Abstract
Diabetes increases the risk of stroke and contributes to poor clinical outcomes in this patient population. Myogenic tone of the cerebral vasculature, including basilar arteries, plays a key role in controlling cerebral blood flow. Increased myogenic tone is ameliorated with ET receptor antagonism in Type 1 diabetes. However, the role of ET-1 and its receptors in cerebrovascular dysfunction in Type-2 diabetes, a common comorbidity in stroke patients, remains poorly elucidated. Therefore, we hypothesized that 1) cerebrovascular dysfunction occurs in the Goto-Kakizaki (GK) model of Type-2 diabetes, and 2) pharmacological antagonism of ETA receptors ameliorates while ETB receptor blockade augments vascular dysfunction. GK or control rats were treated with antagonists to either ETA (Atrasentan, 5mg/kg/d) or ETB (A-192621, 15 or 30 mg/kg/d) receptors for four weeks and vascular function of basilar arteries was assessed using a wire myograph. GK rats exhibited increased sensitivity to ET-1. ETA receptor antagonism caused a rightward shift indicating decreased sensitivity in diabetes while it increased sensitivity to ET-1 in control rats. Endothelium-dependent relaxation was impaired in diabetes. ETA receptor blockade restored relaxation to control values in the GK animals with no significant effect in Wistars and ETB blockade with 30 mg/kg/d A-192621 caused paradoxical constriction in diabetes. These studies demonstrate that cerebrovascular dysfunction occurs and may contribute to altered regulation of myogenic tone and cerebral blood flow in diabetes. While ETA receptors mediate vascular dysfunction, ETB receptors display differential effects. These results underscore the importance of ETA/ETB receptor balance and interactions in cerebrovascular dysfunction in diabetes.
INTRODUCTION
Diabetes exists as an independent risk factor for cardiovascular disease (CVD) (29). Within the diabetic population, CVD is the leading cause of death accounting for 65–75% of all deaths (4). Studies have demonstrated that there is a strong correlation between diabetes and cerebrovascular disorders such as cerebral ischemia and stroke (5, 52). Diabetes not only increases the risk of stroke, but also contributes to increased stroke mortality and impaired recovery after stroke (3, 15, 16).
Regulation of vascular tone is important for maintenance of proper blood flow. In the cerebral circulation, changes in blood flow are buffered by the myogenic response to maintain cerebral blood flow. However, alterations to this system may be detrimental and could contribute to cerebrovascular disease. Studies have demonstrated increased myogenic tone in experimental diabetes (8, 9, 54). In addition to increased basal tone, cerebral arteries from diabetic animals exhibit diminished endothelium derived relaxation (2, 9, 34)
It is well established that the potent vasoconstrictor endothelin-1 (ET-1) is upregulated in the circulation in both clinical and experimental diabetes (7, 51). The effects of endothelin are mediated via two G-protein coupled receptors: ETA and ETB (17). ETA receptors reside on the smooth muscle cell (SMC) and produce vasoconstriction and mediate the proliferative effects of ET-1. Functional studies have suggested that different ETB receptor subtypes may exist (39, 40, 50). However, Mizuguchi et al demonstrated that the observed heterogeneous responses of the ETB receptor are abolished in ETB knockout mice suggesting that one gene is responsible for these actions (38). Inasmuch, ETB receptors do elicit different responses. ETB receptors on endothelial cells promote vasodilation via cGMP while VSMC ETB receptors educe ETA-like responses. Mounting evidence suggests that ET-1 is involved in the pathology of cerebrovascular disease (26). Studies have demonstrated enhanced contractile responses to ET-1 (1, 33) as well as a reduction of increased myogenic tone after ET receptor antagonism (9) in Type 1 diabetes. However, the relative roles of ET-1 and its receptors in cerebrovascular dysfunction in Type-2 diabetes, which is a common comorbidity in stroke patients, are poorly elucidated. Thus, we hypothesized that 1) cerebrovascular dysfunction occurs in the Goto-Kakizaki (GK) model of Type-2 diabetes, and 2) pharmacological antagonism of ETA receptors ameliorates whereas ETB receptor blockade augments vascular dysfunction.
RESEARCH DESIGN AND METHODS
Animals
All experiments were performed on male Wistar (Harlan, Indianapolis, IN) and GK (in-house bred, derived from the Tampa colony) rats (14, 49). The animals were housed at the Medical College of Georgia animal care facility that is approved by the American Association for Accreditation of Laboratory Animal Care. All protocols were approved by the Institutional Animal Care and Use Committee. Animals were fed standard rat chow and tap water ad-libitum until sacrifice at 18 weeks of age. Blood pressure was monitored either by telemetric method (as previously reported) (21) or via the tail cuff method which we have previously validated on telemetry implanted animals (10).
Endothelin receptor blockade
GK rats develop hyperglycemia around 6–8 weeks of age. We have previously shown that by 18 weeks of age, this model displays significant cerebrovascular remodeling and ETA receptor antagonism started after 8 weeks of diabetes at week 14 can ameliorate this response (19). Using the same treatment paradigm, at 14 weeks of age, animals were divided into groups and treated for four weeks with highly selective antagonists for each receptor as follows: ETA receptor blockade – Atrasentan (Abbott Labs) 5 mg/kg/day in drinking water and ETB receptor blockade – A-192621 (Abbott Labs) 15 or 30 mg/kg/day by oral gavage split into two daily doses or vehicle (10). Vehicle for the A-192621 consisted of 83% deionized water, 10% Polyethylene Glycol-400, 5% ethanol, and 2% Cremaphor EL. Tap water was used as vehicle for the Atrasentan.
Surgical procedure
Animals were anesthesized with sodium pentobarbital and exsanguinated via cardiac puncture. The brain was quickly excised for isolation of basilar arteries.
Determination of vascular function
Isometric tension exerted by the vessels was recorded via a force transducer using the wire-myograph technique (Danish Myo Technologies, Denmark). The myograph chambers were filled with Krebs buffer (NaCl 118.3, NaHCO3 25, KCl 4.7, MgSO4 1.2, KH2PO4 1.2, CaCl2 1.5 and Dextrose 11.1 mM), gassed with 95% O2 and 5% CO2 and maintained at 37°C. Vessel segments were mounted in the chamber using 40 µm-thin wires and adjusted to a baseline tension of 0.4g. Cumulative dose response curves to ET-1 (0.1–100 nM) were generated and the force generated was expressed as % change of baseline. In a subset of vehicle-treated animals, basilar arteries were challenged with Sarafotoxin-6-c (S6c, 1–100 nM) with or without preincubation (30 min) with 1 µM BQ-123. In additional vehicle-treated rats, cumulative dose response (1 nM-1 µM) curves to serotonin (5-HT) were generated to determine if hyperreactivity is a general response. Endothelium-dependent relaxation to acetylcholine (Ach, 1 nM-1 µM) was assessed after vessels were constricted to 60% of the baseline tension with 5-HT or directly after ET-1 dose response. Sensitivity (EC50) and maximum response (Rmax) values were calculated from the respective dose-response equations.
