Abstract
Purpose
To detect the testosterone deficiency syndrome in Mongolian men over 40 years old with erectile dysfunction (ED).
Materials and Methods
Total of 309 males over 40 years of age who received medical care at the ADAM Urology and Andrology Clinic from 2010 to 2011 were included in this study. An approval from the Ethics Committee of the Ministry of Health of Mongolia was obtained, and each study participant signed a consent form at the beginning of the study. The participants were assigned to either an ED group or a control group, depending on the results of the international index of erectile function (IIEF)-5 questionnaire. The ED group was further divided into three groups (moderate, severe, and very severe) based on the level of ED. The total testosterone (TT) levels were determined in the blood serum using a competitive enzyme-linked immunesorbent assay (ELISA) analytical system UBI Magiwel™ Testosterone Quantitative test, and free testosterone (FT) calculated as described by the Vermeulen calculation. Test samples were collected between 8:00 and 11:00 am in the mornings and testosterone deficiency syndrome was diagnosed based on the International Society for the Study of the Aging Male guidelines, particularly, if TT was ≤3.46 ng/ml or free testosterone FT was ≤0.072 ng/ml.
Results
ED of moderate, severe, and very severe levels was diagnosed in 199 (64.41%) out of 309 participants. There was an inverse relationship between the main IIEF-5 score and age (r=-0.380, p<0.01). The average TT was 5.75±2.316 ng/ml and FT was 0.091±0.0084 ng/ml. Compared to the ED group, the control group had a higher TT level: 5.6440±1.177 ng/ml and 5.812±2.316 ng/ml, respectively. In the control group, the FT level was 0.061±0.0084 ng/ml, whereas it was 0.041±0.0076 ng/ml in the ED group.
Conclusions
Our study showed that most of the aging males who came to the clinic had moderate to very severe ED (64.55%). The levels of TT (5.644±1.177 ng/ml) and FT (0.041±0.0036 ng/ml) were significantly lower in ED patients (p<0.05). The testosterone deficiency syndrome was detected in 24.27% of the ED group.
Keywords: Erectile dysfunction, Hypogonadism, Testosterone, Prevalence
INTRODUCTION
Erectile dysfunction (ED), also known as impotence, is defined as a consistent or recurrent inability to attain and/or maintain penile erection sufficient for sexual performance.1 According to recent study results, early detection and treatment of ED prevents myocardial infraction and brain stroke or vascular events. ED occurs in more than 50 percent of males over 60 years old, emphasizing the need to diagnose and treat it at an earlier stage.
ED may be assessed in several ways. The most widely used standardized questionnaire is the International Index of Erectile Function (IIEF).2 On the other hand, ED is associated with a decreased level of androgens in aging males; the latter is often referred to as a late onset hypogonadism (LOH) or the testosterone deficiency syndrome (TDS). In simple terms, LOH or TDS can be defined as a decreased serum testosterone level in aging males.3
Testosterone may play a role in the reflex activity of the autonomic nervous system in the pelvis, or may interact with postsynaptic non-genomic receptors suppressing detrusor activity.4
There is not enough evidence-based research related to ED and TDS in Mongolia. Therefore, the influence of testosterone and ED on lower urinary tract symptoms in Mongolian men was investigated. The erectile function levels and forms of sexual function were evaluated by the IIEF-5 score, and LUTS was evaluated with the International Prostate Symptom Score (IPSS) questionnaire.
MATERIALS AND METHODS
1. Subjects
This study was done on 309 men aged over 40 years with a primary complaint of sexual dysfunction such as ED or decreased libido as the main symptom of TDS. Information was collected between May 2010 and November 2012 in the ADAM Urology and Andrology Clinic, Ulaanbaatar of Mongolia. The patients with the following characteristics were excluded from the study: use of androgen therapy or anabolic steroids in the previous 12 months, history of prostate cancer, prostate-specific antigen (PSA) elevated higher than 10 ng/ml with suspicion for prostate cancer by digital rectal examination (DRE), previous prostatic surgery, or hepatic or renal failure.
Socio-demographic characteristics consisted of age and education level. Health-related lifestyle consisted of smoking status and the consumption level of alcohol (high consumption: >30 g/day).
Non-fasting blood samples were taken from the cubital vein between 8:00 AM and 11:00 AM. Measurements of total testosterone (TT; Normal range 3.46~10.0) ng/ml) were performed from blood serum by using enzyme-linked immunesorbent assay (ELISA) technique using the Magiwel by United Biotech Inc. (Mountain View, CA, USA) commercial kit, sex hormone binding globulin (SHBG) by DRG International Inc. (Spring-field, NJ, USA) was measured using an active SHBG two-site immune-radiometric assay.
