Abstract
Close adherence to the recommended treatment regimen is important for the success of recombinant human growth hormone therapy, although nonadherence can be common. Ease of use and safety during use/storage are among several important factors in the design of a growth hormone injection device intended for long-term use. This study was performed to validate the usability and assess the ease of use of a new pen device (SurePal™) that has been developed to support daily administration of the recombinant human growth hormone product, Omnitrope® (somatropin). The primary objectives of the study were to assess if study participants, representing intended users of the pen in clinical practice, were able to perform an injection procedure into an injection pad effectively and safely and disassemble the pen without receiving a needlestick injury. A total of 106 participants (61 adults and 45 children/adolescents) were enrolled at two study centers (one in the US, one in Germany). Results for both primary usability tasks met the predefined acceptance criteria, with >85% of participants successfully performing each task. All of the other tasks/handling steps assessed were also successfully performed by most participants, with high success rates reflected in the high proportion of participants who classified each task as “very easy” or “easy”. After a second use of the device, 87%–97% of participants rated it as “very easy” or “easy” to use. In summary, the new pen device is safe and easy to use for both adults and children, and will help to support effective, long-term daily administration of the recombinant human growth hormone product, Omnitrope®.
Keywords: ease of use, growth hormone, Omnitrope®, SurePal™, pen device
Introduction
Recombinant human growth hormone (rhGH) is used in the treatment of several pediatric conditions characterized by poor growth, including growth hormone deficiency, Turner syndrome, short children born small for gestational age, chronic renal insufficiency, and Prader–Willi syndrome.1–9 It is also used to treat metabolic dysfunction in adult growth hormone deficiency.10 Continuing rhGH treatment in young adults treated for childhood-onset growth hormone deficiency has a beneficial effect on bone mineral density in adult life.11
In order to achieve maximum benefit, treatment with rhGH should begin as soon as possible after diagnosis, and be continued uninterrupted (usually for many years).3,4 An important factor in the success of rhGH therapy is the ability of the patient to comply with the recommended treatment regimen, and failure to comply is associated with reduced linear growth.12 Ensuring compliance can be challenging, given that subcutaneous administration of rhGH is required over the long term. Indeed, noncompliance is fairly common among children and adults receiving this therapy.12–14 Factors affecting adherence include the physical discomfort of injections, the delay before beneficial effects are noticed, and, importantly, the ease of administration for patients or carers.5,15
Many studies have assessed interventions to help improve patient compliance with prescribed medications, with inconsistent results.16 Methods for improving compliance with long-term treatment appear to be mostly complex and not very effective, and research concerning innovations to assist compliance has been identified as a high priority.16 In the setting of rhGH therapy, manufacturers have focused on improvements in injection devices as a means to improve compliance. Studies have identified reliability, ease of use, lack of pain, safety during use/storage, and the number of steps involved in the procedure to be important in the design of a growth hormone injection device intended for long-term use.17–20 Growth hormone injection devices continue to evolve, with conventional syringes and needles having been replaced by more user-friendly pen injection devices. Various types of pen devices have been developed,18,21–25 each with distinct features designed for specific patient needs.
The current study was performed to validate the usability and assess the ease of use of a new pen device (SurePal™, Kundl, Austria). This is a semi-reusable needle-based injection system (the pen itself is reusable, while the drug-containing cartridges are replaced when empty), which has been developed to support daily administration of the rhGH product, Omnitrope® (somatropin, Sandoz, Kundl, Austria).
Materials and methods
The primary objectives of the study were to assess if study participants, representing intended users of the pen in clinical practice, were able to perform an injection procedure into an injection pad effectively and safely and disassemble the pen without receiving a needlestick injury. Both tasks had to be successfully executed by >85% of participants for the usability of the pen to be validated. The required success rate for acceptance was calculated based on the limits of the confidence intervals of the defined sample size. These acceptance criteria were agreed to by the relevant body in the US (US Food and Drug Administration [FDA]) during device development and accepted by the relevant body in Europe (BSI) as part of the CE marking process.