Statistical analysis
Results are given as mean ± SEM. For EC50 and Rmax values, a two-way analysis of variance (ANOVA) was done to analyze disease and treatment effects with a post-hoc Bonferroni test. A repeated measures ANOVA was used to determine group differences (Diabetic vs. Control) across the ET-1 or Ach concentrations. Post-hoc group comparisons at each concentration used a Tukey’s adjustment for the multiple comparisons. Graphpad Prism 4.0 was used for all statistical tests performed.
RESULTS
Animal data
Metabolic parameters of animals are listed in Table 1. Diabetic animals were significantly smaller than control rats in all treatment groups (p<0.001 vs control). Plasma ET-1 levels indicated a disease and drug interaction such that ET-1 was higher in diabetic rats for all treatment groups except for the A-192621 30 mg/kg/day group which was similar in control and diabetic rats. In addition, A-192621 (30 mg/kg/day) treatment caused a significant increase in plasma ET-1 levels in both control and diabetic rats when compared to vehicle, Atrasentan or the A-192621 (15 mg/kg/day) group.
TABLE 1.
Metabolic parameters in treatment groups
| Wistar + Vehicle (n=8) |
Wistar + Atrasentan (n=10) |
Wistar + A-192621 15 mg/kg (n=5) |
Wistar + A-192621 30 mg/kg (n=5) |
|
|---|---|---|---|---|
| Weight (g) | 506 ± 13 | 474 ± 29 | 500 ± 13 | 406 ± 2b |
| Glucose (mg/dl) | 114 ± 5 | 89 ± 3 | 109 ± 4 | 104 ± 5 |
| Insulin (ng/ml) | 2.7 ± 0.6 | 2.1 ± 0.3 | 2.2 ± 0.5 | 2.2 ± .72 |
| ET-1 (fmol/ml) | 0.3 ± 0.1 | 0.4 ± 0.2 | 0.3 ± 0.2 | 1.6 ± 0.5b |
| MAP (mmHg) | 103 ± 5 | 105 ± 5 | 107 ± 2 | 121 ± 3c |
| GK+ Vehicle (n=8) | GK + Atrasentan (n=8) | GK + A-192621 15 mg/kg (n=5) | GK + A-192621 30 mg/kg (n=5) | |
| Weight (g) | 362 ± 8a | 357 ± 10a | 354 ± 6a | 322 ± 10a |
| Glucose (mg/dl) | 207 ± 33a | 172 ± 17a | 304 ± 17a | 173 ± 31a |
| Insulin (ng/ml) | 1.0 ± 0.3a | 0.7 ± 0.1a | 0.9 ± 0.2a | 0.6 ± 0.1a |
| ET-1 (fmol/ml) | 0.9 ± 0.2a | 1.2 ± 0.1a | 1.4 ± 0.2a | 2.3 ± 0.3a,b |
| MAP (mmHg) | 103 ± 4 | 106 ± 3 | 108 ± 3 | 116 ± 2c |
p<0.001 vs Wistar
p<0.001 vs vehicle, Atrasentan or A-192621 15 mg/kg
p<0.01 vs vehicle, Atrasentan or A-192621 15 mg/kg
ET-1-mediated contractility
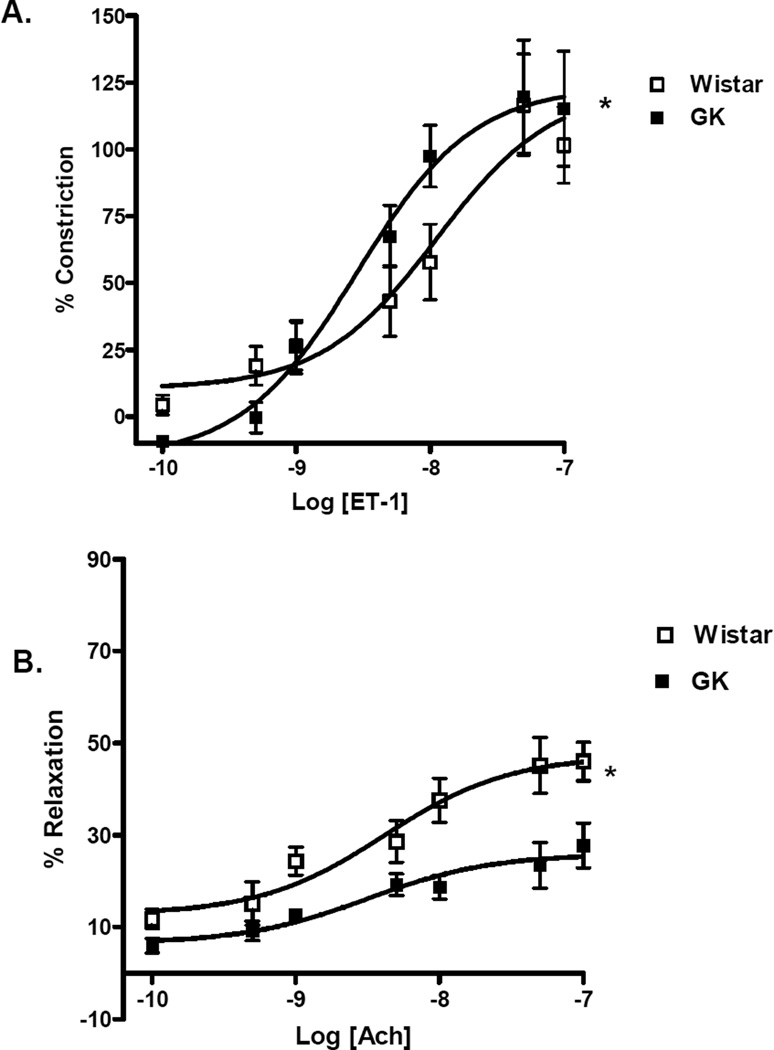
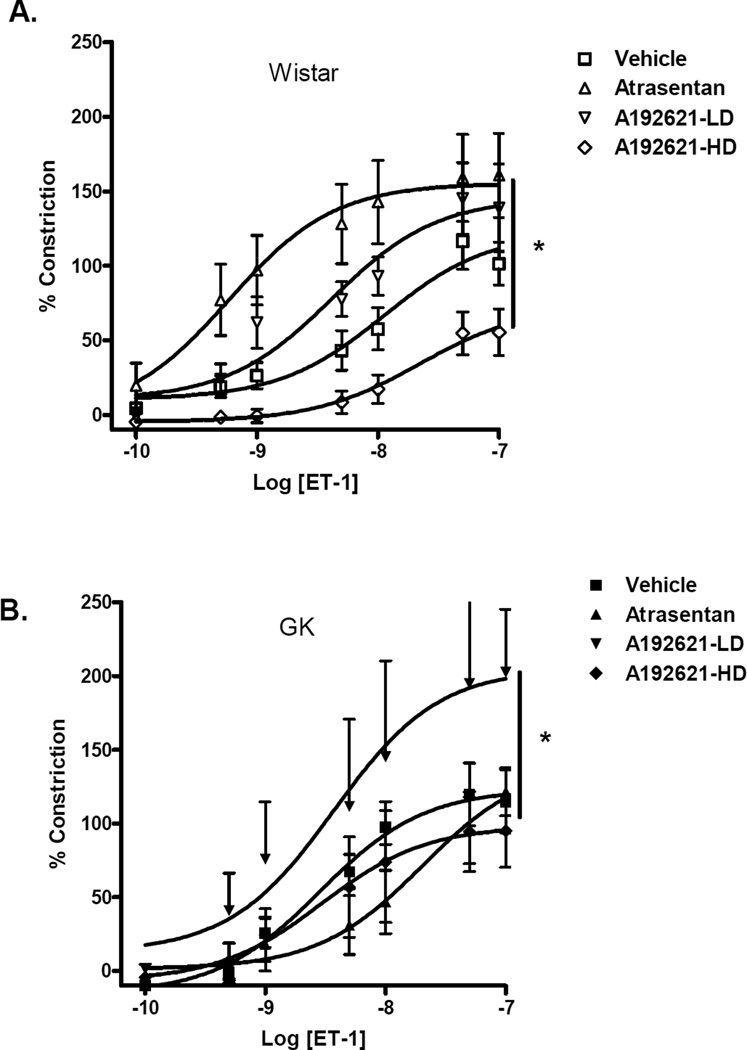
