Abstract
Colorectal cancers develop via two major pathways that Include chromosomal instability and microsatellite Instability. Microsatellite Instability occurs due to deficient DNA mismatch repair (MMR), which can be caused by epigenetic silencing of the MLH1 MMR gene in sporadic colorectal cancers or germline mutations in MMR genes that result in Lynch syndrome. While the molecular origin of deficient MMR differs, sporadic and Lynch syndrome tumors share similar pathological features and have a more favorable stage-adjusted prognosis compared with MMR-proficient cases. While controversy remains, there is evidence to suggest that deficient MMR may predict a lack of benefit from 5-fluorouracil-based adjuvant chemotherapy. The focus of this article is on the MMR phenotype and its prognostic and predictive Implications for the management of patients with colorectal cancer.
Keywords: adjuvant therapy, colorectal cancer, DNA mismatch repair, Lynch syndrome, microsatellite Instability
DNA mismatch repair pathway
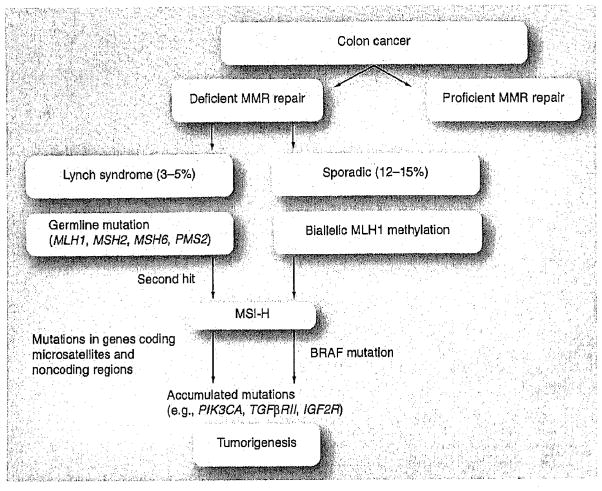
Colorectal cancer (CRC) is the fourth most prevalent cancer and is second only to lung cancer as a cause of cancer-related mortality in the USA [101]. CRC is among the best understood malignancies at the molecular level, yet molecular markers have only recently been shown to impact patient management. The majority of CRCs show chromosomal instability (CIN), leading to aneuploidy, oncogene activation and loss of tumor suppressor genes [1], While the majority of CRCs show CIN, approximately 15% of cancers develop via an alternative pathway of tumorigenesis, which is due to defective function of the DNA mismatch repair (MMR) system [2]. These tumors demonstrate high-frequency microsatellite instability, termed MSI-H, which occurs owing to an inability to repair single-nucleotide DNA mismatches, resulting in inactivating mutations in multiple genes, including TGFβRII, IGFIIR, BAX and others that have coding microsatellite sequences that become frame shifted [3,4]. MSI-H is a hallmark of CRCs with deficient MMR and patients can be subdivided into two molecularly distinct subgroups. These include Lynch syndrome (also referred to as hereditary nonpolyposis CRC), which is characterized by germline mutations in MMR genes (hMLH1, hMSH2, hMSH6, and PMS2), and the more common sporadic CRCs where MMR deficiency is due to hypermethylation of the hMLH1 gene promoter (Figure 1) [3]. Epigenetic inactivation of MLH1 is frequently found in association with a specific pathway of intense DNA hypermethylation in colon cancer known as the CpG-island methylator phenotype (CIMP) [4]. Both CIMP and activating mutations in the BRAF gene (V600E) are strongly correlated with MSI-H owing to methylation of MLH1, which characterizes sporadic cancers but not Lynch syndrome cases (Figure 1). Until recently, MLH1 was the only MMR gene shown to be epigenetically silenced. However, somatic hypermethylation of the MSH2 gene in Lynch syndrome cases was recently reported [5], suggesting a ‘second hit’ to the initial MSH2 germline mutation during tumorigenesis [5]. Despite their different molecular origins, both Lynch syndrome and sporadic MSI-H colon cancers share certain clinical and pathological features, which include proximal tumor site, frequent poor differentiation, diploid DNA content and increased numbers of tumor-infiltrating lymphocytes [6–10]. These features are commonly found in MSI-H cancers but are not exclusive to them. Compared with Lynch syndrome cases, sporadic MSI-H cancers demonstrate older age at diagnosis, a predilection for female gender and an association with cigarette smoking [11]. Evidence indicates that the sessile serrated adenoma may be a precursor lesion for sporadic MSI-H colon cancers [12–15]. The most compelling data linking sessile serrated adenoma to sporadic MSI-H colon cancers are common molecular features that include a high rate of activating mutations in the BRAF gene and CIMP-related silencing of MLH1 [13,14].
Figure 1. Mismatch repair-deficient pathway of colorectal tumorigenesis.
MMR: Mismatch repair; MSI-H: High-frequency microsatellite instability.
