Abstract
Most corals undergo spawning after a particular moon phase, but how moon-related spawning is endogenously regulated in corals remains unknown. The objective of the present study was to evaluate whether dopamine (DA) affects spawning in Acropora tenuis. When pieces of four A. tenuis colonies were reared under a natural photoperiod and water temperature, spawning was observed after the predicted moon phase. After exposure to water containing DA at 0.1 μM, pieces of the same colonies only released 5 to 10 bundles. Co-treatment with DA and pimozide (D1 and D2 receptors antagonist), but not domperidone (D2 receptor antagonist), induced mass release of bundles from the colonies. A cross-experiment revealed high fertilization rates between the control colonies (95%) and between the control and DA-treated colonies (90%), suggesting that gametes developed normally in coral tissue. Therefore, DA appears to have an inhibitory effect on the spawning of A. tenuis.
Synchrony of reproductive events has been reported for multiple phyla and can confer biological advantages such as an increased chance of mating, decreased predation risk, and protection against hybridization1. Mass spawning is a well-known synchronous event that occurs in many hermatypic corals in not only tropical2,3 and subtropical4,5 areas, but also temperate regions6. Because this synchrony in corals occurs during a fixed season of the year in many areas, periodic changes in several environmental factors likely act as reliable cues in regulating the synchronous development and release of gametes. For example, previous studies have reported that steady increases in solar insolation7 and water temperature4 facilitate gamete growth, subsequently scheduling the prospective month(s) for spawning. In most cases, the simultaneous release of gametes is related to the peak period of lunar and tidal stimuli8,9 as well as to the timing of sunset10. Because spawnings exhibit temporal differences across areas, the strongest cue in a specific area appears to be effectively utilized by locally adapted corals.
Environmental factors are perceived by the sensory systems of corals and then transduced as endocrine signals, which actively regulate the synchronization of gametogenesis and spawning. Several reports have shown that coral tissues contain sex steroids11,12,13 as well as a gonadotropin-releasing hormone (GnRH)-like compound14. Estrogens also appear to be involved in reproduction, as seasonal variation in these steroid hormones occur in accordance with reproduction13. To date, however, the key factors (and how they interplay) regulating various reproductive events of corals remain unknown.
Dopamine (DA) is a catecholamine that acts as a neurotransmitter and hormone in animals. In marine invertebrates, DA reportedly controls phototaxis in the larvae of a bryzoan Bugula neritina15, settlement and metamorphosis in the Pacific oyster Crassostrea gigas16, and metamorphosis in the common slipper shell Crepidula fornicata17. In addition, previous studies have documented a positive correlation between gonadal growth and DA concentration in the neural system of the great scallop Pecten maximus18,19. In contrast, DA inhibited the growth and maturation of oocytes in the sea urchin Strongylocentrotus nudus20. These findings imply that DA plays some roles in stimulating or suppressing the reproductive events of invertebrates, although its physiological function appears to differ among species. In vertebrates, including certain fish and tetrapods, however, DA has an inhibitory effect on the release of gonadotropins from the pituitary21,22 and on the release of GnRH from its neuron23,24. Together, these previous results lead to the hypothesis that DA is likely to co-express with sex steroids and a GnRH-like immunoreactive compound in coral tissue during the synchronous growth and release of gametes and that these chemicals regulate the reproductive events of corals either in coordination or antagonistically.
The objective of the present study was to examine whether treatment with DA would affect the final step of reproductive events in the coral Acropora tenuis (Dana, 1846). This species is common in the Indo-Pacific, including Okinawa, Japan, where A. tenuis usually spawns about 1 h after sunset25. Like all Acropora species, A. tenuis produces bundles which mean packaged eggs and sperm sacks in each hermaphrodite polyp. These bundles appear in the polyp mouths (“setting”) about 30 min to 1 h before bundle release (“spawning”). We exposed colonies of A. tenuis to DA alone or in mixture with DA antagonists and compared gamete release, setting, and the occurrence of spawning among treatments. DA antagonists used in the present study were domperidone (DOM) and pimozide (PIM), which antagonize D2 receptor26 and D1 and D2 receptors27, respectively. We also performed cross-experiments among treatment groups, as the methodology for cross-experiments in the genus Acropora has been previously established10,25.
