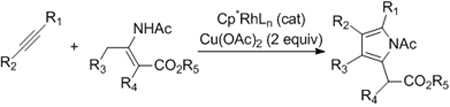
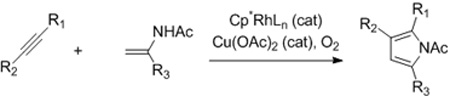
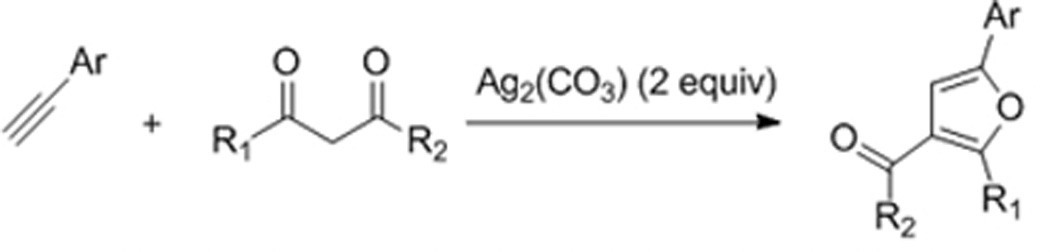
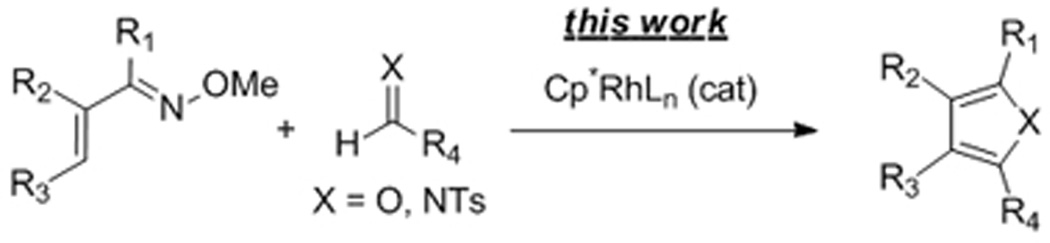
Furans and pyrroles are pervasive in natural products and pharmaceuticals.1 For this reason, the synthesis of these five-membered ring heterocycles has been an intense topic for decades2 with transition metal based C-H bond functionalization strategies recently providing powerful annulative approaches for their assembly.3,4 Specifically, Glorius3a and Fagnou3b have reported on the synthesis of pyrroles by Rh(III)-catalyzed coupling of enamides with internal alkynes (eqs 1 and 2), and most recently, Lei has reported on the synthesis of furans by the silver acetate mediated oxidative coupling of terminal alkynes and 1,3-dicarbonyl compounds (eq 3).3c Herein, we report a new type of catalytic C-H bond functionalization-based annulation enabling the versatile and efficient synthesis of furans and pyrroles from simple electrophilic coupling partners that serve as very different inputs from those used previously (eq 4).5 Additionally, in contrast to the other reported C-H functionalization-based annulations (eqs 1–3), no external oxidant is required.
 |
(1) |
 |
(2) |
 |
(3) |
 |
(4) |
Over the past several years spectacular progress has been achieved on the Rh(III)-catalyzed addition of sp2 C-H bonds across alkenes and alkynes.6,7,8,9 Recently, we and others have also actively explored Rh(III)-catalyzed C-H bond additions across polarized C-N and C-O multiple bonds.10 Our proposed annulation approach for the synthesis of furans and pyrroles in particular requires a Rh(III)-catalyzed alkenyl C-H bond addition to imines and aldehydes. However, few examples of the addition of alkenyl C-H bonds across C-N and C-O multiple bonds have been reported. We first described the addition of alkenyl C-H bonds of enamides to isocyanates,10m and very recently, Shi reported alkenyl C-H bond addition to N-tosyl imines and activated aldehydes using the pyridyl directing group.10f
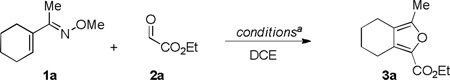
We initiated our investigation by exploring the Rh(III)-catalyzed addition of α,β-unsaturated oxime 1a to ethyl glyoxylate 2a (Table1).11 Although the use of 5 mol % of [Cp*RhCl2]2 and AgSbF6 with commercially available 2a, which is stored as a 50% toluene solution, afforded the desired product 3a in good yield (entry 1),quantitative yield was observed when glyoxylate 2a was used in pure form (entry 2). The reaction time could be shortened to 5 h without a reduction in yield (entry 3). Using 2.0 equiv of aldehyde 2a is crucial because the yield dropped significantly when only 1.0 equiv was employed (entry 4). The use of the pre-formed cationic Rh(III) precursor [Cp*Rh(CH3CN)3(SbF6)2 slightly reduced the yield (entry 5). Lower catalyst loading proved to be effective. At 2.5mol % of catalyst loading, complete conversion to product was maintained (entry 6), while at 1.25 mol % loading only a slight drop in yield was observed (entry 7). Conversion to product was not observed when either [Cp*RhCl2]2 or AgSbF6 alone were used(entries 8 and 9), which suggests that a cationic Rh(III) species is required for this C-H bond functionalization process. Although reaction of oxime 1a with ethyl glyoxylate (2a) proceeds to complete conversion within 5 h at 50 °C (entry 6), a longer reaction time and a higher temperature (75 °C) did not result in any reduction in yield (entry 10). These more forcing conditions were found to be necessary for less reactive oxime substrates (vide infra).
