Abstract
Background
Aggressive surgical resection with intent to cure and surgical debulking procedures are commonly recommended in patients with metastatic pheochromocytoma and paraganglioma. To date there are no data on operative outcomes of patients after surgical resection of metastatic pheochromocytoma and paraganglioma to determine if such an approach is appropriate and what factors may be associated with a favorable outcome.
Study Design
Retrospective analysis of 30 patients with metastatic pheochromocytoma/paraganglioma who underwent surgical treatment. Clinical characteristics and genetic factors were analyzed as predictors of biochemical response to surgery.
Results
Thirty patients underwent a total of 42 operations with a median follow-up time of 24 months (range, 1 to 114). Complete disease resection (R0/R1) was achieved in 18 (42.9%) cases, while 24 cases (57.1%) were debulking (R2) procedures without intent to cure. Complete biochemical remission was achieved in 10 (23.8%) cases and partial biochemical response was achieved in 23 (54.8%) cases. Patients with disease confined to the abdomen were more likely to achieve and maintain a biochemical response postoperatively than those with extra-abdominal disease (P = 0.0003). Debulking operations were significantly less likely to achieve or maintain biochemical palliation, with only 1 patient maintaining a biochemical response 12 months postoperatively (P < 0.0001). Patients were less likely to obtain pharmacologic independence following debulking (P = 0.0003), with only 2 (8.3%) not requiring pharmacotherapy six months after the intervention. Factors not associated with biochemical response to surgery include gender, family history, SDHB mutation status, systemic therapy, and preoperative biochemical profile.
Conclusions
Depending on the extent of disease, patients with metastatic pheochromocytoma/paraganglioma can benefit from aggressive operative intervention and resection with intent to cure. Debulking procedures are unlikely to achieve clinically significant biochemical response, with any biochemical response achieved being very short-lived.
Keywords: pheochromocytoma, paraganglioma, surgery, outcome, metastatic
Introduction
Pheochromocytomas and paragangliomas are tumors arising from chromaffin cells of the adrenals and autonomic paraganglia. According to the 2004 Word Health Organization classification, chromaffin tumors arising directly from the adrenal glands are classified as pheochromocytomas, while those arising from extra-adrenal autonomic paraganglia are classified as paragangliomas (1). Such tumors are often characterized by secretion of catecholamines, with the notable exception of head and neck paragangliomas, which are often biochemically inactive (2,3).
Malignant pheochromocytoma/paraganglioma is defined by the presence of tumor at sites normally devoid of chromaffin cells or by local invasion by the primary tumor (4–6). The rate of metastatic disease is variable, with reports ranging from 2.5% to 40% (6–8). Recurrence, either locoregional or metastatic, usually occurs within 5 years of initial complete resection but has been reported up to 40 years postoperatively (8). Multiple non-anatomic parameters have also been associated with metastatic disease. Pathologic features more often associated with metastatic disease include primary tumor size greater than 6 cm, necrosis, hemorrhage, and high mitotic index (9,10). Possible biochemical indicators of malignant disease include dopamine hypersecretion measured by methoxytyramine, or markedly elevated plasma or urinary metanephrines (10–13). The most well defined genetic risk factor for malignant disease is a mutation in the SDHB gene, which is clinically associated with an earlier onset of disease and more aggressive malignancy (2,14,15).
Surgical resection is the only potentially curative treatment for pheochromocytomas and paragangliomas (16). Initial complete resection with intent to cure (R0) has been shown to improve survival, while surgical debulking is often used in an attempt to achieve biochemical control, improve response to systemic therapies, palliate symptoms, or simply to decrease tumor burden (5,6,17,18). However, there are no data on the benefits of aggressive resection or debulking in the setting of locally invasive, metastatic, or recurrent disease (18–20). Furthermore, there are currently no clinical, genetic, or pathologic parameters that clinicians can rely on to guide operative decision making. The present study seeks to characterize outcomes of patients who underwent operation for locally invasive, metastatic, or recurrent pheochromocytoma/paraganglioma and identify clinical factors that might aid in patient selection and determine patient outcomes.
