Abstract
Amblyopia is a neurological disorder of binocular vision affecting up to 3% of the population resulting from a disrupted period of early visual development. Recently, it has been shown that vision can be partially restored by intensive monocular or dichoptic training (4–6 weeks). This can occur even in adults owing to a residual degree of brain plasticity initiated by repetitive and successive sensory stimulation. Here we show that the binocular imbalance that characterizes amblyopia can be reduced by occluding the amblyopic eye with a translucent patch for as little as 2.5 hours, suggesting a degree of rapid binocular plasticity in adults resulting from a lack of sensory stimulation. The integrated binocular benefit is larger in our amblyopic group than in our normal control group. We propose that this rapid improvement in function, as a result of reduced sensory stimulation, represents a new form of plasticity operating at a binocular site.
Amblyopia (lazy eye) is the most common form of unilateral blindness in the adult population and results from a disruption to normal visual development early in life. Adults with amblyopia are currently offered no treatment in clinical practice, due to the finding that patching of the fellow eye is ineffective after the age of 10 yrs1, presumably due to the lack of plasticity in the adult visual cortex2. However, recent studies have shown that monocular functions of the amblyopic eye can be partly recovered as a result of intensive training of the amblyopic eye, which in turn suggests the existing of some degree of plasticity in adult amblyopes at the monocular site3,4,5,6,7. Additionally, our understanding of the aetiology of amblyopia is evolving and there is now evidence that amblyopia may be the secondary consequence of a loss of binocular function8,9,10,11,12. Accordingly, the affected eye may not be “lazy” but rather actively inhibited by neural inputs from the dominant fellow eye. It has also recently been shown that binocular functions can be restored in adults with amblyopia following an intensive period of dichoptic training aimed at getting the two eyes to work together13,14,15,16,17, suggesting that the binocular visual system also retains a considerable degree of plasticity even in adulthood. However, all of these studies involving either monocular perceptual learning3,4,5,6,7 or dichoptic training13,14,15 take time; the recovery of visual functions can take weeks and could result from the establishment of new and/or stronger synaptic connections in cortex18,19,20 or a long-term regulation of the cortical excitatory/inhibitory balance21,22.
Recently, it has been demonstrated that patching one eye of a binocularly normal subject with a diffuser strengthens that eye's contribution to the binocular percept when the diffuser is removed23. The effect occurs for a number of visual functions (motion, contrast and form judgments) which rely on different regions of the visual cortex24. Here we apply this approach to adults with amblyopia to assess whether adult plasticity can be modulated within a short time period (hrs) to restore binocular function lost during childhood. We show that a short period of patching the amblyopic eye with a diffuser enhances the amblyopic eye's contribution to the binocular percept, suggesting that the binocular plasticity in adults with amblyopia can occur rapidly and is able to reverse well-established neural changes that occurred in childhood.
Results
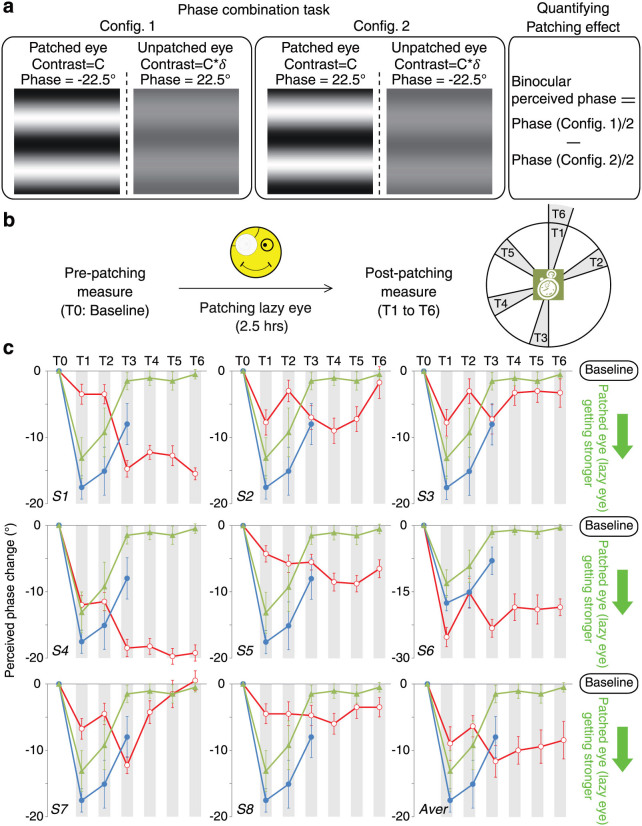
The results are displayed in Figure 1 normalized by subtracting each data point from the pre-patching baseline (zero on the x-axis). Data points below the x-axis indicate a strengthening of the previously patched eye's contribution to the binocular percept. Both the control groups (control group1: blue lines, average results of five normal controls, with a randomly chose eye as the patched eye and the contrast of the stimulus in the unpatched eye being fixed at 64%; control group 2: green lines, average results of four additional normal controls, with the undominant eye chosen as the patched eye and the contrast of the stimulus in the patched eye being fixed at 100%) and amblyopes (red lines, separate panels for eight amblyopes, with the amblyopic eye chosen as the patched eye and the contrast of the stimulus in the patched eye being fixed at 100%) displayed a strengthening of the previously patched eye's contribution to the binocular percept.
