request.
Cytokines Enzyme-linked Immunosorbent Assay Array
The secretory cytokines were detected using the enzyme-linked immunosorbent assay (ELISA)-based Human Antibody Array Assay 3.0 (Panomics, Fremont, CA) according to the manufacturer’s protocol. Briefly, 2 mL fresh supernatants collected from DPSCs treated or not with 250 or 500 µmol/L CQ were incubated with the array membrane followed by incubation with biotin-labeled anticytokine antibodies and streptavidin horseradish peroxidase (HRP). The membranes were exposed using chemiluminescence detection method, and the intensity of the dots on the membranes was quantitated using ImageJ software (National Institutes of Health, Bethesda, MD) (15). The dot intensities were normalized to the number of cells at the time harvesting supernatants.
Alkaline Phosphatase Activity and Alizarin Red S Staining
Confluent DPSCs were cultured in IM containing different amounts of CQ for 4 days followed by an additional 6 days without CQ. To stain for alkaline phosphatase (ALP) activity, ALP staining kit (86R-1KT, Sigma- Aldrich Inc) was used according to the manufacturer’s protocol. To quantify ALP activity, cells were lysed in lysis buffer (50 mmol/L Tris- HCl [pH = 10], 0.5% Triton X-100 (Sigma-Aldrich Inc), and 1% NP- 40). The collected supernatants were incubated with reaction mixture containing 0.2 mol/L glycine-NaOH buffer (pH = 10.4), 16 mmol/L p- NPP substrate (Sigma-Aldrich Inc, Cat #N3254), 1 mol/L MgCl2, and 1 mol/L ZnCl2. The mixture was incubated at 37°C, and the color changes were detected using the microplate reader at 405 nm as described previously (14). Alizarin red S (ARS) staining was