Abstract
Purpose of review
Regulation of intraocular pressure by the conventional (trabecular) outflow pathway is complicated, involving a myriad of mechanical and chemical signals. In most, intraocular pressure is maintained within a tight range over a lifetime. Unfortunately in some, dysfunction results in ocular hypertension and open-angle glaucoma. In the context of established knowledge, this review summarizes recent investigations of conventional outflow function, with the goal of identifying areas for future inquiry and therapeutic targeting.
Recent findings
Mechanical stimulation of conventional outflow cells due to intraocular pressure fluctuations impacts contractility, gene expression, pore formation enzyme activity and signaling. Numerous local signaling mediators in the conventional pathway such as bioactive lipids, cytokines, nitric oxide and nucleotides participate in the regulation of outflow. Interestingly outflow through the conventional pathway is not uniform, but segmental, with passageways constantly changing due to focal protease activity of trabecular cells clearing extracellular matrix materials. The relationship between extracellular matrix expression and trabecular meshwork contractility appears to coordinately impact outflow resistance and is the target of a new class of drugs, the rho kinase inhibitors.
Summary
The conventional outflow pathway is a dynamic, pressure-sensitive tissue that is vulnerable to pathology on many fronts, each representing a therapeutic opportunity.
Keywords: Intraocular Pressure, Outflow facility, Schlemm’s canal, Trabecular Meshwork
Introduction
The principal risk factor and only currently modifiable ocular parameter for millions of patients having primary open-angle glaucoma (POAG) is ocular hypertension (elevated intraocular pressure, IOP). The pathology responsible for ocular hypertension is located in the pressure-dependent, conventional (trabecular) outflow pathway. Over the past 60 years, several key observations implicate trabecular meshwork (TM) dysfunction in POAG. First, untreated POAG patients with ocular hypertension have elevated conventional outflow resistance compared to age-matched controls and surgical removal of TM from POAG eyes, eliminates the extra resistance [1, 2]. Second, activation of TM cells with directed laser energy temporarily reduces outflow resistance and brings down IOP in POAG eyes [3, 4]. Third, water drinking test of POAG patients reveals retarded IOP recovery [5]. Fourth, circadian IOP fluctuations are more dramatic in those with glaucoma, indicating hindered capacitance of TM [6]. Fifth, clinical features of corticosteroid-induced glaucoma mirror those of POAG, particularly effects on outflow facility [7, 8]. Lastly, mutations in a prominent TM gene product, myocilin, result in increased outflow resistance, ocular hypertension and POAG [9, 10].
While it is clear that the pathology responsible for ocular hypertension is located in the conventional outflow pathway, the molecular and cellular mechanisms that underpin the manifestation of disease are largely unknown. Sadly, glaucoma patients do not currently have a daily medication that targets conventional outflow (dys)function. The purpose of the present article is to review the recent knowledge concerning the regulatory mechanisms that underlie IOP control in the conventional outflow pathway, identifying areas for further study and candidates for therapeutic targeting.
Conventional Outflow Regulation
Regulation of conventional outflow resistance is dynamic and likely involves multiple redundant signaling systems and processes. There is much higher incidence of ocular hypertension than hypotony in the general population suggesting that ocular hypertension is better tolerated [11] and further suggesting that the regulatory machinery in the pressure-dependent conventional pathway is biased toward preventing hypotony. Such a design makes sense considering that under conditions of elevated IOP, the visual pathway and thus high acuity vision is maintained. In addition, it is difficult to medically lower IOP in most patients below a critical level (~12–13 mmHg), suggesting that “compensatory systems” are activated as IOP drops [12]. Thus, glaucoma surgeries such as trabeculectomy or the placement of tubes bypass these normal regulatory pathways and are a primary cause of hypotony [13, 14]. Since ocular hypertension is tightly linked to disease progression in POAG [15], current research has been directed at better understanding the mechanical and biochemical elements responsible for maintaining homeostasis in the conventional outflow pathway in health and those that go awry in disease with the goal of future therapeutic intervention.
Location of Outflow Resistance and Funneling
IOP is a byproduct of outflow resistance generation deep in the conventional outflow tract; in the juxtacanalicular tissue (JCT) region, where TM and Schlemm’s canal (SC) inner wall cells interact [2, 16–18]. Importantly, the “extra” resistance found in glaucomatous eyes is also located within the JCT region [2]. However it is not known if the source of resistance is due to TM cells and their extracellular matrix (ECM) or the inner wall of SC. By itself, neither structure appears to generate enough resistance to explain IOP. Recent evidence suggest that the TM and SC cells work together to synergistically regulate resistance likely via a hydrodynamic effect known as “funneling” [19]. Funneling arises because the inner wall is relatively impermeable except at discrete pore sites, and aqueous humor flowing through the JCT must thereby converge or “funnel” to pass through the pores of the inner wall. The convergence of flow caused by funneling reduces the area of the JCT that is actively involved in filtration, thereby increasing its effective hydraulic resistance. Thus, the immediate juxtaposition between the two tissues (facilitated by TM cell tethering to the inner wall), and hence their hydrodynamic coupling, gives rise to the funneling effect such that resistance decreases when they are separated.
Three separate studies, looking at two pharmacological agents (H7 and Y27632) and one experimental phenomenon called “washout” emphasize the importance of separation of TM and SC cells in the JCT. During washout [20], or perfusion with H7 or Y27632 [21, 22] outflow facility increases and alters the distribution of a flow tracers in the conventional tract of living monkeys or bovine eyes, respectively. While tracer is deposited in distinct foci along the inner wall in control eyes, consistent with the distribution of pores, the tracer pattern dramatically changes in treated eyes becoming more uniform over the surface of the inner wall. Moreover, the distance between the cribiform plates and the inner wall in the JCT expands in treated eyes, a phenomenon that is reversible. All three of these observations are in accordance with the loss of TM tethering and consequent disruption of funneling. The funneling model also predicts that changes in pore density of the inner wall will impact funneling and outflow facility. Consistent with the model, perfusion of certain compounds increased both facility and pore density; however, other compounds failed to reveal a relationship. Despite this, two other observations are also consistent with the model: First, if pores are selectively occluded with a flow tracer such as cationic ferritin, outflow facility dramatically decreases [23]. Second, glaucomatous eyes in two studies had significantly lower pore density, suggesting that those with ocular hypertension have a defect in pore formation/closure and thus higher resistance according to the funneling hypothesis [24, 25]. Importantly, recent in vitro studies of SC cells show that pore formation is pressure-dependent and impaired in glaucomatous cells [26].
Local Mediators of Outflow Facility
Due to its unique architecture, directional flow of aqueous humor and close proximity of cells to one another, the conventional outflow pathway utilizes autocrine and paracrine mediators to regulate outflow resistance. Local mediators from several categories including lipid-derived (lysophospholipids, prostaglandins (PG), cannabinoids), cytokines (TGFβ, BMP, Wnt, IL-1, TNFα), nucleotides (ATP/adenosine) and gases (nitric oxide) all impact conventional outflow resistance to varying degrees and in either direction (i.e.: increase or decrease, see table 1).