Methods
Patients
Data pertaining to patient demographics, genetic tests, pathology, radiology, and operative history were reviewed in patients with malignant and metastatic pheochromocytomas and abdominal paragangliomas who were evaluated at the National Institutes of Health (NIH) Warren Magnuson Clinical Center on clinical protocols. All patients underwent genetic testing for mutations and deletions in SDHA, SDHB, SDHC, SDHD, SDHAF2, RET, VHL, and TMEM127. These genetic tests were performed in collaboration with the Mayo Clinic in Rochester, Minnesota. Postoperative follow-up consisted of biochemical testing (plasma catecholamines, metanephrines) and imaging studies (CT, MRI, and FDG-PET imaging) as part of the NIH clinical protocol. Postoperative follow-up consisted of biochemical testing (plasma catecholamines, metanephrines) and interval imaging studies (CT, MRI, and FDG-PET imaging) as part of the NIH clinical protocol. This review led to identification of sixty-one patients that received an operation for biochemically active malignant disease. Of those, thirty patients receiving a total of 42 operations had adequate preoperative data and postoperative follow up to be included in the present study.
Classification of Laboratory Values
Biochemical laboratory values were used as the primary indicator of disease burden, remission, and recurrence. Any biochemical elevation above the upper limit of normal was considered evidence of disease. Seven laboratory values were used as disease surrogates: chromogranin A (upper limit of normal, 225 ng/mL); plasma fractionated metanephrines (61 pg/mL), normetanephrines (112 pg/mL), epinephrine (83 pg/mL), norepinephrine (498 pg/mL), and dopamine (46 pg/mL); and 24 hour urinary fractionated metanephrine and normetanephrine (400 μg/24hrs). Patients are instructed to discontinue use of medications which may result in false positive results prior to laboratory testing with blood pressure monitoring when off medications. Laboratory studies performed within three months of the intervention were used as surrogates for preoperative disease burden. Postoperative values were categorized into three and six month intervals and were recorded for the duration of follow up (median 24 months, range 1–99). Only patients with preoperative lab values and postoperative labs drawn within 6 months of the intervention were included in the study cohort using the same assay, and only those labs with known preoperative and postoperative values were considered for analysis.
Classification of Disease at Presentation
Currently, there is no widely accepted staging system for malignant pheochromocytoma/paraganglioma. For the purposes of this study, the extent of disease was classified based on preoperative anatomic imaging results. Patients were separated into four major subgroups based on anatomic tumor burden: locoregional disease, abdominal metastasis, thoracic metastasis, and bony metastasis. Locoregional disease was considered only in the setting of gross local invasion of surrounding organs or soft tissues. Utilizing these data, patients were further grouped into two categories. Category 1 consisted of patients with tumor confined to the abdomen, either locoregional or metastatic. Category 2 consisted of patients with metastatic disease outside the abdomen.
Statistical Analysis
The primary outcome variables analyzed were duration of biochemical response. Disease-free interval was defined by complete biochemical remission, with all indicative laboratory values returning to the normal range postoperatively. Partial biochemical response was defined as having at least one, but not all, biochemical laboratory values returning to the normal range postoperatively.
Initial data analysis was performed including all 42 operations. To assess whether there was appreciable bias from the use of multiple operations, the analysis was validated using only the initial operation for each of the 30 patients. The association between clinical and genetic factors and length of biochemical response was assessed using Kaplan-Meier survival analysis. The statistical difference between Kaplan-Meier curves was determined using the log-rank test. The Cox proportional hazards model was used to determine the joint significance of factors associated with return of laboratory values to normal levels. The associations between the outcome parameters and clinical variables were determined by Fisher’s exact test when comparing a dichotomous parameter, Mehta’s modification to Fisher’s exact test when comparing to categorical values, and an exact Cochran-Armitage test when comparing categorical results (21). All p-values are two-tailed and presented without any adjustment for multiple comparisons; thus, p<0.005 was used as a cut-off for statistical significance due to the number of tests performed.
Results
Patient Characteristics
Of the 30 patients in the study cohort, 7 patients underwent multiple operations. Only two of the seven patients with multiple operations included had more than two procedures: one patient had six operations over six years, and another patient had three operations in three years. Twelve (40%) patients had a germline mutation in the SDHB gene, with one patient positive for SDHD. The other 17 (56.7%) patients had sporadic tumors not associated with any known genetic abnormality. Additional clinical characteristics of the study cohort are is summarized in Table 1.
Table 1.