performed on DPSCs and MC3T3-E1 cells. Briefly, cells were cultured in IM for 4 weeks and fixed with 1% formalin/phosphate-buffered saline for 10 minutes and stained with 2% ARS solution (pH = 4.1–4.3 with 10% ammonium hydroxide) for 30 minutes at the room temperature. The ARS solution was removed, and cells were washed with ddH2O. The plates were photographed using a camera. For the quantification of ARS staining, stained cells were destained in 10% cetylpryidinium chloride (Sigma- Aldrich Inc) and measured at 652 nm using the microplate reader. The ImageJ software was also used to quantify the staining (15).
Statistical Analysis
Assays were performed in triplicates (MTT assays, qRT-PCR, or ELISA assay). The results are expressed as the mean ± standard deviation. To compare the outcomes, the Student’s t test was performed, and P values <.05 were considered significant.
Results
CQ Inhibits the Proliferation of DPSCs
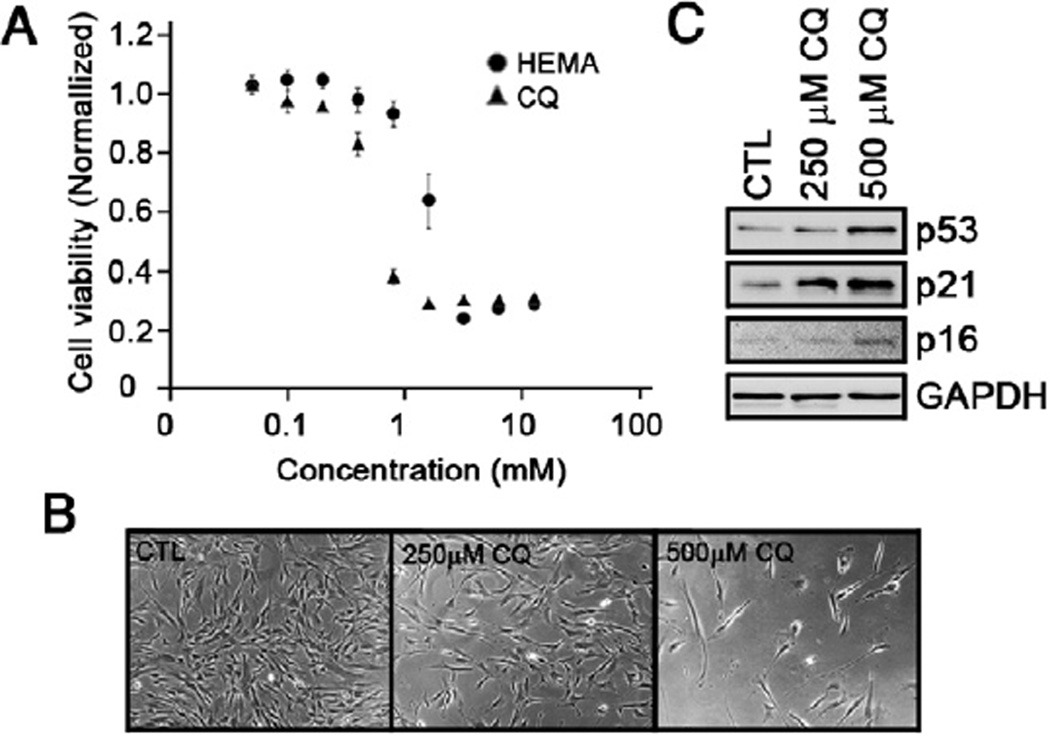
To examine the cytotoxic effects of CQ on DPSCs, the MTT assay was performed with various doses for 3 days, and the cell viability was examined. We found that DPSCs started losing their viability between 200 and 400 µmol/L CQ (Fig. 1A). As a positive control, we also treated DPSCs with HEMA, which is known to cause apoptosis (16). Compared with HEMA, CQ was more cytotoxic to DPSCs (Fig. 1A). When the cells were treated with 250 or 500 µmol/L CQ for 3 days, a loss of cell proliferation was noted (Fig. 1B), and it was associated with enhanced expression of p53, p21WAF1, and p16INK4A (Fig. 1C).
Figure 1.
