Summary
While considerable research has addressed the function of animal vocalizations, the proximate mechanisms driving call production remain surprisingly unclear. Vocalizations may be driven by emotions and the physiological state evoked by changes in the social-ecological environment [1, 2], or animals may have more control over their vocalizations, using them in flexible ways mediated by the animal’s understanding of its surrounding social world [3, 4]. While both explanations are plausible and neither excludes the other, to date no study has attempted to experimentally investigate the influence of both emotional and cognitive factors on animal vocal usage. We aimed to disentangle the relative contribution of both mechanisms by examining howling in captive wolves. Using a separation experiment and by measuring cortisol levels, we specifically investigated whether howling is a physiological stress response to group fragmentation [5] and whether it is driven by social factors, particularly relationship quality [6, 7]. Results showed that relationship quality between the howler and the leaving individual better predicted howling than did the current physiological state. Our findings shed important light on the degree to which animal vocal production can be considered as voluntary.
Video Abstract
Highlights
-
•
We investigated the influence of social and physiological factors on wolf howling
-
•
Wolves howl more to keep contact with affiliated partners and with pack leaders
-
•
Howling is mediated by the social relationship not cortisol level of the howlers
-
•
This pattern indicates that wolves have some voluntary control of their howling
Results
Emerging data suggest that animals are capable of flexibly processing the vocalizations of conspecifics [3]. Communication, however, is an interactive process involving not just comprehension but also production, yet the cognitive mechanisms underlying call production remain ambiguous [8]. A variety of studies, particularly those on the “audience effect,” indicate that animal vocal production can be varied according to social context [3]. Vervet monkeys and meerkats, for example, alarm call less in the absence of conspecifics [9, 10]. Though these effects can be explained through social facilitation, additional research indicates more-subtle forms of socially mediated vocal behavior. Calling probability in male chickens (Gallus gallus) is higher in the presence of females [11], while Thomas Langurs (Presbytis thomasi) will continue alarm calling until all female group members have responded [12]. Such data have led to the hypothesis that senders can produce vocalizations with a certain degree of strategic control that ensures fine-tuned communication [4, 13, 14].
Despite this demonstrated vocal flexibility, lower-level explanations cannot be excluded. Many have argued that vocal production is modulated solely by the current emotional state of the signaler [1, 2]. Accounting for physiological correlates of emotions can be informative and help disentangle the underlying proximate mechanisms. In yellow-bellied marmots (Marmota flaviventris), for example, alarm call production strongly correlates with glucocorticoid production [15]. Experimental manipulations of glucocorticoid synthesis in rhesus macaques (Macaca mulatta) also reduce the probability of alarm call production [16]. Whether the same applies for contact calls produced toward social partners is unclear. Here we investigate the social flexibility of wolf howling and attempt to additionally consider the extent to which this flexibility is modulated by stress hormone levels.
Previous research has suggested that wolf howls serve as communication between temporarily separated pack mates [17] and facilitate reassembly between dispersed individuals [5, 18]. Accordingly, howls function as a long-distance contact call similarly to a number of bird and mammal species [19]. Howls can be emitted by a single wolf or simultaneously by several pack members [20] and seem to be flexibly adjusted to the social environment, since howling patterns differ with pack size or the presence or absence of the dominant individual [6]. It is unclear, however, how fine tuned howling is to the social status (dominance and affiliation) of an absent individual and what role activation of the hypothalamic-pituitary-adrenal axis (HPA) plays.
We investigated whether howling in nine wolves from two packs at the Wolf Science Center, Austria, is influenced by (1) the dominance status of a wolf separated from its pack, (2) its affiliative relationship with the separated individual, and (3) the howler’s HPA stress response. We predicted that if howling is mediated by the social environment, it should occur more in the absence of higher-ranked individuals and preferred social partners. Furthermore, if this relationship is driven by HPA activation, we predicted the amount of howling to be positively correlated with high levels of the corresponding cortisol measures.
To assess the wolves’ dominance status and preferred partners, we collected 10 min focal samples (n = 8/individual) based on which we constructed dominance hierarchies using David’s scores [21] and a standardized sociality index (SI) based on affiliative interactions [22, 23] (see the Supplemental Experimental Procedures available online). In the test condition, each wolf was removed from its pack being taken on 45 min walks (>300 m), rendering the location of the separated individual unpredictable. In the control condition, the wolf was placed in neighboring holding area, where it was visually separated but its location was known to the subjects, and thus communication was thought to be unnecessary and the situation less stressful for the remaining animals.
