Abstract
Toxocariasis is a worldwide zoonosis caused by larvae of ascarid nematodes of dogs or cats, Toxocara canis or T. cati. Diagnosis of human toxocariasis currently relies on serology that uses T. canis excretory-secretory antigen to detect specific IgG antibodies by ELISA. We investigated the serodiagnostic efficacy of ELISA using crude antigen of T. canis larvae (TCLA). Serum specimens of 64 clinically confirmed toxocariasis, 115 healthy controls, and 119 other tissue-invading helminthiases were screened by ELISA using TCLA. The ELISA using TCLA showed 92.2% (59/64 patient samples) sensitivity and 86.6% (103/119) specificity. Its positive diagnostic predictivity was 78.7% and negative predictivity was 97.8%. No serum of healthy controls reacted but that of anisakiasis (45.5%), gnathostomiasis (19.2%), clonorchiasis (15.8%), sparganosis (11.1%), and cysticercosis (6.3%) cross-reacted. Immunoblot analysis on TCLA recognized antigenic proteins of 28- and 30-kDa bands in their dominant protein quantity and strong blotting reactivity. The present results indicate that the ELISA using our TCLA antigen is acceptable by the sensitivity and specificity for serodiagnosis of human toxocariasis. ELISA with TCLA is recommended to make differential diagnosis for patients with any sign of organ infiltration and eosinophilia.
Keywords: Toxocara canis, toxocariasis, serodiagnosis, crude antigen, ELISA
INTRODUCTION
Toxocariasis is a cosmopolitan zoonosis caused by migrating larvae of Toxocara canis or Toxocara cati in human bodies. Humans are commonly infected by accidental ingestion of embryonated Toxocara eggs found in soil, contaminated food, or drinking water. The ingested eggs hatch in the duodenum and the larvae invade into the blood stream. After the invasion in humans, the larvae fail to develop to adult worms and remain as larvae which migrate to different organs and survive for several years. The migrating larvae induce human toxocariasis, which is commonly presented as covert (adult) or common (child) toxocariasis (CT), and less frequently as ocular larva migrans (OLM), and visceral larva migrans (VLM) [1]. Alternatively to the ingestion of eggs, humans can also be infected by eating uncooked or undercooked animal liver or meat containing the infective stage larvae [2,3]. Therefore raw-eating habit of Koreans fosters such kind of food-borne transmission [4-7].
As in all parasitic infections, direct demonstration of adult worms, larvae, or eggs can make a definite diagnosis. However, it is difficult to find out the larvae of T. canis in the tissue by biopsy because of extensive distribution and small size of larvae. Also biopsy is invasive as a diagnostic method. In this context, serology is an alternative standard method for diagnosis of human toxocariasis [8]. The most common serodiagnosis approach is ELISA using specific antigens. A commercial ELISA kit is supplied using excretory-secretory product released by second-stage larvae of T. canis (TES) [9,10]. A seroepidemiological survey using the kit recognized seropositive rate of 5% for toxocariasis in Korean rural adults in 2002 [11] and another investigation indicated 45.5% were confidently diagnosed with toxocariasis among eosinophilia patients in Chungcheongnam-do, the central district of Korea in 2012 [12]. Those serology data suggested high prevalence of hidden toxocariasis in Korea. However, the commercial kit costs much for routine diagnostic service for suspected patients. Recently, Lim [5] commented that toxocariasis is very common but abandoned by physicians in Korea mainly due to their lack of awareness. We infer that toxocariasis is unaware to physicians and neglected by lack of good routine diagnostic service in Korea. Any validated serodiagnostic test which is easy and cheap is necessary for proper management of toxocariasis suspected patients in Korea. The commercial kit has been used only for research purpose.
ELISA using crude antigen of T. canis larvae (TCLA) may help overcome the shortcomings of TES. The present study was undertaken to develop a robust serodiagnostic method for human toxocariasis using TCLA.
MATERIALS AND METHODS
Ethics Statement
The present study protocol was reviewed and approved by the institutional review board (IRB) of the Seoul National University College of Medicine, Seoul, Korea (IRB No.: E-1205-009-408). The IRB waived informed consent from the subjects under the condition of anonymous collection of serum samples from the serum pools of involved authors' institutions. All of the procedures followed the guidelines of the IRB.
The present study used puppies of 2-3 months to collect Toxocara worms by anthelmintic medication in their households. The medication was included in routine health care of puppies which required no permission of institutional animal care and use committee. The owners of the puppies agreed to the medication and worm collection by oral explanation of the study. We supplied levamisole and the owners medicated them. All procedures were made to minimize suffering.