The effects of CQ on proliferation. (A) An MTT assay was performed by treating the DPSCs with different doses of CQ or HEMA for 3 days. The experiment was quadruplicated, and the bar represents the standard error. (B) Photographs of DPSCs treated with indicated doses of CQ for 3 days were taken (200× magnification). (C) CQ-treated DPSCs were harvested and subjected to Western blotting against p53, p21WAF1, p16INK4A, and GAPDH.
CQ Induces Expression of Proinflammatory Cytokines in DPSCs
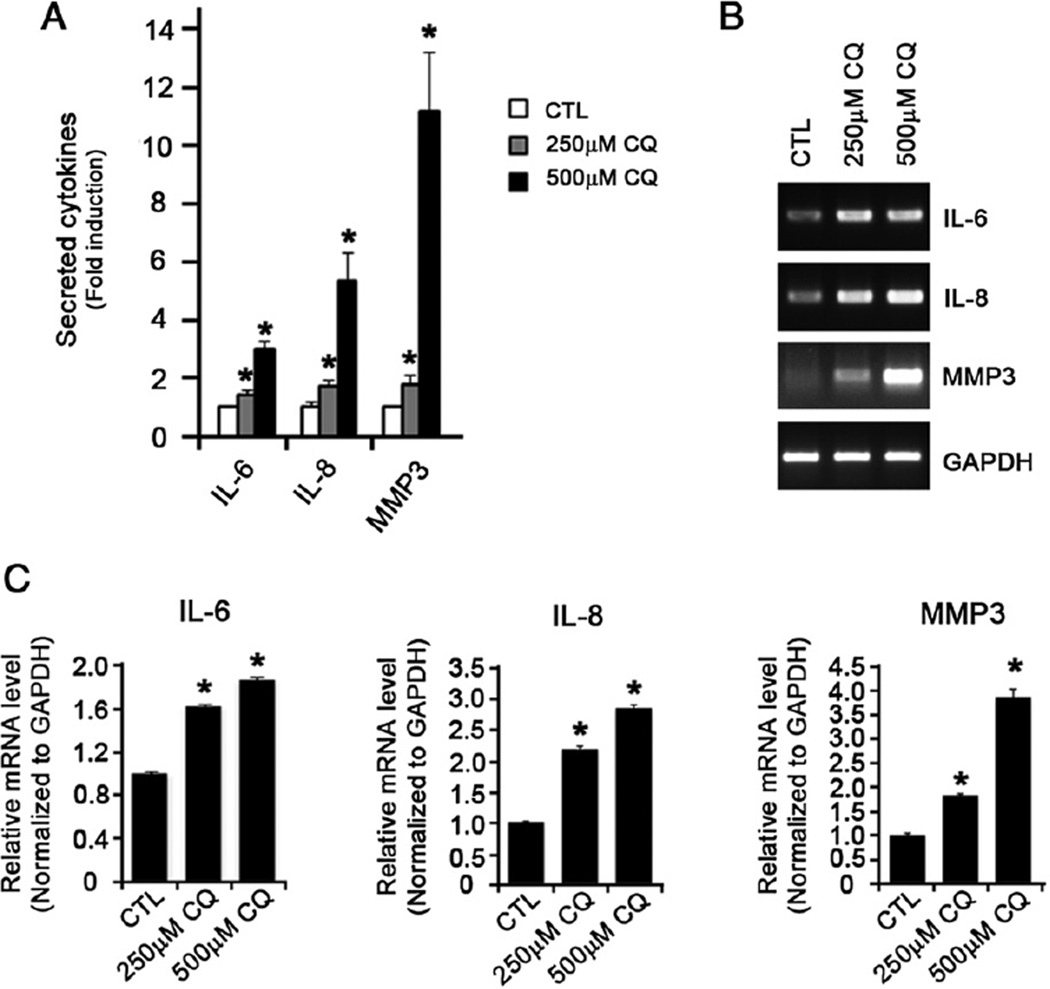
Next, we sought to determine the expression of cytokines by DPSCs when treatedwith CQ. DPSCs were treated with CQ for 3 days, supernatants were collected, and secreted cytokines were screened using the ELISA-based membrane. Among the 36 cytokines that were screened, we found significant increases in the levels of interleukin (IL)-6, IL-8, and matrix metalloproteinase-3 (MMP3) in CQ-treated DPSCs (Fig. 2A). This was also confirmed using conventional RT-PCR (Fig. 2B) and real-time qRT-PCR (Fig. 2C), indicating that CQ enhances the secretion of IL-6, IL-8, and matrix metalloproteinase-3 (MMP3) in DPSCs.
Figure 2.
CQ induces the production of IL-6, IL-8, and MMP3. (A) Supernatants from DPSCs treated or not with 250 or 500 µmol/L CQ for 3 days were collected. The secretory cytokines were detected using ELISA-based Human Antibody Array Assay 3.0. The intensity of the dots indicating the presence of corresponding cytokines was quantified using ImageJ software. The fold induction was indicated in relation to the intensity from the CQ-untreated cells. (B) The same cells were collected and subjected to conventional RT-PCR. (C) Real-time qRT-PCR was also performed in triplicate. GAPDH was used to normalize.
