Abstract
Purpose
Survival of patients with completely resected non–small-cell lung cancer (NSCLC) is unsatisfactory, and in 2002, the benefit of adjuvant chemotherapy was not established. This phase III study assessed the impact of postoperative adjuvant gefitinib on overall survival (OS).
Patients and Methods
Patients with completely resected (stage IB, II, or IIIA) NSCLC stratified by stage, histology, sex, postoperative radiotherapy, and chemotherapy were randomly assigned (1:1) to receive gefitinib 250 mg per day or placebo for 2 years. Study end points were OS, disease-free survival (DFS), and toxicity.
Results
As a result of early closure, 503 of 1,242 planned patients were randomly assigned (251 to gefitinib and 252 to placebo). Baseline factors were balanced between the arms. With a median of 4.7 years of follow-up (range, 0.1 to 6.3 years), there was no difference in OS (hazard ratio [HR], 1.24; 95% CI, 0.94 to 1.64; P = .14) or DFS (HR, 1.22; 95% CI, 0.93 to 1.61; P = .15) between the arms. Exploratory analyses demonstrated no DFS (HR, 1.28; 95% CI, 0.92 to 1.76; P = .14) or OS benefit (HR, 1.24; 95% CI, 0.90 to 1.71; P = .18) from gefitinib for 344 patients with epidermal growth factor receptor (EGFR) wild-type tumors. Similarly, there was no DFS (HR, 1.84; 95% CI, 0.44 to 7.73; P = .395) or OS benefit (HR, 3.16; 95% CI, 0.61 to 16.45; P = .15) from gefitinib for the 15 patients with EGFR mutation-positive tumors. Adverse events were those expected with an EGFR inhibitor. Serious adverse events occurred in ≤ 5% of patients, except infection, fatigue, and pain. One patient in each arm had fatal pneumonitis.
Conclusion
Although the trial closed prematurely and definitive statements regarding the efficacy of adjuvant gefitinib cannot be made, these results indicate that it is unlikely to be of benefit.
INTRODUCTION
Globally, lung cancer remains the most common cancer and is the leading cause of cancer-related mortality in men and women. In 2012, an estimated 20,100 Canadians and 160,340 Americans died of the disease.1,2 Non–small-cell lung cancer (NSCLC) accounts for approximately 85% of all pulmonary neoplasms.3 At initiation of the NCIC CTG BR19 (CTSUBR19) study in 2002, the results of the large platinum-based adjuvant chemotherapy studies in NSCLC were not available and the 5-year survival rate for stage I disease was 60% to 70%, decreasing to 40% for stage II.4 Although some studies demonstrated a biologic effect, adjuvant chemotherapy was not considered standard of care, and practice patterns varied considerably.
It was known that in NSCLC increased epidermal growth factor receptor (EGFR) expression correlated with aggressive biology, poor response to therapy, and poor outcome.5–8 The EGFR pathway was believed to be important in the development and progression of epithelial malignancies and to be a potential target for systemic therapeutics.9 Gefitinib binds reversibly to the internal domain of EGFR and blocks downstream pathways, thereby reducing proliferation, increasing apoptosis, and decreasing angiogenesis and invasion in NSCLC.10 In phase II studies, dramatic responses and improved disease control were observed.11,12 Given the poor survival of patients with completely resected NSCLC, only modest improvements with adjuvant chemotherapy, evidence of gefitinib activity in advanced NSCLC, and gefitinib's acceptable toxicity profile and oral route of administration, this study of adjuvant gefitinib in patients with completely resected NSCLC was undertaken.
PATIENTS AND METHODS
Study Design
This study was a North American, multicenter, prospective, randomized, double-blind, placebo-controlled trial of the EGFR antagonist gefitinib in patients with completely resected stage IB, II, and IIIA NSCLC (American Joint Committee on Cancer/International Union Against Cancer TNM classification, sixth edition)4,13 conducted by NCIC CTG in collaboration with the Clinical Trials Support Unit of the US National Cancer Institute. Within 16 weeks after surgical resection, eligible patients were randomly assigned (1:1) to receive gefitinib or placebo. The study was activated in September 2002. Initially, patients were stratified by stage (IB, II, or IIIA), histologic subtype (squamous v others), postoperative radiotherapy (given v not), and sex. In October 2003, the study was amended to allow, and stratified for, adjuvant chemotherapy (given v not) with random assignment within 26 weeks of surgery. The primary study end point was overall survival (OS). Secondary end points included toxicity, disease-free survival (DFS), and establishment of a tumor bank for biomarker analysis. The protocol was approved by institutional review boards at all study sites, and all patients provided written informed consent. Data were collected, managed, and analyzed by the NCIC CTG.
Eligibility Criteria
Patients ≥ 18 years old with completely resected, histologically proven stage IB, II, or IIIA NSCLC and an Eastern Cooperative Oncology Group performance status of 0 to 2 were eligible. All patients had a presurgical computed tomography (CT) or magnetic resonance imaging scan of the chest and complete mediastinal lymph node resection or nodal sampling (biopsy of nodes ≥ 1.5 cm on presurgical scans was mandatory). A period of no more than 16 weeks between surgery and random assignment was permitted for patients receiving study drug only, and a period of no more than 26 weeks was permitted for those receiving adjuvant chemotherapy. Patients were ineligible if they had undergone only segmentectomy or wedge resection or had prior malignancies within 5 years, clinically significant or untreated ophthalmologic or GI conditions, mixed tumors with small-cell or carcinoid components, more than one discrete area of primary cancer, clinically significant cardiac dysfunction, active infection, or neurologic or psychiatric disorders.
