Abstract
Purpose
Treatment-related stomach cancer is an important cause of morbidity and mortality among the growing number of Hodgkin lymphoma (HL) survivors, but risks associated with specific HL treatments are unclear.
Patients and Methods
We conducted an international case-control study of stomach cancer nested in a cohort of 19,882 HL survivors diagnosed from 1953 to 2003, including 89 cases and 190 matched controls. For each patient, we quantified cumulative doses of specific alkylating agents (AAs) and reconstructed radiation dose to the stomach tumor location.
Results
Stomach cancer risk increased with increasing radiation dose to the stomach (Ptrend < .001) and with increasing number of AA-containing chemotherapy cycles (Ptrend = .02). Patients who received both radiation to the stomach ≥ 25 Gy and high-dose procarbazine (≥ 5,600 mg/m2) had strikingly elevated stomach cancer risk (25 cases, two controls; odds ratio [OR], 77.5; 95% CI, 14.7 to 1452) compared with those who received radiation < 25 Gy and procarbazine < 5,600 mg/m2 (Pinteraction < .001). Risk was also elevated (OR, 2.8; 95% CI, 1.3 to 6.4) among patients who received radiation to the stomach ≥ 25 Gy but procarbazine < 5,600 mg/m2; however, no procarbazine-related risk was evident with radiation < 25 Gy. Treatment with dacarbazine also increased stomach cancer risk (12 cases, nine controls; OR, 8.8; 95% CI, 2.1 to 46.6), after adjustment for radiation and procarbazine doses.
Conclusion
Patients with HL who received subdiaphragmatic radiotherapy had dose-dependent increased risk of stomach cancer, with marked risks for patients who also received chemotherapy containing high-dose procarbazine. For current patients, risks and benefits of exposure to both procarbazine and subdiaphragmatic radiotherapy should be weighed carefully. For patients treated previously, GI symptoms should be evaluated promptly.
INTRODUCTION
Hodgkin lymphoma (HL) is one of the most common malignancies among adolescents and young adults in the United States.1 Major advances in HL treatment in recent decades have led to dramatic improvements in survival, such that the 5-year relative survival after HL is now 86%.2 However, second cancers are a leading cause of morbidity and mortality among the nearly 175,000 HL survivors in the United States today.1,3–6
Emerging evidence suggests that development of secondary GI malignancies is of major concern as HL survivors age, and HL treatments may play a critical role in the development of these malignancies.3,6–17 In an international cancer registry study, the relative risk of stomach cancer among HL survivors diagnosed at age 30 years was increased 9.5-fold compared with the general population.6 The few studies that have investigated stomach cancer risk among cancer survivors with detailed treatment information have suggested an association with subdiaphragmatic irradiation3,7,8,10,12,15 and treatment with alkylating agents (AAs), particularly procarbazine.7,15 However, those investigations did not have sufficient numbers of patients to describe dose-response relationships, investigate interactions between treatments, or explore risks associated with current therapies. We therefore conducted an international, multicenter nested case-control study among 19,882 ≥ 5-year HL survivors to quantify stomach cancer risk in relation to specific HL treatments.
PATIENTS AND METHODS
Patients were selected from a cohort of 19,882 individuals who had survived ≥ 5 years after diagnosis with first primary histologically confirmed HL (Data Supplement). The cohort included 17,447 patients from population-based cancer registries in Denmark (1943 to 1999), Finland (1953 to 2002), Norway (1953 to 2000), Sweden (1958 to 2002), Iowa (United States; 1973 to 2001), and Ontario (Canada; 1964 to 2003) and 2,435 patients from the Netherlands (1965 to 2002), described previously.7
In the population-based cohort of 17,447 patients with HL, the cumulative incidence of second primary invasive stomach cancer was 0.39% (95% CI, 0.28% to 0.50%) at 15 years and 0.92% (95% CI, 0.70% to 1.13%) at 30 years, in analyses with death and other second cancers as competing risks.18 Of the 81 cases of stomach cancer we identified, medical records were available for 72 patients (89%), with all nine excluded patients diagnosed with HL before 1975, likely because older medical records were more prone to have been lost or destroyed. Two controls per case (n = 142) were selected by stratified random sampling from the cohort, individually matched by registry, race, birth date (± 5 years), HL diagnosis date (± 5 years), and survival without subsequent cancer at least as long as the interval from HL to stomach cancer of the matched case. Patients from Norway also were matched on hospital of HL diagnosis (Radium Hospital v other). Medical records were obtained for 96% of initially eligible controls; additional controls were sought to identify two controls per case. One case was excluded because no appropriate controls were found, yielding a study population of 71 cases and 142 matched controls. Detailed data on patient demographics, HL diagnosis, and HL treatments were abstracted onto standardized forms from all available records. For cases, additional records were reviewed to confirm stomach cancer diagnosis and identify the stomach tumor location.
Individual-level data were obtained from a previous Dutch hospital-based case-control study of second primary stomach cancer among ≥ 5-year survivors of HL (18 cases, 48 matched controls),7 yielding a final analytic population of 89 cases and 190 matched controls. The study was approved by the institutional review board at each study center and exempted from review by the National Cancer Institute because analyses used existing deidentified data.
Chemotherapy Data
Abstracted chemotherapy data included dates and routes of administration, reason for treatment (primary or recurrence), and specific regimens or drugs. For AAs and topoisomerase II inhibitors, doses also were recorded. Analyses evaluated the cumulative dose (mg/m2), including all treatments administered before stomach cancer diagnosis (comparable dates for controls).
Radiation Dosimetry
Abstracted radiotherapy details included dates of administration, reason for treatment, beam energy, dose delivered, and field location and configuration. Patients generally were treated with mantle fields, with or without subdiaphragmatic fields (Fig 1), with cumulative target doses of 25 to 45 Gy using conventional fractionation.
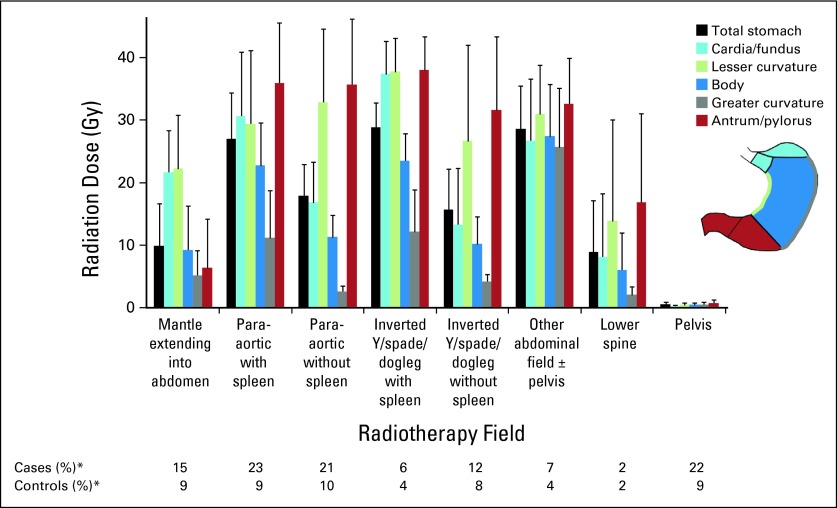
Fig 1.