Figure 1.
(a) A schematic of the experimental protocol. (b) The time line of the patching and testing protocol. (c) Measurement of binocular balance using a binocular combination task after patching of the amblyopic eye for each of eight observers with amblyopia (S1–S8). The red lines with open dots (“ ”) in panel S1–S8 represent the time course of the perceived phase change for each amblyopic observer; the blue lines and filled dots (“
”) in panel S1–S8 represent the time course of the perceived phase change for each amblyopic observer; the blue lines and filled dots (“ ”) represent the average results of five normal controls after patching of one randomly selected eye (control group1); the green lines and filled triangles (“
”) represent the average results of five normal controls after patching of one randomly selected eye (control group1); the green lines and filled triangles (“ ”) represent the average results of four normal controls after patching of the undominant eye (Control group2). The red lines with open dots (“
”) represent the average results of four normal controls after patching of the undominant eye (Control group2). The red lines with open dots (“ ”) in the last panel represent the average results of the eight amblyopes. Displacement below the baseline represents a strengthening of the patched eye contribution to the binocular percept. Error bars represent standard errors.
”) in the last panel represent the average results of the eight amblyopes. Displacement below the baseline represents a strengthening of the patched eye contribution to the binocular percept. Error bars represent standard errors.
The average peak magnitude of this effect was comparable in controls and amblyopes (see the green and red dots and lines in panel “Aver” in Figure 1c) but the time course was not the same. One simple way to compare the patching effect between amblyopes and controls is by calculating the area covered by the phase vs. time curve (i.e., magnitude × time). The average area ratio from amblyopes to controls is 1.91 ± 0.43 (mean ± SEM), which was significantly large than 1 (t(7) = 2.12, p = 0.036, one-tailed), suggesting a larger patching-induced effect in amblyopes than in controls.
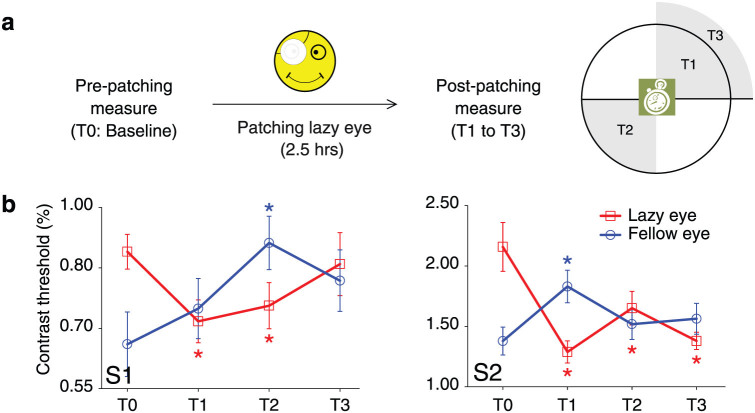
In Figure 2, pre- and post-patching contrast detection thresholds at 0.3 cycle/°, the same spatial frequency that was used for the binocular phase combination task, are shown for both the amblyopic and fellow eye before and after patching of the amblyopic eye for subject S1 and S2 (the corresponding psychometric functions are provided in Fig. S1 in supplementary material). In agreement with the binocular effects, there was a reciprocal change in contrast threshold after patching; the patched eye became more sensitive and the fellow eye less sensitive to contrast. Since 0.3 cycle/° is a quite low spatial frequency, it is interesting to know whether the results generalize to higher spatial frequencies that are more affected in amblyopia. We tested this idea in subject S2 and S3. For subject S2, we found that patching decreased the contrast threshold in the amblyopic eye without significantly changing that of the fellow eye; while for subject S3, contrast threshold in both eyes didn't significantly change (see Fig. S2 in supplementary material). These results, together with the change of binocular perceived phase, suggest that patching effect is intrinsically binocular in nature. However, one should note that, the magnitude of the change in monocular contrast threshold tends not to mirror the change in suprathreshold binocular phase combination.