Table 1.
Summary of Effects of Autocrine/Paracrine Mediators of Conventional Outflow Function (original)
| Local Mediator | Receptor(s) | Impact on C | Species | References |
|---|---|---|---|---|
| PM/PG F2α | PG FP (sv) | ↑ 26%–67% | human | Brubaker et al., 2001; Christiansen et al., 2004; Wan et al., 2007, Toris et al., 2007, Fautsch et al., 2008 |
| PG E2 | EP4 | ↑ 35%–69% | monkey, human | Woodward et al., 2009; Millard et al., 2011 |
| 2-AG/AEA | CB1/CB2 | ↑ 0%–80% | porcine, feline, monkey | Zhong et al., 2005; Njie et al., 2006; Njie et al., 2008; Colasanti, 1990; Chien et al., 2003 |
| LPA | LPAR | ↓ 37% | porcine | Mettu et al., 2004 |
| S1P | S1PR2 | ↓ 31%–41% | porcine, human | Mettu et al., 2004; Stamer et al., 2009; Sumida and Stamer, 2011 |
| TGFβ | RI/RII | ↓ 27%–45% | mouse, rat, porcine, monkey, human | Sheppard et al., 2010; Bhattacharya et al., 2008; Bachmann et al., 2006; Fleenor et al., 2006; Gottanka et al., 2004 |
| IL-1α/β | IL1-R | ↑ 25%–100% | rat, porcine, human | Birke et al., 2011; Bradley et al., 1998; Kee and Seo, 1997; Kelly et al., 2007 |
| TNFα | TNFR | ↑ 20% | porcine | Kelley et al., 2007 |
| Gremlin | BMP RI/RII | ↓ 36% | human | Wordinger et al., 2007 |
| Wnt/sFRP | Fzd | ↓ 55% | human | Wang et al., 2008 |
| Nitric oxide | sGC | ↑ 10%–115% | porcine, human | Dismuke et al., 2008; Ellis et al., 2008; Stamer et al., 2011; Shneemann et al., 2002 |
| ATP/adenosine | A1/KATP | ↑ 28%–71% | bovine, human | Tian et al., 1997; Crosson et al., 2005; Chowdhury et al., 2011 |
Abbreviations: C: outflow facility; PM: Prostamide; PG: Prostaglandin; sv: splice variant; 2-AG: 2-arachindonyl glycerol; AEA: anandamide; LPA: lysophosphatidic acid; S1P: sphingosine-1-phosphate; TGF: transforming growth factor; IL: interleukin; TNF: tumor necrosis factor; sFRP: soluble frizzled-related protein; CB: cannabinoid; R: receptor; BMP: bone morphogenetic protein; Fzd: frizzled receptor; sGC: soluble guanylate cyclase
Over the past decade the involvement of lipid mediation of conventional outflow function has become apparent [27]. For example, prostaglandin E2 and prostaglandin/prostamide F2a, acting at EP4 or FP (and/or FP splice variant) receptors, respectively increase outflow facility [28–33]. Early clinical observations suggesting that PG drugs increase outflow facility was confirmed recently in controlled experimental systems that isolate the conventional outflow pathway. Interestingly, there appears to be two different mechanisms of action of these compounds because effects on outflow are either within minutes or can take days. Acute affects of prostaglandins likely affect the contractility status (relaxation) and/or cell-cell junction assembly/disassembly of cells in the conventional pathway, while long-term effects likely involve alterations in extracellular matrix turnover [28, 34].
Like the PGs, endocannabinoids acting on either CB1 or CB2 receptors increase outflow facility [35–38]. Perfusion of porcine anterior segments with endogenous cannabinoid agonists such as 2-arachidonoylglycerol or anandamide (or synthetic cannabinoids) results in an immediate and significant increase in outflow facility; effects that were blocked in part by CB1 and/or CB2 selective antagonists. In addition to cannabinoid receptors, endogenous ligands and their metabolizing enzymes are also present in the conventional outflow pathway [35, 36, 39].
While the types of lipid mediators described above increase outflow facility, lysophospholipids such as lysophosphatidic acid and sphingosine-1-phosphate (S1P) decrease outflow facility in porcine and human eyes [40, 41]. For example, perfusion of human eyes in organ culture with S1P results in an immediate decrease in outflow facility. The effects of S1P appear to be mediated by both TM and SC cells, involving increased contraction and cell-cell junction assembly, respectively [42]. Use of selective antagonists to S1P receptor subtypes showed that S1P effects on outflow facility are mediated by the S1P2 receptor subtype [43].
To add further complexity to local mediation of conventional outflow, many secreted cytokines impact conventional outflow function. Several cytokines, particularly those that mediate local inflammatory processes have been known to alter conventional outflow homeostasis for some time. For example, TGFβ levels are elevated in the aqueous humor of glaucoma patients and treatment of eyes with TGFβ gradually and substantially decreases outflow facility over the course of days [44] [45]. It appears that TGFβ affects ECM homeostasis in the JCT over time [46]. At least two key types of molecules, BMP4/7 and IL-1, have been shown to antagonize TGFβ signaling and contribute to outflow tissue homeostasis [47, 48]. The lipophilic secreted class of cytokines, Wnts, is expressed and signals in the conventional tract. Hence, an antagonist to Wnt signaling, sFRP increases outflow facility in perfused human anterior segments, while overexpression of sFRP in mice increases IOP [49].
Small signaling molecules such as nucleotides and nitric oxide (NO) modify conventional outflow. For instance, ATP release from TM cells through several different molecular conduits can be induced by stretching of the TM due to IOP fluctuations [50, 51]. Once outside, ATP interacts with the KATP potassium channel to increase outflow facility and/or is rapidly converted to adenosine by ectoenzymes and is free to interact with one of several adenosine receptors [52]. Importantly, adenosine activation of the A1 receptor in the conventional pathway rapidly increases outflow facility in cultured bovine eyes through effects involving MMP activity [53]. Importantly for patients, a synthetic agonist (INO-8875) that specifically activates A1 receptors is currently in clinical trials as a glaucoma therapeutic (clinicaltrials.gov ID# NCT01123785). In addition, the labile gas, nitric oxide, increases outflow facility [54–58]. Using NO-donating compounds or mice overexpressing endothelial nitric oxide synthase, liberation/generation of NO in the conventional tract results in approximately two-fold increase in outflow facility. Possible mechanisms underlying the NO effects are decreased volume, contractility and/or cell-cell junction assembly in conventional outflow cells.