Results
Spawning
Acropora tenuis spawning results from several treatments in 2011 and 2012 are summarized in Table 1. Spawning of A. tenuis occurred under experimental conditions on 16 and 17 June 2011 (1 day before the full moon and on the day of the full moon). About 30–50 min after setting, all four control colony pieces had terminated their spawning within 30–40 min. With the exception of one colony (No. 1), the colonies (Nos. 2–4) of the DA-treatment group released only 5–10 bundles. After treatment with DA + DOM, two colonies (Nos. 1 and 3) failed to release any bundles, while the other two (Nos. 2 and 4) released very few (7–9) bundles.
Table 1. Date and time of setting and spawning and number of bundles released from pieces of Acropora tenuis under each treatment.
| Treatment | ||||||||
|---|---|---|---|---|---|---|---|---|
| Year | Colony No. | Date of spawning | Start time of setting | Start time of spawning | control | dorpamine | dorpamine + DOM (domperidone) | dorpamine + PIM (pigmozide) |
| 2011 | 1 | 17, Jun | 19:15 | 20:00 | Spawning | No bundles | No bundles | - |
| 2 | 16, Jun | 19:15 | 20:00 | Spawning | 10 | 9 | - | |
| 3 | 16, Jun | 19:15 | 20:10 | Spawning | 6 | No bundles | - | |
| 4 | 17, Jun | 19:15 | 20:10 | Spawning | 5 | 7 | - | |
| 2012 | 1 | 31, May | 18:35 | 19:45 | Spawning | 10 | - | Spawning |
| 2 | (No spawning in experimental duration) | No bundles | No bundles | - | No bundles | |||
| 3 | 15, Jul | 18:30 | 20:00 | Spawning | No bundles | - | Spawning | |
| 4 | (No spawning in experimental duration) | No bundles | No bundles | - | No bundles | |||
“Spawning”; more than 50 bundles, “-“; no treatment.
In 2012, spawning of A. tenuis was observed on 31 May (4 days before full moon) and 15 June (4 days after the last quarter moon). On 31 May, pieces of colony No. 1, either without (control) or with DA + PIM treatment, experienced spawning, whereas those treated with DA-alone released only 10 bundles. On 15 June, pieces of colony No. 3 in the control and DA + PIM treatments showed spawning, whereas pieces of the DA colony did not release bundles (Fig. 1). Pieces of colonies Nos. 2 and 4 did not spawn until 30 June, although the presence of developed gonads was confirmed.
Figure 1. Photographs of spawning pieces from No. 3 colony of Acropora tenuis in 2012 under each treatment.