The free testosterone level was calculated by the Vermilion formula and recommended by the International Society for the Study of the Aging Male (ISSAM).
The IIEF-5 was used to evaluate sexual function. Each participant was assigned to either an ED group or a control group, depending on results of the IIEF-5 questionnaire. The evaluation of the degree of ED was considered normal when the IIEF score was 22~25, while 17~21 is mild, 12~16 is mild to moderate, 8~11 is moderate, and 7 or below is severe. To assess the association between ED and lower urinary tract symptoms by the IPSS questionnaire, the Mongolian version of the IPSS was used to evaluate lower urinary tract symptoms. The prostate gland was examined by DRE. Serum PSA levels were measured by the enzyme immune assay method by Human Diagnostic GmbH., (Wiesbaden, Germany) in the same blood sample.
All of the protocols and procedures were approved by the Ethics Committee of the Ministry of Health, Mongolia. Each participant signed a consent form and approved the study protocol before participation.
2. Statistical analyses
Data were processed with IBM SPSS version 19.0 (IBM Corp., Armonk, NY, USA) statistical software to perform descriptive analyses and means comparison of erectile function under each categorical variable with the Mann-Whitney test. The chi-squared test, multiple linear regression test, and logistic regression analyses were also used to determine relationships between the participant's erectile function and LUTS by the IPSS score and testosterone level.
RESULTS
The mean level of serum TT was 6.04±1.83 ng/ml. It was found to be 6.14±1.65 ng/ml in the group aged 40~49, 6.04±2.36 ng/ml in the group aged 50~59, 6.05±1.80 ng/ml in thegroup aged 60~69, and 5.85±1.43 ng/ml in the group aged 70 and above. The mean of the TT in men with ED was 5.6±1.79 ng/ml, which gradually decreased with age.
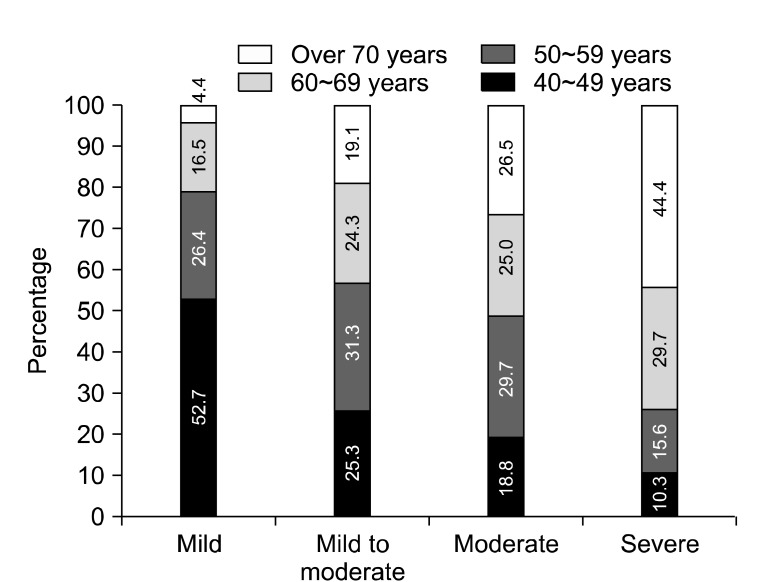
Erectile function: The mean score on the IIEF in the control group was 22.38±3.06 compared to that of the ED group with 11.36±3.47 (p<0.001). The mild form of ED was found in 42 participants (13.59%), mild to moderate in 94 (30.42%), moderate in 58 (18.77%), and severe in 47 (15.21%). Part of the participants (64.41%) were found to have ED at some level from mild to severe based on the IIEF-5 questionnaire score (Fig. 1). The orgasmic function score was found to be 2.29 in the ED group, which was lower than the control group. In addition, the sexual desire score was 4.47 in the control group and 2.75 in the ED group, with a difference of 1.72. There was an inverse correlation between the age group and the IIEF-5 score (r=-0.380, p<0.01).
Fig. 1.
Level of erectile dysfunction in different age groups.
Most participants were heavy smokers and heavy consumers of alcohol. More than half of the participants, 68.3% (n=136) of the ED group and 52.72% (n=58) in the control group, were heavy drinkers. Part of the control group (74.37%; n=148) was smokers compared to 50.91% (n=56) in the ED group. Both alcohol consumption and smoking habits were prevalent in the ED group, but statistically insignificant (p=0.078).