Each wolf was removed from its pack for three test and three matched control trials. The control trials were conducted at the same time 1 day before or after the scheduled walk and lasted for the same time period. The order of individuals taken out for the test trials was randomized. During the first 20 min of the wolf’s absence, we recorded all howls from pack mates. If an individual paused for 1 s between howls, a new howl was counted. After 20 min, we took one saliva sample from each of the remaining animals to measure their circulating cortisol levels (see the Supplemental Experimental Procedures). No special permission for use of animals (wolves) in such sociocognitive studies is required in Austria (Tierversuchsgesetz 2012, TVG 2012).
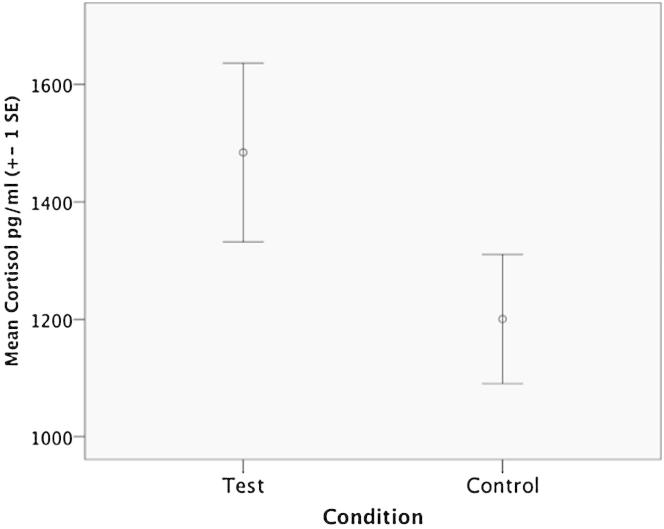
While wolves howled in 26 of 27 experimental trials and in most cases (93%) within the first 20 min after separation (before saliva sampling), they only howled during two control trials, indicating that controls were less stressful for remaining individuals and did not require communication. This contrast was also apparent in a significant difference in cortisol between control and experimental conditions (mean test cortisol = 1,484.2 pg/ml, range = 208–3,715 pg/ml, mean control cortisol = 1,200 pg/ml, range = 256–2,752 pg/ml; likelihood ratio [LR] test, χ2 = 4.3, df = 1, p = 0.039; Figure 1; see the Supplemental Experimental Procedures), suggesting that individuals being taken away was a more agitating event for remaining individuals.
Figure 1.
Cortisol Level in Test and Control Condition
Error bar plot displaying the mean (±1 SE) cortisol level in wolf saliva during control and test experimental conditions.
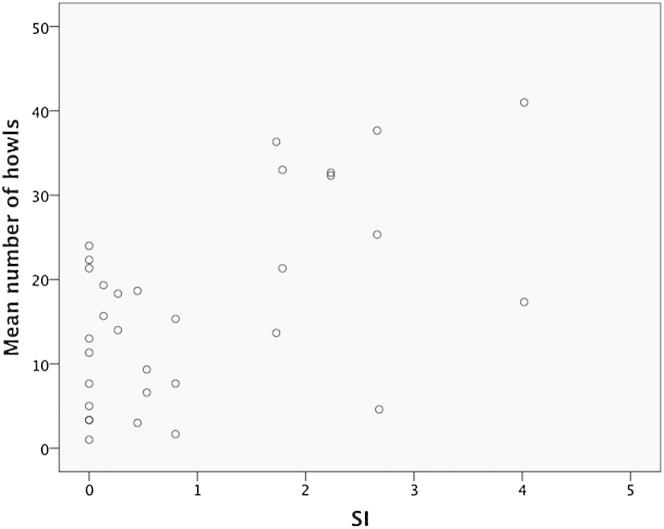
We were specifically interested whether the social status of the removed animal influenced the howling behavior of the remaining pack members. We found that the mean number of howls emitted by the pack mates was significantly affected by the dominance rank of the leaving individual (mean number of howls: LR test, χ2 = 16.1, df = 5, p = 0.006), with more howls being produced when higher-ranked individuals left (see Table 1 and the Supplemental Experimental Procedures), as well as by the number of affiliative interactions between the howler and the removed individual (LR test, χ2 = 10.1, df = 1, p = 0.001; Figure 2; see also Table 2). This effect of affiliation (SI) remained stable when a subset of howls was analyzed disregarding all calls given in response to another howling individual [e.g., all calls given as immediate responses toward howls of the others were omitted (<3 s, based on [24]); mean number spontaneous howls: SI, χ2 = 15.3, df = 1, p < 0.001]. This distinction importantly verifies that an excitement contagion did not drive the observed howling pattern. Interestingly, however, the effect of the rank of the leaving individual on spontaneous howling did not improve the fit of the original full model (model AICc including rank of leaving individual = 99.8 and excluding rank of leaving individual = 87.9; see Table 2), and hence it was subsequently dropped from further likelihood ratio tests, indicating that spontaneous calls were driven more by the affiliative relationship with the leaving individual. An additional analysis accounting for potential affiliative asymmetry between dyads complemented the reported effects detected when the sociality index was employed as an affiliation measure (see the Supplemental Experimental Procedures).