CQ Inhibits Differentiation and Mineralization Potentials of DPSCs
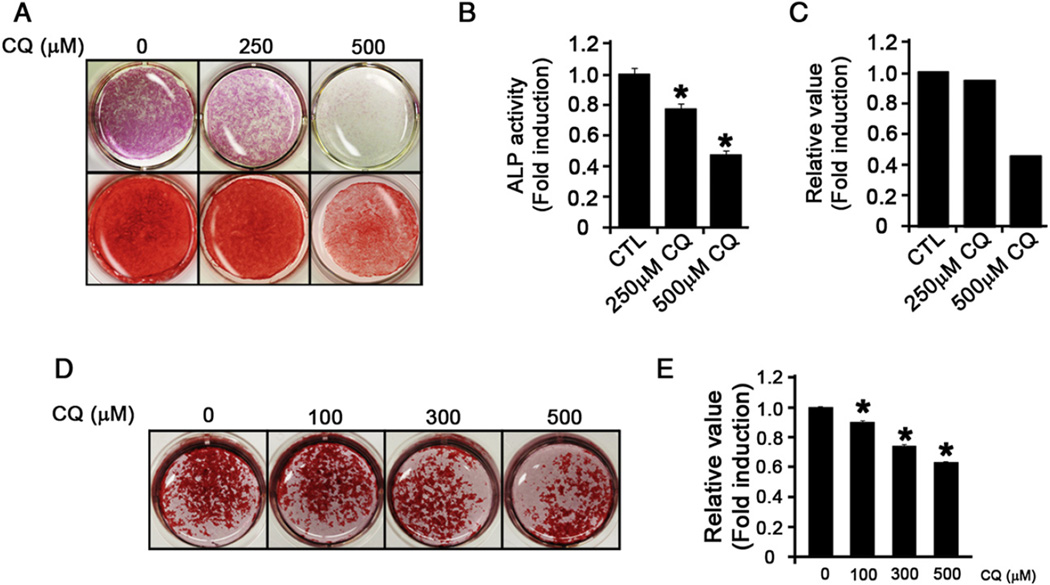
We examined the effects of CQ on the differentiation potential of DPSCs. In the presence of CQ, the diminution of ALP activity was observed in a dose-dependent manner in DPSCs (Fig. 3A, top panel; Fig. 3B). We also evaluated the mineralization potential and observed reduced ARS staining in the presence of CQ (Fig. 3A, bottom panels; Fig. 3C). To further test whether the effects of CQ on mineralization also occur in other cell types, we used a mouse osteoblastic cell line, MC3T3-E1 cells, and found similar results (Fig. 3D and E). Collectively, these data indicate that CQ inhibits the differentiation and mineralization potentials of dental mesenchymal and osteoblastic cells.
Figure 3.
CQ inhibits the differentiation and mineralization of DPSCs. (A) DPSCs treated or not with CQ in IM were cultured for 10 days or 4 weeks, and the differentiation or mineralization potentials were evaluated using ALP (top panels) or ARS staining (bottom panels) methods, respectively. (B) The ALP activity in CQ-treated DPSCs was quantified using a microplate reader at 405 nm. (C) ARS staining was quantified using ImageJ software. (D) A similar experiment was performed using MC3T3-E1 cells, and the ARS staining was performed to examine the mineralization potential. (E) ARS staining was destained and quantified using a microplate reader at 652 nm.
Discussion
The effects of dental material components such as HEMA and triethylene glycol dimethacrylate (TEGDMA) on dental pulp cells are well documented (17). However, there are no reports examining the effects of CQ on the differentiation and mineralization of dental pulp cells. In this study, we showed that CQ inhibits proliferation, enhances the secretion of proinflammatory cytokines, and inhibits the differentiation and mineralization of dental pulp cells in vitro. Our study shows the inhibitory effects of CQ on the proliferation and differentiation capacities of human pulp cells, presumably through the induction of inflammatory reaction.
The concentration of CQ in dental resins is found to be approximately 0.17%–1.03% w/w (18), which is equivalent to 3–5 mmol/L. A previous study suggested that the maximum concentration of 14 mmol/L could potentially be leached out into the oral cavity (6). Using CQ at 250 and 500 µmol/L, which is significantly lower than the maximum leachable amounts of CQ, we showed that CQ inhibits the proliferation of DPSCs, which is associated with the induction of p53, p21WAF1, and p16INK4A (Fig. 1). CQ-mediated inhibition of proliferation seems to be primarily caused by p21WAF1, which was significantly induced at 250 µmol/L CQ compared with p53 and p16INK4A (Fig. 1C). p21WAF1 is a potent cyclin-dependent kinase inhibitor that inhibits cell cycle progression at the G1 phase (19). In line with this finding, previous cell cycle analysis with CQ revealed significant cell cycle arrest at the G1 phase (20), suggesting that p21WAF1 is the primary mediator that causes arrest of the cell cycle and cell division of dental pulp cells by CQ.