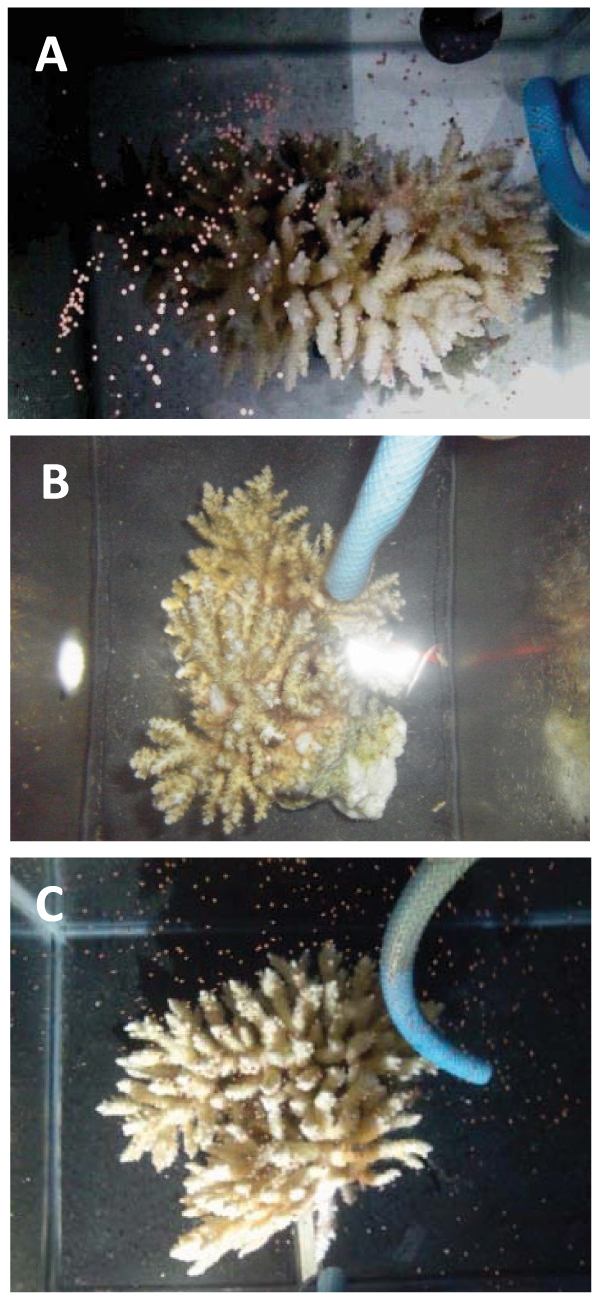
(A), control; (B), DA (dopamine) treatment; (C), DA + PIM (dopamine antagonist, pimozide) treatment.
Fertilization ability and development
Figure 2 presents the fertilization rates from cross-experiments in 2011. A high fertilization rate (95%) was observed in the cross between controls (No. 2 × No. 3). The fertilization rates in crosses between control and DA-treatment colonies were similar to that of the control cross: 95% in the control (No. 3) × DA (No. 2) and 90% in the control (No. 2) × DA (No. 3). All embryos of each cross developed normally, as shown previously28, and became planula larvae within 72 h after insemination.
Figure 2. Relationship between crossed pair and fertilization rate from cross experiments in 2011.
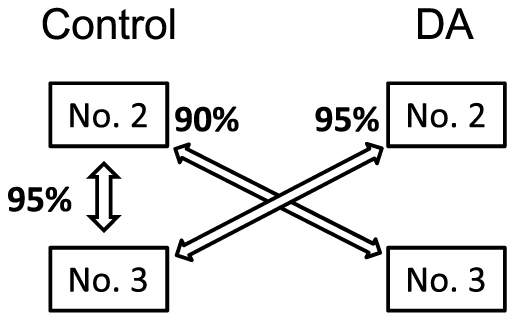
Crossed pair is connected by both directional arrows and values of percentage indicated by side of arrow head indicate fertilization rates. No. 2 and No. 3 show the number of colonies and DA shows dopamine treatment.
Discussion
This is the first report to provide novel insight into regulatory mechanisms from major mass spawner Acropora, which is one of the widespread genera and shows multi-specific synchronous spawning around particular moon phase2,3. When pieces of matured A. tenuis colonies were continuously exposed to seawater containing DA during the final phase of gametogenesis, gamete release was not observed around the predicted spawning lunar phase. In contrast, pieces of control corals under the same rearing conditions experienced spawning around the predicted spawning lunar phase. These results indicate that DA has an inhibitory effect on gamete release from polyps. The role of DA in reproductive activity has been examined in various invertebrates18,20,29,30. High-performance liquid chromatography (HPLC) analyses have revealed that DA levels of the visceral ganglion are correlated with gonadal growth of the snail, P. maximus18. In contrast, DA treatment lowered the incorporation of labeled RNA and protein precursors into cultured gonads in the sea urchin S. nudus, suggesting an inhibitory effect of this neurotransmitter on oocyte growth and maturation20. These findings indicate that DA affects the reproductive activity of invertebrates but that its specific effect varies among species and/or growth stages. In the present study, matured gametes were confirmed in the polyps of DA-treated and control corals until the time of spawning. In addition, DA-treated gametes were successfully fertilized with control gametes, and the subsequent fertilization rates were high. This cross resulted in normal development of planula larvae within 72 h after insemination. Therefore, the inhibitory effect of DA likely occurs at the final stage of gamete release but not at the continuous stages of gametogenesis. Previous studies on seasonal variation in endogenous DA in the gonads of the oyster, C. gigas, have demonstrated that DA levels decreased during the active spawning season31 and increased thereafter32. When artificial spawning was induced in the matured gonad of C. gigas using UV-irradiated seawater, DA decreased during spawning31.