Random Assignment and Treatment Regimen
Patients received either gefitinib 250 mg or placebo orally daily for 2 years. A 50% dose reduction was allowed that, when necessary, was accomplished by alternate-day dosing. No dose reductions less than 50% were allowed. Patients requiring more than 21 days of continuous drug withholding for toxicity were taken off study medication permanently.
Follow-Up
Patients were observed at 1 and 3 months, then every 3 months until 30 months, then every 6 months for years 3, 4, and 5, and then yearly. During the 2 years of drug administration and for 6 months thereafter, patients underwent history, physical examination, hematology, biochemistry, chest x-ray, and adverse event evaluation.
Exploratory Molecular Analyses
DNA was isolated from sections cut from formalin-fixed, paraffin-embedded blocks. Either macrodissection or laser capture microdissection was used to ensure DNA preparations had ≤ 10% contamination with noncancer cell DNA. KRAS mutation analysis was performed using a nested polymerase chain reaction procedure.14 EGFR analyses were performed on all adequate specimens at two different institutions.15,16 When results lacked concordance, the DNA was tested at a third institution.17 Results from the latter laboratory were taken as final (Appendix, online only).
Statistical Analysis
Assuming 60% of patients with stage IB and II disease, the corresponding median survival for patients with stage IB to IIIA was calculated to be approximately 32 months (2.7 years). To have 90% power to detect a hazard ratio (HR) of 0.75 using a one-sided 2.5% level test, a total of 1,242 patients were to be accrued in 3.5 years with 1.8 years of follow-up to reach 537 required events (Appendix).
Primary End Points and Analysis
OS and DFS were defined as the time from random assignment to time of death from any cause and time of documented local or distant recurrence of the initial cancer, respectively. All randomly assigned patients were included in survival analyses on an intent-to-treat basis. Survival was described using the Kaplan-Meier method. A stratified log-rank test was used to compare OS and DFS between arms adjusting for stratification factors. An unadjusted analysis was also performed. For exploratory analyses, a Cox proportional hazards model was derived using a stepwise model-building procedure. All patients receiving one dose of study treatment were included in the safety analysis. Toxicities were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0) and summarized by type and severity. Fisher's exact test was used to compare toxicities between arms. For interim analyses, see the Appendix.
Accrual and Time of Analysis
In December 2004, the phase III study, Iressa Survival Evaluation in Lung Cancer (ISEL), comparing gefitinib with placebo in advanced NSCLC, failed to demonstrate a significant survival benefit.18 In addition, an unplanned interim analysis of trial S0023 demonstrated that maintenance gefitinib after chemoradiation in patients with stage III NSCLC did not improve OS and was potentially detrimental.19 In April 2005, based on these results, the BR19 Data and Safety Monitoring Committee recommended study closure and discontinuation of study medication. The trial committee elected to perform the final analysis once all patients were followed for at least 4 years.
RESULTS
Patient Characteristics
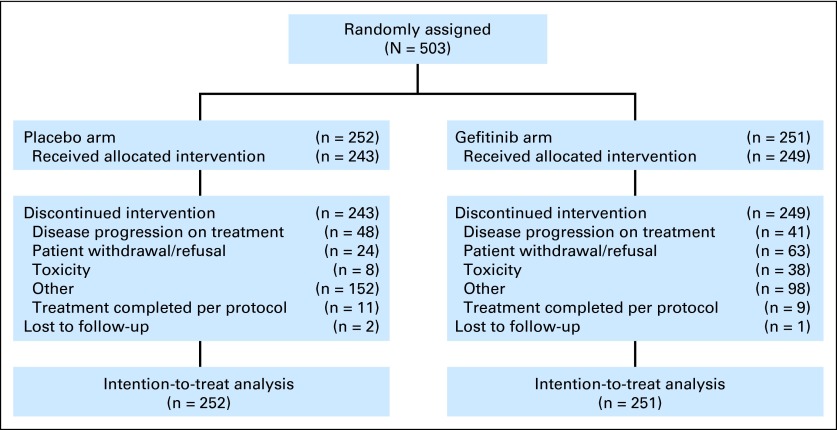
Between September 2002 and April 2005, 503 patients were randomly assigned (251 to gefitinib and 252 to placebo; Fig 1). Eleven patients were ineligible (gefitinib, n = 4; placebo, n = 7) but were included in the intent-to-treat analysis. Reasons included preoperative CT over the deadline (n = 1), stage 1A disease (n = 4), two separate tumors (n = 2), serum creatinine ≥ 1.5× upper limit of normal (n = 1), metastases (n = 1), prior cancer (n = 1), and no preoperative CT (n = 1). Three patients were lost to follow-up. The median follow-up time for all patients was 4.7 years (range, 0.1 to 6.3 years). Baseline characteristics were balanced between arms (Table 1). Only 17% of patients (n = 87) received adjuvant chemotherapy, and 5% (n = 23) received adjuvant radiotherapy. Fifty-two percent, 35%, and 13% of patients had stage IB, II, and IIIA NSCLC, respectively, and 60% and 28% had adenocarcinoma and squamous carcinoma, respectively.
Fig 1.
CONSORT diagram for BR19.
Table 1.