Mean radiation dose to stomach from specific radiotherapy fields for Hodgkin lymphoma with at least some subdiaphragmatic exposure, by stomach site. All radiotherapy fields with exclusively supradiaphragmatic exposure gave mean dose to all stomach sites < 2 Gy, including mantle with lower border at diaphragm (51% cases, 55% controls; mean total stomach dose, 1.3 Gy), mediastinum (21% cases, 24% controls; mean, 0.8 Gy), supraclavicular (10% cases, 7% controls; mean, 0.1 Gy), axilla (17% cases, 16% controls; mean, 0.5 Gy), neck with or without head (37% cases, 27% controls; mean, 0.2 Gy), and other neck/chest (9% cases, 7% controls; mean, 0.4 Gy). (*) Percentages were calculated among patients who received radiotherapy (82 cases, 164 controls). Patients may have received multiple treatment fields.
Radiation doses to the stomach were estimated using a custom-designed dose program, based on measurements in water and anthropomorphic phantoms constructed of tissue-equivalent material.19 Using individual patients' treatment parameters, dose was calculated to 464 points in the stomach based on a typical stomach configuration (Data Supplement),20 summing all radiotherapy treatments received ≥ 5 years preceding stomach cancer diagnosis (comparable dates for controls); only three patients received radiotherapy exclusively < 5 years preceding stomach cancer. Analyses of radiotherapy risks used mean dose to the stomach tumor location (same location for matched controls), specified as cardia, fundus, body, lesser curvature, greater curvature, antrum, or pylorus (Fig 1). For 14 cases (16%) with unspecified tumor location, analyses used mean dose to the entire stomach.
The stomach size, shape, and location exhibit intra- and interindividual variation depending on stomach contents, respiration, abdominal muscle tone, and body build.21 Stomach position was unknown for individual patients in the study and likely varied over the course of radiotherapy. We therefore estimated radiation doses to two alternative stomach configurations for sensitivity analyses (Data Supplement).
Statistical Analysis
The relative risk of stomach cancer was estimated using odds ratios (ORs) derived from conditional regression analyses,22 comparing patients' exposure histories to those of matched controls. Two-sided P values and 95% CIs were based on maximum likelihood methods.
The radiation dose-response relation with stomach cancer was assessed initially in categorical logistic regression analyses, with categories based on the dose distribution in the total study population. Additionally, the excess OR per Gy (EOR/Gy) was estimated using the model OR = exp(Σjαjxj)(1 + βz), where z is radiation dose in Gy, β is EOR/Gy, and xj indicates covariates (eg, chemotherapy). Missing data on radiotherapy dose were handled by including an indicator variable in all analyses.
Chemotherapy-associated stomach cancer risks were assessed initially by estimating the OR for ever having received each specific AA and testing for trend in risk with cumulative dose on a log-linear scale. Additional analyses estimated the OR by dose category, based on the dose distribution in the total study population and taking into account typical doses administered per cycle of commonly used chemotherapy regimens.
Heterogeneity in risks among patient subgroups under a multiplicative model was evaluated by comparing model fit using separate ORs for each subgroup with that using a single estimate. Analyses were performed with SAS software (version 9.2; SAS Institute, Cary, NC) and Epicure (HiroSoft International, Seattle, WA).23
RESULTS
The median age at HL diagnosis was 30 years (range, 11 to 83 years), and 44% of patients were diagnosed in 1975 or later (Table 1). Seventy-four percent of patients had stage I or II HL. Most patients received radiotherapy (92% cases, 86% controls), with or without AA-containing chemotherapy. Cases received subsequent therapy for HL relapse more frequently than controls (47% v 27%). For cases, the median interval from HL to stomach cancer was 15 years, and median age at stomach cancer diagnosis was 50 years. Overall survival after stomach cancer diagnosis was poor (88% of cases were known to have died; median survival time among deceased, 6 months; range, 0 to 4.6 years).
Table 1.
Demographic and Clinical Characteristics of Patients With HL Who Developed Stomach Cancer and Matched Controls
| Characteristic | Cases (n = 89) |
Controls (n = 190) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Study center | ||||
| Denmark | 5 | 6 | 10 | 5 |
| Finland | 10 | 11 | 20 | 11 |
| Iowa | 6 | 7 | 12 | 6 |
| The Netherlands* | 18 | 20 | 48 | 25 |
| Norway | 11 | 12 | 22 | 12 |
| Ontario | 19 | 21 | 38 | 20 |
| Sweden | 20 | 22 | 40 | 21 |
| Sex | ||||
| Male | 55 | 62 | 114 | 60 |
| Female | 34 | 38 | 76 | 40 |
| Year of HL diagnosis | ||||
| < 1970 | 24 | 27 | 47 | 25 |
| 1970 to 1974 | 26 | 29 | 60 | 32 |
| 1975 to 1979 | 17 | 19 | 38 | 20 |
| ≥ 1980 | 22 | 25 | 45 | 24 |
| Age at HL diagnosis, years | ||||
| < 25 | 31 | 35 | 69 | 36 |
| 25 to 39 | 24 | 27 | 53 | 28 |
| 40 to 54 | 19 | 21 | 40 | 21 |
| ≥ 55 | 15 | 17 | 28 | 15 |
| HL histology | ||||
| Nodular sclerosis | 31 | 35 | 68 | 36 |
| Mixed cellularity | 19 | 21 | 47 | 25 |
| Lymphocyte predominant | 12 | 13 | 20 | 11 |
| Lymphocyte depleted | 1 | 1 | 4 | 2 |
| Other/unspecified† | 26 | 29 | 51 | 27 |
| HL stage | ||||
| I | 30 | 34 | 64 | 34 |
| II | 36 | 40 | 76 | 40 |
| III | 19 | 21 | 32 | 17 |
| IV | 4 | 4 | 18 | 9 |
| HL relapse during follow-up | ||||