Mechanoregulation of Outflow Facility
Most people do not develop ocular hypertension and glaucoma, even at advanced ages, suggesting the existence of effective IOP homeostatic mechanisms [59, 60]. Since conventional outflow tissues are constantly subjected to a pressure gradient, it makes sense that biomechanics play a role in outflow regulation. This was demonstrated experimentally several years ago [61, 62] and studies of the mechanistic details in recent years have clarified the process to some extent [59, 60]. Elevated IOP is sensed by cells within the JCT region as mechanical stretch or distortion [63]. TM cells, perhaps with assistance from SC cells, respond by adjusting the outflow resistance, which ultimately corrects the IOP. Changes in cell signaling, gene expression, ECM turnover, and cytoskeletal organization are all associated with, and appear to be involved in, this stretch-induced adjustment of the outflow resistance [60, 64–75]. For example, elevation of IOP in perfused eyes increases the net activity of MMPs within the JCT region, including ADAMTS-4 [76]. In terms of gene expression and signaling, TM cells exposed to mechanical stress increase expression of the micro RNA, miR-24, which regulates a panel of genes including those that process cytokines like TGFβ that regulate outflow facility [77]. Lastly, mechanical stimuli from daily oscillations in IOP, such as ocular pulse, help set contractile tone in the TM [71]. Taken together, mechanical stimulation of conventional outflow cells triggers the coordination of many regulatory processes that contribute to homeostasis.
Trabecular Tone, Cytoskeletal Organization and Outflow Facility
The trabecular meshwork is under constant tension, contracting to counter (i) pull from the ciliary muscle that extends elastic tendons through the TM, anchoring onto the inner wall of Schlemm’s canal and (ii) a constant pressure gradient. The role of trabecular tone or contractility in regulating outflow facility has been known for some time, but the molecular sophistication that underlies its homeostatic role is only recently becoming understood [42, 71, 78–82]. Early on, compounds such as the cytochalasins, which inhibit actin polymerization, were shown to dramatically affect outflow facility [83]; and other direct-acting cytoskeletal agents have similar effects [84]. The therapeutic importance of relaxing the TM is evidenced by clinical trials that are currently underway for several related agents, including Rho kinase inhibitors, that have primary effects on trabecular cytoskeleton/tone that increase outflow facility (figure 1) [85–87]. In contrast, off target effects from agents such as corticosteroids which increase cellular rigidity and promote formation of cytoskeletal structures known as CLANS (cross-linked actin networks) decrease outflow facility [88–92]. The precise mechanism(s) by which cytoskeletal manipulations of TM or SC cells affect outflow resistance remains unclear, but they could be acting directly through cell shape and effect on active flow pathways or through the cellular control of ECM organization via integrins and other ECM interactions [81, 93–95]. Two recent studies highlight the intimate connection between TM cell contractility and ECM-integrin expression [81, 93].
Figure 1. Dose-related intraocular pressure-lowering effects of a rho kinase inhibitor, AR-12286, in ocular hypertensive patients enrolled in phase 2A trial.
Shown are mean change in diurnal intraocular pressure measurements from baseline for each treatment group. bid: twice daily, q.d.: daily
Source: reproduced with permission from Robert D. Williams, Gary D. Novack, Thomas van Haarlem, Casey Kopczynski and AR-12286 Phase 2A Study Group; Ocular Hypotensive Effect of the Rho Kinase Inhibitor AR-12286 in Patients With Glaucoma and Ocular Hypertension; Am J Ophthalmol 2011: 152: 838
ECM-Mediated Resistance and Segmental Outflow
While the relationship between TM contractility and ECM expression/organization is a recent finding, the contribution of glycosaminoglycans (GAGs) and their proteoglycan core proteins to outflow resistance generation has been hypothesized since the 1950s [59, 96]. Hence several ECM components likely participate in the generation of resistance, including versican, a large proteoglycan with numerous GAG side chains, and hyaluronan, which forms long GAG chains and fibrils, and appear to provide much of the outflow resistance [59, 73, 97]. Interestingly, shape changes of TM and SC cells in the JCT is hypothesized to modify the relative orientation of GAGs and proteoglycans within flow channels of the conventional tract, acting to fine tune the resistance to outflow; again emphasizing the relationship between cellular contraction and associated ECM.
In addition to the abundance and position of ECM in flow pathways, the number and location of such flow pathways in the conventional tract also contribute to the regulation of outflow facility. It has long been known that outflow channels are not uniform around the circumference of the eye [97–100]. Rather, outflow is segmental, exhibiting regions of relatively high and low conductance. This variability is reflected by non-uniform pigment, tracer or stiffness patterns in the conventional outflow tissues [97, 101, 102] (figure 2). Some of this segmentation has an anatomical component in that it corresponds closely to the positions of collector channels and some of it does not, but reflects some larger organizational pattern [99]. Interestingly, the distribution of versican is segmental and levels correlate inversely with relative outflow rates [97]. Recent studies suggest that flow pathways are dynamic, constantly changing over time. Moreover, the dynamics may be mediated by local ECM degradation by podosome/invadopodia like structure (PILS) activity of TM cells (figure 3) [103]. This high degree of segmental distribution of flow demands adjustments of theoretical estimates of outflow resistance and further suggests that Schlemm’s canal may not exhibit free radial flow around the eye. It also has obvious implications for surgical stent placement or other trabecular surgeries.
Figure 2. Segmental flow patterns through the human trabecular meshwork.
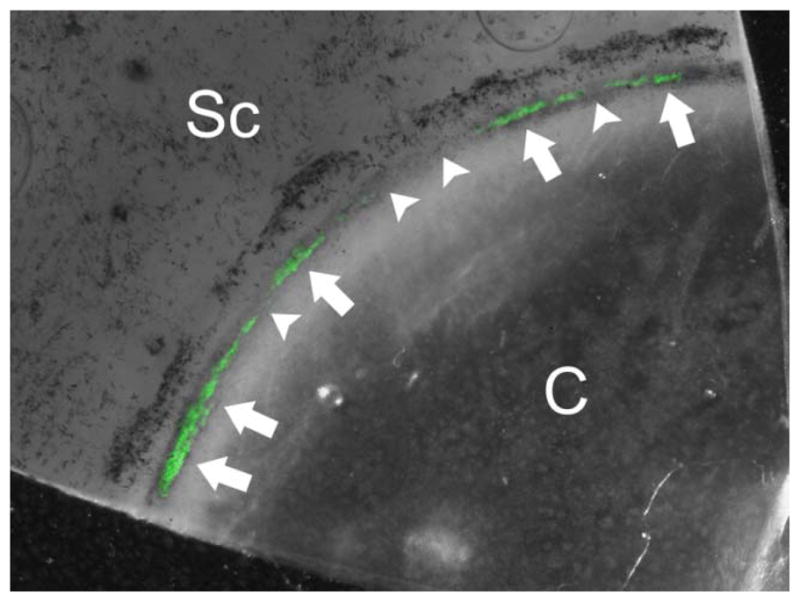
Enucleated human donor eyes were perfused with a bolus of green fluorescent tracer microspheres (200 nm) and perfusion fixed. The trabecular meshwork was imaged en face after removing the iris and ciliary body. The non-uniform pattern of fluorescent tracer decoration suggests segmental flow within the trabecular meshwork. Arrows indicate areas of high flow and arrowheads show areas of low/no flow through the trabecular meshwork. Sc: sclera; C: cornea
Source: image provided by Darryl Overby, Ph.D.