Co-treatment of colony pieces with DA and DOM (a D2 receptor antagonist26) failed to reduce the inhibitory effect of DA on the spawning of A. tenuis. In contrast, treatment with PIM, which antagonizes both the D1 and D2 receptors27, was able to diminish the inhibitory effect of DA. These results imply that the two DA antagonists vary in their effectiveness at blocking the effect of DA on the spawning of corals. Because no other experiments on invertebrates have examined the effects of DOM and/or PIM, whether antagonists exhibit an equivalent effect on other invertebrate species remains unclear. One explanation is that concentration of the antagonists influences on the present results, although we continuously exposed A. tenuis to the same concentration of DOM and PIM (0.1 nM). Period exposing to the antagonists may be also not ruled out, because DOM and PIM were exposed for one month and 10 days, respectively. Alternatively, the fact that only PIM could reduce the inhibitory effect of DA leads us to hypothesize that such function of DA is mediated through D1 receptor in A. tenuis. In this regard, we have cloned D1-like dopamine receptor cDNAs, but not D2 receptor, from the polyps of A. tenuis (see Supplementary Fig. S1–S6 online), supporting our hypothesis.
The presence of free and glucuronided estradiol (E2) has been documented in the tissue of the scleractinian coral Euphyllia ancora which is a gonochoric coral and it is not main member of the synchronous multispecific spawning event throughout the year, and the peaks in these steroids occur just prior to spawning13. The concentration of free E2 was higher than that of glucuronided E2 in the coral tissue during the spawning season, whereas a higher concentration of glucuronided E2 was detected in the seawater during coral spawning13. These authors suggested that increases in free E2 and its glucuronide in coral tissues are involved in gamete release and that E2 glucuronide released in seawater acts as a pheromone for chemical communication among coral colonies during spawning13. In accordance with fluctuation of these steroid hormones, the activity of aromatase, a key enzyme in converting testosterone to E2 that functions in oocyte growth in oviparous vertebrates33, was detected from January to June and dramatically increased toward the day of spawning in May14. Monthly measurement of steroid hormones in the tissue of Montipora verrucosa spawned in June and July using radioimmunoassay revealed that estrone (E1) and E2 increase in April and in February and March, respectively, indicating that estrogens are related to early stages of gametogenesis and protein synthesis12. In addition, it was reported that peaks in E2 occurred during the week prior to spawning and in E1 during spawning12. Similarly, estrogens are also likely involved in regulating gametogenesis and spawning in corals12,14. Here, we demonstrated that DA acts as an inhibitor of the final step of spawning in A. tenuis. Notably, DA levels in the gonads of the scallop Patinopecten yessoensis reportedly decreased after E2 administration32. Taken together, E2 may regulate DA levels in the gonads in not only this scallop, but also in corals.
Presumably, interplay of endogenous players (i.e., estrogens and DA) occurs at the final step of gametogenesis and spawning in corals. Synergistic action by endogenous players may accelerate the spawning of corals. However, E2 levels changed very little in relation to spawning in M. verrucosa12 and in the soft coral Sinularia polydactyla11. In addition, because GnRH-like immunoreactivity was detected from polyps of E. ancora14, corals may have dual neuroendocrine control of reproduction by GnRH and DA, which is the case for several adult teleosts34. Thus, a variety of regulatory mechanisms may control spawning across coral species. Further studies are necessary to better understand the physiological mechanisms of reproductive events in corals.