| No | 47 | 53 | 138 | 73 |
| Yes | 42 | 47 | 52 | 27 |
| HL treatment summary | ||||
| Surgery only | 0 | 0 | 1 | 1 |
| AA plus RT | 49 | 55 | 77 | 41 |
| RT (no AA) | 33 | 37 | 87 | 46 |
| AA (no RT) | 7 | 8 | 25 | 13 |
| HL treatment by treatment course‡ | ||||
| Initial AA plus RT | ||||
| No subsequent AA or RT | 16 | 18 | 34 | 18 |
| Subsequent AA (no RT) | 1 | 1 | 2 | 1 |
| Subsequent RT (no AA) | 3 | 3 | 1 | 1 |
| Subsequent AA plus RT | 2 | 2 | 2 | 1 |
| Initial RT (no AA) | ||||
| No subsequent AA or RT | 27 | 30 | 81 | 43 |
| Subsequent AA (no RT) | 10 | 11 | 19 | 10 |
| Subsequent RT (no AA) | 6 | 7 | 6 | 3 |
| Subsequent AA plus RT | 12 | 13 | 14 | 7 |
| Initial AA (no RT) | ||||
| No subsequent AA or RT | 4 | 4 | 22 | 12 |
| Subsequent AA (no RT) | 3 | 3 | 3 | 2 |
| Subsequent RT (no AA) | 2 | 2 | 0 | 0 |
| Subsequent AA plus RT | 3 | 3 | 5 | 3 |
| AA regimens | ||||
| No AA | 33 | 37 | 88 | 46 |
| MOPP/MVPP without other AA | 24 | 27 | 53 | 28 |
| MOPP/MVPP with DTIC | 10 | 11 | 7 | 4 |
| MOPP/MVPP with other AA except DTIC | 5 | 6 | 10 | 5 |
| COPP/CVPP/PROC with other AA | 9 | 10 | 15 | 8 |
| PROC only | 4 | 4 | 2 | 1 |
| ABVD only | 2 | 2 | 2 | 1 |
| Other | 2 | 2 | 13 | 7 |
| Interval from HL to stomach cancer, years§ | ||||
| 5 to 9 | 14 | 16 | 28 | 15 |
| 10 to 14 | 27 | 30 | 54 | 28 |
| 15 to 19 | 24 | 27 | 55 | 29 |
| 20 to 24 | 10 | 11 | 23 | 12 |
| 25 to 29 | 9 | 10 | 20 | 11 |
| ≥ 30 | 5 | 6 | 10 | 5 |
| Age at stomach cancer diagnosis, years | ||||
| < 40 | 22 | 25 | ||
| 40 to 49 | 20 | 22 | ||
| 50 to 59 | 18 | 20 | ||
| 60 to 69 | 17 | 19 | ||
| ≥ 70 | 12 | 13 | ||
| Stomach cancer histology‖ | ||||
| Adenocarcinoma | 77 | 87 | ||
| Other | 12 | 13 | ||
| Stomach cancer stage | ||||
| I | 17 | 19 | ||
| II | 14 | 16 | ||
| III | 18 | 20 | ||
| IV | 33 | 37 | ||
| Unknown | 7 | 8 | ||
| Stomach cancer site | ||||
| Cardia/fundus | 20 | 22 | ||
| Body | 9 | 10 | ||
| Lesser curvature | 12 | 13 | ||
| Greater curvature | 3 | 3 | ||
| Antrum/pylorus | 31 | 35 | ||
| Unspecified | 14 | 16 | ||
Abbreviations: AA, alkylating agent; ABVD, doxorubicin, bleomycin, vinblastine, dacarbazine; COPP, cyclophosphamide, vincristine, procarbazine, prednisone; CVPP, cyclophosphamide, vinblastine, procarbazine, prednisone; DTIC, dacarbazine; HL, Hodgkin lymphoma; MOPP, nitrogen mustard (mechlorethamine), vincristine, procarbazine, prednisone; MVPP, nitrogen mustard (mechlorethamine), vinblastine, procarbazine, prednisone; PROC, procarbazine; RT, radiotherapy.
Patients from previous study7 were pooled with our study.
Includes Hodgkin's granuloma (12 cases, 23 controls) and unspecified histology (14 cases, 28 controls).
Initial treatment was defined from start of treatment to occurrence of > 3-month period without treatment. One control had initial surgery only. Subsequent AA plus RT may have been administered during same treatment course or sequentially.
Matched time period for controls.
Histologic confirmation in 86 (97%) of 89 cases. Other histology includes carcinoma not otherwise specified (n = 4), neuroendocrine (n = 2), sarcoma (n = 2), unclassified (n = 2), and unknown because diagnosis was based on clinical data/imaging only (n = 2).
Cases received radiotherapy with at least some subdiaphragmatic exposure more frequently than controls (69% v 37%; OR, 6.2; 95% CI, 2.1 to 21.4; Table 2; Fig 1). The highest mean doses to the stomach (≥ 25 Gy) were delivered by para-aortic and inverted Y (or spade/dogleg) fields that included the spleen as well as other abdominal fields. Doses from these fields typically varied three- to eight-fold across the stomach, with medial stomach sites (eg, antrum/pylorus) receiving higher doses than lateral sites.
Table 2.
Risk of Stomach Cancer After HL in Relation to HL Treatment
| HL Treatment | Cases (n = 89) |
Controls (n = 190) |
OR | 95% CI | Ptrenda | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | Mean | No. | % | Mean | ||||
| Radiotherapy fieldsb | |||||||||
| No radiotherapy | 7 | 8 | 26 | 14 | 1.0 | Referent | |||
| Any subdiaphragmatic fields | 61 | 69 | 71 | 37 | 6.2 | 2.1 to 21.4 | |||
| Supradiaphragmatic fields only | 20 | 22 | 90 | 47 | 1.4 | 0.5 to 4.5 | |||
| Radiation dose, Gyc,d | < .001 | ||||||||
| 0 | 9 | 10 | 0.0 | 27 | 14 | 0.0 | 1.0 | Referent | |
| 0.1 to 0.9 | 13 | 15 | 0.3 | 41 | 22 | 0.4 | 1.3 | 0.4 to 4.1 | |
| 1.0 to 4.9 | 13 | 15 | 2.4 | 50 | 26 | 1.9 | 1.0 | 0.3 to 3.5 | |
| 5.0 to 24.9e | 4 | 4 | 18.9 | 20 | 11 | 15.8 | 0.5 | 0.1 to 2.7 | |
| 25.0 to 34.9 | 12 | 13 | 30.4 | 11 | 6 | 31.6 | 4.6 | 1.2 to 20.5 | |
| 35.0 to 39.9 | 24 | 27 | 37.9 | 16 | 8 | 37.9 | 8.2 | 2.6 to 29.7 | |
| ≥ 40 | 12 | 13 | 43.3 | 16 | 8 | 42.6 | 4.2 | 1.2 to 15.6 | |
| AA chemotherapy, No. of cyclesf | .02 | ||||||||
| 0 | 33 | 37 | 0.0 | 88 | 46 | 0.0 | 1.0 | Referent | |
| 1 to 5 | 16 | 18 | 3.4 | 35 | 18 | 2.6 | 1.0 | 0.5 to 2.4 | |
| 6 | 15 | 17 | 6.0 | 29 | 15 | 6.0 | 1.7 | 0.7 to 4.4 | |
| 7 to 10 | 10 | 11 | 8.2 | 22 | 12 | 8.4 | 1.9 | 0.7 to 4.9 | |
| ≥ 11 | 15 | 17 | 19.1 | 16 | 8 | 21.1 | 3.0 | 1.2 to 7.7 | |
| Analyses of specific AAs, mg/m2g | |||||||||
| Procarbazine | 52 | 58 | 8,752.0 | 87 | 46 | 6,528.3 | 1.9 | 1.1 to 3.5 | .003 |
| Nitrogen mustard | 39 | 44 | 62.3 | 77 | 41 | 50.5 | 1.2 | 0.7 to 2.2 | .06 |
| Cyclophosphamide | 10 | 11 | 9,249.5 | 27 | 14 | 7,533.3 | 1.0 | 0.4 to 2.3 | .70 |
| Dacarbazine | 12 | 13 | 2,180.8 | 9 | 5 | 2,295.6 | 9.3 | 2.5 to 45.8 | .008 |
| Lomustine | 5 | 6 | 548.6 | 9 | 5 | 333.0 | 2.3 | 0.7 to 7.9 | .12 |
| Chlorambucil | 7 | 8 | 452.6 | 4 | 2 | 1,357.6 | 3.4 | 0.9 to 14.6 | .59 |