Figure 3. Effects of mechanical stretch on podosome/invadosome-like structures (PILS) component distribution.

TM cells were exposed to ~10% radial stretch for 24 hours (stretch) or were not stretched (control). Component localization was imaged by confocal microscopy examining microtubules (green), MMP-14 (red) and actin microfilaments (blue). Yellow (arrowheads) indicate colocalization of microtubule clusters and MMP-14 at sites of PILS
Source: image provided by Ted Acott, Ph.D
Oxidative Damage and Outflow Dysfunction
TM cells are terminally differentiated and long-lived. In addition to accumulated stress due to normal metabolism over a lifetime, TM cells are subjected to oxidative stress and cellular debris that are continuously introduced via aqueous humor flow. Unfortunately, all four proteolytic enzyme systems responsible for processing cellular material have been shown to be compromised or strained in TM cells with age, in POAG, upon exposure to chronic oxidative stress [104] or upon genetic mutations that produce excess amounts of misfolded protein [105]. The accumulation of nondegradable and oxidized materials in the conventional tract over time has extreme potential to impair normal cellular function including outflow regulation observed in POAG. Accordingly, many hallmarks of oxidative damage in the conventional pathway have been reported in people with POAG; such as decreased antioxidant potential plus increased expression of oxidative stress markers, oxidative DNA damage, peroxidized lipids and mitochondrial reactive oxygen species (ROS) [104]. For example, a recent study of 79 trabeculectomy specimens from patients with POAG showed a 5-fold increase in mitochondrial DNA deletions, a 4-fold decrease in number of mitochondria and 16 fold fewer cells than age-matched controls [106]. Another study showed that in aging and/or chronic exposure to oxidative stress damage may be due to altered iron homeostasis resulting in abnormal production of intralysosomal ROS, possibly leading to lysosomal membrane permeabilization and release of cathepsin-D into the cytosol with consequent TM cell death [107]. Efforts to limit oxidative damage in the conventional pathway are underway. Recently, a mitochondrial targeted antioxidant was shown to attenuate ROS generation in TM cells exposed to oxidative stress in vitro [108]. Moreover, treatment of TM cells with resveratrol, an antioxidant found in red wine and berries, limits appearance of markers of oxidative damage [109]. Taken together, these studies suggest compromised outflow function in POAG may result from diminished capacitance to quench oxidative byproducts, a weakened ability to process cellular material or excessive oxidative load over time.
Conclusion
The pressure-dependent, conventional outflow tract is the diseased tissue responsible for ocular hypertension. Regulation of outflow is dynamic and complicated, involving many signaling molecules and cellular processes that for most people maintain IOP within a narrow range over a lifetime. Understanding the homeostatic mechanisms that regulate conventional outflow will reveal (i) susceptible pathways responsible for ocular hypertension plus (ii) therapeutic targets that have the potential to medically lower IOP below current therapeutic “floor” limits. Most importantly, intervention holds promise to restore function to this vital pressure-responsive and pressure-dampening tissue of the eye.
Key Points.
Molecular/cellular defect(s) in the conventional outflow pathway are responsible for ocular hypertension in glaucoma.
Regulation of conventional outflow facility and thus IOP is dependent upon complex orchestration of many local signaling molecules including bioactive lipids, cytokines, nucleotides and gases.
The contractile tone of the trabecular meshwork is a target for rho kinase inhibitors, a new class of anti-glaucoma medications that increase conventional outflow.
Only a fraction of total flow pathways through the TM are utilized at any one time; these preferential pathways appear to be transient, dynamic and a result of podosome activity.
As the final destination for aqueous humor circulation, the trabecular meshwork is continually challenged with oxidative, mechanical and phagocytic stressors that likely contribute to dysfunction over time.
Acknowledgments
The authors thank Kristin Perkumas for technical assistance in preparing figures and Drs. Catherine Bowes Rickman, Darryl Overby, Vasanth Rao and Paloma Liton for helpful discussions and careful editing of manuscript.
Grant support
National Eye Institute: EY012797 (WDS), EY019696 (WDS), EY003279 (TSA), EY008247 (TSA), EY010572 (TSA) and Research to Prevent Blindness Foundation (WDS and TSA).
Footnotes
Disclosures
Dr. Stamer received an unrestricted grant from Allergan, Inc. that has supported some of the work described. Dr. Acott has no disclosures or conflicts to report.
References
- 1.Grant WM. Clinical tonography. Trans Am Acad Ophthalmol Otolaryngol. 1951;55:774–81. [PubMed] [Google Scholar]
- 2.Grant WM. Experimental aqueous perfusion in enucleated human eyes. Arch Ophthalmol. 1963;69:783–801. doi: 10.1001/archopht.1963.00960040789022. [DOI] [PubMed] [Google Scholar]
- 3.Bradley J, et al. Mediation of laser trabeculoplasty-induced matrix metalloproteinase expression by IL1beta and TNFalpha. Invest Ophthalmol Vis Sci. 2000;41:422–430. [PubMed] [Google Scholar]
- 4.Latina MA, et al. Q-switched 532-nm Nd:YAG laser trabeculoplasty (selective laser trabeculoplasty): a multicenter, pilot, clinical study. Ophthalmology. 1998;105(11):2082–8. doi: 10.1016/S0161-6420(98)91129-0. discussion 2089–90. [DOI] [PubMed] [Google Scholar]
- 5.Susanna R, Jr, et al. The relation between intraocular pressure peak in the water drinking test and visual field progression in glaucoma. Br J Ophthalmol. 2005;89(10):1298–301. doi: 10.1136/bjo.2005.070649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asrani S, et al. Large diurnal fluctuations in intraocular pressure are an independent risk factor in patients with glaucoma. J Glaucoma. 2000;9(2):134–42. doi: 10.1097/00061198-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Armaly MF. Statistical Attributes of the Steroid Hypertensive Response in the Clinically Normal Eye. I. The Demonstration of Three Levels of Response. Invest Ophthalmol. 1965;4:187–97. [PubMed] [Google Scholar]
- 8.Becker B. Intraocular Pressure Response to Topical Corticosteroids. Invest Ophthalmol. 1965;4:198–205. [PubMed] [Google Scholar]
- 9.Stone EM, et al. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275:668–670. doi: 10.1126/science.275.5300.668. [DOI] [PubMed] [Google Scholar]