Isolation and cultivation of T. canis eggs
The fecal materials of puppies after medication were collected and moved to the biosafety level 2 laboratory. The discharged T. canis female worms were isolated and washed in sterile physiologic saline solution, and their uterus was dissected to collect fertilized eggs. The eggs were in vitro cultivated for embryonation of the L2/L3 larvae. The embryonated eggs were digested in 2% NaOH solution for 30 min and incubated in 1% neutral formalin for 21 days at 27℃ with regular inspection. To the egg suspension, an equal volume of 6% pepsin solution was added for 30 min and 1× trypsin was treated until the eggs lost their outer pitted shells, then vortexed with glass beads. The egg suspension was then placed in RPMI 1640 medium containing 100 IU/ml penicillin, 100 µg/ml streptomycin, and 2.5 µg/ml amphotericin B (Gibco BRL Life Technologies, Rockville, Maryland, USA). The suspension was transferred onto 2 layers of gauze and lens paper in a modified Baermann apparatus. The live larvae were collected from the bottom of the apparatus after approximately 4 hr and homogenized in PBS.
Preparation of TCLA
TCLA was obtained from the homogenized larvae in the presence of PBS at pH 7.2 (8.1 mM Na2PO4, 1.5 mM KH2PO4, and 136 mM NaCl) and protease inhibitors (1 mM tosyl-L-lysine chloromethyl ketone, 100 µM iodoacetamide and 10 µg/ml pepstatin A). The larval extract was centrifuged at 13,000 rpm for 30 min at 4℃ and the supernatant was aliquoted and cryopreserved at -70℃ until needed. The protein concentration of the extract was estimated by the BCA™ protein assay kit (Pierce, Rockford, Illinois, USA).
Human serum samples
To evaluate the performance of different antigens for detection of toxocariasis in Korea, we analyzed the assay with different groups of serum samples. A total of 298 serum samples that received from respective hospitals of Korea used in this study. The serum samples were collected from different patients using conventional method by centrifuge 10 min at 3,000 rpm after clotting and stored at -20℃ in the Institute of Endemic Diseases, Seoul National University, Seoul, Korea until assay. Sixty-four serum samples were obtained from patients with toxocariasis which was confirmed by clinical, hematological, past history, and response to anthelminthic medication. Of the 64 toxocariasis patients, 56 were included in previous reports [2,13], and 8 were newly included under diagnosis of OLM. All of the 56 reported cases were confirmed by serological test with the commercial TES ELISA kit (Bordier Affinity Products SA, Crissier, Swizerland) [9,10]. For the analysis of cross-reactions, 119 samples came from patients with other parasite infections. As negative controls, 115 serum samples came from healthy medical school students with no evidence of helminth infections. The serum samples were collected from 2009 to 2011.
Serology by ELISA
ELISA was used to detect specific antibodies in serum of the toxocariasis with two antigens, in-house TCLA and the commercial TES from Bordier's kit (Bordier Affinity Products SA, Crissier, Swizerland) [10]. For standardization of ELISA conditions with TCLA, chequerboard titrations of the antigen at concentrations of 0.5-50 µg of protein/mL and different dilutions of human serum and secondary antibodies were first performed. The wells of polystyrene, flat bottom, 96-well microtitre plates (Costar-Corning, Cambridge, California, USA) were filled with 100 µl of coating buffer containing TCLA and incubated overnight at 4℃. Excess TCLA was removed by washing the wells 5 times with 250 µl of 150 mM phosphate buffered saline (pH 7.2) containing 0.05% (v/v) Tween 20 (PBST). The coated wells were blocked by adding 1% BSA in PBST at 37℃ for 1 hr and washed 5 times with PBST. The plates were incubated for 2 hr at 37℃ with sera diluted 1:100 in PBST (100 µl/well), 1 hr at 37℃ with 6.5 mg/ml anti-human IgG horse-radish peroxidase (HRP; Cappel, West Chester, Pennsylvania, USA) diluted 1:24,000 with PBST (100 µl/well). After each incubation, the plates were washed 5 times with PBST. After adding 100 µl of Tetramethyl Benzidine (TMB; Pierce, Rockford, Illinois, USA) as substrate for 90 sec, the reaction was stopped with 4 N sulphuric acid (H2SO4), and the absorbance was measured at 450 nm. The whole assay was duplicated. Based on absorbance of healthy control and toxocariasis patients, the cut-off value was determined. ELISA with TES followed the vendor's protocol.
SDS-PAGE
The crude antigen of T. canis larvae (150 µl of antigen at a concentration of 1 µg/µl mixed with 150 µl of sample buffer composed of 50 mM Tris-HCl at pH 6.8, 2% SDS, 10% glycerol, 1% β-mercaptoethanol, 12.5 mM ethylenediamine tetraacetic acid and 0.02% bromophenol blue) was boiled for 5 min and then separated on a 10% SDS-PAGE at a constant current of 170 V for 1.5 hr. The individual antigen bands were then visualized by silver staining (Elpisbio, Daejeon, Korea).