Study Cohort Clinical Characteristics
| Sex | |
| Male/female | 17/13 |
| Age at diagnosis, y | |
| Mean ± SD | 32.4 ± 15.5 |
| Range | 8–63 |
| Primary tumor site | |
| Adrenal pheochromocytoma, n (%) | 24 (80) |
| Extra-adrenal paraganglioma, n (%) | 6 (20) |
| Peri-aortic paraganglioma, n | 4 |
| Bladder paraganglioma, n | 2 |
| Symptoms at presentation, n (%) | |
| Persistent hypertension | 22 (73.3) |
| Headache | 13 (43.3) |
| Palpitations | 14 (46.7) |
| Diaphoresis | 11 (36.7) |
| Family history and genetics, n (%)* | |
| Known family history | 6 (20) |
| SDHB | 12 (40) |
| SDHD | 1 (3.3) |
| Sporadic without known mutation | 17 (56.7) |
| Systemic therapy, n (%) | |
| CVD† | 12 (40) |
| XRT‡ | 7 (23.3) |
| I-131 MIBG | 4 (13.3) |
| Multiple therapies, n (%) | 5 (16.7) |
| CVD + XRT, n | 4 |
| CVD + MIGB + XRT, n | 1 |
| Follow-up, mo§ | |
| Median | 24 ± 31.3 |
| Range | 1–114 |
| Patient status at most recent follow-up, n (%) | |
| Alive | 26 (86.7) |
| Deceased | 4 (13.3) |
Testing included frameshifts and deletions in SDHA, SDHB, SDHC, SDHD, SDHAF2, RET, VHL, and TMEM127.
Cyclophosphamide, Vincristine, Dacarbazine.
External beam radiation.
Patient with one month of follow-up had recent surgery with no biochemical response.
Biochemistry
The mean number of labs elevated preoperatively was 3.0 ± 1.8 (range: 1 to 7). The absolute number of elevated preoperative labs was not predictive of biochemical response to surgery (P = 0.89). Preoperative lab value elevations are summarized in detail in Table 2.
Table 2.
Biochemical Information
| No. with elevated levels | Mean ± SD (range) | |
|---|---|---|
| Chromogranin A, ng/mL* (ULN= 225) | 23 | 3,659.2 ± 3,826.9 (269–27,600) |
| Dopamine, serum, pg/mL, (ULN = 46) | 11 | 641.4 ± 792.8 (59–2,077) |
| Epinephrine, serum, pg/mL (ULN = 83) | 6 | 357.0 ± 235.9 (94–616) |
| Norepinephrine, serum, pg/mL (ULN = 498) | 26 | 3,505.9 ± 4,492.2 (518–16,550) |
| Metanephrines, serum, pg/mL (ULN = 61) | 7 | 567.6 ± 632.7 (69–1,892) |
| Normetanephrines, serum, pg/mL (ULN = 112) | 29 | 1,346.4 ± 1,444.6 (115–4,309) |
| Fractionated Metanephrines, urine, μg/24 h (ULN = 400) | 4 | 5,281.3 ± 3,385.9 (915–8,946) |
While no one lab value was elevated for all 30 patients, all patients had at least one elevated lab value.
Only one patient had isolated chromogranin A elevation without elevated catecholamines. ULN, upper limit of normal.
Extent of Disease
Twenty-four of the primary tumors were adrenal pheochromocytomas, while six were abdominal extra-adrenal paragangliomas (Table 1). Of the 42 operations analyzed, 27 were performed on patients with Category 1 disease (12 locally advanced, 15 abdominal metastasis) and 15 were performed on patients with Category 2 disease (5 thoracic metastasis, 10 bony metastasis).
Outcomes
Operative intervention was the primary method of disease control in all 30 patients, though 16 (53.3%) did receive some systemic therapy (Table 1). Of the 42 operations, 14 (33.3%) were for invasive locoregional disease, 19 (45.2%) were for abdominal metastasis, and 9 (21.4%) were for resection of distant metastasis (4 thorax, 5 bone). Thirty-six of the operations were for disease recurrence, while six were first operations for malignant disease at presentation. Of the six patients presenting for initial resection, three had advanced local disease, two had abdominal metastases, and one had bony metastases at the time of surgery. Locoregional operations achieved complete resection in 8/14 cases (R0/R1), with the remaining 6 being debulking procedures. Operations performed for abdominal metastasis were successful in removing all gross disease in 7/19 cases, and extra-abdominal operations were successful in removal of all gross disease in 3/9 cases. Overall, 18/42 operations were R0/R1 resections.