Table 1.
Howls Produced in Relation to Rank
| Rank | Mean number of Howls | Range |
|---|---|---|
| A | 21.3 | 1–41 |
| B | 19.9 | 5–37.6 |
| C | 11.1 | 1.6–18.6 |
| D | 18 | 4.6–24 |
| a | 13.9 | 3–25.3 |
| b | 10.8 | 6.6–18.3 |
Mean number of howls (+range) produced to the different rank categories. Capital letters represent males, and lower case letters represent females.
Figure 2.
The Link between the Dyadic Sociality Index and Number of Howls
Relationship between dyadic sociality index (SI) and number of howls produced by wolves remaining in the pack. See also Figure S2 for power analysis.
Table 2.
Statistical Results for Model Selection
| Howling | Chi Square | p |
| Comparison | ||
| Final model versus null model | 25.9 | <0.001 |
| Spontaneous Howling | Chi Square | p |
| Comparison | ||
| Final model versus null model | 15.3 | <0.001 |
| Howling | AICc | AICc II |
| Full model | 224.3 | 209.9 |
| Cortisol difference | 209.9 | |
| SI | 229.7 | 214.6 |
| Rank out | 238.9 | 225.3 |
| Spontaneous Howling | AICc | AICc II |
| Full model | 115.4 | 99.8 |
| Cortisol difference | 99.8 | |
| SI | 122.1 | 103.5 |
| Rank out | 102 | 87.9 |
Likelihood ratio tests comparing final reduced and null models for response variable: number of howls and spontaneous howls and AICc values for model selection with response variable: number of howls and spontaneous howling. Akaike’s Information Criterion corrected for small samples (AICc) can be used to select the best fitting, most parsimonious model when investigating the influence of multiple fixed explanatory factors. Values represent the AICcs of the model when the specific predictor variable has been omitted. AICcs II represent the refitting of the model when excluding the predictor variable with the lowest AICc values. See also Tables S1–S3.
We also verified that dominance rank did not indirectly drive the observed relationship between positive interactions (SI) and number of howls (LR test, χ2 = 1.24 df = 5, p = 0.9).
The strong dependency of the howling frequencies on the dominance rank and affiliative interactions might suggest that the stress of separation from a leader or a preferred partner (not necessarily kin in our sample) might stimulate howling. While test and control cortisol levels and howling behavior suggest a relationship between physiology and howling, within the test condition we found no effect of cortisol levels on the number of howls or spontaneous vocalizations produced by remaining wolves. Specifically, we found that cortisol difference (test cortisol level minus matched control) did not improve the explanatory power of the full model (all howls: AICc of full model, 224.3, and AICc of reduced model excluding cortisol difference, 209.9; spontaneous vocalizations: AICc of full model, 115.4, and AICc of reduced model excluding cortisol difference, 99.8; see Table 2 for AICc values from sequential model selection procedure and Tables S1 and S2 for model estimates, standard errors, and confidence intervals). Consequently, in both instances, cortisol difference dropped out of the final minimum adequate model. Cortisol levels were also unaffected by the affiliative relationship between wolves (LR test, χ2 = 2.1, p = 0.14; see the Supplemental Experimental Procedures), though the rank of the individual leaving did seem to have a borderline significant effect (LR test, χ2 = 10.8 df = 5, p = 0.05; see the Supplemental Experimental Procedures). A power analysis confirmed that this lack of effect was not merely a side effect of insufficient statistical power (Figure S1).
