Abstract
A new trichostome ciliate, Balantidium honghuensis n. sp., was isolated from the recta of Rana nigromaculata and R. limnocharis during parasite surveys in Honghu Lake, Hubei Province, central China in summer of 2010 and 2011. Its detailed morphometric characters based on LM and SEM studies were described herein. The organism is oval in shape and thickly ciliated. The vestibulum is "V" shaped and occupies about 1/3 to 2/5 of the body length. The vestibular and nearby regions possess strong peripheral fibers which form a marked axial fiber about the cytopharynx. More than 10 contractile vacuoles are distributed along the periphery of the latter body. Comparisions were made between this new species and B. sinensis Nie, 1935. They were discriminated from each other in terms of general body forms, body size, and vestibulum shapes. Besides, special attention was paid to its high-speed daughter swarmers which we believed to be the infective stage of B. honghuensis. Possible infection routes of anura amphibian balantidia were discussed.
Keywords: Balantidium honghuensis, trichostome ciliate, frog, Rana nigromaculata, Rana limnocharis, China
INTRODUCTION
The genus Balantidium Claparède & Lachmann, 1858 has a large number of species that have been reported in marine, freshwater, and terrestrial habitats as endocommensals in the digestive tracts of widely diverse hosts from both invertebrate (coelenterates, platyhelminthes, annelids, molluscs, and arthropods) and vertebrate animals (fish, amphibians, reptiles, and mammals). In the past 7 years, our research group mainly focused on the taxonomy and phylogenetic analysis of banlantidia that inhabiting freshwater fish [1-5] and amphibians [6]. To our knowledge, 19 fish balantidia including 13 freshwater species and 6 marine species and 30 amphibian balantidia containing 26 species from anura amphibians and other 5 species from urodele amphibians (B. elongatum was found in both Rana esculenta and Triton taeniatus) have been discovered to date whose summaries were presented in Li et al. [1] and Li et al. [6], respectively.
Total 13 balantidia species were found in freshwater fishes, among which 8 species, i.e., Balantidium ctenopharyngodoni, B. polyvacuolum, B. fulinensis, B. procyprini, B. semilabeai, B. senilabeai, B. yangtzensis, and B. yinjiangensis, were described in China [7-12]. The other 5 freshwater fish balantidia, i.e., B. piscicola, B. grevolosum, B. strelkovi, B. spinibarbichthys, and B.steinae, were discovered respectively in South America, North America, and USSR [13-15].
As to the anura amphibian balantidia, 26 valid species have been reported, containing B. entozoon, B. elongatum, B. duodeni (syn. B. hyalinum), B. giganteum, B. helenae (syn. B. ovale), B. gracile, B. rotundum, B. falciformis, B. ranarum, B. bicavata, B. amygdalli, B. sushilii, B. sinenesis, B. kirbyi, B. xenopi, B. tigrinae, B. aurangabadensis, B. ranae, B. megastomae, B. cyanophlycti, B. corlissi, B. mininucleatum, B. ganapatii, B. singaporensis, B. claperedei, and B. vanensis [16-26]. Another 5 balantidia species were reported from urodele amphibians, i.e., B. elongatum, B. amblystomatis, B. rayi, B. tylototritonis, and B. andianusis [6,27,28]. Among these mentioned balantidia species, only 2 species were first discovered and named in China. B. sinensis was reported from 2 species of frogs, i.e., R. nigromaculata and R. plancyi, and B. andianusis was described from Chinese giant salamander, Andrias davidianus [6,21]. In the present study, another new Balantidium species inhabiting Rana nigromaculata and R. limnocharis from Honghu Lake in Hubei Province, China is described; this study is expected to contribute to the knowledge of the genus Balantidium.