Baseline Patient Demographics and Clinical Characteristics
| Demographic or Clinical Characteristic | Gefitinib (n = 251) |
Placebo (n = 252) |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Median age, years | 66 | 67 | ||
| Sex | ||||
| Female | 116 | 46 | 116 | 46 |
| Male | 135 | 54 | 136 | 54 |
| Race | ||||
| White | 233 | 93 | 235 | 93 |
| Asian | 6 | 2 | 3 | 1 |
| Other | 12 | 5 | 14 | 6 |
| Histology | ||||
| Adenocarcinoma | 150 | 60 | 149 | 59 |
| Squamous carcinoma | 69 | 27 | 71 | 28 |
| Other | 32 | 13 | 32 | 13 |
| Smoking history | ||||
| Yes | 224 | 89 | 223 | 88 |
| No | 23 | 9 | 19 | 8 |
| Missing | 4 | 2 | 10 | 4 |
| ECOG PS | ||||
| 0-1 | 244 | 97 | 244 | 97 |
| 2 | 7 | 3 | 8 | 3 |
| Stage | ||||
| IB | 133 | 53 | 127 | 50 |
| II | 87 | 35 | 88 | 35 |
| IIIA | 31 | 12 | 36 | 14 |
| Unknown | 0 | 0 | 1 | 0 |
| Type of surgery | ||||
| Pneumonectomy | 30 | 12 | 41 | 16 |
| Other | 221 | 88 | 221 | 84 |
| Prior adjuvant chemotherapy* | ||||
| Yes | 43 | 17 | 44 | 17 |
| No | 208 | 83 | 208 | 83 |
| Prior radiotherapy | ||||
| Yes | 11 | 4 | 12 | 5 |
| No | 240 | 96 | 240 | 95 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; PS, performance status.
Adjuvant chemotherapy included cisplatin 100 mg/m2 on day 1 and vinorelbine 30 mg on days 1 and 8.
Delivery and Toxicity of Therapy
Of 503 randomly assigned patients, 249 received at least one dose of gefitinib, and 243 received one dose of placebo. Median duration of treatment was 4.8 months (range, 1 day to 25 months) for gefitinib and 8.9 months (range, 1 day to 26 months) for placebo. Dose adjustment occurred in 39% of gefitinib-treated patients (96 of 249 patients) and 20% of placebo-treated patients (49 of 243 patients). Drug was held temporarily for 23% of patients on gefitinib and 11% on placebo, with 60% and 35%, respectively, a result of toxicities. Treatment was discontinued as a result of toxicity in 15.3% of patients on gefitinib and 3.3% of patients on placebo. Patient refusal or withdrawal rate after beginning therapy was 24% on gefitinib and 7% on placebo.
Gefitinib patients had a higher incidence of rash, dry skin, diarrhea, anorexia, and nausea but less chest pain, muscle pain, and dyspnea. Adverse events generally were grade 1 to 2. Grade 3 to 4 diarrhea, skin effects, and chest pain were reported in 5% to 8% of both gefitinib- and placebo-treated patients (Table 2). The most common serious adverse event in both cohorts was dyspnea, occurring in 13% and 7% of patients on gefitinib and placebo, respectively. Other serious adverse events were less frequent and occurred in ≤ 5% of patients, with the exception of infection and pain. One patient in each cohort had fatal pneumonitis. Five gefitinib and four placebo patients died of fatal adverse event; three of five deaths in the gefitinib arm were considered drug related.
Table 2.
Serious Adverse Events Graded According to National Cancer Institute Common Terminology Criteria for Adverse Events Version 3.0
| Serious Adverse Events | Gefitinib (n = 249) |
Placebo (n = 243) |
||||||
|---|---|---|---|---|---|---|---|---|
| Grade 3 |
Grade 4 |
Grade 3 |
Grade 4 |
|||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Infection, other | 7 | 3 | 0 | 0 | 3 | 1 | 0 | 0 |
| Rash/acne | 21 | 8 | 0 | 0 | 1 | < 1 | 0 | 0 |
| Dehydration | 2 | 1 | 0 | 0 | 2 | 1 | 0 | 0 |
| Diarrhea | 18 | 7 | 0 | 0 | 5 | 2 | 0 | 0 |
| Nausea | 6 | 2 | 0 | 0 | 1 | < 1 | 0 | 0 |
| Fatigue | 15 | 6 | 2 | 1 | 6 | 2 | 1 | < 1 |
| Dyspnea | 24 | 10 | 7 | 3 | 18 | 7 | 1 | < 1 |
| Vomiting | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pneumonitis | 2 | 1 | 2 | 1 | 2 | 1 | 0 | 0 |
DFS and OS
Among 503 patients, recurrence was documented in 225 (121 patients on gefitinib and 104 patients on placebo). Patterns of disease recurrence were similar between treatment arms for both local (gefitinib, 49.5%; placebo, 49.1%) and distant (gefitinib, 42.1%; placebo, 41.3%) recurrences. The specific sites of disease recurrences are listed in Appendix Table A1 (online only). The median DFS was 4.2 years (95% CI, 3.2 years to not calculable) on gefitinib and not reached on the placebo arm (HR for recurrence, 1.22; 95% CI, 0.93 to 1.61; P = .15; Fig 2A). The stratified Cox regression model found only tumor size ≥ 4 cm (P < .001) to be associated with poor DFS, whereas gefitinib remained not significant and potentially harmful (HR, 1.27; 95% CI, 0.96 to 1.69; P = .096). At data cutoff, 219 of 503 patients had died (116 on gefitinib and 103 on placebo), 72% from progressive disease, 9% from unknown causes, and 19% from other causes. Eighty-five patients (73%) on gefitinib and 73 patients (71%) on placebo died of disease progression. The median survival time on gefitinib was 5.1 years (95% CI, 4.4 years to not calculable) and had not been reached for placebo patients (HR, 1.24; 95% CI, 0.94 to 1.64; P = .14; Fig 2B). The stratified Cox regression model found age ≥ 65 years (HR, 1.42; 95% CI, 1.05 to 1.91; P = .02) and tumor size ≥ 4 cm (HR, 1.72; 95% CI, 1.28 to 2.30; P = .003) to be associated with shorter survival, whereas gefitinib remained not significant and potentially harmful (HR, 1.27; 95% CI, 0.96 to 1.69; P = .097).