Additional objectives of the study included assessment of: success rates for specific tasks/handling steps required to prepare the pen for use; participants’ viewpoints on the ease with which each of these tasks can be performed; and participants’ opinions on the usefulness of the written instructions for use (IFU) that accompany the pen.
Study participants
The study was conducted in two centers, one in the US and one in Germany, and recruited children (or their adult caregivers), adolescents (or their adult caregivers), and adults being treated for growth hormone deficiency. To ensure enough participants, adults with diabetes experienced in the use of pen devices were also included at the German center (six of 15 adults). Participants were subdivided into naïve users (never used an injection device before) and experienced users (patients or caregivers experienced in administering injections, either through use of another growth hormone or insulin injection device). All study participants (or their caregivers) were volunteers and provided written informed consent.
Study device
The pen evaluated in the study was a multidose, semi-reusable needle-based injection system (Figure 1). This new device contains a number of safety features, including a system to ensure that only cartridges of the correct dose can be used with each device, and an optional needle guard. There is also a dose memory function that enables the correct dose to be preset by a doctor or nurse and locked into the device, thereby reducing the risk of incorrect dosing. In addition, there are several features designed to make the device easy and convenient to use, including autopriming and a sliding injection button that reduces the force needed to perform an injection. The device is also designed to minimize drug wastage; if a cartridge in the device does not contain sufficient drug for injection of a complete dose, the device automatically administers the correct amount of additional drug once a new cartridge is inserted (without the need for priming or adjustment of the dose setting). Information on whether the complete dose has been administered is provided to the user through a dose display window; if it does not show zero, the cartridge holds less than the complete dose and a new cartridge should be inserted. The automated nature of this feature makes it more convenient to administer a second injection compared with most other rhGH injection devices, for which the user must calculate the amount of additional dose required and reset their device accordingly.
Figure 1.

The pen device.
Usability tests
Training and test sessions were conducted at both centers as follows. In the US center, 75% of participants received a 15–20-minute training session then waited for at least 2 hours before returning for their test session, while 25% received no training (this was a requirement of the FDA, and participants who were to receive no training were selected at random). In the German center, all participants received training and then immediately commenced the test session. In the test session, participants were asked to perform the tasks/handling steps outlined in Table 1. All participants were allowed to read the IFU document as a supportive measure.
Table 1.
Outline of the usability testing strategy
| Step | US center | German center |
|---|---|---|
| 1 | Introduction and background interview | |
| 2 | 75% received a 15–20-minute training session, 25% received no training | 100% received training and then immediately commenced the test session |
| 3 | Trained patients waited for at least 2 hours before commencing the test session | |
| 4 | Set dose memory | |
| 5 | Preparation of pen (insertion of cartridge) | |
| 6 | Preparation of pen (attach needle and needle hider) | |
| 7 | Injection procedure | |
| 8 | Disassembly | |
| 9 | No drug wastage system including cartridge exchange | |
| 10 | Closing interview | |
The moderator evaluated the performance of each participant by observing the participant conducting different tasks. Participants were asked to comment on the various steps and to rate the ease of performing each step as “very easy”, “easy”, “undecided”, “difficult”, or “very difficult”. Participants were also instructed to rate the helpfulness of the IFU as “very helpful”, “helpful”, “undecided”, “not helpful”, or “not at all helpful”. A note-taker was present during the test session to document all observations and answers by means of a session protocol and a laptop computer. All sessions were recorded on video. Data were summarized using descriptive statistics.
Results
Study participants
A total of 106 participants (61 adults and 45 children/adolescents) were enrolled at the two study centers. Key characteristics of the study participants are summarized in Table 2. Overall, 49% of children/adolescents and 48% of adults had previous experience with either a rhGH or insulin pen.
Table 2.
Key characteristics of study participants
| Children/adolescents (n = 45)* | |
| Gender | |
| Male | 19 (42) |
| Female | 26 (58) |
| Experienced with injections | 22 (49) |
| Received training | 42 (93) |
| Adults (n = 61)† | |
| Gender | |
| Male | 22 (36) |
| Female | 39 (64) |
| Experienced with injections | 29 (48) |
| Received training | 55 (90) |
Notes: Data are n (%).