MATERIALS AND METHODS
The frogs used for this study were captured from Honghu Lake (29°40'-29°58'N; 113°12'-113°26'E), Hubei Province, China in June-July 2010 and 2011. We obtained permits allowing us to capture and sacrifice these specimens. The frogs were transported alive to the laboratory for further examination. All frog samples were dissected as soon as possible. The recta, intestines, and duodena were collected respectively into different Petri dishes and examined with the aid of Stemi SV6/AxioCam MRc5 (Zeiss, Oberkochen, Germany). The ciliates were collected with Pasteur micropipette and washed twice in distilled water.
For light microscopy (LM), specimens were smeared on cover slips, fixed in saturated HgCl2 solution and stained with Heidenhain's or Ehrlich's hematoxylin. Then, they were observed, measured, and photographed with the help of Axioplan 2 imaging and Axiophot 2 (Zeiss, Oberkochen, Germany). All measurements are in micrometers.
For scanning electron microscopy (SEM), the washed specimens were fixed in 2.5% glutaraldehyde in 0.2 M PBS (pH 7.4) on a clean glass slide (1 cm×1 cm), previously treated with 0.1% poly-L-Lysine, and dried completely in air at room temperature. After they were washed with PBS 3 times, they were post-fixed in 1% osmium tetroxide at 4℃ for 1 hr, followed by serial dehydration in acetone and critical point drying using the HCP-2 crtical point dryer (Hitachi Science Systems, Ibaraki, Japan). Then, the glass slide was mounted on an aluminium-stub using a double-sided adhesive tape and sputter-coated with a thin layer gold in IB-3 ion coater (Eiko Engineering, Ibaraki, Japan) before observing and photographing by using a Quanta 200 SEM (FEI, Amsterdam, Netherlands).
RESULTS
Ninety-six (75.6%) of 127 examined R. nigromaculata and 23 (35.4%) of 65 R. limnocharis were found to be infected with Balantidium honghuensis n. sp. These balantidia were present mainly in the recta of frogs.
Balantidium honghuensis n. sp.
Host
Rana nigromaculata Hallowell, 1861 and Rana limnocharis Boie,1834.
Prevalence
75.6% (96 of 127) of R. nigromaculata and 35.4% (23 of 65) R. limnochari were infected.
Locality
Honghu Lake (29°40'-29°58'N; 113°12'-113°26'E), Hubei Province, China.
Habitat
Rectum.
Reference material
Slide 2010W011-13 of Heidenhain's hematoxylin stained specimens, slide 2011W003-5 of Ehrlich's hematoxylin stained specimens have been deposited in Hubei Key Laboratory of Animal Nutrition and Feed Science, Wuhan Polytechnic University, China.
Description
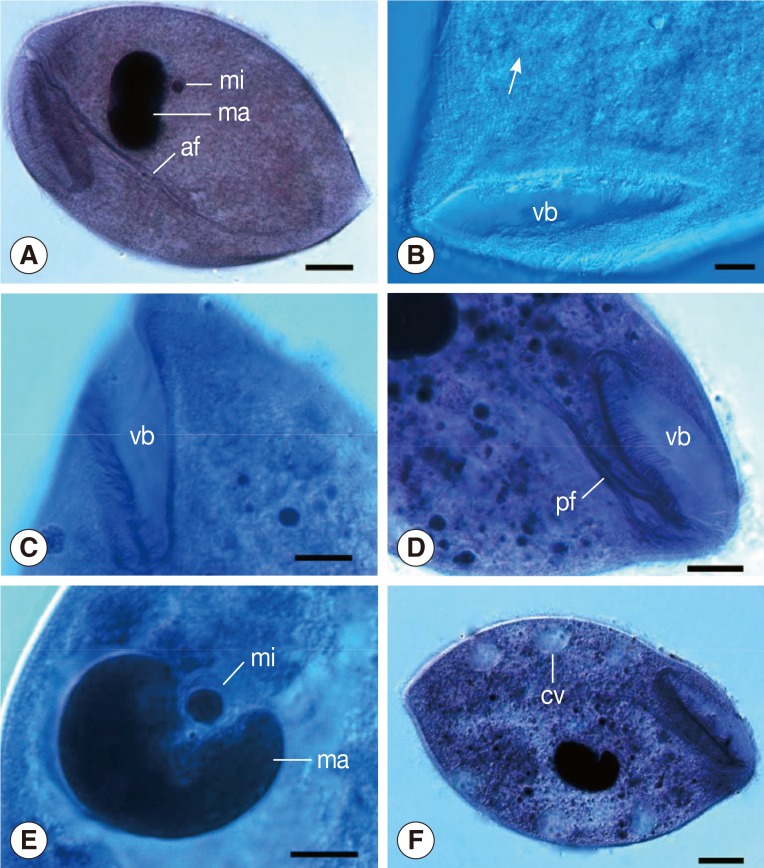