Fig 2.
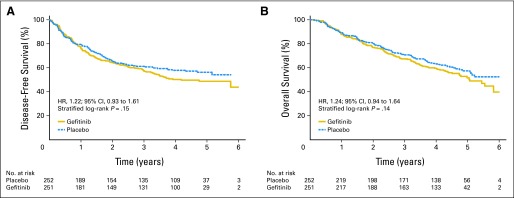
(A) Disease-free survival. (B) Overall survival. HR, hazard ratio.
Exploratory Subgroup Analyses
EGFR.
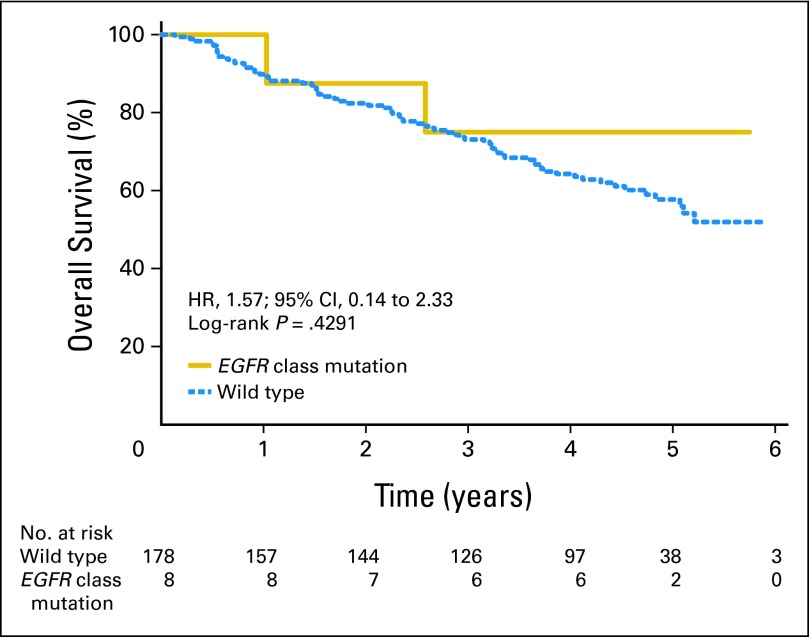
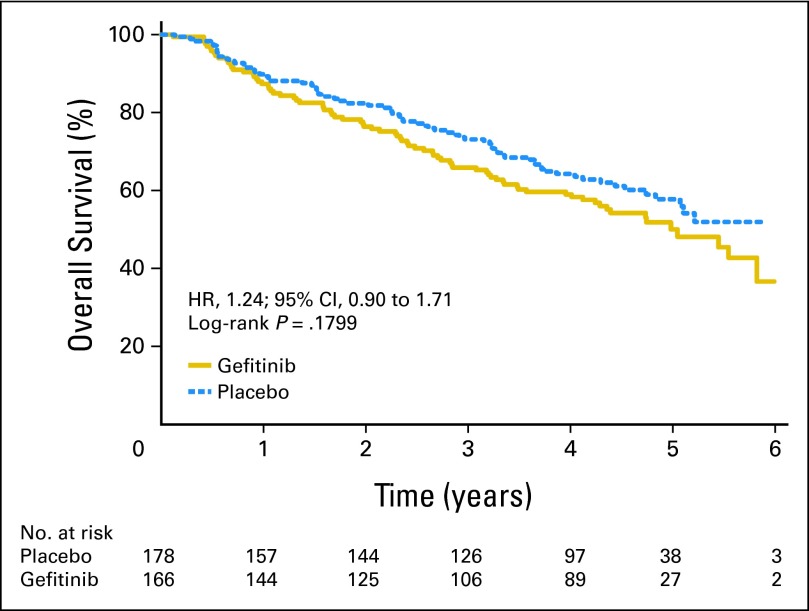
Among 503 patients, EGFR mutation status was determined successfully in 359 tumors (71%; gefitinib, n = 173; placebo, n = 186). Baseline factors were balanced between those with and without EGFR mutation data, except patients who had undergone pneumonectomy (P = .02), and males (P = .06) were more likely to have mutation data. Among patients with known EGFR status, 344 had wild-type status, whereas only 15 (4%) had mutations (all adenocarcinoma). EGFR mutations were more frequent in women (P = .004), nonsmokers (P < .001), and Asians (P = .03). For the 186 patients on placebo, the median DFS and OS (Fig 3) for EGFR wild-type (n = 178) and the EGFR mutant (n = 8) tumors were not reached. EGFR mutation positivity was not a significant prognostic factor for DFS (HR, 0.95; 95% CI, 0.300 to 3.01; P = .93) or OS (HR, 0.57; 95% CI, 0.14 to 2.33; P = .43), but analyses were limited by the low mutation rate. In multivariable Cox regression modeling, EGFR mutation status was not a significant prognostic factor for DFS (HR, 0.92; 95% CI, 0.29 to 2.96; P = .89) or OS (HR, 0.52; 95% CI, 0.13 to 2.12; P = .36). For 344 patients with EGFR wild-type tumors (166 patients on gefitinib and 178 patients on placebo), gefitinib demonstrated no beneficial effect on DFS (HR, 1.28; 95% CI, 0.92 to 1.76; P = .14) or OS (HR, 1.24; 95% CI, 0.90 to 1.71; P = .18; Fig 4). For the 15 patients with EGFR-mutated tumors (seven on gefitinib and eight on placebo), gefitinib demonstrated no beneficial effect on DFS (HR, 1.84; 95% CI, 0.44 to 7.73; P = .40; Fig 5A) or OS (HR, 3.16; 95% CI, 0.61 to 16.45; P = .15; Fig 5B). Cox regression models with gefitinib, EGFR mutations, and their interaction demonstrated no significant interaction for DFS and OS in univariate (P = .60 and P = .25, respectively) or adjusted multivariable analysis (P = .57 and P = .27, respectively).