Basilar arteries of GK rats were hypersensitive to ET-1 (EC50 2.8 ± 1.6 vs 11.3 ± 1.7 nM in controls, p<0.05). (Fig. 1A,Table 2). Dose response curves in both control and diabetic animals showed significant differences between treatment protocols (Fig. 2A and B,Table 2). In control animals, Atrasentan treatment caused a paradoxical leftward shift that was statistically significant compared to vehicle or ETB receptor blockade groups. While A-192621 at 15 mg/kg dose had no effect on responses to ET-1, blockade with 30 mg/kg dose caused a rightward shift of the dose response curve. On the other hand, in the diabetic animals, blockade of the ETA receptor with Atrasentan attenuated the contractile response to ET-1 as indicated by the rightward shift. Interestingly, low dose ETB receptor blockade with 15 mg/kg A-192621 rendered basilar arteries more sensitive to ET-1. A dose response curve to 5-HT in vehicle treated control and diabetic animals showed no difference between Rmax (102 ± 8 in control vs 103 ± 10 in GK rats) or EC50 values (15.3 ± 4.2 control vs 13.2 ± 2.5) in GK rats.
FIG. 1.
Diabetes mediates vascular dysfunction of basilar arteries. (A) GK rats display hypersensitivity (EC50) to ET-1, although the magnitude of constriction did not differ. B. Dose-response curves to acetylcholine demonstrated impaired endothelium dependent relaxation as compared to controls. Results are shown as mean ± SEM, control = 8 and GK=6. *<p<0.01
TABLE 2.
Sensitivity (EC50) and magnitude (Rmax) of vascular responses to ET-1 and ACh in the absence or presence of ET receptor antagonists in basilar arteries of control Wistar and diabetic GK rats.
| Wistar + Vehicle (n=8) |
Wistar + Atrasentan (n=10) |
Wistar + A-192621 15 mg/kg (n=3) |
Wistar + A-192621 30 mg/kg (n=5) |
|
|---|---|---|---|---|
| ET-1 constriction | ||||
| EC50 (nM) | 11.3 ± 1.7 | 0.6 ± 0.2a,* | 4.1 ± 1.8 | 32 ± 2.1a,b |
| Rmax (%baseline) | 99 ± 15 | 159 ± 28 | 139 ± 29a | 56 ± 16 |
| Ach relaxation | ||||
| EC50 (nM) | 4.7 ± 1.5 | 8.1 ± 1.8 | 15.9 ± 4.0 | 2.0 ± 1.6 |
| Rmax (% 5HT) | 56 ± 5 | 46 ± 8 | 53 ± 5 | 90 ± 8a,* |
| GK+ Vehicle (n=6) | GK + Atrasentan (n=6) | GK + A-192621 15 mg/kg (n=3) | GK + A-192621 30 mg/kg (n=6) | |
| ET-1 constriction | ||||
| EC50 (nM) | 2.8 ± 1.6c | 20 ± 2.2 c,d,* | 2.2± 3.6 | 2.6 ± 3.1 |
| Rmax (% baseline) | 115 ± 22 | 128 ± 14 | 203 ± 43e | 95 ± 25f |
| Ach relaxation | ||||
| EC50 (nM) | 2.4 ± 2.0 | 3.8 ± 1.4 | 3.6 ± 4.3 | 4.3 ± 4.6 |
| Rmax (% 5HT) | 26 ± 4c | 64 ± 3c | 27 ± 7c | −2 ± 14d,g,* |
p<0.01 vs vehicle Wistar
p<0.001 vs Atrasentan or A-192621 15 mg/kg Wistar
p<0.01 vs vehicle Wistar
p<0.001 vs GK treatment groups
p<0.05 vs vehicle GK
p<0.05 vs A-192621 15 mg/kg GK
p<0.001 vs Wistar
p<0.0001-disease-treatment interaction in Wistar vs GK rats.