Table 1.
Screening of Reaction Conditions
 | |||||
|---|---|---|---|---|---|
| entry | catalyst | catalyst loading (mol%) |
temp [°C] |
time [h] |
yieldb (%) |
| 1c | [RhCp*Cl2]2, AgSbF6 | 5 | 75 | 20 | 84 |
| 2 | [RhCp*Cl2]2, AgSbF6 | 5 | 75 | 20 | >99 |
| 3 | [RhCp*Cl2]2, AgSbF6 | 5 | 75 | 5 | >99 |
| 4d | [RhCp*Cl2]2, AgSbF6 | 5 | 75 | 5 | 54 |
| 5 | [Cp*Rh(CH3CN)3](SbF6)2 | 5 | 75 | 5 | 87 |
| 6 | [RhCp*Cl2]2, AgSbF6 | 2.5 | 50 | 5 | >99 |
| 7 | [RhCp*Cl2]2, AgSbF6 | 1.25 | 50 | 5 | 89 |
| 8 | [RhCp*Cl2]2 | 5 | 75 | 5 | 0 |
| 9 | AgSbF6 | 20 | 75 | 5 | 0 |
| 10 | [RhCp*Cl2]2, AgSbF6 | 2.5 | 75 | 5 | >99 |
Conditions: 1a (0.10 mmol), purified ethyl glyoxylate 2a (0.20 mmol) in 0.33 mL of DCE.
Determined by 1H NMR analysis relative to 1,3,5-trimethoxybenzene as an external standard.
Commercially available 50% solution of ethyl glyoxylate in toluene used.
1.0 equiv of ethyl glyoxylate was used.
Having defined an effective catalyst system and reaction conditions for the synthesis of furan 3a in high yield, we next explored substrate scope with a range of α,β-unsaturated oximes 1with different substitution patterns (Table 2). Annulation of cyclic and acyclic trisubstituted oximes resulted in fully substituted furan products in good yields (3a–3g), with cyclohexyl (3a, 3e and 3g) and cyclopentyl (3b) derivatives providing access to different bicyclic frameworks. The terminal substituent, R3, is not essential for the success of the reaction thereby enabling the preparation of trisubstituted furans (3h and i). We also explored variation at R1 and established that different alkyl groups work well in this transformation (3e–g). Varying the R2 substituent demonstrated that alkyl, aryl (3h), and benzyl (3i) substituted oximes are effective substrates. However, a substituent at this position is crucial because no product was obtained when R2 was hydrogen. To enhance the practicality of the method, we performed the reaction on the benchtop and found that the yield was comparable to that observed when the reaction was set up in a glovebox under inert atmosphere conditions (see 3a).
Table 2.
Oxime Substrate Scope with Ethyl Glyoxylatea
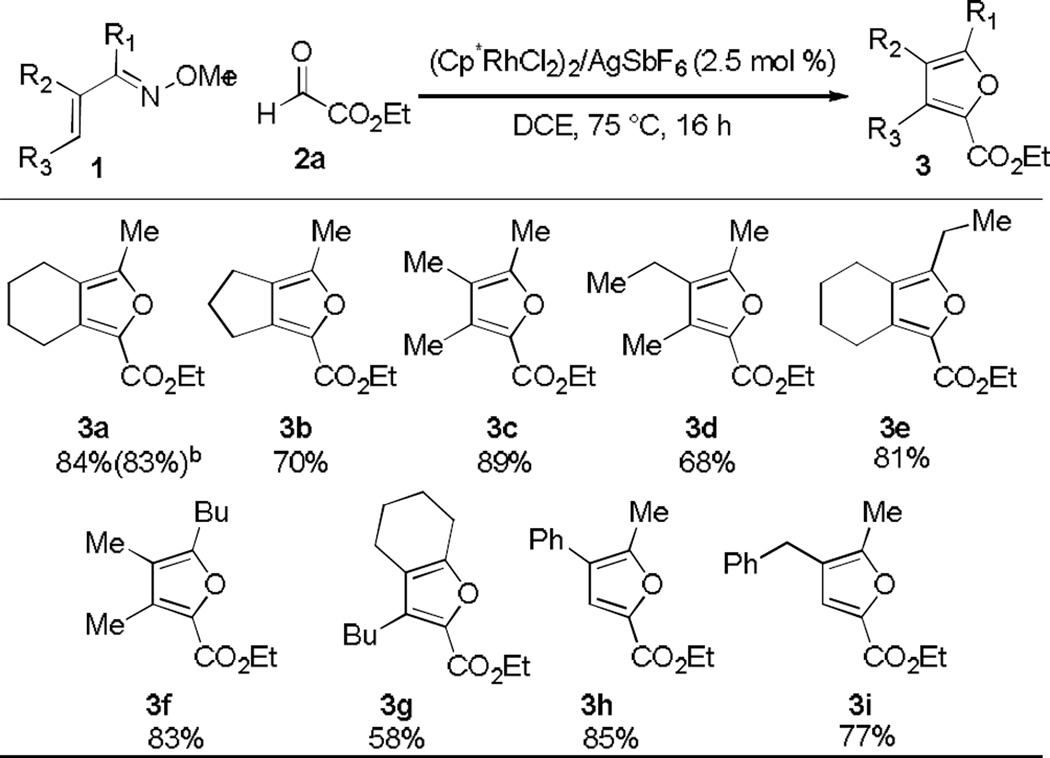 |
Conditions: 1 (0.20 mmol), purified ethyl glyoxylate 2a (0.40 mmol) in 0.67 mL of DCE. The isolated yield of purified material is reported for each product.