Fig 3.
Overall survival in placebo arm in patients with EGFR wild-type versus EGFR-mutant tumors. HR, hazard ratio.
Fig 4.
Overall survival by treatment arm in patients with EGFR wild-type tumors. HR, hazard ratio.
Fig 5.
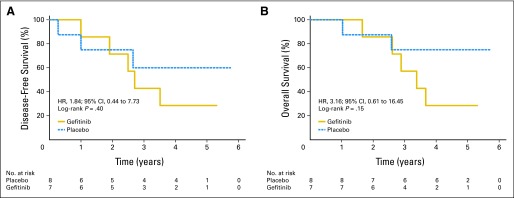
(A) Disease-free survival and (B) overall survival by treatment arm in patients with EGFR exon 19 and 21 mutations. HR, hazard ratio.
KRAS.
Of 503 patients, KRAS mutation status was determined in 350 tumors (gefitinib, n = 169; placebo, n = 181). For the 181 patients on placebo, the median DFS and OS for 128 patients with KRAS wild-type tumors and 53 patients with KRAS mutant tumors were not reached. KRAS mutation status was not a significant prognostic factor for either DFS (HR, 1.09; 95% CI, 0.66 to 1.82; P = .74) or OS (HR, 1.12; 95% CI, 0.67 to 1.86; P = .66). The multivariable Cox regression model adjusted for confounding and prognostic factors found that KRAS mutation status was not associated with DFS (HR, 0.96; 95% CI, 0.57 to 1.61; P = .87) or OS (HR, 0.94; 95% CI, 0.56 to 1.59; P = .83). Among 254 patients with KRAS wild-type tumors (gefitinib, n = 126; placebo, n = 128), gefitinib demonstrated no beneficial effect on DFS (HR, 1.08; 95% CI, 0.74 to 1.59; P = .69) or OS (HR, 1.13; 95% CI, 0.78 to 1.65; P = .51). For 96 patients with KRAS mutated tumors (gefitinib, n = 43; placebo, n = 53), gefitinib demonstrated significant detrimental effect on DFS (HR, 1.77; 95% CI, 1.00 to 3.13; P = .05) and lack of benefit on OS (HR, 1.51; 95% CI, 0.84 to 2.70; P = .16). Cox regression modeling with gefitinib, KRAS status, and their interaction term demonstrated no significant interaction effect (DFS, P = .15; OS, P = .36) and remained nonsignificant in multivariable adjusted models (DFS, P = .12; OS, P = .5).
Impact of Early Trial Closure
To examine the impact of early trial closure on the study results an unplanned subgroup analysis limited to the 397 patients randomly assigned at least 6 months before trial closure (198 on gefitinib and 199 on placebo) was undertaken. Similar to the overall population, gefitinib showed no potential treatment effect over placebo on OS (HR, 1.28; 95% CI, 0.96 to 1.71; P = .097). Similarly, for the 264 patients randomly assigned at least 1 year before trial closure (132 on placebo and 132 on gefitinib), gefitinib showed no potential treatment effect over placebo (HR, 1.24; 95% CI, 0.88 to 1.73; P = .22). The study results do not seem to be related to nonprotocol treatment received after disease progression, which was balanced between arms (Appendix Table A2, online only). Finally, based on a full intent-to-treat analysis, the conditional power of observing a beneficial effect of gefitinib had the study reached its target accrual was only 17.5%.
DISCUSSION
To our knowledge, NCIC CTG BR19 is the first randomized, double-blind, placebo-controlled trial of a targeted agent delivered in the adjuvant setting in completely resected NSCLC. Unfortunately early termination of the study does not allow for statistically robust conclusions. Nevertheless, the sample size is large (503 patients), and the study arms were balanced for the major prognostic factors
Gefitinib was well tolerated with an expected adverse event profile characteristic of EGFR inhibitors, namely rash, dry skin, diarrhea, anorexia, and nausea. The serious adverse event rate was low in both study arms (except dyspnea, which occurred in 7% to 13% of patients and was thought to be a result of surgery), with the majority reported by less than 5% of patients. Although more patients on gefitinib discontinued therapy as a result of treatment toxicity compared with patients on placebo (15.3% v 3.3%, respectively), prolonged administration of gefitinib in the adjuvant setting is tolerable in a majority of patients, although dose adjustments or temporary withholding may be required.
The median follow-up time for all patients accrued was 4.7 years (range, 0.1 to 6.3 years). The median duration of treatment was different between treatment arms (gefitinib, 4.8 months; placebo, 8.9 months) and was attributable to higher treatment toxicity withdrawals, greater number of patients refusing treatment, and greater disease progression and death observed in the gefitinib arm, as compared with placebo. Our results differ from those achieved in both the second-/third-line metastatic (BR.21)20 and maintenance settings (Sequential Tarceva in Unresectable NSCLC [SATURN])21 in biomarker unselected populations, where EGFR inhibition resulted in significant survival improvement. When BR19 was initiated, it was unknown that activating mutations of EGFR were oncogenic drivers and biomarkers of efficacy for EGFR inhibition.22,23 However, the protocol allowed for exploratory analyses, and therefore, KRAS mutation analysis and EGFR analysis of the common exon 19 deletion and exon 21 (L858R) point mutation were undertaken on tissue available from 350 (KRAS) and 359 (EGFR) patients. Baseline characteristics were balanced between those with and without mutation data, suggesting a representative sample of the intent-to-treat population. Having a tumor with a KRAS mutation was not significantly prognostic for DFS or OS, and wild-type KRAS status was not predictive of gefitinib effect on DFS or OS. Patients with KRAS mutation-positive tumors receiving gefitinib had significantly worse DFS, whereas there was no statistically significant effect on OS.