Western blotting
The crude antigen of T. canis larvae was separated by 10% SDS-PAGE (Sodium dodecyl sulphate-polyacrylamide gel electrophoresis) and transferred onto polyvinylidene fluoride (PVDF) membrane. The membrane was cut into strips and blocked with 5% skim milk. The strips were incubated with serum samples (diluted 1:100 in 5% skim milk solution) at 4℃ overnight, and was followed by 1.3 mg/ml polyclonal rabbit anti-human IgG antiserum conjugated with horseradish peroxidase (HRP; Dako, Glostrup, Denmark) which was used at a dilution of 1:2,000 with 5% skim milk. The reaction was visualized using ECL™ Western blotting detection reagents (Amersham, Piscataway, New Jersey, USA).
Statistical analysis
Spearman rank correlation analysis was used to evaluate the correlation between TCLA and TES absorbance. Statistical differences between categorical variables were calculated with a Student's t-test. All analyses were carried out with PASW Statistics, version 18.0 (SPSS Inc., Chicago, Illinois, USA). A P-value of less than 0.05 was considered to indicate statistical significance.
RESULTS
Demography of patients
All of the patients serum samples were collected from the preserved serum bank of patients. Mean age of the patients was 42.4±21.7 years, in range of 14-74. Men were 55 and women were 9 of the 64 patients. Other serum samples were used from the bank of serum confirmed by the Institute of Endemic Diseases, Seoul National University, Seoul, Korea.
Dilution of TCLA and serum
We performed a series of chequerboard titration ELISA with serial dilution of TCLA and serum of patients (data not shown). Based on the titration findings, the best concentration of protein for TCLA was defined as 100 µg/100 µl, and the best dilution of serum was determined as 1:100.
ELISA cut-off criteria
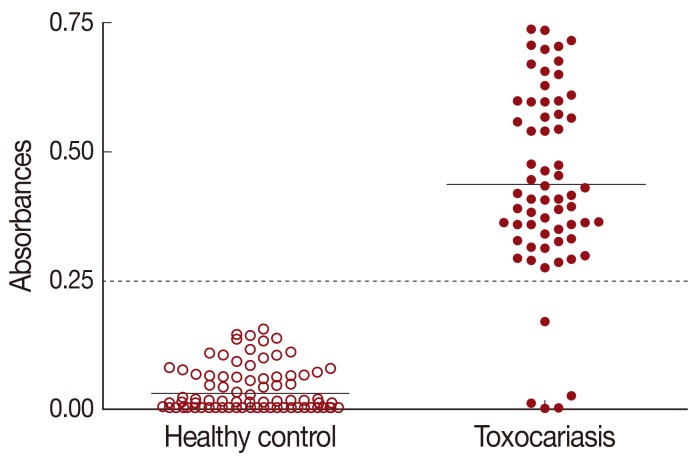
The cut-off value for TCLA ELISA was defined as over the mean absorbance plus 3 SDs (0.030±0.039×3) of the values of 115 healthy controls (Table 1). The absorbances of 64 toxocariasis patients ranged from 0.010 to 0.750 (0.437±0.177). When the absorbance at 450 nm was blanked with the PBS, an absorbance of 0.250 was set as the optimum cut-off value to discriminate the positive and negative reactions (Fig. 1).
Table 1.