Endothelium-dependent relaxation
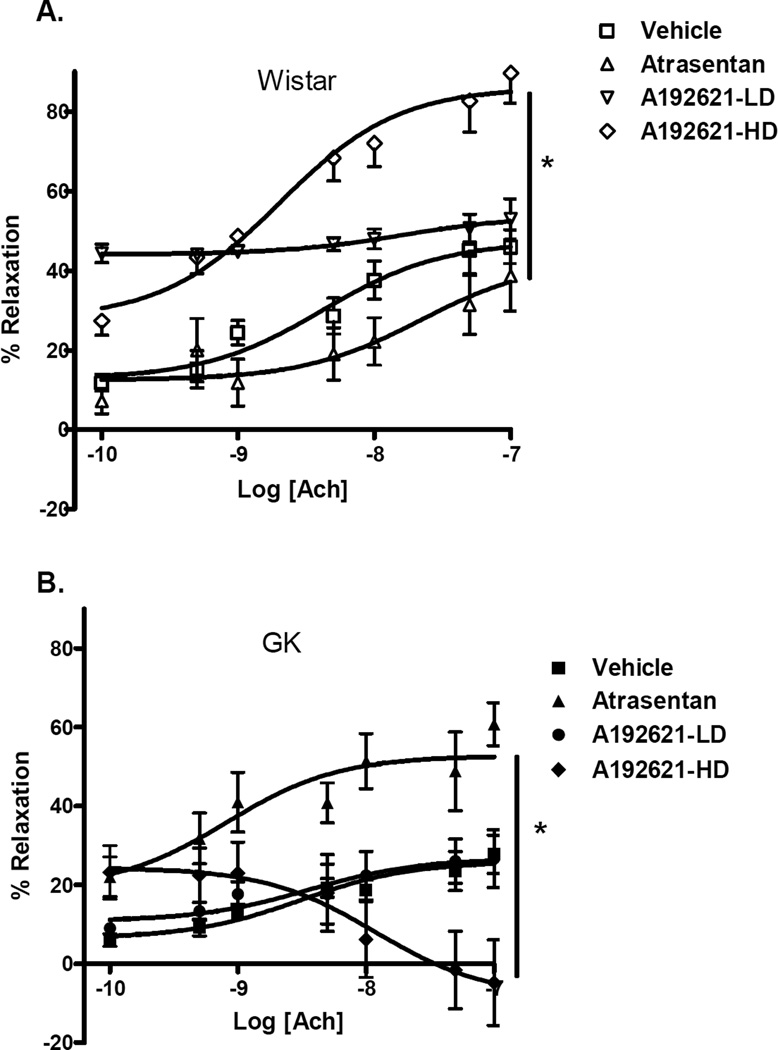
Basilar arteries of GK rats exhibited impaired endothelium relaxation following pre-constriction with 5-HT. Basilar arteries of GKs relaxed only 26 ± 4% in response to acetylcholine whereas the control rat arteries relaxed 56 ± 5% (Fig. 1B,Table 2). In the control group, dose- response curves indicated significant differences between treatment arms (Fig. 3A). While Atrasentan had no effect on relaxation response, ETB receptor antagonism with A-192621 30 mg/kg significantly improved relaxation and A-192621 15 mg/kg group displayed about 40% relaxation across the Ach concentration range. In diabetic rats, however, Atrasentan, significantly improved relaxation. Interestingly, treatment with A-192621 30 mg/kg caused paradoxical constriction while treatment with A-192621 15 mg/kg had no effect (Fig. 3B).
S6c–mediated reactivity
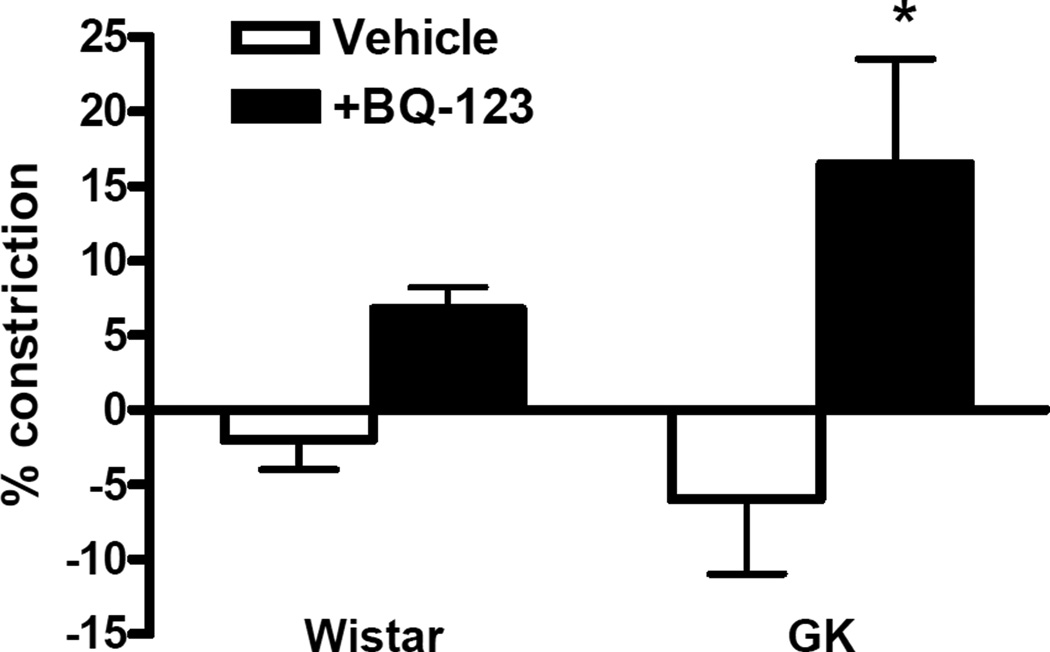
In order to determine whether there is an up-regulation of VSMC ETB receptors, contractile responses to S6c, an ETB receptor selective ligand, were determined (Fig. 4). Since previous studies suggested that a possible ETA-ETB receptor interaction prevents ETB-receptor responses to S6c, these studies were repeated in basilar artery segments preincubated with 1 µM BQ-123. There was a small but significant increase in S6c–mediated constriction in the presence of BQ-123 in diabetic animals suggesting a possible involvement of VSMC ETB receptors in the contractile response.
DISCUSSION
There are three major findings in the current study: first, basilar arteries were hypersensitive to ET-1 in a Type-2 diabetic rat model; second, acetylcholine induced relaxation of basilar arteries was impaired in diabetes; lastly, these effects are primarily mediated by the ETA receptor. These results build upon the past reports that showed cerebrovascular dysfunction in different models of diabetes and provide evidence for the involvement of ET-1 in cerebrovascular dysfunction in a non-obese, spontaneous model of Type 2 diabetes.
While there are many models of diabetes, most are either chemically induced Type-1 models or have co-morbid conditions such as hypertension, obesity and hyperlipidemia. The spontaneously diabetic GK rat is a non-obese, normotensive rat model originally developed from selective inbreeding of glucose intolerant Wistar rats (18). Previous studies have demonstrated that GK rats retain greater than 40% of their beta cell mass and have a reduction of post-prandial glucose when given the insulin secretagogue nateglinide (28, 30, 47, 48). In addition, our laboratory has previously shown that these rats have impaired glucose tolerance as compared to control Wistars (21). Therefore, the GK rat serves as an excellent model for studying the effects of hyperglycemia alone on cerebrovascular function in Type-2 diabetes.