The number of patients with EGFR mutation-positive tumors was low, with only 15 positive tumors (4%) identified despite confirmatory analyses in three different laboratories. Why the mutation rate is low is unknown. A possible explanation is a lower mutation rate in early-stage compared with advanced-stage disease. The small numbers necessitate cautious interpretation of the data. Nevertheless, in this study, the common activating EGFR mutations were not prognostic for DFS (HR, 0.95) but did demonstrate a noticeable prognostic effect on OS (HR, 0.57; 95% CI, 0.14 to 2.33). Gefitinib showed no beneficial effect on DFS (HR, 1.28) or OS (HR, 1.24) for patients with wild-type tumors or on DFS (HR, 1.84) or OS (HR, 3.6) for patients with EGFR mutated tumors. Again, this is surprising because in the metastatic setting the common activating mutations are both prognostic24–26 and predictive of EGFR tyrosine kinase inhibitor effect.27–30 This apparent detrimental effect is not an isolated result confined to this trial. In the Southwest Oncology Group S003 trial, a detrimental effect on OS was seen in the arm receiving adjuvant gefitinib after chemoradiotherapy, and in the Iressa Pan-Asia Study (IPASS) in the metastatic setting, patients with wild-type tumors receiving gefitinib had significantly inferior DFS. What is the possible explanation? First, it is possible that because the study is underpowered, the gefitinib arm did poorly by chance alone. However, the detrimental effect is consistent across all subgroups. Second, because of the small number of mutation-positive patients, they were unable to exert a sufficiently powerful effect on the study results, and therefore, it is merely underpowered with mutation-positive patients. This would negate the effect of EGFR inhibition on the wild-type population seen in both the BR.21 and SATURN studies. Third, duration of treatment with gefitinib may have been too short to see therapeutic benefit; however, our results were not sensitive to treatment duration in patient groups randomly assigned 6 months or more before trial closure. Finally, it may be that the EGFR pathway plays a less important role in early disease and tumors are not as dependent on this pathway as an oncogenic driver as later disease states. These are intriguing questions but none can be answered, even with further analysis. BR19 has left us with unanswered questions, and we look forward to the results of the erlotinib adjuvant trial RADIANT (NCT 00373425) and two adjuvant studies in EGFR mutation-positive patients, the Chinese study (NCT01405079) and the ongoing Japanese study of adjuvant gefitinib versus chemotherapy.
Supplementary Material
Appendix
KRAS and EGFR Analysis
DNA was isolated from sections cut from formalin-fixed, paraffin-embedded blocks. Either macrodissection or laser capture microdissection was used to ensure that DNA preparations had less than 10% contamination with noncancer cell DNA. KRAS mutation analysis was performed using a nested polymerase chain reaction (PCR) procedure.14 Second-round PCR products were screened for the presence of mutations using high-resolution melting analysis on a Corbett Rotorgene 6000 (Qiagen, Venlo, the Netherlands). All samples that were positive for mutations by high-resolution melt analysis were then analyzed by Sanger sequencing on a separate PCR product, both to confirm the presence of the mutation and to determine the codon affected. EGFR mutation analysis was also performed using high-resolution melting screens for exon 19 and 21 mutations, followed by confirmation by Sanger sequencing.15 Because this analysis gave a low frequency of mutations (5%), EGFR mutation analysis was repeated in a second laboratory, using the PCR-restriction fragment length polymorphism and fragment length analyses method for screening and using Sanger sequencing for conformation.16 For samples that were discordant between the two laboratories, reanalysis was performed in a third College of American Pathologists–accredited clinical diagnostic laboratory using the PCR-restriction fragment length polymorphism and fragment length analyses method.17 Results from the latter laboratory were taken as final.
Statistical Analysis
Sample size and duration of study.
The 3-year survival rate for the 60% of patients with stage 1B and II disease together is approximately 63%. The corresponding median survival time for this subgroup of patients is about 54 months (4.5 years). The 3-year survival rate for the 40% of patients with stage IIIA disease is approximately 33%. The corresponding median survival time is about 23 months (1.9 years). To have 90% power to detect a hazard ratio of 0.75, using a one-sided 2.5% level test, after stratifying by the previously mentioned two subgroups, a total of 1,242 patients in 3.5 years with 1.8 years of follow-up were to be accrued. The total number of events required was 537 deaths, and the absolute calculated differences at 3 years were approximately 7% and 10% for the first and second subgroups, respectively.
Primary end points and analysis.
Overall survival, the primary end point of this study, was defined as the time from random assignment to the time of death from any cause. Disease-free survival was defined as the time from random assignment to the time of documented recurrence of the initial cancer. All randomly assigned patients were included in survival analyses based on the intent-to-treat principle. The survival distributions of patients in both treatment groups were described using the Kaplan-Meier method. A stratified log-rank test was used as the primary method to compare the overall survival and disease-free survival between the two arms adjusting for the stratification factors. An unadjusted analysis was also performed. For exploratory analyses, a Cox proportional hazards model was used to adjust the observed treatment effect for the influence of various prognostic factors at study entry and identify factors significantly related to survival outcomes. The final Cox model was determined using a stepwise model-building procedure. All patients who received at least one dose of study treatment were included in the safety analysis. Toxicities were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0). The incidence of toxicities was summarized by type of adverse event and severity. A Fisher's exact test was used to compare toxicities between the two arms.