The organism was oval in shape and thickly ciliated (Figs. 1A, 3A, B). It ranged in length from 129.6 to 180.0 µm (av.=154.1 µm; n=30) and in width from 84.0 to 122.4 µm (av.=101.7 µm; n=30). About 156-190 body kineties (n=12), among which 70-94 are located dorsally and 82-109 kineties ventrally, were arranged in closely set lines parallel to the longitudinal axis and densely packed over the body (Figs. 1B, 4A). The vestibulum was "V" shaped and situated anteroventrally, measuring 49.2-67.3 µm (av.=57.0 µm, n=21) in length that occupied about 1/3 to 2/5 of the body length (Figs. 1B, C, 3A). The width of the vestibulum measured 11.8-19.2 µm (av.=15.5 µm, n=21). The vestibular and nearby regions possessed strong peripheral fibers, deriving from the anterodorsal side and extending straight into the endoplasm (Figs. 1D, 3C, D), forming a marked axial fiber about the cytopharynx, marching a long distance and pointing directly toward the posterior of the body (Figs. 1A, 4B). The macronucleus was ovoid or roughly kidney-shaped with the length ranging 35.3-44.6 µm (av.=39.0 µm; n=20) and the width 19.2-27.6 µm (av.=24.2 µm; n=20). It lay somewhat obliquely near the middle of the body or slightly posterior to it. The micronucleus was spherical and always embedded in the middle concavity of the macronucleus and measured about 3.4-7.2 µm (av.= 5.3 µm; n=20) in diameter (Figs. 1E, 4A, B). More than 10 contractile vacuoles were distributed along the periphery of the latter body with an average of about 11.9 µm in diameter (n=20) (Figs. 1F, 2A, 4A). A marked cytoproct was present at the posterior end of the body (Figs. 3A, 4A).
Fig. 1.
Light microscopic images of B. honghuensis n. sp. (A) Specimens stained with Ehrlich's hematoxylin, showing its general form, macronucleus (ma), micronucleus (mi), and strong axial fiber (af) extending to the posterior body. Scale bar=20 µm. (B) Specimens stained with Heidenhain's hematoxylin, showing the somatic kineties (arrow) around the vestibulum (vb). Scale bar=10 µm. (C) Specimens stained with Heidenhain's hematoxylin, showing the "V" shaped vestibulum (vb). Scale bar=10 µm. (D) Specimens stained with Heidenhain's hematoxylin, showing the peripheral fibres (pf) deriving from the anterodorsal side of vestibular (vb) and nearby regions. Scale bar=15 µm. (E) Specimens stained with Heidenhain's hematoxylin, showing its macronucleus (ma) and micronucleus (mi). Scale bar=10 µm. (F) Specimens stained with Ehrlich's hematoxylin, showing the contractile vacuoles (cv). Scale bar=20 µm.