Identification of colon cancers with deficient MMR in clinical practice
Given the evidence that MSI-H colon cancers have a favorable prognosis and may require different treatment, as discussed in later sections, it is important to identify these tumors in clinical practice. Recognizing MSI-H CRCs requires familiarity with the distinctive clinicopathological features of these tumors. Specifically, clinicians should be alerted to the potential for MSI-H when a poorly differentiated cancer of the proximal colon is diagnosed. In contrast to sporadics, the Amsterdam criteria and the Bethesda guidelines were developed to identify Lynch syndrome patients in clinical practice, and the revised Bethesda criteria aid in the selection of patients with colon cancer for MSI testing. MSI testing is performed on paraffin-embedded tumor tissue using a PCR-based assay for the detection of instability at selected microsatellite loci [6,16]. The use of a reference panel consisting of five mono- and di-nucleotide microsatellite markers was recommended by a National Cancer Institute (NCI) consensus conference [17]. Based upon the number of unstable microsatellite markers, tumors can be grouped into MSI-H (more than two out of five demonstrating instability), MSI-L (low frequency microsatellite instability; one out of five showing instability) or microsatellite stable (MSS) cases (no unstable markers). While MSI testing requires a molecular laboratory, analysis of MMR protein expression by immunohistochemistry (IHC) is an alternative test that is widely available. IHC identifies the loss of the protein product of the affected MMR gene and results are highly concordant with MSI testing [16]. CRCs demonstrating MSI-H or loss of a MMR protein can be collectively referred to as MMR deficient (dMMR), whereas cancers that are MSS/MSI-L or have intact MMR protein expression are MMR proficient (pMMR) and arise via the CIN pathway [1]. Accordingly, the term dMMR can be used interchangeably with MSI-H. MSI-L and MSS cases are generally grouped together as they have similar clinical features and outcomes [17–22]. Since the loss of MLH1 protein expression can be due to methylation or a germline event, IHC testing should be supplemented with MLH1 promoter methylation analysis and/or somatic BRAF (V600E) mutation testing to distinguish sporadic MSI-H CRCs from Lynch syndrome cases [23]. Detection of a BRAF V600E ‘hot-spot’ mutations effectively excludes Lynch syndrome as a cause of dMMR [23]. Loss of MSH2, MSH6 or PMS2 should always raise suspicion for a germline mutation indicating Lynch syndrome. It is critical that patients with suspected hereditary colon cancer be referred for genetic counseling to discuss further evaluation that includes gene sequencing to identify germline mutations as well as the evaluation/screening of family members.
Analysis of all newly diagnosed colon cancers for MMR status has been advocated by some experts and is ongoing at selected institutions. Given the approximate 15% frequency of dMMR, this approach is labor intensive and not cost–effective, yet it can identify previously unrecognized cases of Lynch syndrome in addition to sporadic cases. Predictive models exist for identifying Lynch syndrome cases [24–26]; however, no accepted models are currently available to detect sporadic dMMR cases. The association of MMR status with routine clinicopathological variables was studied in 954 stage II and III colon cancers from completed adjuvant therapy trials. A predictive model showed a low positive predictive value in distal colon cancers, suggesting that screening for MMR should perhaps be limited to proximal tumors [27].
Prognostic impact of MMR
When MSI was first discovered in CRCs in the early 1990s, it was noted that patients with MSI-H tumors had better survival rates compared with those with MSI-L and MSS tumors [6]. MSI-H was also found to be associated with lower tumor stage at diagnosis [28] and was rare in metastatic CRCs [29,30]. An abundant amount of evidence has since accumulated demonstrating the more favorable stage-adjusted survival of colon cancers with dMMR compared with pMMR tumors. These data are largely from retrospective studies and include Phase III clinical trials of 5-fluorouracil (5-FU)-based adjuvant therapy [18,19,31–33] and a population-based study [8]. In a meta-analysis that included 32 studies stratifying survival in CRC patients by MSI status, there were 1277 dMMR CRCs and a 35% reduction in the risk of death was found for patients with dMMR versus pMMR tumors [34]. The overall survival benefit for dMMR cases was maintained when the analysis was restricted to participants in 5-FU-based adjuvant trials (hazard ratio [HR]: 0.69; 95% CI: 0.56–0.85) [34]. However, not all studies demonstrate an association between MMR status and patient survivals [35,36]. In a retrospective analysis of patients treated with 5-FU-based therapy in Phase III adjuvant studies conducted by the National Surgery Breast and Bowel Project (NSABP), no survival differences were found for patients with dMMR versus pMMR colon cancers [35]. A potential factor that may contribute to the discrepant results could be an insufficient number of dMMR tumors since they represent a relatively small subset. Furthermore, tissue availability in retrospective studies is usually incomplete and results in a nonrandom subset of the overall study population, with the potential for selection bias, Another issue is the variability in microsatellite markers used to detect MSI-H cases that may produce false-positive results, which can dilute an already modest prognostic impact [17]. In an effort to validate the prognostic (and predictive) impact of MMR status, data were pooled from stage II and III (lymph node-positive) colon cancer patients participating in the North American and European adjuvant therapy trials [37]. When restricting the analysis to patients not receiving chemotherapy (n = 515), patients with dMMR tumors demonstrated a 49% improvement in disease-free survival (DFS) compared with pMMR cases [37]. The prognostic impact of MMR status was also validated in stage II colon cancer patients (n = 1490) treated in a randomized adjuvant study known as Quick and Simple and Reliable (QUASAR) [38]. In this study, dMMR (13% of patients) was independently associated with better survival (HR: 0.31; 95% CI: 0.15–0.63; p < 0.001) in a multivariate analysis [39]. More recently, data from the Pan European Trial Adjuvant Colon Cancer (PETACC)-3 adjuvant trial demonstrated a significantly improved 5-year relapse-free survival for MSI-H (83%) versus MSS (66%) stage II and III colon cancer patients treated with 5-FU and leucovorin (LV; p = 0.0077) [40]. The survival benefit for MSI-H cases was observed to be greater in stage II than III patients [40]. Since all patients in this study received chemotherapy, the predictive impact of MMR status could not be determined. While the mechanism underlying the better prognosis of dMMR colon cancers is incompletely understood, evidence suggests that the enhanced host-mediated antitumor immune response observed in these tumors may contribute to their more indolent clinical behavior [41–43].
Predictive impact of MMR
Evidence indicates that the MMR status of CRCs may predict the outcome of adjuvant chemotherapy. Whereas a majority of studies demonstrate that patients with dMMR colon cancers do not derive benefit from 5-FU-based adjuvant chemotherapy, those with pMMR tumors receive a significant survival benefit in favor of treatment [33,44–46], Ribic et al. reported the first large, retrospective study demonstrating that dMMR is a predictor of nonresponse to 5-FU in contrast to pMMR in patients with stage II and III colon cancers treated in adjuvant therapy trials [33]. Subsequent retrospective [37,44,46] and prospective [47] studies have since demonstrated consistent results for dMMR as a predictor of nonresponse to 5-FU. Prospective follow-up of patients receiving 5-FU-based adjuvant chemotherapy indicated that the survival benefit of 5-FU treatment was again limited to pMMR tumors [47]. However, conflicting data exist in that some retrospective studies have failed to demonstrate a predictive impact of MMR in randomized 5-FU-based adjuvant trials [35,36], and some earlier reports [48,49] suggested that patients with dMMR colon cancers may receive a greater benefit from 5-FU-based treatment compared with pMMR cases. A meta-analysis that included 454 (14%) stage II and III colon cancers with dMMR from seven studies found that dMMR is predictor of nonresponse to 5-FU compared with pMMR [50]. This result is concordant with an earlier meta-analysis reporting a similar lack of benefit in treated versus untreated dMMR colon cancers (HR: 1.24; 95% CI: 0.72–2.14), although this conclusion was not statistically significant given a modest sample size [34]. It is important to note that preclinical studies using human CRC cell lines demonstrate that 5-FU will selectively kill cells with pMMR compared with cells with dMMR [51]. Furthermore, resistance to 5-FU was overcome by restoring normal MMR function, including demethylating the MLH1 gene [52–54]. By contrast, dMMR colon cancer cells were found to be sensitive to irinotecan [55–57] and oxaliplatin [58]. In an effort to validate the predictive impact of MMR status, Sargent et al. pooled data from colon cancer patients participating in 5-FU-based adjuvant studies, all with untreated control arms, conducted in North American and Europe [37]. This study demonstrated no DFS benefit (HR: 1.39; 95% CI: 0.46–4.15; p = 0.56) from 5-FU-based treatment in dMMR stage II or III tumors compared with untreated control patients [371. Therefore, the overall consensus has been that dMMR is a predictor of nonresponse to 5-FU in colon cancers.
Recent studies have analyzed the predictive impact of MMR status for modern 5-FU-based combination chemotherapy regimens that include irinotecan plus 5-FU and LV. In the CALGB 89803 trial, patients with dMMR stage III colon cancers showed improved 5-year DFS (HR: 0.76; 95% CI: 0.64–0.88 vs HR: 0.57; 95% CI: 0.42–0.71; p = 0.07) when treated with irinotecan plus 5-FU and LV versus those receiving 5-FU/LV. This effect was not observed in pMMR tumors [59]. However, data from the PETACC-3 adjuvant trial failed to show any survival benefit for the addition of irinotecan to 5-FU/LV compared with 5-FU/LV alone in dMMR stage II and III colon cancer patients [40]. Although these studies are entirely contradictory for the predictive impact of MMR status, both demonstrated that the addition of irinotecan does not improve overall survival compared with 5-FU/LV and thus does not have a role in the adjuvant treatment of stage III colon cancer patients despite being an active agent in metastatic disease. While dMMR has been shown to confer resistance to cisplatin, oxaliplatin was effective in dMMR preclinical models as it is differentially recognized by the DNA MMR system [58,60]. To date, very limited data are available concerning MMR status as a predictor of the standard 5-FU plus oxaliplatin (FOLFOX) adjuvant regimen for stage III colon cancer patients [61–64].
Recommendations for use of MMR in clinical decision making
While the use of adjuvant chemotherapy in patients with curatively resected stage II colon cancer is not the standard-of-care and remains controversial, it is estimated that a third of all stage II patients receive adjuvant therapy in the USA. Since only a subset of patients is likely to receive any benefit, there remains a need for molecular markers for risk stratification and to guide adjuvant treatment decisions. Based upon convincing and consistent data from multiple studies, dMMR is a favorable prognostic marker in CRC patients, and a preponderance of evidence indicates that 5-FU is ineffective in dMMR colon cancers. Accordingly, we recommend that patients with dMMR stage II colon cancers not receive adjuvant chemotherapy. This recommendation will spare patients with stage II dMMR tumors from potential treatment-related toxicities and reduced quality of life during chemotherapy where no benefit is anticipated. While dMMR is associated with a favorable prognosis, pMMR alone does not designate a high-risk stage II tumor, nor does it alone provide a rationale for adjuvant chemotherapy. In an ongoing, prospective adjuvant study (Eastern Cooperative Oncology Group [ECOG] 5202) in stage II colon cancer patients, MMR status and chromosome 18q allelic imbalance are used to randomize patients into low-risk (dMMR) and high-risk (pMMR, 18q loss) groups. Since low-risk patients receive observation, this trial will provide prognostic data but not predictive data for MMR and FOLFOX. In stage III colon cancer, insufficient data exist regarding the predictive impact of MMR status for FOLFOX therapy. Until such data are available, the use of MMR status cannot be recommended to inform adjuvant treatment decisions in stage III CRC patients. Therefore, all stage III patients should be treated using the current standard-of-care irrespective of MMR status. Given the available data indicating that stage III colon cancers with dMMR do not benefit from 5-FU alone, neither this drug nor capecitabine are recommended as monotherapy. Finally, most studies have not assessed whether or not the prognostic or predictive impacts of dMMR differ among patients with CRCs due to Lynch syndrome versus sporadic cases due to hypermethylation of the MLH1 gene.
Conclusion
The majority of CRCs demonstrating dMMR are sporadic and develop via a pathway of tumorigenesis that is due to acquired methylation of the MLH1 gene. These tumors are phenotypically similar to Lynch syndrome cases, yet have distinct epidemiological features that include older age at onset and a predilection for female gender. The identification of sporadic dMMR colon cancer patients in clinical practice remains challenging and strategies to improve detection are clearly needed. MMR status in colon cancers has been shown to provide valuable prognostic information and may also predict the outcome of 5-FU-based chemotherapy. In this regard, recent data serve to validate both the prognostic and predictive impact of MMR status in colon cancers for 5-FU-based adjuvant therapy. However, the predictive impacts of MMR status for the standard FOLFOX regimen is unknown and awaits further evaluation. Accordingly, the use of MMR status in decision making regarding adjuvant chemotherapy in stage III colon cancer patients is not recommended at this time. However, MMR status can inform the management of stage II colon cancer patients. Given the favorable prognosis of dMMR stage II colon cancers and the lack of benefit from 5-FU, such patients should not receive adjuvant chemotherapy. In stage II disease, MMR status informs us of whom not to treat.
Future perspective
An increased recognition of colon cancers with dMMR is expected in the near future as strategies to screen tumors are employed and as clinicians gain familiarity with the MMR phenotype. This will enable greater utilization of MMR data for prognostication and clinical decision making. Studies to compare the clinical outcome of colon cancer patients with deficient versus proficient MMR treated with adjuvant FOLFOX are eagerly awaited. However, the ability to distinguish a prognostic versus a predictive effect of dMMR will be limited since all stage III patients should receive treatment with FOLFOX. A goal of future research is to elucidate the genetic, epigenetic and/or immunological mechanisms that may underlie the better prognosis of dMMR CRCs. CRCs with dMMR frequently demonstrate a vigorous host-mediated antitumor immune response, and further characterization of the immune infiltrate and tumor-associated antigens that contribute to this response are needed. An important objective is to exploit dMMR for therapeutic advantage. CRC cell lines with dMMR demonstrate increased sensitivity to PARP inhibition [65], and other promising approaches include the evaluation of BRAF inhibitors in CRCs with activating BRAF mutations and demethylating agents in tumors with MLH1 methylation [52]. A major obstacle to testing novel treatment approaches is the low rate of dMMR in metastatic CRC patients.
Executive summary.
DNA mismatch repair pathway
Approximately 15% of cancers develop via an alternative pathway of tumorigenesis that is due to defective functioning of the DNA mismatch repair (MMR) system.
MMR deficient tumors include those with germline mutations in MMR genes that produce Lynch syndrome and sporadic colon cancers with epigenetic inactivation of the MLH1 gene.
MMR-deficient colon cancers show microsatellite instability (MSI) by PCR-based assya in tumor tissue.
Alternative testing of MMR protein expression by immunohistochemistry.
Colon cancers with deficient MMR from Lynch syndrome or sporadic cases share the same phenotype with a propensity for shared phenotype with propensity for proximal tumor site, poor differentiation, and increased tumor-infiltrating lymphocytes.
Better stage-adjusted prognosis is seen for MMR-deficient cancers.
A predictor of nonresponse to 5-fluorouracil (5-FU)-based adjuvant therapy.
The predictive role for standard 5-FU plus oxaliplatin adjuvant therapy in stage III colon cancer is unknown.
MMR status can inform adjuvant decision making in stage II colon cancer patients.
Clinical utility in stage III colon cancer patients awaits study of the predictive impact of the standard 5-FU plus oxaliplatin regimen.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
Frank Sinicrope is in part supported by the US National Cancer Institute, Senior Scientist Award K05 CA142885-01. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
• ▪ of interest
• ▪▪ of considerable interest
- 1.Grady WM, Carethers JM. Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology. 2008;135(4):1079–1099. doi: 10.1053/j.gastro.2008.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ionov Y, Peinado MA, Malkhosyan S, Shibata D, Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993;363(6429):558–561. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- 3.Herman JG, Umar A, Polyak K, et al. Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc Natl Acad Sci USA. 1998;95(12):6870–6875. doi: 10.1073/pnas.95.12.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toyota M, Ahuja N, Ohe-Toyota M, Herman JG, Baylin SB, Issa JP. CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci USA. 1999;96(15):8681–8686. doi: 10.1073/pnas.96.15.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagasaka T, Rhees J, Kloor M, et al. Somatic hypermethylation of MSH2 is a frequent event in Lynch syndrome colorectal cancers. Cancer Res. 2010;70(8):3098–3108. doi: 10.1158/0008-5472.CAN-09-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993;260(5109):816–819. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- 7.Jass JR, Do KA, Simms LA, et al. Morphology of sporadic colorectal cancer with DNA replication errors. Gut. 1998;42(5):673–679. doi: 10.1136/gut.42.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samowitz WS, Curtin K, Ma KN, et al. Microsatellite instability in sporadic colon cancer is associated with an improved prognosis at the population level. Cancer Epidemiol Biomarkers Prev. 2001;10(9):917–923. [PubMed] [Google Scholar]
- 9.Alexander J, Watanabe T, Wu TT, Rashid A, Li S, Hamilton SR. Histopathological identification of colon cancer with microsatellite instability. Am J Pathol. 2001;158(2):527–535. doi: 10.1016/S0002-9440(10)63994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenson JK, Bonner JD, Ben-Yzhak O, et al. Phenotype of microsatellite unstable colorectal carcinomas: well-differentiated and focally mucinous tumors and the absence of dirty necrosis correlate with microsatellite instability. Am J Surg Pathol. 2003;27(5):563–570. doi: 10.1097/00000478-200305000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Poynter JN, Siegmund KD, Weisenberger DJ, et al. Molecular characterization of MSI-H colorectal cancer by MLHI promoter methylation, immunohistochemistry, and mismatch repair germline mutation screening. Cancer Epidemiol Biomarkers Prev. 2008;17(11):3208–3215. doi: 10.1158/1055-9965.EPI-08-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groff RJ, Nash R, Ahnen DJ. Significance of serrated polyps of the colon. Curr Gastroenterol Rep. 2008;10(5):490–498. doi: 10.1007/s11894-008-0090-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kambara T, Simms LA, Whitehall VL, et al. BRAF mutation is associated with DNA methylation in serrated polyps and cancers of the colorectum. Gut. 2004;53(8):1137–1144. doi: 10.1136/gut.2003.037671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Brien MJ, Yang S, Mack C, et al. Comparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am J Surg Pathol. 2006;30(12):1491–1501. doi: 10.1097/01.pas.0000213313.36306.85. [DOI] [PubMed] [Google Scholar]
- 15.Sugumar A, Sinicrope FA. Serrated polyps of the colon. F1000 Med Reports. 2010;2:89. doi: 10.3410/M2-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16▪.Lindor NM, Burgart LJ, Leontovich O, et al. Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J Clin Oncol. 2002;20(4):1043–1048. doi: 10.1200/JCO.2002.20.4.1043. Mismatch repair (MMR) protein expression is highly concordant with microsatellite instability (MSI) results. [DOI] [PubMed] [Google Scholar]
- 17▪.Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58(22):5248–5257. National Cancer Institute consensus conference on MSI testing in colorectal cancer. [PubMed] [Google Scholar]
- 18.Gafa R, Maestri I, Matteuzzi M, et al. Sporadic colorectal adenocarcinomas with high-frequency microsatellite instability. Cancer. 2000;89(10):2025–2037. [PubMed] [Google Scholar]
- 19.Halling KC, French AJ, McDonnell SK, et al. Microsatellite instability and 8p allelic imbalance in stage B2 and C colorectal cancers. J Natl Cancer Inst. 1999;91(15):1295–1303. doi: 10.1093/jnci/91.15.1295. [DOI] [PubMed] [Google Scholar]
- 20.Thibodeau SN, French AJ, Cunningham JM, et al. Microsatellite instability in colorectal cancer: different mutator phenotypes and the principal involvement of hMLH1. Cancer Res. 1998;58(8):1713–1718. [PubMed] [Google Scholar]
- 21.Sinicrope FA, Rego RL, Halling KC, et al. Thymidylate synthase expression in colon carcinomas with microsatellite instability. Clin Cancer Res. 2006;12(9):2738–2744. doi: 10.1158/1078-0432.CCR-06-0178. [DOI] [PubMed] [Google Scholar]
- 22.Lanza G, Gafa R, Maestri I, Santini A, Matteuzzi M, Cavazzini L. Immunohistochemical pattern of MLH1/MSH2 expression is related to clinical and pathological features in colorectal adenocarcinomas with microsatellite instability. Mod Pathol. 2002;15(7):741–749. doi: 10.1097/01.MP.0000018979.68686.B2. [DOI] [PubMed] [Google Scholar]
- 23▪.Domingo E, Laiho P, Ollikainen M, et al. BRAF screening as a low-cost effective strategy for simplifying HNPCC genetic testing. J Med Genet. 2004;41(9):664–668. doi: 10.1136/jmg.2004.020651. BRAF mutations can distinguish hereditary nonpolyposis colorectal cancer from sporadic colon cancers with deficient MMR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen S, Wang W, Lee S, et al. Prediction of germline mutations and cancer risk in the Lynch syndrome. JAMA. 2006;296(12):1479–1487. doi: 10.1001/jama.296.12.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balmana J, Stockwell DH, Steyerberg EW, et al. Prediction of MLH1 and MSH2 mutations in Lynch syndrome. JAMA. 2006;296(12):1469–1478. doi: 10.1001/jama.296.12.1469. [DOI] [PubMed] [Google Scholar]
- 26.Barnetson RA, Tenesa A, Farrington SM, et al. Identification and survival of carriers of mutations in DNA mismatch-repair genes in colon cancer. N Engl J Med. 2006;354(26):2751–2763. doi: 10.1056/NEJMoa053493. [DOI] [PubMed] [Google Scholar]
- 27▪.Sinicrope F, Foster NR, Sargent DJ, Thibodeau SN, Smyrk TC, O’Connell MJ. Model-based prediction of defective DNA mismatch repair using clinicopathological variables in sporadic colon cancer patients. Cancer. 2010;116(7):1691–1698. doi: 10.1002/cncr.24913. Models using clinicopathological variables to facilitate the identification of sporadic colon cancers with deficient MMR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gryfe R, Kim H, Hsieh ET, et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med. 2000;342(2):69–77. doi: 10.1056/NEJM200001133420201. [DOI] [PubMed] [Google Scholar]
- 29.Haddad R, Ogilvie RT, Croitoru M, et al. Microsatellite instability as a prognostic factor in resected colorectal cancer liver metastases. Ann Surg Oncol. 2004;11(11):977–982. doi: 10.1245/ASO.2004.03.585. [DOI] [PubMed] [Google Scholar]
- 30.Malesci A, Laghi L, Bianchi P, et al. Reduced likelihood of metastases in patients with microsatellite-unstable colorectal cancer. Clin Cancer Res. 2007;13(13):3831–3839. doi: 10.1158/1078-0432.CCR-07-0366. [DOI] [PubMed] [Google Scholar]
- 31.Sinicrope FA, Rego RL, Halling KC, et al. Prognostic impact of microsatellite instability and DNA ploidy in human colon carcinoma patients. Gastroenterology. 2006;131(3):729–737. doi: 10.1053/j.gastro.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Lanza G, Gafa R, Santini A, Maestri I, Guerzoni L, Cavazzini L. Immunohistochemical test for MLH1 and MSH2 expression predicts clinical outcome in stage II and III colorectal cancer patients. J Clin Oncol. 2006;24(15):2359–2367. doi: 10.1200/JCO.2005.03.2433. [DOI] [PubMed] [Google Scholar]
- 33▪▪.Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349(3):247–257. doi: 10.1056/NEJMoa022289. Large-scale study examining the predictive impact of MMR status for adjuvant 5-fluorouracil (5-FU) in stage II and III colon cancer patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34▪▪.Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23(3):609–618. doi: 10.1200/JCO.2005.01.086. Meta-analysis examining the prognostic impact of MSI in colorectal cancer. [DOI] [PubMed] [Google Scholar]
- 35.Kim GP, Colangelo LH, Wieand HS, et al. Prognostic and predictive roles of high-degree microsatellite instability in colon cancer: a National Cancer Institute-National Surgical Adjuvant Breast and Bowel Project Collaborative Study. J Clin, Oncol. 2007;25(7):767–772. doi: 10.1200/JCO.2006.05.8172. [DOI] [PubMed] [Google Scholar]
- 36.Lamberti C, Lundin S, Bogdanow M, et al. Microsatellite instability did not predict individual survival of unselected patients with colorectal cancer. Intl J Colorectal Dis. 2007;22(2):145–152. doi: 10.1007/s00384-006-0131-8. [DOI] [PubMed] [Google Scholar]
- 37▪▪.Sargent DJ, Marsoni S, Monges G, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010;28(20):3219–3226. doi: 10.1200/JCO.2009.27.1825. Validation study showing the prognostic and predictive impact of deficient MMR for 5-FU-based adjuvant chemotherapy in stage II and III colon cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gray R, Barnwell J, McConkey C, Hills RK, Williams NS, Kerr DJ. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007;370(9604):2020–2029. doi: 10.1016/S0140-6736(07)61866-2. [DOI] [PubMed] [Google Scholar]
- 39.Kerr D, Gray R, Quirke P, et al. Quasar Colon Teams: A quantitative multigene RT-PCR assay for prediction of recurrence in stage II colon cancer: selection of the genes in four large studies and results of the independent, prospectively designed QUASAR validation study. J Clin Oncol. 2009;27(Suppl 15):Abstract 4000. [Google Scholar]
- 40.Tejpar S, Bosman F, Delorenzi M, et al. Microsatellite instability (MSI) in stage II and III colon cancer treated with 5FU-LV or 5FU-LV and irinotecan (PETACC 3-EORTC 40993-SAKK 60/00 trial) J Clin Oncol. 2009;27(Suppl 15):Abstract 4001. [Google Scholar]
- 41.Dolcetti R, Guidoboni M, Viel A, Boiocchi M. Correspondence re: Samowitz et al.: Microsatellite instability in sporadic colon cancer is associated with an improved prognosis at the population level. Cancer Epidemiol Biomark Prev. 2001;10:917–923. [PubMed] [Google Scholar]; Cancer Epidemiol Biomarkers Prev. 2002;11(5):499. author reply 499–500. [PubMed] [Google Scholar]
- 42.Sinicrope FA, Rego RL, Garrity-Park MM, et al. Alterations in cell proliferation and apoptosis in colon cancers with microsatellite instability. Intl J Cancer. 2007;120(6):1232–1238. doi: 10.1002/ijc.22429. [DOI] [PubMed] [Google Scholar]
- 43.Drescher KM, Sharma P, Lynch HT. Current hypotheses on how microsatellite instability leads to enhanced survival of Lynch syndrome patients. Clin Develop Immunol. 2010:170432. doi: 10.1155/2010/170432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benatti P, Gafa R, Barana D, et al. Microsatellite instability and colorectal cancer prognosis. Clin Cancer Res. 2005;11(23):8332–8340. doi: 10.1158/1078-0432.CCR-05-1030. [DOI] [PubMed] [Google Scholar]
- 45.Jover R, Zapater P, Castells A, et al. The efficacy of adjuvant chemotherapy with 5-fluorouracil in colorectal cancer depends on the mismatch repair status. Eur J Cancer. 2009;45(3):365–373. doi: 10.1016/j.ejca.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 46.Carethers JM, Smith EJ, Behling CA, et al. Use of 5-fluorouracil and survival in patients with microsatellite-unstable colorectal cancer. Gastroenterology. 2004;126(2):394–401. doi: 10.1053/j.gastro.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 47.Jover R, Zapater P, Castells A, et al. Mismatch repair status in the prediction of benefit from adjuvant fluorouracil chemotherapy in colorectal cancer. Gut. 2006;55(6):848–855. doi: 10.1136/gut.2005.073015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hemminki A, Mecklin JP, Jarvinen H, Aaltonen LA, Joensuu H. Microsatellite instability is a favorable prognostic indicator in patients with colorectal cancer receiving chemotherapy. Gastroenterology. 2000;119(4):921–928. doi: 10.1053/gast.2000.18161. [DOI] [PubMed] [Google Scholar]