Median follow-up was 24 ± 31 months (range, 1 to 114 months). Overall survival in the cohort was 86.7% (26/30) at last follow-up. Ten patients (23.8%) had complete biochemical remission by their first follow-up visit. The mean disease-free interval was 52 ± 29 months (range, 12 to 99), with five patients being disease free throughout the entire follow-up period. Partial biochemical response occurred in 23 (54.8%) cases. The mean duration of partial biochemical response was 32.0 ± 27.8 months (range, 1 to 99).
Amongst the demographic, genetic, and clinical factors analyzed, only preoperative extent of disease was significantly associated with duration of biochemical response to surgery. Patients with Category 1 disease (confined to the abdomen, either locoregional or metastatic) were significantly more likely to achieve biochemical response than patients with Category 2 (extra-abdominal) disease (74.1% vs. 20.0%, P = 0.0009). This response was also substantially more durable in Category 1 patients, with more than 40% maintaining a response three years postoperatively (P = 0.0003, Figure 1). Patients with SDHB mutations appeared to demonstrate a less durable biochemical response, but this result did not achieve statistical significance (P = 0.021, Figure 2). The presence of the classic symptom triad (headache, palpitations, and diaphoresis) was also associated with a shorter duration of biochemical response, but did not reach statistical significance (P = 0.0145). Fourteen patients received some form of systemic therapy, with 12 receiving cyclophosphamide, vincristine, and dacarbazine (CVD) chemotherapy, 4 receiving high dose I-131 MIBG therapy, and 7 receiving external beam radiation. Systemic therapy was not significantly associated with postoperative biochemical response (Table 3).
Figure 1.
Biochemical response in patients stratified by extent of disease prior to surgery
Figure 2.
Biochemical response in patients stratified by SDHB mutation status
Table 3.
Operative Characteristics
| Age at operation, y | |
| Median | 42 ± 14.3 |
| Range | 13–68 |
| Operative category, n (%) | |
| Reoperation | 36 (85.7) |
| First operation | 6 (14.3) |
| Disease resected, n (%) | |
| Locoregional | 14 (33.3) |
| Abdominal metastases | 19 (45.2) |
| Liver | 6 |
| Pelvic | 6 |
| Retroperitoneal | 7 |
| Thoracic metastases, n (%) | 4 (9.5) |
| Bony metastases, n (%) | 5 (11.9) |
| Extent of disease at operation, n (%)* | |