| Multivariate modelh | |||||||||
| Radiation dose, Gyc | < .001 | ||||||||
| < 25 | 39 | 44 | 2.8 | 138 | 73 | 3.1 | 1.0 | Referent | |
| ≥ 25 | 48 | 54 | 37.4 | 43 | 23 | 38.0 | 5.8 | 3.0 to 12.3 | |
| Procarbazine dose, mg/m2i | .009 | ||||||||
| 0 | 37 | 42 | 0.0 | 103 | 54 | 0.0 | 1.0 | Referent | |
| 1 to 5,599 | 12 | 13 | 3,011.8 | 39 | 21 | 3,403.0 | 0.8 | 0.3 to 1.9 | |
| 5,600 to 8,399 | 22 | 25 | 6,775.3 | 29 | 15 | 6,938.9 | 2.9 | 1.2 to 7.0 | |
| ≥ 8,400 | 18 | 20 | 14,994.7 | 19 | 10 | 12,316.5 | 2.3 | 1.0 to 5.5 | |
| Dacarbazine dose, mg/m2 | .04 | ||||||||
| 0 | 77 | 87 | 0.0 | 181 | 95 | 0.0 | 1.0 | Referent | |
| > 0 | 12 | 13 | 2,180.8 | 9 | 5 | 2,295.6 | 8.8 | 2.1 to 46.6 | |
Abbreviations: AA, alkylating agent; ABV, doxorubicin, bleomycin, vinblastine; BEACOPP, bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone; HL, Hodgkin lymphoma; MOPP, nitrogen mustard (mechlorethamine), vincristine, procarbazine, prednisone; OR, odds ratio.
Ptrend in dose was calculated using continuous variables on linear scale for irradiation or log-linear scale for AA chemotherapy.
Details on radiotherapy fields are provided in Figure 1. One case (1%) and three controls (2%) received radiotherapy but did not have detailed information on field type. ORs and 95% CIs were adjusted for No. of cycles of AA-containing regimens.
Radiation dose was estimated to specific site of stomach tumor (matched location for controls).
ORs and 95% CIs were adjusted for No. of cycles of AA-containing chemotherapy. Patients with unknown radiation dose (two cases, nine controls with insufficient details on radiotherapy) were modeled separately with indicator variable and excluded from percentages.
Includes patients with 5.0 to 9.9 (zero cases, five controls), 10.0 to 14.9 (one case, four controls), 15.0 to 19.9 (one case, five controls), and 20.0 to 24.9 Gy (two cases, six controls).
Includes both cyclic and continuous chemotherapy, with 1 month of continuous therapy counted as one cycle. OR (95% CI) was adjusted for radiation dose (unknown; < 25, ≥ 25 Gy).
Each AA was modeled separately with adjustment for radiation dose (unknown; < 25, ≥ 25 Gy). ORs and 95% CIs compare patients who received that AA with referent group of patients who did not. Ptrend uses cumulative dose (mg/m2).
Multivariate model included radiation, procarbazine, and dacarbazine and was also adjusted for unknown radiation dose (two cases, nine controls).
Assuming procarbazine dose of 1,400 mg/m2 per cycle (14 days × 100 mg/m2 per day), categories correspond to zero, one to three, four to five, and ≥ six cycles of MOPP or MOPP-like regimens. Other protocols (eg, MOPP-ABV, BEACOPP) include procarbazine dose of 700 mg/m2 per cycle.
Risk of stomach cancer increased with increasing radiation dose to the stomach tumor location (Ptrend < .001; EOR/Gy, 0.09; 95% CI, 0.04 to 0.21) and with increasing number of AA-containing chemotherapy cycles (Ptrend = .02; Table 2). Compared with patients who did not receive radiotherapy, significantly increased risks were observed for patients who received radiation to the stomach ≥ 25 Gy. Analyses of individual AAs demonstrated statistically significant associations of stomach cancer after procarbazine (Ptrend = .003) or dacarbazine (Ptrend = .008) but not other AAs. Results were similar in a multivariate model including all AAs, although the borderline-significant association of nitrogen mustard dose disappeared after taking into account receipt of procarbazine (data not shown). A multivariate model including only irradiation, procarbazine, and dacarbazine revealed significantly elevated ORs of 5.8 for radiation ≥ 25 Gy; 2.9 and 2.3 for procarbazine 5,600 to 8,399 and ≥ 8,400 mg/m2, respectively; and 8.8 for any dacarbazine.
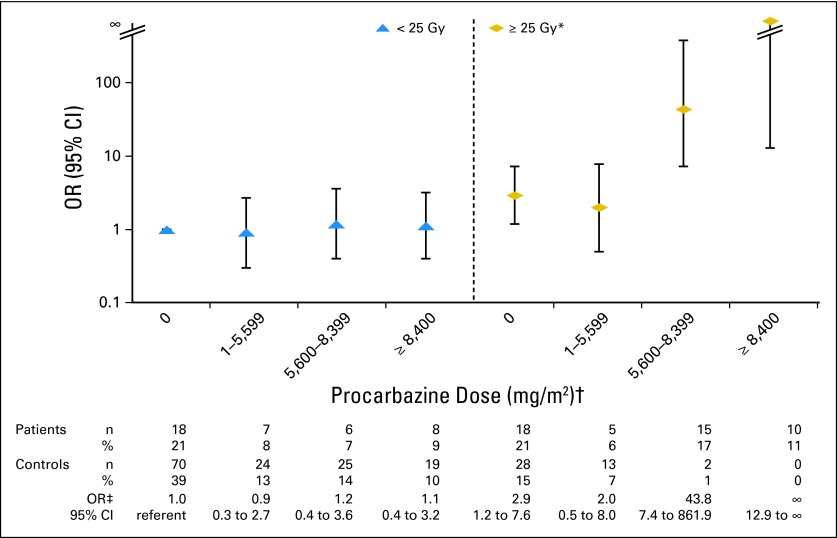
The association between procarbazine and stomach cancer risk was strikingly dependent on the radiation dose to the stomach (Table 3; Fig 2). Patients who received both radiation to the stomach ≥ 25 Gy and procarbazine ≥ 5,600 mg/m2 had 77.5-fold increased risk (95% CI, 14.7 to 1452), based on 25 cases and two controls. No risk was evident in any procarbazine dose category for patients who received radiation to the stomach < 25 Gy. The interaction between procarbazine and radiation to the stomach was similar by age at HL diagnosis, age at stomach cancer diagnosis, and interval from HL to stomach cancer. Additionally, no clear risk pattern emerged regarding the timing of the treatments; risks were elevated among patients who received both exposures ≤ 3 months apart (eight cases, one control), those who received radiation before procarbazine (14 cases, zero controls; median time between exposures, 2.9 years), and those who received procarbazine before radiation (three cases, one control; median time between exposures, 1.6 years). Notably, the radiation-related risk of stomach cancer was increased 2.8-fold (95% CI, 1.3 to 6.4) among patients who received radiation to the stomach ≥ 25 Gy but procarbazine < 5,600 mg/m2 (23 cases, 41 controls). This radiation-related risk was most evident among patients diagnosed with stomach cancer before age 50 years, compared with those age ≥ 50 years (OR, 8.6 v 1.3; Phomogeneity = .05). We estimated the proportion of stomach cancer cases attributable to HL treatment to be 99% (95% CI, 90% to 100%) among patients receiving radiation to the stomach ≥ 25 Gy and procarbazine ≥ 5,600 mg/m2 and 64% (95% CI, 20% to 84%) among patients receiving radiation to the stomach ≥ 25 Gy but procarbazine < 5,600 mg/m2.