ELISA reactions of T. canis larval antigen for healthy control and helminthiases
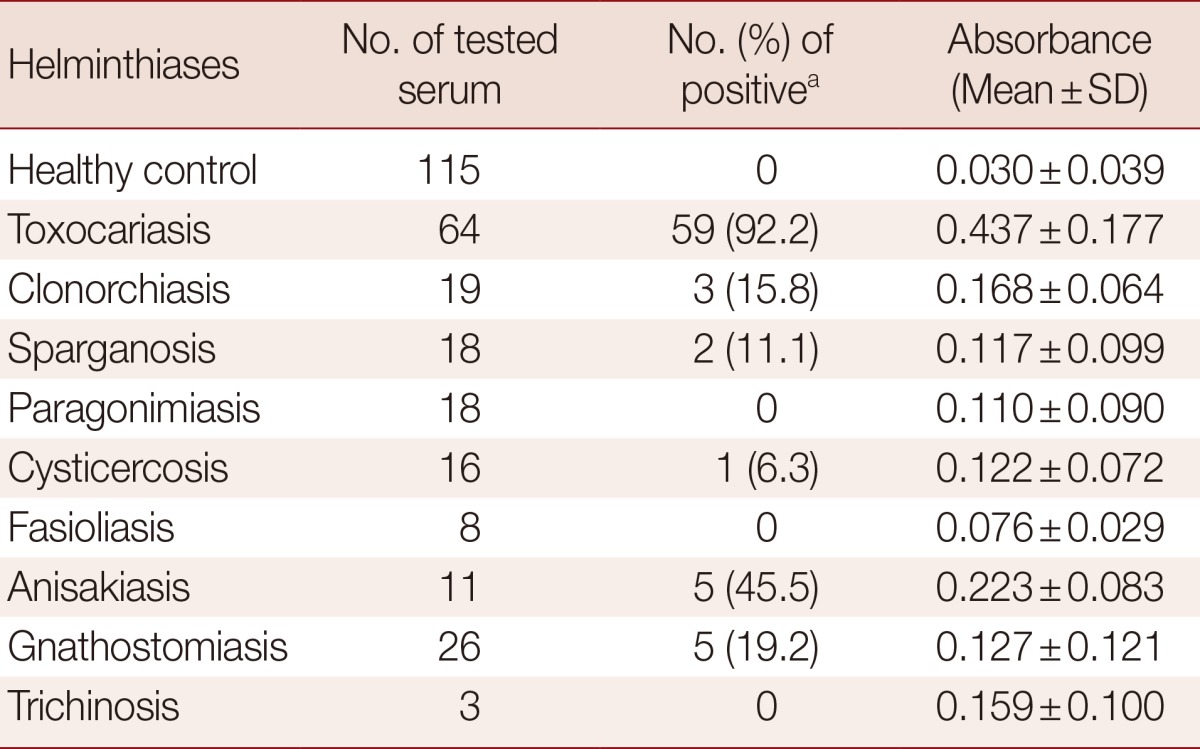
aAbsorbance>0.25.
Fig. 1.
Distribution of ELISA absorbances to TCLA of toxocariasis patients (n=64) and healthy control (n=115). Samples with absorbance of less than 0.25 were defined as negative and those more than 0.25 as positive. Dashed line represents cut-off value. TCLA, crude antigen of Toxocara canis larvae.
Determination of diagnostic values of TCLA ELISA
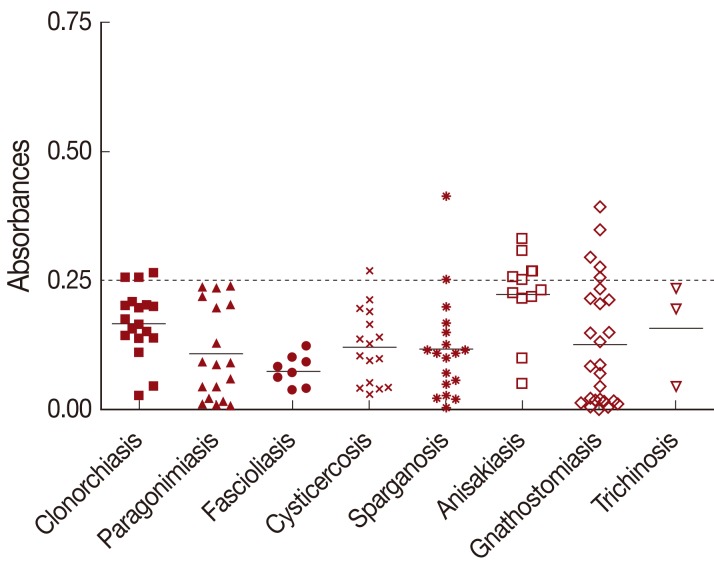
The mean absorbance of IgG ELISA using TCLA against serum specimens of negative control, toxocariasis and other helminthic patients are presented in Table 1 and individual absorbances are plotted in Figs. 1, 2. The ELISA for screening specific serum IgG antibodies for T. canis larval antigen using TCLA gave 92.2% (59/64) sensitivity and 86.6% (103/119) specificity (Table 2). The TCLA cross-reacted with serum samples of clonorchiasis, sparganosis, cysticercosis, anisakiasis and gnathostomiasis (Table 1; Fig. 2). The data exhibited high cross-reactivity with serum of nematode infections, but also some cross-reactivity with that of patients infected with tissue-invading trematodes or cestodes.
Fig. 2.
Cross-reactivity of TCLA to serum samples of clonorchiasis (n=19), paragonimiasis (n=18), fascioliasis (n=8), cysticercosis (n=16), sparganosis (n=18), anisakiasis (n=11), gnathostomiasis (n=26), and trichinosis (n=3). Samples with an absorbance of less than 0.25 were defined as negative and those more than 0.25 as positive. Dashed line represents cut-off value. TCLA, crude antigen of Toxocara canis larvae.