Figure 2. Contrast detection thresholds for a 0.3 cycle/° grating for two amblyopic observers before and after patching of the amblyopic eye.
(a) The time line of the patching and testing protocol. (b) Pre- and post-patching contrast detection thresholds for both the amblyopic and fellow eye. Patching temporarily improved amblyopic eye and diminished fellow eye contrast sensitivity. *, p < 0.05 (compared with the pre-patching baseline); Error bars represent standard errors.
Discussion
The rapid modulation of binocular function demonstrated here furthers our understanding of adult cortical plasticity in two ways. Firstly, it is clear that binocular mechanisms maintain significant plasticity in adulthood, even in patients whose early visual experience prevented normal development of binocular function. Secondly, the duration of intervention required to modulate binocular function, a matter of minutes, strongly implicates changes to the excitatory/inhibitory balance within binocular regions of the visual cortex as the mechanism for plasticity rather than the longer-term establishment of new synaptic connections. The difference between previous reflections of plasticity using perceptual learning that operating on a relatively long time scale and the current reflection of plasticity that operates over a short time scale is not simply one of duration. The former plasticity is initiated by repetitive sensory stimulation and is understood to occur along Hebbian lines presumably involving synaptic long-term establishment of new synaptic connections25. The short-term plasticity reported here occurs from a short period where there is a lack of sensory stimulation. In this way, it is reminiscent of the rapid changes in plasticity that have recently been reported in amblyopic cats as a result of a 10 day period of dark rearing26, both studies involve relatively rapid changes in plasticity associated with a lack of sensory stimulation, the Duffy et al study26 involves both eyes whereas in the present study it only involves one eye. This short-term plasticity may occur from a re-balancing of excitatory/inhibitory signals thought to regulate plasticity changes in cortex27. This may involve a reciprocal change in contrast gain control for the two eyes as a result of disinhibition.
One technical concern is whether amblyopes can successfully perform a phase combination task. Many amblyopes have problem in performing phase task when the input is at medium to high spatial frequencies and low contrasts28,29. However, for low spatial frequencies and high contrasts, they are quite normal28,30. In the binocular phase combination test used here, the grating in the amblyopic eye is of 0.3 cycle/° and 100% contrast. For such suprathreshold stimuli, previous studies have shown that the phase perception is essentially normal in the amblyopic eye31,32. A recent study has demonstrated that amblyopes can still perform the phase task at least up to 2.72 cycle/°33. One simple way to check the ability of phase perception in the amblyopic eye is to measure performance on the phase task just involving the amblyopic eye (i.e. the direct phase judgment test when the fellow eye only sees the background). Theoretically speaking, the perceived phase in this condition should be closed to 22.5° (i.e., the phase of the grating in the amblyopic eye) if the phase perception in the amblyopic eye is normal31. We have now added these results of all the eight amblyopes in Fig. S3 in supplementary. Indeed, the results show it is close to 22.5°, suggesting normal phase performance of the amblyopic eye.
Interestingly, the patching-induced changes in binocular balance occurred in all of our amblyopic observers, no matter whether they had anisometropic, strabismic or mixed amblyopia. The effect was also not related with the presence of stereoscopic function. This indicates that the short-term plasticity reported here is not specific to one particular category of amblyopia and is a general phenomenon associated with the amblyopic visual system. Previous studies on the perceptual learning-induced plasticity also showed that the learning effect was not different between strabismic and anisometropic amblyopes7. Our results, together with these previous studies, suggest that the brain's potential for change exists for all types of amblyopia. It is not surprising that the patching-induced changes we report here do not depend on amblyopes having residual stereo function because there is evidence34,35,36,37 that there is binocular combination, albeit suppressed, in the majority of amblyopes, even those with no measurably stereopsis. Another interesting finding is that the integrated effect is different for normal and amblyopic visual systems, a finding that has also been noted by those who study monocular perceptual learning where more benefits in monocular visual functions were obtained in adult amblyopes than in normal adults3,7,38. For short-term sensory deprivation the binocular plasticity changes in our adult amblyopic group are larger than those in our normal control group, possibly suggesting a larger degree of binocular plasticity for amblyopic visual system.