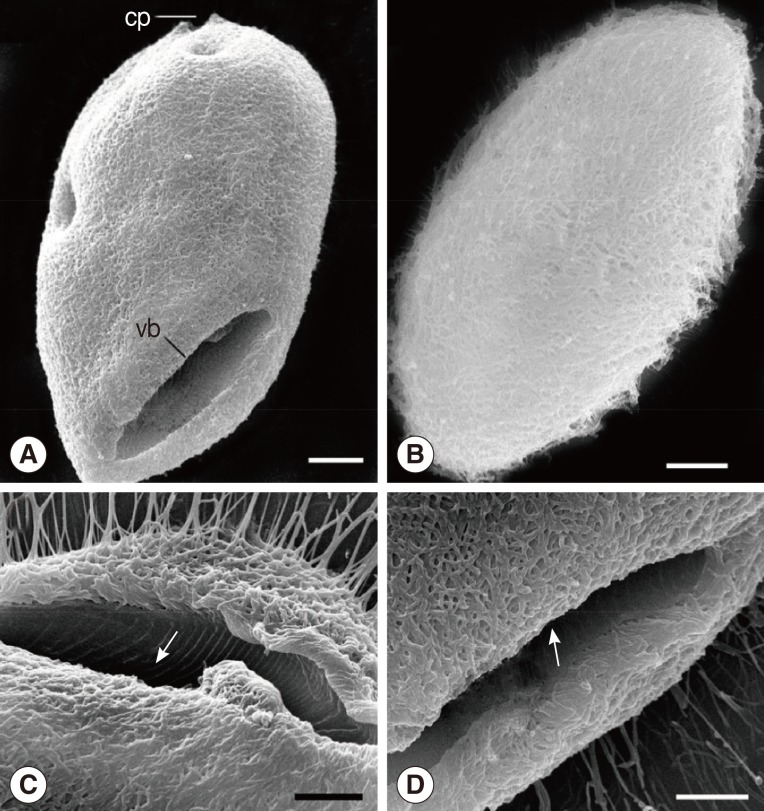
Fig. 3.
SEM images of B. honghuensis. (A) Overview of the ciliate, showing the general form, vestibulum (vb), and cytoproct (cv). Scale bar=20 µm. (B) Overview of the ciliate, showing the body surface of B. honghuensis that is thickly ciliated. Scale bar=20 µm. (C, D) The vestibulum to show the peripheral fibres (arrow) deriving from the anterodorsal side and extending straight into the endoplasm. Scale bar=20 µm.
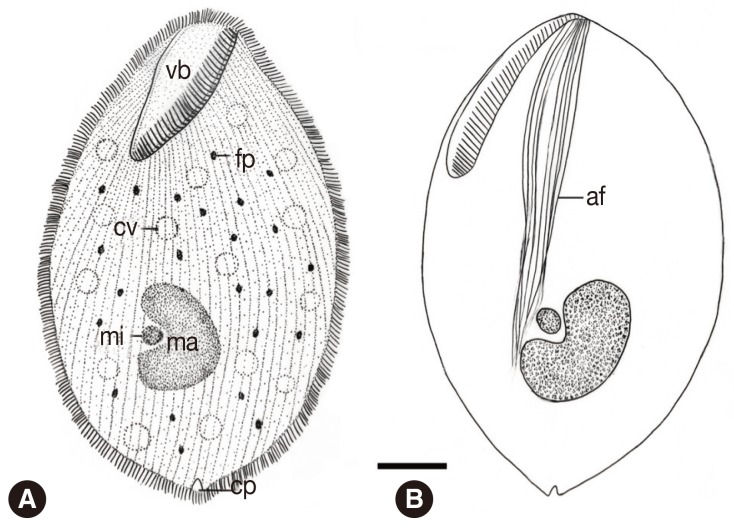
Fig. 4.
Schematic drawing of B. honghuensis, showing the general form and structures from the ventral view: vestibulum (vb), axial fiber (af), food particles (fp), macronucleus (ma), micronucleus (mi), contractile vacuoles, and cytoproct (cv). Scale bar=20 µm.