Conducted in a Schlenk flask under N2 on the benchtop.
Having demonstrated a broad scope for coupling oximes bearing different substitution patterns with glyoxylate 2a, we next explored the possibility of extending this transformation to less activated aldehydes. To apply the transformation to aromatic aldehydes, or even to aliphatic aldehydes, the reversible nature of metal catalyzed C-H bond addition to aldehydes must be considered (Figure 1). In contrast to the reaction of the electron-deficient and destabilized glyoxylate 2a, which likely favors formation of alcohol intermediates such as those illustrated in Figure 1, literature reports suggest that starting materials are generally more stable than the addition products when less electron deficient aldehydes are used.12 For unactivated aldehydes we therefore envisioned that capture of the initial alcohol product by cyclization and aromatization might be necessary to drive the reaction to completion.13
Figure 1.
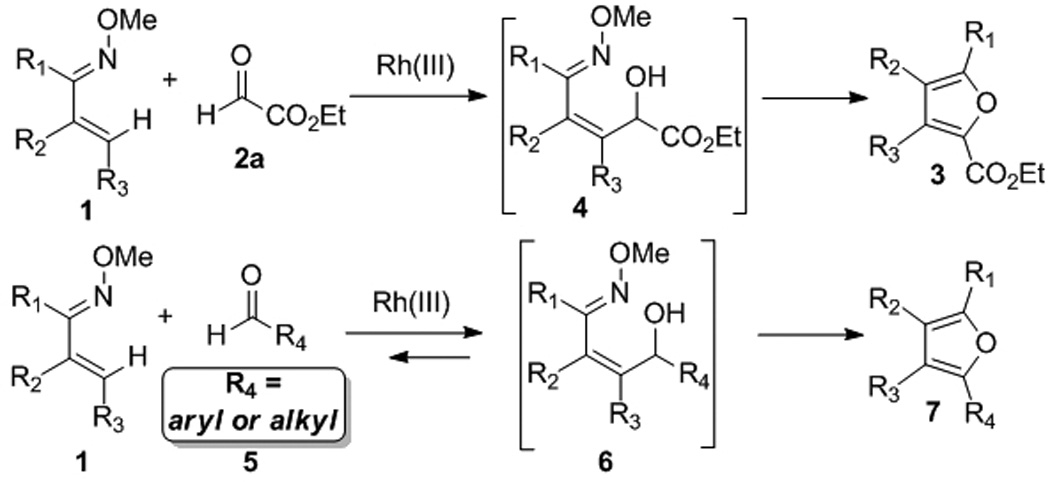
Proposed reaction pathways.
We initially focused on the identification of suitable conditions for the efficient coupling of 4-trifluoromethylbenzaldehyde (5a) and oxime 1b (Table 3). The use of DCE as a solvent, which we have previously found to be one of the most effective for Rh(III) catalyzed C-H additions to polarized C-N and C-O multiple bonds, provided the desired furan 7a in poor yield (entry 1). A range of solvents with different polarities and hydrogen bonding capabilities were therefore evaluated because different solvation properties might be expected to affect the equilibrium between the starting materials and the alcohol intermediate and could also impact the rate of cyclization and aromatization. A variety of alcohols with different steric properties and acidities were first explored, with CF3CH2OH increasing the yield to 50% (entries 2–4). Evaluation of aprotic solvents resulted in an interesting trend. Polar and strongly coordinating solvents, such as CH3CN or DMF resulted in very low yields (entries 5 and 6). However, the use of moderately coordinating solvents significantly improved the yield (entries 7–10). Among the solvents screened, HOAc and THF were the most effective providing 7a in excellent yield (78%).14 THF was chosen for further experimentation because it is more easily removed than HOAc.
Table 3.