- 10.Wilkinson C, et al. Tonography demonstrates reduced facility of outflow of aqueous humor in myocilin mutation carriers. J Glaucoma. 2003;12:237–242. doi: 10.1097/00061198-200306000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Leydhecker W, Akiyama K, Neumann HG. Intraocular pressure in normal human eyes. Klin Monbl Augenheilkd Augenarztl Fortbild. 1958;133(5):662–70. [PubMed] [Google Scholar]
- 12.Palmberg P. Evidence-based target pressures: how to choose and achieve them. Int Ophthalmol Clin. 2004;44(2):1–14. doi: 10.1097/00004397-200404420-00003. [DOI] [PubMed] [Google Scholar]
- 13.Ayyala RS, et al. A clinical study of the Ahmed glaucoma valve implant in advanced glaucoma. Ophthalmology. 1998;105(10):1968–76. doi: 10.1016/S0161-6420(98)91049-1. [DOI] [PubMed] [Google Scholar]
- 14.Azuara-Blanco A, Katz LJ. Dysfunctional filtering blebs. Surv Ophthalmol. 1998;43(2):93–126. doi: 10.1016/s0039-6257(98)00025-3. [DOI] [PubMed] [Google Scholar]
- 15.Leske MC, et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114(11):1965–72. doi: 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Bahler C, et al. Pharmacological disruption of Schlemm’s canal cells and outflow facility in anterior segments of human eyes. Invest Ophthalmol Vis Sci. 2004;45:2246–2254. doi: 10.1167/iovs.03-0746. [DOI] [PubMed] [Google Scholar]
- 17.Johnson M, et al. Modulation of outflow resistance by the pores of the inner wall endothelium. Invest Ophthalmol Vis Sci. 1992;33(5):1670–1675. [PubMed] [Google Scholar]
- 18.Maepa O, Bill A. Pressures in the juxtacanalicular tissue and Schlemm’s canal in monkeys. Exp Eye Res. 1992;54:879–883. doi: 10.1016/0014-4835(92)90151-h. [DOI] [PubMed] [Google Scholar]
- 19.Overby DR, Stamer WD, Johnson M. The changing paradigm of outflow resistance generation: towards synergistic models of the JCT and inner wall endothelium. Exp Eye Res. 2009;88(4):656–70. doi: 10.1016/j.exer.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gong H, Freddo TF. The washout phenomenon in aqueous outflow--why does it matter? Exp Eye Res. 2009;88(4):729–37. doi: 10.1016/j.exer.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu Z, et al. The mechanism of increasing outflow facility by rho-kinase inhibition with Y-27632 in bovine eyes. Exp Eye Res. 2008;86(2):271–81. doi: 10.1016/j.exer.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sabanay I, et al. H-7 effects on the structure and fluid conductance of monkey trabecular meshwork. Archives of Ophthalmology. 2000;118(7):955–62. [PubMed] [Google Scholar]
- 23.Ethier C, Chan D. Cationic ferritin changes outflow facility whereas anionic ferritin does not. Invest Ophthalmol Vis Sci. 2001;42:1795–1802. [PubMed] [Google Scholar]
- 24.Johnson M, et al. The pore density in the inner wall endothelium of Schlemm’s canal of glaucomatous eyes. Invest Ophthalmol Vis Sci. 2002;43(9):2950–5. [PubMed] [Google Scholar]
- 25.Allingham RR, et al. The relationship between pore density and outflow facility in human eyes. Invest Ophthalmol Vis Sci. 1992;33:1661–1669. [PubMed] [Google Scholar]
- 26**.Pedrigi RM, et al. Pores Form in Cultured Schlemm_s Canal Endothelial Cells During Transendothelial Perfusion. Invest Ophthalmol Vis Sci. 2011 ARVO: p. abstract#6265. This is the first demonstration of pore formation by human Schlemm’s canal cells in culture, showing pressure dependence and fewer pores in glaucomatous cells versus normal. [Google Scholar]
- 27.Wan Z, Woodward DF, Stamer WD. Endogenous Bioactive Lipids and the Regulation of Conventional Outflow Facility. Expert Rev Ophthalmol. 2008;3(4):457–470. doi: 10.1586/17469899.3.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bahler CK, et al. Prostaglandins increase trabecular meshwork outflow facility in cultured human anterior segments. Am J Ophthalmol. 2008;145(1):114–9. doi: 10.1016/j.ajo.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29**.Millard LH, Woodward DF, Stamer WD. The Role of the Prostaglandin EP4 Receptor in the Regulation of Human Outflow Facility. Invest Ophthalmol Vis Sci. 2011;52(6):3506–13. doi: 10.1167/iovs.10-6510. This study shows the pivotal role of prostaglandin EP4 receptor activation in the regulation of human pressure-dependent outflow. [DOI] [PubMed] [Google Scholar]
- 30.Wan Z, et al. Bimatoprost, prostamide activity, and conventional drainage. Invest Ophthalmol Vis Sci. 2007;48(9):4107–15. doi: 10.1167/iovs.07-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woodward DF, et al. Prostanoid EP4 receptor stimulation produces ocular hypotension by a mechanism that does not appear to involve uveoscleral outflow. Invest Ophthalmol Vis Sci. 2009;50(7):3320–8. doi: 10.1167/iovs.08-3031. [DOI] [PubMed] [Google Scholar]
- 32.Toris CB, Gabelt BT, Kaufman PL. Update on the mechanism of action of topical prostaglandins for intraocular pressure reduction. Surv Ophthalmol. 2008;53(Suppl 1):S107–20. doi: 10.1016/j.survophthal.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ziai N, et al. The effects on aqueous dynamics of PhXA41, a new prostaglandin F2 alpha analogue, after topical application in normal and ocular hypertensive human eyes. Arch Ophthalmol. 1993;111(10):1351–8. doi: 10.1001/archopht.1993.01090100059027. [DOI] [PubMed] [Google Scholar]
- 34*.Stamer WD, et al. Cellular basis for bimatoprost effects on human conventional outflow. Invest Ophthalmol Vis Sci. 2010;51(10):5176–81. doi: 10.1167/iovs.09-4955. This study details the role of the trabecular meshwork cytoskeleton in bimatoprost effects on cells of the human conventional outflow tract using a novel assay system, cell dielectric spectroscopy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Njie YF, et al. Aqueous humor outflow effects of 2-arachidonylglycerol. Exp Eye Res. 2008;87(2):106–14. doi: 10.1016/j.exer.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Njie YF, et al. N-arachidonylethanolamide-induced increase in aqueous humor outflow facility. Invest Ophthalmol Vis Sci. 2008;49(10):4528–34. doi: 10.1167/iovs.07-1537. [DOI] [PubMed] [Google Scholar]
- 37.Njie YF, et al. Noladin ether acts on trabecular meshwork cannabinoid (CB1) receptors to enhance aqueous humor outflow facility. Invest Ophthalmol Vis Sci. 2006;47(5):1999–2005. doi: 10.1167/iovs.05-0729. [DOI] [PubMed] [Google Scholar]
- 38.Zhong L, et al. CB2 cannabinoid receptors in trabecular meshwork cells mediate JWH015-induced enhancement of aqueous humor outflow facility. Invest Ophthalmol Vis Sci. 2005;46(6):1988–92. doi: 10.1167/iovs.04-0651. [DOI] [PubMed] [Google Scholar]