Previous studies suggest that the application of DA as direct pulp capping materials on exposed pulp induces inflammation (21–23). Our study showed that CQ directly induces the secretion of potent proinflammatory cytokines (eg, IL-6, IL-8, and MMP3) (Fig. 2). These cytokines are frequently noted in inflamed pulp tissues (24–26). Therefore, pulpal inflammation is, in part, mediated by CQ in DAs. The differentiation and mineralization of odontogenic cells are other key steps required to complete the pulpal wound healing processes. When treated with various doses of CQ, all the tested cells including DPSC and MC3T3-E1 exhibited the inhibition of differentiation and mineralization (Fig. 3A–E), suggesting that CQ may be one of the components in DA responsible for the improper pulpal wound healing processes in vivo.
Previous studies showed that even nonirradiated CQ generated ROS intracellularly and caused DNA damages (6). ROS plays a critical role in the differentiation and mineralization of osteogenic cells. It has been shown that H2O2 inhibits osteoblastic differentiation of primary mouse bone marrow stem cells, primary rabbit bone marrow stem cells, and murine osteoblastic cell lines (MC3T3-E1) (27–29). On the other hand, inflammatory cytokines such as IL-6 have been shown to inhibit mineralization both in vitro and in vivo (30, 31). Therefore, it is possible that CQ-mediated inhibition of mineralization occurs either directly via ROS generation or indirectly via inducing inflammation.
Although our study showed the effects of CQ on the proliferation and differentiation capacities of dental pulp cells, it is likely that other components of dental materials also exert adverse effects on the dental pulp cells in vivo. For example, HEMA is known to induce apoptosis (15). The differentiation and mineralization of dental pulp progenitor cells have been recently shown to be inhibited by HEMA and TEGDMA (32). Therefore, the combined effects of these dental materials may be sufficient to suppress pulpal wound healing processes.
Clinically, direct pulp capping with adhesive systems is considered to be not well tolerated because of the induction of pulp inflammation and the lack of dentinal bridge formation even without bacterial presence (33). Our study suggests that CQ may be another contributing factor for inducing such pathological lesions and that the light-curing dental materials containing photoinitiators such as CQ may be not suitable for their use as direct pulp capping materials. Further preclinical and clinical studies warrant a closer examination.
Acknowledgments
Supported in part by the grants (K08DE17121, R03DE30304 to R.H.K., K02DE18959 and R01DE18295 to M.K.K.) from NIDCR/NIH. R.H.K. was supported by the UCLA Academic Senate Faculty Research Grant. M.K.K. was supported by the Jack Weichman Endowment Fund and American Association of Endodontists Foundation grant.
Footnotes
The authors deny any conflicts of interest related to this study.