We and others have observed elevated plasma ET-1 levels in both clinical and experimental diabetes (13, 21, 32, 45). Several studies have demonstrated enhanced contractile responses to ET-1 in aorta and peripheral arteries in diabetes (22, 27, 31). In addition, McIntyre et al reported increased sensitivity to ET-1 in subcutaneous resistance arteries from patients with Type-1 diabetes (37). In the present study, we have demonstrated an exacerbated contractile response of the rat basilar artery to ET-1 in diabetes. Matsumoto et al as well as Alabadi et al have both demonstrated similar results in the rat and rabbit basilar arteries respectively (1, 33). In contrast, Mayhan reported no differences in contractile responses to ET-1 in isolated rat basilar arteries (35). However, these discrepancies may be attributable to the differences in methodologies (e.g. in-vivo vs. in-vitro). Collectively, these previous studies were all performed in chemically induced Type-1 models of diabetes. To our knowledge, the present study is the first demonstration of enhanced cerebrovascular contractile response to ET-1 in a model of Type-2 diabetes.
Previous studies using models of Type-2 diabetes have demonstrated that endothelium derived relaxation is impaired in aortas and the peripheral vasculature (6, 33, 42, 53). However, Type-1 models, such as the STZ rat and alloxan rabbit, have predominated in studies of the cerebral vessels (24, 34, 36). Only in recent years have investigators begun to study the effects of Type-2 diabetes on the cerebral circulation and thus far, there are but a handful of these studies (8, 11, 12, 25, 46). Schwaninger and Karagiannis both reported diminished vasodilation in response to acetylcholine in obese Zucker rats (OZR) (25, 46). However, the OZR is a model of Type-2 diabetes known to have co-morbid conditions such as hypertension and hyperlipidemia which may contribute to altered vascular states. More recently, diabetic db/db mice, characterized by hyperinsulinemia, severe hyperglycemia and obesity, were shown to exhibit similar reductions in endothelium dependent relaxation (8). In the present study, we found that Type-2 diabetes significantly impairs acetylcholine induced relaxation in serotonin preconstricted basilar arteries. ETA receptor antagonism completely restored ACh induced relaxation in diabetic basilar arteries indicating that an activated endothelin system contributes to this impairment. This data, in accordance with previous studies done in varying models of diabetes, suggests that diabetes impairs vascular relaxation without regard to the etiology of the disease or other co-morbid conditions.
The vascular effects of ET-1 are mediated by two distinct receptor subtypes: ETA and ETB. In the present study, we have demonstrated hypersensitivity of the rat basilar artery to ET-1 in diabetes. ETA receptor blockade induced a significant rightward shift of the dose response curve indicating a decrease in sensitivity. Interestingly, in control animals, ETA receptor antagonism increased sensitivity to ET-1. Schilling et al. previously reported decreased sensitivity to ET-1 upon acute blockade of this receptor subtype suggesting that acute vs chronic blockade of the receptors yield different responses (44). Unexpectedly, higher dose blockade of the ETB receptor also produced a rightward shift of the dose response curve in a manner similar to ETA blockade. Previous studies in diabetic rabbit and rat basilar arteries have produced similar results using in-vitro inhibition of ETB receptors (1, 31, 33). Interestingly, Matsumoto et al showed that endothelial denudation of the diabetic basilar artery produced a significant leftward shift of the ET-1 dose response curve (33). In the current study, lower dose ETB blockade also caused a leftward shift possibly suggesting that removal of endothelial ETB receptors alone affects the dilatory actions of ET in diabetes, while complete blockade (both endothelial and VSMC ETB) alters constriction. It has been suggested that ET-1 mediated contraction in the cerebrovasculature may be, in part, mediated through crosstalk of the ET receptor subtypes. Zuccarello et al previously demonstrated that ETB mediated vasoconstriction relies on activation of the smooth muscle as well as the endothelial ETB receptors (55).
Vascular reactivity comprises both constriction and relaxation of blood vessels. In this study, ETA receptor blockade with Atrasentan caused a significant improvement of endothelium-dependent dilatation in diabetic rats and no significant effect in control animals suggesting a greater involvement of ET-1 in the regulation of vascular relaxation in diabetes. More interestingly, ETB receptor antagonism at 30 mg/kg dose resulted in completely opposite results in control vs diabetic rats, i.e, improving relaxation in control animals and causing paradoxical constriction in diabetic animals. Complete reversal of the relaxation response in A-192621(30 mg/kg)-treated GK rats suggests that endothelial ETB receptors are upregulated as a compensatory mechanism to offset impaired relaxation in diabetes and that blockade of these receptors ultimately results in decreased relaxation. Alternatively, these receptors are involved to a greater extent in the regulation of Ach-mediated dilatation in diabetes and again antagonism of this receptor subtype causes paradoxical constriction to Ach.
Collectively, these studies strongly suggest involvement of both endothelial and VSMC ETB receptors in the regulation of vascular function in diabetes. Indeed, it has been demonstrated that diabetes does upregulate vascular ETB receptor expression. Mumtaz et al found increased ETB receptor density in the diabetic rabbit urinary bladder while Ikeda showed a significantly increased ETB gene expression in STZ diabetic rat adrenal glands (23). In 10 week old NOD mice, a Type-1 diabetes model, aortic ETB gene expression was significantly increased while ETA expression was unchanged (41). Conceivably, blockade of ETB receptors in a system which may be shifted more toward contractile responses, would produce effects similar to ETA blockade. It is also possible that ETA-ETB heterodimerization can affect the reactivity studies. Both homo and heterodimerization of the two ET receptors have been reported by functional as well as fluorescence resonance studies (19, 20, 43). It is suggested that when heterodimerized, the ETA receptor overrides ETB receptor activation and thus ETB receptor antagonism provides an ETA blockade-like effect. Harada et al. reported that due to ETA and ETB receptor heterodimerization, ETB receptors do not independently recognize ligands such as ET-1 and S6c unless ETA receptors are blocked with BQ-123. To test this hypothesis, we examined vascular responses to the ETB-selective agonist S6c in the presence and absence of ETA blockade with BQ-123. These studies demonstrated that S6c can induce vasoconstriction in the diabetes group when ETA receptors are antagonized thus indicating increased presence of VSMC ETB receptors.
Though our studies correlate with previously published data, certain limitations must be addressed. First, we examined reactivity of the basilar artery to ET-1 in all groups and to 5-HT only vehicle treated control and GK rats but not in ET receptor antagonist treatment groups. Others have found similar responses to ET-1 in diabetic rat and rabbit basilar arteries (1, 33). Second, due to the limited availability of ETB receptor antagonist as well as the basilar artery segments that can be obtained from one animal, especially in the low dose A-192621 group we had limited number of animals. We treated 5 animals in this group but in 2 animals vessels were completely unresponsive to any stimulus. For the same reasons, reactivity experiments could not be performed with endothelium denuded vessels.
PERSPECTIVES AND SIGNIFICANCE
Diabetes induces vascular dysfunction in isolated rat basilar arteries in the form of increased sensitivity to ET-1 and diminished relaxation capacity to acetylcholine. Inasmuch, these effects appear to be, at least in part, due to an increased contribution of the ETA and smooth muscle ETB receptor activation. These alterations of cerebrovascular function may potentially underlie the increased propensity for cerebrovascular disease in diabetes.