Table 3.
Risk of Stomach Cancer After HL in Relation to Procarbazine and Radiation Dose to Stomach
| Characteristic |
Cases (n = 89) |
Controls (n = 190) |
OR | 95% CI‡ | Pinteraction§ | |||
|---|---|---|---|---|---|---|---|---|
| Radiation Dose (Gy)* | Procarbazine dose (mg/m2)† | No. | %‡ | No. | %‡ | |||
| All patients | < .001 | |||||||
| < 25 | < 5,600 | 25 | 29 | 94 | 52 | 1.0 | Referent | |
| ≥ 25 | < 5,600 | 23 | 26 | 41 | 23 | 2.8 | 1.3 to 6.4 | |
| < 25 | ≥ 5,600 | 14 | 16 | 44 | 24 | 1.2 | 0.5 to 2.7 | |
| ≥ 25 | ≥ 5,600 | 25 | 29 | 2 | 1 | 77.5 | 14.7 to 1,452 | |
| Age at HL diagnosis < 30 years | .06 | |||||||
| < 25 | < 5,600 | 6 | 15 | 44 | 49 | 1.0 | Referent | |
| ≥ 25 | < 5,600 | 13 | 33 | 27 | 30 | 7.1 | 1.9 to 36.8‖ | |
| < 25 | ≥ 5,600 | 6 | 15 | 17 | 19 | 1.9 | 0.5 to 7.3 | |
| ≥ 25 | ≥ 5,600 | 15 | 36 | 2 | 2 | 124 | 15.8 to 3,169 | |
| Age at HL diagnosis ≥ 30 years | .002 | |||||||
| < 25 | < 5,600 | 19 | 40 | 50 | 55 | 1.0 | Referent | |
| ≥ 25 | < 5,600 | 10 | 21 | 14 | 15 | 1.5 | 0.5 to 4.2‖ | |
| < 25 | ≥ 5,600 | 8 | 17 | 27 | 30 | 0.8 | 0.3 to 2.4 | |
| ≥ 25 | ≥ 5,600 | 10 | 21 | 0 | 0 | ∞ | 8.8 to ∞ | |
| Age at stomach cancer diagnosis < 50 years | .13 | |||||||
| < 25 | < 5,600 | 5 | 13 | 44 | 50 | 1.0 | Referent | |
| ≥ 25 | < 5,600 | 13 | 33 | 26 | 30 | 8.6 | 2.3 to 43.9‖ | |
| < 25 | ≥ 5,600 | 7 | 18 | 16 | 18 | 2.9 | 0.8 to 11.6 | |
| ≥ 25 | ≥ 5,600 | 15 | 38 | 2 | 2 | 151 | 17.6 to 4,264 | |
| Age at stomach cancer diagnosis ≥ 50 years | < .001 | |||||||
| < 25 | < 5,600 | 20 | 43 | 50 | 54 | 1.0 | Referent | |
| ≥ 25 | < 5,600 | 10 | 21 | 15 | 16 | 1.3 | 0.4 to 3.6‖ | |
| < 25 | ≥ 5,600 | 7 | 15 | 28 | 30 | 0.6 | 0.2 to 1.7 | |
| ≥ 25 | ≥ 5,600 | 10 | 21 | 0 | 0 | ∞ | 8.9 to ∞ | |
| Interval from HL to stomach cancer < 15 years | .003 | |||||||
| < 25 | < 5,600 | 15 | 37 | 42 | 55 | 1.0 | Referent | |
| ≥ 25 | < 5,600 | 6 | 15 | 13 | 17 | 1.5 | 0.4 to 5.6‖ | |
| < 25 | ≥ 5,600 | 9 | 22 | 22 | 29 | 1.2 | 0.4 to 3.9 | |
| ≥ 25 | ≥ 5,600 | 11 | 27 | 0 | 0 | ∞ | 10.0 to ∞ | |
| Interval from HL to stomach cancer ≥ 15 years | .02 | |||||||
| < 25 | < 5,600 | 10 | 22 | 52 | 50 | 1.0 | Referent | |
| ≥ 25 | < 5,600 | 17 | 37 | 28 | 27 | 4.2 | 1.4 to 14.1‖ | |
| < 25 | ≥ 5,600 | 5 | 11 | 22 | 21 | 1.2 | 0.3 to 4.2 | |
| ≥ 25 | ≥ 5,600 | 14 | 30 | 2 | 2 | 67.0 | 10.5 to 1,407 | |
Abbreviations: ABV, doxorubicin, bleomycin, vinblastine; BEACOPP, bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone; HL, Hodgkin lymphoma; OR, odds ratio.
Radiation dose was estimated to the specific site of the stomach tumor (matched location for controls).
Assuming a procarbazine dose of 1,400 mg/m2 per cycle (14 days × 100 mg/m2 per day), ≥ 5,600 mg/m2 corresponds to ≥ four cycles of MOPP or MOPP-like regimens. Other protocols (eg, MOPP-ABV, BEACOPP) include a procarbazine dose of 700 mg/m2 per cycle.
ORs and 95% CIs were adjusted for receipt of any dacarbazine and unknown radiation dose. Patients with unknown radiation dose were excluded from percentages.
Pinteraction between irradiation and procarbazine was calculated using a likelihood ratio test under the multiplicative model.
Stomach cancer risk among patients who received radiation to stomach tumor site ≥ 25 Gy but procarbazine < 5,600 mg/m2 was compared between patient subgroups using a likelihood ratio test. Age at HL diagnosis: OR < 30, 7.1 v OR ≥ 30, 1.5; Phomogeneity = .11. Age at stomach cancer diagnosis: OR < 50, 8.6 v OR ≥ 50, 1.3; Phomogeneity = .05. Interval from HL to stomach cancer: OR < 15, 4.2 v OR ≥ 15, 1.5; Phomogeneity = .30.
Fig 2.