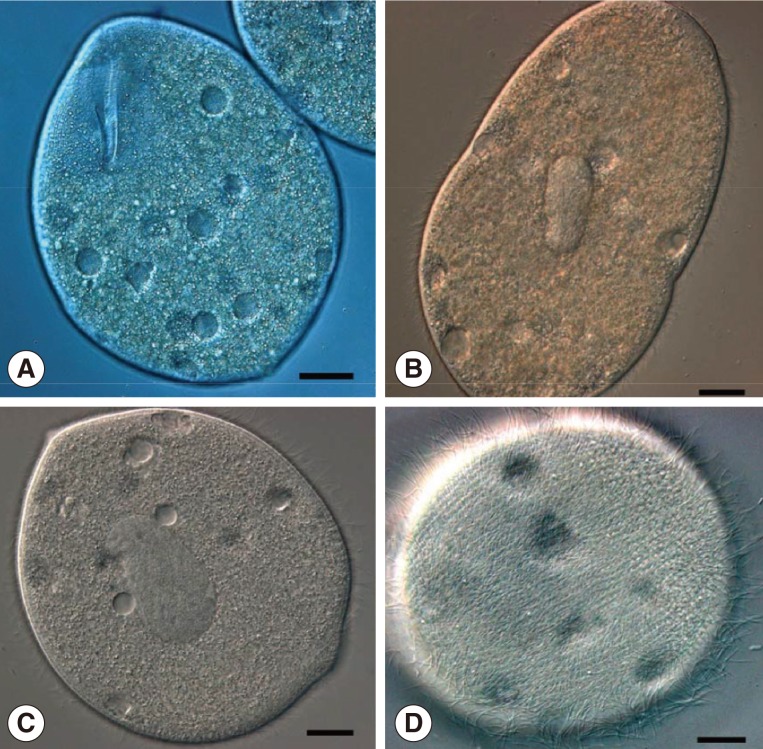
Fig. 2.
Living specimens of B. honghuensis, showing trophozoites and high-speed daughter swarmers. (A) Specimens to show the normal trophozoites of B. honghuensis. Scale bar=20 µm. (B) Images to show the beginning of the division. Scale bar=15 µm. (C, D) High-speed daughter swarmers of B. honghuensis. Scale bar=10 µm.
In addition, we found that a large number of balantidia began to multiply by transverse binary fission after collected from the recta of host frogs and cultured in a Petri dish filled with water from Honghu Lake for about 10 hr at room temperature (Fig. 2A, B). The daughter ciliates were nearly rounded, measuring about 52-70 µm (av.=60 µm; n=10) in diameter. They were high-speed swarmers with thick cilia packing over their round body, which could survive for 36-48 hr in the laboratory (Fig. 2C, D).
DISCUSSION
The occurrence of a new Balantidium species in Chinese anuran amphibians is recorded in this paper. It has been designated B. honghuensis in view of its discovery locality, Honghu Lake in Hubei Province, China.
Among all the described species of Balantidium, B. honghuensis n. sp. most resembled B. sinensis discovered from the intestines of R. nigromaculata Hallowell and R. plancyi Lataste [21]. Both of these 2 balantidia species were found in the recta of R. nigromaculata and they shared some similar features such as possessing more than 10 contractile vacuoles and strong peripheral fibers deriving from the anterodorsal side. Besides, their body dimensions overlapped with each other in some extent (B. honghuensis and B. sinensis: 129.6-180.0×84.0-100.8 µm and 160.0-275.0×70.0-170.0 µm). However, B. honghuensis differed from B. sinensis by possessing a relatively smaller body size and a typical "V"-shaped vestibulum rather than a conspicuous slit-like vestibulum in the latter. Furthermore, in terms of general body forms, the 2 balantidia species could be discriminated from each other: B. honghuensis was oval-shaped with the greatest width at just the middle of the body, while B. sinensis was spindle-like in shape and obviously pointed at the anterior end with the greatest width behind the middle of the body.