FIG. 2.
Effects of chronic ET receptor antagonism on ET-1-mediated contractility in control Wistar (A) and diabetic GK (B) rats. While ETA receptor antagonism improved sensitivity to ET-1 in control animals (n=10), it decreased ET-1 sensitivity in GKs (n=6) as demonstrated by a rightward shift of the dose response curve. ETB blockade with 30 mg/kg A-192621 caused a rightward shift of the dose response almost identical to that caused by Atrasentan. A-192621 15 and 30 mg/kg/day groups are shown as LD (n=3 for both control and GK) and HD (n=5–6 in control and GK), respectively. Results are shown as mean ± SEM, *p<0.001 repeated measures of ANOVA. Significant posthoc comparisons (P<0.05) for control Wistar groups are vehicle vs atrasentan, vehicle vs HD, atrasentan vs LD, atrasentan vs HD, and LD vs HD. For GK groups, vehicle vs atrasentan, vehicle vs LD, atrasentan vs LD, LD vs HD were significantly different.
FIG. 3.
Effects of chronic ET receptor antagonism on endothelium-dependent relaxation in control Wistar (A) and diabetic GK (B) rats. ETA receptor blockade restored maximum ACh induced relaxation in GK basilar arteries to greater than control values. While high dose blockade caused paradoxical vasoconstriction in diabetic animals, it improved the dilatory response in control rats. Results are shown as mean ± SEM, *p<0.001 repeated measures of ANOVA. Significant posthoc comparisons (P<0.05) for control Wistar groups are vehicle vs LD, vehicle vs HD, atrasentan vs LD, atrasentan vs HD and LD vs HD. For GK groups, vehicle vs atrasentan, atrasentan vs LD, and atrasentan vs HD were significantly different.
FIG. 4.
S6c–mediated vascular reactivity. Basilar arteries were stimulated with S6c (1 nM- 1µM) with or without preincubation with 1µM BQ-123 for 30 min. Selective blockade of ETA receptors induced a significant increase in ET-1-mediated constriction in the GK group suggesting presence of ETB receptors on VSMC. P<0.01
Acknowledgements
This work was supported by grants from NIH (HL076236, DK074385), American Heart Association Established Investigator Award and Philip Morris Research Award to Adviye Ergul and American Heart Association SouthEast Affiliate Pre-Doctoral Fellowship to Alex Harris.
REFERENCES
- 1.Alabadi JA, Miranda FJ, Llorens S, Centeno JM, Marrachelli VG, Alborch E. Mechanisms underlying diabetes enhancement of endothelin-1-induced contraction in rabbit basilar artery. Eur J Pharmacol. 2004;486:289–296. doi: 10.1016/j.ejphar.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Arrick DM, Sharpe GM, Sun H, Mayhan WG. Diabetes-induced cerebrovascular dysfunction: role of poly(ADP-ribose) polymerase. Microvasc Res. 2007;73:1–6. doi: 10.1016/j.mvr.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association. National diabetes fact sheet. 2007 http://wwwdiabetesorg/diabetes-statisticsjsp.
- 4.American Heart Association AH. Heart and stroke statistical update. 2007 www.americanheart.org.
- 5.Baliga BS, Weinberger J. Diabetes and stroke: part one--risk factors and pathophysiology. Curr Cardiol Rep. 2006;8:23–28. doi: 10.1007/s11886-006-0006-1. [DOI] [PubMed] [Google Scholar]
- 6.Cheng ZJ, Vaskonen T, Tikkanen I, Nurminen K, Ruskoaho H, Vapaatalo H, Muller D, Park J-K, Luft FC, Mervaala EMA. Endothelial dysfunction and salt-sensitive hypertension in spontaneously diabetic Goto-Kakizaki rats. Hypertension. 2001;37:433–439. doi: 10.1161/01.hyp.37.2.433. [DOI] [PubMed] [Google Scholar]
- 7.Collier A, Leach JP, McLellan A, Jardine A, Morton JJ, Small M. Plasma endothelin-like immunoreactivity levels in IDDM patients with microalbuminuria. Diabetes Care. 1992;15:1038–1040. doi: 10.2337/diacare.15.8.1038. [DOI] [PubMed] [Google Scholar]
- 8.Didion SP, Lynch CM, Baumbach GL, Faraci FM. Impaired endothelium-dependent responses and enhanced influence of Rho-kinase in cerebral arterioles in type II diabetes. Stroke. 2005;36:342–347. doi: 10.1161/01.STR.0000152952.42730.92. [DOI] [PubMed] [Google Scholar]
- 9.Dumont AS, Dumont RJ, McNeill JH, Kassell NF, Sutherland GR, Verma S. Chronic endothelin antagonism restores cerebrovascular function in diabetes. Neurosurgery. 2003;52:653–660. doi: 10.1227/01.neu.0000048187.74897.7e. discussion 659–660. [DOI] [PubMed] [Google Scholar]
- 10.Elgebaly MM, Portik-Dobos V, Sachidanandam K, Rychly D, Malcom D, Johnson MH, Ergul A. Differential effects of ET(A) and ET(B) receptor antagonism on oxidative stress in type 2 diabetes. Vascul Pharmacol. 2007;47:125–130. doi: 10.1016/j.vph.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Erdos B, Simandle SA, Snipes JA, Miller AW, Busija DW. Potassium channel dysfunction in cerebral arteries of insulin-resistant rats is mediated by reactive oxygen species. Stroke. 2004;35:964–969. doi: 10.1161/01.STR.0000119753.05670.F1. [DOI] [PubMed] [Google Scholar]
- 12.Erdos B, Snipes JA, Miller AW, Busija DW. Cerebrovascular dysfunction in Zucker obese rats is mediated by oxidative stress and protein kinase C. Diabetes. 2004;53:1352–1359. doi: 10.2337/diabetes.53.5.1352. [DOI] [PubMed] [Google Scholar]
- 13.Ergul A, Schultz Johansen J, Stromhaug C, Harris AK, Hutchinson J, Tawfik A, Rahimi A, Rhim E, Wells B, Caldwell RW, Anstadt MP. Vascular dysfunction of venous bypass conduits is mediated by reactive oxygen species in diabetes: Role of endothelin-1. J Pharmacol Exp Ther. 2004;313:70–77. doi: 10.1124/jpet.104.078105. [DOI] [PubMed] [Google Scholar]