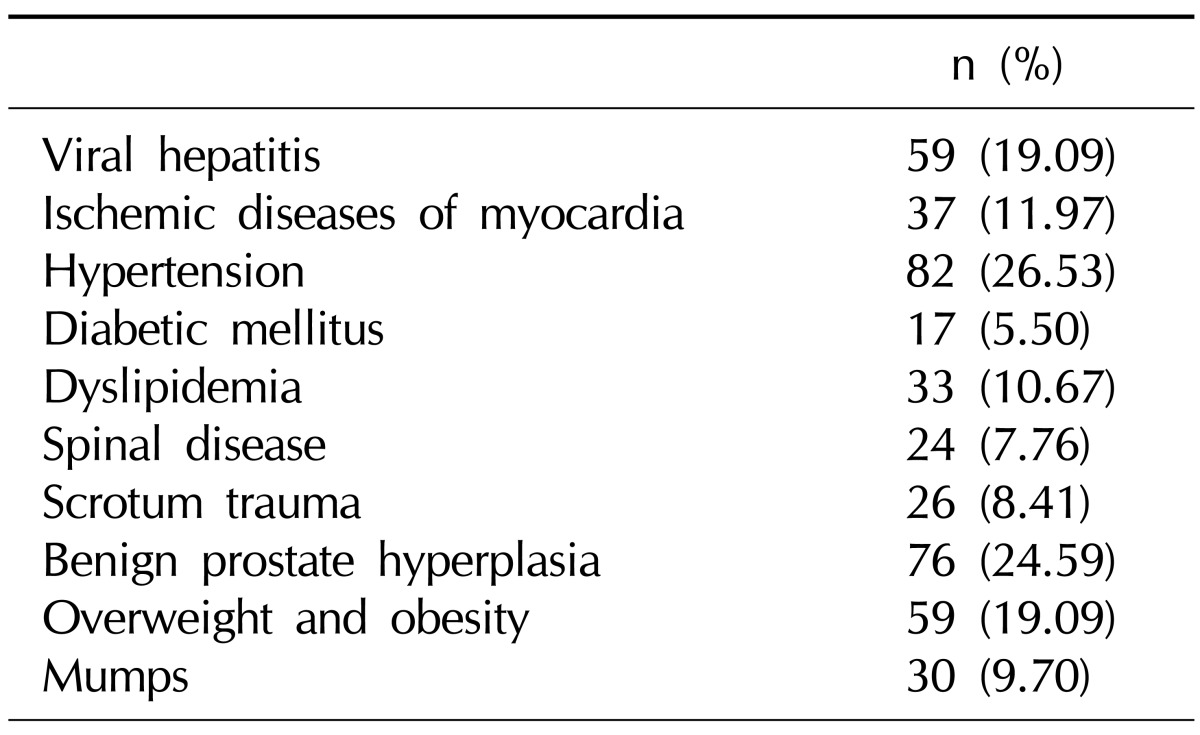
The most common diseases in the ED group were arterial hypertension and benign prostatic hyperplasia shown in the Table 1.
Table 1.
Comorbidities of men with erectile dysfunction
All of the participants underwent a DRE to screen for prostate enlargement or nodules suggesting prostate cancer. 34.54% (n=38) of the participants had a normal prostate size, 52.72% (n=58) had a medium size, and 12.72% (n=14) had an enlarged prostate gland. Thirty-seven men were found to have a prostate gland with an abnormal surface and 5 of the 37 men had a serum level PSA higher than 10 ng/ml and thus were excluded from our study.