Interim Analysis
Two interim analyses (the first after 179 deaths and the second after 358 deaths) were planned to determine whether early termination of the study was necessary if results were extreme. The trial would be stopped if the comparison between the treatment arm and control arm was significant in favor of treatment at a significance level of P = .001 and P = .005 for the first and second interim analyses, respectively. The significance value of P = .023 was used for the final analysis to assure the overall type I error of 0.025 based on the O'Brien-Fleming design truncated at a significant level of P = .001.
Exploratory Analyses: EGFR and KRAS Mutations
Exploratory analyses were performed to characterize the relationships between patients' mutation status and baseline characteristics and outcomes. The χ2 test or Fisher's exact test were used to assess the association between categorical variables. Kaplan-Meier curves were used to estimate the distributions of time-to-event outcomes, the log-rank test was used to test difference between groups, and the Cox regression model was used to correlate mutation status while adjusting baseline characteristics to time-to-event outcomes.
Table A1.
Sites of Disease Recurrence
| Type of Recurrence | Gefitinib (n = 121) |
Placebo (n = 104) |
Total (N = 225) |
|||
|---|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Local recurrence | ||||||
| Local lung recurrence | 32 | 26.4 | 31 | 29.8 | 63 | 28.0 |
| Pleural effusion recurrence | 5 | 4.1 | 6 | 5.8 | 11 | 4.9 |
| Regional lymph node recurrence | 23 | 19.0 | 14 | 13.5 | 37 | 16.4 |
| Total | 60 | 49.5 | 51 | 49.1 | 111 | 49.3 |
| Distant recurrence | ||||||
| Bone recurrence | 11 | 9.1 | 12 | 11.5 | 23 | 10.2 |
| Brain recurrence | 12 | 9.9 | 20 | 19.2 | 32 | 14.2 |
| Distant lung recurrence | 20 | 16.5 | 8 | 7.7 | 28 | 12.4 |
| Liver recurrence | 8 | 6.6 | 3 | 2.9 | 11 | 4.9 |
| Total | 51 | 42.1 | 43 | 41.3 | 94 | 41.7 |
| Other | ||||||
| Other recurrence | 8 | 6.6 | 8 | 7.7 | 16 | 7.1 |
| Death as a result of cancer without documented recurrence | 2 | 1.7 | 2 | 1.9 | 4 | 1.8 |
| Total | 10 | 8.3 | 10 | 9.6 | 20 | 8.9 |
Table A2.
Nonprotocol Cancer Therapy Received After Disease Progression
| Therapy | Gefitinib |
Placebo |
Total No. of Patients* | ||
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||
| All patients | 119 | 53.9 | 102 | 46.1 | 221 |
| Hormonal therapy | 0 | 0 | 1 | 1.0 | 1 |
| Chemotherapy | 59 | 49.6 | 48 | 47.1 | 107 |
| Immunotherapy | 0 | 0 | 1 | 1.0 | 1 |
| EGFR inhibitor | 16 | 13.4 | 19 | 18.6 | 35 |
| BRM | 4 | 3.4 | 2 | 2.0 | 6 |
| HDC/autologous stem-cell transplantation | 0 | 0 | 0 | 0 | 0 |
| Radiation therapy | 59 | 49.6 | 53 | 52.0 | 112 |
| Surgery | 15 | 12.6 | 18 | 17.6 | 33 |
| Other therapy | 10 | 8.4 | 15 | 14.7 | 25 |
Abbreviations: BRM, biologic response modifier; HDC, high-dose chemotherapy.
Four patient deaths were attributable to lung cancer without documented disease progression.
Footnotes
Processed as a Rapid Communication manuscript.
Supported by grants from the Canadian Cancer Society Research Institute (Grants No. 015469 and 021039), the US National Cancer Institute (Grant No. CA077202), Ontario Cancer Research Network (Grant No. 05NOV00186), and AstraZeneca.
Presented, in part, at the 47th Annual Meeting of the American Society of Clinical Oncology, June 3-7, 2011, Chicago, IL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00049543.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: James Jett, Quest Diagnostics (C), Varian Medical Systems (C); Hak Choy, EMD Serono (C), Bayer (C); David Gandara, AstraZeneca (C); Charles Butts, AstraZeneca (C), Roche (C); Joan Schiller, AstraZeneca (C), Genentech (C); Frances A. Shepherd, AstraZeneca (C) Stock Ownership: Frances A. Shepherd, AstraZeneca Honoraria: Glenwood D. Goss, AstraZeneca; Ming-Sound Tsao, Boehringer-Ingelheim, AstraZeneca; Charles Butts, AstraZeneca, Roche; Thomas A. Hensing, Genentech; Frances A. Shepherd, AstraZeneca Research Funding: Glenwood D. Goss, AstraZeneca; Ian Lorimer, AstraZeneca; Ming-Sound Tsao, F. Hoffmann-La Roche; James Jett, Oncimmune, Metabolomix; Hak Choy, Celgene; Thomas A. Hensing, Cerulean; Joan Schiller, AstraZeneca, Genentech Expert Testimony: None Patents: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Glenwood D. Goss, Chris O'Callaghan, Ian Lorimer, Ming-Sound Tsao, Hak Choy, Fadlo Khuri, Kemp Kernstine, Charles Butts, Keyue Ding, Frances A. Shepherd
Financial support: Ian Lorimer
Provision of study materials or patients: Gregory A. Masters, James Jett, Martin J. Edelman, Charles Butts, Frances A. Shepherd
Collection and assembly of data: Glenwood D. Goss, Chris O'Callaghan, Ian Lorimer, Ming-Sound Tsao, James Jett, Rogerio Lilenbaum, Fadlo Khuri, Jonathan Noble, Thomas A. Hensing, Kendrith Rowland, Keyue Ding, Frances A. Shepherd
Data analysis and interpretation: Glenwood D. Goss, Chris O'Callaghan, Ian Lorimer, Ming-Sound Tsao, Gregory A. Masters, Martin J. Edelman, Fadlo Khuri, Katherine Pisters, David Gandara, Kemp Kernstine, Charles Butts, Joan Schiller, Keyue Ding, Frances A. Shepherd