| Category 1 | 27 (64.3) |
| Category 2 | 15 (35.7) |
| Preoperative pharmacotherapy, n (%)† | |
| None‡ | 4 (13.3) |
| 1 Drug | 4 (13.3) |
| 2 Drugs | 20 (66.7) |
| 3+ Drugs | 13 (43.3) |
Category 1 is disease confined to the abdomen, Category 2 is extra-abdominal disease.
Drugs included alpha-blockers, beta-blockers, calcium-channel blockers, and metyrosine.
Pharmacotherapy refused by patient.
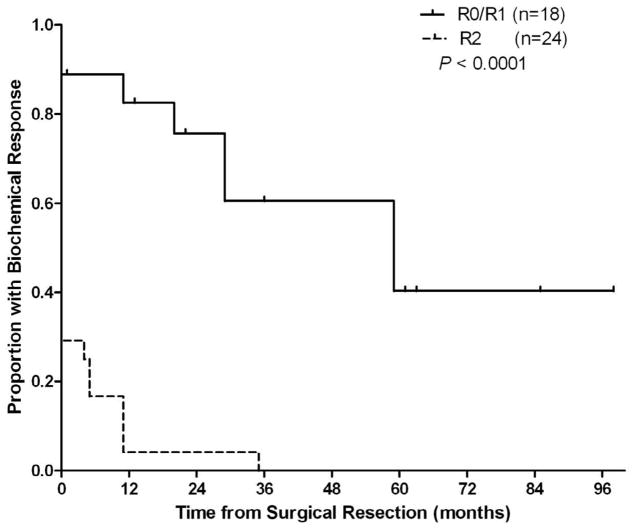
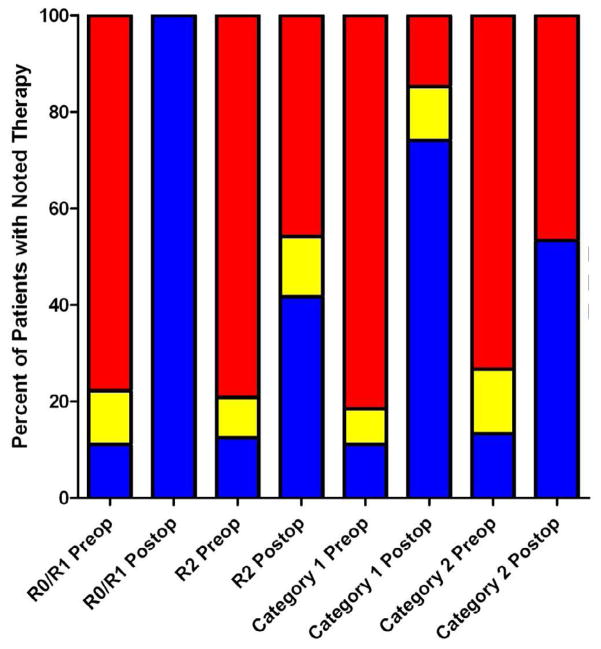
While these clinical factors were associated with clinical response, presence of residual disease postoperatively was the only factor which was found to be significantly associated with duration of biochemical response by the Cox proportional hazards model (Hazard Ratio (HR) =7.42; 95% Confidence Interval for the HR=2.77 to 19.88; p<0.0001). Debulking procedures achieved substantially poorer biochemical response than those operations successfully removing all disease. Of the 18 operations achieving removal of all known disease, 16 (88.9%) led to at least partial biochemical response. Nine of the 10 patients achieving complete biochemical remission were free of all macroscopic disease following the operation based on follow up imaging studies. Of the 24 debulking operations leaving known residual disease, 7 (29.2%) achieved partial biochemical response and 1 (4.2%) achieved complete biochemical remission. The patient achieving complete remission was left with only two rib metastases. Persistence of biochemical response was poor for those 7 patients achieving partial response after debulking, with only 1 patient of the 24 maintaining any response 12 months postoperatively (P < 0.0001, Figure 3). Patients were also less likely to obtain pharmacologic independence following debulking (P = 0.0003), with only 2 (8.3%) not requiring alpha-blockade six months after the intervention (Figure 4). The magnitude of biochemical response for selected subgroups is presented in Figure 5.
Figure 3.
Biochemical response in patients stratified by extent of operative resection
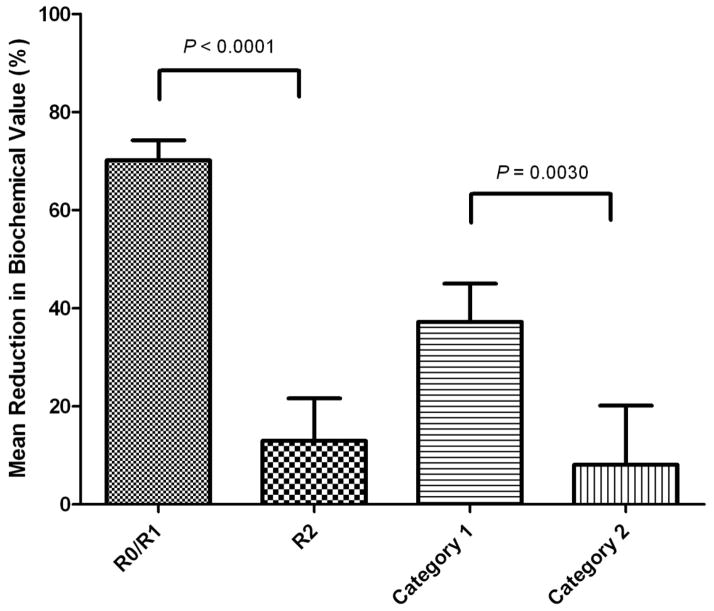
Figure 4.
Mean Laboratory Reduction Achieved by Surgery. R0/R1 mean 70.1% reduction (range, 99.3% reduction to 4% increase); R2 mean 12.9% reduction (range, 99.1% reduction to 236% increase). Category 1 mean 37.2% reduction (range, 99.3% reduction to 236.1% increase); Category 2 mean 8.1% reduction (range 79.2% reduction to 183.5% increase).
Figure 5.