Risk of stomach cancer after Hodgkin lymphoma in relation to radiation dose to stomach and procarbazine dose. OR, odds ratio. (*) Radiation dose was estimated to stomach tumor location (matched location for controls). (†) Assuming procarbazine dose of 1,400 mg/m2 per cycle (14 days × 100 mg/m2 per day), categories correspond to zero, one to three, four to five, and ≥ six cycles of MOPP (mechlorethamine, vincristine, procarbazine, and prednisone) or MOPP-like regimens. Other protocols (eg, MOPP-ABV [MOPP–doxorubicin, bleomycin, and vinblastine], BEACOPP [bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone]) include procarbazine dose of 700 mg/m2 per cycle. (‡) ORs and 95% CIs were adjusted for receipt of any dacarbazine and unknown radiation dose.
Although the number of patients who received dacarbazine was too small for reliable dose-response analysis (12 cases, nine controls), the association between dacarbazine and stomach cancer did not seem to be accounted for or modified by exposure to radiation or procarbazine. Receipt of dacarbazine was associated with 5.4-fold (95% CI, 1.1 to 30.2; seven cases, eight controls) increased risk among patients who received radiation to the stomach < 25 Gy. In addition, although 17 (81%) of the patients who received dacarbazine also were treated with MOPP (mechlorethamine, vincristine, procarbazine, and prednisone), receipt of dacarbazine was associated with 9.5-fold (95% CI, 1.7 to 68.7; six cases, six controls) increased risk among those who received procarbazine < 5,600 mg/m2 (Data Supplement). The dacarbazine-related risk also did not vary significantly by age at HL or stomach cancer diagnosis or interval from HL to stomach cancer.
Our findings were similar in sensitivity analyses restricted to histologically confirmed stomach adenocarcinoma (n = 76) and specified tumor location (n = 75) and systematically excluding each study center one at a time (data not shown). Our findings were also similar when we analyzed radiation dose to two alternative stomach configurations (Data Supplement).
DISCUSSION
In this study with detailed treatment data and long-term patient follow-up, we demonstrated increased risk of stomach cancer with increasing radiation dose to the stomach and increasing number of cycles of AAs. A surprising finding was a striking increase in stomach cancer risk among patients with HL who received radiation to the stomach ≥ 25 Gy and procarbazine ≥ 5,600 mg/m2, irrespective of the time elapsed between exposures. We also observed elevated risk among patients who received radiation to the stomach ≥ 25 Gy in the absence of procarbazine and among patients who received dacarbazine-containing chemotherapy. Half of the cases in our study were diagnosed with stomach cancer at age ≤ 50 years—20 years younger than the median age of stomach cancer diagnosis in the general US population.1 Our findings should therefore raise clinician and patient awareness of the risk of treatment-related stomach cancer in HL survivors, even many years after the completion of therapy.
To our knowledge, our study provides the first robust evidence of a supramultiplicative interaction between chemotherapy and irradiation on risk of subsequent solid cancer. In the previous analysis of the Dutch data, including both HL and testicular cancer survivors (42 cases, 19 of which occurred after HL), stomach cancer risk increased with increasing dose of radiation to the stomach and increasing cumulative dose of procarbazine.7 Additionally, in a recent report from the Childhood Cancer Survivor Study, occurrence of GI malignancies overall (45 cases total, six with stomach cancer) was associated with abdominal irradiation, increasing dose of procarbazine, and receipt of platinum-based chemotherapy.15 However, neither of the previous studies were limited to HL survivors, nor did they have sufficient numbers of exposed patients to evaluate the extent to which a dose-dependent synergistic effect between chemotherapy and irradiation on stomach cancer risk after HL might exist.
The precise biologic mechanism by which ionizing radiation and oral procarbazine could interact to induce stomach cancer is unclear. Procarbazine is a member of the hydrazine/triazene class of AAs that causes DNA methylation after metabolization to methyl diazonium.24 Mice exposed to oral procarbazine experience genotoxicity to the stomach mucosa.25 In vitro studies have demonstrated a number of plausible mechanisms for synergistic effects of chemotherapy and irradiation in disrupting normal cell-cycle progression and DNA repair mechanisms,26,27 but no comparable data exist in humans. Although our data clearly support a synergistic effect of procarbazine and irradiation, with only two controls receiving radiation to the stomach ≥ 25 Gy and procarbazine ≥ 5,600 mg/m2, the exact magnitude of the risk is highly uncertain. Previous reports have demonstrated that chemotherapy and irradiation have an additive effect on lung cancer risk28 or multiplicative effect on sarcoma risk.29 Further research is warranted to understand potential synergistic effects of treatments in long-term toxicities.
Although HL treatment has changed over time, procarbazine remains an important chemotherapeutic agent in clinical practice. Among the patients in our study who received procarbazine, 93% were treated with MOPP or MOPP-like cyclic chemotherapy regimens, the mainstay of HL treatment during the 1970s and 1980s.30 The remaining 7% of patients received continuous procarbazine (as single agent or combined with other agents such as vinblastine). In the 1980s, recognition of the high risks of leukemia and infertility with MOPP led to a decline in its use as first-line therapy.31 Nevertheless, procarbazine remains an important component of combined chemotherapy with the increasing use of BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone),32 especially in Europe, and continued use of MOPP or MOPP-like chemotherapy for relapsed or progressive HL. Although our data support increasing risk of stomach cancer with increasing dose of procarbazine, our sample size was insufficient (five cases, 13 controls) to distinguish risks at varying doses of procarbazine < 5,600 mg/m2 in patients who also received radiation to the stomach ≥ 25 Gy.
Our study also found elevated stomach cancer risk among patients who received dacarbazine, a component of ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) and the most commonly used first-line therapy for HL in the United States today.33,34 The dacarbazine-related stomach cancer risk was evident among patients not treated with subdiaphragmatic radiotherapy or procarbazine ≥ 5,600 mg/m2. However, these findings should be interpreted cautiously because 17 (81%) of the patients who received dacarbazine were also treated with MOPP, and our results could remain confounded by exposure to these other carcinogens. Too few patients received ABVD alone (two cases, two controls) to evaluate stomach cancer risk with this chemotherapy regimen. Dacarbazine is biologically similar to procarbazine and is also broadly carcinogenic in mice and rats.24,35 However, unlike procarbazine, dacarbazine is administered intravenously, and no data exist on the effect of dacarbazine on the stomach in either humans or animals. Because of the prevalence of dacarbazine use in current HL treatment, further investigation of late effects is warranted.
Increased risk of stomach cancer was also observed among patients who received subdiaphragmatic radiation exposure with procarbazine < 5,600 mg/m2, particularly those treated at a younger age. Our findings are consistent with earlier reports of increased stomach cancer risk after radiotherapy for peptic ulcer disease36 or cervical cancer37 or ionizing radiation exposure from the atomic bombs in Japan.38,39 Because current treatment approaches use reduced radiation volumes and doses and improved irradiation techniques,33,34 the percentage of patients with a history of subdiaphragmatic radiotherapy doses ≥ 25 Gy should decrease. However, the absence of significantly increased risk at radiation doses < 25 Gy in this study should be interpreted cautiously because few patients in our study received radiation to the stomach 5 to 24.9 Gy, precluding precise risk estimation in this dose range. Additionally, uncertainties in the radiation dose to the stomach remain because individual patients' stomach position during radiotherapy was unknown. Finally, a linear dose-response relation with stomach cancer has been reported in other settings,36–39 which supports elevated risk even at low radiation doses.