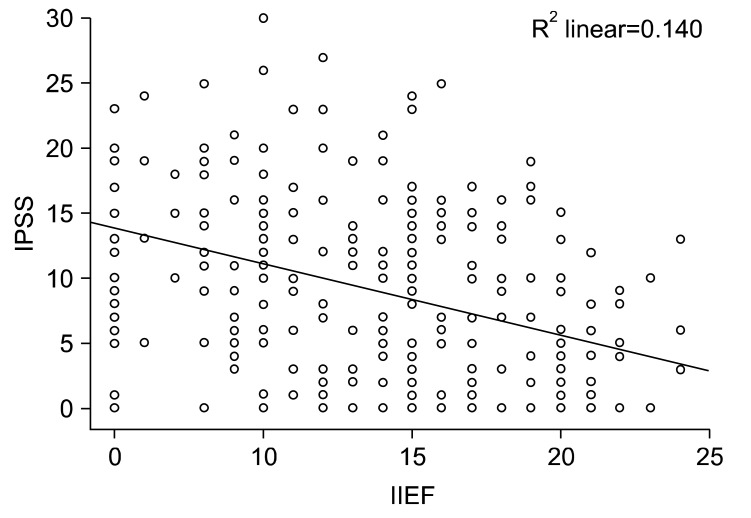
The mean IPSS score was 5.79±5.86 in the control group compared to 10.53±7.22 in the ED group. The IIEF-5 and IPSS scores were correlated (p<0.001) (Fig. 2). The aging male scale (AMS) total scores were 33.59±11.02 and 42.98±11.37 in the ED and control group, respectively. Both the total score and sexual function score of the AMS scale were significantly correlated to the IIEF-5 score (p<0.002).
Fig. 2.
Correlation of international index of erectile function (IIEF) and international prostate symptom score (IPSS) scores.
The mean TT was 6.04±1.83 ng/ml. It was 6.14±1.65ng/ml in the 40~49 age group, 6.04±2.36 ng/ml in those 50~59 years, 6.05±1.80 ng/ml in those 60~69 years, and 5.85±1.43 ng/ml in those 70 years and over. The mean level of the TT was 6.01±2.32 ng/ml in the control group and 5.69±1.159 ng/ml in the ED group.
The c-FT level mean was 0.11±0.06 ng/ml in the control group and 0.081±0.06 ng/ml in the ED group. It was 0.12±0.05 ng/ml in those aged 40~49, 0.11±0.07 ng/ml in those 50~59, 0.10±0.07 ng/ml in those 60~69, and 0.09±0.04 ng/ml in the 70 and over age group. The free testosterone was lower in the ED group (p<0.05), and it declined with age (r=-0.168, p=0.03).
DISCUSSION
Increased testosterone deficiency with aging had been related to health conditions, and it has been extensively studied in the last two decades in many countries. Most population-based studies of testosterone deficiency have been found a prevalence of 5~24% in the aging male population.5-7
In our study, a testosterone deficiency was detected in 24.27% (75) of 309 participants and the TT level was lower than 3.46 ng/ml. This was close to the result of the study done by Haring et al8 in Germany, in which almost 20% of participants were found to have testosterone deficiency.
In a recent report by Hackett et al,9 the mean TT at baseline was 9.1 nmol/L (2.62 ng/ml) and FT was 181 pmol/L in 211 men with type 2 diabetes and after testosterone replacement therapy with long-acting Testosterone Undeconate (TU) improved all domains of sexual function within 30 weeks.
In 2011, Kang et al10 reported a mean TT of 2.71±0.76 ng/ml in 134 men with ED and TDS.
Sexual dysfunction and TDS are age-related. However, there might be differences in the prevalence of onset of testosterone deficiency among different countries because of their unique culture and various conditions. According to the Massachusetts Male Aging Study, 52% of men aged 40 years responded that they had ED. The mean prevalence across all countries was 16%, but was 22% in the US, 13% in the UK, 14% in Mexico and Brazil, 32.4% in Korea, 14% in Germany, and 10% in Italy.11 The results ranged widely depending on the study methodologies, questionnaires, ages of participants, geography, and risk factors.
Total of 64% in 309 Mongolian men over 40 years of age were found to have some degree of ED in this study by the IIEF-5 questionnaire. There was an inverse correlation with a degree of ED score and age group (r=-0.380, p<0.01). ED was defined as a score of <22 on the IIEF-5. The results showed that the total IIEF mean score was 22.38±3.06 compared to 11.36±3.47 (p<0.001) of the ED group. This result was higher than the findings of Lau et al12 of 41.9% of 298 Chinese men dissatisfied with their sexual life.
A study completed in Spain by Martin-Morales et al13 indicated that the prevalence of ED was detected to be 12.1~18.9% of 2,476 men aged 25~70 using the IIEF-5. By age stratum, it occurred in 8.48% of those aged 25~39 years; 13.72%, 40~49 years; 25.5%, 50~59 years; and 48.25% aged 60~70; they had a mild to severe degree of ED.
According to some studies,14,15 the ED prevalence is within the range of 25~52% of men aged over 40 years.
Hypertension, diabetes, inflammatory disease, and prostate disorders were found to be positively associated with ED. A Korean study by Ahn et al16 showed that besides lifestyle factors, age, obesity, and lack of exercise were related to the ED prevalence. Smoking and drinking had no relationship with ED. In the current study, smoking and drinking alcohol and obesity were more prevalent in people with ED, but the difference was not statistically significant.