Reaction Optimization for Aromatic Aldehydesa
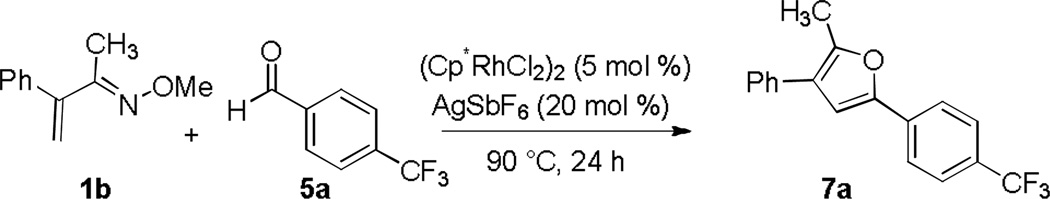 | ||
|---|---|---|
| entry | solvent | yieldb (%) |
| 1 | DCE | 23 |
| 2 | EtOH | trace |
| 3 | i-PrOH | 20 |
| 4 | CF3CH2OH | 50 |
| 5 | CH3CN | trace |
| 6 | DMF | 17 |
| 7 | EtOAc | 63 |
| 8 | Dioxane | 53 |
| 9 | HOAc | 78 |
| 10 | THF | 78 |
Conditions: 1b (0.10 mmol), aldehyde 5a (0.20 mmol) in 0.33 mL of solvent
Determined by 1H NMR analysis relative to 1,3,5-trimethoxybenzene as an internal standard.
Having defined an effective solvent system for the synthesis of furans from aromatic aldehyde 5a, we next explored a wider range of aldehydes. Good functional group compatibility was demonstrated, with aromatic aldehydes substituted with chloro, fluoro, trifluoromethyl, methoxy, nitro, and ester groups all proving to be effective coupling partners. Moreover, substitution at the o-,m-, and p-positions of the aromatic ring was found to be well tolerated (7a–7h). Electron poor aromatic aldehydes generally afford furan products in higher yields (7a, 7d, and 7e), but unactivated aromatic aldehydes are also effective substrates (7b, 7c, and 7h). Moreover, the annulation of aliphatic aldehydes proceeds in moderate yields (7i and j), which is notable because few prior examples of Rh(III)-catalyzed C-H bond additions to aliphatic aldehydes have been reported.6l The reaction is not limited to oxime 1b, but is also effective for other oxime substitution patterns (7k–m).
With successful demonstration of this new type of annulation for the synthesis of diverse furan derivatives, we next investigated the possibility of extending this reaction to pyrrole synthesis. Reaction of oxime 1a with the N-tosyl imine of ethyl glyoxylate 8 in DCE at 90 °C for 16 h afforded pyrrole 9a in reasonable yield (Table 5). The reaction proved to be effective for α,β-unsaturated oximes 1 with different substituents and substitution patterns, but preliminary attempts to extend the reaction to N-Ts imines of aromatic aldehydes have not yet been successful.
Table 5.
Substrate Scope for Pyrrole Synthesisa
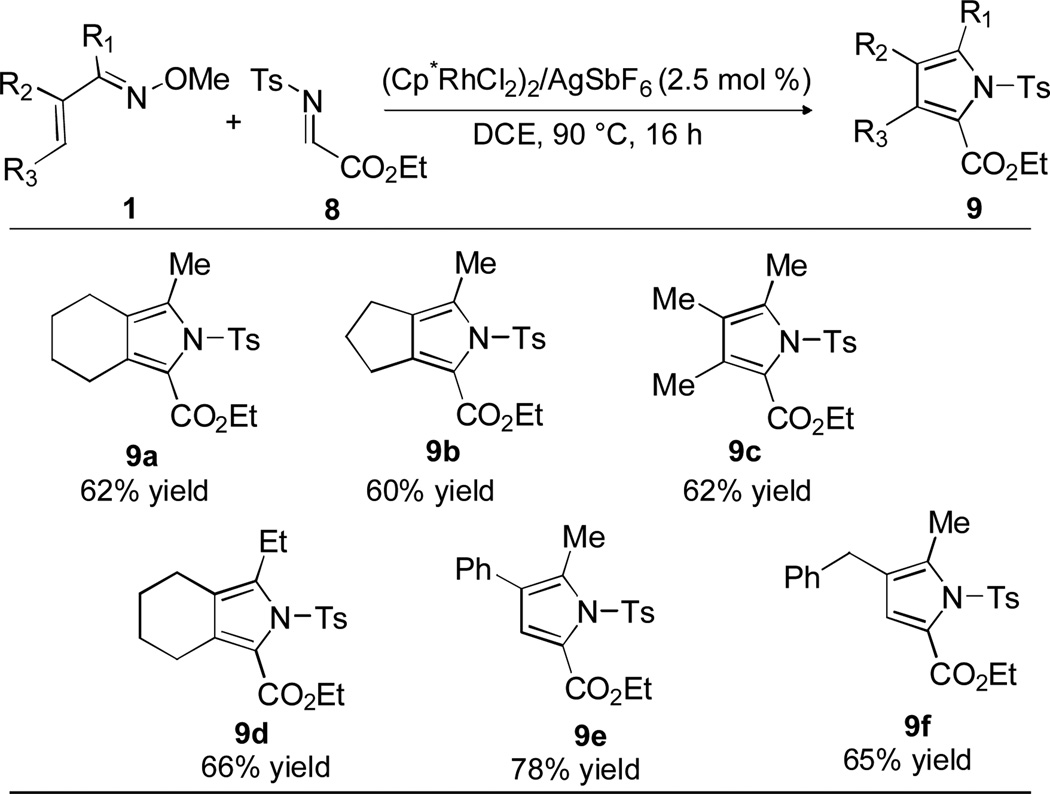 |
Conditions: 1 (0.20 mmol), imine 8 (0.40 mmol) in 0.67 mL of DCE. The isolated yield of purified material is reported for each product.