The primary strengths of this study include investigation of a substantial number of patients exposed to procarbazine (139 v ≤ 30 in previous studies),7,15 abstraction of detailed radiation and chemotherapy dose data from medical records, and individual reconstruction of radiation doses to the stomach tumor location. These detailed treatment data enabled thorough investigation of risks in patients with and without radiotherapy as well as the separation of the effects of specific chemotherapeutic agents. Moreover, because radiation dose varied three- to eight-fold across the stomach, we analyzed dose to the specific location of the stomach tumor to more accurately estimate radiation-related risk.
HL treatment approaches have evolved considerably over the last several decades and have been at the forefront of efforts to balance treatment efficacy with toxicity.40 Our study adds strong evidence to the growing concern that GI malignancies represent an important adverse late effect of treatment for patients with HL and other patients receiving comparable treatments. For current patients, risks and benefits of exposure to both procarbazine and subdiaphragmatic radiotherapy should be weighed carefully. For patients who were treated previously with subdiaphragmatic radiotherapy, symptoms referable to the GI tract should be evaluated promptly. Consideration of expanded screening of HL survivors for second cancers in the highest-risk patients may be warranted,41 along with expanded research of carcinogenic risks with other drugs that are commonly used today, including dacarbazine and platinum agents.15
Supplementary Material
Acknowledgment
We thank Diane Fuchs, Janet Lawler-Heavner, and their staff at Westat (Rockville, MD) for administrative assistance in conducting the field studies and Jeremy Miller (Information Management Services, Silver Spring, MD) for computer programming support.
Footnotes
Supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, and National Cancer Institute Contract No. N01-CP-31157 to Cancer Care Ontario, Toronto, Canada; Danish Cancer Society, Copenhagen, Denmark (Contract No. N01-CP-31019); Finnish Cancer Registry, Helsinki, Finland (Contract No. N01-CP-31154); Information Management Services, Silver Spring, MD (Contract No. N01-CP-31003); Karolinska Institute, Stockholm, Sweden (Contract No. N01-CP-31156); University of Iowa, Iowa City, IA (Contract No. N01-CP-31155); University of Texas MD Anderson Cancer Center, Houston, TX (Contract No. N02-CP-55503); and Westat, Rockville, MD (Contract No. N02-CP-31136). The Dutch study also was supported by the Lance Armstrong Foundation and Contract No. NKI 04-3068 from the Dutch Cancer Society.
Presented in part at the 52nd Annual Meeting of the American Society of Hematology, Orlando, FL, December 4-7, 2010, and Prevention Control Population Research Seminar at Memorial Sloan-Kettering Cancer Center, New York, NY, May 15, 2012.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Lindsay M. Morton, Graça M. Dores, Charles F. Lynch, Marilyn Stovall, Per Hall, Hans H. Storm, Tom Børge Johannesen, Rita E. Weathers, Michael Andersson, Sophie D. Fossa, Michael Hauptmann, Eric J. Holowaty, Heikki Joensuu, Ruth A. Kleinerman, Frøydis Langmark, Eero Pukkala, Leila Vaalavirta, Alexandra W. Van den Belt-Dusebout, Lois B. Travis, Berthe M. Aleman, Flora E. van Leeuwen
Collection and assembly of data: Lindsay M. Morton, Graça M. Dores, Rochelle E. Curtis, Charles F. Lynch, Marilyn Stovall, Per Hall, Hans H. Storm, Tom Børge Johannesen, Susan A. Smith, Rita E. Weathers, Michael Andersson, Sophie D. Fossa, Eric J. Holowaty, Heikki Joensuu, Magnus Kaijser, Ruth A. Kleinerman, Frøydis Langmark, Eero Pukkala, Leila Vaalavirta, Alexandra W. Van den Belt-Dusebout, Lois B. Travis, Berthe M. Aleman, Flora E. van Leeuwen
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2009. http://seer.cancer.gov/csr/1975_2009_pops09/
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Reulen RC, Frobisher C, Winter DL, et al. Long-term risks of subsequent primary neoplasms among survivors of childhood cancer. JAMA. 2011;305:2311–2319. doi: 10.1001/jama.2011.747. [DOI] [PubMed] [Google Scholar]
- 4.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100:1368–1379. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman DL, Whitton J, Leisenring W, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J Natl Cancer Inst. 2010;102:1083–1095. doi: 10.1093/jnci/djq238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodgson DC, Gilbert ES, Dores GM, et al. Long-term solid cancer risk among 5-year survivors of Hodgkin's lymphoma. J Clin Oncol. 2007;25:1489–1497. doi: 10.1200/JCO.2006.09.0936. [DOI] [PubMed] [Google Scholar]
- 7.van den Belt-Dusebout AW, Aleman BMP, Besseling G, et al. Roles of radiation dose and chemotherapy in the etiology of stomach cancer as a second malignancy. Int J Radiat Oncol Biol Phys. 2009;75:1420–1429. doi: 10.1016/j.ijrobp.2009.01.073. [DOI] [PubMed] [Google Scholar]
- 8.Birdwell SH, Hancock SL, Varghese A, et al. Gastrointestinal cancer after treatment of Hodgkin's disease. Int J Radiat Oncol Biol Phys. 1997;37:67–73. doi: 10.1016/s0360-3016(96)00489-0. [DOI] [PubMed] [Google Scholar]
- 9.Metayer C, Lynch CF, Clarke EA, et al. Second cancers among long-term survivors of Hodgkin's disease diagnosed in childhood and adolescence. J Clin Oncol. 2000;18:2435–2443. doi: 10.1200/JCO.2000.18.12.2435. [DOI] [PubMed] [Google Scholar]
- 10.Swerdlow AJ, Barber JA, Hudson GV, et al. Risk of second malignancy after Hodgkin's disease in a collaborative British cohort: The relation to age at treatment. J Clin Oncol. 2000;18:498–509. doi: 10.1200/JCO.2000.18.3.498. [DOI] [PubMed] [Google Scholar]
- 11.van Leeuwen FE, Klokman WJ, Veer MB, et al. Long-term risk of second malignancy in survivors of Hodgkin's disease treated during adolescence or young adulthood. J Clin Oncol. 2000;18:487–497. doi: 10.1200/JCO.2000.18.3.487. [DOI] [PubMed] [Google Scholar]