Recognizing that the association of visceral obesity, high lipids, hypertension, and obesity and hypogonadism with ED is very important for sexual and general health. It should also be noted that testosterone replacement can improve glucose tolerance and visceral fat.17
A study on the potential impact on ED was con-ducted in aging 630 Taiwanese males by Lee et al18 (2010). Vascular risk factors for ED including drinking, cigarette smoking, and hypertension were reviewed, and the men were asked to complete the IPSS questionnaire to evaluate LUTS. In the Taiwanese study, quantitative variables, age, IPSS, prostate different in subjects with and without ED (p<0.05). In a univariate analysis of the association be-tween variables and hypogonadism, we found that age, body mass index (BMI), IIEF-5 score, and prevalence of ED were significantly different in subjects with or without hypogonadism (p<0.05). Some of the results of these findings, such as the BMI and IPSS score, were similar to those of our study.
In 2009, Lee et al19 examined the effects of metabolic components on sexual function in 602 Korean patients with benign prostate hyperplasia. They found that central obesity was more closely related to sexual function than other metabolic components.
In this study, the mean IPSS score was 5.79±5.86 in the control group compared to 10.53±7.22 in the ED group. The IIEF-5 and IPSS scores were correlated (p<0.001) to each other. This was similar to the findings of the study done by Mondul et al,20 who claimed that lower urinary tract symptoms referred to by the IPSS is a relevant factor in ED.
The study of Mehraban et al21 showed that sexual dysfunction, defined as an IIEF score of 20 or less, was present in 68.2% of a group of Italian patients. All of the IIEF domain scores and the overall score were correlated with age (p<0.001) and the IPSS (p<0.001).
Both ED and LUTS are associated with age and may be caused by other co-morbidities.
CONCLUSIONS
The mean IIEF score was 22.38±3.06 in the control group and 11.36±3.47 in the case group (p<0.001). There was a higher incidence of risk factors such as alcohol consumption and smoking, which could cause ED, but the differences were not statistically significant. Enlarged prostate cases were found, and the IPSS score was 10.53±7.22 in the control group and 5.79±5.86 in the case group (p<0.001). The Aging Male Symptoms scale and ED were correlated (p<0.002) with each other. In the current study, 24.27% of men (n=75) were detected to be suffering from ED with age-related testosterone deficiency (<3.46 ng/ml).
ACKNOWLEDGEMENTS
Our deepest appreciation goes to the Asian Research Center and the Korean Foundation for Advanced Studies who generously supported this study. A special acknowledgement goes to all of the supporting organizations, and last but not least, the participants who agreed to take part in this study.
References
- 1.Gooren L, Lim PHC. Testosterone deficiency syndromepractical guidebook for general practitioners. Singapore: Creative plus; 2010. [Google Scholar]
- 2.Shabsigh R, Shah M, Sand M. Erectile dysfunction and men's health: developing a comorbidity risk calculator. J Sex Med. 2008;5:1237–1243. doi: 10.1111/j.1743-6109.2008.00793.x. [DOI] [PubMed] [Google Scholar]
- 3.Haring R, Spielhagen C, Nauck M. Challenges in the measurement of serum testosterone concentrations as a biomarker of men's health. J Lab Med. 2011;35:1–5. [Google Scholar]
- 4.Yassin AA, El-Sakka AI, Saad F, Gooren LJ. Lower urinary-tract symptoms and testosterone in elderly men. World J Urol. 2008;26:359–364. doi: 10.1007/s00345-008-0284-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haring R, Ittermann T, Völzke H, Krebs A, Zygmunt M, Felix SB, et al. Prevalence, incidence and risk factors of testosterone deficiency in a population-based cohort of men: results from the study of health in Pomerania. Aging Male. 2010;13:247–257. doi: 10.3109/13685538.2010.487553. [DOI] [PubMed] [Google Scholar]