Pharmacotherapy breakdown by subgroups
Discussion
Our study examined the results of 42 operations for metastatic pheochromocytoma/paraganglioma and analyzed factors associated with overall survival, disease-free survival, and biochemical response to surgery. Patients who presented with disease confined to the abdomen (Category 1) were significantly more likely to achieve and maintain a biochemical response, and may be more likely to achieve biochemical remission from surgical resection. Unfortunately, the majority of patients have biochemical recurrence within one year and less than 30% maintain biochemical response 5 years after surgery. We also found poor biochemical outcomes from debulking procedures, with less than 5% of debulking procedures leading to even partial normalization of laboratory values 12 months postoperatively (Figure 3). The analysis also showed a statistical trends towards worse operative outcomes in patients with SDHB mutations (Figure 2) and in patients whose initial presentation involved the classic symptom triad of headache, palpitations, and diaphoresis. The very low overall mortality (4/30, 13.3%) and low frequency of complete disease remission (10/42, 23.8%) in our cohort precluded a meaningful Kaplan-Meier analysis of overall survival and disease-free survival, respectively, given the relatively small sample size.
Of these results, the ineffectiveness of surgical debulking procedures is most striking. While the finding that debulking is oncologically inferior to complete resection is intuitive, the low rates of biochemical response after such procedures should be helpful data when considering a patient for such an operation. This is because debulking procedures are often considered mainstays in operative management of patients with hyperfunctioning malignant disease partially due to the presumed decrease in overall catecholamine secretion leading to symptomatic control and improved cardiovascular outcomes (12,17,22–26). It is also often presumed that extra-peritoneal disease is dedifferentiated and less likely to contribute to overall catecholamine secretion, which may lead some to consider aggressive abdominal debulking operations. However, in our cohort, patients with extra-abdominal metastases with gross resection of abdominal disease did not achieve desirable biochemical outcomes.
Our study identified extra-abdominal disease as an indicator of poor surgical outcomes and challenges the broad utility of debulking procedures as an effective way to ameliorate biochemical activity of pheochromocytomas and paragangliomas. We believe these findings contribute to medical decision making and operative planning for these patients. Our data indicate that aggressive debulkings should not be employed purely to decrease biochemical burden unless there is a reasonable chance of complete resection. This is especially true when noting the general effectiveness of pharmacologic therapy in symptom control, as well as the unclear link between catecholamine secretion and cardiovascular outcomes (19,26–29). A more aggressive approach to abdominal debulking may be warranted, even in cases where complete resection is unlikely, if disease has not spread beyond the abdominal cavity. It is important to note, however, that palliative operations indicated for symptoms such as pain or shortness of breath should not be withheld due to these data. Safe, low morbidity operations may alleviate patient discomfort regardless of biochemical response and may be warranted based on clinical judgment.
There are several limitations to our study. This is a retrospective analysis and does not have a control cohort managed without surgical intervention, which limits the strength of our conclusions. The clinical assessment of surgical outcomes would be significantly strengthened by evaluation of a cohort of patients with malignant pheochromocytoma/paraganglioma managed non-operatively, but this is unlikely to exist given the rarity of the disease and the lack of effective treatment alternatives. Our clinical database did not contain any standardized symptom or quality of life review forms, which hinders quality of life assessment of subjective patient variables such as pain. The use of biochemical laboratory values as surrogate for disease burden also has pitfalls. Catecholamine secretion by pheochromocytomas and paragangliomas is cyclical and thus laboratory markers of disease may not be accurate (30). However, catecholamines such as urinary metanephrines have been shown to be good surrogates for tumor burden (22). False-positive biochemical testing results may occur when the elevation in catecholamine and metabolites is less than two times the upper limit of normal during the initial work up of patients to exclude pheochromocytoma/paraganglioma. However, all the patients in this study had malignant disease with biochemical elevation of catecholamine and metabolites, and axial imaging demonstrating the disease before reoperation. Furthermore, only one patient in the study cohort had laboratory elevations below two times the upper limit of normal during follow up. Finally, aggressive debulking of tumor may be clinically warranted as low morbidity, minimally invasive procedures such as radiofrequency ablation are further evaluated, especially in patients with liver metastasis (31).