Methods
Coral maintenance and exposure experiments
Colonies of A. tenuis (20–30 cm in diameter) kept in tanks at Okinawa Churaumi Aquarium (26.69°N, 127.87°E) were used in the present study. After confirming gonadal development in these corals, colonies were transported to Sesoko Station, Tropical Biosphere Research Center, University of the Ryukyus, Japan (26.63°N, 127.86°E) on 20 May 2011 and 24 May 2012.
Experiments began 30 days and 10 days prior to the predicted spawning lunar phase in 2011 and 2012, respectively. The difference in exposure periods between two years was partially due to difficulty of predicted spawning date according to intercalary month and water temperature. DA and its antagonists, DOM (D2 receptor antagonist26) and PIM (D1 and D2 receptors antagonist27), were purchased from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in distilled water as needed. Four colonies of A. tenuis (Nos. 1–4) were selected from the tanks of Okinawa Churaumi Aquarium at random in 2011 and 2012. Each colony was divided into four small pieces and reared separately in 20-L aquaria with running seawater and aeration under a natural photoperiod and water temperature at Sesoko Station. The colony pieces were continuously treated with DA (0.1 μM) or a mixture of DA (0.1 μM) and a DA antagonist. Vehicle-only treatments served as the control group.
Concentrations of DA and antagonists in each aquarium were constantly maintained by a Peristaltic Pump (AC-2110-2, ATTO, Tokyo, Japan). DOM at 0.1 nM and PIM at 0.1 nM were used as the DA antagonist in 2011 and 2012, respectively. Setting and spawning in all colony pieces were checked daily. Because the timings of setting and spawning of A. tenuis in Okinawa have previously been documented25, we also determined whether these timings occurred physiologically in the experimental groups. If spawning was not observed in the colonies, we checked the conditions of gametes in the colonies at 1 week after the main spawning of A. tenuis.
Treatments effects on fertilization ability were checked in the present study in 2011. Cross experiments were performed using gametes from two control pieces (Nos. 2 and 3) and two DA-treatment pieces (Nos. 2 and 3). Gamete bundles from each piece were collected in small tubes (8 mL, 60.582, ASSIST, Tokyo, Japan), and the gametes from two different pieces were mixed without separation of eggs and sperm in cross combinations. Three crosses (control [No. 2] × control [No. 3], control [No. 2] × DA [No. 3], and control [No.3] × DA [No.2]) were established, as the number of bundles produced from the DA-treatment pieces was very small. Sperm concentration was adjusted to more than 106 sperms/mL. Self-fertilizing colonies did not occur in the present study. Twenty eggs from the cross experiments were separately transferred from each combination into Petri dishes 1 h after insemination. The numbers of fertilized and unfertilized eggs were scored at the 16-cell/morula stage 4–5 h after gamete mixing.
Author Contributions
N.I. and A.T. designed the experiments. N.I. carried out the experiments and analyzed the data mainly. C.Y. and Y.T. contributed to the exposure experiments in Sesoko Station. Y.T. performed the molecular and genetic experiments. N.I. and A.T. wrote the manuscript that was edited by all other co-authors.
Supplementary Material
Supplementary Information
Acknowledgments
The authors thank Ms. Hiromi Hannah Yamamoto (Okinawa Churashima Research Center) for providing Acropora tenuis from Okinawa Churaumi Aquarium, and Mr. Yuji Sawada, Department of Chemistry, Biology and Marine Science, University of the Ryukyus, for great assistance with exposure experiments. We thank staff of Sesoko Station, Tropical Biosphere Research Center, University of the Ryukyus, for kind assistance and facility use. This work was supported in part by Grant-in-Aid for Scientific Research on Innovative Areas “Coral reef science for symbiosis and coexistence of human and ecosystem under combined stresses” (Grant No. 20121002) of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan to NI.