References
- 1.Van Landuyt KL, Snauwaert J, De Munck J, et al. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials. 2007;28:3757–3785. doi: 10.1016/j.biomaterials.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 2.Stansbury JW. Curing dental resins and composites by photopolymerization. J Esthet Dent. 2000;12:300–308. doi: 10.1111/j.1708-8240.2000.tb00239.x. [DOI] [PubMed] [Google Scholar]
- 3.Ikemura K, Endo T. A review of the development of radical photopolymerization initiators used for designing light-curing dental adhesives and resin composites. Dent Mater J. 2010;29:481–501. doi: 10.4012/dmj.2009-137. [DOI] [PubMed] [Google Scholar]
- 4.Geurtsen W, Spahl W, Leyhausen G. Variability of cytotoxicity and leaching of substances from four light-curing pit and fissure sealants. J Biomed Mater Res. 1999;44:73–77. doi: 10.1002/(sici)1097-4636(199901)44:1<73::aid-jbm8>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 5.Spahl W, Budzikiewicz H, Geurtsen W. Determination of leachable components from four commercial dental composites by gas and liquid chromatography/mass spectrometry. J Dent. 1998;26:137–145. doi: 10.1016/s0300-5712(96)00086-3. [DOI] [PubMed] [Google Scholar]
- 6.Volk J, Ziemann C, Leyhausen G, Geurtsen W. Non-irradiated campherquinone induces DNA damage in human gingival fibroblasts. Dent Mater. 2009;25:1556–1563. doi: 10.1016/j.dental.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Cavalcanti BN, Rode SM, Marques MM. Cytotoxicity of substances leached or dissolved from pulp capping materials. Int Endod J. 2005;38:505–509. doi: 10.1111/j.1365-2591.2005.00967.x. [DOI] [PubMed] [Google Scholar]
- 8.Atsumi T, Murata J, Kamiyanagi I, et al. Cytotoxicity of photosensitizers camphorquinone and 9-fluorenone with visible light irradiation on a human submandibularduct cell line in vitro. Arch Oral Biol. 1998;43:73–81. doi: 10.1016/s0003-9969(97)00073-3. [DOI] [PubMed] [Google Scholar]
- 9.Atsumi T, Ishihara M, Kadoma Y, et al. Comparative radical production and cytotoxicity induced by camphorquinone and 9-fluorenone against human pulp fibroblasts. J Oral Rehabil. 2004;31:1155–1164. doi: 10.1111/j.1365-2842.2004.01357.x. [DOI] [PubMed] [Google Scholar]
- 10.Pagoria D, Lee A, Geurtsen W. The effect of camphorquinone (CQ) and CQ-related photosensitizers on the generation of reactive oxygen species and the production of oxidative DNA damage. Biomaterials. 2005;26:4091–4099. doi: 10.1016/j.biomaterials.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 11.Atsumi T, Iwakura I, Fujisawa S, Ueha T. The production of reactive oxygen species by irradiated camphorquinone-related photosensitizers and their effect on cytotoxicity. Arch Oral Biol. 2001;46:391–401. doi: 10.1016/s0003-9969(01)00005-x. [DOI] [PubMed] [Google Scholar]
- 12.Engelmann J, Volk J, Leyhausen G, Geurtsen W. ROS formation and glutathione levels in human oral fibroblasts exposed to TEGDMA and camphorquinone. J Biomed Mater Res B Appl Biomater. 2005;75:272–276. doi: 10.1002/jbm.b.30360. [DOI] [PubMed] [Google Scholar]
- 13.Li YC, Huang FM, Lee SS, et al. Protective effects of antioxidants on micronuclei induced by camphorquinone/N, N-dimethyl-p-toluidine employing in vitro mammalian test system. J Biomed Mater Res B Appl Biomater. 2007;82:23–28. doi: 10.1002/jbm.b.30700. [DOI] [PubMed] [Google Scholar]
- 14.Mehrazarin S, Oh JE, Chung CL, et al. Impaired odontogenic differentiation of senescent dental mesenchymal stem cells is associated with loss of Bmi-1 expression. J Endod. 2011;37:662–666. doi: 10.1016/j.joen.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneider CA, Rasband WS, Eliceiri KW. NIH Imag to ImageJ: 25 years of image analysis. Nature Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paranjpe A, Bordador LC, Wang MY, et al. Resin monomer 2-hydroxyethyl methacrylate (HEMA) is a potent inducer of apoptotic cell death in human and mouse cells. J Dent Res. 2005;84:172–177. doi: 10.1177/154405910508400212. [DOI] [PubMed] [Google Scholar]