- 6.Torkler S, Wallaschofski H, Baumeister SE, Völzke H, Dörr M, Felix S, et al. Inverse association between total testosterone concentrations, incident hypertension and blood pressure. Aging Male. 2011;14:176–182. doi: 10.3109/13685538.2010.529194. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell Harman S. Testosterone, sexuality, and erectile function in aging men. J Androl. 2003;24(6 Suppl):S42–S45. doi: 10.1002/j.1939-4640.2003.tb02745.x. [DOI] [PubMed] [Google Scholar]
- 8.Haring R, Völzke H, Felix SB, Schipf S, Dörr M, Rosskopf D, et al. Prediction of metabolic syndrome by low serum testosterone levels in men: results from the study of health in Pomerania. Diabetes. 2009;58:2027–2031. doi: 10.2337/db09-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hackett G, Cole N, Bhartia M, Kennedy D, Raju J, Wilkinson P. Testosterone replacement therapy with long-acting testosterone undecanoate improves sexual function and quality-of-life parameters vs. placebo in a population of men with type 2 diabetes. J Sex Med. 2013;10:1612–1627. doi: 10.1111/jsm.12146. [DOI] [PubMed] [Google Scholar]
- 10.Kang JI, Ham BK, Oh MM, Kim JJ, Moon DG. Correlation between serum total testosterone and the ams and iief questionnaires in patients with erectile dysfunction with testosterone deficiency syndrome. Korean J Urol. 2011;52:416–420. doi: 10.4111/kju.2011.52.6.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosen RC, Fisher WA, Eardley I, Niederberger C, Nadel A, Sand M Men's Attitudes to Life Events and Sexuality (MALES) Study. The multinational Men's Attitudes to Life Events and Sexuality (MALES) study: I. Prevalence of erectile dysfunction and related health concerns in the general population. Curr Med Res Opin. 2004;20:607–617. doi: 10.1185/030079904125003467. [DOI] [PubMed] [Google Scholar]
- 12.Lau JT, Wang Q, Cheng Y, Yang X. Prevalence and risk factors of sexual dysfunction among younger married men in a rural area in China. Urology. 2005;66:616–622. doi: 10.1016/j.urology.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Martin-Morales A, Sanchez-Cruz JJ, Saenz de, Rodriguez-Vela L, Jimenez-Cruz JF, Burgos-Rodriguez R. Prevalence and independent risk factors for erectile dysfunction in Spain: results of the Epidemiologia de la Disfuncion Erectil Masculina Study. J Urol. 2001;166:569–574. doi: 10.1016/s0022-5347(05)65986-1. [DOI] [PubMed] [Google Scholar]
- 14.Meuleman EJ. Prevalence of erectile dysfunction: need for treatment? Int J Impot Res. 2002;14(Suppl 1):S22–S28. doi: 10.1038/sj.ijir.3900793. [DOI] [PubMed] [Google Scholar]
- 15.Jalil H, Mohammad M, Alireza G. Epidiomological study of prevalence and association of sexual dysfunction with potential risk factors. Int J of Urology. 2012;19(Suppl 1):271. [Google Scholar]
- 16.Ahn TY, Park JK, Lee SW, Hong JH, Park NC, Kim JJ, et al. Prevalence and risk factors for erectile dysfunction in Korean men: results of an epidemiological study. J Sex Med. 2007;4:1269–1276. doi: 10.1111/j.1743-6109.2007.00554.x. [DOI] [PubMed] [Google Scholar]
- 17.Corona G, Monami M, Rastrelli G, Aversa A, Tishova Y, Saad F, et al. Testosterone and metabolic syndrome: a meta-analysis study. J Sex Med. 2011;8:272–283. doi: 10.1111/j.1743-6109.2010.01991.x. [DOI] [PubMed] [Google Scholar]
- 18.Lee YC, Liu CC, Huang CN, Li WM, Wu WJ, Yeh HC, et al. The potential impact of metabolic syndrome on erectile dysfunction in aging Taiwanese males. J Sex Med. 2010;7:3127–3134. doi: 10.1111/j.1743-6109.2010.01852.x. [DOI] [PubMed] [Google Scholar]
- 19.Lee SH, Kim JC, Lee JY, Kim JH, Oh CY, Lee SW, et al. Effects of components of metabolic syndrome on sexual function in Korean BPH/LUTS patients. J Sex Med. 2009;6:2292–2298. doi: 10.1111/j.1743-6109.2009.01325.x. [DOI] [PubMed] [Google Scholar]
- 20.Mondul AM, Rimm EB, Giovannucci E, Glasser DB, Platz EA. A prospective study of lower urinary tract symptoms and erectile dysfunction. J Urol. 2008;179:2321–2326. doi: 10.1016/j.juro.2008.01.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehraban D, Naderi GH, Yahyazadeh SR, Amirchaghmaghi M. Sexual dysfunction in aging men with lower urinary tract symptoms. Urol J. 2008;5:260–264. [PubMed] [Google Scholar]