Table 2.
Diagnostic value of T. canis larval antigen by ELISA
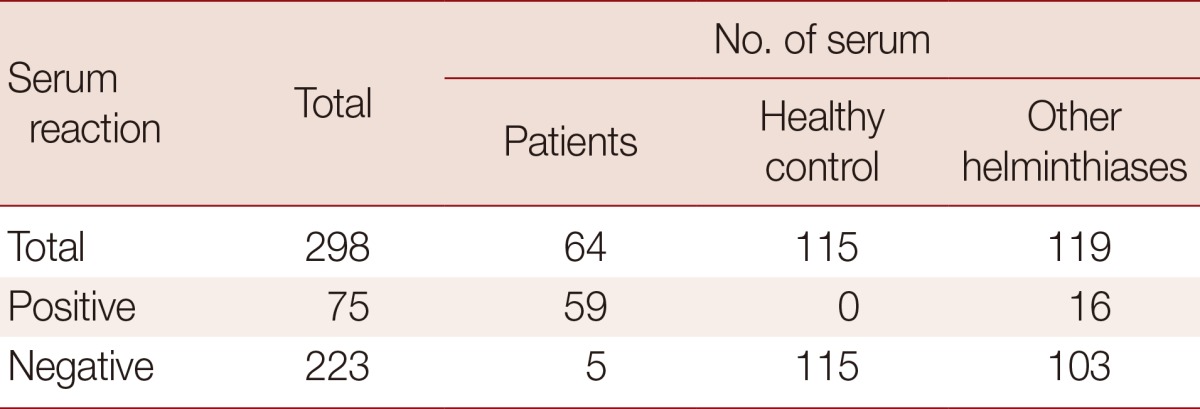
Sensitivity, 92.2% (59/64); specificity, 86.6% (103/119); positive predictive value, 78.7% (59/75); negative predictive value, 97.8% (218/223).
Correlation of ELISA absorbance with TCLA and TES
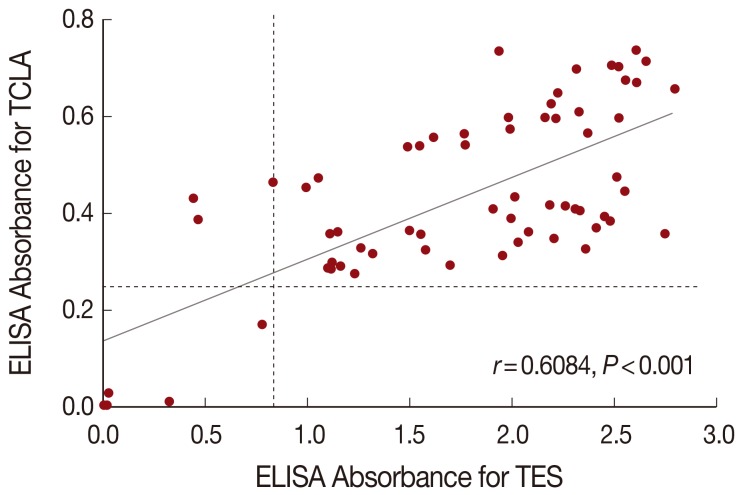
The correlation analysis evaluated quantitative changes of ELISA absorbance with TCLA compared with the absorbance with TES, and the Spearman's correlation coefficient (Spearman's rho, γ) was 0.6084. Of the 64 toxocariasis patients, 59 were positive but 5 were negative both in TES and TCLA ELISA. The ELISA absorbance with TCLA was correlated well (P<0.001) with those with TES (Fig. 3).
Fig. 3.
Correlation of ELISA absorbances between TCLA and TES. The correlation coefficient (Spearman's rho, r) usually presents a weak (r<0.5), moderate (0.5<r<0.8), and strong (r>0.8) relationship. A Student's t-test was used to determine its significance and P-value less than 0.05 was considered statistically significant. Dashed line represents cut-off value. TCLA, crude antigen of Toxocara canis larvae; TES, excretory-secretory product released by second-stage larvae of T. canis.