Occlusion of the amblyopic eye (termed inverse occlusion) is not a new concept in the clinical management of amblyopia. It was first introduced by Bangerter (1953)39 as part of the Pleoptic treatment of amblyopia and although its use was supported in a number of studies40,41,42,43,44,45 the more conventional form of occlusion involving occluding the fellow sighted eye, was found to be more successful44,46,47,48,49,50,51. As a result inverse occlusion has been largely dropped from clinical practice whereas occlusion of the fellow sighted eye (conventional occlusion) has become popular. However, all of these previous studies of inverse occlusion focused exclusively on the monocular function (visual acuity and eccentricity of fixation) of the amblyopic eye and there was no assessment of how inverse occlusion affected binocular function which is the focus of the present investigation. More recently, the binocular status of amblyopia has been better appreciated and found to provide a beneficial approach to therapy. For example, dichoptic training is used to increase the participation of the amblyopic eye to binocular viewing52. This dichoptic approach relies on the common notion of plasticity from enhanced sensory stimulation. However, the current findings which rely on reduced sensory stimulation suggest a new type of plasticity that is both rapid and binocular. The benefits we show for binocular function are very robust but it should be noted that they are not accompanied by consistent changes in monocular contrast sensitivity in all cases, at least over the time scale of our experiments. It could be that the monocular effects, unlike the binocular effects, take more time to develop. The fact that such short-term patching induced binocular plasticity can occur in adults with amblyopia has potential clinical consequences as such an “inverse” patching procedure could provide a promising new approach to re-balancing binocular function in amblyopes thereby reducing the suppressive drive from the fellow eye that underpins the reduced acuity and/or stereopsis experienced by these patients8,9,53. Such an approach could be used alone or in conjunction with dichoptic treatment that aims to reduce suppression by strengthening fusion13,14,15,16,17. It is likely that compliance with patching of the amblyopic eye will be high as it will not interfere with viewing through the fellow eye. These results also suggest that the current treatment approach of patching the fellow sighted eye that has been in operation for the past 300 years, may, in fact, be strengthening rather than reducing the binocular imbalance that characterizes amblyopia.
Methods
Participants
Eight adult amblyopes (mean age: 30.2 ± 10.2 years old; 1 female) were recruited for the main patching experiment. Three of the amblyopic observers also participated in additional measures to assess the effect of amblyopic eye patching on monocular contrast sensitivity. Five normal adults (include the first author, mean age: 30.4 ± 4.9 years old; 2 females) were recruited for the patching experiment as control group1; the first author and three new normal adults (mean age: 26.2 ± 1.2 years old; 2 females) were recruited for the patching experiment as control group2.
This study complied with the Declaration of Helsinki and was approved by the institutional ethics committee of McGill University. With the exception of the first author, all observers were naive to the purpose of the experiment and all observers gave written informed consent. Clinical details of the eight amblyopes are provided in Table S1 in Supplementary online.
Apparatus
Stimuli were generated by a Mac computer using Matlab and PsychToolBox 3.0.9 extensions and dichoptic presentation was achieved using a head mounted display with a separate screen for each eye (eMagin Z800 pro, OLED). The refresh rate of the HMD goggles was 60 Hz with a resolution of 800 × 600, and a mean luminance of 190 cd/m2. Monocular contrast response functions were measured using PsyKinematix software which allows for 10.8 bits of contrast resolution via bitstealing algortihms, and the stimuli were presented on a calibrated Mitsubishi Diamond Pro 2070SB monitor using a Mac computer. More details as to the testing protocol can be obtained on ‘http://psykinematix.kybervision.net/'.
Design
The experiment consisted of three consecutive stages: a pre-patching measurement of binocular balance, a patching stage (2.5 hours) and a post-patching measurement of binocular balance. A transparent patch (transmitting light but not pattern) was used to occlude the amblyopic eye for amblyopes, the randomly-selected eye for observers in control group1 and the undominant eye for observers in control group2 during the patching stage.