In conclusion, a new type of annulation for the synthesis of substituted furans and pyrroles has been developed that proceeds via C-H bond activation. The annulation is initiated by the Rh(III)-catalyzed addition of an alkenyl C-H bond across an aldehyde C=O or imine C=N bond followed by cyclization and aromatization. This operationally simple process is amenable to a number of different substituents on the α,β-unsaturated oxime. Annulations with ethyl glyoxylate and the corresponding N-tosyl imine provide access to 2-carboxyethyl substituted furans and pyrroles, respectively. Moreover, a broad range of aromatic and also aliphatic aldehydes participate in the reaction to provide access to an extensive group of differently substituted furans. We are continuing to investigate this approach for the synthesis of other 5-membered ring heterocycles including azoles
Experimental Section
Representative procedure: In a N2-filled glovebox, [Cp*RhCl2]2 (3.1 mg, 0.0050 mmol, 0.025 equiv), AgSbF6 (6.9 mg, 0.020 mmol, 0.10 equiv), the indicated O-methyl oxime (0.200 mmol, 1.0 equiv) and ethyl glyoxylate (40.8 mg, 0.400 mmol, 2.0 equiv) were added to a screw-capped conical vial with a stir bar followed by addition of DCE (0.66 mL, [O-methyl oxime] = 0.30 M). The vial was sealed with a cap containing a PTFE septum and was removed from the glovebox. The reaction vial was then placed in a temperature-controlled oil bath at 75 °C. After 16 h of stirring, the vial was removed from the oil bath and was cooled to ambient temperature. The mixture was filtered through a pad of celite and the solvent was removed under reduced pressure. The crude residue was loaded onto a silica gel column for chromatographic purification.
Supplementary Material
Table 4.
Substrate Scope for Other Aldehydesa
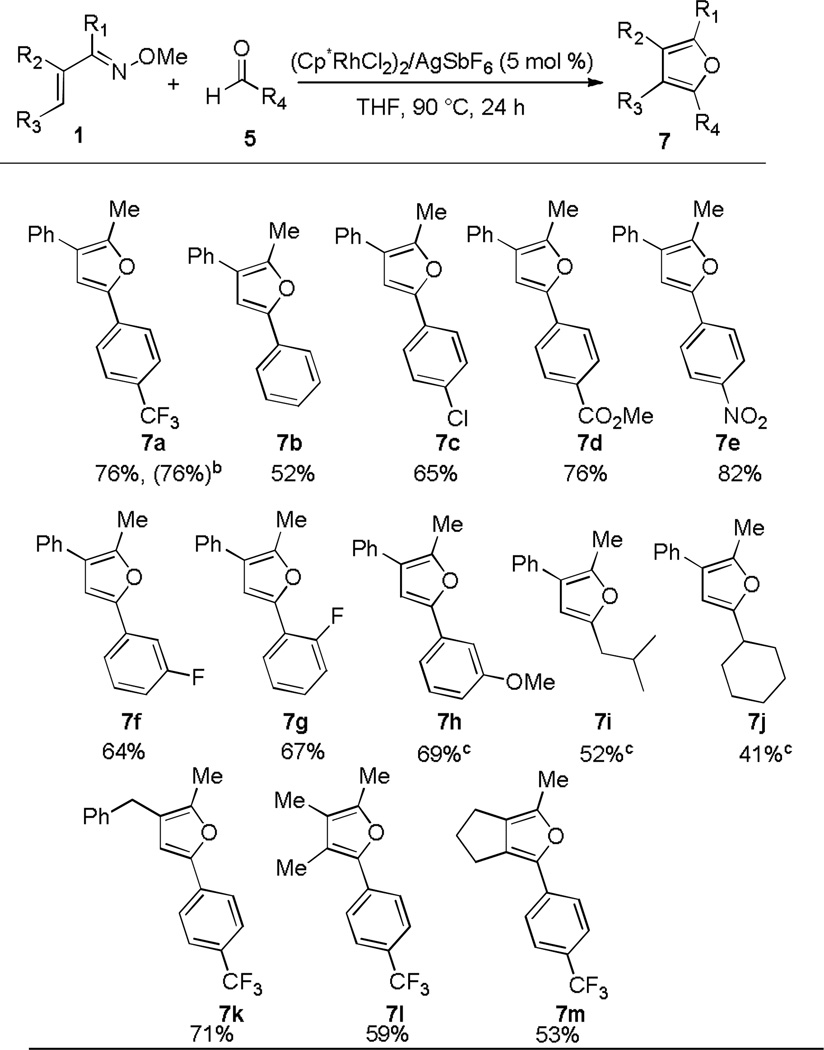 |
Conditions: 1 (0.20 mmol), aldehyde 5 (0.40 mmol) in 0.67 mL of DCE. The isolated yield of purified material is reported for each product.
Conducted in a Schlenk flask under N2 on the benchtop.
Using 10 mol % [Cp*RhCl2]2.
Footnotes
This work was supported by NIH Grant GM069559 (to J.A.E.). R.G.B. acknowledges funding from The Director, Office of Energy Research, Office of Basic Energy Sciences, Chemical Sciences Division, U.S. Department of Energy, under Contract DE-AC02-05CH11231. K.D.H. is grateful to the National Sciences and Engineering Research Council of Canada (NSERC) for a postdoctoral fellowship
Supporting information for this article is available on the WWW under http://www.angewandte.org
Contributor Information
Yajing Lian, Department of Chemistry, Yale University, 225 Prospect St., New Haven, CT 06520 (USA).
Tatjana Huber, Department of Chemistry, Yale University, 225 Prospect St., New Haven, CT 06520 (USA).
Kevin D. Hesp, Department of Chemistry, Yale University, 225 Prospect St., New Haven, CT 06520 (USA)
Robert G. Bergman, Department of Chemistry, University of California, Berkeley, Berkeley, CA 94720-1416 (USA), Lawrence Berkeley National Laboratory, 1 Cyclotron Road, Berkeley, CA 94720 (USA)
Jonathan A. Ellman, Email: jonathan.ellman@yale.edu, Department of Chemistry, Yale University, 225 Prospect St., New Haven, CT 06520 (USA).
References
- 1.a) Katritzky AR. Comprehensive Heterocyclic Chemistry III. 1st ed. New York: Elsevier: Amsterdam; 2008. [Google Scholar]; b) Pozharskii AF, Katritzky AR, Soldatenkov AT. Heterocycles in Life and Society: An Introduction to Heterocyclic Chemistry, Biochemistry, And Applications. 2nd ed. Chichester West Sussex: Wiley; 2011. [Google Scholar]; c) Hou XL, Cheung HY, Hon TY, Kwan PL, Lo TH, Tong SY, Wong HNC. Tetrahedron. 1998;54:1955. [Google Scholar]; d) Lipshutz BH. Chem. Rev. 1986;86:795. [Google Scholar]
- 2.For select reviews, see: Keay BA. Chem. Soc. Rev. 1999;28:209. Dudnik AS, Gevorgyan V. Transition Metal-Catalyzed Synthesis of Monocyclic Five-Membered Aromatic Heterocycles, in Catalyzed Carbon-Heteroatom Bond Formation. Weinheim, Germany: Wiley; 2010. Balme G, Bouyssi D, Monteiro N. Heterocycles. 2007;73:87. For some selected recent examples, see: Donohoe TJ, Bower JF. Proc. Natl. Acad. Sci. U.S.A. 2010;107:3373. doi: 10.1073/pnas.0913466107. Lenden P, Entwistle DA, Willis MC. Angew. Chem. 2011;123:10845. doi: 10.1002/anie.201105795. Angew. Chem. Int. Ed.2011, 50, 10657; Zhang M, Jiang H-F, Neumann H, Beller M, Dixneuf PH. Angew. Chem. 2009;121:1709. doi: 10.1002/anie.200805531. Angew. Chem. Int. Ed.2009, 48, 1681; Hong D, Zhu Y-X, Li Y, Lin X-F, Lu P, Wang Y-G. Org. Lett. 2011;13:4668. doi: 10.1021/ol201891r. Thompson BB, Montgomery J. Org. Lett. 2011;13:3289. doi: 10.1021/ol201133n. Wang T, Chen X-L, Chen L, Zhan Z-P. Org. Lett. 2011;13:3324. doi: 10.1021/ol201054z.
- 3.a) Rakshit S, Patureau FW, Glorius F. J. Am. Chem. Soc. 2010;132:9585. doi: 10.1021/ja104305s. [DOI] [PubMed] [Google Scholar]; b) Stuart DR, Alsabeh P, Kuhn M, Fagnou K. J. Am. Chem. Soc. 2010;132:18326. doi: 10.1021/ja1082624. [DOI] [PubMed] [Google Scholar]; c) He C, Guo S, Ke J, Hao J, Xu H, Chen H-Y, Lei A-W. J. Am. Chem. Soc. 2012;134:5766. doi: 10.1021/ja301153k. [DOI] [PubMed] [Google Scholar]
- 4.For recent general reviews on C-H functionalization with applications to heterocycle synthesis, see: Yamaguchi J, Yamaguchi AD, Itami K. Angew. Chem. 2012;124:9092. doi: 10.1002/anie.201201666. Angew. Chem. Int. Ed.2012, 51, 8960; Colby DA, Bergman RG, Ellman JA. Chem. Rev. 2010;110:624. doi: 10.1021/cr900005n. Chen X, Engle KM, Wang D-H, Yu J-Q. Angew. Chem. 2009;121:5196. doi: 10.1002/anie.200806273. Angew. Chem. Int. Ed.2009, 48, 5094; Lyons TW, Sanford MS. Chem. Rev. 2010;110:1147. doi: 10.1021/cr900184e. Seregin IV, Gevorgyan V. Chem. Soc. Rev. 2007;36:1173. doi: 10.1039/b606984n.
- 5.The connectivity obtained in the initial C-H functionalization step is similar to that observed for an Umpolung process, see: Seebach D. Angew. Chem. 1979;91:259. Angew. Chem. Int. Ed. 1979, 18, 239.
- 6.For recent reviews on Rh(III)-catalyzed C-H functionalization, see: Satoh T, Miura M. Chem.-Eur. J. 2010;16:11212. doi: 10.1002/chem.201001363. Wencel-Delord J, Droge T, Liu F, Glorius F. Chem. Soc. Rev. 2011;40:4740. doi: 10.1039/c1cs15083a. Song G, Wang F, Li X. Chem. Soc. Rev. 2012;41:3651. doi: 10.1039/c2cs15281a.
- 7.For selected Rh(III)-catalyzed oxidative coupling of alkenes, see: Rakshit S, Grohmann C, Besset T, Glorius F. J. Am. Chem. Soc. 2011;133:2350. doi: 10.1021/ja109676d. Patureau FW, Besset T, Glorius F. Angew. Chem. 2011;123:1096. doi: 10.1002/anie.201006222. Angew. Chem. Int. Ed.2011, 50, 1064; Wang F, Song G, Du Z, Li X. J. Org. Chem. 2011;76:2926. doi: 10.1021/jo2002209. Park SH, Kim JY, Chang S. Org. Lett. 2011;13:2372. doi: 10.1021/ol200600p. Li X, Zhao M. J. Org. Chem. 2011;76:8530. doi: 10.1021/jo201530r. Chen J, Song G, Pan C-L, Li X. Org. Lett. 2010;12:5426. doi: 10.1021/ol1022596. Umeda N, Hirano K, Satoh T, Miura M. J. Org. Chem. 2009;74:7094. doi: 10.1021/jo901485v. Ueura K, Satoh T, Miura M. Org. Lett. 2007;9:1407. doi: 10.1021/ol070406h.
- 8.For selected Rh(III)-catalyzed annulation of alkynes, see: Pham MV, Ye B, Cramer N. Angew. Chem. 2012:201206191. doi: 10.1002/anie.201206191. Angew. Chem. Int. Ed.2012, DOI: 10.1002/anie. 201206191; Li B-J, Wang H-Y, Zhu Q-L, Shi Z-J. Angew. Chem. 2012;124:4014. doi: 10.1002/anie.201200271. Angew. Chem. Int. Ed.2012, 51, 3948; Patureau FW, Besset T, Kuhl N, Glorius F. J. Am. Chem. Soc. 2011;133:2154. doi: 10.1021/ja110650m. Guimond N, Gorelsky SI, Fagnou K. J. Am. Chem. Soc. 2011;133:6449. doi: 10.1021/ja201143v. Huestis MP, Chan L, Stuart DR, Fagnou K. Angew. Chem. 2011;123:1374. doi: 10.1002/anie.201006381. Angew. Chem. Int. Ed.2011, 50, 1338; Hyster TK, Rovis T. J. Am. Chem. Soc. 2010;132:10565. doi: 10.1021/ja103776u. Too PC, Wang Y-F, Chiba S. Org. Lett. 2010;12:5688. doi: 10.1021/ol102504b. Umeda N, Tsurugi H, Satoh T, Miura M. Angew. Chem. 2008;120:4083. doi: 10.1002/anie.200800924. Angew. Chem. Int. Ed.2008, 47, 4019.