As to the rounded high-speed daughter ciliates, we think that they ought to be the infective stage of B. honghuensis since no trophozoites could form cysts for a long time after being collected and cultured in a Petri dish filled with water from Honghu Lake. Instead, these trophozoites disappeared completely after 2-day cultivation and were all replaced by their daughter swarmers. On the other hand, it is reasonable to presume that Balantidium species inhabitating anura amphibians complete their transmission through cloaca rather than oral opening considering their special feeding habits. In this case, the aforementioned daughter ciliates should be the just infective stage regardless of the immotile cyst whether it really exists or not. Moreover, the daughter ciliates are high-speed swarmers and could survive for 36-48 hr at room temperature in the laboratory. It is believable that they can remain infective for even a longer time which is enough for them to complete infection in their natural aquatic environment. The infection forms and routes of anura amphibian balantidia are very interesting and will also the focus of our future works.
This study described a new trichostome ciliate, Balantidium honghuensis n. sp., isolated from the recta of Rana nigromaculata and R. limnocharis in Honghu Lake, Hubei Province, central China. Its detailed morphometric characters based on LM and SEM studies were presented. Besides, its high-speed daughter swarmers which we believed to be the infective stage of B. honghuensis were observed.
ACKNOWLEDGMENTS
Financial support for this study was provided by the National Natural Science Foundation of China (Grant No. 31000947) and the Open Fund of Hubei Key Laboratory of Animal Nutrition and Feed Science (Grant No. 2009A001) and the Graduates' Innovation Fund of Wuhan Polytechnic University (Grant No. 2012cx025).
References
- 1.Li M, Li D, Wang J, Zhang J, Gu Z, Gong X. Light and scanning electron microscopic study of Balantidium ctenopharyngodoni Chen, 1955 (Class: Litostomatea) from China. Parasitol Res. 2007;101:185–192. doi: 10.1007/s00436-006-0451-1. [DOI] [PubMed] [Google Scholar]
- 2.Li M, Wang C, Wang J, Li A, Gong X, Ma H. Redescription of Balantidium polyvacuolum Li 1963 (Class: Litostomatea) inhabiting the intestines of Xenocyprinae fishes in Hubei, China. Parasitol Res. 2009;106:177–182. doi: 10.1007/s00436-009-1645-0. [DOI] [PubMed] [Google Scholar]
- 3.Li M, Wang C, Wang J, Yu D, Wang W, Ge X, Xu P. PCR amplification, sequencing and analysis of 18S rDNA of Balantidium ctenopharyngodoni inhabiting grass carp. Acta Hydrobiol Sin. 2011;35:203–209. [Google Scholar]
- 4.Li M, Wang C, Li W, Zeng L, Wang J, Lü Y, Gong X. Ultrastructural study of Balantidium ctenopharyngodoni Chen, 1955 (Class: Litostomatea) Acta Hydrobiol Sin. 2012;36:765–769. [Google Scholar]
- 5.Li W, Wang C, Li M, Huang F, Liu HY. Ultrastructural study of Balantidium polyvacuolum Li, 1963 (Class: Litostomatea) that inhabits Xenocyprinae fish. Acta Hydrobiol Sin. 2012;36:1135–1141. [Google Scholar]
- 6.Li M, Wang J, Zhang J, Gu Z, Ling F, Ke X, Gong X. First report of two Balantidium species from Chinese giant salamander, Andrias davidianus: Balantidium sinensis Nie, 1935 and Balantidium andianusis n. sp.. Parasitol Res. 2008;102:605–611. doi: 10.1007/s00436-007-0795-1. [DOI] [PubMed] [Google Scholar]
- 7.Chen C. The protozoan parasites from four species of Chinese pond fishes: Ctenopharyngodon idellus, Mylopharyngodon piceus, Aristhicthys nobillis and Hypophthalmichthys molithaix. I. The protozoan parasites of Ctenopharyngodon idellus. Acta Hydrobiol Sin. 1955;1:123–164. [Google Scholar]