The effect of patching on binocular sensory balance was quantified using a binocular phase combination task31,32,54. Subjects' binocular sensory balance was measured at 0′ (T1), 9′ (T2) and 30 minutes (T3) after the patch was removed. Three more measurements were made at 40′ (T4), 50′ (T5) and 60′ (T6) after the removal of the patch for amblyopic observers and observers in control group2. For all measurements the contrast of the stimulus in the patched eye was fixed as 100% for amblyopic observers and observers in control group2; the contrast of the stimulus in the unpatched eye was fixed at 64% for observers in control group1. The contrast of the stimulus presented to the other eye was chosen to ensure that both eyes made an approximately equal combination to binocular combination before the patching. These values were based on a series of phase combination measurements made prior to the initiation of the main experiment. Demos of the task and practice trials were provided prior to data collection. Before the measure, subjects also completed a line alignment task to ensure that stimuli presented dichoptically were fused.
Monocular contrast response functions at 0.3 cycle/° (the spatial frequency used in the phase combination task) were measured for both the amblyopic eye (i.e, the patched eye) and fellow eye (i.e., the unpatched eye) in 2 amblyopic subjects, S1 and S2 (on different days to the phase combination measurements). Performance at 5 cycle/° and 3 cycle/° were also measured for subject S2 and S3, respectively.
Procedure
The contributions of each eye to the binocular percept were assessed using a dichoptic phase discrimination paradigm31,32,54 where subjects viewed a periodic 1-D stimulus that was presented with an equal but opposite spatial phase (±ϕ°) in each eye. The binocular percept in this paradigm depends on the internal representations of the two inputs. If each eye contributed equally to binocular vision, the binocular percept will be of a stimulus of 0° phase. Any variance of the binocular balance at one specified interocular contrast ratio can be quantified by the change in the perceived phase. A schematic of this paradigm is provided in Figure 1a. See Supplementary online for more detail.
Author Contributions
J.Z. contributed to the experimental design and carried out the data collection. B.T. contributed to the experimental design and interpretation of the results. R.F.H. helped design the experiment, contributed to the interpretation and wrote the manuscript. No other person was involved in the study.
Supplementary Material
Supplementary
Acknowledgments
We thank Simon Clavagnier and Rong Liu for participating in part of the experiments. This research was supported by the CIHR (# MOP53346) to RFH.
References
- Scheiman M. M. et al. Randomized trial of treatment of amblyopia in children aged 7 to 17 years. Arch Ophthalmol 123, 437–447 (2005). [DOI] [PubMed] [Google Scholar]
- Hubel D. H. & Wiesel T. N. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. The Journal of Physiology 206, 419–436 (1970). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. B., Zhou Y. & Lu Z. L. Broad bandwidth of perceptual learning in the visual system of adults with anisometropic amblyopia. Proc Natl Acad Sci U S A 105, 4068–4073 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y. et al. Perceptual learning improves contrast sensitivity and visual acuity in adults with anisometropic amblyopia. Vision Res 46, 739–750 (2006). [DOI] [PubMed] [Google Scholar]
- Levi D. M. & Li R. W. Perceptual learning as a potential treatment for amblyopia: a mini-review. Vision Res 49, 2535–2549 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R. W., Ngo C., Nguyen J. & Levi D. M. Video-game play induces plasticity in the visual system of adults with amblyopia. PLoS Biology 9, e1001135 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polat U., Ma-Naim T., Belkin M. & Sagi D. Improving vision in adult amblyopia by perceptual learning. Proc Natl Acad Sci U S A 101, 6692–6697 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi H. et al. Neuronal responses in visual area V2 (V2) of macaque monkeys with strabismic amblyopia. Cereb Cortex 21, 2033–2045 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. et al. The role of suppression in amblyopia. Invest Ophthalmol Vis Sci 52, 4169–4176 (2011). [DOI] [PubMed] [Google Scholar]