Discussion
By investigating the influence of physiological correlates of stress (cortisol) and measures of social “value” (rank/preferred partner), we have demonstrated that howling in wolves is not necessarily a byproduct of increased stress hormone level, but instead may be under flexible control of the signaler and used selectively to facilitate reassembly with important individuals.
Previous studies demonstrating the influence of social context on calling probability indicate vocal production in animals may not be totally hardwired and inflexible [1]. While cognitive hypotheses explaining flexibility in animal vocalizations are attractive in providing potential insights into the similarities and differences between human and animal communication, without measurement of physiological correlates of emotions, such as stress, lower level explanations cannot be ruled out. However, our data demonstrate that vocal production in animals may not necessarily be a simple emotional response to changes in the environment, but at least in some situations can be additionally controlled in potentially socially beneficial ways.
In wolf packs, the dominant pair assumes a leading role in terms of decision making, including awakening the pack and initiating social activities, foraging, and travel [25]. Given this central role of dominants, increased howling in their absence may reflect an attempt to maintain contact with these social figures. However, when only spontaneous howls are considered, this effect no longer persisted, indicating that individuals starting a chorus might be influenced more by the relationship with the leaving animal rather than its rank.
When investigating how rank of the removed individual influenced cortisol levels of howlers, we did detect a borderline significant result, suggesting that increased howling may have been mediated by higher stress caused by the absence of the leading animals. This was not entirely unexpected, given that stable social factors such as dominance rank are themselves underwritten by clear physiological markers [26, 27], and hence it is possible that sensitivity to such factors and subsequent variation in behavior may also be mediated by similar physiology.
Social partner preference, however, is a more dynamic and flexible feature of wolf life and thus is more likely to be modulated by cognition. Congruent with this, we detected no significant change in cortisol when preferred social partners were removed from the pack, which, however, led to increased howling. This provides strong support for the hypothesis that wolf howling is potentially a strategically employed vocalization with the goal of ultimately promoting contact with important individuals.
These results are in line with recent findings in other species linking social affiliation and variation in vocal behavior that have already begun to challenge more simplistic explanations for animal calling behavior. Food calling in chimpanzees, for example, has long been considered a purely emotional response to food [28]. However, detailed behavioral observations indicate that chimpanzees do not always food call and male chimpanzees are more likely to produce food calls when close social partners are present in the audience [7]. While promising, in this example, it was not possible to probe the chimpanzee’s underlying emotional state and how this may have impacted food call production. We hope that our approach will encourage further work into the additional influence of physiology underlying animal vocal production (whether cortisol, testosterone, or oxytocin, though see [29]). For instance, recent studies have demonstrated a strong effect of social context on the sympathoadrenergic activity [30, 31], which might mediate context-dependent social behavior and, within this, vocal production. Such an approach is particularly crucial when attempting to assess the influence of various social and ecological factors on vocal behavior and will help disentangle the relative contributions of the underlying cognitive and emotional mechanisms on calling behavior in animals.
Experimental Procedures
Subjects
All nine timber wolves that participated in the study were born in captivity, in five different litters. They were human raised in peer groups and introduced into the pack of older unrelated animals at the age of 5 months.
Observations and Experimental Procedures
Details of the training, observations, and the experimental procedures are given in the Supplemental Experimental Procedures.
Collection and Analyses of Saliva Samples
Twenty minutes after the respective wolf was taken out for a walk, saliva samples were collected from all of the remaining animals. This sampling time was chosen on the basis of a study that indicates that blood-cortisol levels peak 20 min after a dog encounters a stressor [32]. Salivary cortisol metabolite concentrations were measured using an enzyme immunoassay developed by [33], which was validated for dogs [34].
Statistics
Linear mixed effects models were used to investigate the influence of explanatory variables (e.g., difference in cortisol) on response variables (e.g., mean number of howls). Because we had repeated sampling from the same individual as the subject and the removed individual, we fitted them both as random factors [35] using the package lme4 [36].