References
- Domeier M. L. & Colin P. L. Tropical reef fish spawning aggregations: Defined and reviewed. Bull. Mar. Sci. 60, 698–726 (1997). [Google Scholar]
- Babcock R. C. et al. Synchronous spawnings of 105 scleractinian coral species on the Great Barrier Reef. Mar. Biol. 90, 379–394 (1986). [Google Scholar]
- Harrison P. L. et al. Mass spawning in tropical reef corals. Science 223, 1187–1188 (1984). [DOI] [PubMed] [Google Scholar]
- Hayashibara T. et al. Patterns of coral spawning at Akajima Island, Okinawa, Japan. Mar. Ecol. Prog. Ser. 101, 253–262 (1993). [Google Scholar]
- Babcock R. C., Wills B. L. & Simpson C. J. Mass spawning of corals on a high latitude coral reef. Coral Reefs 13, 161–169 (1994). [Google Scholar]
- Nozawa Y., Tokeshi M. & Nojima S. Reproduction and recruitment of scleractinian corals in a high-latitude coral community, Amakusa, southwestern Japan. Mar. Biol. 149, 1047–1058 (2006). [Google Scholar]
- Van Woesik R., Lacharmoise F. & Koksal S. Annual cycles of solar insolation predict spawning times of Caribbean corals. Ecol. Lett. 9, 390–398 (2006). [DOI] [PubMed] [Google Scholar]
- Krupp D. A. Sexual reproduction and early development of the solitary coral Fungia scutaria (Anthozoa: Scleractinia). Coral Reefs 2, 159–164 (1983). [Google Scholar]
- Szmant A. F., Reutter M. & Riggs L. Sexual reproduction of Favia fragum (Esper): Lunar patterns of gametogenesis, embryogenesis and planulation in Puerto Rico. Bull. Mar. Sci. 37, 880–892 (1985). [Google Scholar]
- Hatta M. et al. Reproductive and genetic evidence for a reticulate evolutionary history of mass-spawning corals. Mol. Biol. Evol. 16, 1607–1613 (1999). [DOI] [PubMed] [Google Scholar]
- Slattery M., Hines G. A., Starmer J. & Paul V. J. Chemical signals in gametogenesis, spawning, and larval settlement and defense of the soft coral Sinularia polydactyla. Coral Reefs 18, 75–84 (1999). [Google Scholar]
- Tarrant A. M., Atkinson S. & Atkinson M. J. Estrone and estradiol-17β concentration in tissue of the scleractinian coral, Montipora verrucosa. Comp. Biochem. Physiol. B. 122, 85–92 (1999). [DOI] [PubMed] [Google Scholar]
- Twan W. H., Hwang J. S. & Chang C. F. Sex steroids in scleractinian coral, Euphyllia ancora: implication in mass spawning. Biol. Reprod. 68, 2255–2260 (2003). [DOI] [PubMed] [Google Scholar]
- Twan W. H. et al. The presence and ancestral role of GnRH in the reproduction of scleractinian coral, Euphyllia ancora. Endocrinology 147, 397–406 (2006). [DOI] [PubMed] [Google Scholar]
- Pires A. & Woollacott R. M. Serotonin and dopamine have opposite effects on phototaxis in larvae of the Bryozoan Bugula neritina. Biol. Bull. 192, 399–409 (1997). [DOI] [PubMed] [Google Scholar]
- Bonar D. B., Coon S. L., Walch M., Weiner R. M. & Fitt W. Control of oyster settlement and metamorphosis by endogenous and exogenous chemical cues. Bull. Mar. Sci. 46, 484–498 (1990). [Google Scholar]
- Pechenik J. A., Wei L. & Cochrane D. E. Timing is everything: The effects of putative dopamine antagonists on metamorphosis vary with larval age and experimental duration in the prosobranch gastropod Crepidula fornicate. Biol. Bull. 202, 137–147 (2002). [DOI] [PubMed] [Google Scholar]
- Paulet Y. M., Donval A. & Bekhadra F. Monoamines and reproduction in Pecten maximus, a preliminary approach. Invert. Rep. Dev. 23, 89–94 (1993). [Google Scholar]