Aged 8–16 years in Germany, 8–19 years in the US;
aged ≥18 years in Germany, ≥22 years in the US.
Primary usability tasks
Results for both primary usability tasks met the predefined acceptance criteria, with >85% of participants successfully performing each task (Figure 2). Overall, 92% of participants (92% of adults and 93% of children/adolescents) were able to perform the injection procedure into the injection pad successfully; 92% of participants (95% of adults and 89% of children/adolescents) rated this procedure as very easy or easy. The vast majority of participants (97% of adults and 91% of children/adolescents) were able to complete the task with no help at all, by referring to the IFU or with just verbal hints. The success rate was higher in the US center (98%) than in the German center (89%).
Figure 2.
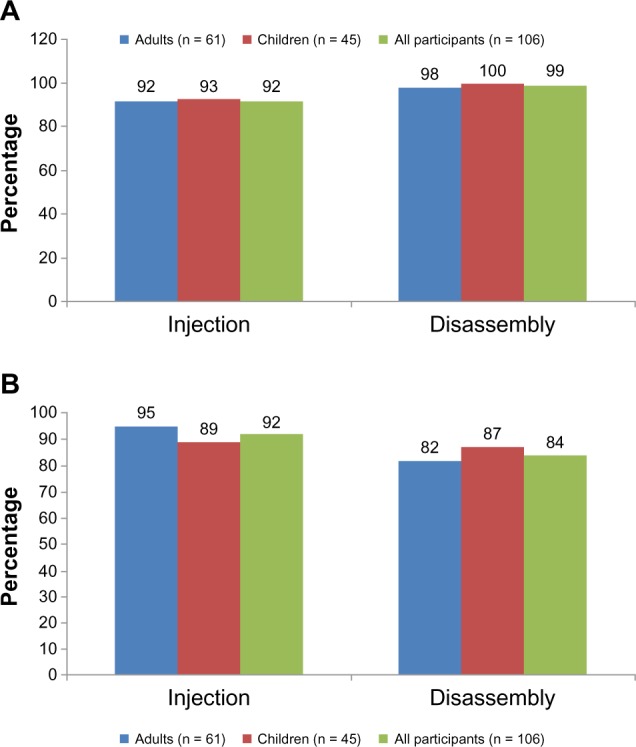
Primary usability tasks. (A) Proportion of patients who successfully performed task. (B) Proportion of patients who rated completing the task as very easy/easy.
In total, 99% of participants (98% of adults and all children/adolescents) were able to disassemble the pen successfully without receiving a needlestick injury; 84% (82% of adults and 87% of children/adolescents) rated disassembly of the pen as very easy or easy. The majority of participants (93% of adults and 89% of children/adolescents) were able to complete the task with no help at all, by referring to the IFU or with just verbal hints. Only one participant (a naïve adult user) received a needlestick injury. The success rate was 98% in the US center and 100% in the German center.
Additional study assessments
Overall, most participants found the pen device easier to use the more they used it. For example, among children/adolescents at the US center, 73% and 87% rated the first and second injection, respectively, as very easy/easy. Similarly, among the German children/adolescents, 70% rated the device as easy to use at first, rising to 97% on subsequent use.
Data for the other tasks/handling steps that were assessed during the study are summarized in Table 3. Overall, 92% of participants (89% of US participants, 93% of German participants) were able to set the dose memory (program the correct dose). The majority of adults (85%) and children/adolescents (84%) were able to complete the task with no help at all, by referring to the IFU or with just verbal hints.
Table 3.
Success rates for additional tasks/handling steps
| Task | Participants successfully completing task, n (%)
|
||
|---|---|---|---|
| Adults† (n = 61) | Children/adolescents* (n = 45) | All participants (n = 106) | |
| Set dose memory | 58 (95) | 39 (87) | 97 (92) |
| Preparation of pen (insertion of cartridge) | 60 (98) | 42 (93) | 102 (96) |
| Preparation of pen (attach needle and needle hider) | 61 (100) | 43 (96) | 104 (98) |
| Low drug-waste system including cartridge exchange | 54 (89) | 37 (82) | 91 (86) |
Notes:
Aged 8–16 years in Germany, 8–19 years in the US;
aged ≥18 years in Germany, ≥22 years in the US.