Acknowledgments
The Wolf Science Center was established by Zsófia Virányi, Kurt Kotrschal, and Friederike Range, and we thank all of our helpers who made this possible and thus indirectly supported this research. We thank Erich Möstl for providing the enzyme immunoassay to analyze the saliva samples and him, Kurt Kotrschal, and Elisabeth Pschernig for troubleshooting during that process. Furthermore, we thank Kurt Kotrschal, Ludwig Huber, Marta Manser, Robert Seyfarth, and three reviewers for valuable and critical comments on a previous version of this manuscript and Andri Manser and Yannick Auclair for statistical advice. We thank Paola Valsecchi for supervising F.M. and for supporting the project. The project was financially supported by Austrian Science Fund (FWF) projects P21244-B17 to F.R. and Z.V. Writing was supported by funding from the European Research Council under the European Union's Seventh Framework Programme (FP/2007-2013)/ERC Grant Agreement no. 311870 to F.R. and by the WWTF project CS11-026 to Z.V. S.W.T. was funded by the University of Zurich.
Published: August 22, 2013
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supplemental Information includes Supplemental Results, Supplemental Experimental Procedures, one figure, and four tables and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2013.06.066.
Supplemental Information
References
- 1.Morton E.S. On the occurrence and significance of motivation-structural rules in some bird and mammal sounds. Am. Nat. 1977;11:855–869. [Google Scholar]
- 2.Tomasello M. MIT Press; Cambridge: 2008. Origins of Human Communication. [Google Scholar]
- 3.Seyfarth R.M., Cheney D.L. Production, usage, and comprehension in animal vocalizations. Brain Lang. 2010;115:92–100. doi: 10.1016/j.bandl.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Slocombe K.E., Zuberbühler K. Chimpanzees modify recruitment screams as a function of audience composition. Proc. Natl. Acad. Sci. USA. 2007;104:17228–17233. doi: 10.1073/pnas.0706741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrington F.H., Asa C.S. Wolf communication: behavior, ecology, and conservation. In: Mech L.D., Boitani L., editors. Wolves: Behavior, Ecology and Conservation. University of Chicago Press; Chicago: 2003. pp. 96–99. [Google Scholar]
- 6.Harrington F.H., Mech L.D. Wolf howling and its role in territory maintenance. Behaviour. 1979;68:207–249. [Google Scholar]
- 7.Slocombe K.E., Kaller T., Turman L., Townsend S.W., Papworth S., Squibbs P., Zuberbühler K. Production of food-associated calls in wild chimpanzees is dependent on the composition of the audience. Behav. Ecol. Sociobiol. 2010;64:1959–1966. [Google Scholar]
- 8.Wheeler B.C., Fischer J. Functionally referential signals: a promising paradigm whose time has passed. Evol. Anthropol. 2012;21:195–205. doi: 10.1002/evan.21319. [DOI] [PubMed] [Google Scholar]
- 9.Cheney D.L., Seyfarth R.M. Vervet monkey alarm calls - manipulation through shared information. Behaviour. 1985;94:150–166. [Google Scholar]
- 10.Townsend S.W., Rasmussen M., Clutton-Brock T., Manser M.B. Flexible alarm calling in meerkats: the role of the social environment and predation urgency. Behav. Ecol. 2012;23:1360–1364. [Google Scholar]
- 11.Marler P.M., Dufty A., Pickert R. Vocal communication in the domestic chicken: II. Is a sender sensitive to the presence and nature of a receiver? Anim. Behav. 1986;34:194–198. [Google Scholar]
- 12.Wich S.A., de Vries H. Male monkeys remember which group members have given alarm calls. Proc. Biol. Sci. 2006;273:735–740. doi: 10.1098/rspb.2005.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crockford C., Wittig R.M., Mundry R., Zuberbühler K. Wild chimpanzees inform ignorant group members of danger. Curr. Biol. 2012;22:142–146. doi: 10.1016/j.cub.2011.11.053. [DOI] [PubMed] [Google Scholar]
- 14.Zuberbühler K. Audience effects. Curr. Biol. 2008;18:R189–R190. doi: 10.1016/j.cub.2007.12.041. [DOI] [PubMed] [Google Scholar]