In summary, our results indicate that patients without extra-abdominal disease are more likely to achieve both remission and a durable biochemical response from aggressive resection of metastatic pheochromocytoma or paraganglioma, and that debulking surgeries are unlikely to lead to sustained control of catecholamine secretion or pharmacologic independence. Based on our results, we believe that debulking procedures in patients with extra-abdominal metastases should be based only on palliating patient symptoms and not on a preoperative goal of biochemical remission. Conversely, a more aggressive operative approach should be offered to patients with exclusively intra-abdominal disease with a goal of postoperative biochemical remission when a complete resection (RO/R1) is possible.
Footnotes
Disclosure Information: Nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.DeLellis RA. Pathology and genetics of tumours of endocrine organs. Lyon: IARC Press; 2004. [Google Scholar]
- 2.Neumann HP, Pawlu C, Peczkowska M, et al. Distinct clinical features of paraganglioma syndromes associated with SDHB and SDHD gene mutations. JAMA. 2004;292:943–951. doi: 10.1001/jama.292.8.943. [DOI] [PubMed] [Google Scholar]
- 3.Schwaber MK, Glasscock ME, Nissen AJ, et al. Diagnosis and management of catecholamine secreting glomus tumors. Laryngoscope. 1984;94:1008–1015. doi: 10.1288/00005537-198408000-00002. [DOI] [PubMed] [Google Scholar]
- 4.O’Riordain DS, Young WF, Jr, et al. Clinical spectrum and outcome of functional extraadrenal paraganglioma. World J Surg. 1996;20:916–921. doi: 10.1007/s002689900139. discussion 922. [DOI] [PubMed] [Google Scholar]
- 5.Wangberg B, Muth A, Khorram-Manesh A, et al. Malignant pheochromocytoma in a population-based study: survival and clinical results. Ann N Y Acad Sci. 2006;1073:512–516. doi: 10.1196/annals.1353.054. [DOI] [PubMed] [Google Scholar]
- 6.Zarnegar R, Kebebew E, Duh QY, Clark OH. Malignant pheochromocytoma. Surg Oncol Clin N Am. 2006;15:555–571. doi: 10.1016/j.soc.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Adjalle R, Plouin PF, Pacak K, Lehnert H. Treatment of malignant pheochromocytoma. Horm Metab Res. 2009;41:687–696. doi: 10.1055/s-0029-1231025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pacak K, Eisenhofer G, Ahlman H, et al. Pheochromocytoma: recommendations for clinical practice from the First International Symposium October 2005. Nature Clin Pract Endocrin Metabol. 2007;3:92–102. doi: 10.1038/ncpendmet0396. [DOI] [PubMed] [Google Scholar]
- 9.Strong VE, Kennedy T, Al-Ahmadie H, et al. Prognostic indicators of malignancy in adrenal pheochromocytomas: clinical, histopathologic, and cell cycle/apoptosis gene expression analysis. Surgery. 2008;143:759–768. doi: 10.1016/j.surg.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Park J, Song C, Park M, et al. Predictive characteristics of malignant pheochromocytoma. Korean J Urol. 2011;52:241–246. doi: 10.4111/kju.2011.52.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.John H, Ziegler WH, Hauri D, Jaeger P. Pheochromocytomas: can malignant potential be predicted? Urology. 1999;53:679–683. doi: 10.1016/s0090-4295(98)00612-8. [DOI] [PubMed] [Google Scholar]
- 12.Khorram-Manesh A, Ahlman H, Nilsson O, et al. Long-term outcome of a large series of patients surgically treated for pheochromocytoma. J Intern Med. 2005;258:55–66. doi: 10.1111/j.1365-2796.2005.01504.x. [DOI] [PubMed] [Google Scholar]
- 13.Eisenhofer G, Lenders JW, Siegert G, et al. Plasma methoxytyramine: a novel biomarker of metastatic pheochromocytoma and paraganglioma in relation to established risk factors of tumour size, location and SDHB mutation status. Eur J Cancer. 2012;48:1739–1749. doi: 10.1016/j.ejca.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amar L, Baudin E, Burnichon N, et al. Succinate dehydrogenase B gene mutations predict survival in patients with malignant pheochromocytomas or paragangliomas. J Clin Endocrin Metabol. 2007;92:3822–3828. doi: 10.1210/jc.2007-0709. [DOI] [PubMed] [Google Scholar]