Overall, 96% of participants were able to reinsert the preassembled cartridge and attach it to the pen correctly (96% of US participants, 97% of German participants). Again, the majority of adults (97%) and children/adolescents (84%) were able to complete the task with no help at all, by referring to the IFU or with just verbal hints.
Ninety-eight percent of participants (100% of US participants, 97% of German participants) were able to attach the needle and needle hider correctly, with 93% of adults and 93% of children/adolescents able to complete the task with no help at all, by referring to the IFU or with just verbal hints; 88% of the participants rated the procedure as being very easy/easy.
Participants’ ability to manage the low drug-wastage system (including cartridge exchange) was also assessed. Overall, 86% successfully managed the system (80% of US participants, 90% of German participants); most (89% of adults and 82% of children/adolescents) were able to complete the task with no help at all, by referring to the IFU or with just verbal hints. Eighty-six per cent rated the procedure as very easy/easy.
Some differences were observed between children/adolescents and adults in the usage of the pen. Many younger participants had less difficulty with the pen than the adults, appearing to remember more from the training sessions and following instructions more carefully. Children/adolescents were less likely to try to perform a task without reading the IFU but were more likely to omit the disinfection step. Some minor differences were also observed between naïve and experienced users, with experienced users generally requiring more assistance than those with no previous experience of injection devices.
Participants at both centers generally had a good impression of the new device. In the US, 100% of the children liked the look of the device, and 90% of adults had a good impression of it. Among the German participants, 90% of children and 74% of adults had a good first impression of the device.
Discussion
This usability study met its primary objectives, in that more than 90% of participants were able to successfully execute the injection procedure and safely disassemble the pen. When analyzed separately, both the adult group and the children/adolescents met the predefined acceptance criteria (>85% success rate) for the two primary usability tasks. Despite the training and test sessions being conducted slightly differently, the success rates for the two primary usability tasks were similar in the US and German centers.
All of the other tasks/handling steps were also successfully performed by most participants. The high success rates for the usability tasks were reflected in the percentage of participants classifying each one as “very easy” or “easy”. Both naïve and experienced participants found the pen easy to use, a property associated with successful adherence to treatment.19,26–28 Experienced participants generally required more assistance than naive subjects; this may be due to the fact that experienced participants had to “unlearn” how to use their old device before learning about the new system. Participants’ perception of ease of use progressed rapidly in line with experience in use of the new device.
An important aspect of this study was the inclusion of caregivers in addition to patients, given that these groups may require/value different features in the device.29 For example, caregivers are more concerned with needlestick injuries than patients.30 Use of the pen was associated with an extremely successful disassembly rate, and only one participant (<1%) received a needlestick injury. Safety during use is an important feature of a growth hormone injection device intended for long-term use.18
While the study met its primary objectives, establishing the validity of this new pen device for the administration of rhGH, several potential limitations do exist; the injection was into a pad rather than a patient, and the study design was open-label and uncontrolled.
In conclusion, the new pen device is safe and easy to use for both adults and children, and will help to support effective, long-term daily administration of the rhGH product, Omnitrope®.
Footnotes
Disclosure
This study was funded by Sandoz Biopharmaceuticals. Organization and overall management of the study was provided by Ellen Schuck (Sandoz Biopharmaceuticals). Medical writing assistance in the preparation of this paper was provided by Tony Reardon of Spirit Medical Communications and funded by Sandoz International GmbH. RR, PS, YH, MC, SL, SM and FL have acted as consultants to Sandoz. MZ is an employee of Sandoz. WB and HS have no conflicts to declare.