- Sengpiel F. & Blakemore C. The neural basis of suppression and amblyopia in strabismus. Eye 10, 250–258 (1996). [DOI] [PubMed] [Google Scholar]
- Sengpiel F., Jirmann K. U., Vorobyov V. & Eysel U. T. Strabismic suppression is mediated by inhibitory interactions in the primary visual cortex. Cereb Cortex 16, 1750–1758 (2006). [DOI] [PubMed] [Google Scholar]
- Sireteanu R., Fronius M. & Singer W. Binocular interaction in the peripheral visual field of humans with strabismic and anisometropic amblyopia. Vision Res 21, 1065–1074 (1981). [DOI] [PubMed] [Google Scholar]
- Hess R. F., Mansouri B. & Thompson B. A new binocular approach to the treatment of Amblyopia in adults well beyond the critical period of visual development. Restor Neurol Neuros 28, 793–802 (2010). [DOI] [PubMed] [Google Scholar]
- Hess R. F., Mansouri B. & Thompson B. A binocular approach to treating amblyopia: Anti-suppression therapy. Optom Vis Sci 87, 697–704 (2010). [DOI] [PubMed] [Google Scholar]
- Hess R. F., Mansouri B. & Thompson B. Restoration of binocular vision in amblyopia. Strabismus 19, 110–118 (2011). [DOI] [PubMed] [Google Scholar]
- Knox P. J., Simmers A. J., Gray L. S. & Cleary M. An exploratory study: prolonged periods of binocular stimulation can provide an effective treatment for childhood amblyopia. Invest Ophthalmol Vis Sci 53, 817–824 (2012). [DOI] [PubMed] [Google Scholar]
- To L. et al. A game platform for treatment of amblyopia. IEEE Transactions on Neural Systems and Rehabilitation Engineering 19, 280–289 (2011). [DOI] [PubMed] [Google Scholar]
- Tsodyks M. & Gilbert C. Neural networks and perceptual learning. Nature 431, 775–781 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotsumoto Y., Watanabe T. & Sasaki Y. Different dynamics of performance and brain activation in the time course of perceptual learning. Neuron 57, 827–833 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. D., Sigman M. & Crist R. E. The neural basis of perceptual learning. Neuron 31, 681–697 (2001). [DOI] [PubMed] [Google Scholar]
- Vogels T. P., Sprekeler H., Zenke F., Clopath C. & Gerstner W. Inhibitory plasticity balances excitation and inhibition in sensory pathways and memory networks. Science 334, 1569–1573 (2011). [DOI] [PubMed] [Google Scholar]
- Froemke R. C., Merzenich M. M. & Schreiner C. E. A synaptic memory trace for cortical receptive field plasticity. Nature 450, 425–429 (2007). [DOI] [PubMed] [Google Scholar]
- Lunghi C., Burr D. C. & Morrone C. Brief periods of monocular deprivation disrupt ocular balance in human adult visual cortex. Curr Biol 21, R538–539 (2011). [DOI] [PubMed] [Google Scholar]
- Zhou J., Clavagnier S. & Hess R. F. Short-term monocular deprivation strengthens the patched eye's contribution to binocular combination. J Vis 13, 12, 1–10 (2013). [DOI] [PubMed] [Google Scholar]
- Seitz A. R. & Dinse H. R. A common framework for perceptual learning. Current opinion in neurobiology 17, 148–153 (2007). [DOI] [PubMed] [Google Scholar]
- Duffy Kevin R. & Mitchell Donald E. Darkness Alters Maturation of Visual Cortex and Promotes Fast Recovery from Monocular Deprivation. Curr Biol 23, 382–386 (2013). [DOI] [PubMed] [Google Scholar]
- Hensch T. K. & Fagiolini M. Excitatory-inhibitory balance and critical period plasticity in developing visual cortex. Progress in brain research 147, 115–124 (2005). [DOI] [PubMed] [Google Scholar]
- Lawden M. C., Hess R. F. & Campbell F. W. The discriminability of spatial phase relationships in amblyopia. Vision Res 22, 1005–1016 (1982). [DOI] [PubMed] [Google Scholar]
- Pass A. F. & Levi D. M. Spatial processing of complex stimuli in the amblyopic visual system. Invest Ophthalmol Vis Sci 23, 780–786 (1982). [PubMed] [Google Scholar]
- Mac Cana F., Cuthbert A. & Lovegrove W. Contrast and phase processing in amblyopia. Vision Res 26, 781–789 (1986). [DOI] [PubMed] [Google Scholar]