- 39.Stamer W, et al. CB1 cannabinoid receptor expression, activation and detection of endogenous ligand in trabecular meshwork and cilary process tissues. Eur J Pharmacol. 2001;431:277–286. doi: 10.1016/s0014-2999(01)01438-8. [DOI] [PubMed] [Google Scholar]
- 40.Mettu P, et al. Role of lysophopholipid growth factors in the modulation of aqueous humor outflow facility. Invest Ophthalmol Vis Sci. 2004;45:2263–2271. doi: 10.1167/iovs.03-0960. [DOI] [PubMed] [Google Scholar]
- 41.Stamer WD, et al. Sphingosine-1-phosphate effects on the inner wall of Schlemm’s canal and outflow facility in perfused human eyes. Exp Eye Res. 2009;89(6):980–8. doi: 10.1016/j.exer.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42*.Sumida GM, Stamer WD. Sphingosine-1-phosphate enhancement of cortical actomyosin organization in cultured human Schlemm’s canal endothelial cell monolayers. Invest Ophthalmol Vis Sci. 2010;51(12):6633–8. doi: 10.1167/iovs.10-5391. This paper documents the cellular basis for of sphingosine-1-phosphate effects on conventional outflow inhibition, involving the stabilization of cortical cellular structures associated with Schlemm’s canal intercellular junctions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43**.Sumida GM, Stamer WD. S1P2 receptor regulation of sphingosine-1-phosphate effects on conventional outflow physiology. Am J Physiol Cell Physiol. 2011;300(5):C1164–71. doi: 10.1152/ajpcell.00437.2010. This study pharmacologically identifies the S1P receptor subtype responsible for sphingosine-1-phosphate ‘s inhibition of conventional outflow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tripathi RC, et al. Aqueous humor in glaucomatous eyes contains an increased level of TGF-beta 2. Exp Eye Res. 1994;59(6):723–7. doi: 10.1006/exer.1994.1158. [DOI] [PubMed] [Google Scholar]
- 45.Gottanka J, et al. Effects of TGF-beta2 in perfused human eyes. Investigative Ophthalmology & Visual Science. 2004;45(1):153–8. doi: 10.1167/iovs.03-0796. [DOI] [PubMed] [Google Scholar]
- 46.Fuchshofer R, Welge-Lussen U, Lutjen-Drecoll E. The effect of TGF-beta2 on human trabecular meshwork extracellular proteolytic system. Exp Eye Res. 2003;77(6):757–65. doi: 10.1016/s0014-4835(03)00220-3. [DOI] [PubMed] [Google Scholar]
- 47.Fuchshofer R, et al. Gene expression profiling of TGFbeta2- and/or BMP7-treated trabecular meshwork cells: Identification of Smad7 as a critical inhibitor of TGF-beta2 signaling. Exp Eye Res. 2009;88(6):1020–32. doi: 10.1016/j.exer.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wordinger RJ, et al. Effects of TGF-beta2, BMP-4, and gremlin in the trabecular meshwork: implications for glaucoma. Invest Ophthalmol Vis Sci. 2007;48(3):1191–200. doi: 10.1167/iovs.06-0296. [DOI] [PubMed] [Google Scholar]
- 49.Wang WH, et al. Increased expression of the WNT antagonist sFRP-1 in glaucoma elevates intraocular pressure. J Clin Invest. 2008;118(3):1056–64. doi: 10.1172/JCI33871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50**.Li A, et al. Cytoskeletal dependence of adenosine triphosphate release by human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2011;52(11):7996–8005. doi: 10.1167/iovs.11-8170. This study demonstrated the dependence of mechanical stimulated ATP release on trabecular meshwork cytoskeleton, having implications for lack of responsiveness of cells in steroid-induced and primary open-angle glaucoma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51*.Li A, et al. Mechanisms of ATP release by human trabecular meshwork cells, the enabling step in purinergic regulation of aqueous humor outflow. J Cell Physiol. 2011 doi: 10.1002/jcp.22715. This study for the first time identifies the molecular pathways, pannexin-1 and connexins, responsible for release of ATP from human trabecular meshwork cells, triggered mechanical strain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52*.Chowdhury UR, et al. ATP-sensitive potassium (KATP) channel activation decreases intraocular pressure in the anterior chamber of the eye. Invest Ophthalmol Vis Sci. 2011;52(9):6435–42. doi: 10.1167/iovs.11-7523. This study demonstrated that opening of an ATP-sensitive potassium channel nearly doubles outflow facility in perfused human eyes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crosson CE, Sloan CF, Yates PW. Modulation of conventional outflow facility by the adenosine A1 agonist N6-cyclohexyladenosine. Invest Ophthalmol Vis Sci. 2005;46(10):3795–9. doi: 10.1167/iovs.05-0421. [DOI] [PubMed] [Google Scholar]
- 54*.Borghi V, et al. A novel nitric oxide releasing prostaglandin analog, NCX 125, reduces intraocular pressure in rabbit, dog, and primate models of glaucoma. J Ocul Pharmacol Ther. 2010;26(2):125–32. doi: 10.1089/jop.2009.0120. This studyreports that a novel bifunctional compound, containing prostaglandin F2α and a nitric oxide donor, lowers introcular pressure better than F2α agonist alone. [DOI] [PubMed] [Google Scholar]
- 55.Dismuke WM, Mbadugha CC, Ellis DZ. NO-induced regulation of human trabecular meshwork cell volume and aqueous humor outflow facility involve the BKCa ion channel. Am J Physiol Cell Physiol. 2008;294(6):C1378–86. doi: 10.1152/ajpcell.00363.2007. [DOI] [PubMed] [Google Scholar]
- 56.Nathanson JA. Nitrovasodilators as a new class of ocular hypotensive agents. J Pharmacol Exp Ther. 1992;260(3):956–65. [PubMed] [Google Scholar]
- 57.Schuman JS, Erickson K, Nathanson JA. Nitrovasodilator effects on intraocular pressure and outflow facility in monkeys. Exp Eye Res. 1994;58(1):99–105. doi: 10.1006/exer.1994.1199. [DOI] [PubMed] [Google Scholar]
- 58**.Stamer WD, et al. eNOS, a Pressure-Dependent Regulator of Intraocular Pressure. Invest Ophthalmol Vis Sci. 2011 doi: 10.1167/iovs.11-7839. in press. Using transgenic mice overexpressing eNOS, this study shows that nitric oxide doubles pressure-dependent outflow, thereby lowering intraocular pressure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Acott TS, Kelley MJ. Extracellular matrix in the trabecular meshwork (Review) Exp Eye Res. 2008;86:543–561. doi: 10.1016/j.exer.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keller K, et al. Extracellular matrix turnover and outflow resistance. Exp Eye Res. 2009;88:676–682. doi: 10.1016/j.exer.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bradley JMB, et al. Effects of mechanical stretching on trabecular matrix metalloproteinases. Invest Ophthalmol Vis Sci. 2001;42:1505–1513. [PubMed] [Google Scholar]
- 62.Borras T, et al. Effects of elevated intraocular pressure on outflow facility and TIGR/MYOC expression in perfused human anterior segments. Invest Ophthalmol Vis Sci. 2002;43(1):33–40. [PubMed] [Google Scholar]
- 63.Johnstone M, Grant M. Pressure-dependent changes in structures of the aqueous outflow system of human and monkey eyes. Am J Ophthalmol. 1973;75:365–383. doi: 10.1016/0002-9394(73)91145-8. [DOI] [PubMed] [Google Scholar]
- 64.Vittal V, et al. Changes in gene expression by trabecular meshwork cells in response to mechanical stretching. Invest Ophthalmol Vis Sci. 2005;46(8):2857–68. doi: 10.1167/iovs.05-0075. [DOI] [PubMed] [Google Scholar]
- 65.Vittitow J, Borras T. Genes expressed in the human trabecular meshwork during pressure-induced homeostatic response. J Cell Physiol. 2004;201(1):126–37. doi: 10.1002/jcp.20030. [DOI] [PubMed] [Google Scholar]
- 66.Aga M, et al. Specialized Podosome- or Invadopodia-like Structures (PILS) for Focal Trabecular Meshwork Extracellular Matrix Turnover. Invest Ophthalmol Vis Sci. 2008;49:5353–5365. doi: 10.1167/iovs.07-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bradley JM, et al. Signaling pathways used in trabecular matrix metalloproteinase response to mechanical stretch. Invest Ophthalmol Vis Sci. 2003;44(12):5174–81. doi: 10.1167/iovs.03-0213. [DOI] [PubMed] [Google Scholar]
- 68.Borras T. Gene expression in the trabecular meshwork and the influence of intraocular pressure. Prog Retin Eye Res. 2003;22(4):435–63. doi: 10.1016/s1350-9462(03)00018-1. [DOI] [PubMed] [Google Scholar]
- 69.Keller KE, Kelley MJ, Acott TS. Extracellular matrix gene alternative splicing by trabecular meshwork cells in response to mechanical stretching. Invest Ophthalmol Vis Sci. 2007;48(3):1164–72. doi: 10.1167/iovs.06-0875. [DOI] [PubMed] [Google Scholar]
- 70.Tumminia SJ, et al. Mechanical stretch alters the actin cytoskeletal network and signal transduction in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1998;39:1361–1371. [PubMed] [Google Scholar]
- 71.Ramos RF, Sumida GM, Stamer WD. Cyclic mechanical stress and trabecular meshwork cell contractility. Invest Ophthalmol Vis Sci. 2009;50(8):3826–32. doi: 10.1167/iovs.08-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fuchshofer R, Tamm ER. Modulation of extracellular matrix turnover in the trabecular meshwork. Exp Eye Res. 2009;88(4):683–8. doi: 10.1016/j.exer.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 73.Keller K, Bradley JM, Acott TS. Differential effects of ADAMTSs -1, -4, and -5 in the Trabecular Meshwork. Invest Ophthalmol Vis Sci. 2009;50:5769–5777. doi: 10.1167/iovs.09-3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baetz NW, et al. Role of aquaporin-1 in trabecular meshwork cell homeostasis during mechanical strain. Exp Eye Res. 2009;89(1):95–100. doi: 10.1016/j.exer.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liton PB, et al. Induction of IL-6 expression by mechanical stress in the trabecular meshwork. Biochem Biophys Res Commun. 2005;337(4):1229–36. doi: 10.1016/j.bbrc.2005.09.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Keller KE, Bradley JM, Acott TS. Differential effects of ADAMTS-1, -4, and -5 in the trabecular meshwork. Invest Ophthalmol Vis Sci. 2009;50(12):5769–77. doi: 10.1167/iovs.09-3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77**.Luna C, et al. MicroRNA-24 regulates the processing of latent TGFbeta1 during cyclic mechanical stress in human trabecular meshwork cells through direct targeting of FURIN. J Cell Physiol. 2011;226(5):1407–14. doi: 10.1002/jcp.22476. This study was the first to link cyclic mechanical stress, the regulation of micro RNAs and TGF pathway in cells that participate in the regulation of pressure-dependent outflow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.WuDunn D. Mechanobiology of trabecular meshwork cells. Exp Eye Res. 2009;88(4):718–23. doi: 10.1016/j.exer.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 79.Zhang M, Maddala R, Rao PV. Novel molecular insights into RhoA GTPase-induced resistance to aqueous humor outflow through the trabecular meshwork. Am J Physiol Cell Physiol. 2008;295(5):C1057–70. doi: 10.1152/ajpcell.00481.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80*.Faralli JA, et al. Integrin-linked kinase regulates integrin signaling in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2011;52(3):1684–92. doi: 10.1167/iovs.10-6397. This study documents the role for integrin-linked kinase in communicating integrin signaling and trabecular cell cytoskeletal organizaton. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81**.Schwinn MK, et al. Heparin II domain of fibronectin mediates contractility through an alpha4beta1 co-signaling pathway. Exp Cell Res. 2010;316(9):1500–12. doi: 10.1016/j.yexcr.2010.03.010. This study reports that control of trabecular meshwork contractility works through alpha4beta1 integrin binding to PPRARI sequence in heparin II domain of fibronectin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rao PV, et al. Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Invest Ophthalmol Vis Sci. 2001;42(5):1029–37. [PubMed] [Google Scholar]
- 83.Kaufman PL, Erickson KA. Cytochalasin B and D dose-outflow facility response relationships in the cynomolgus monkey. Investigative Ophthalmology Visual Science. 1982;23:646–650. [PubMed] [Google Scholar]
- 84.Epstein DL, Rowlette LL, Roberts BC. Acto-myosin drug effects and aqueous outflow function. Invest Ophthalmol Vis Sci. 1999;40(1):74–81. [PubMed] [Google Scholar]
- 85.Chen J, Runyan SA, Robinson MR. Novel ocular antihypertensive compounds in clinical trials. Clin Ophthalmol. 2011;5:667–77. doi: 10.2147/OPTH.S15971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee AJ, Goldberg I. Emerging drugs for ocular hypertension. Expert Opin Emerg Drugs. 2011;16(1):137–61. doi: 10.1517/14728214.2011.521631. [DOI] [PubMed] [Google Scholar]
- 87**.Williams RD, et al. Ocular Hypotensive Effect of the Rho Kinase Inhibitor AR-12286 in Patients With Glaucoma and Ocular Hypertension. Am J Ophthalmol. 2011;152(5):834–841. e1. doi: 10.1016/j.ajo.2011.04.012. This study reports data from a phase 2b clinical trial showing the safety and efficacy of a new class of anti-glaucoma drugs, the rho kinase inhibitors, which target the pressure-dependent outflow pathway. Successfully targeting the diseased conventional outflow pathway with a daily medication, represents a major advance in the treatment of glaucoma. [DOI] [PubMed] [Google Scholar]