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Canadian Cancer Society's Committee on Cancer Statistics. Toronto, Ontario, Canada: Canadian Cancer Society; 2012. Canadian Cancer Statistics 2012. [Google Scholar]
- 2.American Cancer Society. Cancer Facts & Figures 2012. Atlanta, GA: American Cancer Society; 2012. American Cancer Statistics 2012: Lung Cancer. [Google Scholar]
- 3.Travis W, Linder J, Mackay B. Lung Cancer: Principles and Practice. ed 2. Philadelphia, PA: Lippincott Williams & Wilkins; 2000. Classification, histology, cytology and electron microscopy. [Google Scholar]
- 4.Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest. 1997;111:1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 5.Fujino S, Enokibori T, Tezuka N, et al. A comparison of epidermal growth factor receptor levels and other prognostic parameters in non-small cell lung cancer. Eur J Cancer. 1996;32A:2070–2074. doi: 10.1016/s0959-8049(96)00243-2. [DOI] [PubMed] [Google Scholar]
- 6.Moscatello DK, Holgado-Madruga M, Godwin AK, et al. Frequent expression of a mutant epidermal growth factor receptor in multiple human tumors. Cancer Res. 1995;55:5536–5539. [PubMed] [Google Scholar]
- 7.Rusch V, Klimstra D, Venkatraman E, et al. Overexpression of the epidermal growth factor receptor and its ligand transforming growth factor alpha is frequent in resectable non-small cell lung cancer but does not predict tumor progression. Clin Cancer Res. 1997;3:515–522. [PubMed] [Google Scholar]
- 8.Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer. 2001;37(suppl 4):S9–S15. doi: 10.1016/s0959-8049(01)00231-3. [DOI] [PubMed] [Google Scholar]
- 9.Pavelic K, Banjac Z, Pavelic J, et al. Evidence for a role of EGF receptor in the progression of human lung carcinoma. Anticancer Res. 1993;13:1133–1137. [PubMed] [Google Scholar]
- 10.Ciardiello F, Tortora G. A novel approach in the treatment of cancer: Targeting the epidermal growth factor receptor. Clin Cancer Res. 2001;7:2958–2970. [PubMed] [Google Scholar]
- 11.Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected] J Clin Oncol. 2003;21:2237–2246. doi: 10.1200/JCO.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 12.Herbst RS. Dose-comparative monotherapy trials of ZD1839 in previously treated non-small cell lung cancer patients. Semin Oncol. 2003;30:30–38. doi: 10.1053/sonc.2003.50030. [DOI] [PubMed] [Google Scholar]
- 13.American Joint Committee on Cancer. Cancer Staging Handbook. ed 6. New York, NY: Springer; 2002. [Google Scholar]
- 14.Vickers MM, Bar J, Gorn-Hondermann I, et al. Stage-dependent differential expression of microRNAs in colorectal cancer: Potential role as markers of metastatic disease. Clin Exp Metastasis. 2012;29:123–132. doi: 10.1007/s10585-011-9435-3. [DOI] [PubMed] [Google Scholar]
- 15.Smith GD, Chadwick BE, Willmore-Payne C, et al. Detection of epidermal growth factor receptor gene mutations in cytology specimens from patients with non-small cell lung cancer utilising high-resolution melting amplicon analysis. J Clin Pathol. 2008;61:487–493. doi: 10.1136/jcp.2007.051425. [DOI] [PubMed] [Google Scholar]
- 16.Pan Q, Pao W, Ladanyi M. Rapid polymerase chain reaction-based detection of epidermal growth factor receptor gene mutations in lung adenocarcinomas. J Mol Diagn. 2005;7:396–403. doi: 10.1016/S1525-1578(10)60569-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamel-Reid S, Chong G, Ionescu DN, et al. EGFR tyrosine kinase mutation testing in the treatment of non-small-cell lung cancer. Curr Oncol. 2012;19:e67–e74. doi: 10.3747/co.19.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: Results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) Lancet. 2005;366:1527–1537. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 19.Kelly K, Chansky K, Gaspar LE, et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J Clin Oncol. 2008;26:2450–2456. doi: 10.1200/JCO.2007.14.4824. [DOI] [PubMed] [Google Scholar]
- 20.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 21.Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: A multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11:521–529. doi: 10.1016/S1470-2045(10)70112-1. [DOI] [PubMed] [Google Scholar]
- 22.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 23.Sordella R, Bell DW, Haber DA, et al. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science. 2004;305:1163–1167. doi: 10.1126/science.1101637. [DOI] [PubMed] [Google Scholar]
- 24.Eberhard DA, Johnson BE, Amler LC, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 2005;23:5900–5909. doi: 10.1200/JCO.2005.02.857. [DOI] [PubMed] [Google Scholar]
- 25.Soria JC, Mok TS, Cappuzzo F, et al. EGFR-mutated oncogene-addicted non-small cell lung cancer: Current trends and future prospects. Cancer Treat Rev. 2012;38:416–430. doi: 10.1016/j.ctrv.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Shepherd FA, Tsao MS. Unraveling the mystery of prognostic and predictive factors in epidermal growth factor receptor therapy. J Clin Oncol. 2006;24:1219–1220. doi: 10.1200/JCO.2005.04.4420. [DOI] [PubMed] [Google Scholar]
- 27.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 28.Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 29.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 30.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.