- 9.For selected Ru(II)-catalyzed C-H bond functionalization, see: Arockiam PB, Bruneau C, Dixneuf PH. Chem. Rev. 2012 doi: 10.1021/cr300153j. Ackermann L, Lygin AV, Hofmann N. Angew. Chem. 2011;123:6503. doi: 10.1002/anie.201101943. Angew. Chem. Int. Ed.2011, 50, 6379; Ackermann L, Lygin AV. Org. Lett. 2012;14:764. doi: 10.1021/ol203309y. Thirunavukkarasu VS, Donati M, Ackermann L. Org. Lett. 2012;14:3416. doi: 10.1021/ol301387t. Chinnagolla RK, Jeganmohan M. Chem. Commun. 2012;48:2030. doi: 10.1039/c2cc16916a. Muralirajan K, Parthasarathy K, Cheng C-H. Org. Lett. 2012;14:4262. doi: 10.1021/ol302000a. Ackermann L, Wang L, Lygin AV. Chem. Sci. 2012;3:177. Ackermann L, Kozhushkov SI, Yufit DS. Chem. -Eur. J. 2012;18:12068. doi: 10.1002/chem.201200406. Li B, Feng H-L, Wang N-C, Ma J-F, Song H-B, Xu S-S, Wang B-Q. Chem. -Eur. J. 2012;18:12873. doi: 10.1002/chem.201201862.
- 10.For the Rh(III)-catalyzed direct addition of C-H bonds to imines, see: Tsai AS, Tauchert ME, Bergman RG, Ellman JA. J. Am. Chem. Soc. 2011;133:1248. doi: 10.1021/ja109562x. Li Y, Li B-J, Wang W-H, Huang W-P, Zhang X-S, Chen K, Shi Z-J. Angew. Chem. 2011;123:2163. doi: 10.1002/anie.201007464. Angew. Chem. Int. Ed.2011, 50, 2115; Tauchert ME, Incarvito CD, Rheingold AL, Bergman RG, Ellman JA. J. Am. Chem. Soc. 2012;134:1482. doi: 10.1021/ja211110h. Hesp KD, Bergman RG, Ellman JA. Org. Lett. 2012;14:2304. doi: 10.1021/ol300723x. Li Y, Zhang X-S, Li H, Wang W-H, Chen K, Li B-J, Shi Z-J. Chem. Sci. 2012;3:1634. Li Y, Zhang X-S, Zhu Q-L, Shi Z-J. Org. Lett. 2012;14:4498. doi: 10.1021/ol301989n. For the Rh(III)-catalyzed direct addition of C-H bonds to aldehydes, see: Yang L, Correia CA, Li C-J. Adv. Synth. Catal. 2011;353:1269. Li Y, Zhang X-S, Chen K, He K-H, Pan F, Li B-J, Shi Z-J. Org. Lett. 2012;14:636. doi: 10.1021/ol2032784. Park J, Park E, Kim A, Lee Y, Chi KW, Kwak JH, Jung YH, Kim IS. Org. Lett. 2011;13:4390. doi: 10.1021/ol201729w. Sharma S, Park E, Park J, Kim IS. Org. Lett. 2012;14:906. doi: 10.1021/ol2034228. Zhou B, Yang Y, Li Y. Chem. Commun. 2012;48:5163. doi: 10.1039/c2cc31351k. Lian Y, Bergman RG, Ellman JA. Chem. Sci. 2012;3:3088. doi: 10.1039/C2SC20835K. For the Rh(III)-catalyzed direct addition of C-H bonds to other polarized bonds, see: Hesp KD, Bergman RG, Ellman JA. J. Am. Chem. Soc. 2011;133:11430. doi: 10.1021/ja203495c. Du Y, Hyster TK, Rovis T. Chem. Commun. 2011;47:12074. doi: 10.1039/c1cc15843k. Zhu C, Xie W, Falck JR. Chem.-Eur. J. 2011;17:12591. doi: 10.1002/chem.201102475. For a rhenium catalyzed addition of aryl ketimines to aldehydes to give isobenzofurans, see: Kuninobu Y, Nishina Y, Nakagawa C, Takai K. J. Am. Chem. Soc. 2006;128:12376. doi: 10.1021/ja065643e.
- 11.Selected recent examples of using oxime as directing group in the Rh(III)-catalyzed C-H functionalization chemistry, see: Tsai AS, Brasse M, Bergman RG, Ellman JA. Org. Lett. 2011;13:540. doi: 10.1021/ol102890k. Hyster TK, Rovis T. Chem. Commun. 2011;47:11846. doi: 10.1039/c1cc15248c. Zhang X-P, Chen D, Zhao M, Zhao J, Jia A-Q, Li X-W. Adv. Synth. Catal. 2011;353:719. Ng K-H, Zhou Z, Yu W-Y. Org. Lett. 2012;14:272. doi: 10.1021/ol203046n.
- 12.Li H, Li Y, Zhang X-S, Chen K, Wang X, Shi Z-J. J. Am. Chem. Soc. 2011;133:15244. doi: 10.1021/ja205228y. [DOI] [PubMed] [Google Scholar]
- 13.We have recently reported on the cyclative capture of reversibly formed alcohol addition products in the synthesis of furans, see: ref 10l.
- 14.Reaction of oxime 1a with 2a in THF provided furan 3a in 45% yield, and thus DCE remains the optimal solvent for reactions with ethyl glyoxylate.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