References
- 1.American Society of Clinical Endocrinologists Medical guidelines for clinical practice for growth hormone use in adults and children. 2003 update. Endocr Pract. 2003;9:64–76. doi: 10.4158/EP.9.1.64. [DOI] [PubMed] [Google Scholar]
- 2.National Institute for Health and Clinical Excellence Human growth hormone (somatropin) for the treatment of growth failure in children May2010Available from: http://www.nice.org.uk/nicemedia/live/12992/48715/48715.pdf[accessed 21st August 2013]
- 3.GH Research Society Consensus guidelines for the diagnosis and treatment of growth hormone (GH) deficiency in childhood and adolescence: summary statement of the GH Research Society. J Clin Endocrinol Metab. 2000;85:3990–3993. doi: 10.1210/jcem.85.11.6984. [DOI] [PubMed] [Google Scholar]
- 4.Clayton PE, Cuneo RC, Juul A, Monson JP, Shalet SM, Tauber M. Consensus statement on the management of the GH-treated adolescent in the transition to adult care. Eur J Endocrinol. 2005;152:165–170. doi: 10.1530/eje.1.01829. [DOI] [PubMed] [Google Scholar]
- 5.Bondy CA. Care of girls and women with Turner syndrome: a guideline of the Turner Syndrome Study Group. J Clin Endocrinol Metab. 2007;92:10–25. doi: 10.1210/jc.2006-1374. [DOI] [PubMed] [Google Scholar]
- 6.Clayton PE, Cianfarani S, Czernichow P, Johansson G, Rapaport R, Rogol A. Management of the children born small for gestational age through to adulthood: a consensus statement of the International Societies of Pediatric Endocrinology and Growth Hormone Research Society. J Clin Endocrinol Metab. 2007;92:804–810. doi: 10.1210/jc.2006-2017. [DOI] [PubMed] [Google Scholar]
- 7.Mahan JD, Warady BA, Consensus Committee Assessment and treatment of short stature in pediatric patients with chronic kidney disease: a consensus statement. Pediatr Nephrol. 2006;21:917–930. doi: 10.1007/s00467-006-0020-y. [DOI] [PubMed] [Google Scholar]
- 8.Lee PDK, Allen DB, Angulo MA, et al. Consensus statement – Prader-Willi syndrome: growth hormone (GH)/insulin-like growth factor axis deficiency and GH treatment. Endocrinologist. 2000;10(Suppl 1):71–74. [Google Scholar]
- 9.Graber E, Rapaport R. Growth and growth disorders in children and adolescents. Pediatr Ann. 2012;41:e1–e9. doi: 10.3928/00904481-20120307-07. [DOI] [PubMed] [Google Scholar]
- 10.Ho KK. Consensus guidelines for the diagnosis and treatment of adults with GH deficiency II: a statement of the GH Research Society in association with the European Society for Pediatric Endocrinology Lawson Wilkins Society, European Society of Endocrinology, Japan Endocrine Society, and Endocrine Society of Australia. Eur J Endocrinol. 2007;157:695–700. doi: 10.1530/EJE-07-0631. [DOI] [PubMed] [Google Scholar]
- 11.Conway GS, Szarras-Czapnik M, Racz A, Chanson P, Tauber M, Zacharin M. Treatment for 24 months with recombinant human GH has a beneficial effect on bone mineral density in young adults with childhood-onset GH deficiency. Eur J Endocrinol. 2009;160:899–907. doi: 10.1530/EJE-08-0436. [DOI] [PubMed] [Google Scholar]
- 12.Cutfield WS, Derraik JG, Gunn AJ, et al. Non-compliance with growth hormone treatment in children is common and impairs linear growth. PLoS One. 2011;6:e16223. doi: 10.1371/journal.pone.0016223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haverkamp F, Johansson L, Dumas H, et al. Observations of nonadherence to recombinant human growth hormone therapy in clinical practice. Clin Ther. 2008;30:307–316. doi: 10.1016/j.clinthera.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 14.Rosenfeld R, Bakker B. Compliance and persistence in pediatric and adult patients receiving growth hormone therapy. Endocr Pract. 2008;14:143–154. doi: 10.4158/EP.14.2.143. [DOI] [PubMed] [Google Scholar]
- 15.Rappaport R, Mugnier E, Limoni C, et al. A 5-year prospective study of growth hormone (GH)-deficient children treated with GH before the age of 3 years. French Serono Study Group. J Clin Endocrinol Metab. 1997;82:452–456. doi: 10.1210/jcem.82.2.3756. [DOI] [PubMed] [Google Scholar]