- Martinez G. & Revera A. Role of monoamines in the reproductive process of Argopecten pupuratus. Invert. Rep. Dev. 25, 167–174 (1993). [Google Scholar]
- Khotimchenko Y. S. Effect of noradrenaline, dopamine and adrenolytics on growth and maturation of the sea urchin, Strongylocentrotus nudus Agassiz. Int. J. Invert. Rep. Dev. 4, 369–373 (1982). [Google Scholar]
- Peter R. E. & Paulencu C. R. Involvement of the preoptic region in the gonadotropin release-inhibition in the goldfish. Neuroendocrinology 31, 133–141 (1980). [DOI] [PubMed] [Google Scholar]
- Kah O., Dulka J. G., Dubourg P., Thibault J. & Peter R. E. Neuroanatomical substrate for the inhibition of gonadotrophin secretion in goldfish: existence of a dopaminergic preoptico-hypophyseal pathway. Neuroendocrinology 45, 451–458 (1987). [DOI] [PubMed] [Google Scholar]
- Yu K. L. & Peter R. E. Dopaminergic regulation of brain gonadotropin-releasing hormone in male goldfish during spawning behaviour. Neuroendocrinology 52, 276–283 (1990). [DOI] [PubMed] [Google Scholar]
- Yu K. L. & Peter R. E. Adrenergic and dopaminergic regulation of gonadotropin- releasing hormone release from goldfish preoptic-anterior hypothalamus and pituitary in vitro. Gen. Comp. Endocrinol. 85, 138–146 (1992). [DOI] [PubMed] [Google Scholar]
- Fukami H., Omori M., Shimoike K., Hayashibara T. & Hatta M. Ecological and genetic aspects of reproductive isolation by different spawning times in Acropora corals. Mar. Biol. 142, 679–684 (2003). [Google Scholar]
- Dufour S. et al. Dopaminergic inhibition of reproduction in teleost fishes: ecophysiological and evolutionary implications. Ann. NY Acad. Sci. 1040, 9–22 (2005). [DOI] [PubMed] [Google Scholar]
- Beninger R. J. et al. Effects of extinction, pimozide, SCH 23390, and metoclopramide on food-rewarded operant responding of rats. Psychopharmacology. 92, 343–349 (1987). [DOI] [PubMed] [Google Scholar]
- Okubo N. & Motokawa T. Embryogenesis in the reef-building Acropora spp. Zool. Sci. 24, 1169–1177 (2007). [DOI] [PubMed] [Google Scholar]
- Fingerman M. Roles of neurotransmitters in regulating reproductive hormone release and gonadal maturation in decapod crustaceans. Invert. Rep. Dev. 31, 47–54 (1997). [Google Scholar]
- Alfaro J., Zuniga G. & Komen J. Induction of ovarian maturation and spawning by combined treatment of serotonin and a dopamine antagonist, spiperone in Litopenaeus stylirostris and Litopenaeus vannamei. Aquaculture 236, 511–522 (2004). [Google Scholar]
- Osada M., Matsutani K. & Nomura T. Implication of catecholamines during spawning in marine bivalve molluscs. Int. J. Invert. Rep. Dev. 12, 241–252 (1987). [Google Scholar]
- Osada M. & Nomura T. Estrogen effect on the seasonal levels of catecholamines in the scallop Patinopecten yessoensis. Comp. Biochem. Physiol. C. 93, 349–353 (1989). [Google Scholar]
- Mommsen T. P. Fish physiology. Volume XI. The physiology of developing fish. Part A. Eggs and larvae (eds Hoar, W. S. & Randall, D. J.) 348 (Academic Press, 1988).
- Dufour S., Sebert M. E., Weltzien F. A., Rousseau K. & Pasqualini C. Neuroendocrine control by dopamine of teleost reproduction. J. Fish Biol. 76, 129–160 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