- 15.Blumstein D.T., Patton M.L., Saltzman W. Faecal glucocorticoid metabolites and alarm calling in free-living yellow-bellied marmots. Biol. Lett. 2006;2:29–32. doi: 10.1098/rsbl.2005.0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bercovitch F.B., Hauser M.D., Jones J.H. The endocrine stress response and alarm vocalizations in rhesus macaques. Anim. Behav. 1995;49:1703–1706. [Google Scholar]
- 17.Nowak S., Jędrzejewski W., Schmidt K., Theuerkauf J., Myslajek R.W., Jędrzejewska B. Howling activity of free-ranging wolves (Canis lupus) in the Białowieza Primeval Forest and the Western Beskidy Mountains (Poland) J. Ethol. 2007;25:231–237. [Google Scholar]
- 18.Tooze Z.J., Harrington F.H., Fentress J.C. Individually distinct vocalizations in timber wolves, Canis lupus. Anim. Behav. 1990;40:723–730. [Google Scholar]
- 19.Bradbury J., Vehrencamp S. Second Edition. Cambridge University Press; Cambridge: 2011. Principles of Animal Communication. [Google Scholar]
- 20.Joslin P. Movements and home sites of Timber wolves in Algonquin Park. Am. Zool. 1967;7:279–288. [Google Scholar]
- 21.Gammel M.P., de Vries H., Jennings D.J., Carlin C.M., Hayden T.J. David’s score: a more appropriate dominance ranking method than Clutton-Brock et al.’s index. Animal Behavior. 2003;66:601–605. [Google Scholar]
- 22.Silk J.B., Beehner J.C., Bergman T.J., Crockford C., Engh A.L., Moscovice L.R., Wittig R.M., Seyfarth R.M., Cheney D.L. Strong and consistent social bonds enhance the longevity of female baboons. Curr. Biol. 2010;20:1359–1361. doi: 10.1016/j.cub.2010.05.067. [DOI] [PubMed] [Google Scholar]
- 23.McFarland R., Majolo B. Grooming coercion and the post-conflict trading of social services in wild Barbary macaques. PLoS ONE. 2011;6:e26893. doi: 10.1371/journal.pone.0026893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrington F. Chorus howling by wolves: acoustic structure, pack size and the beau geste effect. Bioacoustics. 1989;2:117–136. [Google Scholar]
- 25.Mech L.D. Leadership in wolf, Canis lupus, packs. Canadian Field-Naturalist. 2000;114:259–263. [Google Scholar]
- 26.Creel S. Social dominance and stress hormones. Trends Ecol. Evol. 2001;16:491–497. [Google Scholar]
- 27.Creel S.F. Dominance, aggression, and glucocorticoid levels in social carnivores. J. Mammal. 2005;86:255–264. [Google Scholar]
- 28.Goodall J. Harvard University Press; Cambridge: 1986. The Chimpanzees of Gombe: Patterns of Behavior. [Google Scholar]
- 29.Pfefferle D., Brauch K., Heistermann M., Hodges J.K., Fischer J. Female Barbary macaque (Macaca sylvanus) copulation calls do not reveal the fertile phase but influence mating outcome. Proc. Biol. Sci. 2008;275:571–578. doi: 10.1098/rspb.2007.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wascher C.A.F., Scheiber I.B.R., Kotrschal K. Heart rate modulation in bystanding geese watching social and non-social events. Proc. Biol. Sci. 2008;275:1653–1659. doi: 10.1098/rspb.2008.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wascher C.A.F., Scheiber I.B.R., Weiß B.M., Kotrschal K. Heart rate responses to agonistic interactions in greylag geese, Anser anser. Anim. Behav. 2009;77:955–961. [Google Scholar]
- 32.Hennessy M.B., Williams M.T., Miller D.D., Douglas C.W., Voith V.L. Influence of male and female petters on plasma cortisol and behaviour: Can human interaction reduce the stress of dogs in a public animal shelter? Appl. Anim. Behav. Sci. 1998;61:63–77. [Google Scholar]
- 33.Palme R., Möstl E. Measurement of cortisol metabolites in faeces of sheep as a parameter of cortisol concentration in blood. International Journal of Mammal Biology. 1997;62:192–197. [Google Scholar]
- 34.Patzl M. Veterinärmedizinische Universität Wien; Vienna: 1990. Entwicklung eines Biotin-Streptavidin-Enzymimmunoassays zur Bestimmung von Cortisol in Blut und Speichel von Hunden. [Google Scholar]
- 35.Crawley M.J. Wiley; Chichester: 2002. Statistical Computing: An Introduction to Data Analysis using S-Plus. [Google Scholar]
- 36.Bates, D., and Maechler, M. (2009). me4: linear mixed-effects models using S4 classes. Version 0.999375–31.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.