Immunoblot analysis of TCLA
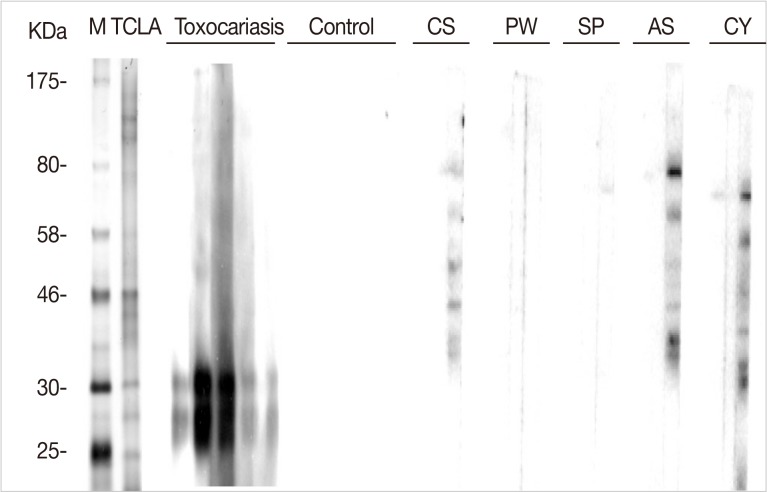
Immunoblot analysis of TCLA showed many protein bands of 25-, 28-, 30-, 35-, 40-, 46-, 56-, 70-, 120-, and 150-kDa. Of them, the 28- and 30-kDa bands presented strong reactivity against human serum specimens from toxocariasis (Fig. 4). No serum samples from healthy control reacted with TCLA, but those from patients of other helminthiases, such as clonorchiasis, anisakiasis, and cysticercosis, cross-reacted with other bands of TCLA (Fig. 4).
Fig. 4.
Western blots of TCLA with serum samples of helminthiasis patients and healthy controls. M, size marker; TCLA, crude antigen of Toxocara canis larvae; CS, clonorchiasis; PW, paragonimaisis; SP, sparganosis; AS, anisakiasis; CY, cysticercosis.
DISCUSSION
We developed a sensitive and specific in house ELISA system for screening of specific serum IgG antibodies to crude antigen of T. canis larvae. The sensitivity and specificity of the ELISA using TCLA were 92.2% and 86.6%, respectively. The positive and negative predictive values were 78.7% and 97.8%, respectively. This diagnostic value is similar with that of the commercial TES ELISA kit, 91% sensitivity and 86% specificity and regarded as acceptable [10]. The current standard test for diagnosis of human toxocariasis is an indirect ELISA using the TES. Absorbance of the present TCLA ELISA was well correlated with that of the TES ELISA, which suggests the TCLA may be used instead of TES for serodiagnosis of human toxocariasis when TES is not available.
The cut-off absorbance for positive reaction in our TCLA ELISA was defined as 0.250 based on mean and 3 standard deviation absorbances between the negative and positive controls. The cut-off value represented good diagnostic validity of our ELISA system. According to the cut-off value, 5 of the 64 clinical toxocariasis patients were serology negative and all of negative controls were negative. The 5 patient samples were also negative by TES ELISA. The 5 patients were all OLM. OLM patients were 8 of the 64 patients, and 3 of them were positive. The serum ELISA is known as less sensitive for diagnosis of OLM than that of other toxocariasis forms [14]. Our study observed that serologic reaction of OLM was very weak by TCLA ELISA. It is hard to explain the negative reaction but they all had ocular lesions without any problem of the liver or lungs. Since their absorbance was low by TES ELISA either, it was likely that the 5 patients failed to produce effective amount of specific antibodies in their serum. In those cases, aqueous humor is recommended to collect when ocular toxocariasis is suspected and higher anti-Toxocara antibody titer in this fluid has been found with OLM [15]. Also it is possible that the patients were misdiagnosed although they were included upon same clinical criteria. However, it is hard to exclude them from the patient pool because of the negative reaction with TES only. If the 5 patients were excluded, the sensitivity of ELISA with TCLA might be 100%. This false negative reaction of the 5 patients limited the sensitivity of the present TCLA ELISA as 92.2%. In our study, 2 of previously confirmed patients were serologically negative with TES, but they were positive with TCLA. The reaction of the 2 serum samples was recognized as false negative with TES of unknown etiology.
All of the 115 healthy controls were negative in our study. The negative serology suggested that the controls were selected from healthy control. The controls were used for real negative ELISA reaction to set the cutoff value for differentiation of positive reactions. Since about 5% were reported seropositive by TES ELISA among healthy population in Korea [11], further studies are warranted for seroepidemiology by TCLA ELISA in Korea.
Serodiagnosis of toxocariasis has some cross-reactions with other helminthiases, which make it less specific. Nevertheless, cross-reactivity with the TES has been reported by many authors in several helminthiases [10,16-20]. They are infections of Trichinella, Strongyloides [10], Fasciola, Ascaris and geohelminthes [16,17,19], filariasis [16], Taenia, Schistosoma and Echinococcus [20]. Especially, the cross-reactions may be a significant problem for serodiagnosis in tropical areas because of frequent polyparasitism [21]. Since the TCLA has more protein molecules than the TES, ELISA using the TCLA is expected less specific. However, the TCLA ELISA was as specific as the TES ELISA. Our study recognized cross-reactions of the TCLA with serum samples of anisakiasis, gnathostomiasis, clonorchiasis, sparganosis, and cysticercosis patients. Among them, cross-reaction was found as high as 45.5% with anisakiasis while that with others remained around 10%. Since Toxocara and Anisakis are member genera of the superfamily Ascaroidea, they may share some cross-reacting antigenic molecules. Therefore, further studies are necessary to assess cross-reactions with other nematodes especially ascarid ones. Fortunately, geohelminthes are almost disappearing and filariasis had been eradicated in Korea. At present it is hard to find polyparasitism in Korea. Therefore TCLA is useful for serodiagnosis of human toxocariasis when a patient is suspected in Korea.
The correlation of ELISA absorbance of the 64 toxocariasis patients between TCLA and TES was analyzed. The Spearman's correlation coefficient (Spearman's rho, γ) was 0.6084 (P<0.001), which indicated the two antigens might produce well-correlated absorbance.
Our TCLA included 2 main antigenic bands of 28- and 30-kDa by Western blotting. The TES was reported that protein molecules smaller than 25-kDa were important for the serodiagnosis [17]. However, the TCLA may have other molecules than the two although they were not strongly visualized by the Western blot. The molecular details of the TCLA should be a subject of further study. In addition, to minimize the cross reactions, the specific fraction of proteins from T. canis larva were synthesized as recombinant proteins and evaluated the validity used for serodiagnosis. Three combined recombinant antigens of T. canis larvae: rTES-26, rTES-30USM, and rTES-120 antigens were applied to screen IgG4 by ELISA, and produced high sensitivity (100%) and specificity (over 92%) [16]. The recombinant antigens should be better for serodiagnosis than the crude antigens. Their application should be studied further.
The present study has some limitations. The specificity or positive predictive value may vary according to number of serum specimens of cross-reacting helminthiases. The serum specimens of other geohelminth infections are required for better evaluation of diagnostic values. Also, it is still plausible that some of the 16 cross-reacting patients of other helminthiases in the present study were true toxocariasis by polyparasitism. Especially those with high absorbance over 0.3 were possibly patients of covert toxocariasis simultaneously. We also have little data on the ELISA absorbance according to the chronological disease course of human toxocariasis. Cured cases may remain positive long by ELISA, which should be evaluated in the future. Of course false reactivity requires more research for diseases which are related with abnormal immunoglobulin production.
In conclusion, the in house ELISA using the TCLA is useful for serodiagnosis of human toxocariasis with 92.2% sensitivity and 86.6% specificity. The present ELISA is recommended to differentiate toxocariasis from other listed diseases of eosinophilia or to diagnose suspected patients in Korea.
ACKNOWLEDGMENTS
The present study was supported by a grant from the Center for Disease Control and Prevention, Ministry of Health and Welfare, Korea (800-2010023). The authors gratefully acknowledge Ms. Hye-Seong Kim for her technical assistance in ELISA.
References
- 1.Smith H, Holland C, Taylor M, Magnaval JF, Schantz P, Maizels R. How common is human toxocariasis? Towards standardizing our knowledge. Trends Parasitol. 2009;25:182–188. doi: 10.1016/j.pt.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Kwon NH, Oh MJ, Lee SP, Lee BJ, Choi DC. The prevalence and diagnostic value of toxocariasis in unknown eosinophilia. Ann Hematol. 2006;85:233–238. doi: 10.1007/s00277-005-0069-x. [DOI] [PubMed] [Google Scholar]