- 15.King KS, Prodanov T, Kantorovich V, et al. Metastatic pheochromocytoma/paraganglioma related to primary tumor development in childhood or adolescence: significant link to SDHB mutations. J Clin Oncol. 2011;29:4137–42. doi: 10.1200/JCO.2011.34.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen WT, Sturgeon C, Clark OH, et al. Should pheochromocytoma size influence surgical approach? A comparison of 90 malignant and 60 benign pheochromocytomas. Surgery. 2004;136:1129–1137. doi: 10.1016/j.surg.2004.05.058. [DOI] [PubMed] [Google Scholar]
- 17.Parenti G, Zampetti B, Rapizzi E, et al. Updated and new perspectives on diagnosis, prognosis, and therapy of malignant pheochromocytoma/paraganglioma. J Oncol. 2012;2012:872713. doi: 10.1155/2012/872713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harari A, Inabnet WB., 3rd Malignant pheochromocytoma: a review. Am J Surg. 2011;201:700–708. doi: 10.1016/j.amjsurg.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Scholz T, Eisenhofer G, Pacak K, et al. Clinical review: Current treatment of malignant pheochromocytoma. J Clin Endocrinol Metab. 2007;92:1217–1225. doi: 10.1210/jc.2006-1544. [DOI] [PubMed] [Google Scholar]
- 20.Ahlman H. Malignant pheochromocytoma: state of the field with future projections. Ann N Y Acad Sci. 2006;1073:449–464. doi: 10.1196/annals.1353.049. [DOI] [PubMed] [Google Scholar]
- 21.Agresti A. Categorical data analysis. New York: Wiley; 1990. [Google Scholar]
- 22.Amar L, Peyrard S, Rossignol P, et al. Changes in urinary total metanephrine excretion in recurrent and malignant pheochromocytomas and secreting paragangliomas. Ann N Y Acad Sci. 2006;1073:383–91. doi: 10.1196/annals.1353.042. [DOI] [PubMed] [Google Scholar]
- 23.Khorram-Manesh A, Ahlman H, Nilsson O, et al. Mortality associated with pheochromocytoma in a large Swedish cohort. Eur J Surg Oncol. 2004;30:556–559. doi: 10.1016/j.ejso.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Agarwal G, Sadacharan D, Kapoor A, et al. Cardiovascular dysfunction and catecholamine cardiomyopathy in pheochromocytoma patients and their reversal following surgical cure: results of a prospective case-control study. Surgery. 2011;150:1202–1211. doi: 10.1016/j.surg.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Naderi N, Amin A, Setayesh A, et al. Pheochromocytoma-induced reverse tako-tsubo with rapid recovery of left ventricular function. Cardiology. 2012;19:527–531. doi: 10.5603/cj.2012.0097. [DOI] [PubMed] [Google Scholar]
- 26.Prejbisz A, Lenders JW, Eisenhofer G, Januszewicz A. Cardiovascular manifestations of phaeochromocytoma. J Hypertens. 2011;29:2049–2060. doi: 10.1097/HJH.0b013e32834a4ce9. [DOI] [PubMed] [Google Scholar]
- 27.Prejbisz A, Lenders JW, Eisenhofer G, Januszewicz A. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2013. Mortality Associated with Phaeochromocytoma. [DOI] [PubMed] [Google Scholar]
- 28.Bravo EL. Pheochromocytoma: an approach to antihypertensive management. Ann N Y Acad Sci. 2002;970:1–10. doi: 10.1111/j.1749-6632.2002.tb04408.x. [DOI] [PubMed] [Google Scholar]
- 29.Zelinka T, Petrak O, Turkova H, et al. High incidence of cardiovascular complications in pheochromocytoma. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2012;44:379–384. doi: 10.1055/s-0032-1306294. [DOI] [PubMed] [Google Scholar]
- 30.Eisenhofer G, Huynh TT, Hiroi M, Pacak K. Understanding catecholamine metabolism as a guide to the biochemical diagnosis of pheochromocytoma. Rev Endocrine Metabol Disorders. 2001;2:297–311. doi: 10.1023/a:1011572617314. [DOI] [PubMed] [Google Scholar]
- 31.McBride JF, Atwell TD, Charboneau WJ, et al. Minimally invasive treatment of metastatic pheochromocytoma and paraganglioma: efficacy and safety of radiofrequency ablation and cryoablation therapy. JVIR. 2011;22:1263–1270. doi: 10.1016/j.jvir.2011.06.016. [DOI] [PubMed] [Google Scholar]