- 14.Farese RV, Standaert ML, Yamada K, Huang LC, Zhang C, Cooper DR, Wang Z, Yang Y, Suzuki S, Toyota T, et al. Insulin-induced activation of glycerol-3-phosphate acyltransferase by a chiro-inositol-containing insulin mediator is defective in adipocytes of insulin-resistant, type II diabetic, Goto-Kakizaki rats. Proc Natl Acad Sci U S A. 1994;91:11040–11044. doi: 10.1073/pnas.91.23.11040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer U, Arnold M, Nedeltchev K, Schoenenberger RA, Kappeler L, Hollinger P, Schroth G, Ballinari P, Mattle HP. Impact of comorbidity on ischemic stroke outcome. Acta Neurol Scand. 2006;113:108–113. doi: 10.1111/j.1600-0404.2005.00551.x. [DOI] [PubMed] [Google Scholar]
- 16.Gentile NT, Seftchick MW, Huynh T, Kruus LK, Gaughan J. Decreased mortality by normalizing blood glucose after acute ischemic stroke. Acad Emerg Med. 2006;13:174–180. doi: 10.1197/j.aem.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Goto K, Hama H, Kasuya Y. Molecular pharmacology and pathophysiological significance of endothelin. Jpn J Pharmacol. 1996;72:261–290. doi: 10.1254/jjp.72.261. [DOI] [PubMed] [Google Scholar]
- 18.Goto Y, Kakizaki M, Masaki N. Production of spontaneous diabetic rats by repetition of selective breeding. Tohoku J Exp Med. 1976;119:85–90. doi: 10.1620/tjem.119.85. [DOI] [PubMed] [Google Scholar]
- 19.Gregan B, Jurgensen J, Papsdorf G, Furkert J, Schaefer M, Beyermann M, Rosenthal W, Oksche A. Ligand-dependent differences in the internalization of endothelin A and endothelin B receptor heterodimers. J Biol Chem. 2004;279:27679–27687. doi: 10.1074/jbc.M403601200. [DOI] [PubMed] [Google Scholar]
- 20.Gregan B, Schaefer M, Rosenthal W, Oksche A. Fluorescence resonance energy transfer analysis reveals the existence of endothelin-A and endothelin-B receptor homodimers. J Cardiovasc Pharmacol. 2004;44:S30–S33. doi: 10.1097/01.fjc.0000166218.35168.79. [DOI] [PubMed] [Google Scholar]
- 21.Harris AK, Hutchinson JR, Sachidanandam K, Johnson MH, Dorrance AM, Stepp DW, Fagan SC, Ergul A. Type 2 diabetes causes remodeling of cerebrovasculature via differential regulation of matrix metalloproteinases and collagen synthesis: role of endothelin-1. Diabetes. 2005;54:2638–2644. doi: 10.2337/diabetes.54.9.2638. [DOI] [PubMed] [Google Scholar]
- 22.Hattori Y, Kawasaki H, Kanno M. Increased contractile responses to endothelin-1 and U46619 via a protein kinase C-mediated nifedipine-sensitive pathway in diabetic rat aorta. Res Commun Mol Pathol Pharmacol. 1999;104:73–80. [PubMed] [Google Scholar]
- 23.Ikeda K, Wada Y, Sanematsu H, Foster HE, Shin D, Weiss RM, Latifpour J. Regulatory effect of experimental diabetes on the expression of endothelin receptor subtypes and their gene transcripts in the rat adrenal gland. J Endocrinol. 2001;168:163–175. doi: 10.1677/joe.0.1680163. [DOI] [PubMed] [Google Scholar]
- 24.Kamata K, Kondoh H. Impairment of endothelium-dependent relaxation of the isolated basilar artery from streptozotocin-induced diabetic rats. Res Commun Mol Pathol Pharmacol. 1996;94:239–249. [PubMed] [Google Scholar]
- 25.Karagiannis J, Reid JJ, Darby I, Roche P, Rand MJ, Li CG. Impaired nitric oxide function in the basilar artery of the obese Zucker rat. J Cardiovas Pharmacol. 2003;42:497–505. doi: 10.1097/00005344-200310000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Khan ZA, Chakrabarti S. Endothelins in chronic diabetic complications. Can J Physiol Pharmacol. 2003;81:622–634. doi: 10.1139/y03-053. [DOI] [PubMed] [Google Scholar]
- 27.Kiff RJ, Gardiner SM, Compton AM, Bennett T. The effects of endothelin-1 and NG-nitro-L-arginine methyl ester on regional haemodynamics in conscious rats with streptozotocin-induced diabetes mellitus. Br J Pharmacol. 1991;103:1321–1326. doi: 10.1111/j.1476-5381.1991.tb09787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitahara Y, Miura K, Takesue K, Mine T, Wada R, Uchida Y, Ito S, Yagihashi S. Decreased blood glucose excursion by nateglinide ameliorated neuropathic changes in Goto-Kakizaki rats, an animal model of non-obese type 2 diabetes. Metabolism. 2002;51:1452–1457. doi: 10.1053/meta.2002.35195. [DOI] [PubMed] [Google Scholar]
- 29.Laakso M. Hyperglycemia and cardiovascular disease in type 2 diabetes. Diabetes. 1999;48:937–942. doi: 10.2337/diabetes.48.5.937. [DOI] [PubMed] [Google Scholar]
- 30.Ladriere L, Bjorkling F, Malaisse WJ. Stimulation of insulin release in hereditarily diabetic rats by mixed molecules formed of nateglinide and a succinic acid ester. Int J Mol Med. 2000;5:63–65. doi: 10.3892/ijmm.5.1.63. [DOI] [PubMed] [Google Scholar]
- 31.Llorens S, Miranda FJ, Alabadi JA, Marrachelli VG, Alborch E. Different role of endothelin ETA and ETB receptors and endothelial modulators in diabetes-induced hyperreactivity of the rabbit carotid artery to endothelin-1. Eur J Pharmacol. 2004;486:43–51. doi: 10.1016/j.ejphar.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Makino A, Oda SI, Kamata K. Mechanisms underlyin increased release of endothelin −1 from aorta in diabetic rats. Peptides. 2001;22:639–645. doi: 10.1016/s0196-9781(01)00374-6. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto T, Yoshiyama S, Kobayashi T, Kamata K. Mechanisms underlying enhanced contractile response to endothelin-1 in diabetic rat basilar artery. Peptides. 2004;25:1985–1994. doi: 10.1016/j.peptides.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Mayhan W. Impairment of endothelium-dependent dilation of cerebral arterioles during diabetes mellitus. Am J Physiol. 1989;256:H621–H625. doi: 10.1152/ajpheart.1989.256.3.H621. [DOI] [PubMed] [Google Scholar]