- 8.Li L. Studies on a new ciliate, Balantidium polyvacuolum sp. nov., from the intestine of fishes. Acta Hydrobiol Sin. 1963;1:81–97. [Google Scholar]
- 9.Feng S. Studies on a new ciliate, Balantidium fulinensis sp. nov., from the intestine of fishes. Acta Hydrobiol Sin. 1992;16:66–70. [Google Scholar]
- 10.Zhao Y, Ma C. A new species of parasitic Balantidium from the freshwater fishes of China (Trichostomatida: Balantidiidae) Acta Zootaxon Sin. 1992;17:1–5. [Google Scholar]
- 11.Feng S, Li L. Parasitic protozoa of fishes from Wuling Mountains Area. II. Notes on two new species of Balantidium. In: Song DX, editor. Invertebrates of Wuling Mountains Area, Southwestern China. Beijing, China: Science; 1997. pp. 162–167. [Google Scholar]
- 12.Wang W, Li L, Yu Y. Parasite fauna of fishes in Wuling Mountains Area. In: Song DX, editor. Invertebrates of Wuling Mountains Area, Southwestern China. Beijing, China: Science; 1997. pp. 73–146. [Google Scholar]
- 13.Entz G. Über organisationsverhältnisse von Nyctotherus piscicola (Daday) Arch Protistenkd. 1913;29:364–386. [Google Scholar]
- 14.Ganthier M. Presence d'un infusoire parasite dans I'estomac d'un saumon de fontaine. C R Séances Soc Biol Fil. 1920;83:1607–1609. [Google Scholar]
- 15.Ha K. New ciliata from the intestine of freshwater fishes of Northern Vietnam. Acta Protozool. 1971;8:261–282. [Google Scholar]
- 16.Bezzenberger E. Über Infusorien aus asiatischen Anuren. Arch Protistenk. 1904;3:138–174. [Google Scholar]
- 17.Bhatia BL, Gulati AN. On some parasitic ciliates from Indian frogs, earthworms and cockroaches. Arch Protistenk. 1972;57:85–120. [Google Scholar]
- 18.Dobell CC. On some parasitic protozoa from Ceylon. Spolia Zeylanica. 1910;7:65–87. [Google Scholar]
- 19.Khan MM, Ip YK. Parasites of toads from Singapore, with a description of Balantidium singaporensis sp. n. (Ciliophora: Balantidiidae) Zool Sci. 1986;3:543–546. [Google Scholar]
- 20.Mahoon MS, Khan MI. Entozoic protozoa of frog Rana cyanophlyctis Schneider. Biologia (Lahore) 1986;32:383–420. [Google Scholar]
- 21.Nie D. Intestinal ciliates of Amphibia of Nanking. Contr Biol Lab Sci Soc China. 1935;11:47–95. [Google Scholar]
- 22.Puytorac PD, Grain J. Structure et ultrastructure de Balantidium xenopi sp. nov. Cilié trichostome parasite du batracien Xenopus fraseri Boul. Protistologica. 1965;1:29–36. [Google Scholar]
- 23.Ray H. On the morphology of Balantidium sushilii n. sp., from Rana tigrina Daud. J Roy Micr Soc. 1932;52:374–382. [Google Scholar]
- 24.Rodriguez JM. On the morphology of Balantidium kirbyi n. sp., from the Plathander. J Parasitol. 1939;25:197–201. [Google Scholar]
- 25.Senler NG, Yildiz I. The Ciliate Fauna in the Digestive System of Rana ridibunda (Amphibia: Anura) I: Balantidium (Balantidiidae, Trichostomatida) Turk J Zool. 2000;24:33–43. [Google Scholar]
- 26.Shete SG, Krishnamurthy R. Observations on the rectal ciliates of the genus Balantidium, Claparede and Lachman, 1858 from Indian amphibians Rana tigrina and R. cyanophlyctis. Arch Protistenk. 1984;128:179–194. [Google Scholar]
- 27.Jírovec O. Über ein neues Balantidium aus dem Darmtraktus von Amblystoma tigrinum. Parasitol Res. 1930;3:17–21. [Google Scholar]
- 28.Pal NL, Dasgupta B. Observations on 2 new species of Balantidium in the Indian salamander Tylototriton verrucosus (Caudata: Salamandridae) Proc Zool Soc (Calcutta) 1978;31:47–52. [Google Scholar]