- 17.Bakopoulou A, Papadopoulos T, Garefis P. Molecular toxicology of substances released from resin-based dental restorative materials. Int J Mol Sci. 2009;10:3861–3899. doi: 10.3390/ijms10093861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taira M, Urabe H, Hirose T, et al. Analysis of photo-initiators in visible-light-cured dental composite resins. J Dent Res. 1988;67:24–28. doi: 10.1177/00220345880670010401. [DOI] [PubMed] [Google Scholar]
- 19.Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 20.Masuki K, Nomura Y, Bhawal UK, et al. Apoptotic and necrotic influence of dental resin polymerization initiators in human gingival fibroblast cultures. Dent Mater J. 2007;26:861–869. doi: 10.4012/dmj.26.861. [DOI] [PubMed] [Google Scholar]
- 21.Pameijer CH, Stanley HR. The disastrous effects of the “total etch” technique in vital pulp capping in primates. Am J Dent. 1998;11:S45–S54. [PubMed] [Google Scholar]
- 22.Costa CA, Hebling J, Hanks CT. Current status of pulp capping with dentin adhesive systems: a review. Dent Mater. 2000;16:188–197. doi: 10.1016/s0109-5641(00)00008-7. [DOI] [PubMed] [Google Scholar]
- 23.Sübay RK, Demirci M. Pulp tissue reactions to a dentin bonding agent as a direct capping agent. J Endod. 2005;31:201–204. doi: 10.1097/01.don.0000137649.24821.91. [DOI] [PubMed] [Google Scholar]
- 24.Yang LC, Tsai CH, Huang FM, et al. Induction of interleukin-6 gene expression by pro-inflammatory cytokines and black pigmented Bacteroides in human pulp cell cultures. Int Endod J. 2003;36:352–357. doi: 10.1046/j.1365-2591.2003.00663.x. [DOI] [PubMed] [Google Scholar]
- 25.Huang GT, Lee HW, Lee HS, et al. Localization of substance P-induced upregulated interleukin-8 expression in human dental pulp explants. Int Endod J. 2008;41:100–107. doi: 10.1111/j.1365-2591.2007.01318.x. [DOI] [PubMed] [Google Scholar]
- 26.De Couto Pita A, Borda E, Ganzinelli S, et al. Cholinoceptor modulation on nitric oxide regulates prostaglandin E(2) and metalloproteinase-3 production in experimentally induced inflammation of rat dental pulp. J Endod. 2009;35:529–536. doi: 10.1016/j.joen.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Liu AL, Zhang ZM, Zhu BF, et al. Metallothionein protects bone marrow stromal cells against hydrogen peroxide-induced inhibition of osteoblastic differentiation. Cell Biol Int. 2004;28:905–911. doi: 10.1016/j.cellbi.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Bai XC, Lu D, Bai J, et al. Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-kappaB. Biochem Biophys Res Commun. 2004;314:197–207. doi: 10.1016/j.bbrc.2003.12.073. [DOI] [PubMed] [Google Scholar]
- 29.Arai M, Shibata Y, Pugdee K, et al. Effects of reactive oxygen species (ROS) on anti-oxidant system and osteoblastic differentiation in MC3T3-E1 cells. IUBMB Life. 2007;59:27–33. doi: 10.1080/15216540601156188. [DOI] [PubMed] [Google Scholar]
- 30.Hughes FJ, Howells GL. Interleukin-6 inhibits bone formation in vitro. Bone Miner. 1993;21:21–28. doi: 10.1016/s0169-6009(08)80117-1. [DOI] [PubMed] [Google Scholar]
- 31.De Benedetti F, Rucci N, Del Fattore A, et al. Impaired skeletal development in interleukin-6-transgenic mice: a model for the impact of chronic inflammation on the growing skeletal system. Arthritis Rheum. 2006;54:3551–3563. doi: 10.1002/art.22175. [DOI] [PubMed] [Google Scholar]
- 32.Bakopoulou A, Leyhausen G, Volk J, et al. Effects of HEMA and TEDGMA on the in vitro odontogenic differentiation potential of human pulp stem/progenitor cells derived from deciduous teeth. Dent Mater. 2011;27:608–617. doi: 10.1016/j.dental.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Modena KC, Casas-Apayco LC, Atta MT, et al. Cytotoxicity and biocompatibility of direct and indirect pulp capping materials. J Appl Oral Sci. 2009;17:544–554. doi: 10.1590/S1678-77572009000600002. [DOI] [PMC free article] [PubMed] [Google Scholar]