- 12.Dores GM, Metayer C, Curtis RE, et al. Second malignant neoplasms among long-term survivors of Hodgkin's disease: A population-based evaluation over 25 years. J Clin Oncol. 2002;20:3484–3494. doi: 10.1200/JCO.2002.09.038. [DOI] [PubMed] [Google Scholar]
- 13.Foss Abrahamsen A, Andersen A, Nome O, et al. Long-term risk of second malignancy after treatment of Hodgkin's disease: The influence of treatment, age and follow-up time. Ann Oncol. 2002;13:1786–1791. doi: 10.1093/annonc/mdf289. [DOI] [PubMed] [Google Scholar]
- 14.Bhatia S, Yasui Y, Robison LL, et al. High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin's disease: Report from the Late Effects Study Group. J Clin Oncol. 2003;21:4386–4394. doi: 10.1200/JCO.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 15.Henderson TO, Oeffinger KC, Whitton J, et al. Secondary gastrointestinal cancer in childhood cancer survivors: A cohort study. Ann Intern Med. 2012;156:757–766. W-260. doi: 10.1059/0003-4819-156-11-201206050-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nottage K, McFarlane J, Krasin MJ, et al. Secondary colorectal carcinoma after childhood cancer. J Clin Oncol. 2012;30:2552–2558. doi: 10.1200/JCO.2011.37.8760. [DOI] [PubMed] [Google Scholar]
- 17.Youn P, Li H, Milano MT, et al. Long-term survival among Hodgkin's lymphoma patients with gastrointestinal cancer: A population-based study. Ann Oncol. 2013;24:202–208. doi: 10.1093/annonc/mds218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gooley TA, Leisenring W, Crowley J, et al. Estimation of failure probabilities in the presence of competing risks: New representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 19.Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: Use in epidemiological studies. Radiat Res. 2006;166:141–157. doi: 10.1667/RR3525.1. [DOI] [PubMed] [Google Scholar]
- 20.Leibel SA, Phillips TL. Textbook of Radiation Oncology. ed 2. Philadelphia, PA: Saunders; 2004. [Google Scholar]
- 21.Dowd SB, Wilson BG. Encyclopedia of Radiographic Positioning. Volume 2. Philadelphia, PA: Saunders; 1995. [Google Scholar]
- 22.Breslow NE, Day NE. Lyon, France: International Agency for Research on Cancer; 1980. Statistical methods, in Cancer Research, Volume 1: The Analysis of Case-Control Studies. [PubMed] [Google Scholar]
- 23.Preston DL, Lubin JH, Pierce DA, et al. Epicure: User's Guide. Seattle, WA: HiroSoft International Corporation; 1993. [Google Scholar]
- 24.Hong WK, Bast RC, Jr, Hait WN, et al. Holland-Frei Cancer Medicine. ed 8. Shelton, CT: People's Medical Publishing House; 2010. [Google Scholar]
- 25.Sasaki YF, Saga A, Akasaka M, et al. Organ-specific genotoxicity of the potent rodent colon carcinogen 1,2-dimethylhydrazine and three hydrazine derivatives: Difference between intraperitoneal and oral administration. Mutat Res. 1998;415:1–12. doi: 10.1016/s1383-5718(98)00002-3. [DOI] [PubMed] [Google Scholar]
- 26.Wilson GD, Bentzen SM, Harari PM. Biologic basis for combining drugs with radiation. Semin Radiat Oncol. 2006;16:2–9. doi: 10.1016/j.semradonc.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Lawrence TS, Blackstock AW, McGinn C. The mechanism of action of radiosensitization of conventional chemotherapeutic agents. Semin Radiat Oncol. 2003;13:13–21. doi: 10.1053/srao.2003.50002. [DOI] [PubMed] [Google Scholar]
- 28.Travis LB, Gospodarowicz M, Curtis RE, et al. Lung cancer following chemotherapy and radiotherapy for Hodgkin's disease. J Natl Cancer Inst. 2002;94:182–192. doi: 10.1093/jnci/94.3.182. [DOI] [PubMed] [Google Scholar]
- 29.Tucker MA, D'Angio GJ, Boice JD, Jr, et al. Bone sarcomas linked to radiotherapy and chemotherapy in children. N Engl J Med. 1987;317:588–593. doi: 10.1056/NEJM198709033171002. [DOI] [PubMed] [Google Scholar]
- 30.Longo DL, Young RC, Wesley M, et al. Twenty years of MOPP therapy for Hodgkin's disease. J Clin Oncol. 1986;4:1295–1306. doi: 10.1200/JCO.1986.4.9.1295. [DOI] [PubMed] [Google Scholar]
- 31.Kaldor JM, Day NE, Clarke EA, et al. Leukemia following Hodgkin's disease. N Engl J Med. 1990;322:7–13. doi: 10.1056/NEJM199001043220102. [DOI] [PubMed] [Google Scholar]
- 32.Engert A, Diehl V, Franklin J, et al. Escalated-dose BEACOPP in the treatment of patients with advanced-stage Hodgkin's lymphoma: 10 years of follow-up of the GHSG HD9 study. J Clin Oncol. 2009;27:4548–4554. doi: 10.1200/JCO.2008.19.8820. [DOI] [PubMed] [Google Scholar]
- 33.Engert A, Plütschow A, Eich HT, et al. Reduced treatment intensity in patients with early-stage Hodgkin's lymphoma. N Engl J Med. 2010;363:640–652. doi: 10.1056/NEJMoa1000067. [DOI] [PubMed] [Google Scholar]
- 34.Townsend W, Linch D. Hodgkin's lymphoma in adults. Lancet. 2012;380:836–847. doi: 10.1016/S0140-6736(12)60035-X. [DOI] [PubMed] [Google Scholar]
- 35.Kaldor JM, Day NE, Hemminki K. Quantifying the carcinogenicity of antineoplastic drugs. Eur J Cancer Clin Oncol. 1988;24:703–711. doi: 10.1016/0277-5379(88)90302-1. [DOI] [PubMed] [Google Scholar]
- 36.Carr ZA, Kleinerman RA, Stovall M, et al. Malignant neoplasms after radiation therapy for peptic ulcer. Radiat Res. 2002;157:668–677. doi: 10.1667/0033-7587(2002)157[0668:mnartf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 37.Boice JD, Jr, Engholm G, Kleinerman RA, et al. Radiation dose and second cancer risk in patients treated for cancer of the cervix. Radiat Res. 1988;116:3–55. [PubMed] [Google Scholar]
- 38.Preston DL, Ron E, Tokuoka S, et al. Solid cancer incidence in atomic bomb survivors: 1958-1998. Radiat Res. 2007;168:1–64. doi: 10.1667/RR0763.1. [DOI] [PubMed] [Google Scholar]
- 39.United Nations Scientific Committee on the Effects of Atomic Radiation. Vienna, Austria: United Nations; 2008. Effects of ionizing radiation, in UNSCEAR 2006 Report, Volume 1: Report to the General Assembly, With Scientific Annexes A and B. [Google Scholar]
- 40.Hodgson DC. Late effects in the era of modern therapy for Hodgkin lymphoma. Hematology Am Soc Hematol Educ Program. 2011;2011:323–329. doi: 10.1182/asheducation-2011.1.323. [DOI] [PubMed] [Google Scholar]
- 41.Ng AK, LaCasce A, Travis LB. Long-term complications of lymphoma and its treatment. J Clin Oncol. 2011;29:1885–1892. doi: 10.1200/JCO.2010.32.8427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.