- 3.Choi D, Lim JH, Choi DC, Paik SW, Kim SH, Huh S. Toxocariasis and ingestion of raw cow liver in patients with eosinophilia. Korean J Parasitol. 2008;46:139–143. doi: 10.3347/kjp.2008.46.3.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noh Y, Hong ST, Yun JY, Park HK, Oh JH, Kim YE, Jeon BS. Meningitis by Toxocara canis after ingestion of raw ostrich liver. J Korean Med Sci. 2012;27:1105–1108. doi: 10.3346/jkms.2012.27.9.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim JH. Foodborne eosinophilia due to visceral larva migrans: a disease abandoned. J Korean Med Sci. 2012;27:1–2. doi: 10.3346/jkms.2012.27.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi D, Lim JH, Choi DC, Lee KS, Paik SW, Kim SH, Choi YH, Huh S. Transmission of Toxocara canis via ingestion of raw cow liver: a cross-sectional study in healthy adults. Korean J Parasitol. 2012;50:23–27. doi: 10.3347/kjp.2012.50.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park MS, Ahn YJ, Moon KR. Familial case of visceral larval migrans of Toxocara canis after ingestion of raw chicken liver. Korean J Pediatr Gastroenterol Nutr. 2010;13:70–74. [Google Scholar]
- 8.Fillaux J, Magnaval JF. Laboratory diagnosis of human toxocariasis. Vet Parasitol. 2013;193:327–336. doi: 10.1016/j.vetpar.2012.12.028. [DOI] [PubMed] [Google Scholar]
- 9.de Savigny DH, Voller A, Woodruff AW. Toxocariasis: serological diagnosis by enzyme immunoassay. J Clin Pathol. 1979;32:284–288. doi: 10.1136/jcp.32.3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacquier P, Gottstein B, Stingelin Y, Eckert J. Immunodiagnosis of toxocarosis in humans: evaluation of a new enzyme-linked immunosorbent assay kit. J Clin Microbiol. 1991;29:1831–1835. doi: 10.1128/jcm.29.9.1831-1835.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park HY, Lee SU, Huh S, Kong Y, Magnaval JF. A seroepidemiological survey for toxocariasis in apparently healthy residents in Gangwon-do, Korea. Korean J Parasitol. 2002;40:113–117. doi: 10.3347/kjp.2002.40.3.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seo M, Yoon SC. A seroepidemiological survey of toxocariasis among eosinophilia patients in Chungcheongnam-do. Korean J Parasitol. 2012;50:249–251. doi: 10.3347/kjp.2012.50.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoon YS, Lee CH, Kang YA, Kwon SY, Yoon HI, Lee JH, Lee CT. Impact of toxocariasis in patients with unexplained patchy pulmonary infiltrate in Korea. J Korean Med Sci. 2009;24:40–45. doi: 10.3346/jkms.2009.24.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Visser L, Rothova A, de Boer JH, van Loon AM, Kerkhoff FT, Canninga-van Dijk MR, Weersink AY, de Groot-Mijnes JD. Diagnosis of ocular toxocariasis by establishing intraocular antibody production. Am J Ophthalmol. 2008;145:369–374. doi: 10.1016/j.ajo.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 15.Felberg NT, Shields JA, Federman JL. Antibody to Toxocara canis in the aqueous humor. Arch Ophthalmol. 1981;99:1563–1564. doi: 10.1001/archopht.1981.03930020437005. [DOI] [PubMed] [Google Scholar]
- 16.Mohamad S, Azmi NC, Noordin R. Development and evaluation of a sensitive and specific assay for diagnosis of human toxocariasis by use of three recombinant antigens (TES-26, TES-30USM, and TES-120) J Clin Microbiol. 2009;47:1712–1717. doi: 10.1128/JCM.00001-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magnaval JF, Fabre R, Maurieres P, Charlet JP, de Larrard B. Application of the western blotting procedure for the immunodiagnosis of human toxocariasis. Parasitol Res. 1991;77:697–702. doi: 10.1007/BF00928685. [DOI] [PubMed] [Google Scholar]
- 18.Yamasaki H, Araki K, Lim PK, Zasmy N, Mak JW, Taib R, Aoki T. Development of a highly specific recombinant Toxocara canis second-stage larva excretory-secretory antigen for immunodiagnosis of human toxocariasis. J Clin Microbiol. 2000;38:1409–1413. doi: 10.1128/jcm.38.4.1409-1413.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romasanta A, Romero JL, Arias M, Sanchez-Andrade R, Lopez C, Suarez JL, Diaz P, Diez-Banos P, Morrondo P, Paz-Silva A. Diagnosis of parasitic zoonoses by immunoenzymatic assays-analysis of cross-reactivity among the excretory/secretory antigens of Fasciola hepatica, Toxocara canis, and Ascaris suum. Immunol Invest. 2003;32:131–142. doi: 10.1081/imm-120022974. [DOI] [PubMed] [Google Scholar]
- 20.Ishida MM, Rubinsky-Elefant G, Ferreira AW, Hoshino-Shimizu S, Vaz AJ. Helminth antigens (Taenia solium, Taenia crassiceps, Toxocara canis, Schistosoma mansoni and Echinococcus granulosus) and cross-reactivities in human infections and immunized animals. Acta Trop. 2003;89:73–84. doi: 10.1016/j.actatropica.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Noordin R, Smith HV, Mohamad S, Maizels RM, Fong MY. Comparison of IgG-ELISA and IgG4-ELISA for Toxocara serodiagnosis. Acta Trop. 2005;93:57–62. doi: 10.1016/j.actatropica.2004.09.009. [DOI] [PubMed] [Google Scholar]