- 16.Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;2:CD000011. doi: 10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed SF, Smith WA, Blamires C. Facilitating and understanding the family’s choice of injection device for growth hormone therapy by using conjoint analysis. Arch Dis Child. 2008;93:110–114. doi: 10.1136/adc.2006.105353. [DOI] [PubMed] [Google Scholar]
- 18.Dumas H, Panayiotopoulos P, Parker D, Pongpairochana V. Understanding and meeting the needs of those using growth hormone injection devices. BMC Endocr Disord. 2006;6:5. doi: 10.1186/1472-6823-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wickramasuriya BP, Casey A, Akhtar S, et al. Factors determining patient choice of device for GH therapy. Horm Res. 2006;65:18–22. doi: 10.1159/000090375. [DOI] [PubMed] [Google Scholar]
- 20.Bhosle M, Klingman D, Aagren M, Wisniewski T, Lee WC. Human growth hormone treatment: synthesis of literature on product delivery systems and administration practices. J Spec Pediatr Nurs. 2011;16:50–63. doi: 10.1111/j.1744-6155.2010.00267.x. [DOI] [PubMed] [Google Scholar]
- 21.Fuchs GS, Mikkelsen S, Knudsen TK, Kappekgaard AM. Ease of use and acceptability of a new pen device for the administration of growth hormone therapy in pediatric patients: an open-label, uncontrolled usability test. Clin Ther. 2009;31:2906–2914. doi: 10.1016/j.clinthera.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Hey-Hadavi J, Pleil A, Deeb LC, et al. Ease of use and preference for a new disposable self-injection pen compared with a reusable pen for administering recombinant human growth hormone: a multicenter, 2-month, single-arm, open-label clinical trial in patient-caregiver dyads. Clin Ther. 2010;32:2036–2047. doi: 10.1016/j.clinthera.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Hokken-Koelega A, Keller A, Rakov V, Kipper S, Dahlgren J. Patient acceptance, ease of use, and preference for norditropin NordiFlex with NordiFlex PenMate: results from an open-label, user survey of everyday use. ISRN Endocrinol. 2011;803948 doi: 10.5402/2011/803948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfutzner A, Hartmann K, Winter F, Fuchs GS, Kappelgaard AM, Rohrer TR. Intuitiveness, ease of use, and preference of a prefilled growth hormone injection pen: a noninterventional, randomized, open-label, crossover, comparative usability study of three delivery devices in growth hormone-treated pediatric patients. Clin Ther. 2010;32:1918–1934. doi: 10.1016/j.clinthera.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Yuen KC, Amin R. Developments in administration of growth hormone treatment: focus on Norditropin® Flexpro®. Patient Prefer Adherence. 2011;5:117–124. doi: 10.2147/PPA.S10985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapoor RR, Burke SA, Sparrow SE, et al. Monitoring of concordance in growth hormone therapy. Arch Dis Child. 2008;93:147–148. doi: 10.1136/adc.2006.114249. [DOI] [PubMed] [Google Scholar]
- 27.Smith SL, Hindmarsh PC, Brook CG. Compliance with growth hormone treatment – are they getting it? Arch Dis Child. 1993;68:91–93. doi: 10.1136/adc.68.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oyarzabal M, Aliaga M, Chueca M, Echarte G, Ulied A. Multicentre survey on compliance with growth hormone therapy: what can be improved? Acta Paediatr. 1998;87:387–391. doi: 10.1080/08035259850156959. [DOI] [PubMed] [Google Scholar]
- 29.Yakushiji F, Fujita H, Terayama Y, et al. The best insulin injection pen device for caregivers: results of injection trials using five insulin injection devices. Diabetes Technol Ther. 2010;12:143–148. doi: 10.1089/dia.2009.0110. [DOI] [PubMed] [Google Scholar]
- 30.Pellissier G, Migueres B, Tarantola A, Abiteboul D, Lolom I, Bouvet E. Risk of needlestick injuries by injection pens. J Hosp Infect. 2006;63:60–64. doi: 10.1016/j.jhin.2005.12.006. [DOI] [PubMed] [Google Scholar]