- 49.Elsaleh H, Joseph D, Grieu F, Zeps N, Spry N, Iacopetta B. Association of tumour site and sex with survival benefit from adjuvant chemotherapy in colorectal cancer. Lancet. 2000;355 (9217):1745–1750. doi: 10.1016/S0140-6736(00)02261-3. [DOI] [PubMed] [Google Scholar]
- 50.Des Guetz G, Schischmanoff O, Nicolas P, Perret GY, Morere JF, Uzzan B. Does microsatellite instability predict the efficacy of adjuvant chemotherapy in colorectal cancer? A systematic review with meta-analysis. Eur J Cancer. 2009;45(10):1890–1896. doi: 10.1016/j.ejca.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 51▪.Carethers JM, Chauhan DP, Fink D, et al. Mismatch repair proficiency and in vitro response to 5-fluorouracil. Gastroenterology. 1999;117(1):123–131. doi: 10.1016/s0016-5085(99)70558-5. Demonstrates the mechanism by which deficient MMR confers resistance to 5-FU in colon cancer cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arnold CN, Goel A, Boland CR. Role of hMLH1 promoter hypermethylation in drug resistance to 5-fluorouracil in colorectal cancer cell lines. Intl J Cancer. 2003;106(1):66–73. doi: 10.1002/ijc.11176. [DOI] [PubMed] [Google Scholar]
- 53.Kat A, Thilly WG, Fang WH, Longley MJ, Li GM, Modrich P. An alkylation-tolerant, mutator human cell line is deficient in strand-specific mismatch repair. Proc Natl Acad Sci USA. 1993;90(14):6424–6428. doi: 10.1073/pnas.90.14.6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Branch P, Aquilina G, Bignami M, Karran P. Defective mismatch binding and a mutator phenotype in cells tolerant to DNA damage. Nature. 1993;362(6421):652–654. doi: 10.1038/362652a0. [DOI] [PubMed] [Google Scholar]
- 55.Vilar E, Scaltriti M, Balmana J, et al. Microsatellite instability due to hMLH1 deficiency is associated with increased cytotoxicity to irinotecan in human colorectal cancer cell lines. Br J Cancer. 2008;99(10):1607–1612. doi: 10.1038/sj.bjc.6604691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Magrini R, Bhonde MR, Hanski ML, et al. Cellular effects of CPT-11 on colon carcinoma cells; dependence on p53 and hMLH1 status. Intl J Cancer. 2002;101(1):23–31. doi: 10.1002/ijc.10565. [DOI] [PubMed] [Google Scholar]
- 57.Rodriguez R, Hansen LT, Phear G, et al. Thymidine selectively enhances growth suppressive effects of camptothecin/irinotecan in MSI* cells and tumors containing a mutation of. MRE11 Clin Cancer Res. 2008;14(17):5476–5483. doi: 10.1158/1078-0432.CCR-08-0274. [DOI] [PubMed] [Google Scholar]
- 58.Fink D, Nebel S, Aebi S, et al. The role of DNA mismatch repair in platinum drug resistance. Cancer Res. 1996;56(21):4881–4886. [PubMed] [Google Scholar]
- 59.Bertagnolli MM, Niedzwiecki D, Compton CC, et al. Microsatellite instability predicts improved response to adjuvant therapy with irinotecan, fluorouracil, and leucovorin in stage III colon cancer: Cancer and Leukemia Group B Protocol 89803. J Clin Oncol. 2009;27(11):1814–1821. doi: 10.1200/JCO.2008.18.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raymond E, Faivre S, Chaney S, Woynarowski J, Cvitkovic E. Cellular and molecular pharmacology of oxaliplatin. Mol Cancer Ther. 2002;1(3):227–235. [PubMed] [Google Scholar]
- 61.Kim ST, Lee J, Park SH, et al. Clinical impact of microsatellite instability in colon cancer following adjuvant FOLFOX therapy. Cancer Chemother Pharmacol. 2010;66(4):659–667. doi: 10.1007/s00280-009-1206-3. [DOI] [PubMed] [Google Scholar]
- 62.des Guetz G, Mariani P, Cucherousset J, et al. Microsatellite instability and sensitivity to FOLFOX treatment in metastatic colorectal cancer. Anticancer Res. 2007;27(4C):2715–2719. [PubMed] [Google Scholar]
- 63.Chua W, Goldstein D, Lee CK, et al. Molecular markers of response and toxicity to FOLFOX chemotherapy in metastatic colorectal cancer. Br J Cancer. 2009;101(6):998–1004. doi: 10.1038/sj.bjc.6605239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zaanan A, Cuilliere-Dartigues P, Guilloux A, et al. Impact of p53 expression and microsatellite instability on stage III colon cancer disease-free survival in patients treated by 5-fluorouracil and leucovorin with or without oxaliplatin. Ann Oncol. 2010;21(4):772–780. doi: 10.1093/annonc/mdp383. [DOI] [PubMed] [Google Scholar]
- 65.Sanchez EV, Chow A, Raskin L, Iniesta MD, Mukherjee B, Gruber SB. Preclinical testing of the PARP inhibitor ABT-888 in microsatellite instable colorectal cancer. J Clin Oncol. 2009;27(Suppl 15):Abstract 11028. [Google Scholar]
Website
- 101.Atlanta: American Cancer Society. Cancer facts & figures. 2010 www5.cancer.org/downloads/STT/Canccr_Facts_and_Figures_2010.pdf.