- 35.Mayhan WG. Constrictor responses of the rat basilar artery during diabetes mellitus. Brain Res. 1998;783:326–331. doi: 10.1016/s0006-8993(97)01387-5. [DOI] [PubMed] [Google Scholar]
- 36.Mayhan WG, Trauernicht AK, Irvine SD. Insulin reverses impaired acetylcholine-induced dilatation of the rat basilar artery during diabetes mellitus. Brain Res. 2001;893:195–201. doi: 10.1016/s0006-8993(00)03314-x. [DOI] [PubMed] [Google Scholar]
- 37.McIntyre C, Hadoke PWF, Williams BC, Lindsay RM, Elliot AI, A MJ. Selective enhancement of sensivity to endothelin-I despite normal endothelium-dependent relaxation in subcutaneour resistancance arteries isolated from patients with Type I diabetes. Clin Sci. 2001;100:311–318. [PubMed] [Google Scholar]
- 38.Mizuguchi T, Nishiyama M, Moroi K, Tanaka H, Saito T, Masuda Y, Masaki T, de Wit D, Yanagisawa M, Kimura S. Analysis of two pharmacologically predicted endothelin B receptor subtypes by using the endothelin B receptor gene knockout mouse. Br J Pharmacol. 1997;120:1427–1430. doi: 10.1038/sj.bjp.0701054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishiyama M, Moroi K, Shan L, Yamamoto M, Takasadi C, Masaki T, Kimura S. Two different endothelin b receptor subtypes mediate contraction of the rabbit saphenous vein. Jpn J Pharmacol. 1995;68:235–243. doi: 10.1254/jjp.68.235. [DOI] [PubMed] [Google Scholar]
- 40.Nishiyama M, Takahara Y, Masaki T, Nakajima N, kimura S. Pharmacological heterogeneity of both ETA and ETB receptors in human saphenous nein. Jpn J Pharmacol. 1995 doi: 10.1254/jjp.69.391. [DOI] [PubMed] [Google Scholar]
- 41.Ortmann J, Traupe T, Nett P, Celeiro J, Hofmann-Lehmann R, Lange M, Vetter W, Barton M. Upregulation of vascular ETB receptor gene expression after chronic ETA receptor blockade in prediabetic NOD mice. J Cardiovasc Pharmacol. 2004;44:S105–S108. doi: 10.1097/01.fjc.0000166230.26583.f8. [DOI] [PubMed] [Google Scholar]
- 42.Pieper GM. Review of alterations in endothelial nitric oxide production in diabetes: protective role of arginine on endothelial dysfunction. Hypertension. 1998;31:1047–1060. doi: 10.1161/01.hyp.31.5.1047. [DOI] [PubMed] [Google Scholar]
- 43.Sauvageau S, Thorin E, Caron A, Dupuis J. Evaluation of endothelin-1-induced pulmonary vasoconstriction following myocardial infarction. Exp Biol Med (Maywood) 2006;231:840–846. [PubMed] [Google Scholar]
- 44.Schilling L, Feger GI, Ehrenreich H, Wahl M. Endothelin-1-induced contraction and relaxation of isolated rat basilar artery: effect of the endothelium. J Cardiovasc Pharmacol. 1995;26(Suppl 3):S197–S199. [PubMed] [Google Scholar]
- 45.Schneider JG, Tilly N, Hierl T, Sommer U, Hamann A, Dugi K, Leidig-Bruckner G, Kasperk C. Elevated plasma endothelin-1 levels in diabetes mellitus. Am J Hypertens. 2002;15:967–972. doi: 10.1016/s0895-7061(02)03060-1. [DOI] [PubMed] [Google Scholar]
- 46.Schwaninger RM, Sun H, Mayhan WG. Impaired nitric oxide synthase-dependent dilatation of cerebral arterioles in type II diabetic rats. Life Sci. 2003;73:3415–3425. doi: 10.1016/j.lfs.2003.06.029. [DOI] [PubMed] [Google Scholar]
- 47.Seica RM, Martins MJ, Pessa PB, Santos RM, Rosario LM, Suzuki KI, Martins MI. [Morphological changes of islet of Langerhans in an animal model of type 2 diabetes] Acta Med Port. 2003;16:381–388. [PubMed] [Google Scholar]
- 48.Seica RM, Suzuki KI, Santos RM, Do Rosario LM. [Impaired insulin secretion in isolated islets of Goto-Kakizaki rats, an animal model of non obese type 2 diabetes, is a primary event] Acta Med Port. 2004;17:42–48. [PubMed] [Google Scholar]
- 49.Standaert ML, Sajan MP, Miura A, Kanoh Y, Chen HC, Farese RV, Farese RV. Insulin-induced activation of atypical protein kinase C, but not protein kinase B, is maintained in diabetic (ob/ob and Goto-Kakazaki) liver Contrasting insulin signaling patterns in liver versus muscle define phenotypes of type 2 diabetic and high fat-induced insulin-resistant states. J Biol Chem. 2004;279:24929–24934. doi: 10.1074/jbc.M402440200. [DOI] [PubMed] [Google Scholar]
- 50.Sudjarwo SA, Hori M, Takai M, Urade Y, Okada T, Karaki H. A novel subtype of endothelin B receptor mediating contraction in swine pulmonary vein. Life Sci. 1993;53:431–437. doi: 10.1016/0024-3205(93)90647-l. [DOI] [PubMed] [Google Scholar]
- 51.Takahashi K, Ghatei MA, Lam HC, O’Halloran DJ, Bloom SR. Elevated plasma endothelin in patients with diabetes mellitus. Diabetologia. 1990;33:306–310. doi: 10.1007/BF00403325. [DOI] [PubMed] [Google Scholar]
- 52.Wannamethee SG, Perry IJ, Shaper AG. Nonfasting serum glucose and insulin concentrations and the risk of stroke. Stroke. 1999;30:1780–1786. doi: 10.1161/01.str.30.9.1780. [DOI] [PubMed] [Google Scholar]
- 53.Witte K, Reitenbach I, Stolpe K, Schilling L, Kirchengast M, Lemmer B. Effects of the endothelin a receptor antagonist darusentan on blood pressure and vascular contractility in type 2 diabetic Goto-Kakizaki rats. J Cardiovas Pharmacol. 2003;41:890–896. doi: 10.1097/00005344-200306000-00009. [DOI] [PubMed] [Google Scholar]
- 54.Zimmermann PA, Knot HJ, Stevenson AS, Nelson MT. Increased myogenic tone and diminished responsiveness to ATP-sensitive K+ channel openers in cerebral arteries from diabetic rats. Circ Res. 1997;81:996–1004. doi: 10.1161/01.res.81.6.996. [DOI] [PubMed] [Google Scholar]
- 55.Zuccarello M, Boccaletti R, Rapoport RM. Does blockade of endothelinB1-receptor activation increase endothelinB2/endothelinA receptor-mediated constriction in the rabbit basilar artery? J Cardiovas Pharmacol. 1999;33:679–684. doi: 10.1097/00005344-199905000-00001. [DOI] [PubMed] [Google Scholar]