- 88*.Filla MS, et al. Dexamethasone-associated cross-linked actin network formation in human trabecular meshwork cells involves beta3 integrin signaling. Invest Ophthalmol Vis Sci. 2011;52(6):2952–9. doi: 10.1167/iovs.10-6618. This study shows data that implicates beta3 integrin signaling in the formation of glucocorticoid-induced CLANS in trabecular meshwork cells, having implications to better understanding/treating steroid-induced glaucoma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89*.O’Reilly S, et al. Inducers of cross-linked actin networks in trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2011;52(10):7316–24. doi: 10.1167/iovs.10-6692. This study documents the efficacy of TGFbeta2 induction of CLAN formation in trabecular meshwork cells. [DOI] [PubMed] [Google Scholar]
- 90.Filla MS, et al. Beta1 and beta3 integrins cooperate to induce syndecan-4-containing cross-linked actin networks in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2006;47(5):1956–67. doi: 10.1167/iovs.05-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Clark AF, et al. Dexamethasone alters F-actin architecture and promotes cross-linked actin network formation in human trabecular meshwork tissue. Cell Motil Cytoskeleton. 2005;60(2):83–95. doi: 10.1002/cm.20049. [DOI] [PubMed] [Google Scholar]
- 92.Clark AF, et al. Glucocorticoid-induced formation of cross-linked actin networks in cultured human trabecular meshwork cells. Investigative Ophthalmology Visual Science. 1994;35:281–294. [PubMed] [Google Scholar]
- 93**.Pattabiraman PP, Rao PV. Mechanistic basis of Rho GTPase-induced extracellular matrix synthesis in trabecular meshwork cells. Am J Physiol Cell Physiol. 2010;298(3):C749–63. doi: 10.1152/ajpcell.00317.2009. This study shows the relationship between cell contractility, Rho GTPase signaling and extracellular matrix expression in trabecular meshwork cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94*.VanderWyst SS, et al. Structural basement membrane components and corresponding integrins in Schlemm’s canal endothelia. Mol Vis. 2011;17:199–209. This study documents collagens, laminins and cognate integrin receptors expressed in the basement membrane of Schlemm’s canal endothelia. [PMC free article] [PubMed] [Google Scholar]
- 95.Gonzalez JM, Jr, et al. Effect of heparin II domain of fibronectin on actin cytoskeleton and adherens junctions in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2006;47(7):2924–31. doi: 10.1167/iovs.06-0038. [DOI] [PubMed] [Google Scholar]
- 96.Keller KE, et al. Effects of modifiers of glycosaminoglycan biosynthesis on outflow facillity in perfusion culture. Invest Ophthalmol Vis Sci. 2008;49:2495–2505. doi: 10.1167/iovs.07-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97**.Keller KE, et al. Segmental versican expression in the trabecular meshwork and involvement in outflow facility. Invest Ophthalmol Vis Sci. 2011;52(8):5049–57. doi: 10.1167/iovs.10-6948. This study identifies the large proteoglycan versican, interacting with long hyaluronan glycosaminoglycan chains, as a central component of the aqueous humor outflow resistance. It also further demonstrates the degree to which outflow is segmental rather than uniform around the circumference of the eye. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hann CR, Bahler CK, Johnson DH. Cationic ferritin and segmental flow through the trabecular meshwork. Invest Ophthalmol Vis Sci. 2005;46(1):1–7. doi: 10.1167/iovs.04-0800. [DOI] [PubMed] [Google Scholar]
- 99.Hann CR, Fautsch MP. Preferential fluid flow in the human trabecular meshwork near collector channels. Invest Ophthalmol Vis Sci. 2009;50(4):1692–7. doi: 10.1167/iovs.08-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Buller C, Johnson D. Segmental variability of the trabecular meshwork in normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 1994;35(11):3841–51. [PubMed] [Google Scholar]
- 101*.Last JA, et al. Elastic modulus determination of normal and glaucomatous human trabecular meshwork. Invest Ophthalmol Vis Sci. 2011;52(5):2147–52. doi: 10.1167/iovs.10-6342. Using atomic force microscopy, this study demonstrates that the cellular/extracellular matrix of the juxtacanalicular region of glaucomatous donor eyes is much more rigid than normal eyes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Johnson DH. Does pigmentation affect the trabecular meshwork? Arch Ophthalmol. 1989;107(2):250–4. doi: 10.1001/archopht.1989.01070010256032. [DOI] [PubMed] [Google Scholar]
- 103.Aga M, et al. Specialized podosome- or invadopodia-like structures (PILS) for focal trabecular meshwork extracellular matrix turnover. Invest Ophthalmol Vis Sci. 2008;49(12):5353–65. doi: 10.1167/iovs.07-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liton PB, Gonzalez P, Epstein DL. The role of proteolytic cellular systems in trabecular meshwork homeostasis. Exp Eye Res. 2009;88(4):724–8. doi: 10.1016/j.exer.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105*.Joe MK, Tomarev SI. Expression of myocilin mutants sensitizes cells to oxidative stress-induced apoptosis: implication for glaucoma pathogenesis. Am J Pathol. 2010;176(6):2880–90. doi: 10.2353/ajpath.2010.090853. Transgenic expression of Y437H mutant myocilin upregulates an endoplasmic reticulum stress marker and depletes antioxidants in “angle” tissues of mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106*.Izzotti A, et al. Mitochondrial damage in the trabecular meshwork of patients with glaucoma. Arch Ophthalmol. 2010;128(6):724–30. doi: 10.1001/archophthalmol.2010.87. This study documented the increased loss of mitochondria and mitochondrial DNA deletion in trabeculeculectomy specimens from glaucomatous patients versus from post mortem donor eyes. [DOI] [PubMed] [Google Scholar]
- 107**.Lin Y, Epstein DL, Liton PB. Intralysosomal iron induces lysosomal membrane permeabilization and cathepsin D-mediated cell death in trabecular meshwork cells exposed to oxidative stress. Invest Ophthalmol Vis Sci. 2010;51(12):6483–95. doi: 10.1167/iovs.10-5410. The present study proposes a mechanism, involving cathepsin-D, by which oxidative stress results in increased cell loss in the conventional outflow pathway in glaucoma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108*.Chen M, et al. Mitochondria-targeted peptide MTP-131 alleviates mitochondrial dysfunction and oxidative damage in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2011;52(10):7027–37. doi: 10.1167/iovs.11-7524. This study shows that several indicators of oxidative damage in trabecular meshwork cells were ameliorated upon treatment with a peptide that preferentially targets the mitochondria. [DOI] [PubMed] [Google Scholar]
- 109.Luna C, et al. Resveratrol prevents the expression of glaucoma markers induced by chronic oxidative stress in trabecular meshwork cells. Food Chem Toxicol. 2009;47(1):198–204. doi: 10.1016/j.fct.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]