- Huang C. B., Zhou J. W., Lu Z. L., Feng L. X. & Zhou Y. F. Binocular combination in anisometropic amblyopia. J Vis 9, 17, 1–16 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. B., Zhou J. W., Lu Z. L. & Zhou Y. F. Deficient binocular combination reveals mechanisms of anisometropic amblyopia: Signal attenuation and interocular inhibition. J Vis 11, 4, 1–17 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J., Klein S. A. & Levi D. M. Binocular combination in abnormal binocular vision. J Vis 13, 1–14 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri B., Thompson B. & Hess R. Measurement of suprathreshold binocular interactions in amblyopia. Vision Res 48, 2775–2784 (2008). [DOI] [PubMed] [Google Scholar]
- Baker D. H., Meese T. S., Mansouri B. & Hess R. F. Binocular summation of contrast remains intact in strabismic amblyopia. Invest Ophthalmol Vis Sci 48, 5332–5338 (2007). [DOI] [PubMed] [Google Scholar]
- Zhou J., Huang P.-C. & Hess R. F. Interocular suppression in amblyopia for global orientation processing. J Vis 13, 19, 1–14 (2013). [DOI] [PubMed] [Google Scholar]
- Baker D. H., Meese T. S. & Hess R. F. Contrast masking in strabismic amblyopia: Attenuation, noise, interocular suppression and binocular summation. Vision Res 48, 1625–1640 (2008). [DOI] [PubMed] [Google Scholar]
- Astle A. T., Webb B. S. & McGraw P. V. The pattern of learned visual improvements in adult amblyopia. Invest Ophthalmol Vis Sci 52, 7195–7204 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangerter A. Aus der Praxis, für die Praxis. Ophthalmologica 125, 398–405 (1953). [DOI] [PubMed] [Google Scholar]
- Andree G. Der Einfluss der inversen Occlusion auf Fixation und Function amblyoper Augen. Albrecht v Graefes Arch Ophthal 170, 257–264 (1966). [DOI] [PubMed] [Google Scholar]
- Arruga A. Effect of occlusion of amblyopic eye on amblyopia and eccentric fixation. Trans Ophthalmol Soc U K 82, 45 (1962). [Google Scholar]
- Cibis L. & Windsor C. Clinical Results With Passive Amblyopia Treatment. Amer Orthopt J 17, 56–61 (1967). [PubMed] [Google Scholar]
- Malik J., Gupta A. K. & Sen D. K. Red filter treatment in eccentric fixation. Brit J Ophthal 52, 839–842 (1968). [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Noorden G. K. Occlusion therapy in amblyopia with eccentric fixation. Arch Ophthal. 73, 776–781 (1965). [DOI] [PubMed] [Google Scholar]
- Wybar K. & Thatacher B. Significance of eccentric fixation in squint. British J Ophthal. 44, 472–491 (1960). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catford G. V. Amblyopic occlusion: the results of treatment. Trans Ophthal Soc UK 87, 179–193 (1967). [PubMed] [Google Scholar]
- Little J. G. & Ogilvie M. Amblyopia: results with conventional therapy. Trans Canadian Ophthal Soc 26, 240–248 (1963). [PubMed] [Google Scholar]
- Mackensen G., Kroner B. & Postic G. Zur Anderung der exzentrischen Fixation unter der Okklusionsbehandlung. Klin Mbl Augenheilk 147, 213–230 (1965). [PubMed] [Google Scholar]
- Malik S. R. K., Gupta A. K. & Grover V. K. Occlusion therapy in amblyopia with eccentric fixation; Comparison of conventional, non-conventional (inverse0, and red-filter occlusion. Brit. J Ophthal. 54, 41–45 (1970). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully J. Early intensive occlusion in strabismus with eccentric fixation. Brit Med J 2, 1610–1612 (1961). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg A. R. & Bohar A. A study on the occlusion therapy of eccentric fixation. Ophthalmologica (Basel) 141, 229–342 (1961). [Google Scholar]
- Li J. et al. Dichoptic training enables the adult amblyopic brain to learn. Curr Biol 23, R308–309 (2013). [DOI] [PubMed] [Google Scholar]
- Narasimhan S., Harrison E. R. & Giaschi D. E. Quantitative measurement of interocular suppression in children with amblyopia. Vision Res 66, 1–10 (2012). [DOI] [PubMed] [Google Scholar]
- Ding J. & Sperling G. A gain-control theory of binocular combination. Proc Natl Acad Sci U S A 103, 1141–1146 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary